Abstract
Background
The amount of initial physiological bone remodeling (IPBR) after implant placement varies and the ways it may play a role in peri‐implantitis development remains unknown. The aim of this retrospective study was to investigate the association between the amount of IPBR during the first year of implant placement and incidence of peri‐implantitis as well as the pattern of progressive bone loss.
Methods
Clinical and radiographic documentation of implants at the time of implant placement (T0), 1 year ± 6 months after crown placement (T1), and at a ≥2‐year follow‐up from implant placement (T2) were retrospectively collected. IPBR was defined as the bone loss occurring from implant placement to the end of the bone remodeling (T1). Cases were grouped into those diagnosed with (test) or without peri‐implantitis (PIm) (control). Linear regression model under generalized estimation equation approach was estimated to assess correlation between marginal bone loss (MBL) rates in both periods (T1‐T0) and (T2‐T1). Receiver operating characteristics curve was estimated to explore an optimal cut‐off point of T1‐T0 MBL to discriminate between PIm and no‐PIm implants.
Results
A total of 45 patients receiving 57 implants without PIm and 40 with PIm were included. There were no associations between PIm and IPBR (p > 0.05), nor between BML of (T2‐T1) and (T1‐T0). However, arch and total follow‐up showed significant influence on the probability of PIm. Splinted implants showed an MBL rate of 0.60‐mm/year higher than non‐splinted implants (p < 0.001) from T1 to T2.
Conclusion
No statistically significant association was found between IPBR and incidence of peri‐implantitis.
Keywords: alveolar bone resorption, dental implants, marginal bone loss, peri‐implantitis
1. INTRODUCTION
Lately, periodontology has become a discipline that is as much about saving dental implants as it is about saving natural teeth. Although dental implants have revolutionized dentistry, they have consequently also created many associated complications such as peri‐implantitis. 1 One of the most important prerequisites for success of the implant therapy is alveolar bone stability, which depends on the quality of biological integration with bone, also known as osseointegration. 2 Early marginal bone loss (MBL) around implants has been attributed to several factors, including but not limited to implant collar design; microgaps and movements 3 ; implant‐abutment junction vertical position 4 ; abutment height 5 ; implant crown/restoration design 6 ; trauma induced by flap elevation 7 ; reduced buccal bone and soft tissue thickness at the implant site 8 ; and possible inflammatory reactions. 9 Additionally, initial bone resorption is expected to occur during the formation of supracrestal fiber height (e.g., biologic width) consisting of the epithelial and connective tissue adhesion forming a mucosal barrier. This phenomenon is considered physiological, and to some extent unavoidable. 10 , 11 Certain implant designs and surgical concepts have been proposed to overcome this initial resorption phenomenon with varied success, including platform switching; implant placement as related to the crestal bone levels; increased peri‐implant soft tissue thickness; and use of long abutments, among others. 12 , 13 , 14 , 15 However, none of the above protocols have been able to completely prevent this early crestal bone resorption.
Galindo‐Moreno and colleagues showed that most of the implants (96%) that exhibited an MBL of >2 mm at 18 months had MBL of at least 0.44 mm or more 6 months post loading. Perhaps if this initial “physiological” bone loss during the healing/remodeling phase exceeds a certain threshold, it may potentially create a niche for pathogenic microorganisms, enabling a more anaerobic environment and promoting progressive bone loss. 16 Conceivably, an early increased peri‐implant bone loss may be indicative of peri‐implantitis development during the remodeling phase. 10 Although a loss of 2 mm of marginal bone during the first year after functional loading has been historically considered a successful outcome, it is critical to revisit and to examine if the 2 mm threshold between physiological and pathological states is still reasonable. 17 , 18 , 19 , 20 Thus, if exceeded a certain limit, it can be hypothesized that early crestal bone remodeling may act like a risk factor for peri‐implantitis. Hence, the aim of this retrospective study was to investigate the association between the amount of initial physiological bone remodeling (IPBR) during the first year of implant placement and incidence of peri‐implantitis, as well as the pattern of progressive bone loss.
2. MATERIALS AND METHODS
This study was approved by the University of Michigan, School of Dentistry, Institutional Review Board for Human Studies (HUM00172687). This retrospective case control investigation included implants placed and maintained at the University of Michigan School of Dentistry. Implants placed from January 1997 to September 2019 were screened. To be included in the present study, the following documentation was needed:
Radiographic documentation: Periapical X‐ray at the time of implant placement (T0), 1 year ± 6 months after crown placement (T1) and from implant placement to the last available follow‐up (T2). If implants with peri‐implantitis were treated, the last X‐ray before treatment was considered as T2.
Clinical documentation: Presence of complete periodontal charts to assess the probing depth (PD) and presence of bleeding on probing 1 year ± 6 months after crown placement and at a ≥ 2‐year follow‐up.
Availability of medical records (to assess presence of diabetes and smoking habits).
Presence of opposing occlusion (teeth/implants).
Single implant restorations, splinted adjacent implants, and implant supported bridges.
2.1. Exclusion criteria
Patients with incomplete charts.
Patients with a <1‐year follow‐up period.
Medically compromised patients (any past records of uncontrolled diabetes, radiation and/or chemotherapy treatment, psychological problems) and severe bruxism cases (diagnosed and/or self‐reported).
Patients treated or maintained in centers outside the University of Michigan School of Dentistry.
Patients with inaccessible files due to bad debt, destroyed records, or decease.
Full‐arch implant restorations, hybrid restorations, and overdentures.
As part of the data collection process, additional information was gathered at the time of implant placement, including: age, tobacco usage, and diabetic history, the number of implants placed and their locations, implant characteristics (brand, length, diameter, implant neck design), mechanism of crown retention (screw or cement‐retained), number of maintenance appointments, type of implant‐abutment connection, apico‐coronal implant position (sub‐, equic‐, or supra‐crestal), as well as timing of bone grafting (prior/during implant placement).
The physical and digital records of implants that fall under the predetermined eligibility criteria were screened and evaluated by two examiners (MVR and AR). Any disagreement that arose during the evaluation and data collection process was resolved through discussion with the supervising investigator (HLW).
Patient enrollment was done through complete‐case analysis. As such all implants that fell into our inclusion criteria were included. Since this is a case control study, the number of implants needed to be matched. After including implants with peri‐implantitis (case group), we consecutively included implants (that respected the inclusion criteria) that did not develop peri‐implantitis.
2.2. Peri‐implantitis and survival rate definition
Presence of peri‐implantitis (PIm): The definition for PIm proposed by the American Academy of Periodontology/European Federation of Periodontology 2017 World Workshop on the Classification of Periodontal and Peri‐implant Diseases and Conditions guidelines 14 was used to classify cases in a binary fashion as either positive or negative for PIm: 0 for peri‐implant health (control group), and 1 for PIm (test group). Because baseline data were available, PIm diagnosis was based on (1) progressive bone loss beyond initial bone remodeling, (2) increased PD compared with previous examinations, and (3) presence of bleeding and/or suppuration on gentle probing. The marginal bone level changes were radiographically examined by two authors (AR, MVR) at the mesial and distal aspects of the affected implants using commercially available software.* If significant differences arose, a third reviewer (HLW) was included for reassessing the radiographs in a joint session and to make a final judgment.
IPBR was defined as the bone loss happening from implant placement to the end of the bone remodeling, generally, 1 year after crown placement (T1).
2.3. Statistical analysis
MBL during the first period (T0 to T1) was considered as the principal predictor on the PI group. Absolute difference T1‐T0 between bone level and yearly rate were defined as follows:
Statistical analysis consists of a description of categorical (absolute and relative frequencies) and continuous (mean, SD, range, and median) variables for the total sample and differentiating by PIm group. At implant level, a multilevel simple binary logistic regression using generalized estimation equations (GEE) was conducted to assess the association between each independent variable and PIm diagnosis (yes/no). Non‐adjusted odds ratio (OR) and 95% confidence intervals were obtained from the Wald Chi‐square statistic. Then, a multiple model was estimated according to the relevant factors and covariates detected in the simple models. Receiver operating characteristics (ROC) curve was estimated to explore an optimal cut‐off point of T1‐T0 MBL to discriminate between PIm and no‐PIm implants. Area under curve (AUC) and 95% confidence interval were obtained.
A linear regression model under GEE approach was estimated to assess correlation between MBL rates of both periods (T1‐T0) and (T2‐T1). Significance level used in analysis was 5% (α = 0.05). Regarding the power analysis, a post‐hoc estimation was obtained. A sample size of 97 independent implants provides 89.4% power at 95% confidence to detect rates at 50% and 80% as significantly different in both groups using a logistic regression model and assuming 95% confidence. However, implants were not independent, and this power must be corrected because of the two‐level structure of data. Each patient provided an average of 1.15 implants and within‐subject correlation CCI = 0.5 (moderate) was assumed, leading to a correcting coefficient D = 1.5. Therefore, 97 dependent implants provide the same power as 68 independent ones, providing power at 74.8% under the same previous conditions.
3. RESULTS
3.1. Clinical characteristics and demographic profiles
A total of 45 patients (17 males and 28 females), averaging 72.2 ± 8.1 years (ranging from 58 to 91 years old) were included in the study. Table 1 provides the demographic and baseline clinical parameters. Overall, the included patient sample hosted a total of 97 implants (57 No PIm and 40 PIm). Implant follow‐up, characteristics of patients and implants, prosthesis, treatment, and time protocols by groups are shown in Table 2. No significant associations between IPBR and the presence of PIm were identified (Table 2). The location of implants showed a weak association (OR = 0.33; p = 0.091). Implants placed in mandible had less risk to develop PIm (67% less). Furthermore, independently from the initial IPBR, 1 additional year of follow‐up increased the risk to have PIm by 18% (OR = 1.18; p = 0.037).
TABLE 1.
Demographic and clinical characteristics
| Characteristics | n (%) |
|---|---|
| No. of patients | 45 |
| Age (years) | 72.2 ± 8.1 |
| Sex | |
| Male | 17 (37.8) |
| Female | 28 (62.2) |
| Diabetes | |
| No | 37 (82.2) |
| Yes | 8 (17.8) |
| Periodontitis | |
| No | 25 (55.6) |
| Yes | 20 (44.4) |
| Smoking | |
| No | 34 (75.6) |
| Yes | 11 (24.4) |
| No. of maintenances per year since implant placement | 3.67 ± 3.75 |
Note: Number of patients (%) or mean ± SD.
TABLE 2.
Characteristics of patients and implants, prosthesis, treatment and time protocols by PI group
| Group | |||||
|---|---|---|---|---|---|
| Parameter | No PI (%) | PI (%) | OR | 95% CI | p‐value |
| N implants | 57 (58.8) | 40 (41.2) | |||
| Age (years) | 72.8 ± 8.0 | 71.4 ± 7.9 | 0.98 | 0.91–1.05 | 0.507 |
| Sex | |||||
| Male | 22 (38.6) | 15 (37.5) | 1 | ||
| Female | 35 (61.4) | 25 (62.5) | 1.05 | 0.35–3.17 | 0.934 |
| Diabetes | |||||
| No | 50 (87.7) | 29 (72.5) | 1 | ||
| Yes | 7 (12.3) | 11 (27.5) | 2.71 | 0.65–11.4 | 0.173 |
| Periodontitis | |||||
| No | 31 (54.4) | 24 (60.0) | 1 | ||
| Yes | 26 (45.6) | 16 (40.0) | 0.80 | 0.26–2.39 | 0.683 |
| Smoking | |||||
| No | 44 (77.2) | 29 (72.5) | 1 | ||
| Yes | 13 (22.8) | 11 (27.5) | 1.28 | 0.36–4.57 | 0.700 |
| N maintenance since IP | 15.3 ± 16.0 | 19.1 ± 11.1 | 1.02 | 0.97–1.07 | 0.463 |
| Tooth type | |||||
| PM | 20 (35.1) | 15 (37.5) | 1 | ||
| M | 37 (64.9) | 25 (62.5) | 0.90 | 0.36–2.27 | 0.825 |
| Arch | |||||
| Maxilla | 5 (8.8) | 9 (22.5) | 1 | ||
| Mandible | 52 (91.2) | 31 (77.5) | 0.33 | 0.09–1.19 | 0.091 |
| Splinted | |||||
| No | 16 (28.1) | 18 (45.0) | 1 | ||
| Yes | 41 (71.9) | 22 (55.0) | 0.48 | 0.15–1.48 | 0.201 |
| Retention | |||||
| Cemented | 48 (84.2) | 32 (80.0) | 1 | ||
| Screw | 9 (15.8) | 8 (20.0) | 1.33 | 0.31–5.70 | 0.698 |
| Level | |||||
| Bone | 48 (84.2) | 38 (95.0) | 1 | ||
| Soft tissue | 9 (15.8) | 2 (5.0) | 0.28 | 0.04–2.13 | 0.219 |
| Stage | |||||
| 1 | 22 (39.3) | 10 (27.8) | 1 | ||
| 2 | 34 (60.7) | 26 (72.2) | 1.68 | 0.53–5.33 | 0.377 |
| Crestal level | 0.235 | ||||
| Equicrestal | 49 (86.0) | 28 (70.0) | 1 | ||
| Supracrestal | 6 (10.5) | 8 (20.0) | 3.50 | 0.59–20.8 | 0.168 |
| Subcrestal | 2 (3.5) | 4 (10.0) | 2.33 | 0.59–9.22 | 0.227 |
| Length (mm) | 0.520 | ||||
| ≤10 mm | 27 (47.4) | 16 (40.0) | 1 | ||
| 10.5–12 mm | 23 (40.4) | 15 (37.5) | 1.10 | 0.36–3.36 | 0.866 |
| >12 mm | 7 (12.3) | 9 (22.5) | 2.17 | 0.56–8.37 | 0.261 |
| Diameter (mm) | 0.558 | ||||
| <4 mm | 13 (22.8) | 10 (25.0) | 1 | ||
| 4–4.5 mm | 21 (36.8) | 19 (47.5) | 1.18 | 0.34–4.03 | 0.796 |
| >4.5 mm | 23 (40.4) | 11 (27.5) | 0.62 | 0.16–2.41 | 0.491 |
| GBR before IP | |||||
| No | 42 (73.7) | 32 (80.0) | 1 | ||
| Yes | 15 (26.3) | 8 (20.0) | 0.70 | 0.20–2.48 | 0.581 |
| GBR at IP | |||||
| No | 41 (71.9) | 35 (87.5) | 1 | ||
| Yes | 16 (28.1) | 5 (12.5) | 0.37 | 0.09–1.58 | 0.177 |
| T1‐T0 follow up (years) | 0.97 ± 0.47 | 1.11 ± 0.58 | 1.68 | 0.63–4.43 | 0.298 |
| T2‐T1 follow up (years) | 3.92 ± 3.01 | 5.60 ± 3.72 | 1.17 | 0.99–1.36 | 0.052 |
| T2‐T0 follow up (years) | 4.89 ± 3.06 | 6.71 ± 3.65 | 1.18 | 1.01–1.38 | 0.037* |
Note: Number of implants (%) or mean ± SD. Results of simple binary logistic regression (odds ratio [OR] and 95% CI) using GEE model.
Abbreviation: IP, implant placement.
p < 0.05.
3.2. Effect of the T1‐T0 MBL on the risk of PI
Multiple models were performed considering absolute MBL (T1‐T0) (Table 3) as independent variable adjusted by position (maxilla/mandible) and total follow‐up (T2‐T0). There was no association between PIm and MBL during the first year after prosthetic placement. However, arch and total follow‐up showed significant influence on the probability of PIm. Each additional year of follow‐up after implant placement increased the risk of having PIm by +20% (OR = 1.20; p = 0.024). Implants placed in the mandible reduced risk of PIm at 73% (OR = 0.27; p = 0.041). ROC curve (Figure 1) was estimated to assess the efficacy of MBL T1‐T0 to discriminate between PIm and no‐PIm implants. The curve (blue) is eventually superposed to the diagonal (green line), suggesting that the discriminant ability of the MBL is not different from chance. The area under curve was estimated at 0.47 (95% CI, 0.35‒0.59) with p‐value = 0.608, concluding the no differentiation from chance.
TABLE 3.
Multiple binary logistic regression on the marginal bone loss at period T1‐T0 [1 year ± 6 months after crown placement (T1)‐ implant placement (T0)] by group
| GROUP | |||||
|---|---|---|---|---|---|
| Parameters | No peri‐implantitis | Peri‐implantitis | OR | 95% CI | p‐value |
| No. of implants | 57 (58.8) | 40 (41.2) | |||
| T1‐T0 MBL (mm) | 0.42 ± 0.34 | 0.35 ± 0.37 | 0.97 | 0.41–2.33 | 0.950 |
| Arch | |||||
| Maxilla | 5 (8.8) | 9 (22.5) | 1 | ||
| Mandible | 52 (91.2) | 31 (77.5) | 0.27 | 0.08–0.95 | 0.041* |
| T2‐T0 follow up (years) | 4.89 ± 3.06 | 6.71 ± 3.65 | 1.20 | 1.02–1.40 | 0.024* |
Note: Mean ± SD. Results of multiple binary logistic regression (odds ratio [OR] and 95% CI) adjusted by follow‐up and arch using GEE model.
p ≤ 0.05.
FIGURE 1.
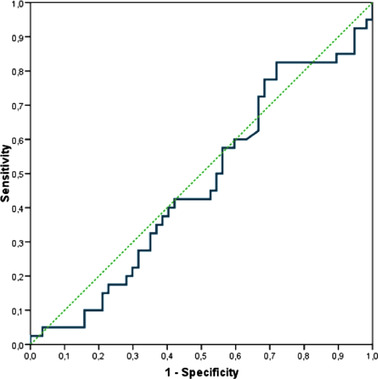
ROC curve to assess the efficacy of marginal bone loss T1‐T0 [1 year ± 6 months after crown placement (T1)‐ implant placement (T0)] to discriminate between implants with and without peri‐implantitis
3.3. Correlation between MBL of (T2‐T1) and (T1‐T0)
Yearly MBL rates from both periods, (T2‐T1) and (T1‐T0), were analyzed to visualize a possible association between time periods. Scatterplot in Figure 2A shows no association between analyzed time periods. When atypical values (implants with yearly rates >2 mm per year) were excluded (Figure 2B), no correlation was found. As graphically expected, results of simple linear regression did not show any significant correlation (b = 0.11; p = 0.291).
FIGURE 2.
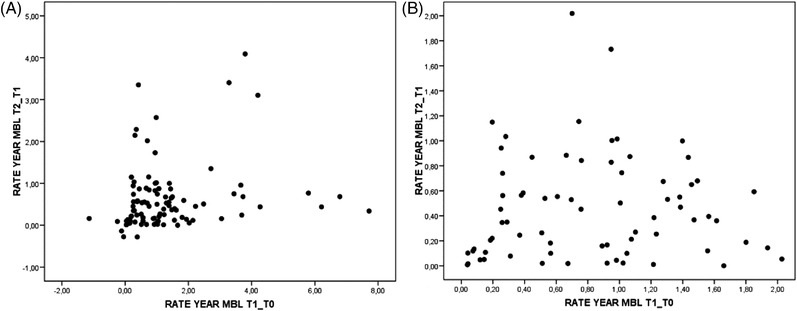
(A) Scatterplot on the association between marginal rates from both periods, (T2‐T1) (≥2‐year follow‐up from implant placement [T2], 1 year ± 6 months after crown placement [T1]), and (T1‐T0) (1 year ± 6 months after crown placement [T1], implant placement [T0]). (B) Same scatterplot after elimination of atypical values (implants with yearly marginal bone loss rates higher than 2 mm)
3.4. Analysis of pathological MBL (T2‐T1)
Results of simple linear regression (B coefficient) using GEE model was performed to study the impact of patients and implants, prosthesis, and treatment variables on the MBL rate (see Table S1 in online Journal of Periodontology). A multiple regression model was built considering the significant /close to significance variables in the simple regression model (Table 4). Splinting or non‐splinting is the most relevant factor in the model. Splinted implants showed an MBL rate of 0.60 mm/year higher than non‐splinted implants (p < 0.001).
TABLE 4.
Marginal bone loss rate at period T2‐T1[≥ 2‐year follow‐up from implant placement (T2)‐ 1 year ± 6 months after crown placement (T1)] by characteristics of patients, implants, prosthesis and treatment
| MBL | |||||
|---|---|---|---|---|---|
| Parameters | N implants | PIm | B | 95% CI | p‐value |
| Smoking | |||||
| No | 73 | 0.52 ± 0.38 | 0 | ||
| Yes | 24 | 0.34 ± 0.20 | −0.26 | −0.59–0.08 | 0.133 |
| No. of maintenances per year since implant placement | 0.007** | ||||
| <2 per year | 33 | 0.29 ± 0.19 | 0 | ||
| 2–3 per year | 25 | 0.50 ± 0.45 | 0.39 | 0.06–0.73 | 0.021* |
| >3 per year | 32 | 0.69 ± 0.80 | 0.72 | 0.18–1.27 | 0.010* |
| Splinted | |||||
| No | 34 | 0.12 ± 0.20 | 0 | ||
| Yes | 63 | 0.70 ± 0.66 | 0.80 | 0.47–1.12 | <0.001*** |
| Level | |||||
| Bone | 86 | 0.43 ± 0.33 | 0 | ||
| Soft tissue | 11 | 0.21 ± 0.14 | 0.16 | −0.23–0.55 | 0.414 |
| Stage | |||||
| 1 | 32 | 0.29 ± 0.24 | 0 | ||
| 2 | 60 | 0.47 ± 0.53 | 0.16 | −0.11–0.43 | 0.255 |
| Crestal level | 0.253 | ||||
| Equicrestal | 77 | 0.39 ± 0.36 | 0 | ||
| Supracrestal | 14 | 0.25 ± 0.18 | −0.15 | −0.41–0.11 | 0.245 |
| Subcrestal | 6 | 0.37 ± 0.47 | 0.05 | −0.27–0.37 | 0.760 |
| T1‐T0 MBL rate (mm/y) | 0.04 | −0.10–0.18 | 0.583 |
Note: Mean ± SD. Results of multiple linear regression (B coefficient) using GEE model.
p ≤ 0.05;
p ≤0.01;
p < 0.001.
The number of maintenance visits showed a relevant association. Implants that underwent 2–3 maintenance visits per year showed a higher rate of MBL/year, at 0.2 mm compared with those with fewer than two visits (p = 0.021). Moreover, implants that had more than three maintenance visits per year showed a higher rate of MBL at 0.4 mm compared with those with fewer than two visits (p = 0.010). After adjusting the model, the rate of MBL in the first period (TI‐T0) remained nonsignificant (p = 0.583).
4. DISCUSSION
The present analysis showed that there was no statistically significant association between IPBR and incidence of PIm. However, each additional year of follow‐up showed a 20% increase in the risk of developing PIm (p = 0.024). Splinted implants had a MBL of 0.60 mm/year, which was higher than non‐splinted implants (0.12 ± 0.20 mm/year) and it is not 0.60 mm higher than in non‐splinted.
Galindo‐Moreno and colleagues analyzed the MBL rates around implants to establish the difference between physiological and pathological bone loss due to peri‐implantitis. 17 , 21 The authors concluded that implants exhibiting increased MBL rates (0.44 mm at 6 months post loading) at early stages could potentially compromise the final implant outcome, with an increased risk for implant failure. In contrast, our results did not show any significant association between IPBR and PIm. This variance in results could be attributed to a few factors such as implant locations, presence of grafted areas, or sample size. Our study evaluated implants placed in both arches while Galindo‐Moreno and coworkers reported on implants placed in the maxillary arch (where our study found more PIm in the maxillary arch). Their measurement timepoints were also comparatively different from the present study. It is important to keep in mind that in the mentioned study, differences in the MBL progression pattern were found in the second period of study; between the T2 observation period (6 months) and the following year (18 months). The present study also showed a 20% increase of PIm in implants during this second temporal frame (12‒24 months). Both studies exhibit a nonlinear trend in the pathological MBL around implants.
Romanos et al. studied the peri‐implant soft tissues around implants with platform switching abutments and found that the supracrestal fiber height (an old term for biologic width) changed significantly based on the location of the implant, either the maxilla or mandible. 21 In maxilla, the supracrestal fiber height was reported to be 6.5 ± 2.5 mm and in the mandible, it was 4.8 ± 1.3 mm. Interestingly, our study showed an association with the arch the implants were placed in, with mandible having a lesser risk of developing PIm. This may be hypothesized to be due to the compact nature of the mandibular bone providing more resistance to the inflammatory infiltrate, in contrast to the trabecular nature of the maxillary bone with marrow spaces. Another retrospective study of 558 implants placed in 172 patients revealed that lower peri‐implant average MBL was associated with type IV bone. 22 They found mean average MBL (mm/yr.) was lowest in type IV bone, followed by bone types III, II, and I. Similar findings were also reported by Lindquist et al. in a 15‐year follow‐up study. 23 On the contrary, Blanes and coworkers did not find significant differences in MBL among bone types but related a tendency to an increased MBL around implants placed in type I bone versus type III bone. 24 Penarrocha‐Diago and collaborators also related an increased MBL in mandible bone in comparison with maxilla bone, regardless of the type of implant used. 25 Many parameters may play an important role in this relationship, not only the bone typology but also the features of the soft tissues overlying the different bone typologies. 26 It is important to keep in mind that pathological MBL is always subsequent to mucositis (soft tissue inflammation) in contraposition to the early physiological MBL. 27
According to our data, a statistically significant difference exists (p < 0.001), with the MBL rate of splinted implants being 0.60 mm/year higher than non‐splinted implants. While this agrees with other studies, 4 it conflicts with other studies, 28 , 29 which reported that the difference of MBL between splinted and non‐splinted implants was clinically insignificant. It is noteworthy that former study had a follow‐up period of 10 years, and only implants placed in the maxillary arch were evaluated. 28
The current analysis has also indicated that implants that went through 2–3 maintenance visits per year showed a higher rate of MBL/year, at 0.2 mm compared with those which had <2 visits (p = 0.021). Even more so, implants that had >3 visits/year showed a higher rate of MBL at 0.4 mm compared with those with <2 visits (p = 0.010). We speculate that this happened in a retrospective fashion, in the same manner that it occurs with patients with periodontitis, where patients with more bone loss had to be kept in a stricter maintenance recall during the follow‐up period. 30 In other words, excessive bone loss resulted in the patient being enrolled into more maintenance visits, and not vice versa.
Our current investigation did not assess the effect of prosthetic abutment height and soft tissue thickness on MBL, both of which have emerged as important factors related to preserving the marginal bone during the early healing phase and could be considered as a limitation to the study. Since the difference between physiologic and pathologic change is in millimeters, the reliability of MBL measurements is of utmost importance, which may be questionable using two‐dimensional periapical radiographs. Several studies evaluating MBL, including ours, have used periapical radiographs for assessments which present certain inherent limitations such as questionable accuracy of measurements and inability to evaluate facial and lingual changes. 26 , 28 , 31 Even so, cone‐beam computerized tomography is not without shortcomings given the artifacts caused by implants. 32
This study also had some design limitations. The first that should be considered is the design of the current study, being a case control, where we included a wide cut‐off point for IPBR (12 months ± 6 months) to have a meaningful sample size. Albeit the sample size of the current study is still considered small. Finally, patient enrollment was done through complete‐case analysis, which may have led to some selection bias to stay consistent with our inclusion criteria.
Further controlled studies, with a higher sample size, with similar/more strict inclusion and exclusion criteria should be designed to avoid all limitations this study might have, if higher level of evidence is sought.
5. CONCLUSIONS
The results of the present study did not show a statistically significant association between the amount of physiological bone remodeling after prosthetic placement and incidence of peri‐implantitis.
Should clinicians wait for a year to determine the implant outcome or does early MBL provide an insight into the implant prognosis? That may not be a straightforward answer, as suggested by the presented evidence, or lack thereof. Clinicians must carefully assess progressive bone loss at various points in time, in addition to clinical parameters such as visual signs of inflammation, PD, line or drop of bleeding on gentle probing, and/or suppuration, to make an informed decision about treatment modalities, including the need for any treatment at all, since peri‐implant health may exist even in the presence of reduced bone support. 33 , 34
CONFLICT OF INTEREST
The authors do not have any financial interests, either directly or indirectly, in the products or information listed in the paper.
AUTHOR CONTRIBUTIONS
Maria Vera Rodriguez, Andrea Ravidà, Muhammad H.A. Saleh, Hom‐Lay Wang, and Pablo Galindo Moreno contributed to the conception and design of the work. Maria Vera Rodriguez, Andrea Ravidà, Muhammad H.A. Saleh, Hussein S. Basma, Himabindu Dukka, and Hadiya Khurshid collected and analyzed the data; and Maria Vera Rodriguez, Andrea Ravidà, Muhammad H.A. Saleh, Hom‐Lay Wang, and Pablo Galindo Moreno led the writing.
Supporting information
Supporting information
Rodriguez MV, Ravidà A, Saleh MHA, et al. Is the degree of physiological bone remodeling a predictive factor for peri‐implantitis? J Periodontol. 2022;93:1273–1282. 10.1002/JPER.21-0723
Footnotes
ImageJ, US National Institutes of Health, Bethesda, MD, USA.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Costa FO, Takenaka‐Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE. Peri‐implant disease in subjects with and without preventive maintenance: a 5‐year follow‐up. J Clin Periodontol. 2012;39:173‐181. [DOI] [PubMed] [Google Scholar]
- 2. Insua A, Monje A, Wang HL, Miron RJ. Basis of bone metabolism around dental implants during osseointegration and peri‐implant bone loss. J Biomed Mater Res A. 2017;105:2075‐2089. [DOI] [PubMed] [Google Scholar]
- 3. Heijdenrijk K, Raghoebar GM, Meijer HJ, Stegenga B, van der Reijden WA. Feasibility and influence of the microgap of two implants placed in a non‐submerged procedure: a five‐year follow‐up clinical trial. J Periodontol. 2006;77:1051‐1060. [DOI] [PubMed] [Google Scholar]
- 4. Ravida A, Saleh MHA, Muriel MC, Maska B, Wang HL. Biological and technical complications of splinted or nonsplinted dental implants: a decision tree for selection. Implant Dent. 2018;27:89‐94. [DOI] [PubMed] [Google Scholar]
- 5. Borges T, Montero J, Leitao B, Pereira M, Galindo‐Moreno P. Periimplant bone changes in different abutment heights and insertion timing in posterior mandibular areas: three‐year results from a randomized prospective clinical trial. Clin Oral Implants Res. 2021;32:203‐211. [DOI] [PubMed] [Google Scholar]
- 6. Katafuchi M, Weinstein BF, Leroux BG, Chen YW, Daubert DM. Restoration contour is a risk indicator for peri‐implantitis: a cross‐sectional radiographic analysis. J Clin Periodontol. 2018;45:225‐232. [DOI] [PubMed] [Google Scholar]
- 7. Saleh MHA, Couso‐Queiruga E, Ravida A, et al. Impact of the periodontal phenotype in premolar and molar sites on bone loss following full thickness mucoperiosteal flap. A 1‐year prospective clinical trial. J Periodontol. 2022. [DOI] [PubMed] [Google Scholar]
- 8. Monje A, Chappuis V, Monje F, et al. The critical peri‐implant buccal bone wall thickness revisited: an experimental study in the Beagle dog. Int J Oral Maxillofac Implants. 2019;34:1328‐1336. [DOI] [PubMed] [Google Scholar]
- 9. Doornewaard R, Sakani S, Matthys C, et al. Four‐implant‐supported overdenture treatment in the maxilla. Part I: a randomized controlled split mouth trial assessing the effect of microthreads and abutment connection type on 4 years peri‐implant health. Clin Implant Dent Relat Res. 2021;23:671‐679. [DOI] [PubMed] [Google Scholar]
- 10. Berglundh T, Lindhe J. Dimension of the periimplant mucosa. Biological width revisited. J Clin Periodontol. 1996;23:971‐973. [DOI] [PubMed] [Google Scholar]
- 11. Ericsson I, Nilner K, Klinge B, Glantz PO. Radiographical and histological characteristics of submerged and nonsubmerged titanium implants. An experimental study in the Labrador dog. Clin Oral Implants Res. 1996;7:20‐26. [DOI] [PubMed] [Google Scholar]
- 12. Saleh MHA, Ravida A, Suarez‐Lopez Del Amo F, Lin GH, Asa'ad F, Wang HL. The effect of implant‐abutment junction position on crestal bone loss: a systematic review and meta‐analysis. Clin Implant Dent Relat Res. 2018;20:617‐633. [DOI] [PubMed] [Google Scholar]
- 13. Kutan E, Bolukbasi N, Yildirim‐Ondur E, Ozdemir T. Clinical and radiographic evaluation of marginal bone changes around platform‐switching implants placed in crestal or subcrestal positions: a randomized controlled clinical trial. Clin Implant Dent Relat Res. 2015;17 Suppl 2:e364‐375. [DOI] [PubMed] [Google Scholar]
- 14. Pico A, Martin‐Lancharro P, Caneiro L, Novoa L, Batalla P, Blanco J. Influence of abutment height and implant depth position on interproximal peri‐implant bone in sites with thin mucosa: a 1‐year randomized clinical trial. Clin Oral Implants Res. 2019;30:595‐602. [DOI] [PubMed] [Google Scholar]
- 15. Linkevicius T, Apse P, Grybauskas S, Puisys A. The influence of soft tissue thickness on crestal bone changes around implants: a 1‐year prospective controlled clinical trial. Int J Oral Maxillofac Implants. 2009;24:712‐719. [PubMed] [Google Scholar]
- 16. Monje A, Insua A, Wang HL. Understanding peri‐implantitis as a plaque‐associated and site‐specific entity: on the local predisposing factors. J Clin Med. 2019;8:279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galindo‐Moreno P, Leon‐Cano A, Ortega‐Oller I, Monje A, O'Valle F, Catena A. Marginal bone loss as success criterion in implant dentistry: beyond 2 mm. Clin Oral Implants Res. 2015;26:e28‐e34. [DOI] [PubMed] [Google Scholar]
- 18. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long‐term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1:11‐25. [PubMed] [Google Scholar]
- 19. Misch CE, Perel ML, Wang HL, et al. Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) Pisa Consensus Conference. Implant Dent. 2008;17:5‐15. [DOI] [PubMed] [Google Scholar]
- 20. Adell R, Lekholm U, Rockler B, Branemark PI. A 15‐year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387‐416. [DOI] [PubMed] [Google Scholar]
- 21. Romanos GE, Traini T, Johansson CB, Piattelli A. Biologic width and morphologic characteristics of soft tissues around immediately loaded implants: studies performed on human autopsy specimens. J Periodontol. 2010;81:70‐78. [DOI] [PubMed] [Google Scholar]
- 22. Ibanez C, Catena A, Galindo‐Moreno P, Noguerol B, Magan‐Fernandez A, Mesa F. Relationship between long‐term marginal bone loss and bone quality, implant width, and surface. Int J Oral Maxillofac Implants. 2016;31:398‐405. [DOI] [PubMed] [Google Scholar]
- 23. Lindquist LW, Carlsson GE, Jemt T. A prospective 15‐year follow‐up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin Oral Implants Res. 1996;7:329‐336. [DOI] [PubMed] [Google Scholar]
- 24. Blanes RJ, Bernard JP, Blanes ZM, Belser UC. A 10‐year prospective study of ITI dental implants placed in the posterior region. I: clinical and radiographic results. Clin Oral Implants Res. 2007;18:699‐706. [DOI] [PubMed] [Google Scholar]
- 25. Penarrocha‐Diago MA, Flichy‐Fernandez AJ, Alonso‐Gonzalez R, Penarrocha‐Oltra D, Balaguer‐Martinez J, Penarrocha‐Diago M. Influence of implant neck design and implant‐abutment connection type on peri‐implant health. Radiological study. Clin Oral Implants Res. 2013;24:1192‐1200. [DOI] [PubMed] [Google Scholar]
- 26. Vervaeke S, Dierens M, Besseler J, De Bruyn H. The influence of initial soft tissue thickness on peri‐implant bone remodeling. Clin Implant Dent Relat Res. 2014;16:238‐247. [DOI] [PubMed] [Google Scholar]
- 27. Renvert S, Giovannoli JL, Roos‐Jansaker AM, Rinke S. Surgical treatment of peri‐implantitis with or without a deproteinized bovine bone mineral and a native bilayer collagen membrane: a randomized clinical trial. J Clin Periodontol. 2021;48:1312‐1321. [DOI] [PubMed] [Google Scholar]
- 28. Vigolo P, Mutinelli S, Zaccaria M, Stellini E. Clinical evaluation of marginal bone level change around multiple adjacent implants restored with splinted and nonsplinted restorations: a 10‐year randomized controlled trial. Int J Oral Maxillofac Implants. 2015;30:411‐418. [DOI] [PubMed] [Google Scholar]
- 29. Tallarico M, Gatti F, Meloni S, et al. To splint or not to splint short dental implants under the same partial fixed prosthesis? One‐year post‐loading data from a multicentre randomised controlled trial. Clinical Trials in Dentistry. 2020;02:47‐58. [Google Scholar]
- 30. Hujoel PP, Leroux BG, Selipsky H, White BA. Non‐surgical periodontal therapy and tooth loss. A cohort study. J Periodontol. 2000;71:736‐742. [DOI] [PubMed] [Google Scholar]
- 31. Blanco J, Pico A, Caneiro L, Novoa L, Batalla P, Martin‐Lancharro P. Effect of abutment height on interproximal implant bone level in the early healing: a randomized clinical trial. Clin Oral Implants Res. 2018;29:108‐117. [DOI] [PubMed] [Google Scholar]
- 32. Vanderstuyft T, Tarce M, Sanaan B, Jacobs R, de Faria Vasconcelos K, Quirynen M. Inaccuracy of buccal bone thickness estimation on cone‐beam CT due to implant blooming: an ex‐vivo study. J Clin Periodontol. 2019;46:1134‐1143. [DOI] [PubMed] [Google Scholar]
- 33. Dukka H, Saleh MHA, Ravida A, Greenwell H, Wang HL. Is bleeding on probing a reliable clinical indicator of peri‐implant diseases? J Periodontol. 2021;92:1669‐1674. [DOI] [PubMed] [Google Scholar]
- 34. Ravida A, Galli M, Siqueira R, Saleh MHA, Galindo‐Moreno P, Wang HL. Diagnosis of peri‐implant status after peri‐implantitis surgical treatment: proposal of a new classification. J Periodontol. 2020;91:1553‐1561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


