Abstract
Music, thanks to its strong evocative power, is considered a powerful mnemonic tool for both normal and clinical populations. However, the mechanisms underpinning the music‐driven benefits on memory remain unclear. In memory research, reward dopaminergic signals have been highlighted as a major modulator of memory traces consolidation. Over the last years, via behavioral and pharmacological approaches, we have investigated the hypothesis that dopaminergic‐dependent musical pleasure is a crucial mechanism underpinning music‐driven memory benefits. Our results show that the pleasure felt during music listening, modulated by both the dopaminergic transmission and participants’ sensitivity to music reward, can increase episodic memory performance for the music itself as well as for nonmusical‐associated information. In this commentary paper, we aim to review the main findings obtained from three different studies, in order to discuss current advances and future directions in this research area.
Keywords: dopamine, episodic memory, music, pleasure, reward
Over the last years, we have investigated the hypothesis that dopaminergic‐dependent musical pleasure is a crucial mechanism underpinning music‐driven memory benefits. Our results show that the pleasure felt during music listening can increase episodic memory performance for the music itself as well as for non‐musical associated information. In this commentary paper, we aim to review the main findings obtained from three different studies, in order to discuss current advances and future directions in this research area.

PLEASURE, MUSIC, AND MEMORY: A STRONG, ANCIENT LINK
In the Greek mythology, the pleasure coming from music was intimately related to memory: the muse of music Euterpe, literally the “giver of delight,” was born from Mnemosine, the muse of memory.
The evocative power of music and its usage as a powerful mnemonic tool constitute a universal for humans. 1 Music is a complex and salient stimulus evolving over time likely to be easily encoded and retrieved. The memory for music itself has been even proposed as a special memory system that can be spared in the presence of memory deficits caused by neurodegenerative diseases, such as Alzheimer's. 2 Furthermore, music has been shown to effectively promote the encoding and retrieval of nonmusical‐associated information, such as verbal 3 material, as well as autobiographical memories. 4 For these reasons, music is considered to be an effective tool for stimulating episodic memory in both healthy and clinical populations.
Conversely, memory is also crucial for the understanding and the appreciation of music. We tend to like more music that is more familiar, and to rate as more familiar the most pleasurable excerpts, even when exposure is controlled. 5 At a neural level, the hippocampus (and its connections with the limbic systems) appears pivotal for orchestrating affective responses to music depending on personal preferences, memory associations, and aesthetic evaluations. 6 Furthermore, neurophysiological findings highlighted that higher degrees of reported music pleasure increase phase synchronization in the gamma band between temporal and frontal regions, crucially involved in both recognition and working memory processes. 7 During music listening, the listener familiarizes with the musical stimulus through learning and memory processes that allow to implicitly extract its regularities and create predictions about future events. 8 Music‐driven pleasurable responses might depend, at least in part, on the resolution of such perceptual expectations, and are reflected by increases in subjective hedonic and motivational ratings, psychophysiological responses, and activity in the dopaminergic mesolimbic system. 9 In particular, musical pleasure is associated with activity in a major structure within brain reward circuits, the ventral striatum (VS), and more specifically the nucleus accumbens (NAcc 10 ). Interestingly, the experience of musical pleasure (the so‐called musical hedonia) can vary across the general population, with some individuals reporting a lower sensitivity to music reward reflected by a decreased brain connectivity between the auditory cortices and reward networks, notably the NAcc. 11
Pleasure can be considered as the ensemble of hedonic responses that, together with the motivational and learning components, constitute the reward experience. 12 In the realm of memory research, reward has been highlighted as a major modulator of memory traces consolidation. Well‐known secondary rewards, such as money, 13 but also more abstract ones, such as curiosity states, have been shown to increase memory performance not only for the rewarding stimulus, but also for associated nonrewarding information. For example, Gruber and colleagues 14 showed that participants better memorized both the information that they were curious about and incidental material (i.e., human faces) presented during curious states. Follow‐up studies also showed the implication of the reward‐motivated learning system when participants successfully learned new information (i.e., pseudo‐words), even when no external performance feedback was presented. 15 At the neural level, this is reflected by activations in the dopaminergic mesolimbic pathway (ventral tegmental area [VTA], VS, and NAcc), as well as in the hippocampus, the last one being crucial for long‐term memory processes. More specifically, midbrain dopamine neurons in the VTA and their projections to the VS (NAcc) both support reward anticipation and modulate activity in the hippocampus. 15 According to the neo‐Hebbian framework for episodic memory, 16 dopamine transmission in the mesolimbic system would promote memory formation and consolidation in the hippocampus by strengthening synaptic long‐term potentiation. Therefore, all the stimuli triggering dopaminergic transmission in the hippocampus could result in long‐term memory improvements, which can be also observed for events that occur after the dopamine release. 17 Overall, this reward‐dependent dopaminergic learning circuit seems to be an important internal loop that could be engaged whenever it is important to encode new and relevant (i.e., valued) information in humans.
Dopamine release has been shown as a necessary condition for pleasurable music responses to take place. 18 Musical pleasure, with its impact on the reward dopaminergic networks, arises, therefore, as a perfect candidate for explaining the positive effect of music on memory. Over the last years, we have provided new insights into this topic by using behavioral and pharmacological approaches. We investigated the hypothesis that the dopaminergic‐dependent musical pleasure is a crucial mechanism underpinning music‐driven memory benefits. In this paper, we aim to review the main findings obtained in order to discuss current advances and future directions in this research area.
FROM PLEASURE TO MEMORY: EXPERIMENTAL EVIDENCE
Through three different studies conducted on three different samples, we investigated in nonmusician, young adult participants whether differences in musical pleasure could result in better memory for music (studies 1 and 2), as well as for associated verbal information (study 3). We investigated the potential role of musical pleasure by (1) measuring the impact of music on subjective hedonic ratings (studies 1, 2, and 3); (2) bearing in mind individual differences in the sensitivity to experience reward in music‐related activities (assessed through the Barcelona Music Reward Questionnaire [BMRQ 19 ]; studies 1, 2, and 3); and (3) designing pharmacological interventions in healthy participants targeting the dopaminergic neurotransmitter system (study 2). For each study, and in order to expose participants to unfamiliar music, we carefully preselected classical music excerpts that were balanced for features, such as arousal, valence, and popularity; this allowed to control for the material, thus avoiding the effects of emotional arousal, valence, or familiarity, and ruling out possible interference of lyrics with memory performance. Furthermore, in order to test the dopamine‐dependent episodic memory performance after a consolidation period, we employed in all the studies a recognition‐recollection paradigm 24 h after the encoding of the material (musical or verbal). Participants were asked to recognize the previously presented items among new ones (i.e., old/new recognition; Figure 1A). If they indicated an item as old, they had to commit an additional choice indicating whether they could really remember it (i.e., conscious recollection of the event and associated contextual information), whether they just had a sense of knowing it (i.e., familiarity), or whether they were just guessing (R/K/G paradigm 20 ).
FIGURE 1.
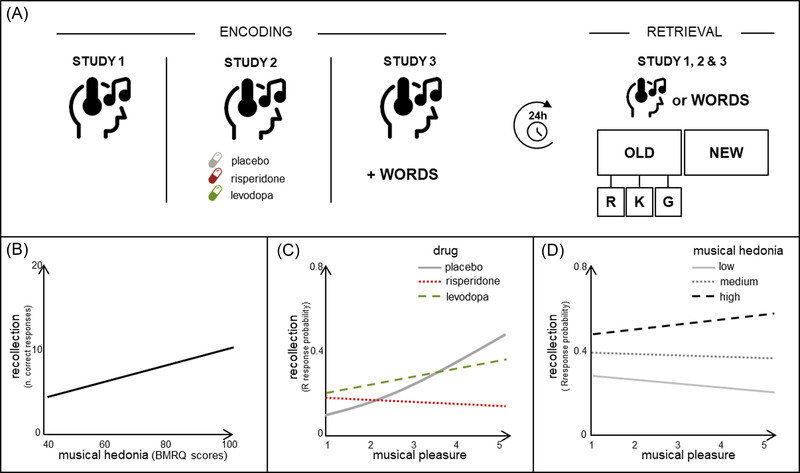
Schematic representation of the experimental paradigms (A) and main findings (B, C, D) of the three studies. (A) The encoding phase (for musical excerpt in studies 1 and 2, and for verbal material associated with musical background in study 3) was always followed, 24 h later, by a retrieval phase with a recognition (old/new)‐recollection (R/K/G) paradigm. Study 1 (B) showed that the higher the musical hedonia (measured through the BMRQ, 19 X axis), the higher the recollection (Y axis, no. of correct R responses) for previously encoded musical excerpts. Panel adapted from Ref. 21. Study 2 (C) revealed that the positive relationship between musical pleasure (i.e., felt during music listening, X axis, from 1 [ = no pleasure] to 5 [ = intense pleasure]) and musical memory (Y axis, no. of R responses probability), confirmed under placebo (gray solid line) and maintained under levodopa (green dashed line), was disrupted under risperidone (red dotted line). Panel adapted from Ref. 22. In study 3 (D), we showed that the higher the musical pleasure (X axis), the higher the recollection (Y axis, no. of R responses probability) for previously encoded words, but specifically for participants with higher musical hedonia (black dashed line). Panel adapted from Ref 23.
In study 1, 21 participants were asked to listen attentively, over three different exposures, to 24 musical excerpts (each one lasting 20 s). For each excerpt, volunteers rated the amount of pleasure felt as well as other emotional‐control measures (i.e., arousal, valence, motivation, and familiarity). The behavioral findings provided the first evidence supporting that music reward experience and memory are tightly interrelated: the greater the pleasure elicited by a particular excerpt, the better the episodic memory for it 24 h later. Importantly, we found that the strength of this effect varied according to individual sensitivity to music reward: the higher the participants’ musical hedonia, the better their musical memory performance (Figure 1B).
In study 2, 22 we investigated the neurochemical brain mechanisms underpinning such music‐pleasure‐driven effect on memory. By employing the same paradigm and material of study 1, we conducted a double‐blind, within‐subject pharmacological intervention. Prior to listening to the music, we orally administered to participants levodopa (i.e., a dopamine precursor likely to increase dopamine transmission in the brain), risperidone (a dopamine R2 receptor antagonist), and placebo (i.e., lactose). In line with study 1, we found that with placebo the greater the reward experienced while listening to music, the better the memory recollection performance the day after. However, and crucially, we showed that such effect was modulated by both dopaminergic signaling and individual differences in music reward sensitivity. More specifically, while greater pleasure was consistently associated with better memory outcomes in participants with higher musical hedonia scores, this effect was dampened when dopaminergic signaling was disrupted (Figure 1C), especially in participants with average or low sensitivity to musical reward. Music‐dependent dopaminergic signaling, which varies across subjects depending on their sensitivity to musical hedonia, is, therefore, a necessary condition for long‐term musical memory processes to take place.
In study 3, 23 we pushed these findings further and behaviorally investigated whether musical pleasure could enhance episodic memory for nonmusical material. Based on previous evidence that music facilitates the encoding of words and that reward promotes memory for relevant and incidental information, we manipulated musical pleasantness to investigate a possible transfer effect of music reward on memory for verbal material. Participants were asked to encode words presented in different auditory contexts: highly pleasant music, slightly pleasant music, and control white noise. Each auditory context was played before and during the verbal encoding, or only before it (i.e., silent encoding). The findings confirmed that musical pleasure constituted a helpful encoding context able to drive memory improvements via reward mechanisms. More specifically, and in line with the previous studies, participants with larger sensitivity to decode music pleasure (i.e., higher musical hedonia) showed an increased recollection performance, especially for words encoded in a highly pleasant musical context (Figure 1D). Interestingly, the effect persisted even when the auditory stimulus was not concurrently present during the encoding.
Taken together, these studies showed that the pleasure felt during music listening, modulated by both the dopaminergic transmission and participants’ sensitivity to music reward, can increase memory performance for the music itself as well as for nonmusical‐associated information.
IMPLICATIONS AND FUTURE DIRECTIONS FOR MUSIC COGNITION RESEARCH
Music is considered among the most pleasurable experiences of human life (see, e.g., Ref. 24). It has a unique capacity for evoking a rich variety of emotions, and it is constantly used for internal mood regulation. Although some authors have postulated that music affects similar brain networks as primary rewards, such as food or sex (see Ref. 9 for a review about the common and distinct neural networks of music and food reward), no clear evolutionary advantage is easily identified, and the evolution of music as a survival adaptation is largely disputed. Over the last years, crucial questions have been raised about the nature of music reward–related signals and their relationship with other core cognitive functions. Contrary to other primary rewards, more abstract rewards, such as aesthetic appreciation (e.g., music), might depend on complex cognitive computations involving attention, learning, valuation, and memory processes, 24 which are regulated by dopaminergic transmission. 25 As increases in dopaminergic transmission have been consistently associated with improvements in memory performance, 26 it appears reasonable to hypothesize that dopaminergic‐dependent musical pleasure can play a major role in regulating human memory.
To the very best of our knowledge, the three studies reviewed here provided the first experimental evidence of a direct implication of music reward in memory formation by reliably showing (1) that musical pleasure can drive memory benefits, and (2) that such an effect is modulated by synaptic dopaminergic availability and individual differences in musical hedonia. Importantly, and in line with studies showing that either extrinsic or intrinsic rewards can promote memory for relevant and incidental information, 14 , 27 we found that the positive effect of musical pleasure could be extended to nonmusical‐associated information (i.e., verbal material). The implication of the dopaminergic‐dependent memory mechanisms was further confirmed by the fact that the effect of the pleasant stimulus on memory lasted over time, even when the music was no longer present. 16 , 17 Furthermore, our findings revealed that musical pleasure mainly stimulated participants’ episodic memory by increasing recollection responses (Figure 1), which specifically depend on the activity of the dopaminergic mesolimbic‐hippocampal loop. 16 , 26
Theoretical and experimental evidence show that perceptual expectations and predictions created during music listening lie at the core of musical pleasure. While extracting musical regularities, the listener's brain creates anticipation about future events. 8 To a certain extent, the feelings of pleasure associated with music could be, therefore, driven by the intrinsic value of successfully anticipating (or not) musical events. Gold et al. 28 showed that increased activity in the NAcc arises from musical reward prediction errors (RPEs) that signal the difference between expected and perceived events during music listening. Importantly, reward prediction mechanisms also promote memory processes. In the context of nondeclarative memory, increases and decreases in synaptic strength have been associated with rewards that were better and worse than expected, respectively. 29 Also, it has been proposed that RPEs also drive declarative, episodic memory processes through the activation of the VS. 30 An interesting line of research would, therefore, investigate the involvement of RPEs in music memory tasks, for example, by employing musical material varying in the degrees of predictability of future musical events (i.e., more certain or uncertain; see, e.g., Ref. 28). If RPEs are a pivotal mechanism underpinning the relationship between musical pleasure and memory, then better‐than‐expected musical events should result in increased subjective ratings of musical pleasure reflected by increased activity in the mesolimbic system, and a better episodic memory performance associated with hippocampal modulation.
The fact that pleasure felt during music listening can help in remembering not only the music, but also a nonmusical‐associated event, suggests that musical pleasure can efficiently tag an episode at the synaptic level, 17 thus making it more memorable. This becomes particularly relevant in daily life, when the complexity of the surrounding environment makes it necessary to attach value to the to‐be‐encoded items in order to successfully retrieve them. 16 In this vein, through reward mechanisms, music might be preferentially associated with events from one's lifetime, thus being particularly helpful in stimulating episodic and autobiographical memory in both healthy and clinical populations. Indeed, music‐related life memories, such as the one from one's adolescence, are often sharp, flashbulb memories particularly resistant to time threats. 4 Music‐driven memory benefits could be associated not only with music capacity to boost the encoding of an event, but also to protect it from interference. Interestingly, it has been reported that when a piece of information is associated with a high reward cue (i.e., money), it becomes more resistant to interference through the activation in the hippocampus and reward‐related areas (such as the VS 31 ). Further interesting research would, therefore, investigate whether music, through pleasurable responses, is efficiently able to protect previously encoded information from forgetting due to interfering information. Furthermore, although our results specifically refer to episodic processes, and given the interdependence between memory systems, 32 it is possible that music‐driven emotional marking could, via hippocampal activation, stimulate other kinds of memory processes. Further research would, therefore, be needed to disentangle whether the effect of musical pleasure on memory could be described for other than episodic processes, such as the encoding and retrieval of semantic, conceptual information.
Interesting studies have shown how eliciting curiosity states, via the activation of the mesolimbic dopaminergic circuit, can increase hippocampal‐dependent memory encoding of the target item. 14 , 33 It might be important to evaluate these mechanisms in the musical domain, also taking into account different developmental periods. For example, adolescence is characterized by higher levels of curiosity and exploratory behavior, accompanied by less cognitive control maturation. 34 Crucially, musical taste especially develops during adolescence, and the music from adolescence is better remembered, and perceived as more emotional and arousing in later life stages. 35 Thus, to a certain extent, the larger impact observed in music and memory during these ages could be due to a hyperhedonia related to the larger activation of the reward‐motivation loop. In addition, the hedonic impact of music in adolescence could determine the future value and interest we will attribute to new music pieces or genres. This could act through top‐down cortical value signals that strategically prioritize the encoding of congruent information (similar pieces of music, styles, etc.) and that triggers internal reward‐learning mechanisms, in turn facilitating music encoding. This is congruent with the idea that value‐based learning gradually grows during life, becoming more strategic with age. 36 Overall, the initial curiosity‐driven music encoding that characterizes later childhood and adolescence might result during adulthood in a more value‐oriented learning, in which music reward is downregulated by top‐down cortical influences (e.g., music long‐term interests). This idea is also convergent with evidence showing the importance of top‐down signals (e.g., knowledge, training, or experience) for aesthetic appreciation and liking. 37 Accordingly, it has been shown that musical expertise increases the top‐down prefrontal modulation on hippocampal activity. 38 New neuroscientific research is, therefore, needed in this arena to understand the mechanisms underlying the relation between music, reward, and memory across different developmental stages and according to musical training.
While opening up the possible role of musical pleasure for memory stimulation in both healthy and clinical populations (such as pathological aging), it might be worthwhile to consider another crucial point. Although many studies in the music psychology and neuroscience research indicated music as an extremely powerful tool for memory enhancement in both normal and clinical populations, the benefits of music on memory often present a high intersubjective variability that may depend, among others, on methodological choices as well as participants’ profiles. 39 In music research, it has become increasingly important to understand how, when, and for whom music can constitute a powerful tool for cognitive stimulation. Critically, our findings suggest that individual differences in sensitivity to music reward can predict the positive effect of music on participants’ memory. In this vein, it has been suggested that the positive effect of music interventions on memory performance in poststroke rehabilitation might be driven to a certain extent by its intrinsic ability to engage motivation‐ and reward‐related dopaminergic networks. 40 Individual scores in musical hedonia, easily assessable through the BMRQ, 19 could, therefore, constitute a useful tool for memory stimulation and rehabilitation paradigms, able to promote finely grained interventions in populations with memory deficits.
Finally, it is also worth mentioning that the research conducted so far has been limited to experimental, high controlled settings. Another, natural next step would be, therefore, to bring this evidence to ecological settings in order to test the relationship between music pleasure and cognition in real‐life experiments (e.g., live concerts) able to account for the complexity of daily life musical experience and episodic encoding.
The work presented so far emphasizes the versatility of dopaminergic transmission in humans 18 and confirms that music can be employed in experimental settings as an ideal tool for the study of abstract reward processes. The direct implication of music pleasure in memory provides evidence that music, via the activation of an ancient brain system related to survival processes, can modulate and improve higher cognitive functions. Crucially, this in turn strengthens the universal value of music 1 and supports its potential effects in human life and well‐being mediated by music‐reward elicited signals.
AUTHOR CONTRIBUTIONS
L.F. and A.R.F. wrote the paper.
COMPETING INTERESTS
The authors declare no competing interests.
PEER REVIEW
The peer review history for this article is available at: https://publons.com/publon/10.1111/nyas.14867.
ACKNOWLEDGMENTS
L.F. is supported by ANR‐20‐CE28‐0008. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. The authors would like to thank all the researchers and coauthors who, through their help, insightful comments, and knowledge from different disciplines, greatly contributed to the development of this work and line of research, in particular: Gemma Cardona, Ernest Mas‐Herrero, Jordi Riba, Pablo Ripollés, Marta Valle, and Robert Zatorre.
Open Access Funding provided by Universita degli Studi di Pavia within the CRUI‐CARE Agreement.
Ferreri, L. , & Rodriguez‐Fornells, A. (2022). Memory modulations through musical pleasure. Ann NY Acad Sci., 1516, 5–10. 10.1111/nyas.14867
REFERENCES
- 1. Patel, A. D. (2010). Music, biological evolution, and the brain. In Bailar (Ed.) M., Emerging disciplines (pp. 91–144) Houston, TX: Rice University Press. [Google Scholar]
- 2. Groussard, M. , Chan, T. G. , Coppalle, R. , & Platel, H. (2019). Preservation of musical memory throughout the progression of Alzheimer's disease? Toward a reconciliation of theoretical, clinical, and neuroimaging evidence. Journal of Alzheimer's Disease, 68, 857–883. [DOI] [PubMed] [Google Scholar]
- 3. Ferreri, L. , Aucouturier, J.‐J. , Muthalib, M. , Bigand, E. , & Bugaiska, A. (2013). Music improves verbal memory encoding while decreasing prefrontal cortex activity: An fNIRS study. Frontiers in Human Neuroscience, 7, 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belfi, A. M. , & Jakubowski, K. (2021). Music and autobiographical memory. Music & Science, 4, 1–5. [Google Scholar]
- 5. Joucla, C. , Nicolier, M. , Giustiniani, J. , Brunotte, G. , Noiret, N. , Monnin, J. , Magnin, E. , Pazart, L. , Moulin, T. , Haffen, E. , Vandel, P. , & Gabriel, D. (2018). Evidence for a neural signature of musical preference during silence. International Journal of Psychophysiology, 125, 50–56. [DOI] [PubMed] [Google Scholar]
- 6. Trost, W. , & Frühholz, S. (2015). The hippocampus is an integral part of the temporal limbic system during emotional processing. Physics of Life Reviews, 13, 87–88. [DOI] [PubMed] [Google Scholar]
- 7. Ara, A. , & Marco‐Pallarés, J. (2020). Fronto‐temporal theta phase‐synchronization underlies music‐evoked pleasantness. Neuroimage, 212, 116665. [DOI] [PubMed] [Google Scholar]
- 8. Meyer, L. B. (2008). Emotion and meaning in music. University of Chicago Press. [Google Scholar]
- 9. Mas‐Herrero, E. , Maini, L. , Sescousse, G. , & Zatorre, R. J. (2021). Common and distinct neural correlates of music and food‐induced pleasure: A coordinate‐based meta‐analysis of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 123, 61–71. [DOI] [PubMed] [Google Scholar]
- 10. Salimpoor, V. N. , Van Den Bosch, I. , Kovacevic, N. , Mcintosh, A. R. , Dagher, A. , & Zatorre, R. J. (2013). Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science, 340, 216–219. [DOI] [PubMed] [Google Scholar]
- 11. Martínez‐Molina, N. , Mas‐Herrero, E. , Rodríguez‐Fornells, A. , Zatorre, R. J. , & Marco‐Pallarés, J. (2016). Neural correlates of specific musical anhedonia. Proceedings of the National Academy of Sciences of the United States of America, 113, E7337–E7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berridge, K. C. , Robinson, T. E. , & Aldridge, J. W. (2009). Dissecting components of reward: ‘Liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adcock, R. A. , Thangavel, A. , Whitfield‐Gabrieli, S. , Knutson, B. , & Gabrieli, J. D. E. (2006). Reward‐motivated learning: Mesolimbic activation precedes memory formation. Neuron, 50, 507–517. [DOI] [PubMed] [Google Scholar]
- 14. Gruber, M. J. , Gelman, B. D. , & Ranganath, C. (2014). States of curiosity modulate hippocampus‐dependent learning via the dopaminergic circuit. Neuron, 84, 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ripollés, P. , Ferreri, L. , Mas‐Herrero, E. , Alicart, H. , Gómez‐Andrés, A. , Marco‐Pallares, J. , Antonijoan, R. M. , Noesselt, T. , Valle, M. , Riba, J. , & Rodríguez‐Fornells, A. (2018). Intrinsically regulated learning is modulated by synaptic dopamine signaling. eLife, 7, e38113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lisman, J. , Grace, A. A. , & Duzel, E. (2011). A neoHebbian framework for episodic memory; Role of dopamine‐dependent late LTP. Trends in Neurosciences, 34, 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moncada, D. , Ballarini, F. , & Viola, H. E. (2015). Behavioral tagging: A translation of the synaptic tagging and capture hypothesis. Neural Plasticity, 2015, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferreri, L. , Mas‐Herrero, E. , Zatorre, R. J. , Ripollés, P. , Gomez‐Andres, A. , Alicart, H. , Olivé, G. , Marco‐Pallarés, J. , Antonijoan, R. M. , Valle, M. , Riba, J. , & Rodríguez‐Fornells, A. (2019). Dopamine modulates the reward experiences elicited by music. Proceedings of the National Academy of Sciences of the United States of America, 116, 3793–3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mas‐Herrero, E. , Marco‐Pallares, J. , Lorenzo‐Seva, U. , Zatorre, R. J. , & Rodríguez‐Fornells, A. (2013). Individual differences in music reward experiences. Music Perception, 31, 118–138. [Google Scholar]
- 20. Yonelinas, A. P. (2002). The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language, 46, 441–517. [Google Scholar]
- 21. Ferreri, L. , & Rodríguez‐Fornells, A. (2017). Music‐related reward responses predict episodic memory performance. Experimental Brain Research, 235, 3721–3731. [DOI] [PubMed] [Google Scholar]
- 22. Ferreri, L. , Mas‐Herrero, E. , Cardona, G. , Zatorre, R. J. , Antonijoan, R. M. , Valle, M. , Riba, J. , Ripollés, P. , & Rodríguez‐Fornells, A. (2021). Dopamine modulations of reward‐driven music memory consolidation. Annals of the New York Academy of Sciences, 1502, 85–98. [DOI] [PubMed] [Google Scholar]
- 23. Cardona, G. , Rodríguez‐Fornells, A. , Nye, H. , Rifà‐Ros, X. , & Ferreri, L. (2020). The impact of musical pleasure and musical hedonia on verbal episodic memory. Scientific Reports, 10, 16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Juslin, P. N. (2013). From everyday emotions to aesthetic emotions: Towards a unified theory of musical emotions. Physics of Life Reviews, 10, 235–266. [DOI] [PubMed] [Google Scholar]
- 25. Sousa, A. M. M. , Zhu, Y. , Raghanti, M. A. , Kitchen, R. R. , Onorati, M. , Tebbenkamp, A. T. N. , Stutz, B. , Meyer, K. A. , Li, M. , Kawasawa, Y. I. , Liu, F. , Perez, R. G. , Mele, M. , Carvalho, T. , Skarica, M. , Gulden, F. O. , Pletikos, M. , Shibata, A. , Stephenson, A. R. , … & Sestan, N. (2017). Molecular and cellular reorganization of neural circuits in the human lineage. Science, 358, 1027–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chowdhury, R. , Guitart‐Masip, M. , Bunzeck, N. , Dolan, R. J. , & Duzel, E. (2012). Dopamine modulates episodic memory persistence in old age. Journal of Neuroscience, 32, 14193–14204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ripollés, P. , Marco‐Pallarés, J. , Alicart, H. , Tempelmann, C. , Rodríguez‐Fornells, A. , & Noesselt, T. (2016). Intrinsic monitoring of learning success facilitates memory encoding via the activation of the SN/VTA‐hippocampal loop. eLife, 5, e17441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gold, B. P. , Mas‐Herrero, E. , Zeighami, Y. , Benovoy, M. , Dagher, A. , & Zatorre, R. J. (2019). Musical reward prediction errors engage the nucleus accumbens and motivate learning. Proceedings of the National Academy of Sciences of the United States of America, 116, 3310–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schultz, W. (2017). Reward prediction error. Current Biology, 27, R369–R371. [DOI] [PubMed] [Google Scholar]
- 30. Ergo, K. , De Loof, E. , & Verguts, T. (2020). Reward prediction error and declarative memory. Trends in Cognitive Sciences, 24, 388–397. [DOI] [PubMed] [Google Scholar]
- 31. Miendlarzewska, E. A. , Bavelier, D. , & Schwartz, S. (2016). Influence of reward motivation on human declarative memory. Neuroscience & Biobehavioral Reviews, 61, 156–176. [DOI] [PubMed] [Google Scholar]
- 32. Henson, R. N. , & Gagnepain, P. (2010). Predictive, interactive multiple memory systems. Hippocampus, 20, 1315–1326. [DOI] [PubMed] [Google Scholar]
- 33. Gruber, M. J. , & Ranganath, C. (2019). How curiosity enhances hippocampus‐dependent memory: The prediction, appraisal, curiosity, and exploration (PACE) framework. Trends in Cognitive Sciences, 23, 1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luciana, M. , & Collins, P. F. (2012). Incentive motivation, cognitive control, and the adolescent brain: Is it time for a paradigm shift? Child Development Perspectives, 6, 392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elvers, P. , Omigie, D. , Fuhrmann, W. , & Fischinger, T. (2015). Exploring the musical taste of expert listeners: Musicology students reveal tendency toward omnivorous taste. Frontiers in Psychology, 6, 1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nussenbaum, K. , Prentis, E. , & Hartley, C. A. (2020). Memory's reflection of learned information value increases across development. Journal of Experimental Psychology: General, 149, 1919–1934. [DOI] [PubMed] [Google Scholar]
- 37. Skov, M. , & Kirk, U. (2022). Expertise and aesthetic liking. In Chatterjee A. & Cardillo E. (Eds), Brain, beauty, and art (pp. 70–74). Oxford University Press. [Google Scholar]
- 38. Gagnepain, P. , Fauvel, B. , Desgranges, B. , Gaubert, M. , Viader, F. , Eustache, F. , Groussard, M. , & Platel, H. (2017). Musical expertise increases top–down modulation over hippocampal activation during familiarity decisions. Frontiers in Human Neuroscience, 11, 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dunn, P. G. , De Ruyter, B. , & Bouwhuis, D. G. (2012). Toward a better understanding of the relation between music preference, listening behavior, and personality. Psychology of Music, 40, 411–428. [Google Scholar]
- 40. Sihvonen, A. J. , Leo, V. , Ripollés, P. , Lehtovaara, T. , Ylönen, A. , Rajanaro, P. , Laitinen, S. , Forsblom, A. , Saunavaara, J. , Autti, T. , Laine, M. , Rodríguez‐Fornells, A. , Tervaniemi, M. , Soinila, S. , & Särkämö, T. (2020). Vocal music enhances memory and language recovery after stroke: Pooled results from two RCTs. Annals of Clinical and Translational Neurology, 7, 2272–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]


