Abstract
Aims
The Heart Failure Association of the European Society of Cardiology (HFA‐ESC) proposed a definition of advanced heart failure (HF) that has not been validated, yet. We assessed its prognostic impact in a consecutive series of patients with high‐risk HF.
Methods and results
The HELP‐HF registry enrolled consecutive patients with HF and at least one high‐risk ‘I NEED HELP’ marker, evaluated at four Italian centres between 1st January 2020 and 30th November 2021. Patients meeting the HFA‐ESC advanced HF definition were compared to patients not meeting this definition. The primary endpoint was the composite of all‐cause mortality or first HF hospitalization. Out of 4753 patients with HF screened, 1149 (24.3%) patients with at least one high‐risk ‘I NEED HELP’ marker were included (mean age 75.1 ± 11.5 years, 67.3% male, median left ventricular ejection fraction [LVEF] 35% [interquartile range 25%–50%]). Among them, 193 (16.8%) patients met the HFA‐ESC advanced HF definition. As compared to others, these patients were younger, had lower LVEF, higher natriuretic peptides and a worse clinical profile. The 1‐year rate of the primary endpoint was 69.3% in patients with advanced HF according to the HFA‐ESC definition versus 41.8% in the others (hazard ratio [HR] 2.23, 95% confidence interval [CI] 1.82–2.74, p < 0.001). The prognostic impact of the HFA‐ESC advanced HF definition was confirmed after multivariable adjustment for relevant covariates (adjusted HR 1.98, 95% CI 1.57–2.50, p < 0.001).
Conclusions
The HFA‐ESC advanced HF definition had a strong prognostic impact in a contemporary, real‐world, multicentre high‐risk cohort of patients with HF.
Keywords: Heart failure, Advanced heart failure, European Society Of Cardiology, Heart Failure Association, Mortality, Hospitalization
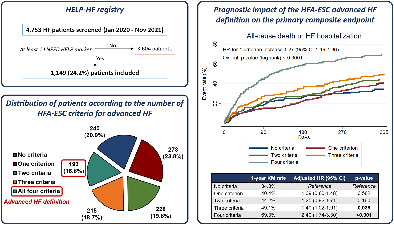
Distribution and prognostic impact of the 2018 Heart Failure Association of the European Society of Cardiology (HFA‐ESC) advanced heart failure (HF) criteria in the HELP‐HF (Assessment of the I Need Help markers in Heart Failure) registry. Consecutive patients with HF who were hospitalized or evaluated as outpatients between January 2020 and November 2021 at four Italian high‐volume centres and had at least one ‘I Need Help’ high‐risk marker (n = 1149) were included in the HELP‐HF registry (upper left panel). Among the included patients, 240 (20.9%) had no criteria for advanced HF according to the updated HFA‐ESC definition, whereas 193 (16.8%) had all four criteria and therefore met the HFA‐ESC definition of advanced HF (lower left panel). A progressively higher risk of the primary composite endpoint of all‐cause mortality or first HF hospitalization at 1 year was observed with an increasing number of HFA‐ESC advanced HF criteria (right panel), and patients meeting the HFA‐ESC definition of advanced HF (i.e. with all four criteria) had the highest risk of adverse outcomes (1‐year rate of the primary composite endpoint 69.3%; adjusted hazard ratio [HR] vs. patients with no criteria 2.40, 95% confidence interval [CI] 1.74–3.30). KM, Kaplan–Meier.
Introduction
Heart failure (HF) remains a major cause of morbidity and mortality. 1 , 2 As a result of its increasing prevalence and of advances in treatment, more patients eventually progress to an advanced stage of the disease, characterized by persistent and severe symptoms and worse prognosis. 3 , 4 , 5 It is estimated that patients with advanced HF represent ∼1%–10% of the overall HF population. 4 , 6 , 7 , 8 , 9 These patients experience refractory symptoms that are disabling for daily life, need multiple hospitalizations for HF, have a poor survival, and may be candidates for long‐term heart replacement therapies (i.e. heart transplantation or left ventricular assist device [LVAD]), if indicated. 2 , 3 , 5 , 9 , 10 , 11 , 12
Despite its major impact on clinical practice for the selection of patients for heart transplantation, LVAD and novel therapies, a proper and timely identification of patients at high risk of major events because of advanced HF remains challenging, and multiple classifications have been developed. 3 , 5 , 11 A useful mnemonic, ‘I NEED HELP’, has been proposed as a 9‐item screening tool including clinical, laboratory and imaging parameters, which could allow an early identification of patients with advanced HF. 4 , 11 , 13 , 14 The Heart Failure Association (HFA) of the European Society of Cardiology (ESC) published in 2018 a position statement with an updated definition of advanced HF, recently endorsed in the latest ESC HF guidelines. 2 , 4 This definition requires the concomitant presence of four criteria despite optimal guideline‐directed treatment: severe and persistent symptoms (criterion 1); severe cardiac dysfunction (criterion 2), defined as a reduced left ventricular ejection fraction (LVEF) ≤30%, isolated right ventricular failure, non‐operable severe valve or congenital abnormalities, or persistently increased levels of natriuretic peptides in the context of HF with mildly reduced ejection fraction (HFmrEF) and preserved ejection fraction (HFpEF); episodes of congestion, low output syndrome, or malignant arrhythmias causing >1 unplanned visit or hospitalization in the last year (criterion 3); and severe impairment of exercise capacity (criterion 4). 4
The aim of our study was to evaluate the clinical characteristics and outcomes of a real‐world, contemporary, multicentre cohort of patients with advanced HF, focusing on the prognostic impact of the updated 2018 HFA‐ESC definition.
Methods
Study design and data collection
The observational, retrospective, multicentre HELP‐HF (Assessment of the I Need Help markers in Heart failure) registry included all consecutive patients who were hospitalized for acute HF or were evaluated as outpatients for chronic HF at four Italian high‐volume centres between 1st January 2020 and 30th November 2021, and had at least one of the following ‘I Need Help’ high‐risk markers: (i) previous or ongoing requirement for inotropes; (ii) persisting New York Heart Association (NYHA) class III or IV and/or persistently high B‐type natriuretic peptide (BNP) or N‐terminal proBNP (NT‐proBNP); (iii) end‐organ dysfunction (worsening renal or liver dysfunction in the setting of HF); (iv) LVEF <20%; (v) recurrent appropriate defibrillator shocks; (vi) more than one hospitalization for HF in the last 12 months; (vii) persisting fluid overload and/or increasing diuretic requirement; (viii) consistently low blood pressure (systolic blood pressure <90–100 mmHg); and (ix) inability to up‐titrate or need to reduce/discontinue prognostic medications (angiotensin‐converting enzyme inhibitor, β‐blocker, angiotensin receptor–neprilysin inhibitor, or mineralocorticoid receptor antagonist). Among the included patients, details on the updated HFA‐ESC criteria for advanced HF were recorded: the presence of each of the four criteria was evaluated and a patient fulfilled the HFA‐ESC definition of advanced HF if all four criteria were met, as described in the 2018 position statement. 4
Institutional review board approval was waived for this registry because of its retrospective design with collection of anonymized data and without any study‐specific intervention. All included inpatients and outpatients were managed and treated as per local clinical practice, in accordance with HF guidelines. 2 De‐identified individual patient data on medical history, clinical presentation, echocardiography and laboratory findings, medical therapy and clinical outcomes were collected. Congestion and perfusion status at clinical presentation was described according to available guidelines and position statements. 2 , 15 Besides the HFA‐ESC definition, American College of Cardiology/American Heart Association (ACC/AHA) stage D and Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) classification were also reported, based on previous definitions. 3 , 8 , 16 , 17 Biventricular function, mitral regurgitation (MR) and tricuspid regurgitation were evaluated and graded as previously described. 2 , 18 Follow‐up was performed by means of medical records (in case of rehospitalizations or outpatient clinical visits) or telephone contact.
Study endpoints
The primary endpoint of the study was the composite of all‐cause mortality or HF hospitalization. Other outcomes of interest were all‐cause mortality and first HF hospitalization as individual endpoints.
Statistical analyses
Continuous variables are presented as mean ± standard deviation or median (interquartile range [IQR]), as appropriate, and were compared with the unpaired Student's t‐test or the Mann–Whitney U test, respectively. Categorical variables are presented as number and percentages and were compared with the χ2 test. Baseline characteristics, echocardiography data, laboratory data, and clinical outcomes were compared between patients fulfilling versus not fulfilling all four HFA‐ESC criteria for advanced HF. The first occurrence of the primary composite endpoint and of all‐cause mortality was evaluated in patients with or without all four HFA‐ESC criteria using the Kaplan–Meier method and compared between groups using the log‐rank test for time to the first event. The occurrence of first HF hospitalization in patients with or without all four HFA‐ESC criteria was evaluated using the Fine–Gray method to account for the competing risk of mortality and was plotted using the cumulative incidence function. For all endpoints, follow‐up was evaluated at the date of the event or the last available follow‐up. Cox proportional hazards regression analysis was also performed to assess the prognostic impact of the HFA‐ESC advanced HF definition on the primary composite endpoint and on all‐cause mortality. The Fine–Gray proportional subdistribution hazards model was used to assess the impact of the HFA‐ESC advanced HF definition on first HF hospitalization, accounting for the competing risk of mortality.
The impact of the HFA‐ESC advanced HF definition on the primary endpoint and individual outcomes was evaluated by means of univariable and multivariable analysis. Covariates with univariable p‐value <0.10 and other selected covariates considered to be relevant according to the investigators' judgment (e.g. age and sex) were included in the multivariable models. Similar Cox regression and Fine–Gray analyses were performed to evaluate the prognostic impact of each HFA‐ESC criterion of advanced HF, of the cumulative number of HFA‐ESC criteria (one, two, three, or four criteria as compared to no criteria), and of INTERMACS 1–3 profile. Imputation for missing values was not performed at multivariable analyses. Results of the Cox regression analyses are reported as unadjusted or adjusted hazard ratio (HR) and 95% confidence interval (CI). Results of the Fine–Gray models are reported as unadjusted or adjusted subhazard ratio (SHR) with 95% CI.
All reported p‐values are 2‐sided, and a p < 0.05 was considered statistically significant. Statistical analyses were performed using STATA version 13.0 (Stata Corp., College Station, TX, USA).
Results
Among 4753 patients with HF screened between January 2020 and November 2021 at the four participating centres, a total of 1149 patients (24.3%) had at least one ‘I NEED HELP’ high‐risk marker and were included in the HELP‐HF registry (Graphical Abstract). Among these 1149 patients, 193 (16.8%) fulfilled all four HFA‐ESC criteria for advanced HF and therefore met the HFA‐ESC advanced HF definition. A total of 215 patients (18.7%) had three criteria, 228 (19.8%) had two criteria, 273 (23.8%) had one criterion and 240 (20.9%) patients had no criteria of the HFA‐ESC definition. Online supplementary Figure S1 details the distribution of the HFA‐ESC advanced HF criteria in the study population.
Baseline patient characteristics and clinical presentation
The mean age of the study population was 75.1 ± 11.5 years and 67.3% of patients were male. At the time of enrolment, 777 patients (67.6%) were hospitalized and 372 (32.4%) were outpatients. Patients with new‐onset (de novo) HF represented 16.3% of the enrolled population.
Baseline characteristics are reported in Table 1 . As compared with patients not fulfilling all four HFA‐ESC criteria for advanced HF, those fulfilling all these criteria were younger (p < 0.001), were more likely to be hospitalized at inclusion (p < 0.001) and with a history of HF hospitalization during the last year (p < 0.001), and less likely to have new‐onset HF (p < 0.001). Furthermore, patients with all four HFA‐ESC criteria had more frequently prior myocardial infarction (p = 0.011) and prior percutaneous valve interventions (p = 0.028), driven by a higher rate of prior mitral transcatheter edge‐to‐edge repair. Patients with the HFA‐ESC advanced HF definition were more likely to have an implantable cardioverter‐defibrillator or cardiac resynchronization therapy‐defibrillator, whereas patients not fulfilling the HFA‐ESC definition were more likely to have a pacemaker (p < 0.001).
Table 1.
Baseline characteristics and clinical presentation
| Overall (n = 1149) | Patients fulfilling all 4 updated 2018 HFA‐ESC criteria for advanced HF (n = 193) | Patients not fulfilling all 4 updated 2018 HFA‐ESC criteria for advanced HF (n = 956) | p‐value | |
|---|---|---|---|---|
| Age (years) | 75.1 ± 11.5 | 71.9 ± 11.4 | 75.7 ± 11.4 | <0.001 |
| Male sex | 773 (67.3) | 140 (72.5) | 633 (66.2) | 0.088 |
| BMI (kg/m2) | 25.7 (22.5–28.4) | 24.8 (22.5–28.4) | 26.0 (23.0–29.7) | 0.034 |
| New‐onset HF | 187 (16.3) | 0 (0.0) | 187 (19.6) | <0.001 |
| Time since HF diagnosis (months) | 30 (3–84) | 65 (24–144) | 24 (1–76) | <0.001 |
| HF hospitalization(s) during last year | 415 (36.1) | 58 (69.9) | 280 (29.3) | <0.001 |
| Type of inclusion | <0.001 | |||
| Outpatient visit | 372 (32.4) | 28 (14.5) | 344 (36.0) | |
| Inpatient hospitalization | 777 (67.6) | 165 (85.5) | 612 (64.0) | |
| Comorbidities | ||||
| Hypertension | 817 (71.1) | 131 (67.9) | 686 (71.8) | 0.278 |
| Diabetes | 447 (38.9) | 82 (42.5) | 365 (38.2) | 0.263 |
| History of AF | 641 (55.8) | 103 (53.4) | 538 (56.3) | 0.458 |
| Prior CAD diagnosis | 504 (43.9) | 98 (50.8) | 406 (42.5) | 0.034 |
| Prior myocardial infarction | 380 (33.1) | 79 (40.9) | 301 (31.5) | 0.011 |
| Prior PCI | 336 (29.2) | 67 (34.7) | 269 (28.1) | 0.067 |
| Prior CABG | 171 (14.9) | 34 (17.6) | 137 (14.3) | 0.242 |
| Prior valve surgery | 139 (12.1) | 27 (14.0) | 112 (11.7) | 0.377 |
| Prior percutaneous valve intervention | 0.028 | |||
| TAVR | 28 (2.4) | 7 (3.6) | 21 (2.2) | |
| Mitral TEER | 49 (4.3) | 15 (7.8) | 34 (3.6) | |
| Known cardiomyopathy | 291 (25.3) | 57 (29.5) | 234 (24.5) | 0.141 |
| Peripheral artery disease | 205 (17.8) | 32 (16.6) | 173 (18.1) | 0.616 |
| Prior stroke or TIA | 173 (15.1) | 27 (14.0) | 146 (15.3) | 0.650 |
| COPD | 266 (23.2) | 37 (19.2) | 229 (24.0) | 0.151 |
| Chronic kidney disease | 650 (56.6) | 121 (62.7) | 529 (55.3) | 0.060 |
| MCI or dementia | 157 (13.7) | 22 (11.4) | 135 (14.1) | 0.315 |
| ADL or IADL impairment | 339 (31.3) | 92 (47.9) | 247 (27.8) | <0.001 |
| NYHA functional class | <0.001 | |||
| I | 48 (4.2) | 2 (1.0) | 46 (4.8) | |
| II | 363 (31.6) | 22 (11.4) | 341 (35.7) | |
| III | 572 (49.8) | 111 (57.5) | 461 (48.2) | |
| IV | 166 (14.5) | 58 (30.1) | 108 (11.3) | |
| Cardiac implantable electronic devices | <0.001 | |||
| Pacemaker | 167 (14.5) | 14 (7.3) | 153 (16.0) | |
| ICD | 183 (15.9) | 49 (25.4) | 134 (14.0) | |
| CRT‐D | 168 (14.6) | 50 (25.9) | 118 (12.3) | |
| CRT‐P | 15 (1.3) | 3 (1.55) | 12 (1.3) | |
| Clinical presentation | ||||
| Cardiogenic shock | 153 (13.3) | 46 (23.8) | 107 (11.2) | <0.001 |
| Acute pulmonary oedema | 153 (13.3) | 41 (21.2) | 112 (11.7) | <0.001 |
| Rales >1/3 lung fields | 490 (42.7) | 104 (53.9) | 386 (40.4) | 0.001 |
| Peripheral oedema | 673 (58.6) | 138 (71.5) | 535 (56.0) | <0.001 |
| Forrester classification | <0.001 | |||
| Class 1 (warm/dry) | 271 (23.6) | 23 (11.9) | 248 (25.9) | |
| Class 2 (warm/wet) | 716 (62.3) | 120 (62.2) | 596 (62.3) | |
| Class 3 (cold/dry) | 40 (3.5) | 12 (6.2) | 28 (2.9) | |
| Class 4 (cold/wet) | 122 (10.6) | 38 (19.7) | 84 (8.8) | |
| Systolic blood pressure (mmHg) | 124 ± 26 | 112 ± 21 | 126 ± 26 | <0.001 |
| Diastolic blood pressure (mmHg) | 71 ± 15 | 68 ± 13 | 72 ± 16 | <0.001 |
| Mean arterial pressure (mmHg) | 89 ± 17 | 83 ± 14 | 90 ± 18 | <0.001 |
| Heart rate (bpm) | 79 ± 20 | 80 ± 18 | 78 ± 21 | 0.415 |
| IV loop diuretics | 778 (67.7) | 144 (74.6) | 634 (66.3) | 0.025 |
| Maximum furosemide dose (mg/day) | 110 (0–500) | 250 (0–1000) | 100 (0–500) | 0.004 |
| Thiazide diuretics | 82 (7.1) | 23 (11.9) | 59 (6.2) | 0.005 |
| Use of inotropes/vasopressors | 277 (24.1) | 94 (48.7) | 183 (19.1) | <0.001 |
| Use of vasodilators | 119 (10.4) | 24 (12.4) | 95 (9.9) | 0.299 |
| Need for temporary MCS | 0.015 | |||
| IABP | 40 (3.5) | 14 (7.3) | 26 (2.7) | |
| Impella | 5 (0.4) | 1 (0.5) | 4 (0.4) | |
| VA‐ECMO | 3 (0.3) | 1 (0.5) | 2 (0.2) | |
| Need for mechanical ventilation | 0.056 | |||
| Non‐invasive | 159 (13.8) | 36 (18.7) | 123 (12.9) | |
| Invasive | 35 (3.1) | 8 (4.2) | 27 (2.8) | |
| Need for CRRT/ultrafiltration | 45 (3.9) | 16 (8.3) | 29 (3.0) | 0.001 |
| Need for ICU admission | 253 (22.0) | 70 (36.3) | 183 (19.1) | <0.001 |
| INTERMACS profile 1–3 | 104 (9.1) | 49 (25.4) | 55 (5.8) | <0.001 |
| ACC/AHA stage D | 185 (16.1) | 90 (46.6) | 95 (9.9) | <0.001 |
Data are presented as n (%), mean ± standard deviation, or median (Q25–Q75).
ACC, American College of Cardiology; ADL, activities of daily living; AF, atrial fibrillation; AHA, American Heart Association; BMI, body mass index; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; CRT‐D, cardiac resynchronization therapy with defibrillator; CRT‐P, cardiac resynchronization therapy with pacemaker; HFA‐ESC, Heart Failure Association of the European Society of Cardiology; HF, heart failure; IABP, intra‐aortic balloon pump; IADL, instrumental activities of daily living; ICD, implantable cardioverter‐defibrillator; ICU, intensive care unit; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; IV, intravenous; MCI, mild cognitive impairment; MCS, mechanical circulatory support; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; TAVR, transcatheter aortic valve replacement; TEER, transcatheter edge‐to‐edge repair; TIA, transient ischaemic attack; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenation.
Details on clinical presentation are reported in Table 1 . At inclusion, patients fulfilling all four HFA‐ESC criteria for advanced HF were more likely to have cardiogenic shock (p < 0.001), acute pulmonary oedema (p < 0.001), rales >1/3 lung fields (p = 0.001), and peripheral oedema (p < 0.001), and had lower blood pressure (p < 0.001). Use of intravenous loop diuretics (p = 0.025), use of inotropes/vasopressors (p < 0.001), need of temporary mechanical circulatory support (p = 0.015), renal replacement therapy or ultrafiltration (p = 0.001), and intensive care unit admission (p < 0.001) were more frequent among patients with the HFA‐ESC advanced HF definition, as compared to those without. Patients with all four HFA‐ESC criteria were more likely to have a INTERMACS profile 1–3 (p < 0.001) or ACC/AHA stage D HF (p < 0.001).
Echocardiographic data and laboratory findings
Echocardiography findings are shown in Table 2 . Median LVEF of the study population was 35% (IQR 25%–50%) and patients with HF with reduced ejection fraction (HFrEF), HFmrEF, and HFpEF were 56.5%, 15.0%, and 28.5%, respectively. Patients fulfilling all four HFA‐ESC advanced HF criteria had lower LVEF (p < 0.001) and were more likely to have HFrEF (p < 0.001), had higher degrees of MR (p = 0.002) and had more frequently right ventricular dilatation (p = 0.001) and dysfunction (p < 0.001).
Table 2.
Echocardiographic data and laboratory findings
| Overall (n = 1149) | Patients fulfilling all 4 updated 2018 HFA‐ESC criteria for advanced HF (n = 193) | Patients not fulfilling all 4 updated 2018 HFA‐ESC criteria for advanced HF (n = 956) | p‐value | |
|---|---|---|---|---|
| Echocardiographic data | ||||
| LVEF (%) | 35 (25–50) | 27 (20–40) | 38 (26–50) | <0.001 |
| LVEF categories | <0.001 | |||
| HFrEF (LVEF <40%) | 649 (56.5) | 144 (74.6) | 505 (52.8) | |
| HFmrEF (LVEF 40%–49%) | 172 (15.0) | 18 (9.3) | 154 (16.1) | |
| HFpEF (LVEF ≥50%) | 328 (28.5) | 31 (16.1) | 297 (31.1) | |
| MR severity | 0.002 | |||
| None/trivial | 83 (7.4) | 7 (3.8) | 76 (8.2) | |
| Mild | 349 (31.3) | 43 (23.2) | 306 (32.9) | |
| Moderate | 452 (40.5) | 85 (46.0) | 367 (39.5) | |
| Severe | 231 (20.7) | 50 (27.0) | 181 (19.5) | |
| RV dilatation | 363 (34.4) | 80 (45.2) | 283 (32.2) | 0.001 |
| RV dysfunction | 482 (43.4) | 117 (63.9) | 365 (39.3) | <0.001 |
| TR severity | 0.096 | |||
| None/trivial | 136 (12.4) | 18 (9.7) | 118 (13.0) | |
| Mild | 372 (34.0) | 65 (35.1) | 307 (33.8) | |
| Moderate | 376 (34.4) | 56 (30.3) | 320 (35.2) | |
| Severe | 209 (19.1) | 46 (24.9) | 163 (18.0) | |
| Laboratory findings | ||||
| Creatinine (mg/dl) | 1.48 (1.08–2.07) | 1.60 (1.22–2.18) | 1.45 (1.06–2.02) | 0.015 |
| eGFR CKD‐EPI (ml/min/1.73 m2) | 41.9 (27.2–60.6) | 37.5 (25.4–59.4) | 42.6 (27.5–61.0) | 0.159 |
| Urea (mg/dl) | 69 (47–109) | 83 (54–117)) | 67 (46–105) | 0.004 |
| NT‐proBNP (pg/ml) | 5254 (2541–12 421) | 7332 (3674–17 281) | 4920 (2320–11 196) | <0.001 |
| BNP (pg/ml) | 648 (298–1248) | 1103 (481–2746) | 607 (285–1220) | 0.004 |
| Haemoglobin (g/dl) | 12.0 (10.6–13.5) | 12.1 (10.3–13.3) | 12.0 (10.6–13.6) | 0.497 |
| Haematocrit (%) | 36.8 (32.7–41.0) | 37.2 (32.3–41.0) | 36.7 (32.8–41.0) | 0.636 |
| Platelet count (109/L) | 203 (159–259) | 194 (151–250) | 206 (161–261) | 0.019 |
| Albumin (g/dl) | 36 (32–39) | 36 (32–39) | 36 (32–39) | 0.816 |
| Sodium (mmol/L) | 140 (137–142) | 139 (136–141) | 140 (137–142) | <0.001 |
| Potassium (mmol/L) | 4.2 (3.8–4.6) | 4.0 (3.7–4.5) | 4.2 (3.8–4.6) | 0.017 |
| AST (IU/L) | 25 (19–37) | 27 (21–40) | 25 (19–36) | 0.020 |
| ALT (IU/L) | 20 (14–33) | 21 (15–35) | 20 (14–33) | 0.322 |
| Total bilirubin (mg/dl) | 0.87 (0.58–1.30) | 1.00 (0.65–1.57) | 0.81 (0.54–1.25) | <0.001 |
| INR | 1.26 (1.10–1.71) | 1.40 (1.18–2.22) | 1.20 (1.10–1.66) | <0.001 |
Data are presented as n (%), or median (Q25–Q75).
ALT, alanine transaminase; AST, aspartate transaminase; BNP, B‐type natriuretic peptide; CKD‐EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; HFA‐ESC, Heart Failure Association of the European Society of Cardiology; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEF, left ventricular ejection fraction; INR, international normalized ratio; MR, mitral regurgitation; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; RV, right ventricular; TR, tricuspid regurgitation.
Regarding laboratory findings (Table 2 ), patients with the HFA‐ESC definition had increased serum creatinine (p = 0.015), increased NT‐proBNP or BNP values (p < 0.001 and p = 0.004, respectively), slightly reduced potassium (p = 0.020), and slightly increased total bilirubin (p < 0.001).
Clinical outcomes
After a median follow‐up of 260 days (IQR 105–390 days), 265 patients (23.1%) died, a first HF hospitalization occurred in 308 patients (26.8%), and a primary composite outcome event occurred in 496 patients (43.2%). The 1‐year rates of the primary composite endpoint were 69.3% in patients with all four HFA‐ESC advanced HF criteria and 41.8% in those without all four HFA‐ESC advanced HF criteria (crude HR 2.23, 95% CI 1.82–2.74, p < 0.001; Figure 1A ). Both individual outcomes, considered separately, were significantly higher in patients fulfilling the HFA‐ESC advanced HF definition compared to the others (Figure 1B and online supplementary Figure S2 ), with a 1‐year all‐cause mortality of 46.5% versus 21.5% and a 1‐year first HF hospitalization rate at competing‐risk analysis of 39.5% versus 26.9% (crude HR 2.68, 95% CI 2.06–3.48, p < 0.001 for mortality; and crude SHR 1.56, 95% CI 1.19–2.05, p = 0.001 for first HF hospitalization). The increased risk of the primary composite endpoint in patients with all four HFA‐ESC advanced HF criteria compared to the others was mainly driven by events occurring during the first 3 months of follow‐up (90‐day rate 44.5% vs. 20.1%, crude HR 2.57, 95% CI 1.98–3.34, p < 0.001). Similar findings were observed for both individual outcomes alone, with 90‐day mortality of 32.0% versus 9.1% and 90‐day first HF hospitalization rate at competing‐risk analysis of 20.6% versus 12.5% in patients with versus without the HFA‐ESC advanced HF definition (crude HR 3.82, 95% CI 2.73–5.34, p < 0.001 for mortality; and crude SHR 1.73, 95% CI 1.19–2.51, p = 0.004 for first HF hospitalization).
Figure 1.
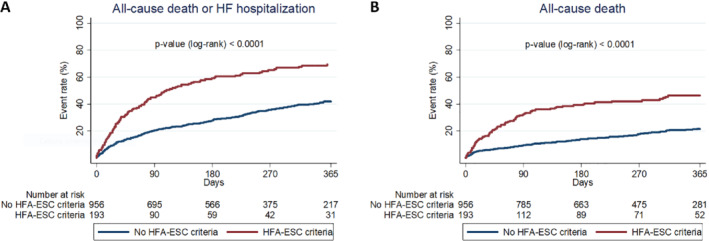
Primary composite endpoint and all‐cause mortality at 1 year in patients with versus without advanced heart failure (HF) definition according to the 2018 Heart Failure Association of the European Society of Cardiology (HFA‐ESC) criteria. The figure shows Kaplan–Meier curves for 1‐year primary composite endpoint of all‐cause mortality or HF hospitalization (A) and Kaplan–Meier curves for 1‐year all‐cause mortality (B) in patients with versus without advanced HF definition according to the 2018 HFA‐ESC criteria (i.e. meeting vs. not meeting all four 2018 HFA‐ESC criteria).
As shown in online supplementary Table S1 , the significant impact of the HFA‐ESC definition on the primary endpoint was confirmed also after multivariable adjustment for age, sex, inpatient versus outpatient status, peripheral artery disease, prior stroke or transient ischaemic attack, history of atrial fibrillation, prior myocardial infarction, chronic obstructive pulmonary disease, NYHA class III or IV, systolic blood pressure and estimated glomerular filtration rate (adjusted HR 1.98, 95% CI 1.57–2.50, p < 0.001). Similar findings were observed for both individual endpoints alone (adjusted HR 2.07, 95% CI 1.54–2.80, p < 0.001 for mortality; and adjusted SHR 1.41, 95% CI 1.05–1.89, p = 0.024 for HF hospitalization; online supplementary Tables S2 and S3 ).
The prognostic impact of each HFA‐ESC criterion of advanced HF on the primary endpoint and on all‐cause mortality was also confirmed at univariable and multivariable analyses (Table 3 ). With respect of the impact of the cumulative number of HFA‐ESC criteria, having only one criterion was not associated with worse outcomes, compared to no criteria, whereas there was a progressively higher risk of the primary endpoint and of mortality in the patients fulfilling two, three, and four criteria (Table 4 ). Although Kaplan–Meier curves for the primary composite endpoint diverged during the first 3–6 months only in patients with three and four criteria (online supplementary Figure S3 ), the 1‐year rate was of 34.8%, 40.4%, 44.2%, 49.1%, and 69.3%, in patients with no criteria, one criterion, two criteria, three criteria and all four criteria, respectively (crude HR for 1‐criterion increase 1.27, 95% CI 1.19–1.36, p < 0.001; overall log‐rank p < 0.0001; Graphical Abstract). Moreover, the risk of the primary endpoint and of mortality was significantly higher among patients with INTERMACS profile 1–3 at clinical presentation, as compared to those with INTERMACS profile 4–7 (online supplementary Table S4 and Figure S4 ).
Table 3.
Impact of each 2018 HFA‐ESC criterion of advanced heart failure on clinical outcomes
| All‐cause death or HF hospitalization (primary endpoint) | All‐cause death | First HF hospitalization | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | SHR (95% CI) | p‐value | |
| Criterion 1 (severe/persistent symptoms) | ||||||
| Univariable analysis | 1.64 (1.38–1.96) | <0.001 | 2.03 (1.59–2.59) | <0.001 | 1.40 (1.12–1.75) | 0.003 |
| Multivariable analysis | 1.44 (1.17–1.77) a | 0.001 | 1.69 (1.27–2.25) b | <0.001 | 1.25 (0.97–1.62) c | 0.089 |
| Criterion 2 (severe cardiac dysfunction) | ||||||
| Univariable analysis | 1.23 (1.03–1.47) | 0.025 | 1.46 (1.14–1.88) | 0.003 | 1.12 (0.89–1.40) | 0.342 |
| Multivariable analysis | 1.18 (0.97–1.43) a | 0.098 | 1.36 (1.03–1.79) b | 0.032 | 1.10 (0.85–1.41) c | 0.486 |
| Criterion 3 (>1 unplanned visit/hospitalization in the last year for episodes of congestion, LCOS or arrhythmias) | ||||||
| Univariable analysis | 1.54 (1.31–1.81) | <0.001 | 1.70 (1.37–2.12) | <0.001 | 1.34 (1.07–1.68) | 0.010 |
| Multivariable analysis | 1.55 (1.28–1.87) a | <0.001 | 1.55 (1.19–2.01) b | 0.001 | 1.33 (1.05–1.67) c | 0.018 |
| Criterion 4 (inability to exercise or low 6MWTD) | ||||||
| Univariable analysis | 1.76 (1.48–2.11) | <0.001 | 2.38 (1.86–3.05) | <0.001 | 1.37 (1.09–1.71) | 0.006 |
| Multivariable analysis | 1.58 (1.29–1.92) a | <0.001 | 2.03 (1.54–2.68) b | <0.001 | 1.24 (0.97–1.59) c | 0.088 |
6MWTD, 6‐min walking test distance; CI, confidence interval; HFA‐ESC, Heart Failure Association of the European Society of Cardiology; HF, heart failure; HR, hazard ratio; LCOS, low cardiac output syndrome; SHR, subhazard ratio.
Adjusted for age, sex, inpatient versus outpatient status, peripheral artery disease, prior stroke or transient ischaemic attack, history of atrial fibrillation, prior myocardial infarction, chronic obstructive pulmonary disease, New York Heart Association class III–IV, systolic blood pressure and estimated glomerular filtration rate.
Adjusted for age, sex, inpatient versus outpatient status, peripheral artery disease, prior stroke or transient ischaemic attack, history of atrial fibrillation, prior myocardial infarction, chronic obstructive pulmonary disease, New York Heart Association class III–IV, systolic blood pressure, heart rate and estimated glomerular filtration rate.
Adjusted for age, sex, history of atrial fibrillation, prior myocardial infarction, chronic obstructive pulmonary disease, New York Heart Association class III–IV, left ventricular ejection fraction <40% and estimated glomerular filtration rate.
Table 4.
Impact of the cumulative number of 2018 HFA‐ESC criteria of advanced heart failure on clinical outcomes
| All‐cause death or HF hospitalization (primary endpoint) | All‐cause death | First HF hospitalization | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p‐value | HR (95% CI) | p‐value | SHR (95% CI) | p‐value | |
| No criteria (reference) | Ref | Ref | Ref | Ref | Ref | Ref |
| One criterion | ||||||
| Univariable analysis | 1.05 (0.78–1.41) | 0.746 | 1.11 (0.71–1.74) | 0.657 | 1.01 (0.71–1.43) | 0.971 |
| Multivariable analysis | 1.09 (0.80–1.48) a | 0.582 | 1.15 (0.71–1.85) b | 0.570 | 1.05 (0.74–1.49) c | 0.792 |
| Two criteria | ||||||
| Univariable analysis | 1.24 (0.92–1.67) | 0.159 | 1.75 (1.13–2.69) | 0.011 | 0.87 (0.60–1.28) | 0.490 |
| Multivariable analysis | 1.25 (0.92–1.71) a | 0.160 | 1.78 (1.13–2.80) b | 0.012 | 0.87 (0.58–1.30) c | 0.501 |
| Three criteria | ||||||
| Univariable analysis | 1.51 (1.13–2.03) | 0.006 | 1.92 (1.25–2.95) | 0.003 | 1.41 (0.98–2.01) | 0.062 |
| Multivariable analysis | 1.40 (1.02–1.91) a | 0.038 | 1.71 (1.08–2.69) b | 0.022 | 1.34 (0.92–1.96) c | 0.132 |
| Four criteria | ||||||
| Univariable analysis | 2.63 (1.98–3.48) | <0.001 | 3.76 (2.52–5.61) | <0.001 | 1.65 (1.15–2.36) | 0.006 |
| Multivariable analysis | 2.40 (1.74–3.30) a | <0.001 | 3.03 (1.93–4.74) b | <0.001 | 1.51 (1.01–2.25) c | 0.046 |
CI, confidence interval; HFA‐ESC, Heart Failure Association of the European Society of Cardiology; HF, heart failure; HR, hazard ratio; SHR, subhazard ratio.
Adjusted for age, sex, inpatient versus outpatient status, peripheral artery disease, prior stroke or transient ischaemic attack, history of atrial fibrillation, prior myocardial infarction, chronic obstructive pulmonary disease, New York Heart Association class III–IV, systolic blood pressure and estimated glomerular filtration rate.
Adjusted for age, sex, inpatient versus outpatient status, peripheral artery disease, prior stroke or transient ischaemic attack, history of atrial fibrillation, prior myocardial infarction, chronic obstructive pulmonary disease, New York Heart Association class III–IV, systolic blood pressure, heart rate and estimated glomerular filtration rate.
Adjusted for age, sex, history of atrial fibrillation, prior myocardial infarction, chronic obstructive pulmonary disease, New York Heart Association class III–IV, left ventricular ejection fraction <40% and estimated glomerular filtration rate.
Discussion
Our study shows that the updated 2018 HFA‐ESC definition of advanced HF identifies a subset of patients with a very high risk of adverse outcomes. One‐year rates of the primary composite endpoint, as well as its single components, all‐cause mortality alone and HF hospitalization alone, were of 69.3%, 46.5% and 39.5% in patients fulfilling the HFA‐ESC definition versus 41.8%, 21.5% and 26.9% in patients evaluated as at high risk according to the ‘I NEED HELP’ classification but without all four HFA‐ESC criteria for advanced HF. The prognostic impact of the HFA‐ESC classification persisted after adjustment for several variables known to be associated with adverse outcomes in HF. Of note, most of the untoward events occurred in the first 3 months after the initial assessment. Thus, the HFA‐ESC definition of advanced HF identifies patients at a very high risk of major events and long‐term heart replacement strategies (i.e. heart transplantation or LVAD), novel therapies or palliative care must be considered as treatment options as soon as possible in these patients. 3 , 11
Several classifications have been proposed to identify patients with advanced HF. 3 , 4 , 5 , 7 , 8 However, a comprehensive but timely definition of these patients remains challenging, since no single parameter can be sufficient to define advanced HF and patients may be identified in a too advanced stage. 5 , 11 In this context, the 2018 HFA‐ESC position statement on advanced HF revised the 2007 diagnostic criteria to provide an updated classification system that takes into account HF symptoms, objective evidence of cardiac dysfunction, recurrent unplanned visits or hospitalizations, and functional impairment. 4 , 19 These updated 2018 HFA‐ESC criteria have recently been included in the 2021 ESC HF guidelines. 2 In our study, we evaluated the prognostic impact of the updated HFA‐ESC classification for advanced HF in a multicentre, contemporary, real‐world cohort of HF patients with at least one high‐risk ‘I NEED HELP’ marker enrolled at four Italian HF centres. The ‘I NEED HELP’ acronym summarizes nine risk factors that have been associated with higher mortality in patients with HF and thus represents a useful screening tool for advanced HF. 2 , 4 , 13 , 14 Our population included both patients hospitalized for acute HF and chronic HF outpatients evaluated at four high‐volume centres between January 2020 and November 2021. As expected, different classification systems identified a variable prevalence of advanced HF among all 4753 screened HF patients, ranging from 24.3% of patients with at least one ‘I NEED HELP’ screening marker to 4.1% of patients fulfilling the stricter HFA‐ESC definition. These figures, coupled with the poor prognosis in the patients fulfilling the HFA‐ESC advanced HF definition, suggest that the ‘I NEED HELP’ acronym could be useful as a screening tool for early identification of high‐risk HF patients, whereas the stricter HFA‐ESC classification could identify the subset of patients who urgently need evaluation for advanced HF therapies or palliative care. Of note, the presence of at least one ‘I NEED HELP’ marker helps to select a HF population with a high probability of advanced HF according to the HFA‐ESC definition, with the latter further stratifying those patients with a particularly increased risk of poor prognosis. Among our 1149 HF patients with at least one ‘I NEED HELP’ criterion, the updated HFA‐ESC classification identified a subset of 193 patients (16.8%) at very high risk of adverse outcomes with rates of 1‐year mortality or HF hospitalization and 1‐year mortality of 69.3% and 46.5%, respectively. Interestingly, several patients presenting with cardiogenic shock (n = 107), INTERMACS profile 1–3 (n = 55) or ACC/AHA stage D (n = 95) did not fulfil all four HFA‐ESC criteria. Despite not meeting the HFA‐ESC advanced HF definition, these patients could have a poor prognosis and, therefore, should not be excluded from timely referral to advanced HF teams or centres if clinically indicated.
Our findings are in line with a recent population‐based cohort study of all Olmsted County reporting 1‐year mortality of 49.9% among 936 patients with advanced HF according to the 2018 HFA‐ESC definition. 9 Similar criteria, NYHA class III or IV, LVEF <30% and HF hospitalization in the previous 6 months, were used in a recent analysis of the GALACTIC‐HF (Global Approach to Lowering Adverse Cardiac Outcomes Through Improving Contractility in Heart Failure) trial to define severe HF. Although the event rates were lower than in our observational study – 42.6 events per 100 patient‐years for the combined endpoint and 21.7 events for 100 patient‐years for all‐cause mortality in this analysis of GALACTIC‐HF – the criteria for severe HF identified patients with approximately twice the risk of major events, compared with the others. 20 Interestingly, the mortality of our cohort identified by the HFA‐ESC classification was higher than that reported in medically‐managed, non‐inotrope‐dependent patients with ambulatory advanced HF enrolled in the MedaMACS (Medical Arm of Mechanically Assisted Circulatory Support) study as well as in the ROADMAP (Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management) trial, 21 , 22 , 23 thus confirming the value of the HFA‐ESC classification. Nevertheless, populations enrolled in trials are highly selected, excluding several patients with high‐risk criteria and major comorbidities such as severe kidney dysfunction, whereas observational cohort studies usually include unselected real‐world patients potentially at higher risk of adverse outcomes.
Of note, the increased risk of clinical events among the patients fulfilling the HFA‐ESC advanced HF definition was mainly observed during the first 3 months after enrolment, with a 90‐day mortality or HF hospitalization rate of 44.5% and a 90‐day mortality of 32.0%. This early high risk of major events is consistent with previous data regarding patients hospitalized for HF 24 , 25 , 26 , 27 , 28 , 29 , 30 and confirms the need to rapidly intensify treatment, including further optimization of guideline‐directed treatment, care of comorbidities (such as iron supplementation in case of iron deficiency), outpatient use of intravenous diuretics and/or inotropes, and – if indicated – non‐pharmacological interventions (e.g. valvular interventions, pulmonary artery pressor sensors, catheter‐based strategies for control of arrhythmias). 2 , 4 , 31 Beyond these strategies, timely referral of these patients to advanced HF centres is of paramount importance to undergo evaluation for long‐term heart replacement therapies, such as heart transplantation or LVAD implantation, when indicated. 4 , 11 Furthermore, a proper identification of patients who are not candidates for early aggressive strategies is fundamental, in order to refer them for palliative or symptom‐focused care and avoid futility or repeated unnecessary hospitalizations, which worsen quality of life and are not cost‐effective. 4
Although most patients included in the HELP‐HF registry had HFrEF (56.5%), a relevant proportion had HFmrEF and HFpEF (15.0% and 28.5%, respectively). The patients fulfilling all four HFA‐ESC criteria for advanced HF had lower median LVEF and more frequently presented with HFrEF, however 25.4% and 16.1% of them had LVEF ≥40% and LVEF ≥50%, respectively. The aforementioned population‐based Olmsted County cohort study found that 57.7% of included patients fulfilling the HFA‐ESC advanced HF definition had HFmrEF or HFpEF. 9 This LVEF distribution resembles that observed in all‐comers, unselected, large populations of patients hospitalized for acute HF, such as the U.S. Get With The Guidelines‐Heart Failure Registry and the European ESC HFA EURObservational Research Programme HF Long‐Term Registry. 25 , 30 , 32 Thus, although treatment strategies have been developed for patients with advanced HF and HFrEF, 2 , 5 , 8 further data are needed also for patients with HFmrEF or HFpEF and advanced HF. 33 , 34 , 35 , 36 , 37
Limitations
The main limitation of our study is represented by its retrospective nature, with all the usual limitations associated with this design. Our findings need to be further validated in larger prospective studies. Furthermore, the clinical events were reported by local investigators and not externally adjudicated, although the reported outcomes (mortality and HF hospitalization) are not likely to be biased. Clinical characteristics and prognosis of patients with advanced HF could be different in cohorts of different ethnicity and race. Finally, in order to enrol a contemporary cohort that could reflect current clinical practice, we decided to restrict the study period (January 2020–November 2021), resulting in a relatively limited follow‐up (median 260 days, IQR 105–390 days).
Conclusions
In our contemporary, real‐world, multicentre cohort of patients with HF and at least one high‐risk ‘I NEED HELP’ marker, the updated 2018 HFA‐ESC definition of advanced HF identified a subset of patients with a worse clinical profile and a poor prognosis. Future studies are needed to further validate the 2018 HFA‐ESC advanced HF classification and optimize the management and outcomes of these patients.
Funding
Conflict of interest: D.S. reports personal fees from Novartis, Merck, GSK and Acceleron, outside the submitted work. M. Merlo reports personal fees for congresses from Novartis, Vifor Pharma, Pfizer, and unrestricted research grant from Pfizer, all outside the submitted work. M. Metra reports consulting honoraria of minimal amount from Abbott, Amgen, Bayer, Edwards Therapeutics, LivaNova and Vifor Pharma for participation in advisory board meetings and executive committees of clinical trials. G.S. reports consulting fees from Novartis, Impulse Dynamics and Biotronik, and speaker fees and honoraria from Novartis, Bayer, AstraZeneca, Boston Scientific, Vifor Pharma, Menarini and Akcea Therapeutics, outside the submitted work. All other authors have nothing to disclose.
Supporting information
Appendix S1. Supporting information
Acknowledgement
Open Access Funding provided by Universita degli Studi di Brescia within the CRUI‐CARE Agreement.
References
- 1. Metra M, Teerlink JR. Heart failure. Lancet. 2017;390:1981–95. [DOI] [PubMed] [Google Scholar]
- 2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 3. Truby LK, Rogers JG. Advanced heart failure. JACC Heart Fail. 2020;8:523–36. [DOI] [PubMed] [Google Scholar]
- 4. Crespo‐Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20:1505–35. [DOI] [PubMed] [Google Scholar]
- 5. Crespo‐Leiro MG, Barge‐Caballero E. Advanced heart failure: definition, epidemiology, and clinical course. Heart Fail Clin. 2021;17:533–45. [DOI] [PubMed] [Google Scholar]
- 6. Xanthakis V, Enserro DM, Larson MG, Wollert KC, Januzzi JL, Levy D, et al. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail. 2016;4:808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bjork JB, Alton KK, Georgiopoulou VV, Butler J, Kalogeropoulos AP. Defining advanced heart failure: a systematic review of criteria used in clinical trials. J Card Fail. 2016;22:569–77. [DOI] [PubMed] [Google Scholar]
- 8. Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin‐Adams M, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21:519–34. [DOI] [PubMed] [Google Scholar]
- 9. Dunlay SM, Roger VL, Killian JM, Weston SA, Schulte PJ, Subramaniam AV, et al. Advanced heart failure epidemiology and outcomes. JACC Heart Fail. 2021;9:722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lombardi CM, Cimino G, Pellicori P, Bonelli A, Inciardi RM, Pagnesi M, et al. Congestion in patients with advanced heart failure. Heart Fail Clin. 2021;17:575–86. [DOI] [PubMed] [Google Scholar]
- 11. Morris AA, Khazanie P, Drazner MH, Albert NM, Breathett K, Cooper LB, et al.; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; and Council on Hypertension. Guidance for timely and appropriate referral of patients with advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2021;144:e238–50. [DOI] [PubMed] [Google Scholar]
- 12. Guglin M, Zucker MJ, Borlaug BA, Breen E, Cleveland J, Johnson MR, et al. Evaluation for heart transplantation and LVAD implantation. J Am Coll Cardiol. 2020;75:1471–87. [DOI] [PubMed] [Google Scholar]
- 13. Baumwol J. “I Need Help” – a mnemonic to aid timely referral in advanced heart failure. J Heart Lung Transplant. 2017;36:593–54. [DOI] [PubMed] [Google Scholar]
- 14. Maddox TM, Januzzi JL, Allen LA, Breathett K, Butler J, Davis LL, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2021;77:772–810. [DOI] [PubMed] [Google Scholar]
- 15. Chioncel O, Parissis J, Mebazaa A, Thiele H, Desch S, Bauersachs J, et al. Epidemiology, pathophysiology and contemporary management of cardiogenic shock – a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020;22:1315–41. [DOI] [PubMed] [Google Scholar]
- 16. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–61. [DOI] [PubMed] [Google Scholar]
- 17. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, et al. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–6. [DOI] [PubMed] [Google Scholar]
- 18. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–71. [DOI] [PubMed] [Google Scholar]
- 19. Metra M, Ponikowski P, Dickstein K, McMurray JJV, Gavazzi A, Bergh CH, et al. Advanced chronic heart failure: a position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2007;9:684–94. [DOI] [PubMed] [Google Scholar]
- 20. Felker GM, Solomon SD, Claggett B, Diaz R, McMurray JJV, Metra M, et al. Assessment of omecamtiv mecarbil for the treatment of patients with severe heart failure: a post hoc analysis of data from the GALCTIC‐HF randomized clinical trial. JAMA Cardiol. 2022;7:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ambardekar AV, Kittleson MM, Palardy M, Mountis MM, Forde‐McLean RC, DeVore AD, et al. Outcomes with ambulatory advanced heart failure from the Medical Arm of Mechanically Assisted Circulatory Support (MedaMACS) Registry. J Heart Lung Transplant. 2019;38:408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Estep JD, Starling RC, Horstmanshof DA, Milano CA, Selzman CH, Shah KB, et al.; ROADMAP Study Investigators . Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients: results from the ROADMAP study. J Am Coll Cardiol. 2015;66:1747–61. [DOI] [PubMed] [Google Scholar]
- 23. Starling RC, Estep JD, Horstmanshof DA, Milano CA, Stehlik J, Shah KB, et al; ROADMAP Study Investigators . Risk assessment and comparative effectiveness of left ventricular assist device and medical management in ambulatory heart failure patients the ROADMAP study 2‐year results. JACC Heart Fail. 2017;5:518–27. [DOI] [PubMed] [Google Scholar]
- 24. Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJV, Granger CB, et al.; Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) Investigators . Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. [DOI] [PubMed] [Google Scholar]
- 25. Kapłon‐Cieślicka A, Benson L, Chioncel O, Crespo‐Leiro MG, Coats AJS, Anker SD, et al.; Heart Failure Association (HFA) of the European Society of Cardiology (ESC) and the ESC Heart Failure Long‐Term Registry Investigators . A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction – insights from the ESC‐HFA EORP Heart Failure Long‐Term Registry. Eur J Heart Fail. 2022;24:335–50. [DOI] [PubMed] [Google Scholar]
- 26. Kimmoun A, Takagi K, Gall E, Ishihara S, Hammoum P, El Bèze N, et al.; METAHF Team . Temporal trends in mortality and readmission after acute heart failure: a systematic review and meta‐regression in the past four decades. Eur J Heart Fail. 2021;23:420–31. [DOI] [PubMed] [Google Scholar]
- 27. Wettersten N, Horiuchi Y, Veldhuisen DJ, Ix JH, Mueller C, Filippatos G, et al. Decongestion discriminates risk for one‐year mortality in patients with improving renal function in acute heart failure. Eur J Heart Fail. 2021;23:1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCallum W, Tighiouart H, Testani JM, Griffin M, Konstam MA, Udelson JE, et al. Acute kidney function declines in the context of decongestion in acute decompensated heart failure. JACC Heart Fail. 2020;8:537–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, et al.; ESC Heart Failure Long‐Term Registry Investigators. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail. 2017;19:1242–54. [DOI] [PubMed] [Google Scholar]
- 30. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction. J Am Coll Cardiol. 2017;70:2476–86. [DOI] [PubMed] [Google Scholar]
- 31. Tomasoni D, Vishram‐Nielsen JKK, Pagnesi M, Adamo M, Lombardi CM, Gustafsson F, et al. Advanced heart failure: guideline‐directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. 2022; 9:1507–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gupta A, Allen LA, Bhatt DL, Cox M, DeVore AD, Heidenreich PA, et al. Association of the Hospital Readmissions Reduction Program implementation with readmission and mortality outcomes in heart failure. JAMA Cardiol. 2018;3:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Longhi S, Saturi G, Caponetti AG, Gagliardi C, Biagini E. Advanced heart failure in a special population: heart failure with preserved ejection fraction. Heart Fail Clin. 2021;17:685–95. [DOI] [PubMed] [Google Scholar]
- 34. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J. 2016;37:1642–50. [DOI] [PubMed] [Google Scholar]
- 36. Pagnesi M, Butler J, Metra M. Ejection fraction in heart failure: just become Emperor's new clothes? Eur J Heart Fail. 2022;24:351–2. [DOI] [PubMed] [Google Scholar]
- 37. Ferreira JP, Packer M, Butler J, Zannad F. Reconsidering the ejection fraction centric view of pharmacologic treatment for heart failure. Eur J Heart Fail. 2022;24:1148–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting information


