Abstract
Handwriting is a vital skill for everyday human activities. It has a wealth of information about writers’ characteristics and can hint toward underlying neurological conditions, such as Parkinson's disease, autism, dyslexia, and attention‐deficit/hyperactivity disorder (ADHD). Many previous studies have reported a link between personality and individual differences in handwriting, but the evidence for the relationship tends to be anecdotal in nature. Using functional magnetic resonance imaging (fMRI), we examined whether the association between personality traits and handwriting was instantiated at the neural level. Results showed that the personality trait of conscientiousness modulated brain activation in the left premotor cortex and right inferior/middle frontal gyrus, which may reflect the impact of personality on orthography‐to‐grapheme transformation and executive control involved in handwriting. Such correlations were not observed in symbol‐drawing or word‐reading tasks, suggesting the specificity of the link between conscientiousness and handwriting in these regions. Moreover, using a connectome‐based predictive modeling approach, we found that individuals’ conscientiousness scores could be predicted based on handwriting‐related functional brain networks, suggesting that the influence of personality on handwriting may occur within a broader network. Our findings provide neural evidence for the link between personality and handwriting processing, extending our understanding of the nature of individual differences in handwriting.
Keywords: brain activation, conscientiousness, functional brain networks, handwriting, personality traits
Handwriting has a wealth of information about writers’ characteristics and can hint towards underlying neurological conditions. Many previous studies have reported a link between personality and individual differences in handwriting, but the evidence for the relationship tends to be anecdotal in nature. Using functional MRI, we examined whether the association between personality traits and handwriting was instantiated at the neural level.

INTRODUCTION
Handwriting is a landmark of human language and motor skills, and it is essential for communication, academic learning, and cognitive development. 1 , 2 It is a complex skill involving the integration of cognitive, linguistic, and motor processes, which are broadly divided into central and peripheral processes. The central process refers to the process of orthographic access via orthographic long‐term memory (the lexical route) or phoneme‐to‐grapheme conversion (the sublexical route). Orthographic working memory is an essential module of the central process that supports the temporary storage of a grapheme for subsequent motor processes. 3 The peripheral process refers to the process of motor output, including allograph selection, motor planning, and execution of motor sequences. 4 , 5 , 6 Previous neuropsychological and brain imaging studies have implicated a complex set of neural underpinnings supporting handwriting processing. 7 , 8 , 9 , 10 For the central component, the left fusiform gyrus has been identified to support long‐term memory for orthographic representation, 11 while the left premotor area extending to Broca's area supports the phoneme‐to‐grapheme conversion for orthographic access. 12 In addition, the intraparietal sulcus and the middle frontal gyrus were found to be responsible for orthographic working memory. 3 , 13 , 14 The peripheral component is mainly involved in the left dorsal premotor cortex (Exner's area), the left inferior/superior parietal lobule, and the cerebellum. Specifically, Exner's area is a writing‐specific brain region that serves as an interface bridging between orthography and motor programs. 13 Moreover, the inferior and superior parietal lobules have been found to serve as the storage for motor programs, while the cerebellum is thought to carry out planning and execution‐specific motor programs during handwriting. 15
Typically, handwriting skill takes about 10 years for an individual to develop. 16 Through this long period of learning and practice, individuals establish a stable personal handwriting style. 17 Many previous studies have explored factors that modulate individual variability in handwriting by using an index of the writing product (e.g., shape and legibility) and writing process (e.g., speed and pause). These factors include cognitive‐linguistic and motor factors, such as orthographic awareness and visuomotor integration during development. 18 , 19 Moreover, previous studies have showed that the physical and mental health status of the writers can influence handwriting. For example, there is evidence showing that handwriting features can hint at underlying neurological conditions, such as Parkinson's disease, 20 , 21 autism, 22 dyslexia, 23 , 24 and ADHD. 25
Another important factor that has been frequently linked to individual differences of handwriting is personality traits. 26 , 27 It has been proposed that a person's personality characteristics may be projected in the way he or she writes, due to the highly individualized nature of handwriting. 26 Indeed, there has been evidence suggesting that some handwriting features indicate specific personality traits. For example, a prior study showed that measures of the rightward slant of handwriting products were related to extraversion and that fast writing speed was related to impulsivity. 27 Additionally, individuals with antisocial personality exhibited differences in the graphic features of handwriting compared with controls. These findings support the association between personality and handwriting. 28 On the other hand, many empirical studies failed to validate the correlation between handwriting and personality. 26 , 29 , 30 Some researchers have proposed that the correlation between handwriting and personality is illusory and actually derives from the semantic association between words used for describing handwriting features (e.g., regular rhythm) and personality traits (e.g., reliable). 30
One major challenge to validate the association between handwriting and personality is related to methodological limitations. For example, traditional analysis of handwriting scripts confronts a major problem of relying on subjective appraisal of the frozen handwritten products, such that the interpretation depends much on the professional skills and the graphological theories applied by the analyst. 31 In this study, we aimed to explore a more comprehensive and objective approach for linking handwriting and personality by characterizing the brain activation patterns during the dynamic handwriting process. We hypothesized that, if personality traits (based on five‐factor model, FFM) influence the way we wrote, this association should be able to be detected by linking personality traits to brain activity patterns that produce distinctive handwriting movements. Considering that previous studies on handwriting have established that different brain regions response to different components of the handwriting processes, identifying the biological markers that instantiate the association between personality traits and handwriting can also help to understand which potential components of handwriting (e.g., orthographic conversion, cognitive control, or motor control) may be modulated by personality traits.
We scanned 50 adult participants with functional MRI while they were performing handwriting tasks using a specially developed fMRI‐compatible touch‐sensitive tablet system. Participants’ personality traits were assessed based on the FFM, a widely used framework describing personality traits along five dimensions: neuroticism (N), extraversion (E), openness (O), agreeableness (A), and conscientiousness (C). 32
We first explored whether personality traits modulated brain activation during the dynamic handwriting process at the group level. We hypothesized that the most plausible brain regions that instantiated the association between personality traits and handwriting should reside within the brain network that was shared by handwriting and personality traits. As previously mentioned, brain regions critically involved in handwriting include premotor cortex, inferior and middle frontal gyri, intraparietal sulcus, fusiform gyrus, and so on. In addition, structural 33 , 34 , 35 , 36 and functional 37 , 38 neuroimaging studies have identified brain substrates that were associated with the FFM personality dimensions, including the prefrontal cortex, precentral gyrus, cingulate gyrus, temporal gyrus, parietal cortex, and visual cortex. Among the five FFM personality dimensions, we considered conscientiousness (related to orderliness and impulse control) to be the most plausible personality trait that modulated brain responses during handwriting, for the following two reasons: (1) conscientiousness is associated with the performance and efficiency of serial order actions 39 and working memory, 40 which are necessarily engaged in handwriting processing; and (2) considerable evidence suggests the frontal cortex as the neural substrate of conscientiousness, 34 , 41 and this region is critically involved in handwriting. 15 Besides, openness to experience is also likely to modulate brain activity of handwriting, as it has been found to be associated with cognitive processes that are involved in handwriting process, including working memory 42 and the efficiency of information processing. 43
We then examined the specificity of correlation between personality and handwriting by testing whether personality traits also modulated brain activation during a symbol‐drawing task (sharing the visual‐motor process with the handwriting task) and a word‐reading task (sharing with the handwriting task the visual‐orthographic as well as incidental phonological and semantic processing of the linguistic stimuli).
Moreover, because the handwriting process encompasses a variety of subcomponents and involves a sophisticated interplay between distributed brain areas, 10 whole‐brain measures of functional brain networks should provide more comprehensive information about handwriting process than activity in a single brain region. Thus, we next used a connectome‐based predictive model (CPM), 44 an influential and extensively validated model approach, to examine whether individuals’ brain networks during the dynamic handwriting process could predict individuals’ personality traits. Such analysis can help validate the application of handwriting in personality measures at the individual level.
MATERIALS AND METHODS
Participants
Fifty adults were recruited to participate in the study (24 males; mean age = 22.30 years, standard deviation [SD] of age = 2.25). All participants were native Chinese speakers and were right‐handed as assessed by a handedness inventory. 45 The participants were physically healthy and reported no history of neurological disease or psychiatric disorder. The study was approved by the Ethics Committee of the Institute of Psychology, Chinese Academy of Sciences, and the experiments were carried out in accordance with the approved guidelines. Free and informed consent was obtained from each participant prior to the experiment.
Task procedure and stimuli during fMRI scans
Handwriting task and symbol‐drawing task
A copying task was used during the fMRI scan, in which the participants were required to write down Chinese characters that were previously presented to them. In this handwriting task, the stimuli were 30 Chinese characters of varying frequency, consisting 15 high‐frequency characters (>500 times per million) and 15 low‐frequency character (<5 times per million), according to the Modern Chinese Frequency Dictionary (1986). 46 The mean number of strokes was 6 (ranging from 4 to 8 strokes) for both the high‐frequency and low‐frequency characters. In the symbol‐drawing task, participants were asked to draw geometric symbols. The stimuli include 15 symbols, including line, circle, triangle, diamond, trapezia, and parallelogram, and combinations of these geometric shapes. During the handwriting and symbol‐drawing tasks, participants were instructed to write or draw at the speed as in their daily life while minimizing movements of their upper arm and forearm.
A block design was employed, consisting of six blocks of copying characters and three blocks of drawing symbols. Each block included five trials. In each trial, a “+” symbol was first presented visually and centrally for 0.3 s, followed by the presentation of a character stimulus for 1 s that was then followed by a response period of 4.7 s. This was the maximum possible time for responding, and it was fixed for each trial. Four blocks of central fixation each with a 12‐s period were also interspersed among the task blocks as a “rest” control condition.
Handwriting and drawing data were recorded using a tablet system specially developed for use in fMRI experiments. 47 Writing latency and duration were analyzed. Writing latency was defined as from the onset of response stimuli to the beginning of writing, and writing duration was defined as from the start of the response to the end of the last written or drawn stroke of the response.
Visual word reading task
Because the character‐handwriting task involves both linguistic and motor components in the task, we designed a visual‐word reading task to examine whether the relationship between handwriting and personality is related to the linguistic processing or is specific to handwriting motor production. Participants were requested to passively read visually presented characters and judge whether the presented character was the same as the prior one by pressing a button using their right hand. A pseudocharacter and a scrambled character conditions were also included as part of a larger study. The pseudocharacters were orthographically legal, but were meaningless and unpronounceable. They were created by replacing one radical of the real characters by another radical. The scrambled characters were orthographically illegal, meaningless, and unpronounceable. They were generated by disarranging the strokes of the real characters, resulting in disorganized visual layouts without any linguistic information. The real‐character reading task shared with the handwriting task the visual‐orthographic processing as well as incidental phonological and semantic processing of the linguistic stimuli, and, therefore, it is suitable to test the specificity of the correlation between personality and handwriting. Conversely, because the pseudocharacters and scrambled character conditions did not involve the linguistic processes that we were interested in, they were not analyzed in the present study.
The stimuli of the reading task were 160 real Chinese characters, which were presented as both handwritten and printed versions. The handwritten version of characters was written by a male expert in handwriting. The mean frequency of the characters was 474.91 times per million according to the Modern Chinese Frequency Dictionary (1986), 46 and the mean number of strokes was 8.16 (ranging from 5 to 12 strokes). The participants underwent four runs of scanning. In each run, there were four blocks for each character condition and each block consisted of 10 trials. In each trial, the visual stimuli were presented for 0.2 s, followed by a blank interval for 1 s. In addition, five 12‐s rest blocks were interspersed into the task blocks.
MRI data acquisition
Imaging was performed using a 3T MRI system (MAGNETOM Prismafit, Siemens, Erlangen, Germany) at the Beijing MRI Center for Brain Research of the Chinese Academy of Sciences. Functional MRI time series data with blood oxygen level–dependent (BOLD) contrast were acquired using a two‐dimensional, T2*‐weighted, gradient echo planar imaging sequence (repetition time [TR] = 1000 ms, echo time [TE] = 30 ms, slice thickness = 2.2 mm, in‐plane resolution = 2.2 mm × 2.2 mm, and flip angle [θ] = 45°). A total of 64 axial slices were collected to cover the whole brain. High‐spatial resolution anatomical images were acquired using a three‐dimensional T1‐weighted, magnetization‐prepared rapid acquisition gradient echo sequence (TR = 2200 ms, TE = 2.08 ms, slice thickness = 1 mm, in‐plane resolution = 1.0 mm × 1.0 mm, and θ = 8°).
Assessment of personality traits
Participants’ personality traits were measured using the shortened Chinese version of the NEO five‐factor inventory (NEO‐FFI), 48 which shows good validity and reliability for the Chinese population. 49 This inventory includes 60 items, with 12 items for each of the five personality dimensions: (1) neuroticism (N), which is associated with the intensity and reactivity of negative emotion; (2) extraversion (E), which is associated with a drive for affiliation and dominance; (3) openness to experience (O), which is characterized by fantasy, interest in novelty, and intellect; (4) agreeableness (A), which is associated with nurturance, kindness, and social harmony; and (5) conscientiousness (C), which is associated with orderliness and impulse control. 32 The scores of the participants’ responses were assessed based on a 5‐point Likert scale, from strongly disagree (1 point) to strongly agree (5 point), thus resulting in score sums between 12 and 60 for each dimension.
Out‐of‐scanner handwriting skill task
To examine the participants’ handwriting performance in a natural state, a pen‐and‐paper writing test was administered after the MRI scan. The task required the participants to copy 40 Chinese characters that contained two radicals arranged in left‐right or top‐down structures. Half of the characters were high‐frequency (>500 times per million), and the other half were low‐frequency (<5 times per million). The mean number of strokes was 9 (6–13 strokes) for the high‐frequency characters and was 8 (5–11 strokes) for the low‐frequency characters. Because the participants used more familiar, natural gestures during the pen‐and‐paper handwriting task, this task enabled better assessment of their handwriting features relative to the in‐scanner handwriting task. Handwriting speed and quality were assessed. Writing speed was defined as the number of characters written per second by calculating the ratio between the number of characters and writing time. Writing quality was evaluated by two independent examiners using a 7‐point Likert scale (1 = very bad and 7 = very good) based on the scripts from the pen‐and‐paper copying tests (natural written scripts). This assessment was based on six dimensions, including stroke form, slant, organization of radicals, neatness, size, and overall appearance. 50 The score was the sum of each dimension's score, and thus the maximum possible score of writing quality was 42.
To examine the relationship between personality traits and handwriting features, we performed regression analysis to test the contribution of personality scores to handwriting performance (speed and quality) in the pen‐and‐paper writing task after controlling for the effect of age and sex.
fMRI data analysis
Preprocessing
Image preprocessing and statistical analyses were conducted using SPM8 freeware (http://www.fil.ion.ucl.ac.uk/spm/, Wellcome Department of Cognitive Neurology, University College London, London). The fMRI time series data for each participant were first corrected for head motion, and the corrected images were coregistered to the associated anatomical imaging data. The anatomical images were segmented and transformed into Montreal Neurological Institute (MNI) stereotactic space, and the resulting transformation parameters were then applied to yield fMRI time series data to be normalized in MNI space with cubic voxels at a spatial resolution of 2 mm × 2 mm × 2 mm. These images were then spatially smoothed using an isotropic Gaussian kernel of 6 mm full‐width at half‐maximum.
Whole brain regression analysis
At the individual level, activation maps contrasting the activity of copying characters (combining high‐frequency and low‐frequency characters) and drawing symbols to a “rest” condition were generated for the handwriting task for each participant using a general linear model (GLM). Similarly, activation maps for the reading task were acquired by contrasting viewing characters (the handwritten characters or the printed characters) to rest. The GLM design matrix included the block design time series convolved with a canonical hemodynamic response function. To minimize residual motion artifacts, head movement parameters (estimated with six degrees of freedom during the motion correction step) were included in the design matrix as nuisance covariates. The data were high‐pass filtered at 0.008 Hz. Then, the individual activation maps were entered into a random‐effects model for group‐level analysis using one‐sample t‐test, resulting in brain activation for handwriting characters, drawing symbols, reading printed characters, and reading handwritten characters, respectively.
Next, multiple regression analysis was applied to examine the brain activation associated with personality traits. To account for the possible shared variance, all the five personal dimensions were entered into the regression model. Moreover, sex, age, and head motion (framewise displacement, FD) were included as covariates. To constrain the search within the brain system for handwriting processing, a mask of significant activation of handwriting and drawing was applied for the regression model. In addition, to examine the specificity of the association between handwriting and personality, multiple regression analysis was also applied for the symbol‐drawing and character‐reading tasks. The voxel‐wise threshold was set at p < 0.001 and p < 0.05, with the family‐wise error (FWE) corrected at the cluster level. To visualize the relationship between the activation of a given region and personality traits, the scatter plots of the correlation between the residuals of contrast estimates of brain regions (excluding the explanatory power of age, sex, FD, and the other four factors) and personality scores were illustrated.
Brain activation–writing performance correlation analyses
To further confirm the correlation between personality traits and brain activation during handwriting, we conducted correlation analyses between brain activity and handwriting performance in the out‐of‐scanner pen‐and‐paper writing task. Specifically, the brain regions showing significant correlation with personality traits were defined as functional regions of interest (ROIs). The residuals of contrast estimates (excluding the explanatory power of age, sex, and FD) were calculated for each ROI, which were then correlated with pen‐and‐paper handwriting speed and quality.
Connectome‐based predictive modeling
Following the guidelines of the CPM approach, 44 several steps are included to establish the prediction model of personality based on functional brain networks associated with handwriting: (1) network construction, that is, establishing a handwriting‐induced functional connectivity (FC) matrix; (2) feature selection and summarization; (3) model building and evaluation of prediction significance; and (4) model validation.
Network construction
Networks were established based on a functional temple of 264 ROIs with 10‐mm diameter spheres. 51 Pearson's correlation coefficients between each pair of nodes were computed using the CONN Functional Connectivity Toolbox, 52 with the effect of the fluctuations in BOLD signals from cerebrospinal fluid, white matter and their derivatives, head motion, and the effects of task were estimated and removed. Correlation coefficients were transformed into Fisher's z‐scores, resulting in undirected and weighted 264 × 264 FC matrices for each participant. The resulting residual time series were band‐pass filtered at 0.008–0.09 Hz to reduce low‐frequency drift and high‐frequency noise effects. 53
Feature selection
Using linear regression analysis, FC edges significantly correlated with personality scores were selected as features, after controlling for age and sex. The significance threshold was set at p < 0.005, uncorrected for multiple comparisons . The edges that showed positive or negative correlation with personality scores were separated into two distinct data sets. Next, the connectivity strength (Fisher's z‐scores) for positive and negative edges was summarized into a single value for each participant.
Model construction
Three linear regression models (positive, negative, and combined positive and negative edges) were established, with personality scores were set as dependent variables and connectivity edges were set as independent variables. A leave‐one‐out cross‐validation method was applied to evaluate the predictive model. One subject was considered a novel observation (test set) and the remaining subjects were used to build the regression models. The correlation coefficients between the observed and predicted scores were calculated for determining the efficiency of predictive accuracy. Finally, a permutation test (1000 iterations) was performed to assess the statistical significance of the true predictive correlation. In each iteration, personality scores were randomly assigned to different subjects and then, a new correlation coefficient between the observed and predicted scores was calculated following the same procedure. The p value of the permutation test was calculated as the proportion of sampled permutations that was greater or equal to the true prediction correlation.
To illustrate the specific functional brain networks that significantly contribute to the prediction model, FC edges that appeared in at least 90% of the iterations were visualized. Those 264 regions (also called nodes in graph theory analysis) contained in this template were assigned to 11 well‐established functional networks defined previously. 51 In addition, one node was identified as a hub in a functional network, if its node degree (defined as the number of edges directly connected to a given node) was 1.5 SD greater than the mean degree across all nodes in this network.
Internal validation of the prediction model
Two internal validation procedures were applied for validating the results. First, in order to determine whether the results were independent of connectivity edges, a more stringent (p < 0.001) and a less conservative (p < 0.01) threshold was used for the edge section procedure. Second, to examine whether the results were influenced by the definition of node, we used another brain template derived from brain structural‐based parcellation to define the ROIs, 54 which includes 210 cortical and 36 subcortical subregions. We repeated the whole CPM analyses based on these changed parameters.
RESULTS
Behavioral results of handwriting tasks and personality measure
For the handwriting task during fMRI scanning, the mean writing latency (SD) and writing duration (SD) was 476.66 ms (141.13 ms) and 2819.10 ms (484.50 ms). For the out‐of‐scanner pen‐and‐paper handwriting task, handwriting speed was 0.40 character/second (SD = 0.07) and writing quality was 25.24 (SD = 6.38).
For personality measure, the participants’ NEO‐FFI scores ranged from 18 to 46 for neuroticism (mean = 33.54), from 25 to 48 for extraversion (mean = 37.78), from 25 to 47 for openness (mean = 38.12), from 28 to 52 for agreeableness (mean = 42.28), and from 28 to 50 for conscientiousness (mean = 40.78).
Regression analysis between personality scores and handwriting performance (speed and quality) in the pen‐and‐paper writing task showed that personality explained no additional variance in writing quality (R 2 change = 0.09, F = 1.55, p = 0.18) or writing speed (R 2 change = 0.03, F = 0.64, p = 0.72) after controlling for the effect of age and sex. This result was in accordance with previous findings, indicating weak correlation between static handwriting features and personality traits. 29
Correlation between personality traits and brain activation during handwriting
The in‐scanner handwriting task activated a distributed network of brain regions, including the bilateral superior/middle/inferior frontal gyrus (SFG/MFG/IFG), precentral gyrus (PreCG), supplementary motor area (SMA), middle/inferior temporal gyrus (MTG/ITG), superior/inferior parietal gyrus (SPL/IPL), postcentral gyrus (PostCG), precuneus, superior/middle/inferior occipital gyrus (SOG/MOG/IOG), fusiform gyrus (FG), lingual gyrus (LG), cuneus, supramarginal gyrus (SMG), cerebellum, insula, and thalamus (Figure 1A).These brain regions were consistent with previous findings of the neural correlates of handwriting. 15 , 55 , 56
FIGURE 1.

Brain activation during handwriting task and its correlation with personality traits. (A) Brain activation associated with handwriting Chinese characters (contrasted with fixation). (B) Correlations between conscientiousness scores and brain activation during handwriting. Abbreviations: IFG, inferior frontal gyrus; L, left; PMd, dorsal premotor cortex; R, right.
We then examined whether personality traits were associated with brain activation during handwriting. Results showed that the scores of conscientiousness were positively correlated with brain activation in the left premotor region (peak at MNI: x = −34, y = −10, z = 46) and right IFG extending to MFG (x = 52, y = 10, z = 28) (Figure 1B). Additionally, openness showed a trend of negative correlation with brain activation in the left cerebellum (x = −40, y = −52, z = −38), but the correlation did not survive multiple comparison corrections after excluding an outlier who showed extremely reduced activation in the left cerebellum.
Brain activation–writing performance correlation analyses
We next tested whether the brain activation in the left premotor region and the right IFG were also correlated with handwriting skill by conducting behavior–brain correlation analyses between activity level in the two regions during handwriting and participants’ out‐of‐scanner handwriting performance (i.e., handwriting speed and handwriting quality). Spearman's rank correlation analysis showed that the activation in the right IFG during handwriting was positively correlated with handwriting quality (r = 0.30, p = 0.037), but not correlated with handwriting speed (r = −0.08, p = 0.565). The activation in the left premotor region during handwriting task was not correlated with handwriting quality (r = 0.10, p = 0.489) or handwriting speed (r = −0.08, p = 0.593).
Specificity of the relation between personality and brain responses during handwriting
We further examined the specificity of the relation between conscientiousness and brain responses during handwriting by testing whether personality traits were related to brain activation during symbol drawing and visual word reading. These two tasks could help to disentangle whether personality traits simply modulated brain activity for visual‐motor execution or visual‐word recognition processing. Results showed that although drawing symbols (Figure 2A) and visual‐character reading (Figure 2B,C) recruited several shared brain regions with the handwriting task, individual differences in conscientiousness were not correlated with brain activation in the premotor or inferior frontal cortices during the symbol‐drawing task or word‐reading task. However, we observed that the score of conscientiousness was positively related to brain activation in the right superior parietal lobule (peak at x = 20, y = −52, z = 66) during reading handwritten characters (Figure 2D). It should be noted that this region did not overlap with the regions identified in the handwriting task.
FIGURE 2.
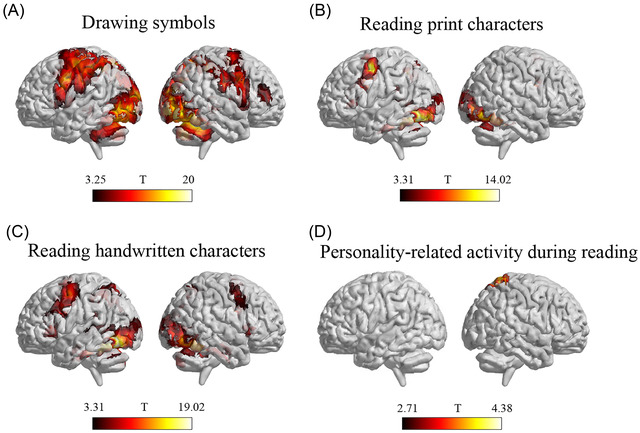
Brain activation during drawing symbols and reading characters and its association with personality traits. (A) Brain activation during drawing symbols. (B) Brain activation during reading printed characters. (C) Brain activation during reading handwritten characters. (D) Brain activity correlated with conscientiousness during reading handwritten characters. Abbreviations: L, left; R, right.
Predicting personality traits using functional brain networks for handwriting
Finally, we explored the extent to which conscientiousness scores could be predicted by individual differences in the handwriting‐evoked FC. The CPM analysis showed that individuals’ scores of conscientiousness (but not other personality traits) were successfully predicted by handwriting‐related brain connectivity patterns (positive network: r = 0.43, p permu = 0.016, negative network: r = 0.36, p permu = 0.035; combined network: r = 0.42, p permu = 0.016) (Figure 3A). Moreover, brain networks that contributed to the prediction mainly involved the default mode network (DMN), cingulo‐opercular network (CON), visual network (VN), and sensorimotor network (SMN) (Figure 3B). Several regions within these networks were identified as hubs, including bilateral inferior and middle frontal gyri, bilateral postcentral gyri, insula, and so on (Table S1). The prediction results based on functional brain networks were further validated by using a more stringent (p < 0.001) and a less conservative (p < 0.01) threshold for edge selection (Figure S1) and using an alternative template for node definition (Figure S2).
FIGURE 3.

Predicting conscientiousness based on functional brain networks during handwriting. (A) Scatter plots for correlations between observed scores and predictive scores of conscientiousness based on the positive, negative, and combined (positive and negative) functional networks. (B) Functional brain networks that contribute to the prediction of conscientiousness. The connectivity matrices between pairs of the 11 brain networks described by Power et al. are shown. 51 The color of each element in the matrices reflects the sum of the weight of all the edges. Abbreviations: AN, auditory network; CON, cingulo‐opercular network; DAN, dorsal attention network; DMN, default mode network; FPN, frontal‐parietal network; SAN, salience network; SCN, subcortical network; SMN, somatosensory motor network; Unc, uncertain; VAN, ventral attention network; VN, visual network.
DISCUSSION
This study provides direct evidence for the link between personality and handwriting at the neural level, advancing our understanding of the individual differences in handwriting. In line with our hypothesis, we found that the personality trait of conscientiousness modulated brain activation in the left premotor cortex and right inferior/middle frontal gyrus during the dynamic handwriting process, suggesting that personality may modulate orthography‐to‐grapheme transcoding and executive control processes involved in handwriting. Such connections were specific to handwriting of characters, but not observed during drawing symbols or visual‐word reading. Moreover, individuals’ conscientiousness scores could be predicted based on handwriting‐related functional brain networks, indicating that the association between individual differences in personality and handwriting was also instantiated at the brain‐network level.
The association between premotor activation during handwriting and conscientiousness
Conscientiousness is a personality trait characterized by orderliness, industriousness, self‐control, and responsibility. 57 It is one of the most reliable predictors of occupational performance, 58 academic outcomes, 59 and physical health. 60 In accordance with our hypothesis, conscientiousness modulated brain activation during handwriting. More specifically, the levels of conscientiousness were positively correlated with brain activation in left premotor region during handwriting, suggesting that this region may be essential brain loci for the link between personality traits and handwriting.
The activation peak of the left premotor region in our study is close to Exner's area, 13 a key region specific for handwriting. 13 , 15 , 55 Roux et al. pointed out that Exner's area was the brain basis of the interface between orthographic representation and motor programs, which may govern allographic motor specifications by sending information downward to the hand motor area, ultimately resulting in the variation of handwriting processing or products. In addition, Exner's area has been proposed to be the neural substrate of orthographic working memory that houses the temporary store of orthographic representation for the process of orthography‐to‐grapheme transformation, as it is sensitive to letter length or stroke number during handwriting. 13 , 61 Accordingly, conscientiousness may influence handwriting process through targeting both the central and peripheral components of handwriting.
Besides, Exner's area has also been found to be engaged in reading in both alphabetic languages and Chinese. 62 In our study, the copying task necessarily involves an initial reading process before handwriting. Thus, it is possible that the activation in Exner's area may reflect the modulation of conscientiousness to the reading process. However, this possibility can be ruled out by the control analysis of the supplementary reading task, in which we did not detect the correlation between brain activation in Exner's area during the reading task and conscientiousness.
The association between right IFG activation during handwriting and conscientiousness
We also found that conscientiousness modulated neural responses of handwriting in the right IFG. The involvement of the right IFG has been occasionally evidenced in handwriting, but its exact function remains unclear. 15 Our data also showed that the activation of the right IFG during handwriting was correlated with handwriting quality of the out‐of‐scanner writing task, confirming the role of the IFG in handwriting processing. This region has frequently been implicated in inhibition control processing, which is a critical aspect of executive control, 63 , 64 such as inhibition actions for inappropriate response given the operative goal. 65 In line with this view, previous studies have demonstrated the association between poor executive control and handwriting impairment in children. 66 In our study, individuals with higher conscientiousness may exert greater effort on regulating executive function of inhibition, producing greater activity in the right IFG during handwriting.
An alternative explanation for the role of the right IFG is phonological processing. A prior study by Gimenez et al. showed that, in beginning readers/writers, handwriting quality was correlated with gray matter volume of the right IFG and brain activation of this region during a phonological processing task. 67 The authors argued for a role of the right IFG in phonological processing, which may be essential in the development of complex motor skills required in handwriting. Compared to Gimenez et al.’s study, our fMRI task is a handwriting task that emphasizes more the motor‐related execution components. The right IFG may play a well‐established role in executive function that affects the formation of handwriting visual features.
Conscientiousness is predicted by brain functional networks of handwriting
At the brain‐network level, we also identified functional networks associated with the dynamic handwriting process whose strengths successfully predicted individual differences in conscientiousness, including the CON, DMN, VN, and SMN. This finding suggests that the influence of personality on handwriting may occur within a broader network engaged in handwriting. We found the CON, an essential executive control network, contributed significantly to the prediction of conscientiousness. This result is also in line with the previous finding of resting‐state FC that conscientiousness is associated with executive control networks. 37 , 41 The DMN has been suggested to support self‐related cognitive processes 68 and working memory. 69 Recent evidence indicates that the reconfiguration of the DMN serves handwriting speed control. 10 The VN and SMN are important brain networks for visual‐orthographic and motor processes in handwriting. The connectivity between the SMN and VN was enhanced with the increase of motor speed demand during handwriting. 10 Together, conscientiousness may reflect how individuals are able to efficiently utilize brain networks for high‐level cognitive control and task‐specific visual and motor processes engaged in handwriting processing.
The specificity of the association between conscientiousness and handwriting
Interestingly, we found that conscientiousness is the only trait reliably associated with, and predicted by, brain activity/connectivity patterns during handwriting. One explanation for the specificity of the relation between conscientiousness and neural responses to handwriting is that conscientiousness may be more closely related to the cognitive processes that are involved in handwriting relative to other personality traits. This result is in line with previous findings showing that conscientiousness is the only personality dimension that modulates multicomponent behavior. 70 During handwriting, the correct orthographic units have to be retrieved in the correct order and put into a tuned motor system for stroke‐by‐stroke execution, which requires ongoing monitoring to inhibit erroneous responses. Given that conscientiousness is conceptualized as a trait akin to orderliness and impulse control, individual differences in brain activation associated with the self‐controlled and goal‐directed handwriting process may be more easily captured by individual differences in conscientiousness than other personality traits. This explanation is supported by the functional role of brain regions that we found to be modulated by conscientiousness. Particularly, the right IFG may subserve the executive control and goal‐directed process during handwriting.
We also found a trend of correlation between openness and brain activation in the left cerebellum during handwriting, but the result did not survive multiple comparison correction. This result is partially in accordance with our hypothesis. We hypothesized that openness is likely to modulate brain activity in handwriting because it has been found to modulate the cognitive processes of working memory 42 and the efficiency of information processing. 43 Considering that the cerebellum has been frequently reported to support handwriting 9 , 15 and its function has been typically linked with motor control, the result suggests that openness may modulate the motor component of handwriting. Further studies are required to validate this finding using more stringent statistical criteria.
Future directions
Our findings open up myriad opportunities for future research. First, the personality measure in our study is derived from a self‐report inventory based on the five‐factor personality model. Whether the associations between personality and brain responses to handwriting can be generalized to other personality models requires validation in future studies. A second important question is about the developmental mechanisms that give rise to the link between personality traits and handwriting. Of particular interest is the period from childhood through late adolescence, when individual handwriting traits come to be established. 71 Future studies with a longitudinal design should be able to resolve this question by elaborating both the consistency and the dynamic change of personality and handwriting over time.
CONCLUSIONS
To conclude, this study revealed that a personality trait (conscientiousness) modulated brain activation in the left premotor cortex and right inferior/middle frontal gyrus during the dynamic handwriting processing. We also established a prediction model based on handwriting‐related brain networks that could reliably predict conscientiousness scores individually. These findings suggest that the association between personality traits and handwriting is instantiated at both regional and brain‐network levels, extending our understanding of the nature of individual differences in handwriting and opening up the possibility of characterizing a person's personality by analyzing brain patterns during the dynamic handwriting process (“neurographology”).
AUTHOR CONTRIBUTIONS
Y.Y. and M.X. conceived and designed the research. Y.Y., J.J.L., J.Z., and H.Y.B. performed the experiments. Y.Y., J.J.L., K.Z., and H.S.R.K. performed the data analyses. Y.Y., J.Z., K.Z., H.S.R.K., and M.X. cowrote the paper. Y.Y., J.J.L., J.Z., K.Z., H.S.R., H.Y.B., and M.X. commented on the manuscript.
COMPETING INTERESTS
The authors declare no competing interests.
PEER REVIEW
The peer review history for this article is available at: https://publons.com/publon/10.1111/nyas.14871.
Supporting information
Table S1. The hubs in the positive or negative functional brain networks correlated with conscientiousness scores.
Figure S1. The prediction results based on functional brain networks were further validated by using different edge selection thresholds. Scatter plots of the correlations between observed consciousness scores and predicted scores by positive (red), negative (blue), and combined (yellow) networks using an edge‐selection threshold of p < 0.001 (A) and p < 0.01 (B).
Figure S2. The prediction results based on functional brain networks were validated by using the template of Fan et al. (2016) for node definition. Scatter plots of the correlations between observed consciousness scores and predicted scores by positive (red), negative (blue), and combined (yellow) networks.
ACKNOWLEDGMENTS
This work was supported by the National Science and Technology Innovation 2030 Major Program (2021ZD0203803), the Beijing Natural Science Foundation (No. 5222027), the National Natural Science Foundation of China (No. 31800954, 32171054), Shenzhen Innovation in Science and Technology Foundation for the Excellent Youth Scholars (RCYX20210706092043066), Shenzhen‐Hong Kong Institute of Brain Science‐Shenzhen Fundamental Research Institutions (2021SHIBS0003), and the Scientific Foundation of Institute of Psychology, Chinese Academy of Sciences (E2CX3625CX).
Yang, Y. , Li, J. , Zhang, J. , Zhou, K. , Kao, H. S. R. , Bi, H.‐Y. , & Xu, M. (2022). Personality traits modulate the neural responses to handwriting processing. Ann NY Acad Sci., 1516, 222–233. 10.1111/nyas.14871
Contributor Information
Yang Yang, Email: yangyang@psych.ac.cn.
Min Xu, Email: xumin@szu.edu.cn.
REFERENCES
- 1. Palmis, S. , Danna, J. , Velay, J.‐L. , & Longcamp, M. (2017). Motor control of handwriting in the developing brain: A review. Cognitive Neuropsychology, 34(3–4), 187–204. [DOI] [PubMed] [Google Scholar]
- 2. Tan, L. H. , Spinks, J. A. , Eden, G. F. , Perfetti, C. A. , Siok, W. T. , & Desimone, R. (2005). Reading depends on writing, in Chinese. Proceedings of the National Academy of Sciences of the United States of America, 102(24), 8781–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapp, B. , Purcell, J. , Hillis, A. E. , Capasso, R. , & Miceli, G. (2016). Neural bases of orthographic long‐term memory and working memory in dysgraphia. Brain, 139(2), 588–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Galen, G. P. (1991). Handwriting: Issues for a psychomotor theory. Human Movement Science, 10(2–3), 165–191. [Google Scholar]
- 5. Purcell, J. , Turkeltaub, P. E. , Eden, G. F. , & Rapp, B. (2011). Examining the central and peripheral processes of written word production through meta‐analysis. Frontiers in Psychology, 2, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kandel, S. , Peereman, R. , Grosjacques, G. , & Fayol, M. (2011). For a psycholinguistic model of handwriting production: Testing the syllable‐bigram controversy. Journal of Experimental Psychology: Human Perception and Performance, 37(4), 1310. [DOI] [PubMed] [Google Scholar]
- 7. Longcamp, M. , Lagarrigue, A. , Nazarian, B. , Roth, M. , Anton, J. L. , Alario, F. X. , & Velay, J. L. (2014). Functional specificity in the motor system: Evidence from coupled fMRI and kinematic recordings during letter and digit writing. Human Brain Mapping, 35(12), 6077–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rapp, B. , & Lipka, K. (2011). The literate brain: The relationship between spelling and reading. Journal of Cognitive Neuroscience, 23(5), 1180–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang, Y. , Zuo, Z. , Tam, F. , Graham, S. J. , Tao, R. , Wang, N. , & Bi, H.‐Y. (2019). Brain activation and functional connectivity during Chinese writing: An fMRI study. Journal of Neurolinguistics, 51, 199–211. [Google Scholar]
- 10. Li, J. , Hong, L. , Bi, H.‐Y. , & Yang, Y. (2021). Functional brain networks underlying automatic and controlled handwriting in Chinese. Brain and Language, 219, 104962. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura, K. , Honda, M. , Hirano, S. , Oga, T. , Sawamoto, N. , Hanakawa, T. , Inoue, H. , Ito, J. , Matsuda, T. , & Fukuyama, H. (2002). Modulation of the visual word retrieval system in writing: A functional MRI study on the Japanese orthographies. Journal of Cognitive Neuroscience, 14(1), 104–115. [DOI] [PubMed] [Google Scholar]
- 12. Omura, K. , Tsukamoto, T. , Kotani, Y. , Ohgami, Y. , & Yoshikawa, K. (2004). Neural correlates of phoneme‐to‐grapheme conversion. Neuroreport, 15(6), 949–953. [DOI] [PubMed] [Google Scholar]
- 13. Roux, F. E. , Dufor, O. , Giussani, C. , Wamain, Y. , Draper, L. , Longcamp, M. , & Démonet, J. F. (2009). The graphemic/motor frontal area Exner's area revisited. Annals of Neurology, 66(4), 537–545. [DOI] [PubMed] [Google Scholar]
- 14. Rapp, B. , & Dufor, O. (2011). The neurotopography of written word production: An fMRI investigation of the distribution of sensitivity to length and frequency. Journal of Cognitive Neuroscience, 23(12), 4067–4081. [DOI] [PubMed] [Google Scholar]
- 15. Planton, S. , Jucla, M. , Roux, F.‐E. , & Démonet, J.‐F. (2013). The “handwriting brain”: A meta‐analysis of neuroimaging studies of motor versus orthographic processes. Cortex, 49(10), 2772–2787. [DOI] [PubMed] [Google Scholar]
- 16. Graham, S. , Berninger, V. , Weintraub, N. , & Schafer, W. (1998). Development of handwriting speed and legibility in grades 1–9. Journal of Educational Research, 92(1), 42–52. [Google Scholar]
- 17. Kao, H. S. R. (1992). The psychology of calligraphy art. Hong Kong: Hong Kong Cultural Education Press. [Google Scholar]
- 18. Cornhill, H. , & Case‐Smith, J. (1996). Factors that relate to good and poor handwriting. American Journal of Occupational Therapy, 50(9), 732–739. [DOI] [PubMed] [Google Scholar]
- 19. Weintraub, N. , & Graham, S. (2000). The contribution of gender, orthographic, finger function, and visual‐motor processes to the prediction of handwriting status. Occupational Therapy Journal of Research, 20(2), 121–140. [Google Scholar]
- 20. Wu, T. , Zhang, J. , Hallett, M. , Feng, T. , Hou, Y. , & Chan, P. (2016). Neural correlates underlying micrographia in Parkinson's disease. Brain, 139(1), 144–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosenblum, S. , Samuel, M. , Zlotnik, S. , Erikh, I. , & Schlesinger, I. (2013). Handwriting as an objective tool for Parkinson's disease diagnosis. Journal of Neurology, 260(9), 2357–2361. [DOI] [PubMed] [Google Scholar]
- 22. Fuentes, C. T. , Mostofsky, S. H. , & Bastian, A. J. (2009). Children with autism show specific handwriting impairments. Neurology, 73(19), 1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kandel, S. , Lassus‐Sangosse, D. , Grosjacques, G. , & Perret, C. (2017). The impact of developmental dyslexia and dysgraphia on movement production during word writing. Cognitive Neuropsychology, 34(3–4), 219–251. [DOI] [PubMed] [Google Scholar]
- 24. Sumner, E. , Connelly, V. , & Barnett, A. L. (2013). Children with dyslexia are slow writers because they pause more often and not because they are slow at handwriting execution. Reading and Writing, 26(6), 991–1008. [Google Scholar]
- 25. Graham, S. , Fishman, E. J. , Reid, R. , & Hebert, M. (2016). Writing characteristics of students with attention deficit hyperactive disorder: A meta‐analysis. Learning Disabilities Research and Practice, 31(2), 75–89. [Google Scholar]
- 26. Klimoski, R. J. , & Rafaeli, A. (1983). Inferring personal qualities through handwriting analysis. Journal of Occupational Psychology, 56(3), 191–202. [Google Scholar]
- 27. Williams, M. , Berg‐Cross, G. , & Berg‐Cross, L. (1977). Handwriting characteristics and their relationship to Eysenck's extraversion‐introversion and Kagan's impulsivity‐relfectivity dimensions. Journal of Personality Assessment, 41(3), 291–298. [DOI] [PubMed] [Google Scholar]
- 28. Gawda, B. (2008). A graphical analysis of handwriting of prisoners diagnosed with antisocial personality. Perceptual and Motor Skills, 107(3), 862–872. [DOI] [PubMed] [Google Scholar]
- 29. Neter, E. , & Ben‐Shakhar, G. (1989). The predictive validity of graphological inferences: A meta‐analytic approach. Personality and Individual Differences, 10(7), 737–745. [Google Scholar]
- 30. King, R. N. , & Koehler, D. J. (2000). Illusory correlations in graphological inference. Journal of Experimental Psychology: Applied, 6(4), 336. [DOI] [PubMed] [Google Scholar]
- 31. Eysenck, H. J. , & Gudjonsson, G. (1986). An empirical study of the validity of handwriting analysis. Personality and Individual Differences, 7(2), 263–264. [Google Scholar]
- 32. McCrae, R. R. , & Costa, J. (2008). The five‐factor theory of personality. New York: Guilford Press. [Google Scholar]
- 33. Kapogiannis, D. , Sutin, A. , Davatzikos, C. , Costa, P. , & Resnick, S. (2013). The five factors of personality and regional cortical variability in the Baltimore longitudinal study of aging. Human Brain Mapping, 34(11), 2829–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeYoung, C. G. , Hirsh, J. B. , Shane, M. S. , Papademetris, X. , Rajeevan, N. , & Gray, J. R. (2010). Testing predictions from personality neuroscience: Brain structure and the Big Five. Psychological Science, 21(6), 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riccelli, R. , Toschi, N. , Nigro, S. , Terracciano, A. , & Passamonti, L. (2017). Surface‐based morphometry reveals the neuroanatomical basis of the five‐factor model of personality. Social Cognitive and Affective Neuroscience, 12(4), 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Owens, M. M. , Hyatt, C. S. , Gray, J. C. , Carter, N. T. , MacKillop, J. , Miller, J. D. , & Sweet, L. H. (2019). Cortical morphometry of the five‐factor model of personality: Findings from the Human Connectome Project full sample. Social Cognitive and Affective Neuroscience, 14(4), 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rueter, A. R. , Abram, S. V. , MacDonald, A. W. , Rustichini, A. , & DeYoung, C. G. (2018). The goal priority network as a neural substrate of conscientiousness. Human Brain Mapping, 39(9), 3574–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sampaio, A. , Soares, J. M. , Coutinho, J. , Sousa, N. , & Gonçalves, Ó. F. (2014). The Big Five default brain: Functional evidence. Brain Structure & Function, 219(6), 1913–1922. [DOI] [PubMed] [Google Scholar]
- 39. Stock, A.‐K. , & Beste, C. (2015). Conscientiousness increases efficiency of multicomponent behavior. Scientific Reports, 5(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dima, D. , Friston, K. J. , Stephan, K. E. , & Frangou, S. (2015). Neuroticism and conscientiousness respectively constrain and facilitate short‐term plasticity within the working memory neural network. Human Brain Mapping, 36(10), 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toschi, N. , Riccelli, R. , Indovina, I. , Terracciano, A. , & Passamonti, L. (2018). Functional connectome of the five‐factor model of personality. Personality Neuroscience, 1, E2, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DeYoung, C. G. , Shamosh, N. A. , Green, A. E. , Braver, T. S. , & Gray, J. R. (2009). Intellect as distinct from openness: Differences revealed by fMRI of working memory. Journal of Personality and Social Psychology, 97(5), 883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeYoung, C. G. , Peterson, J. B. , & Higgins, D. M. (2005). Sources of openness/intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. Journal of Personality, 73(4), 825–858. [DOI] [PubMed] [Google Scholar]
- 44. Shen, X. , Finn, E. S. , Scheinost, D. , Rosenberg, M. D. , Chun, M. M. , Papademetris, X. , & Constable, R. T. (2017). Using connectome‐based predictive modeling to predict individual behavior from brain connectivity. Nature Protocols, 12(3), 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Snyder, P. J. , & Harris, L. J. (1993). Handedness, sex, familial sinistrality effects on spatial tasks. Cortex, 29(1), 115–134. [DOI] [PubMed] [Google Scholar]
- 46. Wang, H. , Chang, B. , Li, Y. , Lin, L. , Liu, J. , Sun, Y. , Yu, Y. , Zhang, J. , & Li, D. (1986). Modern Chinese Frequency Dictionary. Beijing Language Institute Press. [Google Scholar]
- 47. Tam, F. , Churchill, N. W. , Strother, S. C. , & Graham, S. J. (2011). A new tablet for writing and drawing during functional MRI. Human Brain Mapping, 32(2), 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Costa Jr, P. T. , & McCrae, R. R. (1992). Four ways five factors are basic. Personality and Individual Differences, 13(6), 653–665. [Google Scholar]
- 49. Yang, J. , McCrae, R. R. , Costa, P. T. , Dai, X. , Yao, S. , Cai, T. , & Gao, B. (1999). Cross‐cultural personality assessment in psychiatric populations: The NEO‐PI‐R in the People's Republic of China. Psychological Assessment, 11(3), 359–368. [Google Scholar]
- 50. Yang, Y. , Tam, F. , Graham, S. J. , Sun, G. , Li, J. , Gu, C. , Tao, R. , Wang, N. , Bi, H.‐Y. , & Zuo, Z. (2020). Men and women differ in the neural basis of handwriting. Human Brain Mapping, 41(10), 2642–2655. 10.1002/hbm.24968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Power, J. D. , Cohen, A. L. , Nelson, S. M. , Wig, G. S. , Barnes, K. A. , Church, J. A. , Vogel, A. C. , Laumann, T. O. , Miezin, F. M. , Schlaggar, B. L. , & Petersen, S. E. (2011). Functional network organization of the human brain. Neuron, 72(4), 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connectivity, 2(3), 125–141. [DOI] [PubMed] [Google Scholar]
- 53. Jiang, R. , Zuo, N. , Ford, J. M. , Qi, S. , Zhi, D. , Zhuo, C. , Xu, Y. , Fu, Z. , Bustillo, J. , Turner, J. A. , Calhoun, V. D. , & Sui, J. (2020). Task‐induced brain connectivity promotes the detection of individual differences in brain–behavior relationships. Neuroimage, 207, 116370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fan, L. , Li, H. , Zhuo, J. , Zhang, Y. , Wang, J. , Chen, L. , Yang, Z. , Chu, C. , Xie, S. , Laird, A. R. , Fox, P. T. , Eickhoff, S. B. , Yu, C. , & Jiang, T. (2016). The human brainnetome atlas: A new brain atlas based on connectional architecture. Cerebral Cortex, 26(8), 3508–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Planton, S. , Longcamp, M. , Péran, P. , Démonet, J.‐F. , & Jucla, M. (2017). How specialized are writing‐specific brain regions? An fMRI study of writing, drawing and oral spelling. Cortex, 88, 66–80. [DOI] [PubMed] [Google Scholar]
- 56. Purcell, J. , Turkeltaub, P. E. , Eden, G. F. , & Rapp, B. (2011). Examining the central and peripheral processes of written word production through meta‐analysis. Frontiers in Psychology, 2, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roberts, B. W. , Lejuez, C. , Krueger, R. F. , Richards, J. M. , & Hill, P. L. (2014). What is conscientiousness and how can it be assessed? Developmental Psychology, 50(5), 1315–1330. [DOI] [PubMed] [Google Scholar]
- 58. Wilmot, M. P. , & Ones, D. S. (2019). A century of research on conscientiousness at work. Proceedings of the National Academy of Sciences of the United States of America, 116(46), 23004–23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Noftle, E. E. , & Robins, R. W. (2007). Personality predictors of academic outcomes: Big Five correlates of GPA and SAT scores. Journal of Personality and Social Psychology, 93(1), 116–130. [DOI] [PubMed] [Google Scholar]
- 60. Moffitt, T. E. , Arseneault, L. , Belsky, D. , Dickson, N. , Hancox, R. J. , Harrington, H. , Houts, R. , Poulton, R. , Roberts, B. W. , Ross, S. , Sears, M. R. , Thomson, W. M. , & Caspi, A. (2011). A gradient of childhood self‐control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences of the United States of America, 108(7), 2693–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chen, H.‐Y. , Chang, E. C. , Chen, S. H. Y. , Lin, Y.‐C. , & Wu, D. H. (2016). Functional and anatomical dissociation between the orthographic lexicon and the orthographic buffer revealed in reading and writing Chinese characters by fMRI. Neuroimage, 129, 105–116. [DOI] [PubMed] [Google Scholar]
- 62. Nakamura, K. , Kuo, W.‐J. , Pegado, F. , Cohen, L. , Tzeng, O. J. , & Dehaene, S. (2012). Universal brain systems for recognizing word shapes and handwriting gestures during reading. Proceedings of the National Academy of Sciences of the United States of America, 109(50), 20762–20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hampshire, A. , Chamberlain, S. R. , Monti, M. M. , Duncan, J. , & Owen, A. M. (2010). The role of the right inferior frontal gyrus: Inhibition and attentional control. Neuroimage, 50(3), 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Aron, A. R. , Robbins, T. W. , & Poldrack, R. A. (2014). Inhibition and the right inferior frontal cortex: One decade on. Trends in Cognitive Sciences, 18(4), 177–185. [DOI] [PubMed] [Google Scholar]
- 65. Sharp, D. J. , Bonnelle, V. , Boissezon, X. D. , Beckmann, C. F. , James, S. G. , Patel, M. C. , Mehta, M. A. , & Smith, E. E. (2010). Distinct frontal systems for response inhibition, attentional capture, and error processing. Proceedings of the National Academy of Sciences of the United States of America, 107(13), 6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosenblum, S. (2018). Inter‐relationships between objective handwriting features and executive control among children with developmental dysgraphia. PLoS One, 13(4), e0196098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gimenez, P. , Bugescu, N. , Black, J. , Hancock, R. , Pugh, K. , Nagamine, M. , Kutner, E. , Mazaika, P. , Hendren, R. , & McCandliss, B. (2014). Neuroimaging correlates of handwriting quality as children learn to read and write. Frontiers in Human Neuroscience, 8, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kam, J. W. , Lin, J. J. , Solbakk, A.‐K. , Endestad, T. , Larsson, P. G. , & Knight, R. T. (2019). Default network and frontoparietal control network theta connectivity supports internal attention. Nature Human Behaviour, 3(12), 1263–1270. [DOI] [PubMed] [Google Scholar]
- 69. Vatansever, D. , Menon, D. K. , Manktelow, A. E. , Sahakian, B. J. , & Stamatakis, E. A. (2015). Default mode dynamics for global functional integration. Journal of Neuroscience, 35(46), 15254–15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Stock, A.‐K. , & Beste, C. (2015). Conscientiousness increases efficiency of multicomponent behavior. Scientific Reports, 5(1), 15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hamstrabletz, L. , & Blote, A. W. (1993). A longitudinal study on dysgraphic handwriting in primary school. Journal of Learning Disabilities, 26(10), 689–699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The hubs in the positive or negative functional brain networks correlated with conscientiousness scores.
Figure S1. The prediction results based on functional brain networks were further validated by using different edge selection thresholds. Scatter plots of the correlations between observed consciousness scores and predicted scores by positive (red), negative (blue), and combined (yellow) networks using an edge‐selection threshold of p < 0.001 (A) and p < 0.01 (B).
Figure S2. The prediction results based on functional brain networks were validated by using the template of Fan et al. (2016) for node definition. Scatter plots of the correlations between observed consciousness scores and predicted scores by positive (red), negative (blue), and combined (yellow) networks.


