Abstract
Objective
Novel and minimally invasive neurotechnologies offer the potential to reduce the burden of epilepsy while avoiding the risks of conventional resective surgery. Few neurotechnologies have been tested in randomized controlled trials with pediatric populations, leaving clinicians to face decisions about whether to recommend these treatments with insufficient evidence about the relevant risks and benefits. This study specifically explores the preferences of clinicians for treating pediatric drug‐resistant epilepsy (DRE) with novel neurotechnologies.
Methods
A discrete‐choice experiment (DCE) was designed to elicit the preferences of clinicians with experience in treating children with DRE using novel neurotechnological interventions. The preferences for six key attributes used when making treatment decisions (chances of clinically significant improvement in seizures, major and minor risks from intervention, availability of evidence, financial burden for the family, and access to the intervention) were estimated using a conditional logit model. The estimates from this model were then used to predict the adoption of existing novel neurotechnological interventions.
Results
Sixty‐eight clinicians completed the survey: 33 neurosurgeons, 28 neurologists, and 7 other clinicians. Most clinicians were working in the United States (74%), and the remainder (26%) in Canada. All attributes, apart from the nearest location with access to the intervention, influenced preferences significantly. The chance of clinically significant improvement in seizures was the most positive influence on clinician preferences, but low‐quality evidence and a higher risk of major complications could offset these preferences. Of the existing neurotechnological interventions, vagus nerve stimulation was predicted to have the highest likelihood of adoption; deep brain stimulation had the lowest likelihood of adoption.
Significance
The preferences of clinicians are drive primarily by the likelihood of achieving seizure freedom for their patients, but preferences for an intervention are largely eradicated if only low quality of evidence supporting the intervention is available. Until better evidence supporting the use of potentially effective, novel neurotechnologies becomes available, clinicians are likely to prefer more established treatments.
Keywords: clinician preferences, discrete choice experiment, neurotechnology, pediatric epilepsy
Key points.
Clinicians are highly motivated to reduce seizures in children with drug‐resistant epilepsy (DRE) but require evidence about relevant risks and benefits of treatment.
In the absence of robust evidence for novel neurotechnologies, it is likely that clinicians will favor more established treatments.
Clinicians consider financial burdens, but may be less influenced by other factors such as access to treatment, which are critical to caregivers of children with DRE.
1. INTRODUCTION
Epilepsy is a common chronic condition of the central nervous system causing seizures affecting up to 1% of children in North America. 1 , 2 , 3 In most cases, epilepsy can be treated successfully using antiseizure medications (ASMs). However, about one fourth of children treated with ASMs either fail to become or remain seizure‐free, a condition known as drug‐resistant epilepsy (DRE). 4 , 5 There are many potential negative consequences for children with DRE during their development, which include an elevated risk of mortality, 6 , 7 and the potential long‐term impacts on quality of life associated with difficulties with learning and development, psychological concerns, disability, and social isolation. 8 , 9
Epilepsy surgery is an option for some children, but relies on the proper selection of suitable candidates. If carefully selected, up to 70% of children with DRE can achieve seizure freedom after surgical intervention. 5 If seizure freedom is achieved, many of the negative consequences of DRE for the child can be minimized or avoided. 5 , 10 , 11 However, conventional surgery is an irreversibly invasive, open procedure that requires a large scalp incision, temporary removal of sections of the skull, excision of the epileptogenic focus within the brain, and—in extreme cases—the removal or disconnection of the affected hemisphere of the brain. This invasiveness comes with significant risks including infection, bleeding, injury to the areas of the brain proximal to the resection, impairment of memory, stroke, and sometimes death. 12 Recovery from conventional surgery may be a lengthy process involving an extended stay in hospital and rehabilitation after discharge. Furthermore, conventional resective surgery can only be offered to patients in whom the seizure‐initiating brain regions have been accurately identified and found to be distant to eloquent brain regions. 13
In response to the risks of conventional surgery and a paucity of alternatives, a range of less‐invasive technologies have emerged that could offer an alternative to conventional surgery for children with DRE, or offer hope to those not candidates for resective surgery. These include ablative neurotechnologies such as stereotactic radiosurgery, magnetic resonance imaging (MRI)–guided laser interstitial thermal therapy (MRgLITT), and neuromodulatory neurotechnologies such as vagus nerve stimulation (VNS), deep brain stimulation (DBS), and responsive neurostimulation (RNS). Although these novel neurosurgical treatments offer the potential to reduce seizure frequency for a child while avoiding the risks, and for some interventions, the irreversibility of conventional surgery, many are adopted into practice without the rigors of a randomized clinical trial in this age group. 14 Therefore, when children with DRE and their caregivers and clinicians are deciding whether or not to try a novel neurotechnology, they are often facing these decisions with insufficient evidence about the relevant risks and benefits of treatment.
A detailed understanding is needed of the factors that clinicians consider regarding whether to recommend a novel neurotechnological intervention for children with DRE, and how they balance the potential risks, benefits, and consequences for children with DRE and their caregivers. This information will inform the design of future research to support evidence‐based decision‐making by clinicians around the use of novel neurotechnologies for children with DRE. Consequently, the aim of this study was to quantify the relative preferences of clinicians who treat children with DRE for novel neurotechnologies that vary in their potential risks, benefits, and delivery, and to use these preferences to predict the likely adoption of these novel neurotechnologies.
2. MATERIALS AND METHODS
2.1. Approach
Pediatric epilepsy clinicians, including neurologists and neurosurgeons, in Canada and the United States completed a self‐administered, web‐based discrete‐choice experiment (DCE) survey. The DCE is an established choice‐based survey technique that has been used increasingly in health care to evaluate the trade‐offs that people might be willing to make between treatments and services. DCEs have been used extensively to examine both the preferences for and acceptability of new treatments and services before they are introduced. 15 DCEs rely on the ability to describe products or services according to the most important characteristics to consumers, known as attributes, which have levels that can represent different alternatives. In health care, an attribute might be an outcome, like the chance of cure, and the levels might take the form of different probabilities. The value of a product or service is then estimated as the weighted sum of the levels of these characteristics. 16
DCEs rely on key assumptions of random utility theory: first that an individual presented with two or more options will choose the option with the highest perceived utility (or value) and, second, that when comparing two options A and B, the probability that option A is chosen over option B is proportional to the extent to which option A is valued over option B. 17 These choice‐based techniques, which requires participants to trade off different combinations of attributes of treatments or services, allows the relative preference for each of the attributes to be estimated.
2.2. Survey development
The DCE was integrated into a three‐part questionnaire. The first included questions on respondents' general knowledge of, and familiarity with, several neuromodulatory and ablative neurotechnological interventions. The second contained the choice experiment with instructions on how to complete choices, the hypothetical clinical scenario, and the attributes and levels included in the choice set. In the third section, respondents were asked to provide socioeconomic information, including gender, age group, race/ethnicity, country of practice, years of experience, and specialty. Respondents had the opportunity to revise their answers. The questionnaire is available from the corresponding authors upon request.
2.3. Attribute and level selection
The DCE survey was developed using qualitative methods to understand the attributes and levels that define novel neurotechnologies for children with DRE, in line with international guidelines. 18 , 19 , 20 Specifically, we conducted four focus groups with clinicians (pediatric neurosurgeons and neurologists) with experience treating children with DRE; the focus groups were conducted at national or international conferences and included clinicians from six Canadian provinces and ten US states, providing broad geographic representation of both countries. The full analysis and derivation of attributes are described in detail elsewhere, including the demographics of focus group participants. 21 Briefly, focus groups were recorded and transcribed, and then analyzed using inductive thematic analysis, which resulted in six core attributes for the DCE (Table 1). The levels for each attribute were based on the range of plausible values for neurotechnologies available from a literature review, 14 the qualitative analysis of focus groups, 21 and the expert opinion of stakeholder groups. After selecting an initial set of attributes, we undertook further pilot testing with clinicians and discussions with expert stakeholder groups composed of pediatric neurosurgeons and pediatric neurologists to narrow the list of attributes, ensure that no critical attributes were omitted, and refine the wording of attributes and levels. The DCE had a combination of six attributes: five attributes had four levels and one attribute had three levels, resulting in a possible 3072 choices.
TABLE 1.
The attributes and levels included in the DCEs and the sources of evidence for their derivation
| Attributes | Levels | Sources/evidence |
|---|---|---|
| 1. Chance of clinically significant improvement in seizures | 1. No clinically significant improvement in seizures | Attribute: Focus groups 21 |
| 2. Less than 50% chance of clinically significant improvement in seizures | Levels: Expert opinion/ Literature 24 | |
| 3. 50%–79% chance of clinically significant improvement in seizures | ||
| 4. 80%–100% chance of clinically significant improvement in seizures | ||
| 2. Minor risk of complications from intervention | 1. No additional risk of minor neurologic complications from treatment | Attribute: Focus groups 21 |
| 2. Minor neurologic complications occur in 5 in 100 patients | Levels: Expert opinion/Literature 25 | |
| 3. Minor neurologic complications occur in 10 in 100 patients | ||
| 4. Minor neurologic complications occur in 15 in 100 patients | ||
| 3. Major risk of complications from intervention | 1. No additional risk of major neurologic complications from treatment | Attribute: Focus groups 21 |
| 2. Major neurologic complications occur in 1 in 100 patients | Levels: Expert opinion/Literature 25 | |
| 3. Major neurologic complications occur in 5 in 100 patients | ||
| 4. Major neurologic complications occur in 10 in 100 patients | ||
| 4. Availability of evidence for the intervention | 1. The efficacy of this intervention has been shown in clinical trials. | Attribute: Focus groups 21 |
| 2. The efficacy of this intervention has been shown in case reports/case series. | Levels: Expert opinion | |
| 3. You might have anecdotal evidence relayed from colleagues. | ||
| 5. Financial burden on family | 1. None | Attribute: Focus groups 21 |
| 2. Low | Levels: Expert opinion | |
| 3. Medium | ||
| 4. Extreme | ||
| 6. Access to the intervention | 1. This intervention is available in your institution | Attribute: Focus groups 21 |
| 2. This intervention is available in another institution in your province/state | Levels: Expert opinion | |
| 3. This intervention is only available in another province/state |
2.4. Experimental design
To generate a manageable set of choices for participants to complete without compromising the statistical modeling of preference estimates, we used Ngene software to create an efficient experimental design. 22 Given the risk of attrition of busy physician participants especially with the extra burdens of the coronavirus disease 2019 (COVID‐19) pandemic, we generated a design that required as few choices per participant as possible. This resulted in 42 different choice sets, which were divided into six blocks of seven questions. Each participant was randomized to one of the six survey versions and therefore made seven choices.
2.5. Construction of tasks
Each participant was asked to consider the following scenario: “Imagine you have a patient with pediatric DRE, for whom you have to make a treatment decision/recommendation. Your patient is a child who has been treated for several years with multiple antiseizure medications and despite this they continue to have frequent seizures that affect their quality of life.” They were then asked to choose between two full profiles describing unnamed hypothetical neurotechnological interventions. An opt‐out option of “Continue with current standard of care” was included as the choice to not to use a novel neurotechnology intervention at any given point in time was a realistic alternative. An example choice set is shown in Figure 1.
FIGURE 1.
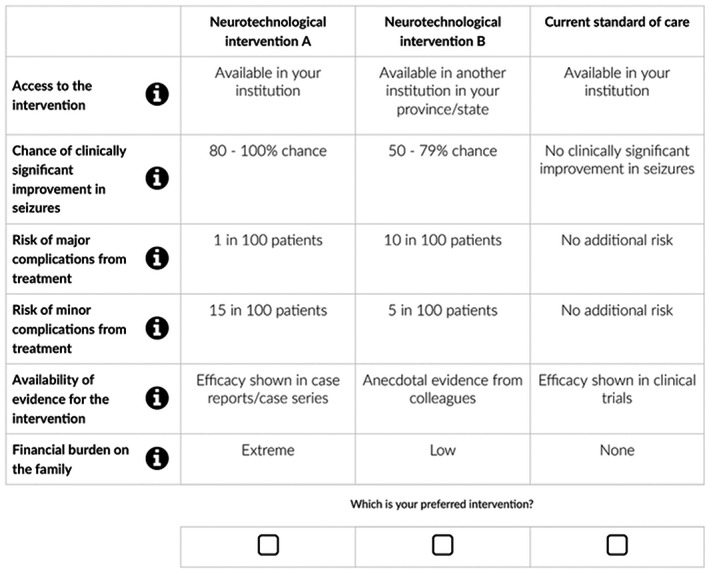
Example choice set from the DCE.
2.6. Piloting
The survey was piloted internally for language, formatting, and coding errors with our team of investigators and collaborators, which included ethicists, pediatric neurosurgeons, and pediatric neurologists. In addition, the survey was piloted with a group of neurosurgeons who provided feedback on wording and the experience of completing the survey. A general population pilot sample, recruited using Amazon's Mechanical Turk (MTurk) platform, was used to test the survey for face validity (n = 72).
2.7. Sampling and recruitment
An invitation email with a description of the survey and a link to the questionnaire was sent to the following physician groups in September 2020: the Canadian Pediatric Neurosurgery Study Group (~35 members), Canadian League Against Epilepsy and Canadian Pediatric Epilepsy Network (>200), members of the American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Pediatric Neurosurgery (>200), 23 and the Child Neurology Society (n > 2000).
Ethics approval was granted by the University of British Columbia Behavioral Research Ethics Board (H18‐02783).
2.8. Data analysis
2.8.1. Understanding clinician preferences
The DCE data were analyzed using a conditional logit model in STATA 15.6 software. 17 The model assumes that the utility function of an individual can be defined using the levels of the attributes in each choice set. The model provides coefficients that represent estimates of the mean effect of each attribute level in predicting individual preferences along with their statistical significance. The higher the value of a coefficient, the higher the importance of the related attribute level for respondents considering the decision. A positive coefficient indicates that the respondent attaches positive value to that level, whereas a negative coefficient indicates that the respondent attached a negative value to that level. We effects coded attribute levels to estimate a coefficient for each level of each attribute. Effects coding gives attributes a central utility of zero; the coefficients (utilities) are estimates of relative preference that must be interpreted relative to the magnitude of the coefficients for other attributes.
2.8.2. Predicting adoption of interventions using preferences
The estimated coefficients for each attribute level can be used to estimate the utility (or value) of existing neurotechnologies, for example, DBS. The first step of this process is to select a level from each of the six attributes included in the DCE that corresponds best with DBS. The exponentiated sum of the estimated coefficients for the levels that are selected for DBS can be considered the utility of DBS. To compare adoption of DBS with another option, for example, current standard of care drug therapy, the process is repeated to estimate the utility of that option using the attribute levels that best correspond with the current standard of care (drug therapy). The predicted probability of adoption, or that DBS would be chosen ahead of no change from the current standard of care (drug therapy), can then be calculated as the exponent of the utility for DBS divided by the sum of the exponents of utilities for DBS and no change from the current standard of care (drug therapy). The attribute levels for each of the available neurotechnological interventions were chosen in consultation with a neurosurgeon on our team (PJM), and are shown in the supporting material (Table A1).
Using this method, we first predicted the potential adoption of seven existing neurotechnologies: VNS, RNS, DBS, transcranial direct current stimulation (tDCS), stereotactic radiosurgery, MRgLITT, and magnetic resonance imaging (MRI)–guided focused ultrasound (MRgFUS) compared with the current standard of care (drug therapy). We then predicted adoption of each neurotechnology in scenarios where we assumed that all treatments within the ablative or neuromodulatory classes of neurotechnology were available. For the neuromodulatory class, we predicted adoption in a scenario where VNS, RNS, DBS, or tDCS was available. For the ablative class we predicted adoption in a scenario where stereotactic radiosurgery, MRgLITT, or MRgFUS was available.
To explore the sensitivity of our predictions of adoption of existing neurotechnologies to the assumptions we made about the level of each attribute that best represents each neurotechnology, we asked two additional members of our team (GMI, RN) and the team member who chose the original levels (PJM) to provide estimates of their confidence in the choice of attribute level for each treatment. This information was used to construct a distribution of chosen attribute levels for each treatment. Predictions of adoption were then estimated using attribute levels selected using 1000 bootstrap samples from these distributions, using the method described earlier.
3. RESULTS
3.1. Sample
Sixty‐eight clinicians completed the survey (Table 2): 33 neurosurgeons (49%), 28 neurologists (41%), 6 nurses, and 1 clinical coordinator in pediatric epilepsy clinics. Most respondents were practicing in the United States (74%) and in academia (91%), identified as male (66%), and as White (78%). The sample represented a range of ages and years in practice.
TABLE 2.
Sample characteristics
| N | % | |
|---|---|---|
| Country | ||
| Canada | 50 | 74% |
| United States | 18 | 26% |
| Profession | ||
| Neurologist/Epileptologist | 28 | 41% |
| Neurosurgeon | 33 | 49% |
| Other | 7 | 10% |
| Practice | ||
| Academic | 62 | 91% |
| Private | 1 | 1% |
| Private/Academic | 5 | 7% |
| Years in practice | ||
| ≤5 | 12 | 18% |
| 6–15 | 28 | 41% |
| 16–25 | 17 | 25% |
| >25 | 11 | 16% |
| Age | ||
| ≤35 | 7 | 10% |
| 36–45 | 22 | 32% |
| 46–55 | 25 | 37% |
| 56–75 | 14 | 21% |
| Race/ethnicity | ||
| Asian | 8 | 12% |
| Mixed race | 1 | 1% |
| White | 53 | 78% |
| Prefer not to say | 1 | 1% |
| African Canadian | 2 | 3% |
| Missing | 3 | 4% |
| Self‐identified gender | ||
| Man | 45 | 66% |
| Woman | 23 | 34% |
3.2. Clinician preferences
The estimated preferences for attribute levels (Figure 2) showed that clinicians had the strongest preferences for increasing the chance of clinically significant improvement in seizures and for evidence of the efficacy of the intervention from more robust sources. The full table of coefficients is available in the supporting material (Table A2).
FIGURE 2.

Preferences of clinicians for different features of novel neurotechnologies for DRE.
Failing to increase the chance of a clinically significant improvement in seizures above 50% was associated with a significantly negative impact on preferences (−2.942, 95% confidence interval [CI] −1.971, −3.913). Increasing the chance of clinically significant improvement in seizures above 50% was associated with a strong and statistically significant positive preference (50–79: 1.577, 95% CI 1.197, 1.957; and 80–100: 1.831, 95% CI 1.382, 2.281). The availability of evidence for the intervention from clinical trials was associated with a large, statistically significant positive preference (1.181, 95% CI 0.634, 1.729), whereas having only anecdotal evidence was associated with a large, statistically significant negative preference (−1.169, 95% CI ‐0.856, −1.482). The availability of evidence from case reports or series did not significantly influence preferences either positively or negatively.
We found evidence that certain levels of risk of major neurological complications could contribute positively to preferences at low levels (1 in 100 patients) or negatively to preferences at higher levels (10 in 100), with a negative gradient of preferences as risk increased. There was also a preference for eliminating the risk of minor neurological complications. In addition, there appeared to be a threshold of tolerance for the financial burden that treatment could place on families; an extreme financial burden was associate with a negative, statistically significant preference against an intervention (−0.532, 95% CI −0.186, −0.878). Access to the intervention—defined as the nearest location that an intervention was available—for a patient did not significantly influence preferences in this study.
We found no clear evidence of differences in patterns of preferences between participants from the United States and Canada (Figure A1). The preference of participants from the United States was somewhat more strongly influenced by the financial burden on families compared to their Canadian counterparts, who in turn appeared to be more heavily influenced by the available evidence (Figure A1). Similarly, there was no clear difference in patterns of preferences between neurosurgeons and neurologists or other clinicians (Figure A2). Neurosurgeons may place somewhat greater importance on the potential risk of major complications than other clinicians, whereas other clinicians placed greater importance than neurosurgeons on the financial burden on the family.
3.3. Predictions of adoption
Once we calculated coefficients that represent the relative strength of clinicians' preferences for each level of each attribute, we used this information to make predictions about the likely adoption of existing neurotechnologies.
First, we predicted the likelihood of each treatment being used compared with the current standard of care (Figure 3). We predict that VNS would have the highest likelihood of adoption (98%, 95% CI 97%, 100%) and DBS would have the lowest (66%, 95% CI 45%, 87%). However, predicted adoption for all other treatments were within the range of 78% to 94%, and CIs were wide; therefore, we could only be confident that the adoption of VNS was likely to be significantly higher than DBS.
FIGURE 3.

Predicted adoption of each neurotechnology compared with remaining on current standard of care drug therapy.
Second, we modeled predicted adoption within neuromodulatory and ablative classes of neurotechnology, assuming all treatments within that class were available (Figure 4). Within the neuromodulatory class, we predicted that VNS would be strongly favored, with a predicted adoption of 78% (95% CI 60%, 97%). All other options had predicted likelihoods of adoption between 3% and 11% and overlapping CIs. Adoption within the ablative class was lower, ranging from 16% for MRgFUS (95% CI 6%, 26%) to 44% (95% CI 25%, 64%) for stereotactic radiosurgery. The CIs for all treatments overlapped, suggesting no significant difference between options.
FIGURE 4.

Predicted likelihood of adoption of neurotechnologies within the neuromodulatory and ablative categories.
3.4. Sensitivity analysis
Introducing uncertainty about the choice of levels used to describe each treatment increased all confidence intervals around our predictions of adoption. Because the distribution of chosen attribute levels for each treatment was based on a small sample, the estimates of three team members, we expected CIs to be wide. However, confidence intervals widened most for stereotactic radiosurgery, MRgFUS, and tDCS, suggesting that there was most uncertainty about the levels that best represent these treatments (Figure A3). VNS remained the neurotechnology expected to have the highest likelihood of adoption, followed by RNS, MRgLITT, and DBS. The adoption estimates for stereotactic radiosurgery reduced from 94% to 83% (rank second to fifth), and increased from 66% to 91% (rank seventh to fourth) for DBS. Within the neuromodulatory class (Figure A4), VNS still had the highest predicted likelihood of adoption, followed by RNS, but DBS was predicted to have a higher uptake than tDCS. Within the ablative class, MRgLITT had a higher predicted uptake than stereotactic radiosurgery, but MRgFUS remained the option with the lowest predicted likelihood of adoption.
4. DISCUSSION
In this study exploring clinician preferences for novel neurotechnologies to treat pediatric DRE we found that preferences are driven primarily by the likelihood of achieving seizure freedom. However, preferences for an intervention would be largely eradicated by low quality of evidence supporting that intervention. Clinicians also consider the impact of the intervention on their patients, with evidence suggesting that the potential risks of treatment and the financial impact on the patient's family are also important in their decisions. The location of where the treatment could be offered (i.e., locally vs the need for travel) did not impact clinician preference for an intervention. The pattern of preference for potentially effective treatments with high‐quality evidence translated to predictions that clinicians would be more likely to prefer more established treatments until better evidence for potentially effective, novel neurotechnologies is available.
To our knowledge this is the first study looking at the trade‐offs clinicians are willing to make when considering different neurotechnologies to treat pediatric DRE. The finding that the potential benefit of a novel neurotechnology does not offset lack of evidence for certain new treatments is important in the context of a recent review highlighting a need for more evidence before novel neurotechnological interventions are incorporated into clinical practice. 14 Our predictions of lower adoption of treatments with lower‐quality available evidence is consistent with the finding that a lag between clinical research and practice is due to a lack of high‐quality evidence from robust study designs such as randomized controlled trials. This finding is also consistent with previous research that found that general population preferences for new treatments are diminished when the implications of newness in terms of lower levels of robust evidence are communicated. 26 Our finding that the risks of major and minor complications seem less important than potential benefits might reflect that all recommended treatment options have inherent risks, whether associated with treatment or uncontrolled epilepsy.
The finding that the clinicians in this study valued the potential effectiveness of neurotechnologies and the availability of high‐quality evidence is reassuring and expected. These preferences naturally place novel neurotechnologies at a relative disadvantage, particularly in Canada and for some programs in the United States, where public or private insurance funding for interventions requires robust evidence across multiple aspects of value and safety before funding is approved. It is likely that novel neurotechnologies become available and accumulate evidence more quickly in the United States, where there is greater pressure than in Canada for health care providers and insurers to compete and offer newer, more innovative treatments. This was discussed by clinicians in the focus group study that informed the present one. 27 In interpreting the results, it is important to bear in mind that the features of novel neurotechnologies are a critical component in clinician decision‐making, but that they are embedded in a broad context of considerations that are either prerequisites for treatment (e.g., appropriateness of a treatment option), or do not vary between interventions (such as trust in the clinician and the clinician‐patient relationship) that have been described elsewhere. 21 , 27 Finally, there is evidence that patients and clinicians have discordant preferences for treatment, which has been documented in a review of DCEs, 28 and in our previous research. 21 , 27 , 29 , 30 Therefore, it is important to understand how treatment preferences of children with DRE and their caregivers may differ from those of clinicians. Future research to quantify the preferences of these groups is needed for comparison. Understanding the preferences of clinicians and children and their caregivers is valuable for the design of studies involving novel neurotechnologies; understanding how individuals consider choices and make trade‐offs—for either technological or other interventions including diet or alternative approaches—is also invaluable for the design of clinical studies and trials, recruitment and participation in research, and for the improvement of treatment delivery. 31 , 32 , 33
One key strength of this study is the validity of the group of clinicians. Our clinician team members supported the recruitment effort, providing us with access to mailing lists of several pediatric neurosurgery and pediatric neurology societies in the United States and Canada. The commitment of our team to recruitment yielded enough responses to run the analysis with a sample of clinicians that are actively involved in making these types of decisions when treating children with DRE. In exploratory sensitivity analyses, we found that the results are consistent between subgroups of participants such as neurosurgeons and other clinicians, and clinicians in the United States and Canada. Although the sample size of these subgroups precludes making formal comparisons, the trends nonetheless indicate that our results have applicability to both countries and different health care systems: the main difference between the US and Canadian samples was a stronger preference to financial burden on the family in the former, which provides evidence of the face validity of the findings. A further strength of this study was the use of qualitative methods to derive attributes in the development of the DCE, in line with best practices. 19 , 20
There are limitations to the study that warrant consideration. First, we acknowledge that the DCE explored preferences for alternative hypothetical scenarios or stated preferences. As with any DCE, it is not known how these reflect the actual choices clinicians would make in practice—revealed preferences—or whether these choices were made in a way that is more consistent with the underlying theory. However, the results have face validity in the direction and strength of coefficients, and with the predictions of adoption. A recent review also reports evidence that DCEs can generate reasonable estimates of revealed preferences from stated preferences. 34 Furthermore, when we predicted the likelihood of adoption using the results of the DCE, the predictions assumed that we could select the most appropriate level of each attribute to describe each of the neurotechnologies across experts. However, we explored the sensitivity of our estimates of adoption to variation in the levels selected, and the analyses suggested that the relative order of preference for neurotechnologies was robust.
5. CONCLUSIONS
Clinicians are currently making decisions with children with DRE and their caregivers about whether to use novel neurotechnologies in the absence of robust evidence about their risks, benefits, and consequences. We found that although achieving seizure freedom is a core goal, clinicians would be discouraged by low quality of evidence and, to a lesser extent, potential financial consequences for the patient and their families. It seems reasonable that potential medical benefits for a child hold greater importance than financial burden in clinical decision‐making, but we note that inequities of access could be perpetuated in such a scenario. Nevertheless, based on the preferences elicited in this study, it is equally reasonable and ethically appropriate that clinicians favor more established treatments while better evidence accumulates for newer neurotechnologies for children with DRE.
AUTHORS’ CONTRIBUTIONS
JI, PJM, MH, MC, and KJK formulated the idea for the study. MH, GA, MA, and AS conducted the statistical analysis. MH, GA, and MA prepared the manuscript. GA, PJM, MA, LC, WC, MC, VH, GI, KJK, AL, RN, ER, AS, MH, and JI provided input into the study design and the revision of the manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. JI, PJM, and MH are the guarantors.
CONFLICT OF INTEREST
George Ibrahim has received support from and has served as a paid consultant for LivaNova Inc. All other authors have no conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (NIH) BRAIN Initiative on Neuroethics [RF1 # MH117805 01; JI, Principal Investigator; PJM, co‐Principal Investigator]. JI is a Distinguished University Scholar and UBC Distinguished Scholar in Neuroethics. PJM was the Alcan Chair in Neurosciences at UBC during the course of this work. MH is supported by a Michael Smith Foundation for Health Research Scholar Award (16813). ER receives salary support from the Fonds de recherche du Québec – Santé. LC is a Dorothy Foehr Huck and J. Lloyd Huck Chair in Neuroethics at Pennsylvania State University.
Apantaku GO, McDonald PJ, Aguiar M, Cabrera LY, Chiong W, Connolly MB, Clinician preferences for neurotechnologies in pediatric drug‐resistant epilepsy: A discrete choice experiment. Epilepsia. 2022;63:2338–2349. 10.1111/epi.17328
Glory O. Apantaku and Patrick J. McDonald Joint first authors.
Mark Harrison and Judy Illes Joint last authors; Equal authors listed in alphabetical order.
Funding information
National Institutes of Health (NIH) BRAIN Initiative on Neuroethics (RF1 # MH117805 01; JI, Principal Investigator; PJM, co‐Principal Investigator).
Contributor Information
Mark Harrison, Email: mark.harrison@ubc.ca.
Judy Illes, Email: jilles@mail.ubc.ca.
REFERENCES
- 1. Aaberg KM, Gunnes N, Bakken IJ, Søraas CL, Berntsen A, Magnus P, et al. Incidence and prevalence of childhood epilepsy: a Nationwide cohort study. Pediatrics. 2017;139(5):e20163908. [DOI] [PubMed] [Google Scholar]
- 2. Russ SA, Larson K, Halfon N. A National Profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129(2):256–64. [DOI] [PubMed] [Google Scholar]
- 3. Prasad AN, Sang X, Corbett BA, Burneo JG. Prevalence of childhood epilepsy in Canada. Can J Neurol Sci J Can Sci Neurol. 2011;38(5):719–22. [DOI] [PubMed] [Google Scholar]
- 4. Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, et al. Definition of drug resistant epilepsy: consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–77. [DOI] [PubMed] [Google Scholar]
- 5. Muh CR. Current and emerging surgical therapies for severe pediatric epilepsies. Semin Pediatr Neurol. 2016;23(2):143–50. [DOI] [PubMed] [Google Scholar]
- 6. Verducci C, Hussain F, Donner E, Moseley BD, Buchhalter J, Hesdorffer D, et al. SUDEP in the north American SUDEP registry: the full spectrum of epilepsies. Neurology. 2019;93(3):e227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callenbach PMC, Westendorp RGJ, Geerts AT, Arts WFM, Peeters EAJ, van Donselaar CA, et al. Mortality risk in children with epilepsy: the Dutch study of epilepsy in childhood. Pediatrics. 2001;107(6):1259–63. [DOI] [PubMed] [Google Scholar]
- 8. Engel J. What can we do for people with drug‐resistant epilepsy? Neurology. 2016;87(23):2483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar G. Evaluation and management of drug resistant epilepsy in children. Curr Probl Pediatr Adolesc Health Care. 2021;51(7):101035. [DOI] [PubMed] [Google Scholar]
- 10. Titus JB, Lee A, Kasasbeh A, Thio LL, Stephenson J, Steger‐May K, et al. Health‐related quality of life before and after pediatric epilepsy surgery: the influence of seizure outcome on changes in physical functioning and social functioning. Epilepsy Behav EB. 2013;27(3):477–83. [DOI] [PubMed] [Google Scholar]
- 11. Hannan S, Cross JH, Scott RC, Harkness W, Heyman I. The effects of epilepsy surgery on emotions, behavior, and psychosocial impairment in children and adolescents with drug‐resistant epilepsy: a prospective study. Epilepsy Behav EB. 2009;15(3):318–24. [DOI] [PubMed] [Google Scholar]
- 12. Joudi Mashhad M, Harati H, Parooie F, Salarzaei M. Epilepsy surgery for refractory seizures: a systematic review and meta‐analysis in different complications. Egypt J Neurol Psychiatry Neurosurg. 2020;56(1):35. [Google Scholar]
- 13. Dlugos DJ. The early identification of candidates for epilepsy surgery. Arch Neurol. 2001;58(10):1543–6. [DOI] [PubMed] [Google Scholar]
- 14. Kaal KJ, Aguiar M, Harrison M, McDonald PJ, Illes J. The clinical research landscape of pediatric drug‐resistant epilepsy. J Child Neurol. 2020;35(11):763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Bekker‐Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ. 2012;21(2):145–72. [DOI] [PubMed] [Google Scholar]
- 16. Louviere J, Hensher D, Swait J, Adamowicz W. Stated Choice Methods: Analysis and Applications. Cambridge: Cambridge University Press; 2000. 10.1017/CBO9780511753831 [DOI] [Google Scholar]
- 17. McFadden D. Conditional logit analysis of qualitative choice behavior. Frontiers in Econometrics. New York: Academic Press; 1974. p. 105–42. [Google Scholar]
- 18. Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–13. [DOI] [PubMed] [Google Scholar]
- 19. Hollin IL, Craig BM, Coast J, Beusterien K, Vass C, DiSantostefano R, et al. Reporting formative qualitative research to support the development of quantitative preference study protocols and corresponding survey instruments: guidelines for authors and reviewers. Patient‐Patient‐Centered Outcomes Res. 2020;13(1):121–36. [DOI] [PubMed] [Google Scholar]
- 20. Coast J, Al‐Janabi H, Sutton EJ, Horrocks SA, Vosper AJ, Swancutt DR, et al. Using qualitative methods for attribute development for discrete choice experiments: issues and recommendations. Health Econ. 2012;21(6):730–41. [DOI] [PubMed] [Google Scholar]
- 21. Apantaku G, Aguiar M, Kaal KJ, McDonald PJ, Connolly MB, Hrincu V, et al. Understanding attributes that influence physician and caregiver decisions about neurotechnology for pediatric drug‐resistant epilepsy: a formative qualitative study to support the development of a discrete choice experiment. The Patient. 2021;15:219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rose JM, Bliemer M. Ngene [Internet]. ChoiceMetrics. Available from: http://www.choice‐metrics.com/
- 23. Neurosurgical Subspecialty Sections of the AANS and CNS [Internet]. Available from: https://www.aans.org/. Accessed 2022.
- 24. McHugh JC, Singh HW, Phillips J, Murphy K, Doherty CP, Delanty N. Outcome measurement after vagal nerve stimulation therapy: proposal of a new classification. Epilepsia. 2007;48(2):375–8. [DOI] [PubMed] [Google Scholar]
- 25. Hader WJ, Tellez‐Zenteno J, Metcalfe A, Hernandez‐Ronquillo L, Wiebe S, Kwon C‐S, et al. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54(5):840–7. [DOI] [PubMed] [Google Scholar]
- 26. Harrison M, Marra CA, Bansback N. Preferences for “new” treatments diminish in the face of ambiguity. Health Econ. 2017;26(6):743–52. [DOI] [PubMed] [Google Scholar]
- 27. McDonald PJ, Hrincu V, Connolly MB, Harrison MJ, Ibrahim GM, Naftel RP, et al. Novel neurotechnological interventions for pediatric drug‐resistant epilepsy: physician perspectives. J Child Neurol. 2021;36(3):222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrison M, Milbers K, Hudson M, Bansback N. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open. 2017;7(5):e014719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hrincu V, McDonald PJ, Connolly MB, Harrison MJ, Ibrahim GM, Naftel RP, et al. Choice and trade‐offs: parent decision making for Neurotechnologies for pediatric drug‐resistant epilepsy. J Child Neurol. 2021;36(11):943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Udwadia FR, McDonald PJ, Connolly MB, Hrincu V, Illes J. Youth weigh in: views on advanced neurotechnology for drug‐resistant epilepsy. J Child Neurol. 2021;36(2):128–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aguiar M, Harrison M, Munro S, Burch T, Kaal KJ, Hudson M, et al. Designing discrete choice experiments using a patient‐oriented approach. Patient ‐ Patient‐Centered Outcomes Res. 2021;14(4):389–97. [DOI] [PubMed] [Google Scholar]
- 32. Dobra RA, Boeri M, Elborn S, Kee F, Madge S, Davies JC. Discrete choice experiment (DCE) to quantify the influence of trial features on the decision to participate in cystic fibrosis (CF) clinical trials. BMJ Open. 2021;11(3):e045803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Queally M, Devane D, Griffin MD, Gillespie P, Pandit A. Using a discrete choice experiment to examine the factors influencing clinical trial participation in a nationally representative sample. 2021. Available from: https://www.researchsquare.com. Accessed 2022.
- 34. Quaife M, Terris‐Prestholt F, Di Tanna GL, Vickerman P. How well do discrete choice experiments predict health choices? A systematic review and meta‐analysis of external validity. Eur J Health Econ. 2018;19:1053–66. 10.1007/s10198-018-0954-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


