Abstract
Aim
The plant‐based polyphenol‐rich extract TOTUM‐63 improves glucose homeostasis in various preclinical models of obesity and type 2 diabetes (T2D). A pilot exploratory study showed that TOTUM‐63 has good safety and tolerability profiles, and beneficial effects on postprandial glucose control in healthy individuals with overweight. The aim of this study was to assess the effects of TOTUM‐63 on glycaemic control in individuals with prediabetes or early stage newly‐diagnosed T2D (which does not require pharmacological treatment).
Materials and Methods
This study was a multicentre, randomized, double‐blind, placebo‐controlled trial. Individuals with prediabetes or early stage newly‐diagnosed T2D and with overweight/abdominal obesity received TOTUM‐63 (5 g/day) or placebo for 6 months. The primary outcome was the change in fasting blood glucose.
Results
Fifty‐one participants (age: 57.1 ± 10 years; body mass index: 31.3 ± 5.7 kg.m2; 35 women and 16 men) completed the study (n = 38 TOTUM‐63, n = 13 placebo). After 6 months, blood glucose concentration after fasting and after the 2‐h oral glucose tolerance test was reduced in the TOTUM‐63‐treated group compared with the placebo group (placebo‐corrected difference between baseline and month 6: −0.71 mmol/L, p < .05, and −1.93 mmol/L, p < .05, respectively). TOTUM‐63 was safe and well tolerated and significantly reduced body weight gain (−1.9 kg; p < .05), waist circumference (−4.5 cm; p < .001), circulating triglycerides (−0.54 mmol/L; p < .01) and low‐density lipoprotein‐cholesterol (−0.38 mmol/L; p < .05) compared with placebo.
Conclusions
TOTUM‐63 lowered fasting blood glucose in participants with impaired fasting glycaemia and glucose intolerance. Moreover, TOTUM‐63 showed a good safety and tolerability profile and improved several metabolic syndrome features. Therefore, TOTUM‐63 is a promising candidate for T2D prevention.
Keywords: botanical extract, diabetic, glycaemia, metabolic syndrome, prediabetic, TOTUM‐63, weight management
1. INTRODUCTION
Prediabetes is an increasingly recognized metabolic state defined by an intermediate hyperglycaemia with glucose levels above the normal range but below diabetes diagnosis level. The prediabetes diagnostic criteria and terminology vary among organizations. The American Diabetes Association (ADA) and the World Health Organization (WHO) define prediabetes based on the detection of impaired glucose tolerance [2‐h blood glucose concentration between 7.8 and 11.0 mmol/L during an oral glucose tolerance test (OGTT)] and impaired fasting glucose (5.6‐6.9 mmol/L for ADA and 6.1‐6.9 mmol/L for WHO). Patients with prediabetes are at higher risk of type 2 diabetes (T2D) and cardiovascular diseases. 1 According to the study by the International Diabetes Federation for the 9th edition of the Diabetes Atlas, the global diabetes prevalence in 2021 was estimated at 10.5% (i.e. 537 million people worldwide). 2 Many studies showed that lifestyle or pharmacological interventions are useful to prevent prediabetes progression to T2D or even to reverse the intermediate hyperglycaemic state and restore normal glucose levels. 3 However, follow‐up studies highlighted poor long‐term adherence to lifestyle changes. Moreover, to date, no drug has been approved for prediabetes treatment or T2D prevention. 4 Therefore, new strategies targeted to patients with prediabetes or first‐stage T2D are critically needed, in association with lifestyle changes, to prevent or delay T2D appearance.
TOTUM‐63 is a patented blend of plant‐based biomolecules with a high content of polyphenolic compounds. Preclinical studies in rodent models showed that TOTUM‐63 can prevent and even reverse the development of high fat diet (HFD)‐induced excessive weight gain and glucose homeostasis impairment through pleiotropic effects in various metabolic organs. 5 , 6 , 7 In HFD‐fed rats, TOTUM‐63 exerted positive effects on body weight and glucose homeostasis that were additive to those induced by a physical activity programme, suggesting complementary benefits of these interventional strategies. 6 The first exploratory clinical trial in 14 men with overweight showed that TOTUM‐63 is safe and well tolerated, and improved the participants' glucose and insulin responses to a high‐carbohydrate breakfast test. 5 Therefore, TOTUM‐63 might be an interesting non‐pharmacological strategy to improve glucose homeostasis in people with dysglycaemia for whom pharmacological treatments are not recommended. The primary aim of this randomized placebo‐controlled trial was to assess, for the first time, the effects of TOTUM‐63 supplementation for 6 months on glucose metabolism in subjects with prediabetes or early stage newly diagnosed untreated T2D.
2. MATERIALS AND METHODS
2.1. Study design
This was a randomized (3:1 active compound to placebo), double‐blind, placebo‐controlled, parallel‐group study. This unequal randomization ratio was chosen because this study was the first proof‐of‐concept randomized clinical trial in the target population to gain information on TOTUM‐63 effects. 8 The primary goal was to assess the effect of 6‐month TOTUM‐63 supplementation on fasting plasma glucose (FPG) in subjects with prediabetes or untreated T2D. Secondary outcomes included evaluation of fasting insulinaemia, HOMA‐IR, glycated haemoglobin (HbA1c), 2‐h OGTT glycaemia (tested with 75 g of glucose), body weight, body mass index, waist circumference, hip circumference, fasting blood triglyceride concentration, total cholesterol, high‐density lipoprotein‐cholesterol, low‐density lipoprotein (LDL)‐cholesterol, non‐esterified fatty acids, systolic and diastolic blood pressure, and safety parameters. The study was registered at ClinicalTrials.gov (identifier ID: NCT02868177).
The procedures were in accordance with the ethical standards of the responsible institutional or regional committees on human experimentation and with the Declaration of Helsinki 1975, as revised in 1983.
The study was supervised by Biofortis (Saint‐Herblain, France) who managed all administrative and logistic procedures. This multicentre trial was carried out in four countries (France, Ireland, Serbia and Slovenia) and at seven sites: Clinical Investigation Unit Biofortis (Saint‐Herblain, France), Institut Pasteur de Lille/Service de Nutrition (Lille, France), Atlantia Food Clinical Trials (Cork, Ireland), Clinical Center of Vojvodina (Nova Sad, Serbia), Institute for Endocrinology (Belgrade, Serbia), Clinical Center of Kragujevac, (Kragujevac, Serbia), and University Medical Center Ljubljana (Ljubljana, Slovenia). The study was performed between October 2016 and April 2019.
The study design included a screening visit (V0): clinical examination and 2‐h OGTT (two time points: 0 and 120 min). One to 3 weeks after the V0, a randomization visit (V1) took place during which the clinical examination, blood sampling and 2‐h OGTT (five time points: 0, 30, 60, 90 and 120 min) were performed. The randomization method is detailed in Section 2.2. The experimental phase (placebo or active product) lasted 6 months. One intermediate follow‐up visit (V2) was planned after 3 months of intervention (blood sampling). The final visit (V3; clinical examination, blood sampling and 2‐h OGTT with five time points) was performed at the study end, after 6 months of intervention. A French translation of the self‐administered International Physical Activity Questionnaire‐short form (IPAQ‐sf), based on a 7‐day recall, was filled in by participants at each visit. A 3‐day food survey was completed in the last week before V1, V2 and V3 (one weekend day and at least the day before the visit). Routine laboratory safety assessments were performed according to the standard laboratory procedures of the accredited laboratory at each study centre. The other laboratory analyses were done at local laboratories: all blood testing at V0, safety parameters at V3, non‐esterified fatty acids and HbA1c at V1, V2 and V3. Other measurements and analyses (efficacy parameters) were performed at Biofortis Central Laboratory.
2.2. Randomization
At V1, each subject was randomly assigned to TOTUM‐63 or placebo (3:1) using the blocked randomization table generated by the SAS® software (version 9.3 or higher; SAS Institute Inc., Cary, NJ, USA) after checking the participant's eligibility once the results were available for inclusion (after the V0 screening visit). This minimized the selection bias. Product allocation depended only on the subject inclusion sequence in the study. The investigator allocated the product in accordance with the randomization number assigned by the computer to the participants at V1. The randomization list (identification of active/placebo batches) was prepared before the study start by a person not related to the clinical phase or data management. Therefore, during the whole study and in the absence of unblinding, investigators and participants were not aware of the product they tested/took. Every effort was made to maintain blinding during the study. The product labelling did not show any difference between the test and placebo products.
2.3. Inclusion and exclusion criteria
The main inclusion criteria were: age between 35 and 75 years (limits included); prediabetes or T2D that did not require immediate pharmacological therapy according to the current guidelines; waist circumference >94 cm for men and >80 cm for women; reported body weight variations <5% in the 3 months before randomization; no significant change in food habits or physical activity in the 3 months before randomization, and agreement to keep them unchanged throughout the study (no hyper‐hypocaloric diet, no start‐stop of physical activity planned in the next 7 months); FPG >6.1 mmol/L; 2‐h OGTT glycaemia >7.8 mmol/L; HbA1c <7%; and fasting blood triglycerides >1.5 g/L.
The main exclusion criteria were: metabolic disorder (e.g. treated diabetes); uncontrolled thyroid disorder or other metabolic disorders; uncontrolled hypertension (systolic blood pressure ≥160 mmHg and/or diastolic blood pressure ≥100 mmHg); severe chronic disease or gastrointestinal disorders found to be incompatible with the study participation by the investigator; current antidiabetic or lipid‐lowering treatment; ongoing treatment with drugs that could affect glucose and/or lipid homeostasis parameters; regular intake of dietary supplements or ‘functional foods’ rich in plant stanol or sterol, long chain omega‐3 fatty acids or other substances intended to lower LDL‐cholesterol, triglycerides or glycaemia; treatment or dietary supplements that could significantly affect the parameter(s) monitored during the study according to the investigator; dietary supplement intake in the last month before V1; intake of more than three (for men) and two (for women) standard alcoholic drinks per day; extreme eating habits (e.g. vegetarian or vegan).
2.4. Intervention
Participants started taking the assigned product the day after V1. The test product was a food supplement packaged in capsules that contained 623.85 mg of TOTUM‐63, a patented blend of biomolecules extracted from five plants (Chrysanthellum indicum subsp. afroamericanum B.L. Turner, Cynara scolymus L., Vaccinium myrtillus L., Olea europaea L. and Piper nigrum L.). The chemical characterization of the batch used in this study was performed with the Folin‐Ciocalteu method to estimate the total polyphenols, the AOAC Method 2017 to estimate total fibre, and high‐performance liquid chromatography‐ultraviolet/visible/mass spectrometry to quantify the other compounds of interest (Table S1). The placebo contained an equal amount of maltodextrin (623.75 mg/capsule). Participants took eight capsules per day (623.75 mg of TOTUM‐63 or maltodextrin/capsule, thus 4.99 g of TOTUM‐63 or placebo per day): three capsules just before breakfast, two capsules just before lunch and three capsules just before dinner for 6 months.
2.5. Statistics
Statistical analyses were performed with the Stata software (version 13; StataCorp, College Station, TX, USA).
Sample size was estimated according to (a) the CONSORT 2010 statement, extension to randomized pilot and feasibility trials, 9 and (b) the Cohen's d value that defines effect‐sizes as small (d = 0.2), medium (d = 0.5) and large (d = 0.8; ‘grossly perceptible and therefore large’). 10 Sample size was calculated to highlight an effect‐size equal to at least 1 standard‐deviation. For a two‐sided type I error at 5% and a statistical power of ~90%, at least 13 participants in the placebo group and 44 in the TOTUM‐63 group were needed. To take into account participants’ lost to follow‐up (~20%), it was proposed to include 66 participants in total.
All tests were two‐sided, with a type I error set at 0.05. Continuous data were expressed as mean ± SD or median (IQR) if not normally distributed. Statistical analyses were performed with linear mixed models to consider the variability between and within participants (subjects as random‐effect) and to measure the following fixed effects: group (placebo vs. TOTUM‐63), time (V1 and V3), and their interaction. The baseline value of the dependent variable was considered as an adjustment covariate. The normality of residuals was studied using the Shapiro‐Wilk test. When appropriate, a logarithmic transformation of the dependent variable was proposed to achieve the normality and satisfy the statistical assumptions. Descriptive statistics, including the percentage of participants with at least one adverse event and the number of events per participant, were generated in function of the adverse event severity level, link with the research procedure or study product, and body system. For each severity level and link to the research procedure or study product, the percentage of subjects showing at least one adverse event was compared between groups using the chi‐squared or Fisher's exact test (if n < 5). The number of adverse events per participants was compared between groups using a Poisson regression with robust standard errors.
3. RESULTS
Among the 157 subjects screened for eligibility, 91 were excluded (not meeting the inclusion criteria: n = 85; eligible but refused to participate: n = 6). The remaining 66 participants were randomly allocated to the placebo (n = 15) or TOTUM‐63 (n = 51) group. Eight participants withdraw prematurely from the study for personal reasons. Seven participants were excluded from the analysis during the blind data review for major deviations from the protocol. Two of them were excluded for wrong inclusion and five were excluded for poor respect of the instructions and low compliance, mainly related to large differences between visit dates for personal reasons (n = 4), or initiation of a treatment forbidden by the exclusion criteria during the study (n = 1). Finally, 51 participants (13 in the placebo and 38 in the TOTUM‐63 group) were included in the analysis. Figure 1 shows the flowchart of the participants' inclusion/exclusion process.
FIGURE 1.
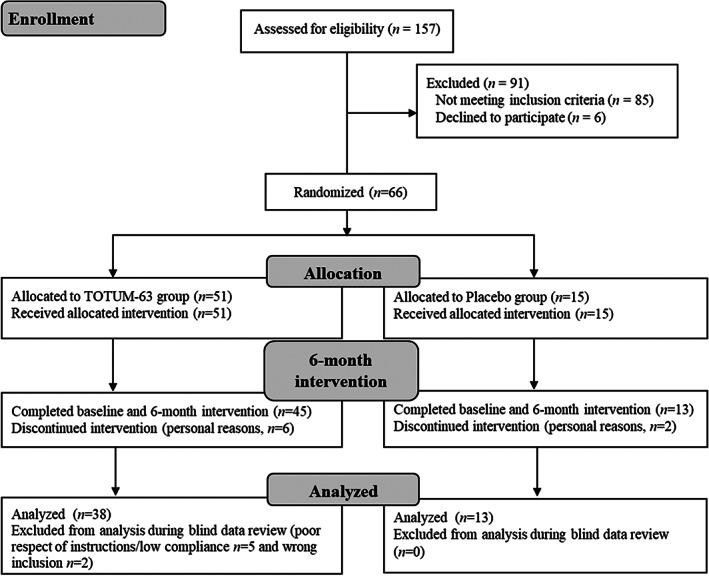
Flowchart of the participants' inclusion/exclusion process
3.1. Baseline characteristics
At baseline (V1) (Table 1), all participants had overweight or obesity, prediabetes or newly‐diagnosed T2D, and hypertriglyceridaemia. There was no significant difference in the baseline characteristics between placebo and TOTUM‐63 groups.
TABLE 1.
Baseline participants' characteristics a
| Variable | Placebo (n = 13) | TOTUM‐63 (n = 38) | p value |
|---|---|---|---|
| Age, years | 56.2 ± 12.9 | 57.4 ± 9.3 | .72 |
| Sex, n (%) | .73 | ||
| Women | 10 (76.9) | 25 (65.8) | |
| Men | 3 (23.1) | 13 (34.2) | |
| Smoking status, n (%) | .71 | ||
| Current smokers | 2 (15.4) | 9 (23.7) | |
| Non‐smokers | 11 (84.6) | 29 (76.3) | |
| Prediabetes/T2D, n (%) | .21 | ||
| Prediabetes | 6 (46) | 25 (66) | |
| T2D | 7 (54) | 13 (34) | |
| Body weight, kg | 89.5 ± 15.1 | 85.0 ± 16.5 | .37 |
| BMI, kg/m2 | 33.3 ± 6.1 | 30.6 ± 5.0 | .13 |
| Waist circumference, cm | 103.9 ± 11.0 | 103.6 ± 12.3 | .94 |
| Hip circumference, cm | 113.0 ± 13.2 | 107.0 ± 9.6 | .15 |
| Waist to hip ratio | 0.92 ± 0.06 | 0.97 ± 0.87 | .73 |
| Fasting plasma glucose, mmol/L | 6.99 ± 1.49 | 6.89 ± 1.14 | .83 |
| 2‐h OGTT glycaemia, mmol/L | 10.70 ± 2.40 | 10.10 ± 3.17 | .48 |
| Triglycerides, mmol/L | 1.66 ± 0.73 | 2.11 ± 0.93 | .09 |
| Systolic blood pressure, mmHg | 131.9 ± 12.2 | 130.8 ± 14.1 | .79 |
| Diastolic blood pressure, mmHg | 82.3 ± 15.2 | 82.3 ± 9.4 | 1.00 |
| Physical activity score (MET min/week) | 2044 ± 2644 | 2646 ± 3819 | .53 |
Values are the mean ± SD except for sex: n (%); smoking status: n (%) and Prediabetes/T2D: n (%).
Abbreviations: BMI, body mass index; MET, metabolic equivalent of task; OGTT, oral glucose tolerance test; T2D, type 2 diabetes.
3.2. Dietary intake and physical activity
Comparison of data collected in the dietary surveys did not reveal any significant difference between groups (placebo and TOTUM‐63) concerning the change of total energy intake between V1 and V2 (−12.5 ± 457.9 vs. 35.7 ± 482.1 kcal/day, respectively, p = NS) and between V1 and V3 (−131.9 ± 631.2 vs. −10.7 ± 528.7 kcal/day, respectively, p = NS). Similarly, the IPAQ‐sf (physical activity) score changes between V1 and V2 (−51.3 ± 2457.4 vs. 75.1 ± 4238.4 MET‐min/week respectively, p = NS) and between V1 and V3 (−513.3 ± 3375.2 vs. −103.3 ± 2283.5 MET‐min/week respectively, p = NS) were comparable between groups.
3.3. TOTUM‐63 improves glucose homeostasis
The mean difference in FPG change from baseline to month 6 (V1‐V3) between the TOTUM‐63 and placebo groups was 0.71 mmol/L (Figure 2A; −0.24 ± 1.04 vs. 0.47 ± 0.90 mmol/L respectively, p < .05). The FPG difference between groups was confirmed by multivariate analysis following adjustment for body mass index (not shown, p = .02). Similarly, the mean between‐group difference in the V1‐V3 change of 2‐h OGTT glycaemia was 1.93 mmol/L (Figure 2B; −0.12 ± 2.53 vs. 1.81 ± 3.58 mmol/L, respectively, p < .05).
FIGURE 2.
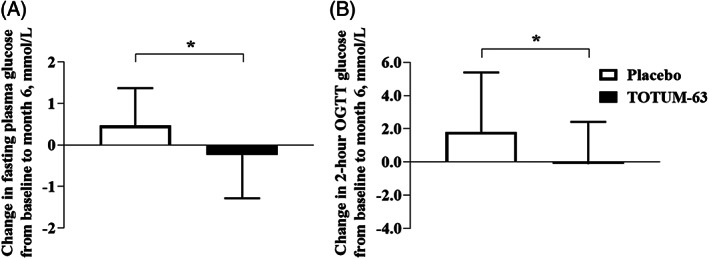
Mean absolute changes in (A) fasting blood glucose and (B) 2‐h oral glucose tolerance test (OGTT) glucose concentration from baseline to month 6. Data are the mean absolute change ± SD. Statistical analyses were performed with linear mixed models with baseline values as the adjustment covariate. *p < .05
3.4. TOTUM‐63 improves body weight, waist circumference, lipid profile and systolic blood pressure
The mean differences between the TOTUM‐63 and placebo group in the V1‐V3 change of body weight and waist circumference were 1.9 kg (−0.07 ± 2.58 vs. 1.83 ± 2.05 kg, respectively, p < .05) and 4.48 cm (−1.67 ± 4.51 vs. 2.81 ± 2.34 cm, respectively, p < .001) (Table 2). The mean between‐group differences in the V1‐V3 change of triglycerides, total cholesterol and LDL‐cholesterol were 0.54 mmol/L (p < .01), 0.6 mmol/L (p < .05) and 0.38 mmol/L (p < .05), respectively. The mean between‐group difference in the V1‐V3 change of systolic blood pressure was 10.5 mmHg (p < .01). Conversely, V1‐V3 changes in HOMA‐IR, HbA1c, high‐density lipoprotein‐cholesterol and diastolic blood pressure from baseline to month 6 were not different between groups.
TABLE 2.
Changes in anthropometric and metabolic parameters between baseline and month 6 a
| Placebo (n = 13) | TOTUM‐63 (n = 38) | Intervention effects | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | Placebo change | TOTUM‐63 change | p value | |
| Body weight, kg | 89.5 ± 15.1 | 91.4 ± 15.5 | 85.0 ± 16.5 | 84.9 ± 16.7 | 1.83 ± 2.05 | −0.07 ± 2.58 | .02 |
| Body mass index, kg/m2 | 33.3 ± 6.1 | 33.9 ± 6.2 | 30.6 ± 5.0 | 30.6 ± 5.2 | 0.7 ± 0.7 | −0.01 ± 0.9 | .01 |
| Waist circumference, cm | 103.9 ± 11.0 | 106.7 ± 11.3 | 103.6 ± 12.2 | 101.9 ± 12.9 | 2.8 ± 2.34 | −1.7 ± 4.51 | .001 |
| Hip circumference, cm | 113.0 ± 13.2 | 113.8 ± 13.9 | 107.0 ± 9.6 | 107.1 ± 9.6 | 0.8 ± 3.0 | 0.1 ± 4.2 | .62 |
| Fasting plasma glucose, mmol/L | 6.99 ± 1.49 | 7.47 ± 1.62 | 6.89 ± 1.14 | 6.66 ± 0.83 | 0.48 ± 0.90 | −0.24 ± 1.04 | .026 |
| Fasting insulinaemia, mU/L | 21.2 ± 19.5 | 18.6 ± 13.5 | 21.0 ± 13.6 | 18.8 ± 10.8 | −2.7 ± 8.0 | −2.1 ± 14.0 | .76 |
| HOMA‐IR score | 7.41 ± 9.21 | 6.45 ± 5.81 | 6.50 ± 4.65 | 5.54 ± 3.18 | −0.96 ± 3.85 | −0.97 ± 5.10 | .42 |
| HbA1c, % | 6.23 ± 0.46 | 6.40 ± 0.67 | 5.92 ± 0.51 | 5.93 ± 0.57 | 0.17 ± 0.37 | 0.01 ± 0.34 | .20 |
| 2‐h OGTT glycaemia, mmol/L | 10.77 ± 2.45 | 12.53 ± 3.44 | 10.13 ± 3.17 | 10.01 ± 3.07 | 1.81 ± 3.58 | −0.12 ± 2.53 | .049 |
| Triglycerides, mmol/L | 1.66 ± 0.73 | 1.83 ± 0.68 | 2.11 ± 0.93 | 1.75 ± 0.97 | 0.18 ± 0.62 | −0.36 ± 0.72 | .008 |
| Total cholesterol, mmol/L | 5.24 ± 1.07 | 5.57 ± 1.21 | 5.58 ± 0.92 | 5.31 ± 0.87 | 0.33 ± 0.93 | −0.27 ± 0.78 | .02 |
| LDL‐cholesterol, mmol/L | 3.22 ± 0.92 | 3.43 ± 0.93 | 3.46 ± 0.78 | 3.28 ± 0.70 | 0.21 ± 0.65 | −0.17 ± 0.65 | .04 |
| HDL‐cholesterol, mmol/L | 1.24 ± 0.24 | 1.29 ± 0.29 | 1.15 ± 0.36 | 1.21 ± 0.33 | 0.05 ± 0.20 | 0.06 ± 0.16 | .59 |
| NEFA, mmol/L | 0.49 ± 0.20 | 0.47 ± 0.20 | 0.56 ± 0.20 | 0.46 ± 0.18 | −0.02 ± 0.19 | −0.10 ± 0.23 | .30 |
| Systolic blood pressure, mmHg | 131.9 ± 12.2 | 139.5 ± 20.4 | 130.8 ± 14.1 | 127.7 ± 12.4 | 7.5 ± 11.9 | −3.0 ± 10.2 | .004 |
| Diastolic blood pressure, mmHg | 82.3 ± 15.2 | 84.2 ± 11.6 | 82.3 ± 9.3 | 80.6 ± 7.4 | 1.8 ± 7.1 | −1.7 ± 9.0 | .12 |
Values are the mean ± SD. Statistical analyses were performed with linear mixed models and baseline values as the adjustment covariate.
Abbreviations: HbA1c, glycated haemoglobin; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NFEA, non‐esterified fatty acids; OGTT, oral glucose tolerance test.
3.5. TOTUM‐63 is safe and well tolerated
The safety outcomes are summarized in Table 3. In the safety population, 84 adverse events in 42 participants were reported (i.e. a mean of 1.27 adverse events per participant, in both groups). The only serious adverse event (tachycardia with chest pain) was not linked to the product according to the investigator. Ten adverse events (gastrointestinal problems: abdominal pain, diarrhoea or nausea) for which a link with the study product could not be excluded were recorded. The percentages of participants with serious adverse events (p = 1.00), severe adverse events (p = .22), moderate or mild adverse events (p = .63), adverse events for which a link with the research (p = 1.00) or with the study product (p = .67) was not excluded were comparable between groups.
TABLE 3.
Adverse events in the safety population a
| Placebo (N = 15) | TOTUM‐63 (N = 51) | ||||
|---|---|---|---|---|---|
| Adverse events | Concerned participants: n (%) | Number of events | Concerned participants: n (%) | Number of events | p value |
| All adverse events | 11 (73.3) | 19 | 31 (60.8) | 65 | .37 |
| All serious adverse events | 0 (0) | 0 | 1 (2) | 1 | 1.00 |
| Event intensity | |||||
| Mild | 7 (46.7) | 7 | 15 (29.4) | 16 | .23 |
| Moderate | 8 (53.3) | 8 | 27 (52.9) | 47 | 1.00 |
| Severe | 2 (13.3) | 4 | 2 (3.9) | 2 | .22 |
| Link with the research | |||||
| Excluded | 10 (66.7) | 17 | 27 (52.9) | 59 | .35 |
| Not excluded | 2 (13.3) | 2 | 6 (11.8) | 6 | .87 |
| Link with the study product | |||||
| Excluded | 10 (66.7) | 18 | 27 (52.9) | 56 | .35 |
| Not excluded | 1 (6.7) | 1 | 7 (13.7) | 9 | .67 |
| Action taken | |||||
| Not applicable | 1 (6.7) | 1 | 10 (19.6) | 12 | .43 |
| No action | 9 (60.0) | 16 | 27 (52.9) | 43 | .63 |
| Product definitively stopped | 2 (13.3) | 2 | 4 (7.8) | 4 | .61 |
| Product interrupted | 0 (0) | 0 | 3 (5.9) | 6 | 1.00 |
| Adverse event course | |||||
| End of adverse event | 11 (73.3) | 16 | 26 (51.0) | 54 | .13 |
| Adverse event induced another adverse event | 0 (0) | 0 | 2 (3.9) | 2 | 1.00 |
| Still in progress at the study end | 2 (13.3) | 3 | 8 (15.7) | 9 | .82 |
| Adverse events by body system | |||||
| Cardiovascular | 0 (0.0) | 0 | 3 (5.9) | 4 | 1.00 |
| Respiratory | 2 (13.3) | 2 | 2 (3.9) | 2 | .22 |
| Gastrointestinal | 2 (13.3) | 2 | 12 (23.5) | 14 | .50 |
| Neurologic/Psychiatric | 2 (13.3) | 2 | 10 (19.6) | 15 | .72 |
| ENT | 5 (33.3) | 5 | 11 (21.6) | 12 | .35 |
| Mucocutaneous | 1 (6.7) | 1 | 1 (2.0) | 1 | .41 |
| Musculoskeletal | 3 (20.0) | 5 | 8 (15.7) | 10 | .70 |
| Other | 2 (13.3) | 2 | 6 (11.8) | 7 | .87 |
AE, adverse event; ENT, ear nose throat system.
4. DISCUSSION
Our results show that the supplementation of TOTUM‐63 for 6 months reduced FPG in individuals with prediabetes or early stage newly diagnosed untreated T2D, compared with placebo. Similarly, the OGTT response was improved, confirming the beneficial impact of this polyphenol‐rich supplement on the whole‐body glucose homeostasis in these subjects with glucose intolerance. Moreover, TOTUM‐63 reduced body weight, waist circumference, lipid profile and blood pressure compared with placebo. Long‐term supplementation with TOTUM‐63 was safe and well tolerated.
The main goal of this study was to assess the effect of TOTUM‐63 supplementation on FPG in subjects with prediabetes or early stage newly‐diagnosed untreated T2D. At the end of the 6‐month intervention, FPG was increased by 0.48 mmol/L in the placebo group, and decreased by 0.24 mmol/L in the TOTUM‐63 group, resulting in a significant placebo‐corrected difference of −0.72 mmol/L (−9.3%) from baseline. Glucose homeostasis worsening in the absence of any intervention is a well‐known feature in this high‐risk population. Indeed, approximately 5‐10% of individuals with prediabetes progress to T2D every year. 11 In our population, the combination of abdominal obesity, hypertriglyceridaemia, impaired glucose tolerance and impaired fasting glucose greatly increased the risk of T2D. In this context, TOTUM‐63 effect on FPG is a clinically‐relevant finding, and is in line with those obtained in interventional studies based on lifestyle interventions with/without drugs. For instance, the Diabetes Prevention Program study (n = 3234 individuals with elevated fasting and post‐load blood glucose concentration) 12 showed that at 6 months (early response), FPG was decreased by −0.06 mmol/L in the standard lifestyle recommendations plus placebo group and by −0.22 mmol/L in the standard lifestyle recommendations plus metformin and intensive programme of lifestyle modifications groups. The effect of TOTUM‐63 on FPG was strengthened by its positive impact on the 2‐h OGTT glycaemia, confirming previous results obtained in an exploratory single‐arm clinical trial in men with overweight and normal glycaemia. This previous study observed an improvement of the glucose and insulin responses to a carbohydrate tolerance test following 4 weeks of supplementation with TOTUM‐63. 5 Moreover, a preclinical study in HFD‐fed rats showed that TOTUM‐63 has additive beneficial effects on glycaemic control when combined with physical activity. 6 Hence, the association of TOTUM‐63 supplementation and lifestyle interventions may be interesting because it could lead to higher benefits for individuals with prediabetes and reduce T2D risk.
More experiments are needed to elucidate fully the mechanisms underlying beneficial effects of TOTUM‐63 on glucose homeostasis in people with prediabetes or T2D. Preclinical studies in HFD‐fed rodents showed positive effects on FPG and glucose tolerance, associated with pleiotropic effects on various metabolic organs. 5 , 6 , 7 In a study conducted in HFD‐fed mice, the hyperinsulinaemic‐euglycaemic clamp method showed that TOTUM‐63 supplementation improves insulin sensitivity. This effect was associated with enhanced glucose uptake and insulin signalling in various peripheral organs, particularly in skeletal muscle, the main tissue involved in insulin‐mediated glucose disposal. 7 Moreover, TOTUM‐63 anti‐inflammatory properties could also play some role in the improved insulin sensitivity, as the plant extract could also counteract obesity‐associated metaflammation in HFD‐fed mice, 7 which is implicated in the dysregulation of various metabolic pathways. 13 TOTUM‐63 effect on glucose homeostasis could also involve other mechanisms and different tissues. Indeed, in mice, TOTUM‐63 can reduce energy assimilation in the intestine. 5 , 7 This effect might partly explain the beneficial effects of TOTUM‐63 on body weight. The reduction of fat mass and body weight might contribute to some of the beneficial effects of TOTUM‐63 on metabolic homeostasis; however, weight‐matched analyses in HFD‐fed mice revealed that the effect of TOTUM‐63 on glucose control would probably not be explained by its anti‐obesity effects. 7 In the present study, a sensitivity analysis of the fasting blood glucose concentration and 2‐h OGTT glycaemia after adjustment for body weight confirmed that the effects of TOTUM‐63 were independent of body weight changes (not shown, p = .02 and p = .03 for fasting blood glucose and 2‐h OGTT glycaemia, respectively). The effects of TOTUM‐63 might also be because of a positive impact on gut microbiota. Indeed, two independent preclinical studies showed that TOTUM‐63 improves HFD‐induced gut microbiota dysbiosis in rodents. 5 , 6 Based on the growing evidence that gut dysbiosis is implicated in the pathogenesis of metabolic disorders, 14 the beneficial effect of TOTUM‐63 on gut microbiota would probably contribute to the improvement of glucose homeostasis and of other metabolic parameters, possibly via the many polyphenolic compounds present in this supplement. 15 However, because of the many potentially bioactive molecules present in TOTUM‐63, it is very difficult to identify the specific effects of a given molecule (or secondary metabolites). Moreover, these different molecules may have many potential interactions, with possible inhibitory and/or synergistic outcomes. 16 , 17 Nevertheless, it has been already shown that some of the main TOTUM‐63 components, particularly oleuropein or chlorogenic acid, are implicated in glucose homeostasis regulation. The acute intake of oleuropein in healthy subjects lowers postprandial glycaemia. 18 Similarly, acute or chronic administration of chlorogenic acid in healthy volunteers or patients with impaired glucose tolerance improves glucose homeostasis. 19 , 20 , 21 Therefore, oleuropein and chlorogenic acid are major candidates to explain partly the glucose homeostasis changes observed in the TOTUM‐63 group. Many other compounds included in the formulation of TOTUM‐63 (e.g. luteolin, apigenin 22 , 23 , 24 , 25 and various anthocyanins 26 , 27 , 28 , 29 ) also have interesting effects on glucose homeostasis and could contribute, alone or in combination, to the beneficial effects of TOTUM‐63, probably via their impact on the gut microbiota. 30 , 31 Moreover, we cannot exclude the involvement of potentially non‐identified biomolecules or secondary metabolites derived from microbial activity or liver metabolism.
Prediabetes and T2D are often associated with other metabolic abnormalities, such as obesity, hypertension and dyslipidaemia, 32 thus requiring different concomitant treatments (polypharmacy). The use of combination therapies in individuals with T2D has greatly increased in the last two decades. 33 In this context, plant‐based active substances that contain various bioactive phytochemicals might act simultaneously on different therapeutic targets. 34 , 35 In the present study, besides its glucose‐lowering properties, TOTUM‐63 supplementation had additional beneficial effects on body weight/waist circumference, LDL‐cholesterol/triglycerides and systolic blood pressure in participants with prediabetes/untreated T2D. This strongly suggests that TOTUM‐63 supplementation is a highly relevant interventional strategy in this population. Moreover, the present study also confirmed TOTUM‐63 long‐term safety and good tolerability in a population with prediabetes/untreated T2D, as already observed in a previous shorter‐term exploratory study in volunteers with overweight. 5 Considering the crucial issue of the tolerability linked to the intrinsic side effects of antidiabetic drugs and/or polypharmacy in patients with T2D, good tolerability of TOTUM‐63 is important and clinically relevant.
The main limitation of the present study is the relatively low number of subjects (n = 13 in the placebo and n = 38 in the TOTUM‐63 group). The drop‐out rate was higher than expected (13 vs. 38 analysed instead of 13 vs. 44 anticipated). However, as the sample size was estimated with a statistical power of ~90%, the statistical power remained >80% to detect significant differences, despite this higher than anticipated drop‐out rate. The low number of participants did not allow subgroup analyses to be performed (e.g. prediabetes vs. T2D, men vs. women). Future clinical studies must: (a) confirm the present results in a larger sample, and (b) thoroughly investigate the mechanisms underlying the beneficial effects of TOTUM‐63 in humans, by assessing key biomarkers and parameters associated with glucose control improvement.
As a reminder, there is now a growing consensus that the prevalence of prediabetes is a health care challenge and that interventions are required to delay or prevent progression to T2D. While T2D prevention is a target priority for the WHO, there is currently no approved medication for prediabetes treatment. In this context and as a conclusion, this study showed that TOTUM‐63, a new plant‐based polyphenol‐rich active substance, has positive effects on glucose homeostasis, and on body weight, waist circumference, lipid profile and blood pressure in individuals with prediabetes or early stage untreated T2D and overweight/obesity. TOTUM‐63 is therefore a promising candidate for T2D risk prevention and is currently tested in a larger clinical study (ClinicalTrials.gov NCT04423302).
AUTHOR CONTRIBUTIONS
Pascal Sirvent, Murielle Cazaubiel, Sebastien L. Peltier and Jean‐Marie Bard: designed the research; Vivien Chavanelle, Yolanda F. Otero, Maxime Bargetto, Florian Le Joubioux, Murielle Cazaubiel and Jean‐Marie Bard: conducted the research; Pascal Sirvent, Vivien Chavanelle, Yolanda F. Otero, Maxime Bargetto, Florian Le Joubioux, Nathalie Boisseau, Thierry Maugard, Murielle Cazaubiel, Bruno Pereira, Bruno Guigas, Samy Hadjadj, Sebastien L. Peltier, André Marette and Jean‐Marie Bard: analysed the data; Pascal Sirvent and Jean‐Marie Bard: wrote the manuscript; Vivien Chavanelle, Yolanda F. Otero, Maxime Bargetto, Florian Le Joubioux, Nathalie Boisseau, Thierry Maugard, Murielle Cazaubiel, Bruno Pereira, Bruno Guigas, Samy Hadjadj, Sebastien L. Peltier, André Marette and Jean‐Marie Bard: revised the manuscript; Pascal Sirvent and Jean‐Marie Bard: had primary responsibility for final content; and all authors: read and approved the final manuscript. Supported in part by BPI (Banque Publique d'Investissement) France and by Valbiotis. The study design, implementation, analysis, and interpretation were carried out jointly by the funders and principal investigator.
CONFLICT OF INTEREST
Pascal Sirvent, Vivien Chavanelle, Yolanda F. Otero, Maxime Bargetto, Florian Le Joubioux and Murielle Cazaubiel are Valbiotis employees. Sebastien L. Peltier is Valbiotis CEO. All other authors declare that they have no competing interests.
5.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14817.
ACKNOWLEDGMENTS
The authors thank Odd‐Erik Johansen (Nestle Health Science) for scientific assistance and advice in the process of writing and formatting this article and Elisabetta Andermarcher for the careful reading of this article.
Sirvent P, Chavanelle V, Otero YF, et al. TOTUM‐63, a plant‐based polyphenol‐rich extract, improves glycaemic control in subjects with prediabetes or early stage newly‐diagnosed type 2 diabetes in a randomized, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2022;24(12):2331‐2340. doi: 10.1111/dom.14817
Clinical Trial Registry number: NCT02868177 https://clinicaltrials.gov/ct2/show/NCT02868177.
Funding information BPI (Banque Publique d'Investissement) France; Valbiotis
DATA AVAILABILITY
Data described in the manuscript, code book and analytic code will be made available upon request pending approval by the corresponding author.
REFERENCES
- 1. Beulens J, Rutters F, Ryden L, et al. Risk and management of pre‐diabetes. Eur . J Prev Cardiol. 2019;26(2_suppl):47‐54. [DOI] [PubMed] [Google Scholar]
- 2. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country‐level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sallar A, Dagogo‐Jack S. Regression from prediabetes to normal glucose regulation: state of the science. Exp Biol Med. 2020;245(10):889‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kerrison G, Gillis RB, Jiwani SI, et al. The effectiveness of lifestyle adaptation for the prevention of prediabetes in adults: a systematic review. J Diabetes Res. 2017;2017:8493145‐8493120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chavanelle V, Otero YF, Le Joubioux F, et al. Effects of Totum‐63 on glucose homeostasis and postprandial glycemia: a translational study. Am J Physiol Endocrinol Metab. 2021;320(6):E1119‐E1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dupuit M, Chavanelle V, Chassaing B, et al. The TOTUM‐63 supplement and high‐intensity interval training combination limits weight gain, improves glycemic control, and influences the composition of gut mucosa‐associated bacteria in rats on a high fat diet. Nutrients. 2021;13(5):1569‐1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van der Zande HJP, Lambooij JM, Chavanelle V, et al. Effects of a novel polyphenol‐rich plant extract on body composition, inflammation, insulin sensitivity, and glucose homeostasis in obese mice. Int J Obes. 2021;45(9):2016‐2027. [DOI] [PubMed] [Google Scholar]
- 8. Peckham E, Brabyn S, Cook L, Devlin T, Dumville J, Torgerson DJ. The use of unequal randomisation in clinical trials — an update. Contemp Clin Trials. 2015;45(Pt A):113‐122. [DOI] [PubMed] [Google Scholar]
- 9. Eldridge SM, Chan CL, Campbell MJ, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t‐tests and ANOVAs. Front Psychol. 2013;4:863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high‐risk state for diabetes development. Lancet. 2012;379(9833):2279‐2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177‐185. [DOI] [PubMed] [Google Scholar]
- 14. Yang G, Wei J, Liu P, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism. 2021;117:154712. [DOI] [PubMed] [Google Scholar]
- 15. Anhe FF, Varin TV, Le Barz M, et al. Gut microbiota Dysbiosis in obesity‐linked metabolic diseases and prebiotic potential of polyphenol‐rich extracts. Curr Obes Rep. 2015;4(4):389‐400. [DOI] [PubMed] [Google Scholar]
- 16. Caesar LK, Cech NB. Synergy and antagonism in natural product extracts: when 1 + 1 does not equal 2. Nat Prod Rep. 2019;36(6):869‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Y, Zhang Z, Li S, Ye X, Li X, He K. Synergy effects of herb extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia. 2014;92:133‐147. [DOI] [PubMed] [Google Scholar]
- 18. Carnevale R, Silvestri R, Loffredo L, et al. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br J Clin Pharmacol. 2018;84(7):1566‐1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuniga LY, Aceves‐de la Mora MCA, Gonzalez‐Ortiz M, Ramos‐Nunez JL, Martinez‐Abundis E. Effect of chlorogenic acid administration on glycemic control, insulin secretion, and insulin sensitivity in patients with impaired glucose tolerance. J Med Food. 2018;21(5):469‐473. [DOI] [PubMed] [Google Scholar]
- 20. Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78(4):728‐733. [DOI] [PubMed] [Google Scholar]
- 21. Thom E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long‐term in overweight and obese people. J Int Med Res. 2007;35(6):900‐908. [DOI] [PubMed] [Google Scholar]
- 22. Bumke‐Vogt C, Osterhoff MA, Borchert A, et al. The flavones apigenin and luteolin induce FOXO1 translocation but inhibit gluconeogenic and lipogenic gene expression in human cells. PLoS One. 2014;9(8):e104321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang M, Jiang ZH, Li CG, et al. Apigenin prevents metabolic syndrome in high‐fructose diet‐fed mice by Keap1‐Nrf2 pathway. Biomed Pharmacother. 2018;105:1283‐1290. [DOI] [PubMed] [Google Scholar]
- 24. Jung UJ, Cho YY, Choi MS. Apigenin ameliorates dyslipidemia, hepatic steatosis and insulin resistance by modulating metabolic and transcriptional profiles in the liver of high‐fat diet‐induced obese mice. Nutrients. 2016;8(5):305‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ren B, Qin W, Wu F, et al. Apigenin and naringenin regulate glucose and lipid metabolism, and ameliorate vascular dysfunction in type 2 diabetic rats. Eur J Pharmacol. 2016;773:13‐23. [DOI] [PubMed] [Google Scholar]
- 26. Promyos N, Temviriyanukul P, Suttisansanee U. Investigation of anthocyanidins and anthocyanins for targeting alpha‐glucosidase in diabetes mellitus. Prev Nutr Food Sci. 2020;25(3):263‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Y, Zhang JL, Zhou Q. Targets and mechanisms of dietary anthocyanins to combat hyperglycemia and hyperuricemia: a comprehensive review. Crit Rev Food Sci Nutr. 2022;62(4):1119‐1143. [DOI] [PubMed] [Google Scholar]
- 28. Oliveira H, Fernandes A, FB N, Mateus N, de Freitas V, Fernandes I. Anthocyanins as antidiabetic agents‐in vitro and in Silico approaches of preventive and therapeutic effects. Molecules. 2020;25(17):3813‐3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Les F, Casedas G, Gomez C, Moliner C, Valero MS, Lopez V. The role of anthocyanins as antidiabetic agents: from molecular mechanisms to in vivo and human studies. J Physiol Biochem. 2021;77(1):109‐131. [DOI] [PubMed] [Google Scholar]
- 30. Anhe FF, Nachbar RT, Varin TV, et al. A polyphenol‐rich cranberry extract reverses insulin resistance and hepatic steatosis independently of body weight loss. Mol Metab. 2017;6(12):1563‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anhe FF, Roy D, Pilon G, et al. A polyphenol‐rich cranberry extract protects from diet‐induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64(6):872‐883. [DOI] [PubMed] [Google Scholar]
- 32. Menke A, Knowler WC, Cowie CC. In: Cowie CC, Casagrande SS, et al., eds. Physical and Metabolic Characteristics of Persons With Diabetes and Prediabetes. Bethesda (MD): Diabetes in America ; 2018. [PubMed] [Google Scholar]
- 33. Janssen VE, Visseren FL, de Boer A, et al. Combined use of polypill components in patients with type 2 diabetes mellitus. Eur J Prev Cardiol. 2018;25(14):1523‐1531. [DOI] [PubMed] [Google Scholar]
- 34. Rouhi‐Boroujeni H, Heidarian E, Rouhi‐Boroujeni H, Deris F, Rafieian‐Kopaei M. Medicinal plants with multiple effects on cardiovascular diseases: a systematic review. Curr Pharm Des. 2017;23(7):999‐1015. [DOI] [PubMed] [Google Scholar]
- 35. Nyakudya TT, Tshabalala T, Dangarembizi R, Erlwanger KH, Ndhlala AR. The potential therapeutic value of medicinal plants in the management of metabolic disorders. Molecules. 2020;25(11):2669‐2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book and analytic code will be made available upon request pending approval by the corresponding author.


