Abstract
Background and purpose
The aim was to assess the safety and efficacy of nusinersen in adult 5q spinal muscular atrophy (SMA) patients.
Methods
Patients older than 15 years and followed for at least 6 months with one motor scale (Hammersmith Functional Motor Scale Expanded, HFMSE; Revised Upper Limb Module, RULM) in five referral centers were included. The clinical and patients' global impression of change (CGI‐C and PGI‐C) were recorded in treated patients at the last visit. Functional scales (Egen Klassification, EK2; Revised Amyotrophic Lateral Sclerosis Functional Rating Scale, ALSFRS‐R) and the percentage predicted forced vital capacity were collected when available.
Results
Seventy‐nine SMA patients (39 treated with nusinersen) were included. Compared with untreated patients, treated patients showed a significant improvement of 2 points (±0.46) in RULM (p < 0.001) after 6 months. After a mean follow‐up of 16 months, nusinersen treatment was associated with a significant improvement in HFMSE (odds ratio [OR] 1.15, p = 0.006), the 6‐min walk test (OR = 1.07, p < 0.001) and the EK2 (OR = 0.81, p = 0.001). Compared with untreated patients, more treated patients experienced clinically meaningful improvements in all scales, but these differences were statistically significant only for RULM (p = 0.033), ALSFRS‐R (p = 0.005) and EK2 (p < 0.001). According to the CGI‐C and PGI‐C, 64.1% and 61.5% of treated patients improved with treatment. Being a non‐sitter was associated with less response to treatment, whilst a longer time of treatment was associated with better response. Most treated patients (77%) presented at least one adverse event, mostly mild.
Conclusions
Nusinersen treatment is associated with some improvements in adult SMA patients. Most severely affected patients with complex spines are probably those with the most unfavorable risk–benefit ratio.
Keywords: adults, cohort study, nusinersen, spinal muscular atrophy, treatment
INTRODUCTION
5q spinal muscular atrophy (SMA) is a genetic neurodegenerative disease caused by a homozygous deletion or mutation in the survival motor neuron 1 (SMN1) gene, affecting the lower motor neurons. This results in progressive tetraparesis, affecting first the lower limbs and later the upper limbs, followed by respiratory insufficiency, dysarthria and dysphagia [1, 2]. According to the age of symptom onset and to the highest acquired motor milestone, SMA children are typically classified as type 1–3. SMA type 1 patients will never be able to sit unsupported, whilst SMA type 2 patients will never be able to walk independently [1, 2]. SMA types, and therefore the disease severity, are largely explained by the number of SMN2 gene copies, which are also capable of producing a small amount of SMN protein [3]. Thus, whilst SMA type 1 patients will usually die during childhood, most type 2 and 3 patients will reach adulthood with a variable degree of disability [4]. The rare type 4 patients typically start after 30 years old and will not present any noteworthy disability [1]. Due to disease progression, the SMA type, defined in infancy, does not reliably inform about functionality in adulthood. Therefore, adult SMA patients are functionally classified as non‐sitters, sitters and walkers [2].
Nusinersen, an antisense oligonucleotide, was approved for the treatment of SMA after having been shown to improve survival and motor function in infants and children in two randomized placebo‐controlled clinical trials [5, 6]. Conversely, in the adolescent and adult population, the evidence is based on real‐world studies suggesting that nusinersen improves motor scales compared with historical cohorts [7]. However, fewer studies have focused on functional and patients’ reported outcome (PRO) data on nusinersen efficacy [8, 9], despite its importance for regulatory agencies. Considering the high frequency of adverse events (AEs) associated with repeated lumbar punctures and the high costs of treatment, it is of utmost importance to add real‐world evidence of nusinersen potential risks and benefits in the adult population. Therefore, the objective of this study was to report the safety as well as motor and functional outcomes in a multicenter Spanish cohort of treated and non‐treated adult SMA patients.
METHODS
Study design and participants
Nusinersen was approved in Spain for the treatment of SMA patients in March 2018, with some restrictions posed by a protocol of the Spanish health department [10]. Briefly, very severe (defined as Egen Klassification [EK2] > 47 or requiring non‐invasive ventilation [NIV] for more than 16 h a day) or mild (type 3 patients with Hammersmith Functional Motor Scale Expanded (HFMSE) > 54 or type 4) SMA patients were usually excluded from treatment.
For this prospective observational study, SMA patients from five centers in Spain were included (Hospital la Fe, Hospital Sant Joan de Deu, Hospital de Bellvitge, Hospital Virgen de la Arrixaca, Hospital de Basurto). The inclusion criteria were (i) genetically confirmed SMA (either homozygous deletion or compound heterozygous mutation in SMN1); (ii) older than 15 years at the baseline visit; (iii) longitudinal data on at least one motor scale at the time of the study closure (August 2020). Patients meeting the criteria established by the health department were routinely offered nusinersen treatment. The final decision to start the treatment was made by the patient after discussion with the neurologist of pros and cons. After the protocol approval, prospective data of treated and untreated patients were collected at baseline, 6 months later and every 6–12 months later on. When available, retrospective data of untreated patients were also collected from October 2015.
Procedures
Treated patients were injected with the 12 mg loading doses of nusinersen (at days 0, 14, 28 and 65) and maintenance doses every 4 months, as per label. Conventional and imaging‐guided [11] (including ultrasound, fluoroscopy and computed tomography) lumbar punctures were performed by experienced neurologists and neuroradiologists, respectively. All treated patients received at least the loading doses of nusinersen, except one patient [11] who was discontinued after the second dose of nusinersen due to the lack of lumbar access and was excluded from efficacy analysis.
All patients received the same multidisciplinary care in their respective centers, regardless of whether they were treated or not.
Clinical variables and outcomes
Age, gender and age at symptom onset, as well as the presence of severe scoliosis (>45° Cobb angle) and/or scoliosis surgery were recorded in all the patients upon recruitment. Patients were classified as type 1 to 4 as defined elsewhere [1], as well as in functional subgroups [2]: walkers (able to walk at least five steps without assistance), sitters (able to sit without assistance nor head support for more than 10 s) and non‐sitters. The use of NIV, gastrostomy and salbutamol was also recorded at baseline in all patients.
Motor and functional scales were administered by experienced and/or trained neurologists and physiotherapists. All efforts were made to keep the same evaluator for every patient throughout the study. All centers collected motor scales and pulmonary tests, but functional scales were missing in some centers. Moreover, not all scales are applicable to all patients (see below). Consequently, the number and characteristics of SMA patients varies for each scale.
The HFMSE consists of 33 items, with a maximum of 66 points (higher scores indicating better function), and it is designed for the assessment of sitters and walkers [17]. Based on natural history data and patient interviews, a score change of more than 2 points is considered to be clinically meaningful [12].
The Revised Upper Limb Module (RULM) includes 20 items with a maximum score of 37 (higher scores indicating better function) [13]. It has been validated in both ambulant and non‐ambulant patients, and a score change of 2 points or more is usually considered to be clinically meaningful [14].
The 6‐min walk test (6MWT) measures the distance a patient is able to walk within 6 min, and it is therefore only applicable to walkers. Based on previous clinical trial data in Duchenne patients, a change of 30 m or more was considered to be clinically meaningful [15].
The EK2 is a functional scale that includes 17 items in eight daily‐life categories (wheelchair use, wheelchair transfers, trunk mobility, eating, swallowing, breathing, coughing, fatigue). Each item is scored from 0 to 3 for a maximum of 51 points (higher scores indicating worse function). It has been designed for and validated in a non‐ambulant SMA population [16, 17].
The Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS‐R) is a functional scale that includes 12 items on four domains (bulbar, upper limbs, lower limbs, respiratory). Each item is scored from 0 to 4 for a maximum of 48 points (higher scores indicating better function). It was designed for amyotrophic lateral sclerosis patients but it has also been used and validated in adult SMA patients [16, 18].
According to their specific validity, the 6MWT was assessed in walkers, the HFMSE in walkers and sitters and the EK2 in sitters and non‐sitters. The RULM, ALSFRS‐R and the percentage predicted forced vital capacity (FVC%) were assessed in all subgroups of patients.
Furthermore, the clinical and the patients' global impression of change (CGI‐C and PGI‐C) were obtained in all treated patients at the last visit. For the CGI‐C, neurologists were asked to respond to the following question about each patient: “compared to his/her condition right before treatment, how much has the patient changed?” For the PGI‐C, patients were asked to respond to the following question: “compared to your condition before treatment, how are you doing overall?” Responses were collected in a semi‐quantitative manner from very much worse (−3) to very much improved (+3), with 0 being no change.
The primary end‐points were HFMSE and RULM, since they were mandatory in the national protocol [10]. All other measures were considered secondary end‐points.
To assess safety, the following items were recorded systematically at each visit: patient‐reported AEs, categorized by severity and relationship to treatment according to MedDRA (version 21.1); the start of NIV or placement of gastrostomy; abnormal routine laboratory findings.
Statistical analysis
Data were summarized as means, standard deviations, medians, and first and third quartiles for the continuous variables, and as relative and absolute frequencies for the categorical variables.
Two time points were chosen for all patients to assess the short‐ and long‐term response to nusinersen: at 6 months and at the last visit available. Rank‐based regression models were used to analyze the effect of treatment on the visit scores at 6 months. For these models, the baseline scores and the treatment with nusinersen were included as predictive variables. To analyze the effect of treatment on the visit scores at the last visit, mixed ordinal regression models were used. Since the last visit comprises different time intervals in each patient and the effect of treatment is expected to increase with time [14, 19], both the follow‐up time (in months) and the interaction between time and treatment were included as predictive variables. Convergence problems appeared in the fitted ordinal regression models of two scales (ALSFRS‐R and RULM), due to our limited sample size. Bayesian modeling adjustment with a weakly informative prior (N[0, 3]) was used in those cases. For each model, only the estimate of the effect of treatment is shown (Tables 2 and 3).
TABLE 2.
Raw score differences between baseline and 6‐month visits in treated and untreated patients and the estimated effect of nusinersen according to the multivariable model, after adjusting for baseline values
| Test | Raw scores | Estimate | SE | p | |
|---|---|---|---|---|---|
| Untreated | Treated | ||||
| HFMSE (n = 44) | 0.16 (1.83) | 2.43 (4.52) | 2 | 1.12 | .082 |
| RULM (n = 71) | −0.58 (2.27) | 1.67 (3.28) | 2 | 0.46 | <.001 * |
| 6MWT (n = 17) | 19.94 (70.03) | 23.22 (62.75) | −6.27 | 46.12 | .894 |
| FVC% (n = 40) | −1.09 (5.65) | 2.6 (8.29) | 3.19 | 2.11 | .139 |
| ALSFRS‐R (n = 42) | −0.08 (1.24) | 0.77 (1.59) | 3.42 | 3.03 | .999 |
| EK2 (n = 30) | 1.07 (2.83) | −2.72 (2.74) | −4 | 3.19 | .221 |
Note: In bold, statistically significant results.
Abbreviations: ALSFRS‐R, Amyotrophic Lateral Sclerosis Functional Scale Revised; EK2, Egen Klassifikation 2; FVC%, percentage predicted forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; 6MWT, 6‐min walk test.
p < 0.001.
TABLE 3.
Effect of the interaction “treatment and follow‐up time” in the different outcomes at the last visit available for each scale
| OR | Lower 95 | Upper 95 | p | |
|---|---|---|---|---|
| HFMSE (n = 50) | 1.15 | 1.041 | 1.271 | 0.006 |
| RULM (n = 75) | 1.022 | 0.961 | 1.091 | – |
| 6MWT (n = 22) | 1.071 | 1.065 | 1.078 | <0.001 * |
| FVC% (n = 48) | 1.002 | 0.9 | 1.116 | 0.975 |
| ALSFRS‐R (n = 5) | 1.036 | 0.94 | 1.144 | – |
| EK2 (n = 38) | 0.809 | 0.712 | 0.92 | 0.001 |
Note: In bold, statistically significant results. p values are lacking in variables calculated with Bayesian models.
Abbreviations: ALSFRS‐R, Amyotrophic Lateral Sclerosis Functional Scale Revised; EK2, Egen Klassifikation 2; FVC, percentage predicted forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; 6MWT, 6‐min walk test.
p < 0.001.
For the calculation of the responders' rate, several definitions of responder were used. First, the percentage of treated and untreated patients who improved by at least the minimal clinically important difference (MCID) established for each scale was calculated. For the EK2 and ALSFRS‐R scales a change ≥2 points was considered as clinically meaningful, based on the investigators' experience. Chi‐squared tests were used to assess the differences in responder rates as defined above. Secondly, the percentage of treated patients who experienced at least mild improvements (1 point) according to the CGI‐C and the PGI‐C was measured.
The concordance between CGI‐C and PGI‐C was also assessed using the Bangdiwala's observer agreement chart for ordinal variables [20]. A weight of 1 was settled on for complete agreement and a weight of 0.5 for partial agreement, defined as a difference of 1 point between CGI‐C and PGI‐C. Differences between scores of >1 point were considered as disagreement. The agreement was quantified as moderate when B = 0.50–0.69, strong when rs = 0.70–0.89 and very strong when rs = 0.90–1.00.
Finally, an ordinal multivariable model was used to assess those variables predicting improvement according to the CGI‐C.
All analyses were pre‐specified before looking at the data. p values <0.05 were considered statistically significant. All the statistical analyses and graphs were performed with R software (version 4.0.3).
RESULTS
Population characteristics
The study included 79 SMA patients (39 treated with nusinersen). Their demographic and clinical characteristics are summarized in Table 1. Treated patients were somewhat older (33 vs. 30 years old) and more frequently male (51% vs. 42%) and type 3 (74% vs. 42%). Untreated patients were more frequently non‐sitter (50% vs. 26%) and NIV users (38% vs. 23%), despite a shorter disease duration (25 vs. 29 years). Both subgroups had a similar rate of concomitant salbutamol treatment.
TABLE 1.
Demographic and baseline clinical characteristics of SMA patients included in the study
| Variable | Non‐treated (n = 40) | Treated (n = 39) | ||
|---|---|---|---|---|
| Age (years) | Mean (SD) | 30.34 (14.05) | 33.35 (13.35) | |
| Median (1st, 3rd quartile) | 26.98 (18.59, 38.17) | 31.42 (21.85, 44.03) | ||
| Male sex | N (%) | 17 (42.5%) | 20 (51.28%) | |
| SMA type | 2a | N (%) | 14 (35%) | 8 (20.51%) |
| 2b | 6 (15%) | 2 (5.13%) | ||
| 3a | 8 (20%) | 15 (38.46%) | ||
| 3b | 9 (22.5%) | 14 (35.9%) | ||
| 4 | 3 (7.5%) | 0 (0%) | ||
| SMN2 copies | 1 | N (%) | 1 (2.5%) | 0 (0%) |
| 2 | 2 (5%) | 5 (12.82%) | ||
| 3 | 27 (67.5%) | 23 (58.97%) | ||
| 4 | 10 (25%) | 11 (28.21%) | ||
| Disease duration (years) | Mean (SD) | 24.97 (12.25) | 28.84 (13.53) | |
| Median (1st, 3rd quartile) | 22.82 (16.85, 34.3) | 27.8 (17.94, 38.83) | ||
| Functional status | N (%) | |||
| Non‐sitter | 20 (50%) | 10 (25.64%) | ||
| Sitter | 9 (22.5%) | 16 (41.03%) | ||
| Walker | 11 (27.5%) | 13 (33.33%) | ||
| NIV use | N (%) | |||
| No | 24 (61.54%) | 30 (76.92%) | ||
| 8 h | 14 (35.9%) | 9 (23.08%) | ||
| 24 h | 1 (2.56%) | 0 (0%) | ||
| Gastrostomy | N (%) | 1 (2.5%) | 0 (0%) | |
| Severe scoliosis | N (%) | 27 (67.5%) | 20 (51.28%) | |
| Salbutamol | N (%) | 22 (55%) | 19 (48.72%) | |
| HFMSE (n = 50) | Mean (SD) | 29.95 (25.51) | 25.9 (20.11) | |
| Median (1st, 3rd quartile) | 25 (4, 57) | 21 (7, 47) | ||
| RULM (n = 75) | Mean (SD) | 18.29 (14.35) | 20.64 (10.97) | |
| Median (1st, 3rd quartile) | 14.25 (5, 35.75) | 20.75 (12, 29.38) | ||
| 6MWT (n = 22) | Mean (SD) | 432.44 (127.61) | 269.75 (123.41) | |
| Median (1st, 3rd quartle) | 460.5 (390.25, 498.75) | 280.5 (179.75, 368.38) | ||
| FVC% (n = 48) | Mean (SD) | 59.58 (39.33) | 72.86 (37.73) | |
| Median (1st, 3rd quartile) | 44.5 (28.9, 87.75) | 76.5 (39.5, 104.75) | ||
| ALSFRS‐R (n = 52) | Mean (SD) | 26.62 (11.09) | 31.38 (8.36) | |
| Median (1st, 3rd quartile) | 29 (20, 31) | 32 (25, 38.5) | ||
| EK2 (n = 38) | Mean (SD) | 23.23 (9.47) | 14.8 (9.17) | |
| Median (1st, 3rd quartile) | 22 (18, 28.75) | 9 (8.25, 23) | ||
| Follow‐up (months) | Mean (SD) | 15.8 (9.55) | 16.06 (5.74) | |
| Median (1st, 3rd quartile) | 14.47 (11.2, 17.98) | 15.37 (11.55, 22.33) | ||
| Number of visits | Mean (SD) | 2.6 (1.13) | 3.21 (1.34) | |
| Median (1st, 3rd quartile) | 2 (2, 3) | 3 (2, 4) | ||
Notes: SMA type 2a: those who were able to sit unsupported, but not to stand or walk with help. SMA type 2b: those who were able to stand or walk with help. SMA type 3a: those able to walk without help, in whom the disease started before 36 months of age. SMA type 3b: those able to walk without help, in whom the disease started after 36 months of age.
Abbreviations: 6MWT, 6‐min walk test; ALSFRS‐R, Amyotrophic Lateral Sclerosis Functional Scale Revised; EK2, Egen Klassifikation 2; FVC%, percentage predicted forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; NIV, non‐invasive ventilation; RULM, Revised Upper Limb Module; SMA, 5q spinal muscular atrophy.
Overall, better baseline scores were found in treated versus untreated patients (Table 1) except in the 6MWT (because none of the type 4 patients were treated) and in the HFMSE (because it was not assessed in non‐sitters). Treated patients received a mean of six doses of nusinersen and 45% of them required imaging‐guided lumbar puncture.
Treatment effect at 6 months
At 6 months, an improvement in treated patients was predominant in all scales and tests, whilst in untreated patients scores usually worsened or remained stable except for the 6MWT (Figure 1). Nusinersen treatment improved 2 points (±0.46) in RULM (p < 0.001) according to the model, after adjusting by baseline scores. Differences in other scales were not statistically significant (Table 2).
FIGURE 1.
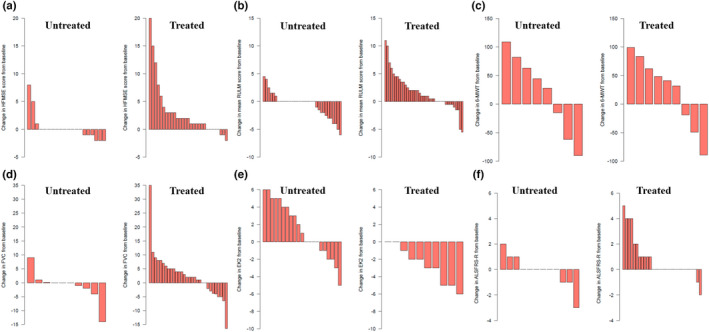
Individual changes in scores from baseline to T1 (6 months) in treated and untreated patients in the different tests: (a) HFMSE; (b) RULM; (c) 6MWT; (d) FVC%; (e) EK2; (f) ALSFRS‐R. ALSFRS‐R, Amyotrophic Lateral Sclerosis Functional Scale Revised; EK2, Egen Klassifikation 2; FVC%, percentage predicted forced vital capacity; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; 6MWT, 6‐min walk test [Colour figure can be viewed at wileyonlinelibrary.com]
Treatment effect at the last visit
Both treated and untreated patients were followed up for a mean of 16 months (Table 1), although more visits were performed for treated patients (3.21 vs. 2.6). At the last visit, after adjusting for the baseline values and follow‐up time, the effect of treatment was associated with a significant improvement in HFMSE (OR = 1.15, 95% confidence interval [CI] 1.04, 1.27, p = 0.006), 6MWT (OR = 1.07, 95% CI 1.06, 1.08, p < 0.001) and EK2 (OR = 0.81, 95% CI 0.71, 0.92, p = 0.001) and a non‐statistically significant improvement was found in all other scales (Table 3).
Responders and variables predicting response
According to the MCID of each scale a variable percentage of treated (25%–80%) and untreated (0%–57%) patients experienced clinically meaningful improvements at the last visit (Table 4). Clinically meaningful improvements were more frequent in treated patients in all scales, although these differences were statistically significant only for RULM, ALSFRS‐R and EK2 (Table 4).
TABLE 4.
Percentage of patients experiencing clinically meaningful impairments (as defined in Methods) on each scale at the last visit
| Treated | Untreated | p | |
|---|---|---|---|
| HFMSE (n = 44) | 25% | 9.5% | 0.3 |
| RULM (n = 72) | 50% | 22.9% | 0.033 |
| 6MWT (n = 17) | 75% | 57% | 0.85 |
| ALSFRS‐R (n = 42) | 25.7% | 0% | 0.005 |
| EK2 (n = 31) | 80% | 22.7% | <0.001 * |
Abbreviations: ALSFRS‐R, Amyotrophic Lateral Sclerosis Functional Scale Revised; EK2, Egen Klassifikation 2; HFMSE, Hammersmith Functional Motor Scale Expanded; RULM, Revised Upper Limb Module; 6MWT, 6‐min walk test.
p < 0.001.
According to the CGI‐C and PGI‐C, 64.1% and 61.5% of treated patients improved, whilst 0% and 2.5% of patients respectively deteriorated (Figure 2). There was a high agreement between CGI‐C and PGI‐C (unweighted agreement 0.6, weighted agreement 0.8). A CGI‐C of 3 (very much improved) was scored in two SMA type 3a patients. A sitter with four SMN2 copies, who was able to stand still with help but had lost her ability to walk some years before, improved 20 points in HFMSE, 10 points in RULM and was able to walk unaided 30 m in the 6MWT after 14 months of treatment. Another walker with three SMN2 copies, who had been deteriorating the year before treatment start and was close to losing ambulation, improved 24 points in HFMSE, 7 points in RULM and 183 m in 6MWT after 14 months of treatment (Figure S1).
FIGURE 2.
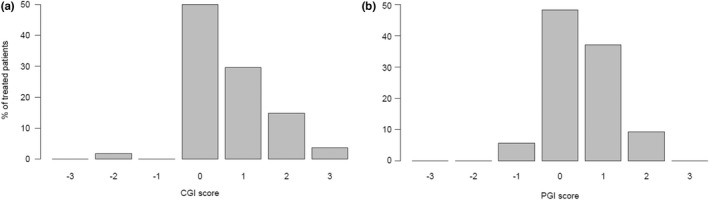
Graphical representation of the clinical global impression of change (CGI‐C) and the patients’ global impression (PGI‐C) scores
According to the multivariable model (Table 5), being a non‐sitter (compared with walker) was associated with less response to treatment, as assessed with the CGI‐C, whilst a longer time of treatment was associated with better response.
TABLE 5.
Multivariable model assessing the effect of several variables in the response to treatment, as defined per the clinical global impression of change scale
| Estimate | SE | p | |
|---|---|---|---|
| Age | −0.043 | 0.035 | .226 |
| Disease duration | 0.047 | 0.034 | .175 |
| SMN2 copy number | 0.183 | 0.369 | .623 |
| Sitter | −0.382 | 0.36 | .297 |
| Non‐sitter | −0.912 | 0.406 | .032 |
| Treatment duration | 0.054 | 0.024 | .035 |
Note: In bold, statistically significant results.
Adverse events
Thirty treated patients (77%) presented at least one AE during the follow‐up. Overall, 55 AEs were reported, mostly related with the administration procedure: 45 were mild (post lumbar puncture syndrome and back pain) and 10 were moderate (seven post lumbar puncture syndrome, two urinary retention due to neurogenic bladder, one radial neurapraxia). Two patients (5%) discontinued treatment due to AEs (repeated post lumbar puncture syndromes) and another due to technically challenging lumbar punctures. One treated patient started NIV during follow‐up, after a respiratory infection that required hospitalization. No clinically relevant laboratory changes were found.
DISCUSSION
This multicenter study provides class III evidence that nusinersen improves motor function in at least a subset of SMA patients and causes frequent, usually mild, AEs.
Since the approval of nusinersen, several real‐world studies have suggested its efficacy in adult SMA patients, at least in a subset of patients [7]. However, these studies showed some limitations. First, only short‐term results were reported and a direct comparison with a control group of untreated patients was lacking. Whilst any improvement in a neurodegenerative disease could be regarded as a treatment effect, the scarcity of natural history data, the phenotypic variability, slow disease progression and the limited sensitivity of available outcome measures are major barriers to interpreting short‐term results [21]. Thus, individual improvements in some motor scales in a time frame less of than 2 years are not infrequent in the untreated adult population [22, 23, 24]. Moreover, in the last years, other treatments (such as salbutamol or pyridostigmine) used off‐label for the treatment of SMA patients could have a positive effect in motor scales [25], compared with historical controls.
Secondly, previous research has largely overlooked the particularities of adult patients. For example, patients were stratified following the classical children classification instead of as functional subgroups, as previously recommended [21, 26, 27], and HFMSE was a common outcome for all patients in those studies, despite not being designed to assess non‐sitter patients [16, 27]. Surprisingly, functional scales and PROs have scarcely been used to describe treatments effects, despite being validated in adult patients and their importance in the clinical practice and for regulatory agencies [16].
This multicenter study used real‐world data to assess nusinersen efficacy and safety, whilst overcoming some previous limitations. Namely, a control group with natural history data was included for direct comparison and, importantly, a similar percentage of patients were treated with salbutamol in both the control and the nusinersen group. Moreover, patients were categorized in functional subgroups, in which validated motor and functional scales as well as PROs were appropriately used. Finally, the statistical approach was designed to control for common pitfalls in real‐world studies, such as selection bias and the variability in the follow‐up.
Overall, our results support previous evidence suggesting the efficacy of nusinersen. This effect was statistically significant for RULM after 6 months of treatment, and for HFMSE, 6MWT and EK2 after a mean follow‐up of 16 months. Overall, the effect of treatment in motor scales, as shown in our models, seems to be modest, in line with previous studies [7]. Interestingly, the greatest effect was found in EK2, a bedside functional scale for the assessment of non‐ambulant patients. This could reflect its ability to detect mild functional changes in non‐ambulant patients and to measure the effect of nusinersen on fatigability, which might not be captured by HFMSE and RULM. However, direct comparisons between scales should be interpreted with caution because not all scales are applicable to the same patients. Remarkably, those outcome measures applicable to all functional subgroups (RULM, ALSFRS‐R and FVC%) failed to show statistically significant improvements, suggesting that the measurement of treatment effect in real‐world studies is also hindered by the huge heterogeneity of SMA patients. Thus, whenever possible, functional stratification should be considered in studies addressing adult SMA patients [27].
Previous studies have reported a 30%–60% of responders, according to the predefined MCID of motor scales [14, 19, 28]. However, the responder rate in those studies should also be interpreted with caution, since two important biases could lead to underestimation and overestimation.
On the one hand, both HFMSE and RULM show floor and ceiling effects [16, 29], which could reduce their sensitivity to detect changes. The use of functional scales showing higher sensitivity to changes, such as EK2 or ALSFRS‐R, could increase the responder rate. Thus, in our study, the rate of responders ranged from 25% of treated patients, according to HFMSE, to 80% of treated patients, according to EK2.
On the other hand, as mentioned above, a non‐negligible proportion of untreated adult patients may show “significant improvements” when followed for less than 2 years. Thus, the comparison with a control group is essential to interpret the results. In our study, the responder rate was greater in all scales in treated versus untreated patients, although this difference was statistically significant only for RULM and EK2.
Finally, according to CGI‐C and PGI‐C, about 60% of treated patients experienced improvements considered as clinically meaningful. This figure coincides with another recent study, which found 64% of responders according to patients' impression [9]. However, these measures might overestimate the effect of treatment in a real‐world study since they cannot be compared with a natural history group.
It has been claimed that the mild improvements found in the adult SMA population after nusinersen treatment could be due to a placebo effect [30]. Whilst a placebo effect might indeed explain some improvements, increasing evidence also supports a physiological effect of nusinersen. First, most reports show consistent positive results [7]. Secondly, although most patients in our study experience only mild improvements, about 25% of them experienced moderate or even remarkable improvements. In a neurodegenerative disease, any strong improvement is unexpected and is hardly explained by a placebo effect. Thirdly, longer treatment duration was associated with greater response in our and previous studies [14, 19].
Despite the consistent results of nusinersen, the response seems variable amongst patients. In clinical trials, shorter disease duration, better baseline functionality and more SMN2 copies have been associated with better response in children [5, 6, 31]. In adults, higher baseline scores in motor scales have been found to correlate with greater response [14, 19], although the floor effect of motor scales in patients with lower functionality [16] could explain these findings. Our multivariable model, based on the CGI‐C (which captures changes far beyond motor scales), confirmed that non‐sitters are less likely to improve, whilst age, disease duration and the SMN2 copy number did not seem to influence the response.
Moreover, when deciding to start a treatment, the potential benefit must be balanced against the risks and the costs of treatment. In keeping with previous studies [14, 19, 28, 30, 32], Spanish patients reported frequent treatment‐related AEs (77%). Whilst most were mild and transient, some of them were permanent (e.g., neurogenic bladder) or led to short‐term treatment discontinuation in 7.7% of patients. Most AEs were related to the administration procedure and could therefore be more frequent and severe in patients with complex spines [30], in whom transforaminal approaches are frequently tried [33]. The use of non‐traumatic needles and ultrasound‐guided parasagittal approaches [11] could help to reduce the frequency and severity of AEs.
Whilst the decision to start any treatment should be made at an individual level, our and previous studies suggest that non‐sitters are less likely to improve with nusinersen, being also probably those with greater risks of serious AEs. Therefore, the risk–benefit balance of nusinersen in these patients should be evaluated carefully, especially considering the availability of oral alternatives.
Our work has several limitations, which are common in real‐world studies in rare diseases. A greater sample size would have been desirable to be able to stratify the results according to the functional subgroups and to increase the power of the multivariable analysis. Moreover, despite a common protocol, there was some methodological heterogeneity amongst centers, especially regarding retrospective data, and some baseline patients' characteristics were unbalanced between treated and untreated groups. Nevertheless, the statistical analysis was designed to minimize all these limitations, for example by adjusting by baseline scores and the follow‐up time. Finally, 16 months of follow‐up may be insufficient to detect changes in both treated and untreated patients as previous studies have suggested [14, 18, 23].
In conclusion, our multicenter real‐world study provides class III evidence that nusinersen treatment associates with mild motor and functional improvements in up to 60% of adult SMA patients, but also causes frequent mild AEs. Most severely affected patients with complex spines are probably those with the most unfavorable risk–benefit ratio. Collaborative long‐term studies are warranted to confirm this, helping to personalize therapeutic decisions in adult SMA patients.
AUTHOR CONTRIBUTIONS
Juan F. Vázquez‐Costa: Conceptualization (lead); data curation (equal); formal analysis (equal); writing – original draft (lead); writing – review and editing (equal). Mónica Povedano: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Andrés E. Nascimiento‐Osorio: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Antonio Moreno Escribano: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Solange Kapetanovic Garcia: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Raul Dominguez: Data curation (equal). Jessica M. Exposito: Data curation (equal). Laura González: Data curation (equal). Carla Marco: Data curation (equal). Julita Medina Castillo: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Nuria Muelas: Writing – review and editing (equal). Daniel Natera de Benito: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Nancy Carolina Ñungo Garzón: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Inmaculada Pitarch Castellano: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Teresa Sevilla: Writing – review and editing (equal). David Hervás: Formal analysis (equal); writing – review and editing (equal).
FUNDING INFORMATION
This study has received funding from FUNDAME (FUN‐000‐2017‐01), from CUIDAME (PIC188‐18), from the Instituto de Salud Carlos III (JR19/00030 PI JFVC, 19/01178 PI TS), and from Generalitat Valenciana (PROMETEO/ 2018/135, PI TS). The Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER) is the initiative from the ISCIII. TS and JFVC are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO‐NMD). Sponsors did not participate in the study design, data acquisition and analysis, data interpretation or in writing the article.
CONFLICT OF INTEREST
This study has received funding from FUNDAME (FUN‐000‐2017‐01) and CUIDAME (PIC188‐18). Dr Vázquez‐Costa is funded by grants of the Instituto de Salud Carlos III (JR19/00030, PI Vázquez) and received personal fees from Biogen and Roche outside the submitted work. Dr Nascimento‐Osorio received personal fees from Avexis, Biogen and Roche outside the submitted work; principal investigator for ongoing Biogen and Roche clinical trials. Dr N. Muelas and Dr A. Moreno received personal fees from Biogen outside the submitted work. Dr M Povedano received personal fees from Biogen and Roche outside the submitted work. Dr Solange Kapetanovic Garcia, Dr Raul Dominguez, Dr Jessica M. Exposito, Dr Laura González, Dr Carla Marco, Dr Julita Medina Castillo, Dr Daniel Natera de Benito, Dr Nancy Carolina Ñungo Garzón, Dr David Hervás have nothing to disclose. Dr Pitarch‐Castellano received personal fees from Avexis, Biogen and Roche outside the submitted work; principal investigator for ongoing Biogen clinical trial.
ETHICS STATEMENT
The study was approved by the Ethics Committee for Biomedical Research of Instituto de Investigación Sanitaria la Fe and Fundació Sant Joan de Déu. All the participants gave written informed consent.
Supporting information
Figure S1 Supinfo
ACKNOWLEDGEMENTS
Patients and patients' associations (FundAME, GaliciAME and ForzAME) are thanked for their collaboration in this study. Fernando Mora, M Carmen Baviera, Sandra Roca and Obdulia Moya are also thanked for their participation in patients' assessment.
Vázquez‐Costa JF, Povedano M, Nascimiento‐Osorio AE, et al. Nusinersen in adult patients with 5q spinal muscular atrophy: A multicenter observational cohorts' study. Eur J Neurol. 2022;29:3337‐3346. doi: 10.1111/ene.15501
DATA AVAILABILITY STATEMENT
JFVC and DH had full access to the database population used to create the study population. All data supporting our findings are available on reasonable request.
REFERENCES
- 1. Wadman RI, Wijngaarde CA, Stam M, et al. Muscle strength and motor function throughout life in a cross‐sectional cohort of 180 patients with spinal muscular atrophy types 1c–4. Eur J Neurol. 2018;25(3):512‐518. [DOI] [PubMed] [Google Scholar]
- 2. Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103‐115. [DOI] [PubMed] [Google Scholar]
- 3. Calucho M, Bernal S, Alías L, et al. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord [Internet]. 2018;28(3):208‐215. doi: 10.1016/j.nmd.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 4. Wijngaarde CA, Stam M, Otto LAM, et al. Population‐based analysis of survival in spinal muscular atrophy. Neurology. 2020;94(15):E1634‐E1644. [DOI] [PubMed] [Google Scholar]
- 5. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later‐onset spinal muscular atrophy. New Engl J Med [Internet]. 2018;378(7):625‐635. doi: 10.1056/NEJMoa1710504 [DOI] [PubMed] [Google Scholar]
- 6. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile‐onset spinal muscular atrophy. New Engl J Med [Internet]. 2017;377(18):1723‐1732. doi: 10.1056/NEJMoa1702752 [DOI] [PubMed] [Google Scholar]
- 7. Coratti G, Cutrona C, Pera MC, et al. Motor function in type 2 and 3 SMA patients treated with nusinersen: a critical review and meta‐analysis. Orphanet J Rare Dis [Internet]. 2021;16(1):430. https://pubmed.ncbi.nlm.nih.gov/34645478/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osmanovic A, Ranxha G, Kumpe M, et al. Treatment expectations and patient‐reported outcomes of nusinersen therapy in adult spinal muscular atrophy. J Neurol [Internet]. 2020;267(8):2398‐2407. https://pubmed.ncbi.nlm.nih.gov/32361837/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer T, Maier A, Uzelac Z, et al. Treatment expectations and perception of therapy in adult patients with spinal muscular atrophy receiving nusinersen. Eur J Neurol [Internet] 2021;28(8):2582‐2595. doi: 10.1111/ene.14902 [DOI] [PubMed] [Google Scholar]
- 10. Agencia española de medicamentos y productos sanitarios. Informe de Posicionamiento Terapéutico de nusinersen (Spinraza®) en atrofia muscular espinal [Internet]. 2018:1‐10. https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT‐nusinersen‐Spinraza‐atrofia‐muscular‐espinal.pdf
- 11. Veiga‐Canuto D, Cifrián‐Pérez M, Pitarch‐Castellano I, Vázquez‐Costa JF, Aparici F. Ultrasound‐guided lumbar puncture for nusinersen administration in spinal muscular atrophy patients. Eur J Neurol [Internet]. 2021;28(2):676‐680. https://pubmed.ncbi.nlm.nih.gov/33051940/ [DOI] [PubMed] [Google Scholar]
- 12. Pera MC, Coratti G, Forcina N, et al. Content validity and clinical meaningfulness of the HFMSE in spinal muscular atrophy. BMC Neurol [Internet]. 2017;17(1):1‐10. doi: 10.1186/s12883-017-0790-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mazzone ES, Mayhew A, Montes J, et al. Revised upper limb module for spinal muscular atrophy: development of a new module. Muscle Nerve [Internet]. 2017;55(6):869‐874. 10.1002/mus.25430 [DOI] [PubMed] [Google Scholar]
- 14. Hagenacker T, Wurster CD, Günther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non‐interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19(4):317‐325. [DOI] [PubMed] [Google Scholar]
- 15. Mcdonald CM, Henricson EK, Abresch RT, et al. The 6‐minute walk test and other clinical endpoints in Duchenne muscular dystrophy: reliability, concurrent validity, and minimal clinically important differences from a multicenter study. Muscle Nerve [Internet]. 2013;48(3):357‐368. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3826053/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vázquez‐Costa JF, Povedano M, Nascimiento‐Osorio AE, et al. Validation of motor and functional scales for the evaluation of adult patients with 5q spinal muscular atrophy. medRxiv [Internet]. 2021;2021.06.12.21258357. doi: 10.1101/2021.06.12.21258357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steffensen BF, Mayhew A, Aloysius A, et al. Egen classification revisited in SMA. Neuromuscul Disord. 2008;18:740‐741. [Google Scholar]
- 18. Walter MC, Wenninger S, Thiele S, et al. Safety and treatment effects of nusinersen in longstanding adult 5q‐SMA Type 3—a prospective observational study. J Neuromuscul Dis [Internet]. 2019;6(4):453‐465. https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JND‐190416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maggi L, Bello L, Bonanno S, et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry [Internet]. 2020;91(11):1166‐1174. http://jnnp.bmj.com/ [DOI] [PubMed] [Google Scholar]
- 20. Muñoz SR, Bangdiwala SI. Interpretation of kappa and B statistics measures of agreement. J Appl Stat. 1997;24(1):105‐112. [Google Scholar]
- 21. Wan HWY, Carey KA, D'Silva A, et al. Health, wellbeing and lived experiences of adults with SMA: a scoping systematic review [Internet]. Orphanet J Rare Dis. 2020;15(1):70. https://pubmed.ncbi.nlm.nih.gov/32164772/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coratti G, Lucibello S, Pera MC, et al. Gain and loss of abilities in type II SMA: a 12‐month natural history study. Neuromuscul Disord. 2020;30(9):765‐771. [DOI] [PubMed] [Google Scholar]
- 23. Coratti G, Pera MC, Lucibello S, et al. Age and baseline values predict 12 and 24‐month functional changes in type 2 SMA. Neuromuscul Disord. 2020;30(9):756‐764. [DOI] [PubMed] [Google Scholar]
- 24. Coratti G, Messina S, Lucibello S, et al. Clinical variability in spinal muscular atrophy type III. Ann Neurol. 2020;88(6):1109‐1117. [DOI] [PubMed] [Google Scholar]
- 25. Frongia AL, Natera‐De Benito D, Ortez C, et al. Salbutamol tolerability and efficacy in patients with spinal muscular atrophy type II. Neuromuscul Disord [Internet]. 2019;29:517‐524. www.sciencedirect.comwww.elsevier.com/locate/nmd [DOI] [PubMed] [Google Scholar]
- 26. Walter MC, Chiriboga C, Duong T, et al. Improving care and empowering adults living with SMA: a call to action in the new treatment era. J Neuromuscul Dis [Internet]. 2021;8(4):543‐551. https://www.medra.org/servlet/aliasResolver?alias=iospress&doi=10.3233/JND‐190416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sansone VA, Walter MC, Attarian S, et al. Measuring outcomes in adults with spinal muscular atrophy—challenges and future directions—meeting report. J Neuromuscul Dis. 2020;7(4):523‐534. [DOI] [PubMed] [Google Scholar]
- 28. de Wel B, Goosens V, Sobota A, et al. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J Neurol [Internet]. 2020;268(3):923‐935. https://pubmed.ncbi.nlm.nih.gov/32935160/ [DOI] [PubMed] [Google Scholar]
- 29. Wijngaarde CA, Stam M, Otto LAM, et al. Muscle strength and motor function in adolescents and adults with spinal muscular atrophy. Neurology [Internet]. 2020;95(14):e1988–e1998. https://n.neurology.org/content/95/14/e1988 [DOI] [PubMed] [Google Scholar]
- 30. Moshe‐Lilie O, Visser A, Chahin N, Ragole T, Dimitrova D, Karam C. Nusinersen in adult patients with spinal muscular atrophy: observations from a single center. Neurology [Internet]. 2020;95(4):E413–6. https://pubmed.ncbi.nlm.nih.gov/32665408/ [DOI] [PubMed] [Google Scholar]
- 31. de Vivo DC, Bertini E, Swoboda KJ, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the phase 2 NURTURE study. Neuromuscul Disord [Internet]. 2019;29(11):842‐856. doi: 10.1016/j.nmd.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yeo CJJ, Simeone SD, Townsend EL, Zhang RZ, Swoboda KJ. Prospective cohort study of nusinersen treatment in adults with spinal muscular atrophy. J Neuromuscul Dis. 2020;7(3):257‐268. [DOI] [PubMed] [Google Scholar]
- 33. Bortolani S, Stura G, Ventilii G, et al. Intrathecal administration of nusinersen in adult and adolescent patients with spinal muscular atrophy and scoliosis: transforaminal versus conventional approach. Neuromuscul Disord. 2019;29(10):742‐746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Supinfo
Data Availability Statement
JFVC and DH had full access to the database population used to create the study population. All data supporting our findings are available on reasonable request.


