Abstract
Objectives
The survival rates for patients affected by aneurysmal subarachnoid hemorrhage (aSAH) have increased in recent years; however, many patients continue to develop cognitive dysfunctions that affect their quality of life. The commonly used outcome measures often fail to identify these cognitive dysfunctions. This study aimed to evaluate the long‐term outcomes at 1 and 3 years after aSAH to assess changes over time and relate outcomes to patient characteristics and events during the acute phase.
Materials and Methods
This prospective observational study included patients that experienced aSAH. Patients were assessed according to the extended Glasgow Outcome Scale, Life Satisfaction Questionnaire, Mayo‐Portland Adaptability inventory‐4, and Mental Fatigue scale.
Results
Patients were assessed after 1 year (n = 62) and 3 years (n = 54). At 3 years, the extended Glasgow Outcome Scale score improved in 15% and worsened in 12% of the patients. Mental fatigue was observed in 57% of the patients at 1 year. Patients <60 years of age at the time of aSAH had more self‐assessed problems, including pain/headache (p < .01), than patients >60 years of age. Patients with delayed cerebral ischemia during the acute phase reported more dissatisfaction at 3 years, whereas no significant result was seen at 1 year.
Conclusions
Cognitive dysfunction, especially mental fatigue, is common in patients with aSAH, which affects quality of life and recovery. Patient outcome is a dynamic process developing throughout years after aSAH, involving both improvement and deterioration. This study indicates the importance of longer follow‐up periods with broad outcome assessments.
Keywords: aneurysmal subarachnoid hemorrhage, cognitive dysfunction, long‐term outcome, mental fatigue
1. INTRODUCTION
Although the incidence of aneurysmal subarachnoid hemorrhage (aSAH) is relatively low (6–9/100,000), the economic burden imposed by aSAH on the affected patients and society is high. 1 , 2 Younger age at onset in combination with the relatively high mortality and morbidity rates results in years of life lost, similar to that in ischemic or hemorrhagic stroke. 3 However, in recent decades, the mortality rate has decreased owing to improvements in treatment strategies and risk factor control. 4 Risk factors for poor outcomes after aSAH (death or low functional status) are related to poor neurological status at admission, radiological grading of initial bleeding, age, development of delayed cerebral ischemia (DCI), and hydrocephalus with an external ventricular drain (EVD). 5 , 6 , 7
Survivors of aSAH commonly develop cognitive deficits, which may negatively influence their quality of life in the short and long term. 8 , 9 , 10 Even patients who are considered to have good functional outcomes can have subtle, yet burdensome cognitive symptoms affecting their ability to return to the life as it was before aSAH. 11 , 12 , 13 Commonly used outcome measures, such as the extended Glasgow Outcome Scale (GOSE) 14 or modified Ranking Scale (mRS), 15 are not always sufficient for detecting these cognitive deficits. A recent systematic review of 65 studies that included 6832 patients evaluated the presence of cognitive deficits in survivors of aSAH. 16 The results indicated that 40%–70% of all patients who had survived an aSAH experienced a wide range of cognitive impairments.
The primary aim of this study was to prospectively evaluate long‐term outcomes after aSAH in a well‐defined cohort. Patients were followed up from the time of ictus and assessed at 1 and 3 years after the aSAH using several outcome assessment tools. In addition to GOSE, we used three outcome assessment tools, namely the Fugl‐Meyer Life Satisfaction Questionnaire (LiSAT‐11), 17 , 18 the Mayo‐Portland Adaptability inventory‐4 (MPAI‐4), 19 and the Mental Fatigue Scale (MFS). 20 These outcome assessment tools were chosen because they all have well‐documented psychometric properties, and together, they cover the most important physical, cognitive, emotional, behavioral, and social problems that patients may encounter after aSAH. Secondary aims were to assess the decline and improvement over time (between 1 and 3 years) and relate outcomes to patient characteristics and events during the acute phase.
2. MATERIALS AND METHODS
2.1. Study design and population
This was a prospective observational cohort study that adhered to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines. 21 Patients with aSAH admitted to Sahlgrenska University Hospital, Gothenburg, Sweden, between May 2015 and October 2016 were consecutively screened for eligibility. Follow‐up was performed at 1 and 3 years after the onset of aSAH. The inclusion criteria consisted of an age ≥18 years and a confirmed diagnosis of an aSAH on digital subtraction angiography. Patients with a previous history of stroke or other brain injuries were excluded as were those with arterial fibrillation and/or pacemakers because heart rate variability was assessed during the acute phase after the aSAH as part of another study. 22 , 23
Patients were treated in a neurointensive care unit (NICU) in accordance with a local protocol, mostly consistent with the American Heart Association/American Stroke Association guidelines for the management of aSAH. 24 Cerebral aneurysms were usually secured within 24 h after admission with either surgical clipping or endovascular coiling. Nimodipine (Nimotop®, Bayer) was intravenously administered according to the local protocol. Hydrocephalus was treated with an EVD. DCI was defined as the presence of focal neurological impairment or global neurological impairment defined as a two‐point drop on the Glasgow Coma Scale (GCS) 25 lasting for at least 1 h, and/or the occurrence of a cerebral infarction on computer tomography or magnetic resonance imaging, not attributed to other causes. 26
2.2. Ethics approval
The study adheres to the Declaration of Helsinki, and the study protocol was approved by the Ethical Regional Board of Gothenburg, Sweden (053‐15, T 213‐18). Written informed consent was obtained from all patients or their next of kin before their participation in the study.
2.3. Data collection
Data regarding age, sex, medical history, and imaging were all documented at admission or collected from the case records. The GCS was scored at the emergency department of the primary receiving hospital. Patients were then scored upon admission to the NICU by the attending neurosurgeon according to the Hunt and Hess classification 27 and the World Federation of Neurological Surgeons scale. 28 The amount of subarachnoid blood on initial imaging was evaluated and scored according to the modified Fisher's scale. 29 Treatment of the aneurysm with surgery or endovascular coiling, development of hydrocephalus, and treatment with EVD or development of DCI during the NICU period were documented. Aneurysm location was dichotomized into anterior or posterior circulation. Patients were observed upon follow‐up and scored using the GOSE 14 at 1 and 3 years after ictus with a structured telephone interview. In addition to GOSE, we used three different self‐assessment questionnaires, namely the LiSAT‐11, 18 MPAI‐4, 19 and MFS. 20 The questionnaires were sent by mail, and the patients were reminded to return the questionnaires up to three times when necessary.
2.4. Outcome measures
The GOSE is an 8‐point scale representing levels of function (neurological deficits and day‐to‐day living) ranging from death (1 point) to good recovery (8 points). GOSE 8 (good recovery) indicates full recovery or minor symptoms that do not affect activities of daily living (ADLs). The GOSE was further dichotomized into favorable outcome (GOSE 5–8) and unfavorable outcome (GOSE 1–4). 30 The self‐assessment questionnaires used for this study are shown in Table 1.
TABLE 1.
Self‐assessment questionnaires
| Questionnaire and question content | Answer options |
|---|---|
Life Satisfaction Questionnaire 11 (LiSat‐11)
a
|
|
|
The Mayo‐Portland Adaptability Inventory (MPAI‐4) c Part A—Abilities (Question 1 to 12) 1.Mobility, 2. Use of hands, 3. Vision 4. Audition, 5. Dizziness, 6. Motor speech, 7A. Verbal communication, 7B. Nonverbal communication, 8. Attention/concentration, 9. Memory, 10. Fund of information, 11. Novel problem‐solving, 12. Visuospatial abilities Part B—Adjustment (Questions 13 to 21) 13. Anxiety, 14. Depression, 15. Irritability, anger, aggression 16. Pain/headache, 17. Fatigue, 18. Sensitivity to mild symptoms, 19. Inappropriate social interaction, 20. Impaired self‐awareness, 21. Family/significant relationships Part C—Participation (Question 22 to 29) 22. Initiation, 23. Social contacts (not family), 24. Leisure and recreational activities, 25. Self‐care, 26. Residence, 27. Transportation, 28A. Paid employment, 28B. Other employment, 29. Managing money and finances |
For questions 1 to 22, 25–27, 29: 0 = none, 1 = mild problems no interference with activities, 2 = mild problems with interference 5–24% of the time, 3 = moderate problems with interference 25–75% of the time, 4 = severe problems with interference >75% of the time For questions 23 and 24‐ 0 = normal involvement/participation, 1 = mild difficulty with involvement/participation, 2 = mildly limited 75–95% normal involvement/participation, 3 = moderately limited, 25–75% normal involvement/participation. 4 = no or rare involvement/participation For question 28A, 28B 0 = Full time, 1 = Part time (3–30 hours/wk) without support, 2 = Full‐time or part‐time with support, 3 = sheltered work/supervised environment, 4 = unemployment/inactive |
Mental Fatigue Scale (MFS)
d
|
0. No problems/discomfort 0.5 No to some mild problems/discomfort 1.0 Mild problems/discomfort 1.5 Mild to moderate problems/discomfort 2.0 Moderate problems/discomfort 2.5 Moderate–severe problems/discomfort 3.0 Severe problems/discomfort A total score of ≥10.5 indicate mental fatigue |
Note: Self‐assessment questionnaires used for evaluation at 1 and 3 years.
Life Satisfaction Questionnaire (LiSAT‐11) according to Fugl‐Meyer assessment.
Activity of daily living.
Mayo‐Portland Adaptability Inventory‐4 in which parts A to C were used in this study.
Mental Fatigue Scale.
Outcomes at 1 and 3 years and differences between the two outcomes were studied using the GOSE and all items included in the LiSAT‐11, MPAI‐4, and MFS. For statistical analysis, the answer options of the LiSAT‐11 were dichotomized as “not satisfied” (very dissatisfied, dissatisfied, and rather dissatisfied) and “satisfied” (rather satisfied, satisfied, and very satisfied). For the MPAI‐4, the answers were dichotomized as “no problems” (no to mild problems with no interference with activities) and “problems” (mild problems with interference with activities to moderate and severe problems). For the MPAI‐4 questionnaire, part A (abilities) and part B (adjustments), all items were analyzed. In part C (participation), questions regarding employment and all of part D were excluded from statistical analysis owing to few answers. The maximum score for the MFS is 42, with a score ≥10.5 indicating mental fatigue. The MFS was therefore dichotomized as <10.5 (no mental fatigue) and ≥10.5 (mental fatigue). 20 , 31 In addition, the cohort was dichotomized by age of onset of aSAH (<60 years and ≥60 years), as well as the presence or absence of DCI.
2.5. Statistical analysis
Patient characteristics are presented as the median (range) for continuous data and the number and percentage for categorical variables. To compare ordered categorical variables between two groups, the Mantel–Haenszel chi‐square test was used. For continuous variables, Fisher's non‐parametric permutation test and for dichotomous variables, Fisher's exact test were used. To evaluate the association between two ordered or continuous variables, the Spearman correlation coefficient was calculated. All tests of significance were two‐sided and conducted at a significance level of 0.05. All analyses were performed with SAS version 9.4.
3. RESULTS
3.1. Patient selection
From May 2015 to October 2016, a total of 105 patients with aSAH were admitted to our institution. Patients were consecutively screened for eligibility with the exception of holiday periods. Four patients met exclusion criteria, four died shortly after admission, and from four consent could not be obtained, resulting in the inclusion of 64 patients. Additionally, two patients were lost to follow‐up; hence, 62 patients were assessed at 1 year and 54 patients at 3 years. A flowchart of the patient selection process is shown in Figure 1. Data of patient characteristics are shown in Table 2.
FIGURE 1.
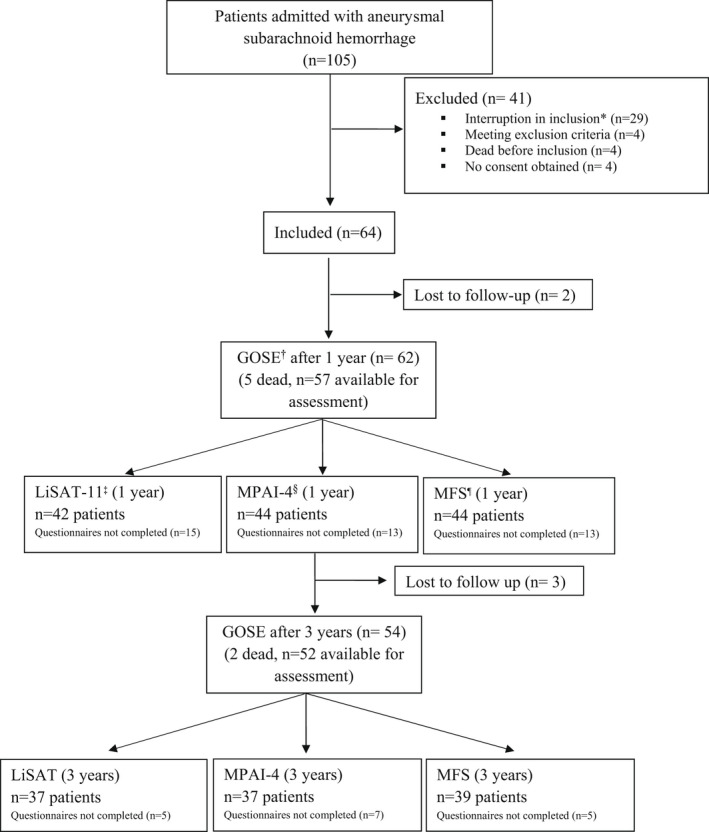
Consort flow chart. *Interruption in inclusion for logistics reasons during holidays. †Glasgow Outcome Scale extended (GOSE). ‡Life Satisfaction Checklist (LiSAT‐11) according to Fugl‐Meyer assessment. §Mayo‐Portland Adaptability Inventory‐4 (MPAI‐4). ¶Mental Fatigue Scale (MFS).
TABLE 2.
Patient characteristics
| Characteristic | GOSE | GOSE only | LiSat‐11 | MPAI‐4 | MFS | Age <60 years | Age ≥60 years |
|---|---|---|---|---|---|---|---|
| n = 62 | n = 16 | n = 42 | n = 44 | n = 44 | n = 37 | n = 25 | |
| Age, years | |||||||
| Median (range) | 58 (36–78) | 60 (47–75) | 57 (36 –76) | 56 (36–76) | 57 (36–76) | 53 (36–59) | 67 (60–78) |
| Sex | |||||||
| Female, n (%) | 46 (74) | 12 (75) | 32 (76) | 33 (75) | 33 (75) | 29 (78) | 17 (68) |
| GCS | |||||||
| Median (range) | 13 (4–15) | 13 (5–15) | 14 (4–15) | 14 (4–15) | 14 (4–15) | 13 (5–15) | 14 (5–15) |
| GCS score | |||||||
| 3–8, n (%) | 11 (18) | 6 (37) | 4 (10) | 5 (11) | 4 (9) | 6 (16) | 5 (20) |
| 9–12, n (%) | 8 (13) | 2 (13) | 5 (12) | 5 (11) | 6 (14) | 7 (19) | 1 (4) |
| 13–15, n (%) | 43 (69) | 8 (50) | 33 (78) | 34 (78) | 34 (78) | 24 (65) | 19 (76) |
| Hunt & Hess a scale | |||||||
| Median (range) | 3 (1–5) | 3 (2–5) | 3 (1–5) | 3 (1–5) | 2 (1–5) | 3 (2–5) | 2 (1–5) |
| 1–2, n (%) | 27 (44) | 5 (31) | 20 (48) | 21 (48) | 22 (50) | 12 (32) | 15 (60) |
| 3–5, n (%) | 35 (56) | 11 (69) | 22 (52) | 23 (52) | 22 (50) | 25 (68) | 10 (40) |
| WFNS b | |||||||
| Median (range) | 2 (1–5) | 3 (1–5) | 2 (1–5) | 2 (1–5) | 2 (1–5) | 2 (1–5) | 2 (1–5) |
| 1–3, n (%) | 43 (69) | 8 (50) | 33 (79) | 34 (77) | 34 (77) | 24 (65) | 19 (76) |
| 4–5, n (%) | 19 (31) | 8 (50) | 9 (21) | 10 (23) | 10 (23) | 13 (35) | 6 (24) |
| Modified Fisher scale c | |||||||
| Median (range) | 4 (0–4) | 4 (0–4) | 3 (0–4) | 3 (0–4) | 3 (0–4) | 3 (0–4) | 4 (0–4) |
| 0–2, n (%) | 6 (10) | 2 (13) | 4 (10) | 4 (9) | 4 (9) | 3 (8) | 3 (12) |
| 3–4, n (%) | 56 (90) | 14 (87) | 38 (90) | 40 (91) | 40 (91) | 34 (91) | 22 (88) |
| Aneurysm location, n (%) | |||||||
| Anterior circulation | 45 (73) | 12 (75) | 30 (71) | 31 (70) | 31 (70) | 27 (73) | 18 (72) |
| Posterior circulation | 17 (27) | 4 (25) | 12 (29) | 13 (30) | 13 (30) | 10 (27) | 7 (28) |
| Aneurysm treatment, n (%) | |||||||
| Surgical clipping | 17 (27) | 7 (44) | 8 (19) | 9 (20) | 8 (18) | 10 (27) | 5 (20) |
| Endovascular coiling | 45 (73) | 9 (56) | 34 (81) | 35 (80) | 36 (82) | 27 (73) | 19 (76) |
| DCI, n (%) | 16 (26) | 3 (19) | 12 (29) | 12 (27) | 13 (30) | 12 (32) | 4 (16) |
| EVD, n (%) | 32 (52) | 12 (75) d | 18 (43) | 19 (43) | 19 (43) | 19 (51) | 13 (52) |
| GOSE median (range) | 5 (1–8) | 3 (1–7) e | 6 (3–8) | 6 (3–8) | 6 (3–8) | 5 (1–8) | 5 (1–8) |
| 1, n (%) | 5 (8) | 5 (31) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 4 (16) |
| 2–4, n (%) | 13 (21) | 7 (44) | 5 (12) | 6 (14) | 6 (14) | 9 (24) | 4 (16) |
| 5–8, n (%) | 44 (71) | 4 (25) | 37 (88) | 38 (86) | 38 (86) | 27 (73) | 17 (68) |
Note: Characteristics of patients available for follow‐up at 1 year and included patients at 1 year for each questionnaire. GOSE only, patients scored according to GOSE but have not answered any questionnaire.
Abbreviations: DCI, Delayed cerebral ischemia; EVD, External ventricular drainage; GCS, Glasgow Coma Scale; GOSE, Glasgow Outcome Scale Extended; LiSat‐11, Life Satisfaction Checklist according to Fugl‐Meyer assessment; MFS, Mental Fatigue Scale; MPAI‐4, Mayo‐Portland Adaptability Inventory‐4.
Hunt and Hess scale, scored from 1, indicating mild headache, to 5, indicating coma.
World Federation of Neurological Surgeons scale, scored from 1 (GCS 15) to 5 (GCS 3–6)
Modified Fisher scale; amount of blood in the subarachnoid space, scored from 0, indicating no blood detected, to 4, indicating thick subarachnoid hemorrhage with intraventricular blood
Significance between GOSE only and GOSE with questionnaires, p‐value .04
Significance between GOSE only and GOSE with questionnaires, p‐value <.001
3.2. Follow‐up at 1 year
For the 62 patients eligible for follow‐up after 1 year, the median (range) GOSE score was 5 (1–8), whereas no patient had a GOSE score of 2. The mortality rate was 8%. Eight patients (13%) were shunt‐dependent at 1 year and demonstrated significantly poorer outcomes (GOSE 1–4) (p = .039).
The LiSAT‐11 test was completed by 42 patients, MPAI‐4 by 44 patients, and MFS by 44 patients (Figure 1). Patients who did not provide answers had lower GOSE scores. Furthermore, the median (range) GOSE scores of patients who did and did not answer the LiSAT‐11, MPAI‐4, and MFS questionnaires were 6 (3–8) and 3 (1–7), respectively (p < .0001 for all).
According to the LiSAT‐11 questionnaire, 79% of the patients graded “life as whole” as satisfying after 1 year, while 21% reported that they were dissatisfied (Table 3). The items for which >25% of the patients reported dissatisfaction included vocation, economy, leisure, and sexual life. Mild‐to‐severe problems were identified in items from the MPAI‐4 questionnaire; part A‐ability, part B‐adjustment, and part C‐participation (Table 3). The highest incidence of reported problems in the MPAI‐4 was related to fatigue, which was reported by 55% of patients. Additionally, mental fatigue assessed from the MFS questionnaire was observed in 57% of patients (Table 3).
TABLE 3.
Outcome at 1 year
| Questionnaire | Answer option n (%) | Answer option n (%) | Total answers |
|---|---|---|---|
| LiSAT‐11 a | 6–4: Very satisfied/satisfied/rather satisfied | 3–1: Rather dissatisfied/dissatisfied/very dissatisfied | |
| Life as a whole | 33 (79) | 9 (21) | 42 |
| Vocation | 25 (64) | 14 (36) | 39 |
| Economy | 29 (69) | 13 (31) | 42 |
| Leisure | 28 (68) | 13 (32) | 41 |
| Contacts | 32 (76) | 10 (24) | 42 |
| Sexual Life | 22 (59) | 16 (42) | 38 |
| ADL b | 38 (90) | 4 (10) | 42 |
| Family life | 40 (95) | 2 (5) | 42 |
| Partner Relationship | 31 (86) | 5 (14) | 36 |
| Somatic Health | 33 (79) | 9 (21) | 42 |
| Psychological Health | 32 (78) | 9 (22) | 41 |
| MPAI‐4 c | 0‐1 = no problems/mild problems no interference with activities | 2–4 = mild problems with interference/moderate problems/severe problems | Total answers |
|---|---|---|---|
| Part A—ability | |||
| Mobility | 32 (73) | 12 (27) | 44 |
| Use of hands | 33 (77) | 10 (23) | 43 |
| Vision | 39 (88) | 5 (11) | 44 |
| Audition | 39 (88) | 5 (11) | 44 |
| Dizziness | 32 (76) | 10 (24) | 42 |
| Motor speech | 37 (84) | 7 (16) | 44 |
| Verbal communication | 34 (79) | 9 (21) | 43 |
| Nonverbal communication | 37 (88) | 5 (12) | 42 |
| Attention/concentration | 30 (71) | 12 (29) | 42 |
| Memory | 30 (70) | 13 (30) | 43 |
| Fund of information | 38 (88) | 5 (12) | 43 |
| Problem solving | 33 (79) | 9 (21) | 42 |
| Visuospatial abilities | 35 (83) | 7 (17) | 42 |
| Part B—adjustment | |||
| Anxiety | 32 (73) | 12 (29) | 44 |
| Depression | 29 (66) | 15 (34) | 44 |
| Irritability/anger | 34 (79) | 9 (21) | 43 |
| Pain/headache | 34 (79) | 9 (21) | 43 |
| Fatigue | 20 (45) | 24 (55) | 44 |
| Sensitivity to mild symptoms | 32 (76) | 10 (24) | 42 |
| Inappropriate social interaction | 37 (90) | 4 (10) | 41 |
| Impaired self‐awareness | 38 (88) | 5 (12) | 43 |
| Family/significant relationships | 25 (68) | 12 (32) | 37 |
| Part C—participation | |||
| Initiation | 31 (70) | 13 (30) | 44 |
| Social contact | 35 (80) | 9 (20) | 44 |
| Leisure/recreational activity | 31 (72) | 12 (28) | 43 |
| Self‐care | 41 (93) | 3 (7) | 44 |
| Residence | 34 (77) | 10 (23) | 44 |
| Transportation | 35 (81) | 8 (19) | 43 |
| Managing money and finance | 40 (93) | 3 (7) | 43 |
| MFS d | <10.5 No mental fatigue | ≥10.5 Mental Fatigue | Total answers |
|---|---|---|---|
| 19 (43) | 25 (57) | 44 |
Note: Dichotomized outcome at 1 year according to Life Satisfaction Questionnaire (LiSAT‐11), Mayo‐Portland Adaptability Inventory (MPAI‐4), and Mental Fatigue Scale (MFS). Items for which >25% of the patients reported dissatisfaction/problems are shown in bold.
Life Satisfaction Questionnaire according to Fugl‐Meyer assessment. Answer options for LiSAT‐11 dichotomized to 6–4 (Very Satisfied/Satisfied/Rather Satisfied) and 3–1 (Rather Dissatisfied/Dissatisfied/Very dissatisfied).
Activities of daily living.
Mayo‐Portland Adaptability Inventory‐4, dichotomized to 0–1 (no problems, to mild problems with, no interference with activities) and 2–4 (mild problems with interference/moderate problems/severe problems).
Mental Fatigue Scale with mental fatigue ≥10.5 versus no mental fatigue <10.5 in total points.
3.3. Follow‐up at 3 years compared to follow‐up at 1 year
In 73% of patients, the GOSE score remained unchanged between 1 and 3 years. Meanwhile, 15% of patients improved, and 12% declined according to their GOSE score. Additionally, the mortality rate at 3 years was 11%. Comparing the results of the LiSAT‐11 and MPAI‐4 at 1 and 3 years for all items, the condition of some patients improved whereas that of others worsened. Items for which >25% of patients indicated a change are shown in Figure 2. Additionally, items for which the largest proportion of patients indicated an improvement in leisure (46%), somatic health (43%), and vocation (38%), and a decline in memory (37%), attention/ concentration (35%), and life as a whole (34%). All results are shown in Table S1A–C in the appendix. The total incidence of mental fatigue according to the MFS was 57% (25/44) at 1 year and 54% (21/39) at 3 years.
FIGURE 2.
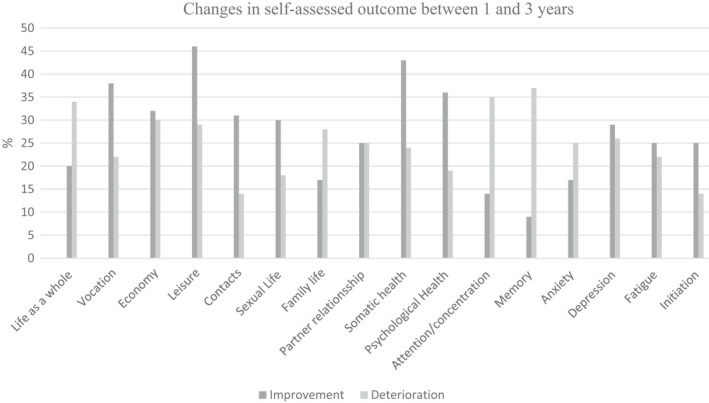
Changes in self‐assessed outcome between 1 and 3 years according to LiSAT‐11 (Life Satisfaction Questionnaire according to Fugl Meyer) and MPAI‐4 (Mayo‐Portland Adaptability Inventory‐4). Patient outcome difference (improvement or deterioration) between 1 and 3 years after aneurysmal subarachnoid hemorrhage. Items for which more than 25% of patients indicated a change, improvement or deterioration, are presented.
3.4. Follow‐up related to age
Patients <60 years of age at the time of aSAH constituted the majority of patients at the 1‐ and 3‐year follow‐ups (60% and 61%, respectively). No difference in the GOSE score was observed between the age groups at 1 or 3 years. The median (range) GOSE score at 1 year was 5 (1–8) for both age groups and that at 3 years was 6 (1–8).
Significant differences between patients <60 years of age and those ≥60 years of age for the items in the LiSAT‐11 and MPAI‐4 questionnaires are shown in Figure 3. For all items in which a significant change was observed, patients <60 years of age more frequently reported self‐assessed dissatisfaction or problems. At 3 years, the only difference that remained between the age groups was that patients ≥60 years of age reported significantly fewer problems with pain/headache and leisure (p < .01 and p < .018) than younger patients. All results are shown in Table S2:1–2:3 in the appendix. In patients <60 years of age who could be assessed at both 1 and 3 years, the incidence of mental fatigue was 42% and 33%, respectively. For patients ≥60 years, the corresponding incidence of mental fatigue was 47% and 53%, respectively.
FIGURE 3.
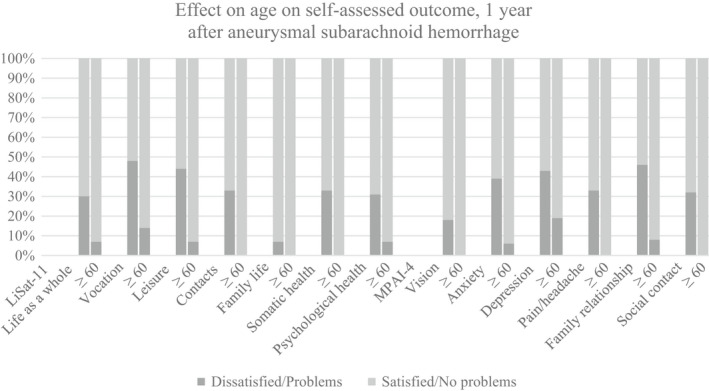
The effect on age on self‐assessed outcome according to LiSAT‐11(Life Satisfaction Questionnaire according to Fugl Meyer) and MPAI‐4 (Mayo‐Portland Adaptability Inventory‐4) 1 year after aneurysmal subarachnoid hemorrhage. Items with a significant difference (p<0.05) between patient groups (<60 years and ≥60 years of age). Answer options for LiSAT‐11 dichotomized as satisfied (Very Satisfied/Satisfied/Rather Satisfied) and dissatisfied (Rather Dissatisfied/Dissatisfied/Very dissatisfied). MPAI‐4, dichotomized as no problems (no problems to mild problems no interference with activities) and problems (mild problems with interference/moderate problems/severe problems).
3.5. Follow‐up related to DCI during the acute phase
During the acute phase after aSAH, 26% of patients experienced DCI. The median (range) GOSE score at 1 year was 5 (1–8) for patients who had DCI and 6 (1–8) for those who did not; at 3 years, the score was 6 (1–8) for both groups. No significant differences in items in the LiSAT‐11 and MPAI‐4 were observed at 1 year between those who did and did not develop DCI. Significant differences at 3 years in different items in the LiSAT‐11 and MPAI‐4 questionnaires are shown in Figure 4. In all cases, patients who developed DCI during the acute phase reported greater dissatisfaction or experienced more problems at 3 years. All results are shown in Table S3:1–3:3 in the appendix. Among patients who experienced DCI and who could be assessed at both 1 and 3 years, the incidence of mental fatigue was 60% and 50%, respectively. Among patients that did not experience DCI, the incidence of mental fatigue was 59% for both years.
FIGURE 4.
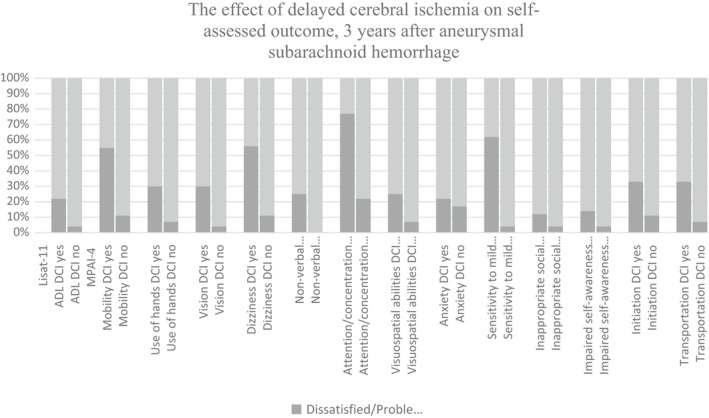
The effect of delayed cerebral ischemia (DCI) on self‐assessed outcome according to LiSAT‐11(Life Satisfaction Questionnaire according to Fugl Meyer) and MPAI‐4 (Mayo‐Portland Adaptability Inventory‐4), 3 years after aneurysmal subarachnoid hemorrhage. Items with a significant difference (p < 0.05) between patients diagnosed with DCI or not (non‐DCI) at 3 years are presented. Answer options for LiSAT‐11 dichotomized as satisfied (Very Satisfied/Satisfied/Rather Satisfied) and dissatisfied (Rather Dissatisfied/Dissatisfied/Very dissatisfied). MPAI‐4, dichotomized as no problems (no problems to mild problems no interference with activities) and problems (mild problems with interference/moderate problems/severe problems). ADL=activity of daily living, DCI = delayed cerebral ischemia, nDCI= no delayed cerebral ischemia.
4. DISCUSSION
The main results of this prospective observational study are that patients with aSAH experience physical, cognitive, emotional, behavioral, and social deficits affecting quality of life for at least 3 years after the hemorrhage. We found that recovery was a dynamic process and patient conditions were likely to improve or deteriorate during the first years. Furthermore, age at the time of aSAH as well as development of DCI during the acute phase affected recovery and life satisfaction.
Obvious and discrete cognitive deficits after aSAH have been reported to affect quality of life in several previous studies. 7 , 9 However, most published studies were retrospective and assessed patients at only one time point. 16 In the present prospective study, we observed that patient conditions both improved and deteriorated regarding a variety of factors between 1 and 3 years post‐hemorrhage, indicating a dynamic recovery pattern after aSAH. Particularly, fatigue was a very common concern in our study, which is in accordance with other studies. 32 , 33 , 34
Rehabilitation in the first months after an aSAH often focuses on helping the patient manage their ADLs, return home and, if possible, return to work. Less attention has been paid to more diffuse, cognitive symptoms that may manifest later. Sonessons et al reported that patients scored higher in areas such as “independence” and “material and physical well‐being” compared to “intellectual development.” 35 Perssons et al also showed comparably higher scores in areas concerning ADLs than those related to emotional problems. 36
A consensus has not yet been reached on the ways to measure outcomes after aSAH, the questionnaires to be used, or an appropriate time frame. Thus, comparing results among studies is challenging. In the present study, in addition to the GOSE, three questionnaires (LiSAT‐11, MPAI‐4, and MFS) were selected to evaluate several self‐assessed aspects of quality of life, from functional abilities to cognitive dysfunction. For most patients, all questionnaires were considered easy to complete without the assistance of an instructor. As discussed by Stienen et al, a broad array of scales exists to evaluate outcomes, but no standardized outcome evaluation method has still established for patients with aSAH. 30 Follow‐up assessments using only one questionnaire have been considered too narrow, and important deficits may likely to be missed. 7 , 37 The Montreal Cognitive Assessment is a commonly used scale to measure outcomes; however, this scale requires the assistance of a test instructor and is thus not feasible for easy follow‐up at home. 38
In the present study, 79% of the patients graded “life as a whole” as satisfying after 1 year, which is in line with a previous study conducted at our hospital. 39 However, when asked about more specific functions, dissatisfaction was observed. Specifically, a vast majority of the patients were satisfied with their ADLs and family life. However, many patients indicated problems with both vocation and economy.
In a previous study, the LiSAT‐11 questionnaire was used to assess a representative Swedish sample of 1207 women and 1326 men, aged 18–64 years. A total of 96% of the sample answered that they were very to rather satisfied with life as a whole, compared to 79% in this study of patients that had an aSAH. Questions related to life as a whole, sexual life, partner relationship, contact with friends, and psychological health were not significantly related to age. Moreover, patients' vocational and financial situations affected life satisfaction even in the representative population. 40 , 41 Specifically, vocationally active subjects were more satisfied with life as a whole compared to those who were unemployed, on sickness benefit, or receiving disability pension. The financial situation was also significantly related to life satisfaction. 40 , 41
Fatigue was frequently reported by patients in the present study, which is in accordance with the findings of other investigations. 8 , 11 , 33 , 42 Fatigue was considered a mild‐to‐severe problem in 55% of the patients according to the MPAI‐4 questionnaire, and 57% of patients had a score indicating mental fatigue in the MFS questionnaire at the 1‐year follow‐up. Mental fatigue is a common and often long‐term disabling condition that may occur after the onset of different types of neurological problems, SAH being a less studied cause. 33 As shown by Western et al, even patients with seemingly good recovery can have mental fatigue that severely affects their quality of life. 11 As fatigue is a more hidden issue that is difficult to discover based on symptoms, it can be speculated that patients may be quite stigmatized. Moreover, while appreciating a good recovery from a severe disease, patients may still significantly experience fatigue in their daily lives. This indicates that assessment of fatigue is important and should be included in the outcome measures after aSAH.
In most published studies on outcomes, the diversity in groups of patients, such as the effect of age or events occurring in the acute phase on outcome, is not addressed. Previous studies have shown that higher age is a risk factor for poor overall outcome. 43 In contrast, our data showed that younger patients at ictus (aged <60 years) had significantly more self‐assessed dissatisfaction or problems than older patients (aged ≥60 years) at the 1‐year follow‐up. After 3 years, the differences between the age groups were reduced. This could be because the older group had other expectations of life, as most would soon retire and have fewer obligations.
The development of DCI during the acute phase is a feared complication after aSAH. However, in our study, no significant differences were found between patients with or without DCI, according to the questionnaires at the 1‐year follow‐up. At the 3‐year follow‐up, however, patients diagnosed with DCI during the acute phase experienced significantly more problems and were more dissatisfied than those who did not develop DCI. This could be because at 1 year, all patients were more generally affected by the aSAH, and at 3 years, the effect of DCI as a secondary stroke became more evident. These results are similar to the findings of Walter et al, where patients who developed DCI had more problems after 24 months, but not after 12 months. 44 These findings emphasize the importance of long‐term follow‐up to assess the needs of late rehabilitation efforts, especially for patients that experienced DCI.
When comparing patients' scores between 1 and 3 years, improvement and decline were both observed in all questionnaires. Improvement was more common in functional areas, such as somatic health, leisure, and vocation. In contrast, decline was mostly noted in areas concerning cognitive function, such as memory and attention/concentration.
In this study, we showed that the outcomes after aSAH were not static. Patients often experience cognitive dysfunction, with mental fatigue being a common problem, and improve several years after ictus. Previous thoughts on rehabilitation and outcome often focused on the importance of the first months after aSAH, with less attention paid to the long‐term effects. Our study demonstrated the importance of extending the time course for follow‐up after aSAH, which is consistent with the results of Hammer et al, Rackauskaite et al, and Wilson et al. 45 , 46 , 47 Additionally, Stienen et al also recommended performing outcome assessment at 3 and 12 months after aSAH, although our results indicated that the follow‐up period should be even longer. 30 The dynamics of recovery in the years following an aSAH gives hope to patients, their families, and healthcare workers involved in rehabilitation and follow‐up for this patient population.
This study was limited by the low number of patients and the large drop‐out rate of patients who did not answer the questionnaires. However, because the demographical data, severity of hemorrhage, and the incidence of DCI were comparable to those reported in other recently published studies on patients with aSAH, we consider our cohort to be representative of the aSAH population. 48 , 49
This study is strengthened by the prospective follow‐up. The patients included were identified and enrolled during the acute phase and contacted at two time points: 1 and 3 years after their stroke. Thus, no selection bias was present, which would have been a risk if patients had been identified retrospectively. However, some patients dropped out and did not return the questionnaires. We observed that the median GOSE score differed significantly between patients who returned the questionnaires and those who did not, which is important to keep in mind when interpreting the results of the present study.
The at‐home completion of the questionnaires is both a strength and a limitation of the study. Patients did not have to visit a healthcare facility and had more time to answer all questions. However, patients with poor outcomes or more cognitive problems may have experienced more difficulties with understanding all the questions or had problems with attention and concentration, thus leaving questions unanswered or not returning questionnaires at all.
We found the MPAI‐4 questionnaire, in which the last parts had to be excluded from analysis owing to the low response rate, to be too extensive and possibly more suitable for follow‐up at a hospital or primary care facility. With future studies, perhaps an online or computer‐based follow‐up form might help patients complete the questionnaires without the need for paper work.
5. CONCLUSION
In conclusion, we showed that cognitive dysfunction and mental fatigue affecting patients' quality of life were common after aSAH, and the outcome was not static. Patient conditions both improved and deteriorated during the first years after aSAH. Our results emphasize the importance of prolonged follow‐up after aSAH. Moreover, younger patients may require more support early after aSAH. Finally, this study suggested that measures assessing fatigue should be included in the outcome assessment. The dynamics of recovery in the years following aSAH warrant further investigation with more long‐term follow‐up using broad outcome measures.
Funding information
This study was financed by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF Agreement (ALFGBG‐772521, ALFGBG‐720511, ALFGBG‐74160), and the Healthcare Board, Region Västra Götaland (VGFOUREG‐939556 and VGFOUREG‐856851).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/ane.13674.
ETHICS APPROVAL AND PATIENT CONSENT
The study adheres to the Declaration of Helsinki, and the study protocol was approved by the Ethical Regional Board of Gothenburg, Sweden (053‐15, T 213‐18). Written informed consent was obtained from all patients or from their next of kin before their participation in the study.
Supporting information
Table S1‐S3
ACKNOWLEDGMENTS
The authors thank Christopher Backström of Statistiska Konsultgruppen for help with the statistical analysis. We would like to thank Editage (www.editage.com) for English language editing. We also greatly appreciate Ingrid Eiving for her valuable contributions.
Wenneberg, S. B. , Block, L. , Sörbo, A. , Naredi, S. , Oras, J. , Hendén, P. L. , Ljungqvist, J. , Liljencrantz, J. & Hergès, H. O. (2022). Long‐term outcomes after aneurysmal subarachnoid hemorrhage: A prospective observational cohort study. Acta Neurologica Scandinavica, 146, 525–536. 10.1111/ane.13674
[Correction added on 14 October 2022 after first online publication: Figures and tables are changed.]
DATA AVAILABILITY STATEMENT
Deidentified data may be shared upon reasonable request.
REFERENCES
- 1. Neifert SN, Chapman EK, Martini ML, et al. Aneurysmal subarachnoid hemorrhage: the last decade. Transl Stroke Res. 2021;12(3):428‐446. [DOI] [PubMed] [Google Scholar]
- 2. Buunk AM, Spikman JM, Metzemaekers JDM, van Dijk JMC, Groen RJM. Return to work after subarachnoid hemorrhage: the influence of cognitive deficits. PLoS One. 2019;14(8):e0220972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(10069):655‐666. [DOI] [PubMed] [Google Scholar]
- 4. Etminan N, Chang HS, Hackenberg K, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta‐analysis. JAMA Neurol. 2019;76(5):588‐597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jaja BNR, Saposnik G, Lingsma HF, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: the SAHIT multinational cohort study. BMJ. 2018;360:j5745. [DOI] [PubMed] [Google Scholar]
- 6. Rubbert C, Patil KR, Beseoglu K, et al. Prediction of outcome after aneurysmal subarachnoid haemorrhage using data from patient admission. Eur Radiol. 2018;28(12):4949‐4958. [DOI] [PubMed] [Google Scholar]
- 7. Geraghty JR, Lara‐Angulo MN, Spegar M, Reeh J, Testai FD. Severe cognitive impairment in aneurysmal subarachnoid hemorrhage: predictors and relationship to functional outcome. J Stroke Cerebrovasc Dis. 2020;29(9):105027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al‐Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519‐e536. [DOI] [PubMed] [Google Scholar]
- 9. Eagles ME, Tso MK, Macdonald RL. Cognitive impairment, functional outcome, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2019;124:e558‐e562. [DOI] [PubMed] [Google Scholar]
- 10. Persson HC, Törnbom M, Winsö O, Sunnerhagen KS. Symptoms and consequences of subarachnoid haemorrhage after 7 years. Acta Neurol Scand. 2019;140(6):429‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Western E, Nordenmark TH, Sorteberg W, Karic T, Sorteberg A. Fatigue after aneurysmal subarachnoid hemorrhage: clinical characteristics and associated factors in patients with good outcome. Front Behav Neurosci. 2021;15:633616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Passier PE, Visser‐Meily JM, Rinkel GJ, Lindeman E, Post MW. Life satisfaction and return to work after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2011;20(4):324‐329. [DOI] [PubMed] [Google Scholar]
- 13. Mayer SA, Kreiter KT, Copeland D, et al. Global and domain‐specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology. 2002;59(11):1750‐1758. [DOI] [PubMed] [Google Scholar]
- 14. Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow outcome scale and the extended Glasgow outcome scale: guidelines for their use. J Neurotrauma. 1998;15(8):573‐585. [DOI] [PubMed] [Google Scholar]
- 15. Rankin J. Cerebral vascular accidents in patients over the age of 60. II Prognosis. Scott Med J. 1957;2(5):200‐215. [DOI] [PubMed] [Google Scholar]
- 16. Nussbaum ES, Mikoff N, Paranjape GS. Cognitive deficits among patients surviving aneurysmal subarachnoid hemorrhage. A contemporary systematic review. Br J Neurosurg. 2020;35:1‐18. [DOI] [PubMed] [Google Scholar]
- 17. Ekstrand E, Lexell J, Brogårdh C. Test‐retest reliability of the life satisfaction questionnaire (LiSat‐11) and association between items in individuals with chronic stroke. J Rehabil Med. 2018;50(8):713‐718. [DOI] [PubMed] [Google Scholar]
- 18. Fugl‐Meyer AR, Bränholm I‐B, Fugl‐Meyer KS. Happiness and domain‐specific life satisfaction in adult northern swedes. Clin Rehabil. 1991;5(1):25‐33. [Google Scholar]
- 19. Malec JF. The Mayo‐Portland participation index: a brief and psychometrically sound measure of brain injury outcome. Arch Phys Med Rehabil. 2004;85(12):1989‐1996. [DOI] [PubMed] [Google Scholar]
- 20. Johansson B, Starmark A, Berglund P, Rodholm M, Ronnback L. A self‐assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Inj. 2010;24(1):2‐12. [DOI] [PubMed] [Google Scholar]
- 21. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495‐1499. [DOI] [PubMed] [Google Scholar]
- 22. Bjerkne Wenneberg S, Lowhagen Henden P, Oras J, et al. Heart rate variability monitoring for the detection of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Acta Anaesthesiol Scand. 2020;64:945‐952. [DOI] [PubMed] [Google Scholar]
- 23. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43(6):1711‐1737. [DOI] [PubMed] [Google Scholar]
- 25. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81‐84. [DOI] [PubMed] [Google Scholar]
- 26. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391‐2395. [DOI] [PubMed] [Google Scholar]
- 27. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968;28(1):14‐20. [DOI] [PubMed] [Google Scholar]
- 28. Drake CG. Report of world Federation of Neurological Surgeons Committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68(6):985‐986. [DOI] [PubMed] [Google Scholar]
- 29. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 30. Stienen MN, Visser‐Meily JM, Schweizer TA, Hänggi D, Macdonald RL, Vergouwen MDI. Prioritization and timing of outcomes and endpoints after aneurysmal subarachnoid hemorrhage in clinical trials and observational studies: proposal of a multidisciplinary research group. Neurocrit Care. 2019;30(Suppl 1):102‐113. [DOI] [PubMed] [Google Scholar]
- 31. Johansson B, Rönnbäck L. Evaluation of the mental fatigue scale and its relation to cognitive and emotional functioning after traumatic brain injury or stroke. Int J Phys Med Rehabil. 2014;2(1):e1000182. [Google Scholar]
- 32. Western E, Sorteberg A, Brunborg C, Nordenmark TH. Prevalence and predictors of fatigue after aneurysmal subarachnoid hemorrhage. Acta Neurochir. 2020;162(12):3107‐3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sorbo A, Eiving I, Lowhagen Henden P, Naredi S, Ljungqvist J, Odenstedt HH. Mental fatigue assessment may add information after aneurysmal subarachnoid hemorrhage. Brain Behav. 2019;9(7):e01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samuelsson J, Jakobsson H, Rentzos A, Jakola AS, Nilsson D. Neurological outcome, mental fatigue, and occurrence of aneurysms >15 years after aneurysmal subarachnoid hemorrhage. World Neurosurg. 2021;151:e122‐e127. [DOI] [PubMed] [Google Scholar]
- 35. Sonesson B, Kronvall E, Säveland H, Brandt L, Nilsson OG. Long‐term reintegration and quality of life in patients with subarachnoid hemorrhage and a good neurological outcome: findings after more than 20 years. J Neurosurg. 2018;128(3):785‐792. [DOI] [PubMed] [Google Scholar]
- 36. Persson HC, Carlsson L, Sunnerhagen KS. Life situation 5 years after subarachnoid haemorrhage. Acta Neurol Scand. 2018;137(1):99‐104. [DOI] [PubMed] [Google Scholar]
- 37. Rautalin IM, Sebök M, Germans MR, et al. Screening tools for early neuropsychological impairment after aneurysmal subarachnoid hemorrhage. Neurol Sci. 2020;41(4):817‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695‐699. [DOI] [PubMed] [Google Scholar]
- 39. Sörbo AK, Blomqvist M, Emanuelsson IM, Rydenhag B. Psychosocial adjustment and life satisfaction until 5 years after severe brain damage. Int J Rehabil Res. 2009;32(2):139‐147. [DOI] [PubMed] [Google Scholar]
- 40. Melin R, Fugl‐Meyer KS, Fugl‐Meyer AR. Life satisfaction in 18‐ to 64‐year‐old swedes: in relation to education, employment situation, health and physical activity. J Rehabil Med. 2003;35(2):84‐90. [DOI] [PubMed] [Google Scholar]
- 41. Fugl‐Meyer AR, Melin R, Fugl‐Meyer KS. Life satisfaction in 18‐ to 64‐year‐old swedes: in relation to gender, age, partner and immigrant status. J Rehabil Med. 2002;34(5):239‐246. [DOI] [PubMed] [Google Scholar]
- 42. Kutlubaev MA, Barugh AJ, Mead GE. Fatigue after subarachnoid haemorrhage: a systematic review. J Psychosom Res. 2012;72(4):305‐310. [DOI] [PubMed] [Google Scholar]
- 43. Catapano JS, Rumalla K, Srinivasan VM, et al. Treatment of octogenarians and nonagenarians with aneurysmal subarachnoid hemorrhage: a 17‐year institutional analysis. Acta Neurochir. 2021;163:2941‐2946. [DOI] [PubMed] [Google Scholar]
- 44. Walter J, Grutza M, Vogt L, Unterberg A, Zweckberger K. The neuropsychological assessment battery (NAB) is a valuable tool for evaluating neuropsychological outcome after aneurysmatic subarachnoid hemorrhage. BMC Neurol. 2020;20(1):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hammer A, Ranaie G, Yakubov E, et al. Dynamics of outcome after aneurysmal subarachnoid hemorrhage. Aging (Albany NY). 2020;12(8):7207‐7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rackauskaite D, Svanborg E, Andersson E, Lowhagen K, Csajbok L, Nellgard B. Prospective study: long‐term outcome at 12‐15 years after aneurysmal subarachnoid hemorrhage. Acta Neurol Scand. 2018;138(5):400‐407. [DOI] [PubMed] [Google Scholar]
- 47. Wilson DA, Nakaji P, Albuquerque FC, McDougall CG, Zabramski JM, Spetzler RF. Time course of recovery following poor‐grade SAH: the incidence of delayed improvement and implications for SAH outcome study design. J Neurosurg. 2013;119(3):606‐612. [DOI] [PubMed] [Google Scholar]
- 48. Gerner ST, Reichl J, Custal C, et al. Long‐term complications and influence on outcome in patients surviving spontaneous subarachnoid hemorrhage. Cerebrovasc Dis. 2020;49(3):307‐315. [DOI] [PubMed] [Google Scholar]
- 49. Pace A, Mitchell S, Casselden E, et al. A subarachnoid haemorrhage‐specific outcome tool. Brain. 2018;141(4):1111‐1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S3
Data Availability Statement
Deidentified data may be shared upon reasonable request.


