Abstract
BCG, the attenuated strain of Mycobacterium bovis, has been widely used as a vaccine against tuberculosis and is thus an important candidate as a live carrier for multiple antigens. With the aim of developing a recombinant BCG (rBCG) vaccine against diphtheria, pertussis, and tetanus (DPT), we analyzed the potential of CRM197, a mutated nontoxic derivative of diphtheria toxin, as the recombinant antigen for a BCG-based vaccine against diphtheria. Expression of CRM197 in rBCG was achieved using Escherichia coli-mycobacterium shuttle vectors under the control of pBlaF*, an upregulated β-lactamase promoter from Mycobacterium fortuitum. Immunization of mice with rBCG-CRM197 elicited an anti-diphtheria toxoid antibody response, but the sera of immunized mice were not able to neutralize diphtheria toxin (DTx) activity. On the other hand, a subimmunizing dose of the conventional diphtheria-tetanus vaccine, administered in order to mimic an infection, showed that rBCG-CRM197 was able to prime the induction of a humoral response within shorter periods. Interestingly, the antibodies produced showed neutralizing activity only when the vaccines had been given as a mixture in combination with rBCG expressing tetanus toxin fragment C (FC), suggesting an adjuvant effect of rBCG-FC on the immune response induced by rBCG-CRM197. Isotype analysis of the anti-diphtheria toxoid antibodies induced by the combined vaccines, but not rBCG-CRM197 alone, showed an immunoglobulin G1-dominant profile, as did the conventional vaccine. Our results show that rBCG expressing CRM197 can elicit a neutralizing humoral response and encourage further studies on the development of a DPT vaccine with rBCG.
Many currently used vaccines require multiple doses to achieve maximum protection, which has led to reduced coverage of vaccination campaigns, especially in developing countries. The use of live viral or bacterial carriers for heterologous antigen presentation, such as vaccinia virus, Salmonella, and Mycobacterium bovis BCG (Bacille Calmette-Guérin), has been intensively investigated in an effort to reduce the number of doses required for immunization. M. bovis BCG is the most widely used live vaccine, having been administered as an antituberculosis vaccine to over 3 billion individuals. It has several features that have encouraged its use as a live carrier for recombinant antigens, such as low production cost, possibility of administration at birth with very strong adjuvant activity, induction of immunity after a single dose, and low frequency of side effects. The induction of humoral and cellular immune responses against antigens from several pathogens by recombinant BCG (rBCG) strains has been reported, such as rBCG expressing antigens from human immunodeficiency virus (25, 29), simian immunodeficiency virus (30), Leishmania major (1, 6), Plasmodium falciparum (13), Streptococcus pneumoniae (18), and Borrelia burgdorferi (26).
The conventional diphtheria-pertussis-tetanus (DPT) vaccine was shown to be extremely efficient, and the recently developed acellular DPT vaccine showed lower reactogenicity. However, both DPT and acellular DPT vaccines require multiple doses to attain complete protection, and the acellular DPT vaccine is expensive. The expression of DPT antigens in live carriers such as BCG could thus provide a single-dose vaccine against these pathogens. Tetanus and pertussis antigens have been expressed in rBCG, inducing significant immune responses (2, 5, 21), but expression of diphtheria antigens in an rBCG vaccine has not yet been described.
Diphtheria toxin (DTx) is a secreted molecule of 58.35 kDa produced by Corynebacterium diphtheriae and composed of two functional subunits: subunit A encompasses the catalytic domain responsible for ADP-ribosylation of elongation factor 2, which blocks protein synthesis of target cells, and subunit B is responsible for binding to the cell surface receptors and transferring subunit A into the cytoplasm (28). Immunity against diphtheria is obtained by the induction of a neutralizing Th2-dominant (mainly immunoglobulin G1 [IgG1]) humoral immune response against DTx. The conventional vaccine consists of the alum-adsorbed, formaldehyde-treated toxin (diphtheria toxoid), administered to children in three doses at 1, 3, and 5 months, followed by boosters at 1.5 and 5 years of age. CRM197 (cross-reacting material), a mutant DTx devoid of toxic activity, carries a unique glycine-to-glutamic acid substitution at residue 52 within the catalytic domain, which eliminates its toxic activity (8). It is used in several systems as the protein carrier for conjugated polysaccharide vaccines (15, 24). Native CRM197 induces lower antibody levels than diphtheria toxoid, but its immunogenicity is improved after a mild formaldehyde treatment (12).
Expression and purification of recombinant CRM197 in E. coli has been described (3). Expression of this antigen or its fragments in the recombinant Salmonella enterica serovar Typhi CVD 908-htrA vaccine strain has proved to be compromised by the insolubility of the heterologous proteins (22). Solubilization by using the hemolysin A secretion system from E. coli resulted in low expression levels, and all constructs failed to induce immune responses. Recently, a Staphylococcus carnosus strain expressing the receptor-binding domain of DTx was shown to induce neutralizing antibodies after nine doses of 3 × 108 CFU (7).
In this study, we analyzed the potential of CRM197, as the antigen in an rBCG vaccine against diphtheria, with the long-term goal of developing an rBCG DPT vaccine. Here we describe the successful expression of CRM197 in rBCG using E. coli/mycobacterium vectors, under the control of the pBlaF* promoter, an upregulated β-lactamase promoter isolated from Mycobacterium fortuitum. We also describe efficient priming of the DTx-neutralizing humoral response in mice immunized with rBCG-CRM197.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and vaccine preparation.
All cloning steps were performed in E. coli DH5α grown in Luria-Bertani medium supplemented with ampicillin (100 μg/ml) or kanamycin (20 μg/ml). The BCG Moreau strain was used to generate the rBCG strains. Liquid cultures of the BCG strains were regularly grown in Middlebrook 7H9 medium supplemented with albumin-dextrose-catalase (ADC; Difco, Detroit, Mich.), with or without kanamycin (20 μg/ml), at 37°C using stationary tissue culture flasks. The rBCG strains were cultured in Ungar's medium (16) for the heterologous protein localization assays. BCG was transformed by electroporation as previously described (29) and plated onto Middlebrook 7H10 agar plates supplemented with oleic acid-ADC (Difco) containing kanamycin (20 μg/ml). Plates were incubated at 37°C for 3 weeks before expansion of the transformed colonies in liquid media. rBCG vaccines were prepared from mid-log-phase liquid cultures of selected clones. The liquid cultures were centrifuged at 4,000 × g, resuspended in 10% glycerol, and maintained at −80°C until used. The numbers of CFU in the frozen stocks were previously determined by growing the thawed vaccine preparations on Middlebrook 7H10 plates containing kanamycin (20 μg/ml) at several dilutions. Immediately before vaccination, cells were thawed and diluted in saline to reach the appropriate concentrations.
Construction of the CRM197 expression vectors.
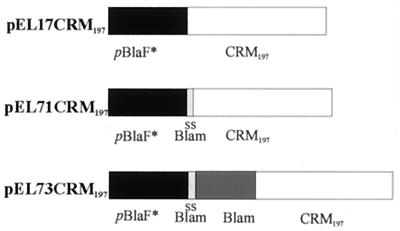
pJEM17, pLA71, and pLA73 contain the E. coli and mycobacterium origins of replication, a kanamycin resistance gene, the pBlaF* promoter, its ATG initiation codon, and a multicloning site (19, 27). pJEM17 expresses the native inserted gene, and pLA71 and pLA73 place the heterologous gene in fusion with either the β-lactamase signal sequence or the whole β-lactamase-encoding gene (Fig. 1). For the construction of pEL17CRM197, the CRM197 gene was PCR amplified from pSM308, a Bacillus subtilis plasmid, without its signal sequence using the primers 5′TAG TAG GGA TCC TGG CGC TGA TGA TGT TGT TGA T3′ and 5′TAG TAG GGA TCC GAT ATC TCA GCT TTT GAT TTC AAA AAA TAG C3′. Underlining and italics indicate BamHI and EcoRV restriction sites, respectively. The amplified fragment (1,604 bp) was digested with BamHI and subcloned into pBCSK+ (Stratagene, La Jolla, Calif.). The BamHI/EcoRV fragment was further cloned into pJEM17 digested with the same restriction enzymes, resulting in pEL17CRM197. For construction of pEL71CRM197 and pEL73CRM197, the CRM197 gene without its signal sequence was amplified by PCR with the primers 5′TAG TAG GGA TCC TAC GTA CGG GCG CTG ATG ATG TTG TTG AT3′ and 5′TAG TAG GGA TCC GCG GCC GCT CAG CTT TTG ATT TCA AAA AAT AGC3′. Underlining, italics, and bold type indicate BamHI, SnaBI, and NotI restriction sites, respectively. The amplified fragment was digested with BamHI and subcloned into pUC18 (New England Biolabs, Beverly, Mass.). The fragment was further cloned into pLA71 and pLA73 digested with SnaBI and NotI, resulting in pEL71CRM197 and pEL73CRM197, which have the CRM197 gene inserted in frame with β-lactamase fragment sequences.
FIG. 1.
Schematic representation of the promoter and antigen regions of shuttle vectors pEL17CRM197, pEL71CRM197, and pEL73CRM197. All vectors contain E. coli and mycobacterial origins of replication, a kanamycin resistance gene (Kanr), pBlaF*, and CRM197. pEL71CRM197 also has the β-lactamase signal sequence (ss Blam), while pEL73CRM197 has the complete β-lactamase (Blam) sequence fused to the CRM197 sequence.
Western blotting.
Kanamycin-resistant BCG clones were grown in 50-ml Middlebrook 7H9-ADC liquid cultures supplemented with kanamycin (20 μg/ml). Cells from 25 ml of these cultures were harvested at mid-log phase by centrifugation, washed once with 5 ml of Tris-EDTA, resuspended in 0.5 ml of Tris-EDTA, and disrupted on ice for 2 min using a GE 100 ultrasonic processor at half-maximum constant output. The protein concentration in the culture lysates was determined with a protein assay (Bio-Rad, Hercules, Calif.), using bovine serum albumin (BSA) as a standard. Approximately 50-μg aliquots of protein extracts were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12% gel). The proteins were then electrotransferred onto a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.), and the membrane was saturated with 5% nonfat dry milk in phosphate-buffered saline (PBS) containing 0.1% Tween 20 (vol/vol) (Sigma, St. Louis, Mo.) (PBS-T). Horse anti-diphtheria toxoid serum, routinely produced by Instituto Butantan (São Paulo, São Paulo, Brazil), was adsorbed against BCG to remove antibodies against mycobacterial antigens according to the method described by Gruber and Zingales (10) and used for detection of CRM197 or diphtheria toxoid in the immunoblots (1:1,000). Horseradish peroxidase (HRP)-conjugated anti-horse antibody (Sigma) was used as secondary antibody, and detection was performed with an ECL kit (Amersham-Pharmacia, Little Chalfont, Buckinghamshire, England).
Localization of heterologous proteins in rBCG.
Clones of the rBCG strains expressing the heterologous protein were grown in 30-ml cultures of Ungar's medium supplemented with kanamycin (20 μg/ml). The cells were harvested at mid-log phase by centrifugation. The proteins from the culture supernatants were precipitated with acetone. The cell pellet was resuspended in PBS, with adjustment of cell density to equivalent values, and sonicated for 2 min as described above. Membranes were solubilized by the addition of 2% (vol/vol) Triton X-114. Insoluble material (cell wall-enriched fractions) were separated by centrifugation at 27,000 × g, and the supernatant was subjected to detergent phase partitioning, separating the membrane and cytosol fractions, as described elsewhere (26). Samples from each fraction were subjected to SDS-PAGE and immunoblotting as described above.
Immunizations.
Male 4-week-old BALB/c mice from Instituto Butantan were immunized intraperitoneally (i.p.) with 107 CFU of BCG, rBCG-CRM197, or a mixture of 5 × 106 CFU of rBCG-CRM197 and 5 × 106 CFU of rBCG-FC (rBCG expressing tetanus toxin fragment C) (Mazzantini et al., unpublished data) in 500 μl of apyrogenic saline. The conventional DT vaccine (1 Lf [limit of flocculation] of alum-adsorbed diphtheria toxoid and 0.25 Lf of tetanus toxoid per mouse) produced by Instituto Butantan was used as a positive control. Blood was collected from the retro-orbital plexus, and pooled sera were analyzed by enzyme-linked immunosorbent assay (ELISA) for antibodies against diphtheria toxoid.
ELISA.
Serum antibody responses to rBCG immunizations and controls were quantified by ELISA. Briefly, Polysorp 96-well plates (Nunc International, Rochester, N.Y.) were coated with diphtheria toxoid (Instituto Butantan) (100 μl; 2 μg/ml in carbonate-bicarbonate buffer, pH 9.6; 4°C overnight), washed three times with PBS-T, blocked with 10% nonfat dry milk in PBS, and then incubated with serial dilutions of mouse sera in PBS–1% BSA at 37°C for 1 h. The plates were washed as described above and incubated with HRP-conjugated goat anti-mouse IgG (1:2,000) (Sigma) in PBS–1% BSA at 37°C for 1 h. Antibody isotyping was performed using goat anti-mouse IgG1, IgG2a, IgG2b, IgG3, IgM, and IgA (1:2,000) (Sigma) and HRP-conjugated anti-goat (1:10,000) antibodies (Sigma). Following washing, antibodies were visualized by adding OPD substrate (100 μl; 0.04% o-phenylenediamine in citrate phosphate buffer [pH 5], containing 0.01% H2O2). After color development (15 min), the reaction was interrupted by addition of 8 M H2SO4 (50 μl), and the A492 was determined. Absorbance values were plotted against serum dilutions.
Vero cell method potency test.
The in vitro Vero cell method based on the protocol described by Gupta and colleagues (11) was used for titration of diphtheria antitoxin. Briefly, sera in twofold dilutions in 96-well plates were incubated in the presence of 0.003 Lf of DTx (which is neutralized by 0.008 IU of standard anti-DTx/ml) per ml in Dulbecco's modified Eagle's medium (Sigma) containing 10% fetal bovine serum (Cultilab; Campinas, São Paulo, Brazil) for 1 h at room temperature. Vero cells (1.5 × 104) were added to each well and incubated for 96 h at 37°C in a 5% CO2 incubator. Cells were washed with PBS and then fixed and stained with 20% formaldehyde–1% crystal violet. The highest dilution of serum able to protect cells from DTx killing was used for calculation of the anti-DTx dose. Values were multiplied by 10, according to the correlation established for in vivo and in vitro testing in Instituto Butantan, in order to compare our results with values required for approval of the conventional vaccine, which is performed in vivo. Each serum was analyzed in duplicate, and controls for DTx and standard anti-DTx were included in all experiments.
RESULTS
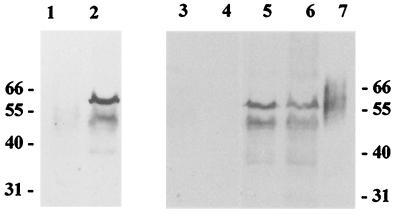
Expression and localization of CRM197 in rBCG. We have constructed a series of plasmid vectors for expression of CRM197 in BCG under the control of the pBlaF* promoter, either directly at the multicloning site in pEL17CRM197, in fusion with the β-lactamase signal sequence in pEL71CRM197, or with the whole β-lactamase sequence in pEL73CRM197 (Fig. 1). BCG was transformed with each of the three constructs, generating rBCG(pEL17CRM197), rBCG(pEL71CRM197), and rBCG(pEL73CRM197), respectively. Expression of the antigen in the different strains was analyzed by immunoblotting. Figure 2 shows that CRM197 was expressed in significant amounts by all constructs, but mainly bands with the predicted size for CRM197 (58 kDa) and bands possibly originating from proteolysis of the former one were detected. These results indicated that the protein fusions in rBCG(pEL71CRM197) and rBCG(pEL73CRM197) were not stable and were probably cleaved near the fusion point between the β-lactamase fragments and CRM197. Total cell extracts were further subjected to cellular fractionation to determine the localization of CRM197 in the rBCG strains through immunoblotting. Surprisingly, in all the rBCG-CRM197 strains, CRM197 was mostly localized to the detergent-insoluble fraction, which is enriched in cell wall components (data not shown), even though the pEL17CRM197 construct does not carry any signal sequence for protein export.
FIG. 2.
Expression of CRM197 in rBCG. Total cell extracts of rBCG (50 μg) were analyzed by Western blotting using anti-diphtheria toxoid antiserum. Lanes: 1, rBCG(pJEM17); 2, rBCG(pEL17CRM197); 3, rBCG(pLA71); 4, rBCG(pLA73); 5, rBCG(pEL71CRM197); 6, rBCG(pEL73CRM197); 7, control diphtheria toxoid. Molecular weights (in thousands) are indicated on the left and right.
Immune response to rBCG expressing CRM197.
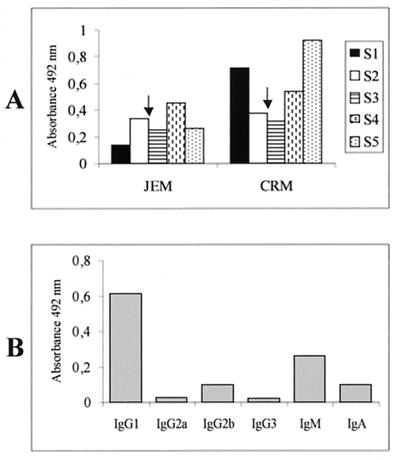
Eight BALB/c mice were immunized i.p. with 107 CFU of rBCG(pEL17CRM197), using rBCG(pJEM17) as a negative control, and given boosters under the same conditions after 9 weeks. An initial increase in anti-diphtheria toxoid antibody level was observed in the sera of mice immunized with rBCG(pEL17CRM197), and this level decreased to control levels in the following months (Fig. 3A). A more important increase was observed 2 months after the booster dose. Comparable results were obtained in mice immunized with rBCG(pEL71CRM197), since expression and localization of CRM197 were similar in all constructs (results not shown). At 20 weeks after priming (S5), the isotype profile of the sera of rBCG(pEL17CRM197)-immunized mice showed mainly IgG1 induction (Fig. 3B); IgG1 is considered the main antibody isotype responsible for DTx neutralization. Despite showing the expected isotype profile, these sera failed to neutralize DTx in the in vitro Vero cell neutralization assay.
FIG. 3.
Antibody response induced by rBCG-CRM197. (A) BALB/c mice were immunized i.p. with rBCG(pJEM17) or rBCG(pEL17CRM197) (107 CFU), receiving a booster dose under the same conditions after 9 weeks (indicated by arrows). S1, 4 weeks; S2, 8 weeks; S3, 12 weeks; S4, 16 weeks; S5, 20 weeks. Pooled sera were collected at the indicated times and analyzed for anti-diphtheria toxoid antibodies by ELISA. (B) Sera from mice immunized with rBCG(pEL17CRM197) (S5) were subjected to anti-diphtheria toxoid immunoglobulin isotyping by ELISA. Sera were diluted 1/20.
Induction of DTx-neutralizing antibodies after rBCG priming.
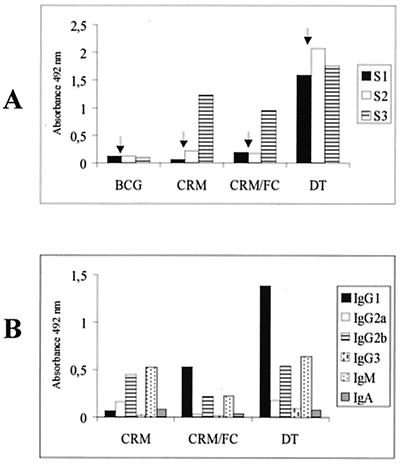
In order to determine if mice immunized with rBCG(pEL17CRM197) were primed to respond to an infection with C. diphtheriae, we administered a subimmunizing dose (1/25) of the conventional alum-adsorbed DT vaccine at 3 weeks after priming. This subimmunizing dose was determined as the highest dose that does not elicit any anti-diphtheria toxoid antibody response (data not shown). Five mice were primed with rBCG(pEL17CRM197) or with a mixture of rBCG(pEL17CRM197) and rBCG(pRL17FC) (rBCG expressing tetanus toxin fragment C in a vector derived from pJEM17) (Mazzantini et al., unpublished). BCG and the conventional DT vaccine were used as negative and positive controls, respectively. The mixture of rBCG expressing CRM197 and rBCG expressing FC was tested because of our long-term goal for the development of an rBCG DPT vaccine. Figure 4A shows the induction of significant levels of anti-diphtheria toxoid antibodies by rBCG(pEL17CRM197) and by its combination with rBCG(pRL17FC), at 7 weeks after priming (S3). A large increase in antibody levels was observed after the subimmunizing dose, reaching around 70% of that obtained with the conventional DT vaccine. Similar results were obtained in different experiments. Isotyping of the antibodies present in the sera of immunized mice, showed that rBCG(pEL17CRM197) induced mainly IgM, while its combination with rBCG(pRL17FC) induced mainly IgG1, with a profile similar to that induced by conventional DT vaccine (Fig. 4B). The sera were then tested for their neutralizing activity, and, as expected, only the sera from mice immunized with the combination of rBCG-CRM197 and rBCG-FC were able to neutralize DTx (anti-DTx level = 0.16 IU/ml). Mice immunized with either control BCG or rBCG(pEL17CRM197) did not produce any detectable neutralizing activity. Control sera from mice immunized with the conventional DT vaccine produced at Instituto Butantan exhibited a strong neutralizing activity (anti-DTx level = 2.56 IU/ml).
FIG. 4.
Antibody response induced by rBCG-CRM197: effect of a subimmunizing dose of DT. (A) BALB/c mice were immunized i.p. with BCG, rBCG(pEL17CRM197) (107 CFU), a mixture of rBCG(pEL17CRM197) and rBCG(pRL17FC) (5 × 106 CFU each), or the conventional DT vaccine, receiving a subimmunizing dose of DT at 3 weeks (indicated by arrows). Pooled sera were collected at the indicated times and analyzed for anti-diphtheria toxoid antibodies by ELISA. S1, 3 weeks; S2, 5 weeks; S3, 7 weeks. (B) S3 sera were subjected to anti-diphtheria toxoid immunoglobulin isotyping by ELISA. Sera were diluted 1/40.
DISCUSSION
In order to analyze the potential of CRM197 in an rBCG vaccine against diphtheria we attempted the expression of CRM197 under the control of the pBlaF* promoter in fusion with different fragments of β-lactamase or not fused. Strong promoter activity of pBlaF* in rBCG has been described (19, 27), and fusion with fragments of the β-lactamase protein has led to export of the reporter antigen. Expression was observed in all constructs, but only bands corresponding to CRM197 alone or its degradation products were observed, indicating that cleavage in the region of the fusion was probably taking place. This phenomenon has previously been observed with the same mycobacterial expression system (19). Surprisingly, most of the protein was localized in the fraction enriched in bacterial cell wall, even when no export signal sequences were present. The intrinsic ability of DTx to directly interact with the lipid membrane (4, 20) could be responsible for this unexpected result. Furthermore, CRM197 was shown to bind more strongly to the lipid bilayer than DTx (23). Alternatively, CRM197 could be localized in inclusion bodies, which would precipitate together with cell wall components in the fractionation experiments. Since entrapment of recombinant proteins in inclusion bodies is normally associated with very high expression levels, we consider the latter possibility unlikely, because the expression of CRM197 is not very high (as analyzed by SDS-PAGE and Coomassie blue staining; results not shown). Furthermore, examples of recombinant antigens localized in inclusion bodies in rBCG were not found in the literature.
Immunization of mice with rBCG expressing the native CRM197 gene in the rBCG(pEL17CRM197) strain followed by a booster dose at 2 months under the same conditions was able to elicit more important antibody responses only at long intervals after priming (Fig. 3). At this point, rBCG(pEL17CRM197) induced mainly IgG1 (Th2 response) anti-diphtheria toxoid antibodies, and this is the antibody isotype considered responsible for the neutralization of DTx. However, the antibody levels induced in the sera of immunized mice were insufficient to neutralize toxin activity, or the antibodies could have been of a nonneutralizing type.
It has been observed that mycobacteria of the M. tuberculosis complex (to which BCG belongs) induce strong cellular responses soon after infection, but humoral responses appear late during the development of the disease. Immunizations with rBCG expressing bacterial antigens are usually followed during 4 to 6 months (5, 18, 26). We also observed a gradual increase in the humoral response up to 5 months after priming. On the other hand, it has been proposed that rBCG could elicit a priming effect, which may enable the induction of a memory response triggered by an infection (9). We thus analyzed the effect of a subimmunizing dose of the conventional DT vaccine at shorter intervals after rBCG priming, in an effort to mimic an infection. A similar strategy has been used in mice immunized with rBCG expressing FC (5). Indeed, mice immunized with rBCG(pEL17CRM197) or its combination with rBCG(pRL17FC), induced a strong humoral response 3 weeks after a subimmunizing dose of DT (7 weeks after priming) (Fig. 4A). Interestingly, the induction of an IgG1-predominant (Th2 response) and neutralizing-antibody response was achieved only with the combination of rBCG strains expressing the diphtheria and tetanus antigens. These results indicate an adjuvant effect of rBCG-FC on the immune response induced by rBCG-CRM197. It was recently shown that rBCG expressing E. coli heat labile enterotoxin (LT-Bh) induced a primary response shifted towards IgG2a, characteristic of the Th1 response typically associated with mycobacterial infections (14). We could detect a Th2-dominant response (mainly IgG1 antibodies) at long intervals after priming with rBCG-CRM197 (Fig. 3B) or within a shorter time in animals injected with a subimmunizing dose of the conventional DT vaccine after priming with a mixture of rBCG-CRM197 and rBCG-FC (Fig. 4B). These results might indicate that the antigen is more driving in the induction of the immune response, with the detection of a Th1-dominant response for LT-Bh and a Th2-dominant response for CRM197.
Priming with a combination of rBCG strains expressing the diphtheria and tetanus antigens, followed by a subimmunizing dose of DT, induced an antibody response with neutralizing activity against DTx (0.16 IU/ml), as did the conventional DT vaccine (2.56 IU/ml), although at lower levels. Quality control of diphtheria vaccines is normally performed with sera from immunized guinea pigs, and the level of DTx neutralization required for vaccine certification is 0.5 IU/ml after a single dose. The diphtheria vaccine produced by Instituto Butantan consistently shows induction of neutralizing activity well above the minimum requirements (results not shown). Gupta and collaborators (12) described substantial differences between in vitro neutralization tests performed with sera from mice and guinea pigs immunized with CRM197, showing significantly lower titers for mouse sera. Since our results were obtained with mice, the antibody levels induced by rBCG priming might be close to that required for approval of the conventional vaccines against diphtheria.
The expression of CRM197 in rBCG was investigated with the aim of developing an rBCG DPT vaccine. The expression of FC has been achieved by several groups using different mycobacterial vectors, showing the induction of a neutralizing humoral response (2, 5). rBCG expressing the S1 subunit of pertussis toxin (PT) in fusion with FC has been shown to induce a specific T-cell response against PT, as well as a tetanus toxin-neutralizing humoral response (2). Furthermore, we have recently shown that rBCG expressing the genetically detoxified S1 subunit of PT-9K/129G in fusion with the β-lactamase signal sequence induces a cellular response which protects mice against an intracerebral challenge with live Bordetella pertussis (21). We now demonstrate that rBCG expressing CRM197 can induce a neutralizing humoral response against DTx. Taken together, these results further encourage studies on the development of a one-dose rBCG vaccine eliciting protective immunity against diphtheria, pertussis, and tetanus. Enhancement of the immune response elicited by rBCG-CRM197 will be investigated and could perhaps be achieved when the vaccine is administered in combination with rBCG expressing tetanus and pertussis antigens. The administration of rBCG vaccines to humans would also require improvements, such as the elimination of antibiotic resistance markers and stable expression of the antigens through insertion of sequences into the mycobacterial genome, goals that are currently being pursued in our laboratory.
ACKNOWLEDGMENTS
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), CNPq, and Fundação Butantan.
We thank Marisa G. Trevelin, Solange R. Silva, and Sebastiana V. Oliveira for technical assistance and Fátima A.M. Oliveira for secretarial assistance.
REFERENCES
- 1.Abdelhak S, Louzir H, Timm J, Blel L, Benlasfar Z, Lagranderie M, Gheorghiu M, Dellagi K, Gicquel B. Recombinant BCG expressing the leishmania surface antigen Gp63 induces protective immunity against Leishmania major infection in BALB/c mice. Microbiology. 1995;141:1585–1592. doi: 10.1099/13500872-141-7-1585. [DOI] [PubMed] [Google Scholar]
- 2.Abomoelak B, Huygen K, Kremer L, Turneer M, Locht C. Humoral and cellular immune responses in mice immunized with recombinant Mycobacterium bovis bacillus Calmette-Guérin producing a pertussis toxin-tetanus toxin hybrid protein. Infect Immun. 1999;67:5100–5105. doi: 10.1128/iai.67.10.5100-5105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishai W R, Rappuoli R, Murphy J R. High-level expression of a proteolytically sensitive diphtheria toxin fragment in Escherichia coli. J Bacteriol. 1987;169:5140–5151. doi: 10.1128/jb.169.11.5140-5151.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blewitt M G, Chung L A, London E. Effect of pH on the conformation of diphtheria toxin and its implications for membrane penetration. Biochemistry. 1985;24:5458–5464. doi: 10.1021/bi00341a027. [DOI] [PubMed] [Google Scholar]
- 5.Cassatt D R, de la Cruz V F, Burlein J E, Koenig S, Stover C K. Protection of mice against tetanus challenge using an experimental tetanus vaccine based on recombinant BCG. In: Ginsberg H S, Brown F, Channock R M, Lerner R A, editors. Vaccines, '93. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 385–389. [Google Scholar]
- 6.Connell N D, Medina A E, McMaster W R, Bloom B R, Russell D G. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guérin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fromen-Romano C, Drevet P, Robert A, Ménez A, Léonetti M. Recombinant Staphylococcus strains as live vectors for the induction of neutralizing anti-diphtheria toxin antisera. Infect Immun. 1999;67:5007–5011. doi: 10.1128/iai.67.10.5007-5011.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giannini G, Rappuoli R, Ratti G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM 45 and CRM 197. Nucleic Acids Res. 1984;12:4063–4069. doi: 10.1093/nar/12.10.4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gicquel B. BCG as a vector for the construction of multivalent recombinant vaccines. Biologicals. 1995;23:113–118. doi: 10.1006/biol.1995.0021. [DOI] [PubMed] [Google Scholar]
- 10.Gruber A, Zingales B. Alternative method to remove antibacterial antibodies from antisera used for screening of expression libraries. BioTechniques. 1995;19:28. [PubMed] [Google Scholar]
- 11.Gupta R K, Higham S, Gupta C K, Rost B, Siber G R. Suitability of the Vero cell method for titration of diphtheria antitoxin in the United States potency test for diphtheria toxoid. Biologicals. 1994;22:65–72. doi: 10.1006/biol.1994.1009. [DOI] [PubMed] [Google Scholar]
- 12.Gupta R K, Collier R J, Rappuoli R, Siber G R. Differences in the immunogenicity of native and formalinized cross reacting material (CRM197) of diphtheria toxin in mice and guinea pigs and their implications on the development and control of diphtheria vaccine based on CRMs. Vaccine. 1997;15:1341–1343. doi: 10.1016/s0264-410x(97)00034-0. [DOI] [PubMed] [Google Scholar]
- 13.Haeseleer F, Pollet J F, Haumont M, Bollen A, Jacobs P. Stable integration and expression of the Plasmodium falciparum circumsporozoite protein coding sequence in mycobacteria. Mol Biochem Parasitol. 1993;57:117–126. doi: 10.1016/0166-6851(93)90249-w. [DOI] [PubMed] [Google Scholar]
- 14.Hayward C M M, O'Gaora P, Young D B, Griffin G E, Thole J, Hirst T R, Castello-Branco L R R, Lewis D J M. Construction and murine immunogenicity of recombinant bacille Calmette Guérin vaccines expressing the B subunit of Escherichia coli heat labile enterotoxin. Vaccine. 1999;17:1272–1281. doi: 10.1016/s0264-410x(98)00350-8. [DOI] [PubMed] [Google Scholar]
- 15.Heath P T. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J. 1998;17:117–122. doi: 10.1097/00006454-199809001-00005. [DOI] [PubMed] [Google Scholar]
- 16.Hemert P F. Vaccine production as a unit process. In: Hockenhull D J D, editor. Progress in industrial microbiology. Edinburgh, United Kingdom: Churchill Livingstone; 1974. pp. 227–233. [Google Scholar]
- 17.Himmelrich H, Lo-Man R, Winter N, Guermonprez P, Sedlik C, Rojas M, Monnaie D, Gheorghiu M, Lagranderie M, Hofnung M, Gicquel B, Clément J- M, Leclerc C. Immune responses induced by recombinant BCG strains according to level of production of a foreign antigen: MalE. Vaccine. 2000;18:2636–2647. doi: 10.1016/s0264-410x(00)00070-0. [DOI] [PubMed] [Google Scholar]
- 18.Langermann S, Palaszynski S R, Burlein J E, Koenig S, Hanson M S, Briles D E, Stover C K. Protective humoral response against pneumococcal infection in mice elicited by recombinant bacille Calmette-Guérin vaccines expressing pneumococcal surface protein A. J Exp Med. 1994;180:2277–2286. doi: 10.1084/jem.180.6.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim E M, Rauzier J, Timm J, Torrea G, Murray A, Gicquel B, Portnoi D. Identification of Mycobacterium tuberculosis DNA sequences encoding exported proteins by using phoA gene fusions. J Bacteriol. 1995;177:59–65. doi: 10.1128/jb.177.1.59-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montecucco C, Schiavo G, Tomasi M. pH dependence of the phospholipid interaction of diphtheria toxin fragments. Biochem J. 1985;231:123–128. doi: 10.1042/bj2310123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nascimento I P, Dias W O, Mazzantini R P, Miyaji E N, Gamberini G, Quintilio W, Gebara V C, Cardoso D, Ho P L, Raw I, Winter N, Gicquel B, Rappuoli R, Leite L C C. Recombinant Mycobacterium bovis BCG expressing pertussis toxin subunit S1 induces protection against an intracerebral challenge with live Bordetella pertussis in mice. Infect Immun. 2000;68:4877–4883. doi: 10.1128/iai.68.9.4877-4883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orr N, Galen J E, Levine M. Expression and immunogenicity of a mutant diphtheria toxin molecule, CRM197, and its fragments in Salmonella typhi vaccine strain CVD 908-htrA. Infect Immun. 1999;67:4290–4294. doi: 10.1128/iai.67.8.4290-4294.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papini E, Colonna R, Schiavo G, Cusinato F, Tomasi M, Rappuoli R, Montecucco C. Diphtheria toxin and its mutant CRM197 differ in their interaction with lipids. FEBS Lett. 1987;215:73–78. doi: 10.1016/0014-5793(87)80116-3. [DOI] [PubMed] [Google Scholar]
- 24.Rennels M B, Edwards K M, Keyserling H L, Reisinger K S, Hogerman D A, Madore D V, Chang I, Paradiso P R, Malinoski F J, Kimura A. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101:604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 25.Stover C K, de la Cruz V F, Fuerst T R, Burlein J E, Benson L A, Bennett L T, Bansal G P, Young J F, Lee M H, Hatfull G F, Snapper S B, Barletta R G, Jacobs W R, Jr, Bloom B R. New use of BCG for recombinant vaccines. Nature. 1991;351:456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- 26.Stover C K, Bansal G P, Hanson M S, Burlein J E, Palaszynski S R, Young J F, Koenig S, Young D B, Sadziene A, Barbour A G. Protective immunity elicited by recombinant bacille Calmette-Guérin (BCG) expressing outer surface protein A (OspA) lipoprotein: a candidate Lyme disease vaccine. J Exp Med. 1993;178:197–209. doi: 10.1084/jem.178.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timm J, Perilli M G, Duez C, Trias J, Orefici G, Fattorini L, Amicosante G, Oratore A, Joris B, Frere J M, Pusgley A P, Gicquel B. Transcription and expression analysis, using lacZ and phoA gene fusions, of Mycobacterium fortuitum beta-lactamase genes cloned from a natural isolate and a high-level beta-lactamase producer. Mol Microbiol. 1994;12:491–504. doi: 10.1111/j.1365-2958.1994.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Ness B G, Howard J B, Bodley J W. ADP-ribosylation of elongation factor 2 by diphtheria toxin. J Biol Chem. 1980;255:10710–10720. [PubMed] [Google Scholar]
- 29.Winter N, Lagranderie M, Rauzier J, Timm J, Leclerc C, Guy B, Kieny M P, Gheorghiu M, Gicquel B. Expression of heterologous genes in Mycobacterium bovis BCG: induction of a cellular response against HIV-1 Nef protein. Gene. 1991;109:47–54. doi: 10.1016/0378-1119(91)90587-2. [DOI] [PubMed] [Google Scholar]
- 30.Winter N, Lagranderie M, Gangloff S, Leclerc C, Gheorghiu M, Gicquel B. Recombinant BCG strains expressing the SIVmac251nef gene induce proliferative and CTL responses against nef synthetic peptides in mice. Vaccine. 1995;13:471–478. doi: 10.1016/0264-410x(94)00001-4. [DOI] [PubMed] [Google Scholar]