Abstract
Objective
To assess the diagnostic performance of preoperative application of the Enzian classification (cEnzian) using surgical findings as reference standard.
Design
A prospective international non‐interventional study.
Setting
Twelve endometriosis centres in four European countries (Austria, Germany, Switzerland and Czech Republic).
Population
1062 women with endometriosis surgery.
Methods
Extent of endometriosis was preoperatively classified using the cEnzian classification based on gynaecological examination and/or transvaginal ultrasound (TVS) and/or magnetic resonance imaging (MRI). After subsequent surgery, the surgeon classified the intraoperative findings using the Enzian classification.
Main outcome measures
Sensitivity, specificity, PPV, NPV, LR+, LR− and accuracy were calculated. Conditional frequencies of intraoperative Enzian codings and the corresponding 95% confidence intervals were computed for each preoperative coding and visualised in plots.
Results
Although overall consistency of cEnzian and Enzian was poor (35.14%, 95% confidence interval 32.26–38.03), high specificities and negative predictive values (NPVs) of the cEnzian compartments could be demonstrated. Looking at the individual parts of the Enzian classification, the poorest diagnostic performance was detected for compartment B and the highest PPVs were found for category 3 lesions (>3 cm), independent of the compartment.
Conclusions
Using the Enzian classification in a non‐invasive setting is a useful tool providing us with an ‘at a glance’ summary of the diagnostic workup regarding deep endometriosis with high specificities and NPVs. An attempt to merge the two new endometriosis classification systems (#Enzian and AAGL 2021) seems reasonable taking into consideration the respective advantages of each other.
Keywords: classification, endometriosis, Enzian
Tweetable Abstract
The Enzian classification is a useful tool for an ‘at a glance’ description of preoperative workup regarding DE.
1. INTRODUCTION/BACKGROUND
Finding an adequate classification system for endometriosis has been a long‐lasting and ongoing process. In 2017, a consensus statement of the World Endometriosis Society (WES) was the first global attempt to guide the usage of existing endometriosis classification systems. 1 The recommendation was that surgeons should use a uniform classification toolbox including the revised American Society for Reproductive Medicine (r‐ASRM) classification 2 and, where appropriate, the Enzian classification 3 for deep endometriosis (DE) and the Endometriosis Fertility Index (EFI) 4 for fertility prediction.
All these above‐mentioned classification systems are based on findings during a surgical procedure—usually laparoscopy. This is plausible, as a laparoscopic workup with histological verification was considered the gold standard of endometriosis diagnostics for a long time. 5 , 6 However, the most accurate preoperative assessment of the extent of the disease is crucial for successful surgical therapy. Furthermore, new, alternative medical therapies and advances in diagnostic imaging techniques have raised the importance of using non‐invasive diagnostic tools prior to proceeding with surgery of potentially high morbidity.
The question arises as to how accurate current preoperative diagnostics in endometriosis can assess the extent of the disease using an existing classification system. For the Enzian classification, a few small single‐centre diagnostic studies have been published so far, based either on sonography 7 , 8 or magnetic resonance imaging. 9 , 10 , 11 All of these studies showed promising results for widespread use in a non‐invasive setting.
The objective of this large multicentre study was to understand the diagnostic performance of the preoperative application of the Enzian classification as a summary of non‐invasive workup regarding deep endometriosis (using surgical findings as reference standard).
2. METHODS
The cEnzian study is an international prospective non‐interventional study which investigates whether the findings during subsequent surgery can be predicted by the preoperative application of the Enzian classification (version 2012) based on findings in imaging techniques and gynaecological clinical examination. The main research aim was not to perform a diagnostic accuracy study for the single diagnostic modalities, but to assess the diagnostic performance of the complete preoperative evaluation in a large prospective study. The preoperative application of the classification has been named cEnzian (for clinical Enzian) and is mostly identical to the known Enzian 2012 classification (Figure S1). 3 , 12 Only the parameter ‘FU’—representing intrinsic ureteric endometriosis in the Enzian version of 2012—was omitted in the cEnzian, as the preoperative differentiation between intrinsic and extrinsic ureteral endometriosis is usually not possible. For sizing, the largest diameter of the endometriotic lesion was used. If multiple compartments (A, B, C) were involved in one lesion, the size in each compartment was evaluated and coded individually. Precise instructions for using the cEnzian and Enzian classification, including complete definitions of the compartments (Table S5), were printed on each case report form. In 2017, the time of study planning, gathering information on writing these instructions was an enormous effort, because detailed instructions on usage of the latest Enzian classification (version 2012) have never been published and could only be achieved in close cooperation with the creators of the classification. To maybe provide a better reading flow for readers inexperienced in the Enzian classification, we put short definitions of the compartments at the beginning of the respective parts in the Results section.
Women aged 18 years or older with a planned endometriosis surgery were recruited from 12 centres in four European countries (Austria, Germany, Switzerland and Czech Republic). All of the participating centres are specialised endometriosis clinics certified by EuroEndoCert. The surgery itself was not a study procedure, as it was planned independently of the study depending on the patient's complaints. Cancellation of the planned surgery or a time interval of more than 6 months between preoperative evaluation and surgery were defined as dropout criteria. Women without preoperative signs for deep endometriosis could be included in the study as well. A recruitment period of 2 years with at least 1000 included women was planned—with the expectation of a 10% drop‐out rate.
The actual recruitment phase was from January 2018 to October 2019. Following the informed consent and study inclusion, common diagnostic measures were carried out routinely. Depending on the use or need, ‘gynaecological examination with palpation’ and/or ‘transvaginal ultrasound (TVS)’ and/or ‘magnetic resonance imaging (MRI)’ were performed. After each examination modality, the corresponding section on the CRF was filled in. The final cEnzian was the summary of the results of all the diagnostic measures preoperatively performed. The examiners for palpation and sonography were gynaecologists/gynaecologic surgeons. MRI ratings were assigned by a gynaecologist together with a specialised radiologist of the respective centre. For imaging evaluation (both sonography and MRI), anatomical structures were defined according to the IDEA consensus of 2016. 13 In a study of Indrielle‐Kelly et al. 14 it was shown that the IDEA consensus can be properly used for MRI as well. In case of discrepancies between diagnostic modalities, the responsible gynaecologist in the study centre determined the final cEnzian coding in the relevant compartment, taking into account the respective experience in the different diagnostic modalities. An intraoperative examination under anaesthesia directly before the start of surgery was not considered in the cENZIAN assignment. Immediately after surgery, the surgeon recorded his or her intraoperative impressions in the postoperative section of the questionnaire (Enzian 2012 classification). The histological workup had no influence on the determination of postoperative codings. Due to the local requirements of national societies (recommendation that examiner and surgeon should be the same person if possible) and ethical concerns, the blinding of surgeons was not possible. The completed data sheets were returned pseudonymised to the leading study centre in Linz, Austria.
The study was approved by the institutional ethics committee of the Johannes Kepler University (Nr. 1002/2017) and registered in the German Clinical Trials Register (ID: DRKS00013614). The STROBE checklist was used for reporting. 15 There was no patient or public involvement (PPI) in this research. Terminology used in this publication is as advised by an international working group of AAGL, ESGE, ESHRE and WES. 16
2.1. Statistical analysis
The entire dataset was analysed using descriptive statistics. The compartments A/B/C were analysed based on the ratings from 0 to 3 as well as the binary categorization ‘affected’/‘not affected’ (presence or absence of a DE lesion in a certain compartment). Ratings of the different locations in the F‐compartment (FA, FB, FI, FO) are binary (‘affected’ or ‘not affected’, no classification by size). The surgery‐based Enzian classification was used as the gold standard for the evaluation of the preoperative classification. For each compartment, sensitivity (SENS), specificity (SPEC), positive predictive value (PPV), negative predictive value (NPV), likelihood ratio of a positive test result (LR+), likelihood ratio of a negative test result (LR−) and accuracy (ACC) were calculated. Taking compartment A as an example, sensitivity describes the correct detection of DE by the cEnzian in compartment A, whereas specificity states the correct exclusion of DE by the cEnzian in this compartment. Furthermore, PPV and NPV were calculated for each numeric coding (0–3) in compartments A, B and C. Normal approximation was used to compute 95% confidence intervals. Receiver operating curves (ROCs) could not be determined due to the binary outcomes with no underlying continuous variable.
Regarding the exact severity grades (0–3) in the compartments A, B and C, conditional frequencies of intraoperative Enzian codings and the corresponding 95% confidence intervals (95% CI) were computed for each preoperative coding and visualised in plots (e.g. frequency of an intraoperative A0, A1, A2 and A3 in the case of a preoperatively coded A0). Heatmaps have been provided to visualise the association between the cEnzian and Enzian classification.
Associations between nominal variables were tested using Fisher's exact test. As mentioned above, all the preoperative examinations were performed in specialised endometriosis centres by, however, clinicians with different experience in endometriosis diagnostics. To estimate effects of potential covariates (e.g. BMI) while taking into account the potential heterogeneity in endometriosis diagnosis experience of raters or centres, two mixed effects logistic regression models for overall exact consistency with random effects for raters and centres, respectively, were fitted.
Missing values were not replaced. ‘Not assessable’ codings in single compartments of the postoperative Enzian classification were transformed to missing values, whereas a ‘not assessable’ coding in a cEnzian compartment was not transformed to a missing value but scored as a mismatch (worst‐case scenario for the preoperative ‘not assessable’ codings in the diagnostic performance analysis). The term ‘valid ratings’ refers to the number of ratings without missing values. Data analysis was performed using the statistics software R. 17 Level of significance was set to 5%.
3. RESULTS
In all, 1144 women were enrolled in the study. After taking into account all of the drop‐out criteria, the number of cases was reduced to 1062. The reasons for the drop‐outs are listed in Figure S2. The median age in the final dataset was 32 years (interquartile range [IQR] 27–37) showing a median BMI of 23.03 (IQR 20.70–26.43).
Of all the women included, 1048 (98.68%), 1057 (99.53%) and 168 (15.82%) were examined with a gynaecological examination (including palpation), ultrasound and MRI, respectively. Except for one case, every woman included in the study had at least one imaging modality (TVS or MRI) and not only a gynaecological examination with palpation. Preoperatively, deep endometriosis was suspected in 41.34% (n = 439) of patients. After surgery, 53.11% (n = 564) were classified as having deep endometriosis. The distribution of all coded cEnzian and Enzian values is provided in Table S2. In 539 patients (50.75%), the cEnzian rater and the Enzian rater were the same person.
3.1. Overall consistency of cEnzian and Enzian
In total, cEnzian and Enzian ratings were exactly the same for 369 women (35.14% of 1050 valid ratings, 95% CI 32.26–38.03)—meaning that in these patients all compartments were coded with exactly the same values in the preoperative and postoperative classification. The number of matches increased to 423 women (40.29%, 95% CI 37.32–43.25) after categorising the numerical ratings in compartments A/B/C into ‘affected’ (combining values 1, 2 and 3) and ‘not affected’ (0 coded).
A further analysis with non‐consideration of the FA compartment (corresponding to adenomyosis) was performed as well. The number of matches was then 486 and 548, respectively, for exact and categorised comparison. Focusing only on valid cases with intraoperatively confirmed deep endometriosis (n = 553), exact consistency was only seen in 59 women (10.67%, 95% CI 8.10–13.24) and categorised consistency in 113 patients (20.43%, 95% CI 17.07–23.79).
3.2. Compartment A
Compartment A describes DE of the rectovaginal septum and/or the vagina and/or generally DE medial of the uterosacral ligaments retrocervically (without rectal wall). A total of 824 of 1058 valid numerical cEnzian ratings (77.88%) concerning compartment A were exactly equal to the postoperative allocated Enzian A rating. When excluding the cases without intraoperatively confirmed DE in compartment A (corresponding to the exclusion of Enzian A0 ratings), only 115 of 277 ratings were exactly equal (41.52%). Figure 1 shows the conditional frequencies of intraoperative Enzian codings with their 95% confidence intervals for each preoperative coding (cEnzian A0, A1, A2 and A3) and the corresponding heat map.
FIGURE 1.
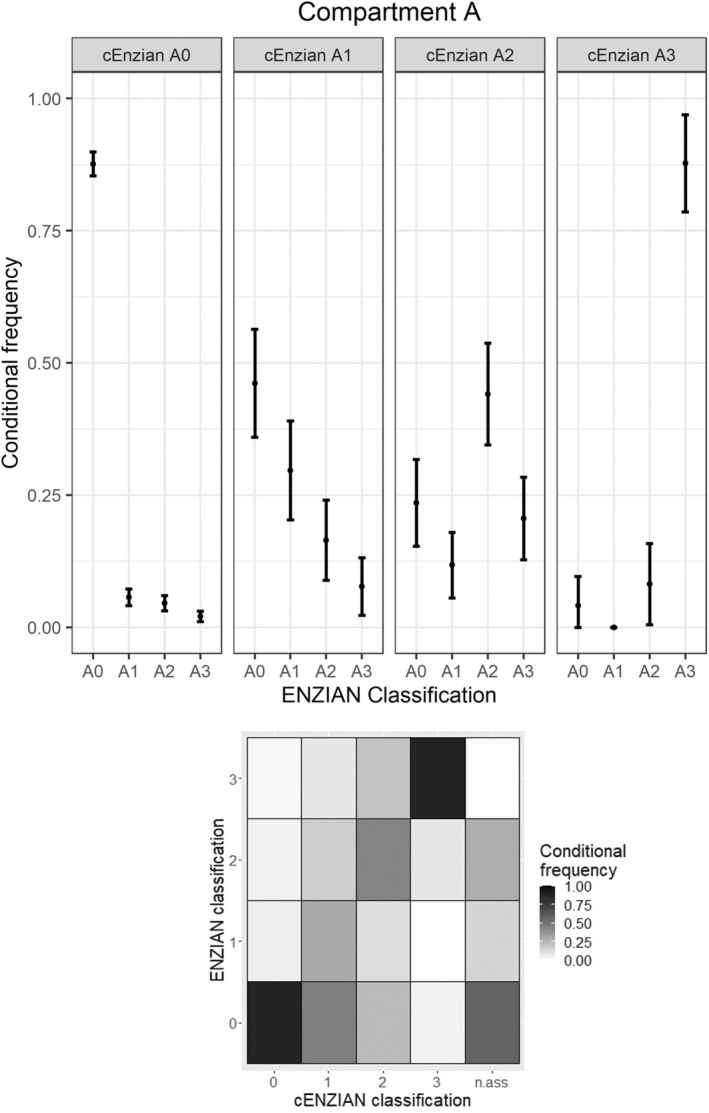
Concordance in compartment A.
Categorising the numerical ratings of compartment A into ‘A affected’ (A1, A2 or A3 coded) and ‘A not affected’ (A0 coded) increases the number of matches to 883 of 1058 ratings (83.46%). The presence of DE in compartment A could be preoperatively detected with a sensitivity of 62.82% (95% CI 59.90–65.73) and its non‐occurrence with a specificity of 90.78% (95% CI 89.04–92.52). The corresponding PPV and NPV were 71.90% and 87.64%, respectively. The further calculated PPVs for the exact cEnzian‐codings A1, A2 and A3 were 29.67%, 44.12% and 87.76%, respectively. These numbers for compartment A are also displayed in Table 1. The low PPVs of cEnzian A1 and A2 with a wide spread of corresponding Enzian codings are also clearly visible in Figure 1.
TABLE 1.
Test measures of the cEnzian classification
| Compartment A | Compartment B | Compartment C | FA | FB | FI | FO/F() a | |
|---|---|---|---|---|---|---|---|
| Exact matches—Total |
824/1058 (77.88%) |
642/1056 (60.80%) |
899/1054 (85.29%) |
809/1062 (76.18%) |
1015/1062 (95.57%) |
1001/1062 (94.26%) |
1025/1062 (96.52%) |
| Exact matches—Only DE |
115/277 (41.52%) |
123/455 (27.03%) |
52/174 (29.89%) |
389/546 (71.25%) b |
19/59 (32.20%) |
23/76 (30.26%) |
23/53 (43.40%) |
| Categorised matches |
883/1058 (83.46%) |
731/1056 (69.22%) |
938/1054 (88.99%) |
NA | NA | NA | NA |
| Sensitivity |
62.82% (59.90–65.73) |
46.59% (43.58–49.60) |
52.30% (49.28–55.31) |
71.25% (68.52–73.97) |
32.20% (29.39–35.01) |
30.26% (27.50–33.03) |
43.40% (40.42–46.38) |
| Specificity |
90.78% (89.04–92.52) |
86.36% (84.29–88.43) |
96.25% (95.10–97.40) |
81.40% (79.05–83.74) |
99.30% (98.80–99.80) |
99.19% (98.65–99.73) |
99.31% (98.81–99.81) |
| PPV | |||||||
| Categorised | 71.90% | 72.11% | 75.83% | 80.21% | 73.08% | 74.19% | 76.67% |
| 1: DE <1 cm | 29.67% | 34.38% | 39.29% | NA | NA | NA | NA |
| 2: DE 1–3 cm | 44.12% | 45.10% | 30.43% | NA | NA | NA | NA |
| 3: DE >3 cm | 87.76% | 68.75% | 58.70% | NA | NA | NA | NA |
| NPV | 87.64% | 68.47% | 91.17% | 72.79% | 96.14% | 94.86% | 97.09% |
| LR+ | 6.81 | 3.42 | 13.95 | 3.83 | 46.14 | 37.30 | 62.55 |
| LR− | 0.41 | 0.62 | 0.50 | 0.35 | 0.68 | 0.70 | 0.57 |
Note: 95% confidence intervals are provided for sensitivity and specificity.
NA, not applicable.
‘FO’ (Enzian 2012) corresponding to ‘F()’ in the #Enzian classification (2021).
Total intraoperative FA‐codings (adenomyosis) in the denominator.
3.3. Compartment B
Compartment B includes DE of the uterosacral ligaments (from the uterine base to the pelvic sidewall), the cardinal ligament and the pelvic sidewall. The calculated values for compartment B can be found in Table 1. In analogy to Figure 1, Figure 2 shows the conditional frequencies of intraoperative Enzian codings with their 95% confidence intervals for each preoperative B coding and the corresponding heat map.
FIGURE 2.
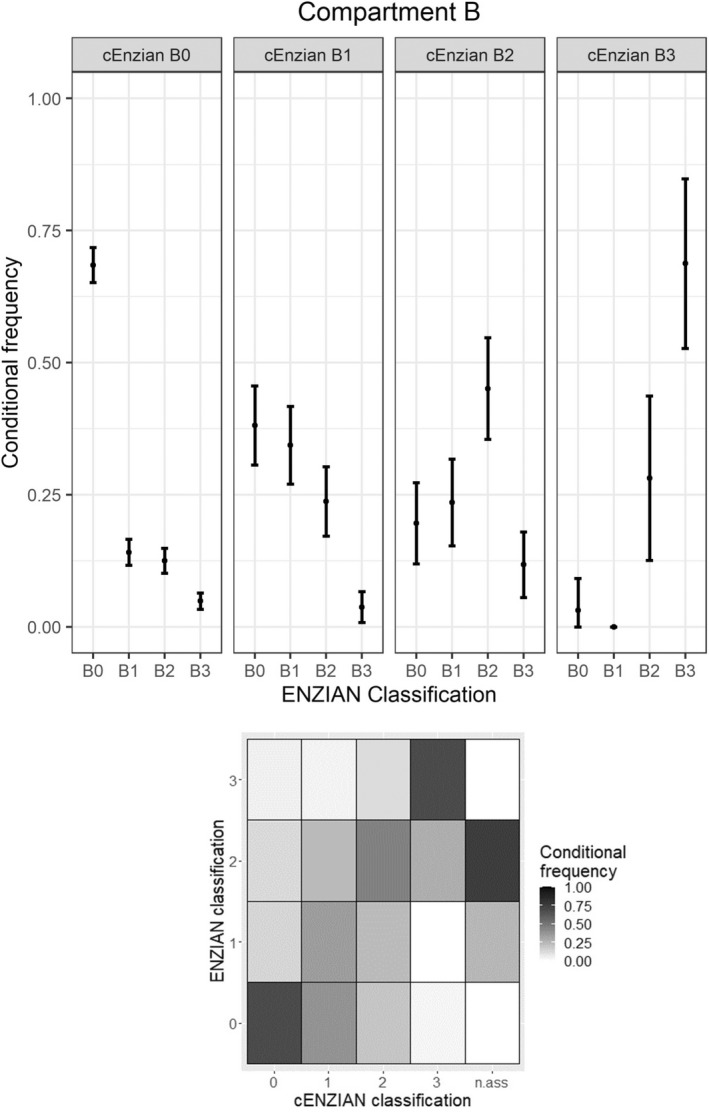
Concordance in compartment B.
Looking only at the cases with intraoperatively coded deep endometriosis in compartment B, the number of exact numerical matches was very low (27.03%). Using all cases, categorisation into ‘B affected’ and ‘B not affected’ improved the consistency to 69.22%. The low number of exact matches is also reflected in the poor PPVs for B1, B2 and B3 (34.38%, 45.10% and 68.75%, respectively). In comparison with compartments A and C, compartment B shows the lowest sensitivity (46.59%, 95% CI 43.58–49.60) and specificity (86.36%, 95% CI 84.29–88.43).
3.4. Compartment C
Respective numbers for compartment C (DE of the rectum) are listed in Table 1 and conditional frequencies of intraoperative Enzian codings including the heat map are shown in Figure 3. Specificity was the highest (96.25%, 95% CI 95.10–97.40) of the three major compartments, crowning the generally high specificity of the cEnzian compartments and resulting in a good NPV of 91.17% for compartment C. On the other hand, poor exact PPVs for C‐lesions <1 cm (C1, 39.29%) and 1–3 cm (C2, 30.43%) were observed.
FIGURE 3.
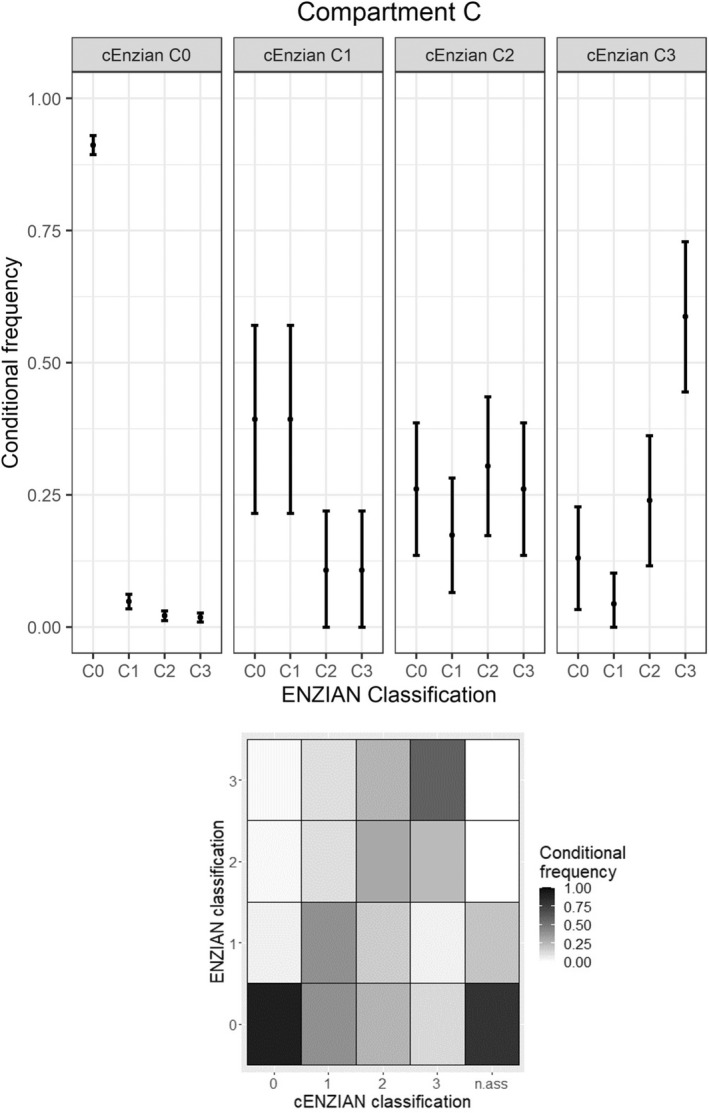
Concordance in compartment C.
3.5. Compartment F
Codings regarding adenomyosis (FA) had the highest (but still poor) sensitivity of all compartments (71.25%). The other F‐variables (FB, FI, FO) showed a low sensitivity with a high specificity (>99%). Exact values are listed in Table 1.
3.6. Influence of diagnostic modalities used
The measures of diagnostic accuracy were also computed for each diagnostic method (clinical examination, sonography, MRI) and reported in Table S3. As expected, no postoperative ‘FI’‐coding could be detected preoperatively by gynaecological examination. Sensitivity for the B‐compartment was remarkably poor for ‘sonography’ (20.14%) and not much better for ‘gynaecological examination (38.32%). The lowest sensitivity overall (besides FI by gynaecological examination) was seen for FB‐coding by gynaecological examination (18.97%). Preoperative codings by MRI showed the highest sensitivities in comparison with gynaecological examination and sonography (except for FA and FO). Overall, cases with MRI showed better overall consistencies than cases without MRI (Fisher's exact test, P = 0.02). The distributions of the different examination modalities for each numerical Enzian coding are displayed in Figures S3–S5.
3.7. Inter‐rater and inter‐centre variability
Two separate mixed effects logistic regression models were fitted to estimate effects of predictive factors on the probability of overall exact consistency with random effects for study centres and cEnzian raters, respectively, to take into account heterogeneity with respect to study centres or cEnzian raters. A negative effect of the presence of deep endometriosis and a positive effect of rater relationship (whether the cEnzian and Enzian raters were the same person or different ones) was significant in both regression models (decreasing and increasing the odds ratio of overall consistency, respectively). Overall consistency was not significantly different for patients with preoperative evaluation and surgery more than 90 days apart compared with those with both within 90 days. Details on the mixed effects logistic regression models are provided in Table S4.
4. DISCUSSION
4.1. Main findings
To our knowledge, the cEnzian study is by far the largest prospective clinical study on the preoperative application of the Enzian classification for endometriosis. Although the overall consistency of cEnzian and Enzian was poor, high specificities and good negative predictive values of the cEnzian compartments could be observed in a real‐world clinical setting. Looking at the individual parts of the Enzian classification, the poorest diagnostic performance was detected for compartment B and the highest PPVs were found for category 3 lesions (>3 cm), independently of the compartment.
4.2. Interpretation
The presented poor exact overall consistency between the preoperative and postoperative classifications (35.14% in the whole study population and 10.67% in women with DE) can probably be explained to a large extent by the fact that the Enzian classification is composed of multiple compartments with multiple numerical codings, resulting in over 2000 possible combinations for the Enzian version of 2012. Given this high number of possible combinations for the whole classification, it seems to make more sense to assess the Enzian compartments separately. This is reflected by the fact that none of the previous studies, conducted on the preoperative prediction of the Enzian classification (based on sonography or MRI), evaluated the overall agreement, but only the different compartments. 7 , 8 , 9 , 10 , 11
In 2021, a single‐centre study by Hudelist et al. 7 compared preoperatively assigned Enzian codings using transvaginal sonography (TVS) with the intraoperatively assigned ones. The preoperative estimation of deep endometriosis in 195 women using the Enzian classification was satisfactory for the authors, only assessment of deep endometriotic lesions in compartment B was less precise. When looking at the performance of sonography in detail, the published data of Hudelist et al. on the sensitivities and specificities of TVS regarding Enzian compartments A (SENS 84%, SPEC 85%), B (SENS 91%, SPEC 73%) and C (SENS 92%, SPEC 95%) showed clearly better sensitivities in comparison with the cEnzian study. This difference could be explained by the fact that the mentioned study was a single‐rater study with an expert in TVS for mapping endometriosis. It is known that the operator's experience in performing TVS is crucial for detecting deep endometriosis via sonography. 18 Another prospective study by Goncalves et al. 8 on the replacement of diagnostic laparoscopy by transvaginal ultrasound showed a substantial correlation between the compartments A (weighted Kappa = 0.827), B (weighted Kappa = 0.670) and C (weighted Kappa = 0.670), obtained either by TVS with bowel preparation or diagnostic laparoscopy.
Di Paola et al. in 2013, 9 Burla et al. in 2019, 10 and Thomassin‐Naggara et al. in 2020 11 used MRI to assign a preoperative Enzian coding. In the retrospective study of Di Paola,9 which included 82 women with histopathologically confirmed deep endometriosis, MRI‐based Enzian codings showed a good accuracy in the detection of lesions in the different Enzian compartments (81% for compartment A, 89% for B, 82% for C, 100% for FA and 37% for FB). The study of Burla et al. 10 was also retrospective, containing 63 patients. Sensitivities and negative predictive values for compartment A, B, C, FA, FB and FI were 95.2% and 91.7%, 78.4% and 56%, 91.4% and 89.7%, 57.1% and 94.1%, 85.7% and 98.3%, and 73.3% and 92.2%, respectively. The authors concluded that the Enzian classification may be used as an anatomical land map and valuable communication tool between radiologists and gynaecologists. The third retrospective MRI‐based prediction study by Thomassin‐Naggara et al. 11 included 150 women showing concordance with the surgical Enzian codings for compartment A in 78.7%, for compartment B in 34.7% and for compartment C in 82.7%. Similar to our study, MRI seems to have the worst diagnostic performance in compartment B (except the study results of Di Paola 2013).
Besides the study of Hudelist et al., 7 none of the listed studies investigated the exact numerical ratings. Comparing the results of the cEnzian study and the data of Hudelist et al., the numeric values of 0 (no DE) and 3 (>3 cm) in the different compartments could be predicted reliably, while the codings of 1 (<1 cm) and 2 (1–3 cm) showed poor positive predictive values. This might open the discussion that, in a future revision of the Enzian classification, a fusion of these two size groups should be taken into consideration. A classification that already uses this 3‐cm cut‐off is the new AAGL 2021 endometriosis classification. 19
4.3. Strengths and limitations
The strengths of the cEnzian study are the prospectively collected data, the large study population recruited by multiple competence centres for endometriosis and the acquisition of real‐world data. Nevertheless, some limitations of the study should be considered as well:
For statistical analysis, the surgery‐based Enzian classification was used as a reference. However, as there is a lack of validation studies on the Enzian classification regarding its clinical validity, accuracy and reproducibility, this should be considered a weakness of the study. Furthermore, in a study from Goncalves et al. 8 the accuracy to detect rectosigmoid endometriosis was shown to be better for preoperative TVS than for diagnostic laparoscopy.
The cEnzian raters are all clinicians in the competence centres for endometriosis, nevertheless the expertise of each rater is different. As mentioned above, especially in the field of mapping endometriosis by transvaginal ultrasound, a certain expertise is necessary. As a regional feature, it needs to be mentioned that in German‐speaking countries, TVS for mapping and surgical planning is usually performed by the responsible gynaecologists/gynaecologic surgeons themselves and not by a professional subgroup of ‘gynaecological sonographers’.
As rater blinding was not possible for ethical reasons (provision of all available relevant preoperative information to the surgeon), an influence of the Enzian codings by the present cEnzian counterpart could not be excluded (bias towards increasing the ENZIAN prediction quality). A hint towards this effect is reflected in the positive effect of rater agreement in the mixed effects logistic regression model.
A new revised version of the Enzian classification (the ‘#Enzian’ classification) was released in 2021 20 —providing a more comprehensive classification for endometriosis with compartments for peritoneal endometriosis (compartment P), ovarian endometriosis (O) and adhesions of the tubo‐ovarian unit (T). Some minor changes to the ABCF‐compartments were done as well (e.g. separate listing of compartment B by left and right side) without relevant impact on the study results presented. Definition for ‘FU’‐coding was changed to ‘intrinsic or extrinsic involvement of the ureters with hydroureteric changes or hydronephrosis’ and can now be used in a preoperative setting as well (in contrast to the 2012 version).
5. CONCLUSION
Using the Enzian classification in a preoperative or non‐invasive setting is a useful tool, providing us with a short, ‘at a glance’ summary of the diagnostic workup regarding deep endometriosis. Due to the high specificities and NPVs shown for the different compartments, adequate planning of a surgery seems to be possible and invasive procedures may be avoided in the case of negative results. Preoperative cEnzian ratings for compartment B are of limited value and the clinical relevance of a subdivision into endometriotic nodules <1 cm and 1–3 cm should be evaluated to consider a possible fusion into a single group. In general, an attempt to merge the two new endometriosis classification systems (#Enzian and AAGL 2021) seems reasonable—with consideration of the respective advantages of each other. If the availability of an expert sonographer is limited, preoperative MRI should be considered.
AUTHOR CONTRIBUTIONS
SE: project development, data collection, data analysis, manuscript writing. PO: project development, manuscript editing, supervision. KN: data collection, manuscript editing. BS: data collection, manuscript editing. JD: data collection, manuscript editing. LW: data collection, manuscript editing. BK: data collection, manuscript editing. KNG: data collection, manuscript editing. SK: data collection, manuscript editing. DS: data collection, manuscript editing. MM: data collection, manuscript editing. HK: data collection, manuscript editing. PH: data analysis, manuscript editing. HW: data analysis, manuscript editing. OS: manuscript editing, supervision. SS: project development, data collection, data analysis, manuscript editing.
CONFLICT OF INTERESTS
None declared. Completed disclosure of interest forms are available to view online as supporting information.
ACKNOWLEDGEMENTS
None.
DETAILS OF ETHICS APPROVAL
The study was approved by the institutional ethics committee of the Johannes Kepler University (submission number 1002/2017, date of approval 20 December 2017).
Supporting information
Appendix S1
Data S1
Enzelsberger S‐H, Oppelt P, Nirgianakis K, Seeber B, Drahoňovský J, Wanderer L, et al. Preoperative application of the Enzian classification for endometriosis (The cEnzian Study): A prospective international multicenter study. BJOG. 2022;129:2052–2061. 10.1111/1471-0528.17235
Funding information
The study was supported by a research grant from Stiftung Endometriose Forschung. This financial support had no influence on study design, data collection, analysis, interpretation, writing or decision to submit for publication
Clinical Trial Registration: DRKS—German Clinical Trials Register, ID: DRKS00013614, http://www.drks.de/DRKS00013614
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, et al. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod. 2017;32(2):315–24. [DOI] [PubMed] [Google Scholar]
- 2. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67(5):817–21. [DOI] [PubMed] [Google Scholar]
- 3. Stiftung‐Endometriose‐Forschung . Enzian 2012. Consensus meeting, Annual Conference of the Stiftung Endometriose Forschung (Foundation for Endometriosis Research). Weissensee, Austria: Foundation for Endometriosis Research; 2011. [Google Scholar]
- 4. Adamson GD, Pasta DJ. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil Steril. 2010;94(5):1609–15. [DOI] [PubMed] [Google Scholar]
- 5. Dunselman GA, Vermeulen N, Becker C, Calhaz‐Jorge C, D'Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–12. [DOI] [PubMed] [Google Scholar]
- 6. Hirsch M, Begum MR, Paniz E, Barker C, Davis CJ, Duffy J. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG. 2018;125(5):556–64. [DOI] [PubMed] [Google Scholar]
- 7. Hudelist G, Montanari E, Salama M, Dauser B, Nemeth Z, Keckstein J. Comparison between sonography‐based and surgical extent of deep endometriosis using the Enzian classification – a prospective diagnostic accuracy study. J Minim Invasive Gynecol. 2021;28:1643–9.e1. [DOI] [PubMed] [Google Scholar]
- 8. Goncalves MO, Siufi Neto J, Andres MP, Siufi D, de Mattos LA, Abrao MS. Systematic evaluation of endometriosis by transvaginal ultrasound can accurately replace diagnostic laparoscopy, mainly for deep and ovarian endometriosis. Hum Reprod. 2021;36(6):1492–500. [DOI] [PubMed] [Google Scholar]
- 9. Di Paola V, Manfredi R, Castelli F, Negrelli R, Mehrabi S, Pozzi MR. Detection and localization of deep endometriosis by means of MRI and correlation with the ENZIAN score. Eur J Radiol. 2015;84(4):568–74. [DOI] [PubMed] [Google Scholar]
- 10. Burla L, Scheiner D, Samartzis EP, Seidel S, Eberhard M, Fink D, et al. The ENZIAN score as a preoperative MRI‐based classification instrument for deep infiltrating endometriosis. Arch Gynecol Obstet. 2019;300(1):109–16. [DOI] [PubMed] [Google Scholar]
- 11. Thomassin‐Naggara I, Lamrabet S, Crestani A, Bekhouche A, Wahab CA, Kermarrec E, et al. Magnetic resonance imaging classification of deep pelvic endometriosis: description and impact on surgical management. Hum Reprod. 2020;35(7):1589–600. [DOI] [PubMed] [Google Scholar]
- 12. Enzian 2012 classification. Available from: https://www.endometriose‐sef.de/aktivitaeten/klassifikation‐enzian/
- 13. Guerriero S, Condous G, van den Bosch T, Valentin L, Leone FP, Van Schoubroeck D, et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: a consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet Gynecol. 2016;48(3):318–32. [DOI] [PubMed] [Google Scholar]
- 14. Indrielle‐Kelly T, Fruhauf F, Fanta M, Burgetova A, Lavu D, Dundr P, et al. Diagnostic accuracy of ultrasound and MRI in the mapping of deep pelvic endometriosis using the International Deep Endometriosis Analysis (IDEA) Consensus. Biomed Res Int. 2020;2020:3583989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. [DOI] [PubMed] [Google Scholar]
- 16. International Working Group of AAGL, ESGE, ESHRE and WES , Tomassetti C, Johnson NP, Petrozza J, Abrao MS, Einarsson JI, et al. An International Terminology for Endometriosis, 2021. Facts Views Vis Obgyn. 2021;13(4):295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. R‐Core‐Team . R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 18. Tammaa A, Fritzer N, Strunk G, Krell A, Salzer H, Hudelist G. Learning curve for the detection of pouch of Douglas obliteration and deep infiltrating endometriosis of the rectum. Hum Reprod. 2014;29(6):1199–204. [DOI] [PubMed] [Google Scholar]
- 19. Abrao MS, Andres MP, Miller CE, Gingold JA, Rius M, Neto JS, et al. AAGL 2021 endometriosis classification: an anatomy‐based surgical complexity score. J Minim Invasive Gynecol. 2021;28:1941–50.e1. [DOI] [PubMed] [Google Scholar]
- 20. Keckstein J, Saridogan E, Ulrich UA, Sillem M, Oppelt P, Schweppe KW, et al. The #Enzian classification: a comprehensive non‐invasive and surgical description system for endometriosis. Acta Obstet Gynecol Scand. 2021;100:1165–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request. The data are not publicly available due to privacy or ethical restrictions.


