Abstract
This article presents a review after an exhaustive search that yielded 23 works carried out in the last decade for the availability of optical microscopes with open hardware as a low‐cost alternative to commercial systems. These works were developed with the aim of covering needs within several areas such as: Bio Sciences research in institutions with limited resources, diagnosis of diseases and health screenings in large populations in developing countries, and training in educational contexts with a need for high availability of equipment and low replacement cost. The analysis of the selected works allows us to classify the analyzed solutions into two main categories, for which their essential characteristics are enumerated: portable field microscopes and multipurpose automated microscopes. Moreover, this work includes a discussion on the degree of maturity of the solutions in terms of the adoption of practices aligned with the development of Open Science.
Research Highlights
Concise review on low‐cost microscopes for developing Open Science, exposing the role of smartphone‐based microscopy. The work classifies microscopes in two main categories: (1) portable field microscopes, and (2) multipurpose automated microscopes.
Keywords: automated microscope, digital image processing, low‐cost microscopy, open hardware, open science, open source, smartphone‐based microscopy
Taxonomy of low‐cost microscopes.
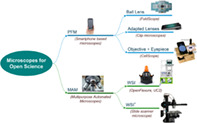
1. INTRODUCTION
Nowadays, the optical microscope is a helpful tool in many disciplines related to public health for the diagnosis of diseases (e.g., infectious, parasitic, cancer, etc.) and environmental studies (e.g., quality of aquatic ecosystems). In addition to its great usefulness in these areas of knowledge, it is an important training and dissemination instrument within the life sciences. However, its operation presents a relevant limitation due to its high cost when it comes to its application in institutions with tight budgets or extensive health plans devoted to the population in developing countries with high incidence of diseases (e.g., at the tropics). It should be noted that light microscopy solutions can be prohibitive when remote points‐of‐care (POC) need to be equipped. Under these conditions the current advances in this century related to digital image processing and computer vision have driven the development of optical microscopy solutions to facilitate their availability and reduce their cost by using open hardware and software (OHS).
The availability of open‐source libraries such as OpenCV (opencv.org) for developing of image processing and artificial vision software has catalyzed the proposal of an enormous number and quality of projects in these fields of knowledge since their inception. Their popularity has also been inspired by the constant improvement of digital image sensors which has made low‐cost projects associated with image acquisition systems possible, for example with a ubiquitous device like a simple USB webcam. Moreover, another boosting factor is the growing computing capacity of digital systems, along with the popularity of programming languages such as Java and Python which speed up the application development cycle compared to compiled languages such as C/C++. In this way, a scenario has been reached in which the appearance of new open‐source tools has been promoted, among which those aimed at Machine Learning such as Scikit‐learn (scikit-learn.org), TensorFlow (www.tensorflow.org), PyTorch (pytorch.org), etc. stand out. In particular, the field of digital microscopy is not exempt from the appearance of open‐source tools such as Fiji/ImageJ (imagej.net), which have become very popular in the scientific community and continue to develop and expand with the addition of new plugins. Finally, the continuous progress of telecommunications has enabled the deployment of web services for remotely processing the digital image and video supplied by clients with limited resources, promoting new scenarios for telemicroscopy.
The appearance of open hardware platforms such as Arduino and Raspberry Pi (Rpi), together with compatible input/output devices has led to an explosion of open projects in which these affordable platforms act as intelligent controllers. The aforementioned hardware is used in both, educational and specialized projects, sharing practices committed to the spirit of the Maker philosophy and Open science (Chagas, 2018; Dosemagen et al., 2017; Hatch, 2014). In particular, the availability of digital cameras compatible with open hardware platforms (e.g., Rpi HQ Camera) has also facilitated the use of such hardware in projects related to image processing and computer vision. The small size factor of these platforms, together with the availability of wireless communications, facilitate the development of new mobile devices capable of interconnection, even in local and wide area networks. The use of open hardware facilitates the development of compatible devices and interface boards to provide broad‐spectrum generic functionalities at low prices. The list of compatible devices consists of: DC motors, A/D sensors, digital cameras, wireless communications, etc.
Most projects related to open hardware require specific housing and couplings crafting, very often with the aid of Do‐it‐yourself tools. Low‐cost 3D printing (under 500 USD) by material extrusion or fused deposition modeling (FDM) has facilitated the task of building both the physical structure and the couplings required to integrate the components of a functional prototype.
2. OPEN PROJECTS ON LOW‐COST OPTICAL MICROSCOPES
In the technological context previously exposed, some projects aim to add new functionalities to commercial systems through open, low‐cost solutions. For instance, new microscopy modalities as epifluorescence, stage/focus automation, and so on (Dacal et al., 2021; Hasan et al., 2016; Salido et al., 2020; Stewart & Giannini, 2016). On the contrary, the goal of this paper was to analyze microscopy solutions built from scratch. With this objective in mind, we have identified two categories that differ on the orientation that drives their purpose, which are: mobility and flexibility. As a result, we can consider portable field microscopes (PFM) when mobility is necessary and low‐cost multipurpose automated microscopes (MAM) when flexibility is the primary objective of the solution. Both strategies differ, although they have some common attributes, like its low cost and its ability to equip a research laboratory or a POC at any time and place.
This manuscript presents the common characteristics of the two aforementioned categories after the analysis of a representative number of works carried out in the field during the last decade. In the next sections, PFMs and MAMs are described and divided into several subcategories which share some aspects. The description of each subcategory includes its Pros (strengths) and Cons (limitations).
2.1. Portable field microscopes
This type of microscopes is used in applications of field work requiring the continuous displacement of the equipment, such as:
Biomedical sciences. For instance, in field POCs during health screenings to diagnose neglected tropical diseases (e.g., schistosomiasis, trichuriasis, etc.) and some cancer types (e.g., cervix, oral, etc.) in developing countries or zones affected by war or humanitarian emergencies (Coulibaly et al., 2016; Naqvi et al., 2020; Rajchgot et al., 2017; Saeed & Jabbar, 2018).
Environmental sciences. As in the detection of alterations in biomarkers related to the presence of pollutants in different terrestrial and aquatic ecosystems (Salido et al., 2020).
Multidisciplinary education and Citizen Science promotion. At different educational levels in which it is not possible or advisable to use fragile and expensive equipment (Cybulski et al., 2014; Garcia‐Soto et al., 2021; Grier et al., 2018; Kim et al., 2016). Moreover, in the COVID‐19 pandemic scenario, portable low‐cost imaging systems had helped students in online learning environments (Yu et al., 2022).
These microscopes will meet the following characteristics:
Light weighted. They are small and light for easy transportation.
Simple optical systems adapted to the task. They are usually composed of small, cheap lenses that sacrifice quality at the cost of drastic reduction of space and replacing cost even if the whole microscope needs to be replaced. Moreover, they aim to reduce the risks of expenses derived from damage in the equipment caused by the conditions of the working environment. The housing and couplings of the components constituting its physical structure can be easily obtained using rapid prototyping tools such as 3D printing.
Simplicity of operation. They do not typically have automatic displacement systems which hinders the acquisition of whole slide images (WSI). Therefore, the movement of the slide is manual in almost all cases.
Low energy consumption. They must be able to function over a long period of time without the significant need for energy. The need for energy is usually limited to the existence of an independent lighting system. In some cases, they have the option of working in brightfield with a mirror and sunlight.
Independent imaging system. This type of microscope depends on independent image capture systems that often consist of a mobile phone camera in order to reduce their cost. This ensures the universality of the solution in which software as a mobile app can be incorporated for visualization, processing, decision‐making assistance, and connectivity with remote systems, both to have access to mobile services and for the provision of live video streams. In fact, the use of mobile phones as a digital microscopy image capture system (separate from the microscope itself) has been reported as an efficient low‐cost solution for tele‐diagnosis in isolated areas with limited communication infrastructures (Bellina & Missoni, 2009; Morrison & Gardner, 2014).
A PFM is often referred to as a smartphone‐based microscope whenever the observation of the sample requires using a mobile phone equipped with camera and visualization software. In this case, the structure of the optical system can adopt one of the configurations shown in Figure 1 (left column) taken by courtesy of Switz et al. (2014). The central and right columns in Figure 1 display the effect of the optical system on the resolution and distortion in the field of view (FOV) while observing a sample.
FIGURE 1.
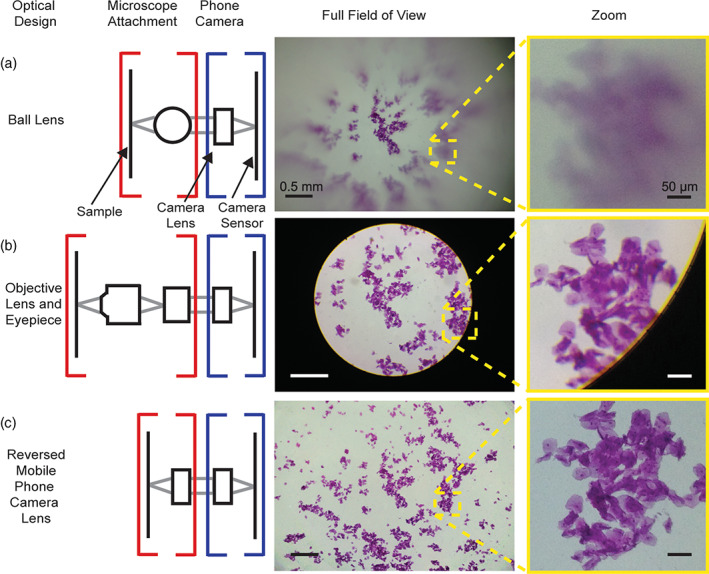
Comparison of optical systems in mobile phone microscopes (CC‐BY 4.0 Switz et al., 2014. DOI: 10.1371/journal.pone.0095330.g001)
In the next sections, the three subcategories or subtypes that we distinguish in PFM microscopes depending on their optical system will be described.
2.1.1. PFM with ball lens
These works are, to a certain extent, the heirs of the work carried out in the last quarter of the seventeenth century by the pioneering microscopist Antonj van Leeuwenhoek. Thanks to his skill in lens making acquired in the family business, he was able to observe microbial life forms, which he called animalcules. With his microscopes up to 275× magnification and spatial resolution of 1.4 μm, he laid the foundations for modern microbiology (Wollman et al., 2015).
The lens achieves the magnification required to capture images by the camera sensor in the smartphone. They are extremely cheap because of the low price of the beads used as lenses (i.e., borosilicate glass and sapphire), the simple housing of such lenses, and their easy fitting.
Pros
Very cheap, with simple adapted housings and couplings (e.g., clip type).
Compactness and portability.
Possibility of large magnification values limited by the lens diameter (e.g., 100× to 350× with diameters from 3 to 1 mm, respectively).
Cons
Auto‐focus (AF) operation based on the capability of the smartphone camera.
Simple illumination systems.
Reduced resolution and considerable image distortion.
Field of view (FOV) below the capability of the camera sensor.
Difficulty in obtaining whole slide imaging (WSI) by manual movement.
In the category of PFMs, the Foldscope project (www.foldscope.com) developed at Stanford (USA) deserves a special mention (Cybulski et al., 2014). Foldscope (see Figure 2), inspired by the figures of origami, is a paper microscope made at an extremely low cost—under 10 USD—that can be used for various modalities of microscopy as shown in Figure 3. It can be operated as an educational tool but also in POCs in remote locations and with scarce resources for diagnosis of tropical diseases and others illness of great incidence in the population. In a standard Foldscope configuration, a 140× magnification can be attained. It allows three possible ways of visualization: eye, projection, and smartphone. In the latter case, the phone can be securely attached through a magnetic ring coupling and the lighthing can be improved with a LED module.
FIGURE 2.
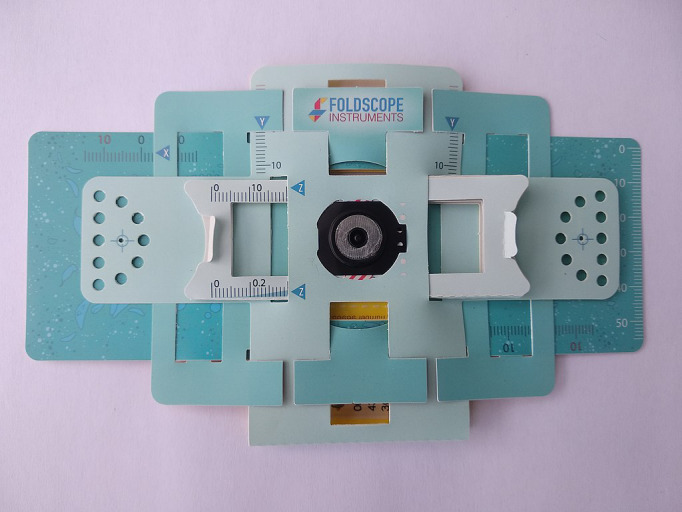
Foldscope, the origami‐based paper microscope (CC‐BY‐SA 4.0 Sockenpaket, Wikimedia commons)
FIGURE 3.
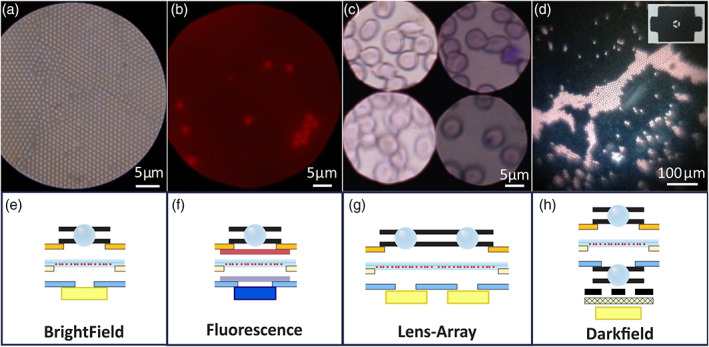
Foldscope imaging modalities showing the respective arrangements of ball lenses (CC‐BY 4.0, Cybulski et al., 2014; DOI: 10.1371/journal.pone.0098781.g002)
2.1.2. PFM with adapted lenses
This configuration is obtained by means of a simple optical system without an eyepiece, located between the sample and the smartphone camera (see in Figure 4 the example extracted from the work of Orth et al. (2018)). In applications in which an extended FOV is required, some arrangements are proposed with an identical lens as the present in the smartphone camera but reversed, as indicated in Figure 1c (bottom row). However, when greater magnification is required, a choice consist of an arrangement of several lenses as a ball lens (BL) next to a convex flat lens in front of the smartphone camera as in the work by Rabha et al. (2019). Some of these microscopes are being commercially available becoming a success story, like the DIPLE® microscope (Cesaretti et al., 2020) patented by SmartMicroOptics srl (smartmicrooptics.com). DIPLE® uses a magnetic coupling between the magnifying lens (ranging from 35× to 150×) and the supporting stage for the microscope slides. In the design of DIPLE®, the phone is not connected to the lens, and the distance lens‐sample is regulated with a screw, in order to allow a fine regulation. Such a fine control of the working distance is very important, in particular with high magnification. Moreover, the high‐end version of DIPLE® allows 2D slide scan using a manual screw driven system. Other designs in this category are: PNNL Smartphone Microscope, Nurugo Micro, TinyScope Microscope, etc. The common characteristics to this type of configuration are summarized below, though the optical characteristics are very dependent on the equipped lenses.
FIGURE 4.
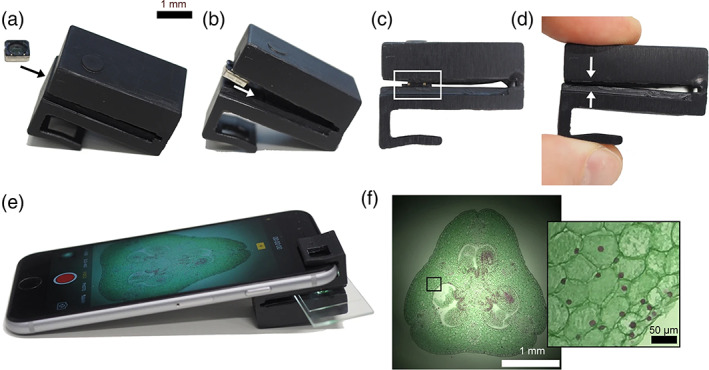
Mobile phone microscope assembly process and sample view. (a) Insert the mobile phone camera lens (objective lens) into the microscope clip. (b) Push objective lens further into the clip until it fits into the recess. (c) Gently push the objective lens assembly into the recess. The white boxed region shows the objective lens assembly sitting in the friction‐fit recess. (d) Gently squeeze microscope clip so that the opposite sides of the slide holder come into contact. This pushes the objective lens assembly into its final position in the microscope clip recess. (e) Insert sample slide and attach the clip to a mobile phone as shown. Open the camera app (or other 3rd party camera app), switch to video mode and activate the flash to view the sample in brightfield mode. In this example, the sample is Lilium ovary (Southern Biological). Exposure time: 1/4808s, ISO 25. (f) Brightfield image of Lilium ovary using “Photo” mode with flash. Inset: Magnified image of boxed region. (CC‐BY 4.0, Orth et al., 2018; DOI: 10.1038/s41598‐018‐21543‐2).
Pros
Inexpensive optical system with simple housing and coupling (e.g., magnetic or clip type, as shown in Figure 4).
High magnification available.
Compactness and portability.
Cons
Image distortion because of the optical system simplicity.
AF based on smartphone camera capability.
Limited illumination systems.
Difficulty in obtaining automatic WSI because of the slide manual‐driven movement.
2.1.3. PFM with adapted objective and eyepiece
The objective of this configuration is to fit a higher quality optical system constituted by lens and eyepiece adapted to the smartphone camera. The increased complexity of the optical system makes it necessary to use more complex adapted housing and couplings than the presented in the previous category. Some solutions are capable of slide displacement (x–y) for limited WSI capture. The cost of the microscopes included in this category—under 250 USD—is a 10 factor higher than in the previous ones. However, it is still quite cheap compared to end market alternatives, around 1000 USD.
Pros
Good resolution and low distortion image.
Flexible illumination systems.
Ease of 2D manual motion of the slide.
Manual focus control.
Cons
Manual stage and focus control.
FOV under the actual sensor image capability.
Since these microscopes have a more ambitious scope in their capabilities, some of them have derived in commercial products, as the marketed by iolight ltd. (iolight.co.uk) and CellScope Inc. (acquired by Jhonson & Jhonson). The CellScope developed by Skandarajah et al. (2014) included in the manuscript (see Figure 5) is not commercially available, however it constitutes still a good representative in this category. In this type of microscopes, the use of a more complex optical system improves the image quality obtained with an over cost with respect to solutions with simpler optics.
FIGURE 5.
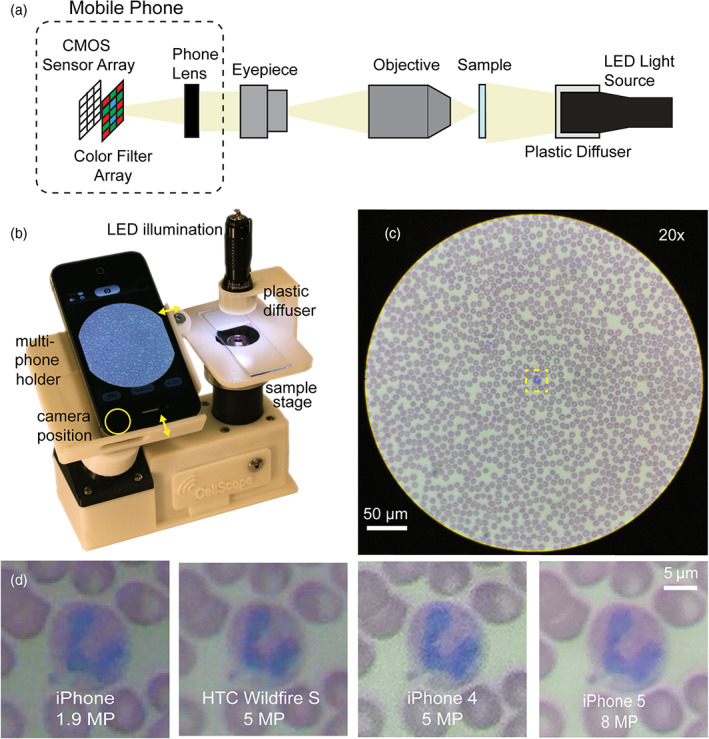
CellScope a multi‐phone mobile microscope and resulting images of the same sample with different smartphone models (CC‐BY 4.0, Skandarajah et al., 2014; DOI: 10.1371/journal.pone.0096906.g001)
2.2. Low‐cost MAM
This type of microscope emerges as a low‐cost (under 1000 USD) and high‐availability alternative to professional systems, in applications where a quality certification is not mandatory, with a cost 10–100 times higher. Unlike mobility‐oriented systems, they are equipped with dedicated affordable digital image acquisition systems incorporated to the microscope itself (e.g., CMOS cameras), but with a need for a separate device for image display (e.g., laptop, smartphone or similar). If the stage 2D displacement (x–y) and the zoom (z) are automated, the same device devoted to display the image is responsible for sending the motion orders carried out by user commands and the acquired image postprocessing. Open hardware such as the control boards Raspberry Pi (Rpi) and Arduino are frequently utilized for both the control of the electromechanical system and the illumination of the sample. 3D printing is a very convenient tool for fabricating the necessary components for these microscopes, such as housings and couplings, during the component's integration into the system.
MAM systems are more difficult to replicate than PFM systems because of their increased complexity. To facilitate this task, in these projects, it is necessary to provide:
the lists of compatible commercial off‐the‐shelf components (COTS),
the 3D models for printing and/or manufacturing of auxiliary structural elements (e.g., STL—Standard Tessellation Language—files),
the detailed guides of the whole assembly of components and their integration, and
the source code of software required for the system control and the sample observation.
Therefore, the construction and assembly of this type of microscope is more complex than for PFMs, since it requires some expertise and experience in the development of devices with open hardware and the proper use of DIY tools.
This category of microscope can be considered a true automated digital image system, in which both the illumination system and the 2D movements of the stage (x–y) and the focus axis (z) are controlled. In general, these microscopes are designed prioritizing their versatility to offer the most used optical microscopy modalities (i.e., bight‐/darkfield, fluorescence, etc.). For this reason, we also call them low‐cost Multi‐purpose Automated Microscopes (MAM). Depending on the automated range of motion they possess, we differentiate two subcategories of MAM devices: local observation microscopes—less than a whole slide—(MAM‐WSI−) and slide scanner microscopes with high observation range—even beyond the size of a standardized slide—(MAM‐WSI+).
There is a set of computational microscopy solutions that is gaining interest, such as Fourier ptychography (Aidukas et al., 2019; Dong et al., 2014) and digital inline holography (Amann et al., 2019). Although these techniques do not have their emphasis on the automation of the 3D motions of the microscope (x–y/z), they represent a high degree of automation in the acquisition and preprocessing of images.
The software that accompanies the system becomes paramount, since it is responsible for both the control of electromechanical subsystems and the automation of the digital imaging system. Some important software modules include focus stacking, scanning control and tile stitching to acquire a whole slide image. Digital imaging software can be as simple as a visualization program and an image recording, but the more sophisticated ones contemplate high‐level processing tasks such as: medical diagnostic assistance, image interpretation (e.g., detection and recognition), tele microscopy, etc.In the next section, we provide a more in‐depth description of the subcategories of MAMs: MAM‐WSI− and MAM‐WSI+.
2.2.1. Local observation microscopes (MAM‐WSI −)
They are systems in which versatility prevails. Therefore, they have a wide range of applications ranging from the equipment for analysis tasks in POCs and laboratories with limited budgets, to an affordable training tool for educational purposes in academic institutions.
Their optical and illumination systems consist of standardized COTS components, which allows the integration of elements of comparable quality to that of commercial systems. These microscopes are built with electromechanical systems and control hardware popularized in the development of open hardware projects, such as stepper motors, position sensors, and other devices connected to Arduino/Rpi controller boards. Figure 6a,b shows the OpenFlexure (Collins et al., 2020), one of the microscopes within this category that has achieved a prominent popularity and public diffusion for constituting a practical paradigm of the Open Source‐Hardware philosophy to develop Open Science and continuous improvements (Collins et al., 2021; McDermott et al., 2021).
FIGURE 6.
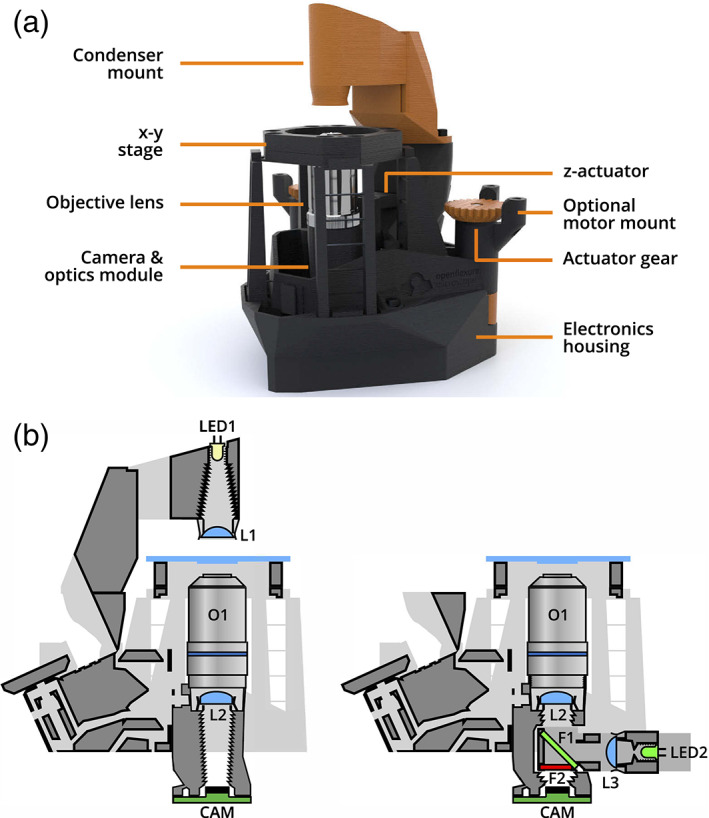
(a) Overview of the OpenFlexure microscope design, in transmission bright‐field configuration (CC‐BY 4.0, Collins, Knapper, Stirling, Mduda, Mkindi, Mayagaya, Mwakajinga, Nyakyi, Sanga, Carbery, White, Dale, Lim, Baumberg, Cicuta, McDermott, Vodenicharski and Bowman, “Robotic microscopy for everyone: the OpenFlexure microscope,” Biomed. Opt. Express 11, 2447–2460 (2020); DOI: 10.1364/BOE.385729). (b) Cross‐section schematics of trans−/epi‐illumination (left/right) configurations (CC‐BY 4.0, Collins, Knapper, Stirling, Mduda, Mkindi, Mayagaya, Mwakajinga, Nyakyi, Sanga, Carbery, White, Dale, Lim, Baumberg, Cicuta, McDermott, Vodenicharski and Bowman, “Robotic microscopy for everyone: the OpenFlexure microscope,” Biomed. Opt. Express 11, 2447–2460 (2020); DOI: 10.1364/BOE.385729)
OpenFlexure is a system that under a structure created by 3D printing provides modularity and flexibility to offer different microscopy modalities and automation possibilities for the stage (x–y) and focus (z) in its motorized version. This platform can be configured as an inverted or upright microscope including computational illumination with a LED array to provide different types of imaging as follows: bight‐/dark‐field, Rheinberg, differential phase contrast, ptychography. In addition to the quality of the result, the great success of this project is due to the amount of free open information provided which facilitates the replication of this system (openflexure.org, gitlab.com/openflexure).
In this section, we also want to highlight the UC2 project (you.see.too, github.com/openUC2/UC2-GIT), born as a low‐cost open standard platform to configure versatile custom microscopy systems, with a physical structure built by 3D printing (Diederich et al., 2020). UC2 emerges with a clear educational vocation, as it is a low‐cost tool that facilitates rapid experience in the design and application of different microscopy modalities. Figure 7 shows the common workflow of an application built with UC2.
FIGURE 7.
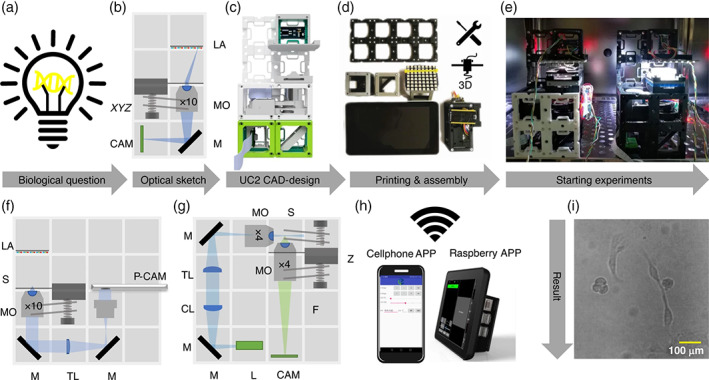
Common workflow to build a UC2 application: (a) starting with a biological question/idea in need of an imaging device drafted in (b) (inverted incubator microscope) and transferred using UC2 components from the CAD library in (c). After printing and assembling it (d) the device will be placed in its working environment (e.g., incubator) (e) ready to acquire long‐term image series of e.g. MDCK cells visualized in (i). Remote‐control is granted using “smart components” (e.g., cellphone, raspberry pi) in (h). Reusing components allows the conversion into a cellphone‐microscope (f) or light sheet microscope (g) within minutes. CL: Cylindrical lens, TL: Tube lens, L: Laser, LA: LED‐array, M: Mirror, MO: Microscope objective, P‐CAM: Detector (smartphone or raspberry pi), S: Sample positioning stage, F: Emission filter, Z: Focusing stage. (CC‐BY 4.0, Diederich et al., 2020; DOI: 10.1038/s41467‐020‐19,447‐9)
Pros
Small size of the entire system that facilitates field work.
Flexibility for different illuminations modalities.
Automated stage motion (x‐y) control.
Automated focus control (z).
Cons
Limited range of automated scanning for the sample slide (WSI−).
Although, they can use some ingenious solutions like in OpenFlexure (Sharkey et al., 2016), their short‐range capability for the motion of the stage constitutes their main limitation. Therefore, the automation of the stage is crucial in these systems, since the precision of their positioning and the required cost to achieve it are opposite. Thus, systems that rely on very precise electromechanical COTS components increase their cost, making their inclusion in the category of low‐cost microscopes debatable.
2.2.2. Slide scanner microscopes (MAM‐WSI +)
The above‐mentioned motion capability of the microscope stage for local viewing is insufficient for applications that need whole‐slide scanning or even larger regions, such as Petri dishes and tissue microarrays. The subcategory of microscopes included in this section seeks to meet these needs. Therefore, these microscopes must be equipped with automatic positioning systems of wide range (greater than 10 cm). These positioning systems are responsible for the elevated cost of commercial systems. This makes it difficult to propose low‐cost alternatives with acceptable performance. To achieve an affordable cost, some developments of open projects with this purpose rely on electromechanical positioning systems derived or adapted from entry 3D printers, as shown in Figure 8a after the work by Salido et al., 2021.
FIGURE 8.
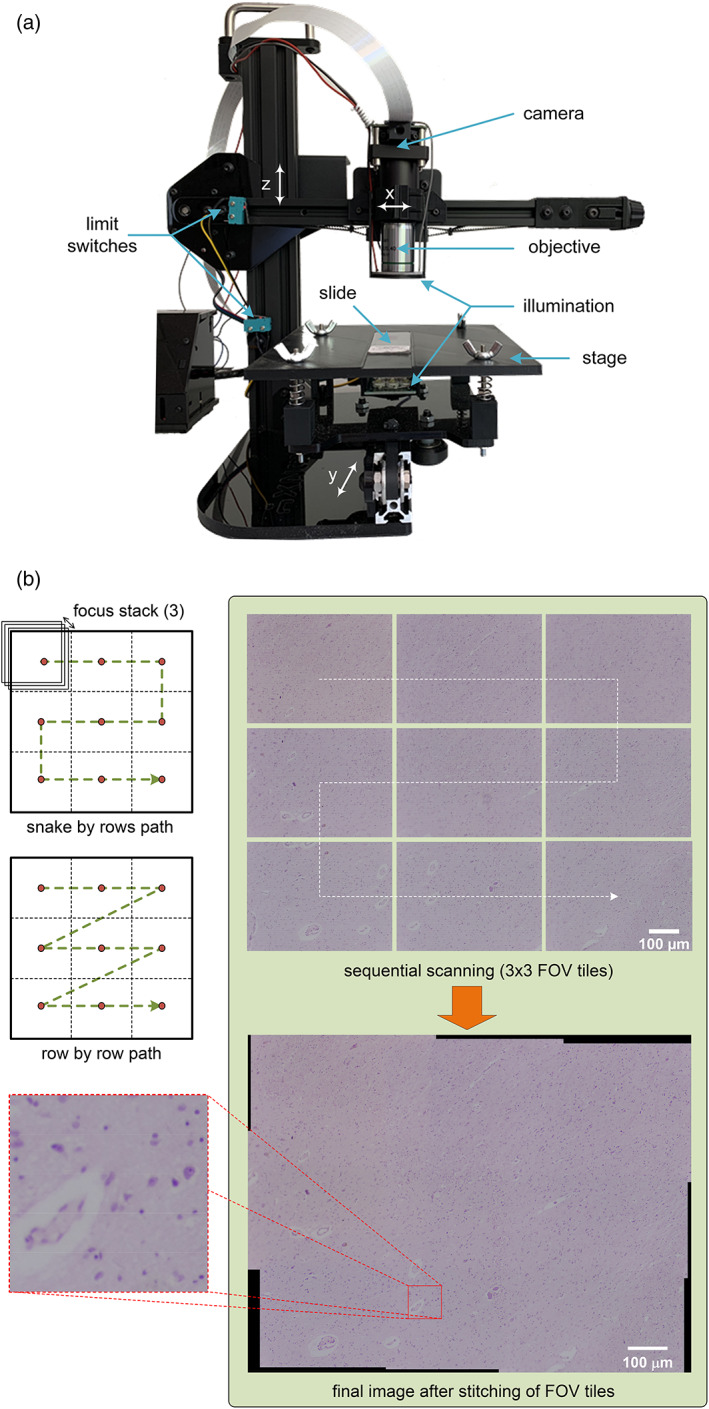
(a) MicroHikari3D automated microscopy platform for whole slide scanning; (b) example of sequential scanning and final image after stitching of tiles with MicroHikari3D
Within this type of microscope, there is no solution with a level of maturity comparable to that reached in other categories such as the Foldscope and OpenFlexure projects. To reach a similar level of maturity, it is necessary for the proposed projects to advance in the modularity of their solutions. In addition, they need to improve the availability of auxiliary support material to facilitate the reproducibility of the prototypes, along with detailed instructions for their assembly. It should also be noted that MAMs require the most complex development of software for image acquisition and processing with high quality end results. Thus, in these projects, the software modules for focus stacking, scan control, and mosaic stitching are paramount. Figure 8a,b shows a microscope belonging to the MAM‐WSI+ category, and the final image by prior whole slide sequential scanning followed by stitching of the individual tiles acquired for every single FOV (Salido et al., 2021).
Pros
Automated 3D motion for stage and focus (x–y/z).
Long range scanning to acquire WSI and bigger regions (e.g., Petri dishes).
Flexible illumination systems.
Cons
Moderate to high assembly complexity.
Compactness and portability.
3. DISCUSSION
To carry out the review presented in this manuscript, we did a bibliographic investigation. This research tried to identify published works under open access, in the last decade, on low‐cost microscopy with truly impact promoting the Open‐Science development. A fundamental requirement was that the papers were available under Open Access modality and that they had somehow high/moderate impact by citation in works on the field. Although the number of works under study was higher, Table 1 displays the final 23 selected papers. In this list, the work by Breslauer et al. (2009) is prior to the fixed time range, however it was included because of its high impact and for constituting one of the pioneering works in the area under study. The 23 papers were classified attending the criteria described in the previous sections. Thus, 13 of 23 papers correspond to category PFM with subcategories BL (4 papers), adapted lenses (AL) (4 papers) and objective plus eyepiece (OE) (5 papers). The other 10 of 13 papers under study correspond to MAM category, with subcategories WSI− (7 papers) and WSI+ (3 papers). The results of the qualitative analysis are described as follows:
- There are a considerable number of early works carried out in the category of PFM. The most plausible reasons for this proliferation are:
- The availability of COTS components as lenses and their cheap price (e.g., BLs).
- Simplicity of crafting coupling and housing for optical, image, and illumination systems using 3D printing.
- Ease of system mounting over mobile phone cameras.
- Quite low cost (in many cases under 20 USD) even including the mobile phone.
The optical quality of PFM is quite limited but suitable for applications in educative contexts and diffusion of Bio Sciences. Moreover, they have been applied for medical diagnosis and screening of neglected tropical diseases, although their results showed low sensitivity and high specificity.
The category of MAM microscopes faces a major challenge with the automatic control of stage movement and autofocus (x–y/z) when using low‐cost COTS components. Therefore, the projects classified as MAM‐WSI− have reached a greater degree of maturity and flexibility because of their more restricted motion. In this category, the more mature projects are OpenFlexure and UC2.
The degree of adoption of Open Science practices relying on open hardware/software in the different projects is uneven, but in each category of microscopes it is possible to identify these prominent representatives: Foldscope (PFM with BL [PFM‐BL]) and OpenFlexure (MAM‐WSI−). The prominence of the selected techniques described in this paper relies on not only the widespread use (number of references) but also the existence of a user community behind as in the case of the Openflexure. In the case of Foldscope, although it is not a strictly open‐source solution, it constitutes a cost‐effective microscope with over a million of copies around the world that allowed to create a global community of microscopists to share experiences and data, promoting Open‐Science.
Currently, the adoption of Open Science practices in projects related to MAM‐WSI+ is in a quite immature state, with laudable efforts that require greater modularity and generality to create synergies with others. In general, the final costs in these projects are underestimated, since only the cost of the components of the prototype is considered in their evaluation excluding the great investment in engineering required for its development.
-
It is observed a wide range of real applications for the developed microscopes. In the case of PFM, the verified applications for the equipment proposed in works of our study are classified in three groups:
- Educative purpose. They are used as training tools in subjects related to life sciences.
- Assessment of water quality. It is achieved through the detection of parasites capable of causing infections and quantifying the species of microalgae present.
- Diagnosis of diseases caused by parasites transmitted by water, food, and insects (e.g., malaria, schistosomiasis, American trypanosomiasis—Chagas—etc.).
Regarding the MAM, its validity has been verified in applications that can be grouped depending on the range of movement of the microscope stage:- Generic multimodal microscopy.
- Physiological imaging of incubated cells in microplates (e.g., pharmacological response).
- Tissue microarray observation for pathological analysis (e.g., breast cancer).
TABLE 1.
Selected works for the review
| Type | Year | Authors | DOI |
|---|---|---|---|
| PFM with adapted objective and eyepiece (PFM‐OE) | 2009 | Breslauer, Maamari, Switz, Lam and Fletcher | 10.1371/journal.pone.0006320 |
| PFM with ball lens (PFM‐BL) | 2011 | Smith, Chu, Espenson, Rahimzadeh, Gryshuk, Molinaro, Dwyre, Lane, Matthews and Wachsmann‐Hogiu | 10.1371/journal.pone.0017150 |
| PFM with adapted lens (PFM‐AL) | 2014 | Switz, D'Ambrosio and Fletcher | 10.1371/journal.pone.0095330 |
| PFM‐BL | 2014 | Cybulski, Clements and Prakash | 10.1371/journal.pone.0098781 |
| PFM‐OE | 2014 | Skandarajah, Reber, Switz and Fletcher | 10.1371/journal.pone.0096906 |
| PFM‐AL | 2016 | Coulibaly, Ouattara, D'Ambrosio, Fletcher, Keiser, Utzinger, N'Goran, Andrews and Bogoch | 10.1371/journal.pntd.0004768 |
| PFM‐OE | 2016 | Kim, Gerber, Chiu, Lee, Cira, Xia and Riedel‐Kruse | 10.1371/journal.pone.0162602 |
| PFM‐AL | 2017 | Sung, Campa and Shih | 10.1364/boe.8.005075 |
| PFM‐BL | 2018 | Orth, Wilson, Thompson and Gibson | 10.1038/s41598‐018‐21543‐2 |
| PFM‐BL | 2018 | Zeng, Jin, Li, Liu, Li, Li and Li | 10.1016/j.sna.2018.03.009 |
| PFM‐OE | 2018 | Sun and Hu | 10.1016/j.bios.2017.08.025 |
| PFM‐AL | 2019 | Rabha, Sarmah and Nath | 10.1111/jmi.12829 |
| PFM‐OE | 2020 | Zhu, Pirovano, O'Neal, Gong, Kulkarni, Nguyen, Brand, Reiner and Kang | 10.1364/boe.11.000089 |
| MAM‐WSI− | 2018 | Lu, Liu, Xiao, Hu, Zhang, Xu, Chu, Xu and Smith | 10.1371/journal.pone.0194063 |
| MAM‐WSI− | 2019 | Guo, Bian, Jiang, Murphy, Zhu, Wang, Song, Shao, Zhang and Zheng | 10.1364/ol.45.000260 |
| MAM‐WSI+ | 2019 | Gürkan and Gürkan | 10.1109/access.2019.2914958 |
| MAM‐WSI− | 2020 | Collins, Knapper, Stirling, Mduda, Mkindi, Mayagaya, Mwakajinga, Nyakyi, Sanga, Carbery, White, Dale, Lim, Baumberg, Cicuta, McDermott, Vodenicharski and Bowman | 10.1364/boe.385729 |
| MAM‐WSI− | 2020 | Courtney, Alvey, Merces, Burke and Pickering | 10.1098/rsos.191949 |
| MAM‐WSI− | 2020 | Diederich, Lachmann, Carlstedt, Marsikova, Wang, Uwurukundo, Mosig and Heintzmann | 10.1038/s41467‐020‐19,447‐9 |
| MAM‐WSI− | 2020 | Li, Krishnamurthy, Li, Vyas, Akireddy, Chai and Prakash | 10.1101/2020.12.28.424613 |
| MAM‐WSI+ | 2020 | Katunin, Cadby and Nikolaev | 10.1101/2020.07.02.185454 |
| MAM‐WSI− | 2021 | Wincott, Jefferson, Dobbie, Booth, Davis and Parton | 10.12688/wellcomeopenres.16536.1 |
| MAM‐WSI+ | 2021 | Merces, Kennedy, Lenoci, Reynaud, Burke and Pickering | 10.12688/10.1016/j.ohx.2021.e00189 |
4. CONCLUSION
This work constitutes a review of the projects carried out over the last decade on the development of open optical microscopy solutions based on smartphones and open hardware/software for the development and promotion of Open Science. After the mentioned review, in this manuscript we offer a classification of the microscopy solutions into two broad categories: PFM, and MAM. Within the first category, we distinguished three subcategories based on the type of optical system they use: BL, AL, and constituted by adapted OE. We divided the MAM category into two subcategories, depending on the range of motion for the automated stage (WSI− and WSI+). Because of PFM simplicity, they are earlier solutions with a considerable number of available successful instances at an extremely low cost. Their main application states in the educational field for promoting Bio Sciences. Despite their high specificity values, their application in healthcare in POCs for neglected disease detection should still improve results in terms of sensitivity. The development of MAM microscopes has been boosted in recent years with the advances in 3D printing technologies as a prototyping tool and improvements in automated motion control with low‐cost open hardware. However, this type of microscopes with scanning capabilities for whole slide imaging still needs to improve its modularity to bring together the efforts in a common line of work.
The two works that stand out in each one of the categories for adopting Open Science practices and constituting solutions of significant impact are Foldscope (PFM‐BL) and OpenFlexure (AMM‐WSI−), because they had created a global community around them. For instance, Foldscope is being followed by 62,793 users in on facebook, and 6073 on twitter (see social communities links in foldscope.com and openflexure.org).
AUTHOR CONTRIBUTIONS
Gabriel Cristobal: Conceptualization; supervision; validation. Gloria Bueno: Conceptualization; methodology; resources. Jesus Ruiz‐Santaquiteria: Validation. Jesus Salido: Conceptualization; investigation; methodology.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported in part by Junta de Comunidades de Castilla‐La Mancha under project HIPERDEEP (Ref. SBPLY/19/180501/000273).
Salido, J. , Bueno, G. , Ruiz‐Santaquiteria, J. , & Cristobal, G. (2022). A review on low‐cost microscopes for Open Science. Microscopy Research and Technique, 85(10), 3270–3283. 10.1002/jemt.24200
Review Editor: Alberto Diaspro
Funding information Junta de Comunidades de Castilla‐La Mancha, Grant/Award Number: SBPLY/19/180501/000273
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the repositories linked to any of the works included in this review.
REFERENCES
- Aidukas, T. , Eckert, R. , Harvey, A. R. , Waller, L. , & Konda, P. C. (2019). Low‐cost, sub‐micron resolution, wide‐field computational microscopy using opensource hardware. Scientific Reports, 9, 7457. 10.1038/s41598-019-43845-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann, S. , von Witzleben, M. , & Breuer, S. (2019). 3D‐printable portable open‐source platform for low‐cost lens‐less holographic cellular imaging. Scientific Reports, 9, 11260. 10.1038/s41598-019-47689-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellina, L. , & Missoni, E. (2009). Mobile cell‐phones (M‐phones) in telemicroscopy: Increasing connectivity of isolated laboratories. Diagnostic Pathology, 4, 19. 10.1186/1746-1596-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslauer, D. N. , Maamari, R. N. , Switz, N. A. , Lam, W. A. , & Fletcher, D. A. (2009). Mobile phone based clinical microscopy for global health applications. PLoS One, 4, e6320. 10.1371/journal.pone.0006320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesaretti, M. , Gal, J. , Bouveyron, C. , Diaspro, A. , Fontas, E. , Antonini, A. , Anty, R. , Iannelli, A. , & Patouraux, S. (2020). Accurate assessment of nonalcoholic fatty liver disease lesions in liver allograft biopsies by a smartphone platform: A proof of concept. Microscopy Research and Technique, 83(9), 1025–1031. 10.1002/jemt.23478 [DOI] [PubMed] [Google Scholar]
- Chagas, A. M. (2018). Haves and have nots must find a better way: The case for open scientific hardware. PLoS Biology, 16, e3000014. 10.1371/journal.pbio.3000014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, J. T. , Knapper, J. , McDermott, S. J. , Ayazi, F. , Bumke, K. E. , Stirling, J. , & Bowman, R. W. (2021). Simplifying the OpenFlexure microscope software with the web of things. Royal Society Open Science, 8, 1–11. 10.1098/rsos.211158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, J. T. , Knapper, J. , Stirling, J. , Mduda, J. , Mkindi, C. , Mayagaya, V. , Mwakajinga, G. A. , Nyakyi, P. T. , Sanga, V. L. , Carbery, D. , White, L. , Dale, S. , Lim, Z. J. , Baumberg, J. J. , Cicuta, P. , McDermott, S. , Vodenicharski, B. , & Bowman, R. (2020). Robotic microscopy for everyone: The OpenFlexure microscope. Biomedical Optics Express, 11, 2447–2460. 10.1364/BOE.385729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly, J. T. , Ouattara, M. , D'Ambrosio, M. V. , Fletcher, D. A. , Keiser, J. , Utzinger, J. , N'Goran, E. K. , Andrews, J. R. , & Bogoch, I. I. (2016). Accuracy of mobile phone and handheld light microscopy for the diagnosis of schistosomiasis and intestinal protozoa infections in Côte d'Ivoire. PLoS Neglected Tropical Diseases, 10, e0004768. 10.1371/journal.pntd.0004768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney, A. , Alvey, L. M. , Merces, G. O. T. , Burke, N. , & Pickering, M. (2020). The Flexiscope: A low cost, flexible, convertible and modular microscope with automated scanning and micromanipulation. Royal Society Open Science, 7, 191949. 10.1098/rsos.191949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski, J. S. , Clements, J. , & Prakash, M. (2014). Foldscope: Origami‐based paper microscope. PLoS One, 9, e98781. 10.1371/journal.pone.0098781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacal, E. , Bermejo‐Peláez, D. , Lin, L. , Álamo, E. , Cuadrado, D. , Martínez, Á. , Mousa, A. , Postigo, M. , Soto, A. , Sukosd, E. , Vladimirov, A. , Mwandawiro, C. , Gichuki, P. , Williams, N. A. , Muñoz, J. , Kepha, S. , & Luengo‐Oroz, M. (2021). Mobile microscopy and telemedicine platform assisted by deep learning for the quantification of Trichuris trichiura infection. PLoS Neglected Tropical Diseases, 15, e0009677. 10.1371/journal.pntd.0009677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich, B. , Lachmann, R. , Carlstedt, S. , Marsikova, B. , Wang, H. , Uwurukundo, X. , Mosig, A. S. , & Heintzmann, R. (2020). A versatile and customizable low‐cost 3D‐printed open standard for microscopic imaging. Nature Communications, 11, 5979. 10.1038/s41467-020-19447-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, S. , Guo, K. , Nanda, P. , Shiradkar, R. , & Zheng, G. (2014). FPscope: A field‐portable high‐resolution microscope using a cellphone lens. Biomedical Optics Express, 5, 3305–3310. 10.1364/boe.5.003305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosemagen, S. , Liboiron, M. , & Molloy, J. (2017). Gathering for Open Science hardware 2016. Journal of Open Hardware, 1, 1–5. 10.5334/joh.5 [DOI] [Google Scholar]
- Garcia‐Soto, C. , Seys, J. J. C. , Zielinski, O. , Busch, J. A. , Luna, S. I. , Baez, J. C. , Domegan, C. , Dubsky, K. , Kotynska‐Zielinska, I. , Loubat, P. , Malfatti, F. , Mannaerts, G. , McHugh, P. , Monestiez, P. , van der Meeren, G. I. , & Gorsky, G. (2021). Marine citizen science: Current state in Europe and new technological developments. Frontiers in Marine Science, 8, 1–13. 10.3389/fmars.2021.621472 [DOI] [Google Scholar]
- Grier, Z. , Soddu, M. F. , Kenyatta, N. , Odame, S. A. , Sanders, J. I. , & Wright, L. P. (2018). A low‐cost do‐it‐yourself microscope kit for hands‐on science education. In Gregory G. G. (Ed.), Optics Education and Outreach V. SPIE. 10.1117/12.2320655 [DOI] [Google Scholar]
- Guo, C. , Bian, Z. , Jiang, S. , Murphy, M. , Zhu, J. , Wang, R. , Song, P. , Shao, X. , Zhang, Y. , & Zheng, G. (2019). OpenWSI: A low‐cost, high‐throughput whole slide imaging system via single‐frame autofocusing and open‐source hardware. Optics Letters, 45, 260. 10.1364/ol.45.000260 [DOI] [Google Scholar]
- Gürkan, G. , & Gürkan, K. (2019). Incu‐stream 1.0: An open‐hardware live‐cell imaging system based on inverted bright‐field microscopy and automated mechanical scanning for real‐time and long‐term imaging of microplates in incubator. IEEE Access, 7, 58764–58779. 10.1109/ACCESS.2019.2914958 [DOI] [Google Scholar]
- Hasan, M. M. , Alam, M. W. , Wahid, K. A. , Miah, S. , & Lukong, K. E. (2016). A low‐cost digital microscope with real‐time fluorescent imaging capability. PLoS One, 11, e0167863. 10.1371/journal.pone.0167863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch, M. (2014). The maker movement manifesto: Rules for innovation in the new world of crafters, hackers, and tinkerers. McGraw‐Hill Education. [Google Scholar]
- Katunin, P. , Cadby, A. and Nikolaev, A. (2020) An open‐source experimental framework for automation of high‐throughput cell biology experiments. bioRxiv . 10.1101/2020.07.02.185454. [DOI] [PMC free article] [PubMed]
- Kim, H. , Gerber, L. C. , Chiu, D. , Lee, S. A. , Cira, N. J. , Xia, S. Y. , & Riedel‐Kruse, I. H. (2016). LudusScope: Accessible interactive smartphone microscopy for life‐science education. PLoS One, 11, e0162602. 10.1371/journal.pone.0162602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Krishnamurthy, D. , Li, E. , Vyas, P. , Akireddy, N. , Chai, C. and Prakash, M. (2020) Squid: Simplifying quantitative imaging platform development and deployment. bioRxiv . 10.1101/2020.12.28.424613. [DOI]
- Lu, Q. , Liu, G. , Xiao, C. , Hu, C. , Zhang, S. , Xu, R. X. , Chu, K. , Xu, Q. , & Smith, Z. J. (2018). A modular, open‐source, slide‐scanning microscope for diagnostic applications in resource‐constrained settings. PLoS One, 13, e0194063. 10.1371/journal.pone.0194063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott, S. , Ayazi, F. , Collins, J. , Knapper, J. , Stirling, J. , Bowman, R. and Cicuta, P. (2021) Multi‐modal microscopy imaging with the OpenFlexure Delta Stage. arXiv arXiv:2112.05804. [DOI] [PubMed]
- Merces, G. O. , Kennedy, C. , Lenoci, B. , Reynaud, E. G. , Burke, N. , & Pickering, M. (2021). The incubot: A 3D printer‐based microscope for long‐term live cell imaging within a tissue culture incubator. HardwareX, 9, e00189. 10.1016/j.ohx.2021.e00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison, A. , & Gardner, J. (2014). Smart phone microscopic photography: A novel tool for physicians and trainees. Archives of Pathology & Laboratory Medicine, 138(8), 1002. 10.5858/arpa.2013-0425-ED [DOI] [PubMed] [Google Scholar]
- Naqvi, A. , Manglik, N. , Dudrey, E. , Perry, C. , Mulla, Z. D. , & Cervantes, J. L. (2020). Evaluating the performance of a low‐cost mobile phone attachable microscope in cervical cytology. BMC Women's Health, 20, 60. 10.1186/s12905-020-00902-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth, A. , Wilson, E. R. , Thompson, J. G. , & Gibson, B. C. (2018). A dual‐mode mobile phone microscope using the onboard camera flash and ambient light. Scientific Reports, 8, 3298. 10.1038/s41598-018-21543-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabha, D. , Sarmah, A. , & Nath, P. (2019). Design of a 3D printed smartphone microscopic system with enhanced imaging ability for biomedical applications. Journal of Microscopy, 276, 13–20. 10.1111/jmi.12829 [DOI] [PubMed] [Google Scholar]
- Rajchgot, J. , Coulibaly, J. T. , Keiser, J. , Utzinger, J. , Lo, N. C. , Mondry, M. K. , Andrews, J. R. , & Bogoch, I. I. (2017). Mobile‐phone and handheld microscopy for neglected tropical diseases. PLoS Neglected Tropical Diseases, 11, e0005550. 10.1371/journal.pntd.0005550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed, M. A. , & Jabbar, A. (2018). “Smart diagnosis” of parasitic diseases by use of smartphones. Journal of Clinical Microbiology, 56. 10.1128/jcm.01469-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salido, J. , Sánchez, C. , Ruiz‐Santaquiteria, J. , Cristóbal, G. , Blanco, S. , & Bueno, G. (2020). A low‐cost automated digital microscopy platform for automatic identification of diatoms. Applied Sciences, 10, 6033. 10.3390/app10176033 [DOI] [Google Scholar]
- Salido, J. , Toledano, P. T. , Vallez, N. , Deniz, O. , Ruiz‐Santaquiteria, J. , Cristobal, G. , & Bueno, G. (2021). MicroHikari3D: An automated DIY digital microscopy platform with deep learning capabilities. Biomedical Optics Express, 12, 7223–7243. 10.1364/BOE.439014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey, J. P. , Foo, D. C. W. , Kabla, A. , Baumberg, J. J. , & Bowman, R. W. (2016). A one‐piece 3D printed flexure translation stage for open‐source microscopy. Review of Scientific Instruments, 87, 025104. 10.1063/1.4941068 [DOI] [PubMed] [Google Scholar]
- Skandarajah, A. , Reber, C. D. , Switz, N. A. , & Fletcher, D. A. (2014). Quantitative imaging with a mobile phone microscope. PLoS One, 9, e96906. 10.1371/journal.pone.0096906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, Z. J. , Chu, K. , Espenson, A. R. , Rahimzadeh, M. , Gryshuk, A. , Molinaro, M. , Dwyre, D. M. , Lane, S. , Matthews, D. , & Wachsmann‐Hogiu, S. (2011). Cell‐phone‐based platform for biomedical device development and education applications. PLoS One, 6, e17150. 10.1371/journal.pone.0017150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, C. , & Giannini, J. (2016). Inexpensive, open source epifluorescence microscopes. Journal of Chemical Education, 93, 1310–1315. 10.1021/acs.jchemed.5b00984 [DOI] [Google Scholar]
- Sun, D. , & Hu, T. Y. (2018). A low cost mobile phone dark‐field microscope for nanoparticle‐based quantitative studies. Biosensors and Bioelectronics, 99, 513–518. 10.1016/j.bios.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung, Y. , Campa, F. , & Shih, W.‐C. (2017). Open‐source do‐it‐yourself multi‐color fluorescence smartphone microscopy. Biomedical Optics Express, 8, 5075–5086. 10.1364/BOE.8.005075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switz, N. A. , D'Ambrosio, M. V. , & Fletcher, D. A. (2014). Low‐cost mobile phone microscopy with a reversed mobile phone camera lens. PLoS One, 9, e95330. 10.1371/journal.pone.0095330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wincott, M. , Jefferson, A. , Dobbie, I. M. , Booth, M. J. , Davis, I. , & Parton, R. M. (2021). Democratising microscopi: A 3d printed automated XYZT fluorescence imaging system for teaching, outreach and fieldwork. Wellcome Open Research, 6, 63. 10.12688/wellcomeopenres.16536.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman, A. J. M. , Nudd, R. , Hedlund, E. G. , & Leake, M. C. (2015). From animaculum to single molecules: 300 years of the light microscope. Open Biology, 5, 150019. 10.1098/rsob.150019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, A. , Wisinski, J. , Osmundson, T. , Sanderfoot, A. , Cooper, S. , & Klein, J. (2022). Instructional innovations in college‐level molecular bioscience labs during the pandemic‐induced shift to online learning. Education in Science, 12, 230. 10.3390/educsci12040230 [DOI] [Google Scholar]
- Zeng, Y. , Jin, K. , Li, J. , Liu, J. , Li, J. , Li, T. , & Li, S. (2018). A low cost and portable smartphone microscopic device for cell counting. Sensors and Actuators A: Physical, 274, 57–63. 10.1016/j.sna.2018.03.009 [DOI] [Google Scholar]
- Zhu, W. , Pirovano, G. , O'Neal, P. K. , Gong, C. , Kulkarni, N. , Nguyen, C. D. , Brand, C. , Reiner, T. , & Kang, D. (2020). Smartphone epifluorescence microscopy for cellular imaging of fresh tissue in low‐resource settings. Biomedical Optics Express, 11, 89–98. 10.1364/boe.11.000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in the repositories linked to any of the works included in this review.


