Abstract
Background and purpose.
The senescence-accelerated mouse P1 (SAMP1) suffers from humoral immune deficiency, arterial stiffness and accelerated aging. In contrast, the microRNA-150 knockout (miR-150-KO) mice show enhanced humoral immune function including increased B cell population and elevated serum immunoglobulin levels and enjoy extended lifespan. The purpose of this study was to investigate whether transplantation of bone marrow cells (BMCs) from miR-150-KO mice affects immune deficiency and arterial stiffening in SAMP1 mice.
Methods and Results.
Pulse wave velocity and blood pressure were increased significantly in SAMP1 mice (10 months), indicating arterial stiffening and hypertension. Interestingly, transplantation of BMCs from miR-150-KO mice significantly attenuated arterial stiffening and hypertension in SAMP1 mice within eight weeks. BMC transplantation from miR-150-KO mice partially rescued the downregulation of B lymphocytes, largely restored serum IgG and IgM levels, decreased inflammatory cytokine and chemokine expression, and attenuated macrophage and T cell infiltration in aortas in SAMP1 mice. BMC transplantation nearly abolished the upregulation of collagen 1, TGFβ1, Scleraxis, MMP-2 and MMP-9 expression and the downregulation of elastin levels in aortas in SAMP1 mice. FISH staining confirmed existence of the transplanted BMCs at end of the experiment. In cultured endothelial cells, IgG-deficient medium invoked upregulation of inflammatory cytokine/chemokine expression which can be rescued by treatment with IgG.
Conclusions.
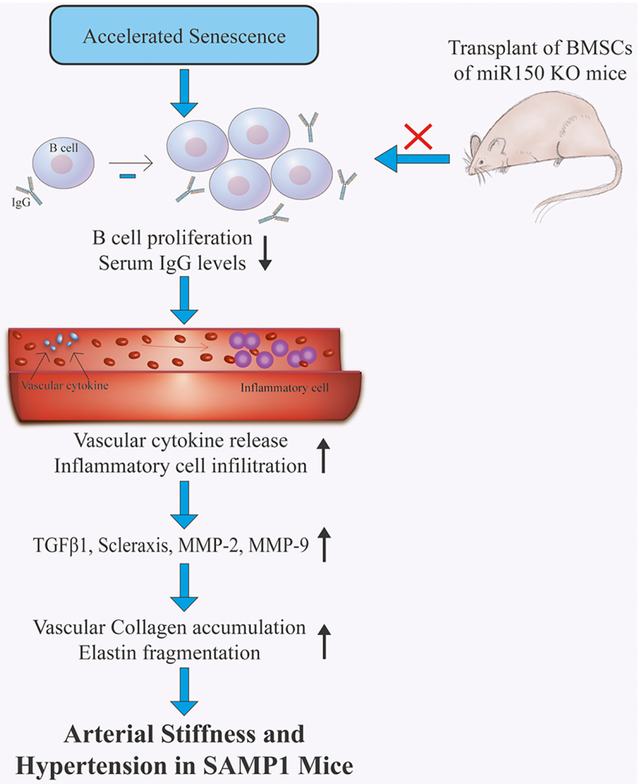
Accelerated senescence caused arterial stiffening via impairing the humoral immune function in SAMP1 mice. BMC transplantation from miR-150-KO mice attenuated arterial matrix remodeling and stiffening and hypertension in SAMP1 mice partly via improving the humoral immune function which attenuates vascular inflammation.
Keywords: matrix remodeling, B cell, IgG, macrophage, inflammation, FISH
Graphics Abstract
Introduction
Large conduit arteries (e.g., aorta) lose elasticity with aging leading to central arterial stiffening and hypertension [1; 2]. To address this important topic, NHLBI supported 11 R01 awards under a Request for Application (RFA) HL-10-027 to investigate the “Cellular and Molecular Mechanisms of Arterial Stiffening and its Relationship to Development of Hypertension” [1]. One of the major findings of this NHLBI initiative is that increased arterial stiffness precedes the development of systemic hypertension in several different animal models [1]. Clinical and population studies also showed the same temporal sequence [3; 4]. Therefore, aging-associated arterial stiffness is the cause rather than the consequence of hypertension [2; 5]. The prevalence of arterial stiffening and hypertension is increased in the aged population[6][9]. Aging-related hypertension is characterized by an increase in systolic blood pressure [2; 7].
Arterial stiffening is associated with structural remodeling and functional impairments in arterial walls, which increases the cardiovascular morbidity and mortality in humans [8]. It has been reported that arterial stiffening is an independent predictor of cardiovascular risk in patients with hypertension, diabetes and end-stage renal diseases [9; 10]. Arterial stiffening is characterized by the decreased ratio of elastin/collagen which impairs arterial compliance [11]. Increased pulse wave velocity (PWV) is the gold standard for the diagnosis of arterial stiffness in patients [12; 13]. A longitudinal study indicated that age is an important determinant of PWV [4]. Prevalence of arterial stiffness and hypertension is increased while the immune function is impaired in the aged population [2; 14–19]. Several studies showed that aging is associated with a decline in the immune function [17–19], which largely increases the risk of autoimmune disorders and cancer [20]. As age advances, the immune system undergoes profound remodeling and functional deterioration [21]. Serum concentrations of normal IgG and IgM are reduced in the elderly [22; 23].
The senescence-accelerated mouse (SAM) was originally developed through a litter of AKR/J mice showing early aging phenotypes in the Jackson Laboratory. It consists of nine senescence-accelerated mouse prone strains (SAMP) and three senescence-resistant strains (SAMR). The SAMP1 mouse is one of the nine SAMP mouse strains, which show spontaneous and accelerated aging processes, impaired immune function, and shortened life span [24]. The immune function is impaired with age in SAMP1 mice, especially the compromised antibody production [25]. In this study, we examined whether accelerated senescence affects arterial compliance using the SAMP1 mouse model.
MiR-150 plays a crucial role in B cell differentiation and development via targeting c-Myb [26–28]. MiR-150 blocks B cell development and decreases B cell numbers. On the other hand, miR-150 knockout increased B cell proliferation and maturation and enhanced the humoral immune response [26]. Serum levels of IgG and IgM were largely increased in miR-150 knockout mice [26].
The immune dysfunction is critical in the initiation and propagation of the inflammatory process in blood pressure elevation and arterial injury [29; 30]. Recent reports demonstrated that inflammation was involved in the process of arterial wall stiffness [2]. Inhibition of inflammatory factors, such as TNF-α and IL-6, attenuates arterial stiffness [2]. Inflammation induces fragmentation of elastin, proliferation of smooth muscle cells, and changes in the composition of the extracellular matrix in arterial wall leading to arterial remodeling (hypertrophy) and stiffness [1; 31]. Aging is associated with increased production of proinflammatory cytokines from disease-free arteries [2].
SAMP1 mice suffer from humoral immune deficiency and arterial stiffness while miR-150 KO mice exhibit enhanced humoral function. In this study, we investigated whether transplantation of bone marrow cells (BMCs) from miR-150 KO mice affects the immune function and arterial stiffness in SAMP1 mice. We hypothesize that transplantation of BMCs from miR-150 KO mice would improve humoral immune dysfunction and arterial stiffness in SAMP1 mice.
Methods
Expended methods can be found in the Online Supplemental Methods and Data.
Animal Study Protocols
This study was carried out according to the Guidelines of the National Institute of Health on the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee (IACUC) of University of Oklahoma Health Science Center. Four genotypes of animals were used in this study. The female SAMP1 mice and the female AKR/J control mice, the male miR-150 knockout mice and the male wild-type control mice (C57BL/6, 6–8 mice/group). All mice were housed in cages at room temperatures (25±1°C) and were provided with Purina laboratory chow (No. 5001) and tap water. Pulse wave velocity (PWV) and blood pressure were measured during the treatment period. After 8 weeks, all animals were perfused transcardially with heparinized-PBS following euthanasia with ketamine (90 mg/kg) and xylazine (10 mg/kg, IP). The aortas were then quickly removed, washed, and cut into pieces for subsequent analyses.
Bone marrow transplantation
The intra-bone marrow-bone marrow transplantation (IBM-BMT) was carried out according to the method reported previously (Supplemental Fig. S1).
Western blot and immunohistochemical analysis
Western blot and immunohistochemical analysis were performed as described in our recent studies [32; 33]. For detailed procedures, refer to the Online Supplemental Methods.
Fluorescent in situ hybridization (FISH)
The bone marrow cells were collected from the tibia and femur and harvested by the standard methods. Briefly, bone marrow cells were smeared on slides and prepared according to the manufacturer’s instructions. Mouse chromosome Y probe, labeled with Green 5-Fluorescein dUTP (Empire Genomics, New York, US), were denatured at 73 °C for 5 min and applied to the sections. Slides were hybridized for 16 h at 37 °C in a humidified chamber. The sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) mounting medium (Santa Cruz Biotechnology). Images were captured using Olympus IX73 fluorescent microscope (Olympus Corp., Tokyo, Japan).
Statistical Analysis
Quantitative data were presented as the Mean ± SE. Data were analyzed by one-way analysis of variance (ANOVA) followed by the Bonferroni post-test using Prism software (GraphPad). The sample values were first determined to be normally distributed by the Kolmogorov-Smirnov test, and then the comparisons between two groups were performed using an unpaired Student’s t-test. For all analysis, p<0.05 were considered statistically significant.
Results
SAMP1 mice developed arterial stiffness and hypertension
To assess arterial compliance, we measured pulse wave velocity (PWV) in 10 month-old SAMP1 mice and the age-matched AKR/J mice (controls). PWV and systolic blood pressure was increased significantly in SAMP1 mice compared with the age-matched AKR/J mice (Supplemental Fig. S2A, 2B), suggesting that SAMP1 developed arterial stiffness and hypertension.
Aortic matrix remodeling in SAMP1 mice
The Masson trichrome staining method was used to stain the collagen changes in aorta. The result showed that collagen deposition (blue staining) was increased in the aorta in SAMP1 mice compared with that of the AKR/J mice (Supplemental Fig. S2C, E). To further evaluate the vascular stiffness, we assessed the elastin level using Vehoeff’s elastic stain kit. The elastin laminae in the aorta was partially broken (fragmentation), showing lost wave shapes (the dark color indicates the elastin) in SAMP1 mice (Supplemental Fig. S2D, F, G). Thus, senescence may impair arterial matrix structure. In contrast, the elastin in the aorta in AKR/J mice was intact. The elastin /collagen ratio was decreased in SAMP1 mice (Supplemental Fig. S2H). Collage deposition and elastin fragmentation lead to arterial matrix remodeling which contributes to arterial stiffness.
The protein level of collagen 1 and elastin was further quantified using Western blot. The collagen 1 level was increased while the elastin level was decreased in the aorta in SAMP1 mice compared with that of the AKR/J mice (Supplemental Fig. S3A, B). The ratio of elastin/collagen 1 was decreased in aorta in SAMP1 mice. Protein expression of transforming growth factor-β1 (TGF-β1), a stimulator of collagen synthesis, was also increased significantly in the aorta in SAMP1 mice (Supplemental Fig. S3A, B).
Transplantation of BMCs from miR-150 KO mice partly rescued downregulation of B cells in bone marrow and peripheral blood in SAMP1 mice.
Because miR-150 KO enhances the humoral immune function, we assessed whether BMCs transplantation (BMT) from miR-150 KO mice affects arterial stiffness and hypertension in SAMP1 mice. Briefly, SAMP1 mice were transplanted with BMCs from miR-150 KO mice and the WT C57BL/6 mice, respectively (Supplemental Fig. S1). Lymphocyte population was gated from BMCs and PBCs according to forward scatter (FSC) characteristics and side scatter (SSC) characteristics. Gated lymphocytes were then separated in CD19+ B cells and CD3+ T cells. The percentages of CD19+ B cells in the bone marrow and blood were decreased in SAMP1 mice compared with that of AKR/J mice (Fig. 1). BMC transplantation from miR-150 KO mice partially rescued the downregulation of B cells in the bone marrow and blood, albeit not to the control level (AKR/J mice). The percentage of CD3+ T cells was also assessed. There was no significant difference in the percentage of CD3+ cells between the SAMP1 mice and the AKR/J mice (Fig. 1). BMT tended to increase percentage of T cells vs. the SAMP1+WT BMC group.
Figure 1.
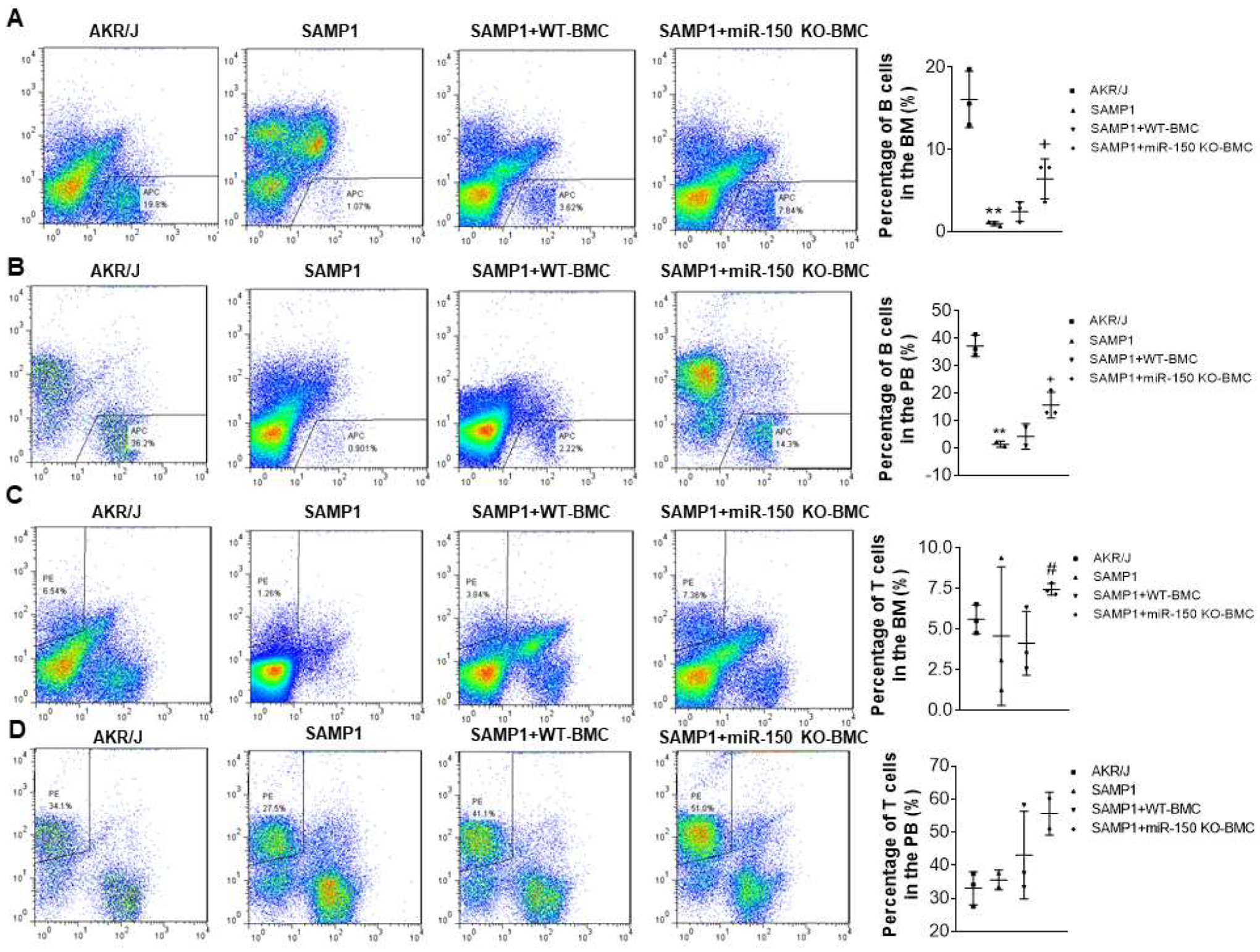
Flowcytometry analysis of B cells and T cells percentage in the bone marrow (BM) and peripheral blood (PB). Representative B cell plots and B cell percentages in the BM (A) and PB (B). Representative T cell plots and T cell percentages in the BM (C) and PB (D). Data = mean ± SEM (n=3). The data were analyzed using one-way ANOVA analysis with Bonferroni correction. ** P<0.01 Vs AKR/J mice; + P<0.05, ++P<0.01 Vs SAMP1 mice; #P<0.05 Vs SAMP1+WT-BMC mice.
Transplantation of BMCs from miR-150 KO mice rescued downregulation of IgG/IgM levels in serum in SAMP1 mice.
Serum levels of IgG and IgM were decreased in SAMP1 mice compared with those of the AKR/J mice (Fig. 2). Transplantation of BMCs from miR-150 KO mice significantly increased serum levels of IgG and IgM in SAMP1 mice, although they did not reach the control level. BMCs from WT mice slightly, but did not significantly, increase the serum levels of IgG and IgM in SAMP1 mice (Fig. 2).
Figure 2.
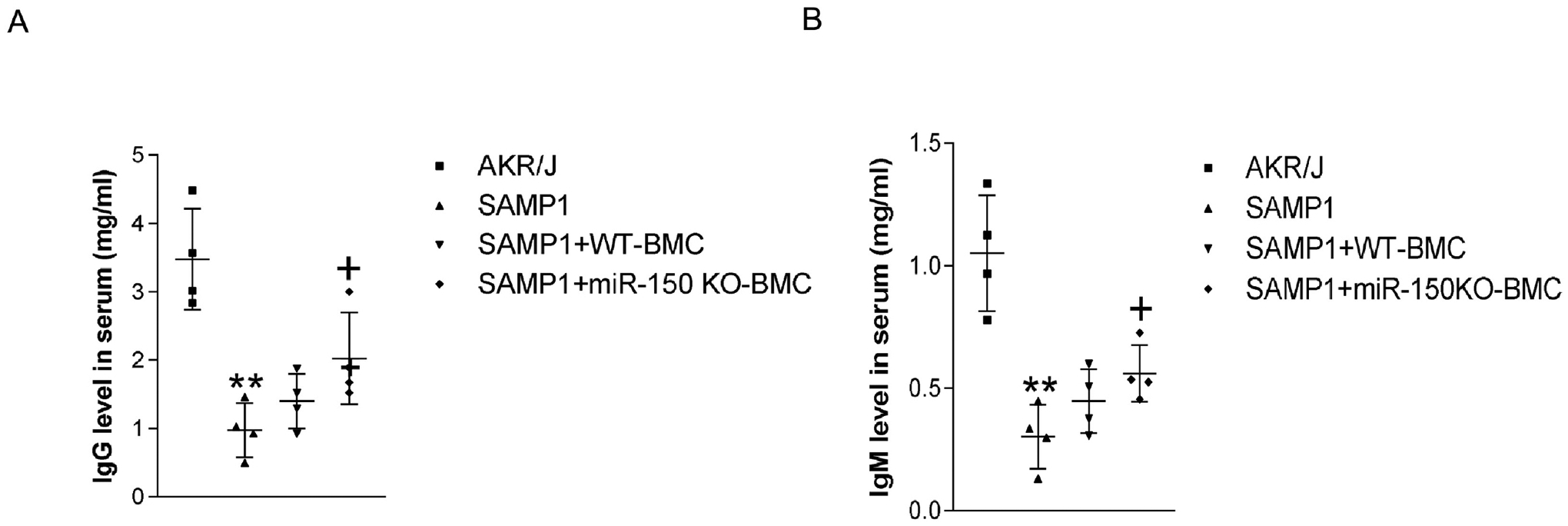
Transplantation of BMCs from miR-150 KO mice increased IgG and IgM level in serum in SAMP1 mice. (A). IgG level in the serum. (B). IgM level in the serum. Data = mean ± SEM (n=4). One-way ANOVA analysis with Bonferroni correction was performed. ** P<0.01 Vs AKR/J mice; + P<0.05 Vs SAMP1 mice.
Transplantation of bone marrow cells (BMCs) from miR-150 KO mice attenuated arterial stiffness and hypertension in SAMP1 mice
PWV and BP were higher in SAMP1 mice compared with those of AKR/J mice (10 months old) (Fig. 3). Interestingly, BMC transplantation from miR-150 KO mice significantly attenuated the elevation of PWV and BP in SAMP1 mice within 2 weeks (Fig. 3). Treatment with BMCs from WT mice slightly, but not significantly, decreased PWV in SAMP1 mice (Fig. 3). These results indicated that transplantation of BMCs from miR-150 KO mice effectively attenuated arterial stiffness and hypertension in SAMP1 mice.
Figure 3.
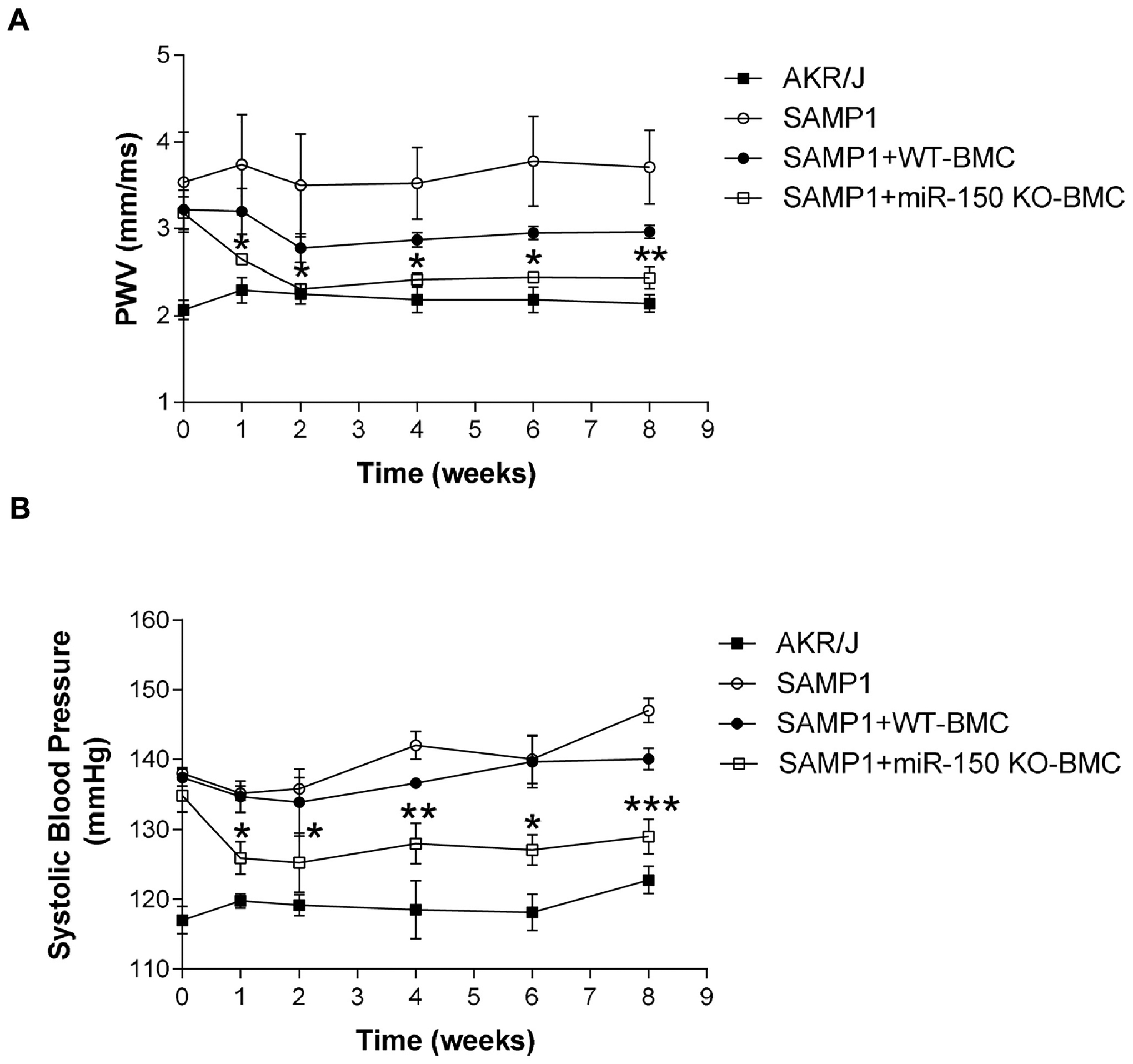
Transplantation of bone marrow cells (BMCs) from miR-150 KO mice attenuated arterial stiffness and hypertension in SAMP1 mice. (A) The time course of PWV changes. (B) The time course of systolic blood pressure changes. Data = mean ± SEM (n=4–6).
One-way ANOVA analysis with Bonferroni correction was performed. *P<0.05, **P<0.01, ***P<0.001 Vs SAMP1 mice.
Transplantation of BMCs from miR-150 KO mice abolished upregulation of collagen levels and downregulation of elastin levels in aortas in SAMP1 mice.
Masson trichrome staining indicated that BMC transplantation from miR-150 KO mice largely decreased collagen staining density and collagen-positive area in aortas in SAMP1 mice (Fig. 4A, B). Aortas from the BMC transplantation group exhibited reduced elastin fragmentation (elastin breaks) and preserved elastic laminar waviness, suggesting that the BMC transplantation from miR-150 KO mice effectively prevented elastin degradation in this animal model (Fig. 4A, B). Thus, BMC transplantation improves aortic matrix remodeling.
Figure 4.
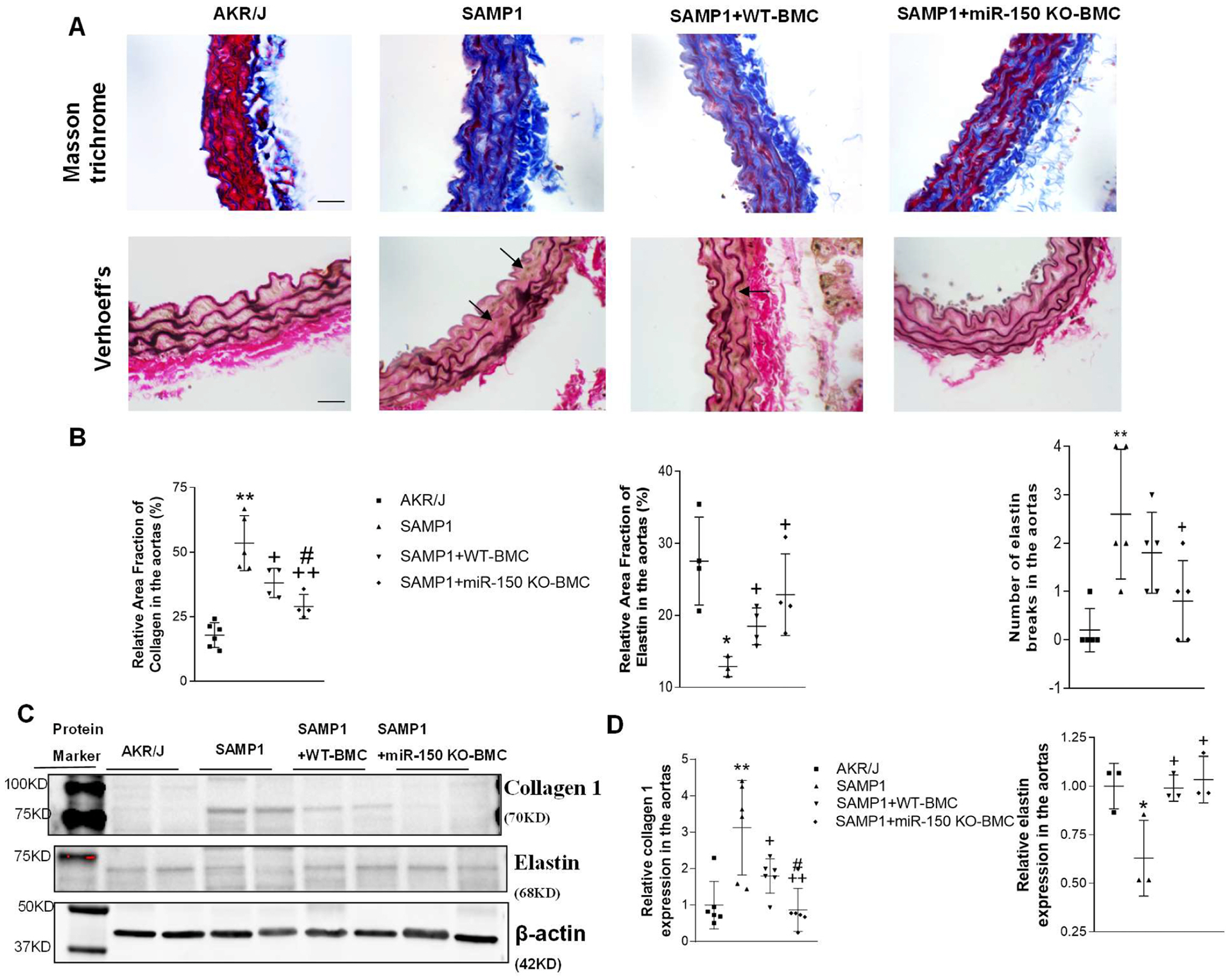
Transplantation of BMCs from miR-150 KO mice abolished upregulation of collagen levels and downregulation of elastin levels in aortas in SAMP1 mice. The analysis was performed at 8 weeks after the BMT. (A). Upper panel, representative Masson-stained sections showing collagen levels in aorta. The blue staining indicates the collagen staining. Lower panel, representative Verhoeff’s-stained sections showing elastic lamella changes. The dark staining indicates the elastin staining. Arrows point to the elastin break in the aortic sections. Scale bar indicates 100 μm. (B) Relative area fraction of collagen and elastin in the aorta, and the number of elastin lamella breaks in the aortic sections. (C) Representative Western blot bands of collagen I and elastin. (D) Quantitative analysis of collagen 1 and elastin in the aorta. The target bands were first normalized with β-actin and then calculated as fold changes vs. the AKR/J group. Data = mean ± SEM (n=3–6). One-way ANOVA analysis with Bonferroni correction was performed. *P<0.05, **P<0.01 Vs AKR/J mice; +P<0.05, ++P<0.01 Vs SAMP1 mice; #P<0.05 Vs SAMP1+WT-BMC mice.
The western blot analysis showed that elastin expression was decreased, but collagen 1 expression was increased in the aortas of SAMP1 mice compared with that in the AKR/J mice (Fig. 4C, D). BMC transplantation from miR-150 KO rescued downregulation of elastin levels and upregulation of collagen 1 levels (Fig. 4C,D). BMC transplantation from WT mice also improved downregulation of elastin levels and upregulation of collagen 1 levels in SAMP1mice.
Transplantation of BMCs from miR-150 KO mice attenuated expression of TGF-β1, Scleraxis, MMP-2 and MMP-9 in aortas of SAMP1 mice
The expression of profibrotic TGF-β1 was upregulated in aortas in SAMP1 mice which was abolished by transplantation of BMCs from the miR-150 KO mice (Supplemental Fig. S4A,B). Scleraxis, a transcription factor of collagen gene expression, upregulates collagen 1 expression in the tendon, ligaments, and cardiac fibroblasts [34]. Interestingly, scleraxis expression was increased significantly in the aortas of SAMP1 mice, which was largely attenuated by BMCs transplantation from the miR-150 KO mice (Supplemental Fig. S4A,B).
MMPs play an important role in physiological homeostasis and pathological vascular extracellular matrix remodeling. MMP-2 and MMP-9 are the key enzymes that break down elastin in the aorta [35]. MMP-2 and MMP-9 expression levels were increased in the aortas of SAMP1 mice compared with the AKR/J mice (Supplemental Fig. S4A,B). Interestingly, upregulation of MMP-2 and MMP-9 levels were rescued in SAMP1 mice following transplantation with BMCs from miR-150 KO (Supplemental Fig. S4A,B).
Transplantation of BMCs from miR-150 KO mice effectively mitigated vascular inflammatory activation in SAMP1 mice
Vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) are the markers of immune-inflammatory activation. The protein expressions of VCAM-1 (Supplemental Fig. S5A,B), ICAM-1 (Supplemental Fig. S5A,C), TNF-α (Supplemental Fig. S5A,D), MCP-1(Supplemental Fig. S5A,E), and IL-6 (Supplemental Fig. S5A,F) were increased significantly in the aorta in SAMP1 mice compared with AKR/J mice. Thus, accelerated senescence activated vascular inflammatory responses. Transplantation BMCs from miR-150 KO mice largely decreased the inflammatory cytokines and chemokines (Supplemental Fig. S5A,B), indicating effective mitigation of inflammatory activation. In contrast, BMCs transplantation from WT mice only slightly decreased these inflammatory factors in the aorta in SAMP1 mice.
Transplantation of BMCs from miR-150 KO mice decreased the infiltration of the inflammatory cells in aortas of SAMP1 mice
We next evaluated inflammatory cells infiltration in vascular wall by immunostaining of CD68 (macrophages) and CD3 (T cells) antibodies, respectively. The numbers of CD68+ (Fig. 6A, B) and CD3+ (Fig. 5A, C) cells were increased in aortas of SAMP1 mice versus AKR/J mice, indicating that accelerated senescence leads to vascular inflammation. BMC transplantation from miR-150 KO mice abolished macrophage and T cells infiltration in aortas of SAMP1 mice (Fig. 5A–C). BMCs from the miR-150 KO mice were more effective in decreasing the number of inflammatory/immune cells compared with the BMCs from the WT mice (Fig. 5).
Figure 6.
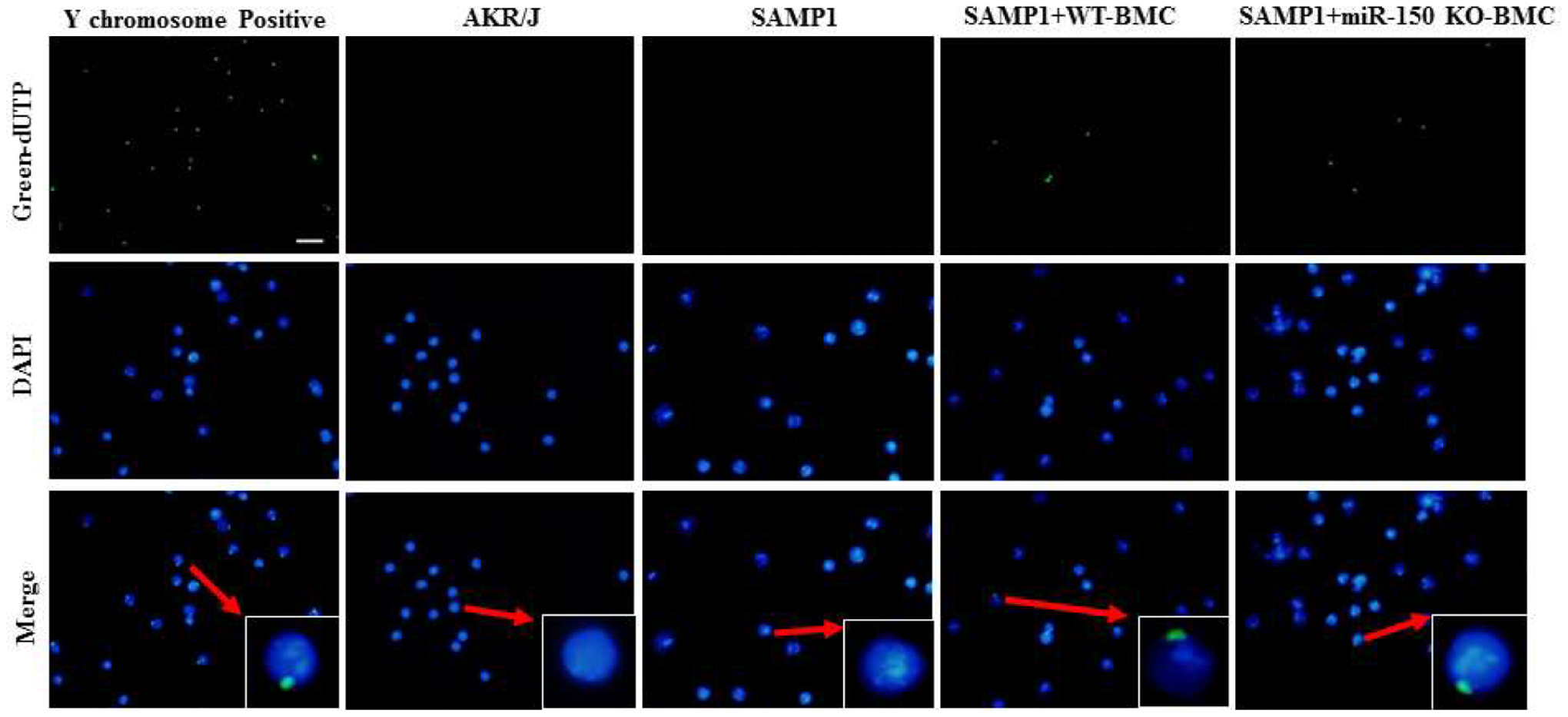
Representative sections of FISH staining showing that BMCs from male miR-150 KO mice were detected in the BM of female SAMP1 mice at 8 weeks after BMT. The Y chromosomepositive cells (green) and DAPI (blue) for nuclei in the BM were examined at 8 weeks after BMT. Merged images show that Y chromosome is localized in the nucleus. Scale bar indicates 100 μm.
Figure 5.
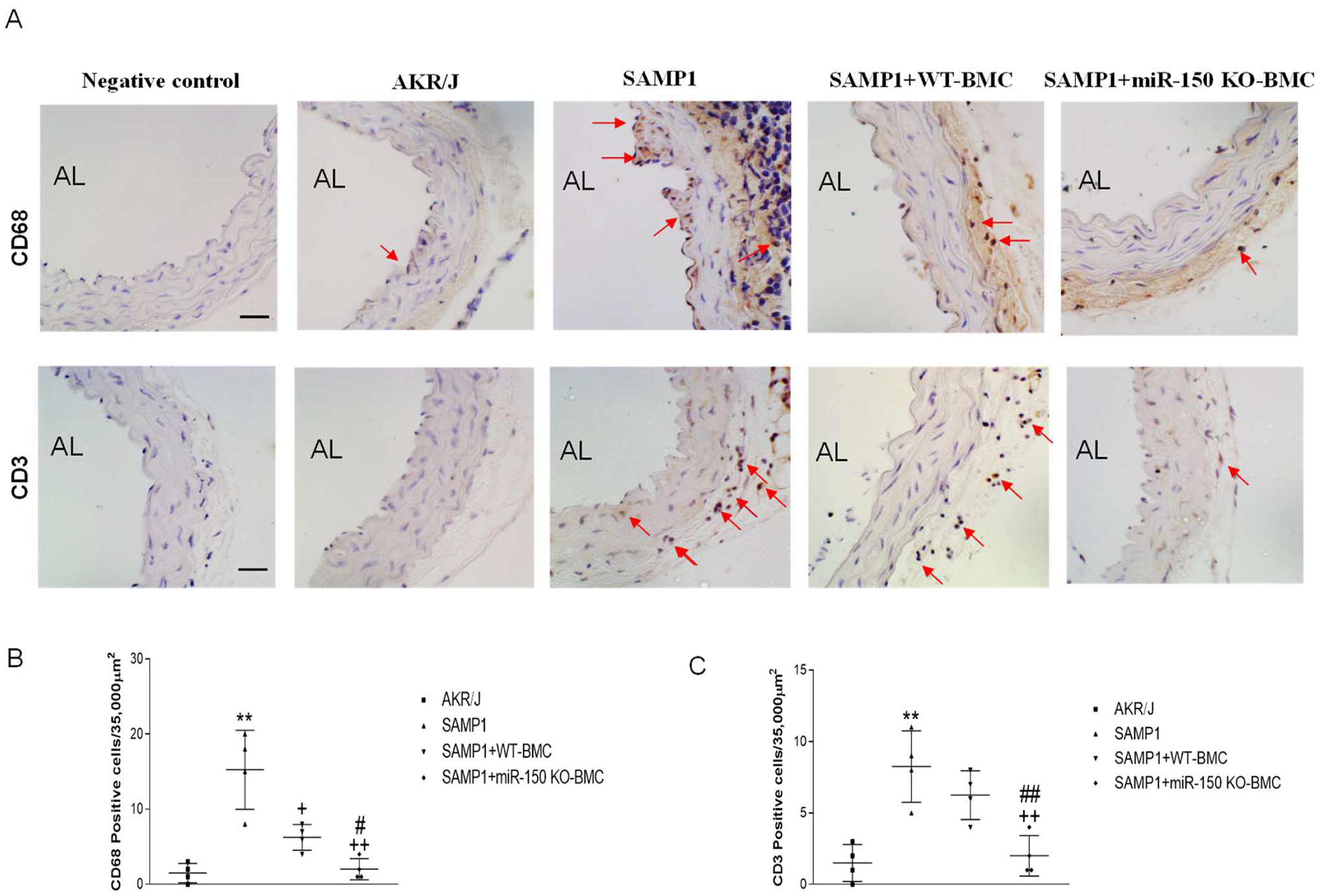
Transplantation of BMCs from miR-150 KO mice attenuated the infiltration of the inflammatory cells in aortas in SAMP1 mice. The analysis was performed at 8 weeks after the BMT. (A) Representative photomicrographs of CD68 (macrophages) and CD3 (T cells) immunostaining in aorta sections. Arrows point to positive staining (brown). Negative controls show sections stained without first antibodies. Semiquantitative analysis of infiltration of CD68+ cells (B) and CD3+ cells (C) in aorta sections. Scale bar indicates 100 μm. Data = mean ± SEM (n=4). One-way ANOVA analysis with Bonferroni correction was performed. **P<0.01 Vs AKR/J mice; +P<0.05, ++P<0.01 Vs SAMP1 mice; #P<0.05, ##P<0.01 Vs. SAMP1+WT-BMC mice.
BMCs from miR-150 KO mice still existed in the bone marrows in SAMP1 mice at 8 weeks after BMC transplantation
To evaluate whether transplanted BMCs still exist in the bone marrow at 8 weeks after transplantation, bone marrows of female SAMP1 mice transplanted with BMCs from male miR-150 KO mice were collected and stained using the Y chromosome FISH method (Fig. 6). The bone marrow of male mice was used as a positive control which demonstrated that 100% of the cells are stained positive for Y chromosome (Fig. 6). As expected, no Y chromosome-positive cells were observed in bone marrow of female SAMP1 or AKR/J mice that did not undergo BMC transplantation. In female SAMP1 mice transplanted with BMCs from male miR-150 KO mice, many Y chromosome-positive cells were detected in the bone marrow cells (Fig. 6). Y chromosome staining was found in the nuclei of bone marrow cells. These results confirmed that transplanted BMCs still existed in bone marrow at 8 weeks after BMC transplantation.
Supplement with IgG protein blocked IgG deficiency-induced inflammatory activation in vascular endothelial cells
IgG is the major immunoglobulin in the serum. We investigated whether removal of IgG from serum affects inflammatory factor expression levels in vascular endothelial cells. Endothelial cells were treated with ultra-low IgG serum with or without bIgG for 12 hours, then the cells were collected. Western blot analysis showed that protein expressions of VCAM-1, ICAM-1, TNF-α and MCP-1 were increased significantly in endothelial cells cultured in ultra-low IgG serum, demonstrating for the first time that IgG deficiency activated the inflammatory responses (Supplemental Fig. S6). Supplement with bIgG completely blocked IgG deficiency-induced inflammatory activation in vascular endothelial cells (Supplemental Fig. S6).
Discussion
This study reported that that accelerated senescence is associated with arterial remodeling and stiffness in SAMP1 mice (Figs. 3 and 4, Supplemental Fig. S2). We found that the humoral immune system is impaired by accelerated senescence in SAMP1 mice as evidenced by diminished B cell populations in bone marrow and peripheral blood (Fig. 1) and downregulation of IgG and IgM levels in serum (Fig. 2A,B).
Emerging evidence suggests that microRNAs (miRNAs) are involved in the regulation of immune function [36]. MiR-150 is specifically expressed in mature B lymphocytes [26]. Knockout of miR-150 leads to B cell expansion and significant increases in serum levels of normal immunoglobulins including IgG/IgM in mice [26]. Knockdown of miR-150 in bone marrow-derived mononuclear cells (MNCs) can induce mobilization of the cells, so the cells can easily migrate to the injury site for tissue repair [37]. Because miR-150 KO expands the B cell population and increases serum IgG/IgM levels [26], we investigated whether transplant of bone marrow cells (BMCs) from miR-150 KO mice would improve the humoral immune insufficiency in SAMP1 mice.
An intriguing finding of this study is that BMC transplantation from miR-150 KO mice improved the downregulation of the B cell population and serum IgG/IgM levels in SAMP1 mice (Figs. 1, 2). Importantly, transplantation of BMCs from the miR-150 KO mice effectively attenuated arterial stiffness in SAMP1 mice (Fig. 3). The beneficial effects of BMCs transplantation from miR-150 KO mice may be partly attributed to improvement in immune function which attenuated vascular inflammatory activation and inflammatory cell infiltration (Fig. 5, Supplemental Fig. S5), leading to attenuation of arterial stiffening and hypertension in SAMP1 mice. Bone marrow transplantation (BMT) is a useful approach for treating hematopoietic disorders and autoimmune diseases. It was reported that intra-bone marrow–bone marrow transplantation (IBM-BMT) was the most effective method for allogeneic BMT [38]. BMT would replace the recipient’s hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) with donor-derived HSCs and MSCs. Immunological functions would also be restored after IBM-BMT. Newly developed T cells, B cells, and antigen-presenting cells are found in the recipient mice which receive IBM–BMT [39].
Physiologically, normal IgG is maintained at a constant level in the blood. Different from pathogenic IgG (induced by specific pathogens), normal IgG plays an important role in maintaining normal physiological function. In this study, we found that serum IgG levels decreased in SAMP1 mice (Fig. 2). To further investigate whether IgG deficiency directly affects the inflammatory response in vascular endothelial cells, we treated the endothelial cells with IgG-deficient serum in the in vitro study. Interestingly, IgG deficiency elicited inflammatory activation in endothelial cells as evidenced by significant increases in the expression of ICAM-1, VCAM-1, MCP-1 and TNF-α (Fig. S4). Importantly, the upregulation of these inflammatory cytokines/chemokines can be rescued by adding IgG in the serum. This important finding provides the first evidence that IgG is a critical regulator/modulator of the inflammatory status in endothelial cells. Therefore, we believe that BMT from miR-150 KO mice attenuated inflammatory activation and inflammatory/immune cell infiltration in SAMP1 mice partly by increasing serum IgG levels in SAMP1 mice (Figs. 2 and 5, Supplemental Fig. S5). It was shown that Intravenous immunoglobulins (ivIg), preparations of normal polyspecific IgG obtained from large pools of plasma from healthy subjects, can modulate the cellular immune system [40; 41]. In addition to its use as substitute therapy for primary and secondary antibody deficiencies, ivIg is used increasingly in patients with autoimmune and systemic inflammatory diseases [42; 43] as well as a supportive therapy of immunodeficient patients [44]. Recent studies showed that treatment with human IgG also decreased infiltration of macrophages and key inflammatory markers in skeletal muscle in a mouse model of Duchenne muscular dystrophy [45].
IgG is the major serum isotype among the antibodies. In aged humans and mice, B cell function declines as evidenced by decreases in immunoglobulin (Ig) class switch to IgG [16]. In this study, we found that the B cell population was significantly decreased in SAMP1 mice (Fig. 1). BMT from miR-150 KO mice partly rescued the downregulation of the B cell population in the blood and bone marrow in SAMP1 mice. The downregulation of IgG and IgM levels in the serum was also largely restored in SAMP1 mice due to BMT from miR-150 KO mice. Our previous study also showed that normal IgG could downregulate intracellular superoxide levels and attenuate migration and permeability in aortic endothelial cells isolated from a hypertensive patient [46]. It was reported that normal IgG exhibits important anti-inflammatory and immunomodulatory effects on various diseases [47; 48]. Thus, BMT from miR-150 KO mice improved arterial stiffening and hypertension in SAMP1 mice partly by increasing the B cell population and serum IgG/IgM levels (Supplemental Fig. S7).
On the other hand, B cell numbers and serum IgG are increased above normal levels in some forms of clinical hypertension [49], which are likely an immune responses to pathogenic factors released due to vascular tissue injuries associated with hypertension. Under this circumstance, B lymphocytes may produce “pathogenic” antibodies in response to antigens released from injured vessels. “Pathogenic” IgG can accumulate in the injured site which causes vascular inflammation including infiltration of immune or inflammatory cells contributing to the pathogenesis of hypertension. In an animal model of AngII-induced hypertension, for example, AngII and/or AngII-induced vascular injures result in accumulation of pathogenic IgG in vessels which evokes generation and release of cytokines and chemokines leading to vascular infiltration of T cells and myeloid cells [50]. In this case, inhibition of AngII-induced increases in B cells and IgG levels would attenuate hypertension [51]. Hypertension may be linked to overactivation of B cells and increased production of autoantibodies (anti-AT1R, anti-α1AR and anti-β1AR) [50]. It was reported that suppression of plasma B cells attenuates hypertension in an experimental model of autoimmune disease [52]. It should be mentioned, however, that hypertension is a complicated syndrome which is likely attributed to numerous pathological disorders. Thus, the mechanism of hypertension varies with pathological factors. With natural aging, the humoral immune system is compromised and the normal IgG level is downregulated in humans [22; 23]. Aging-associated hypertension is primarily systolic hypertension due to large conduit artery stiffness [1; 2], which may be different from other forms of hypertension that are mainly attributed to increased peripheral vascular resistance.
Recent studies have shed new light on the importance of inflammation in the pathogenesis of arterial stiffening [1; 2; 53; 54]. Song et al reported the association of adipocytokines with carotid intima media thickness and arterial stiffness in obstructive sleep apnea patients [55]. Inflammation upregulates TGFβ1 and MMP levels, induces collagen accumulation and elastin fragmentation, proliferation of smooth muscle cells, and changes in the composition of the extracellular matrix in arterial wall leading to arterial remodeling and stiffness [1; 2; 31]. In this study, we found inflammatory activation in aortas in SAMP1 mice as evidenced by significant increases in aortic expression of VCAM1, ICAM1, TNFα, MCP-1, and IL-6 (Fig. S5). Adhesion molecules, VCAM-1 and ICAM-1, which are related to leukocyte attachment, rolling, and trans-endothelial migration, play a significant role in the recruitment of inflammatory cells. Arterial stiffness is associated with increased levels of TNF-α and IL-6, as well as VCAM-1 and ICAM-1 [1; 2], suggesting an important role of inflammatory factors in the pathogenesis of arterial stiffness. MCP-1 is a potent attractor for recruitment of monocytes/macrophages and immune cells, which accumulate in the injured region in various vascular diseases, such as atherosclerosis, arterial stiffening and hypertension. Notably, the inflammatory activation was effectively suppressed by BMT from miR-150 KO mice as evidenced by significant decreases in the inflammatory markers (Supplemental Fig. S5). In consistence with inflammatory activation, the infiltration of macrophages and T cells was remarkably increased in the aorta in SAMP1 mice (Fig. 5). BMT from miR-150 KO mice reduced vascular inflammation as evidenced by diminished inflammatory and immune cell infiltration in the aorta in SAMP1 mice. BMT from miR-150 KO mice rescued upregulation of TGF-β1, scleraxis and MMP expression which decreases collagen deposition and elastin fragmentation and consequently arterial remodeling and stiffening in SAMP1 mice. The beneficial effect of BMT from miR-150 KO mice may be partly attributed to suppression of inflammation in SAMP1 mice. Inflammation activates NADPH oxidases and increases superoxide or reactive oxygen species (ROS) generation leading to oxidative stress which impairs vascular endothelial function and causes arterial remodeling [2; 46; 56; 57]. Oxidative stress activates extracellular matrix metalloproteinases (MMPs) which leads to arterial remodeling (e.g., collagen accumulation and elastin fragmentation) and ultimately arterial stiffening [2; 46; 56; 57].
Macrophages can develop polarized functions (M1 and M2) which are important for orchestrating appropriate inflammatory responses to environmental challenges [58; 59]. Classical activation (M1) eliminates pathogens or damaged tissues while alternative activation (M2) promotes regulation and tissue repair [58; 59]. M1 polarized macrophages release pro-inflammatory cytokines and mediators leading to inflammation, while M2 polarized macrophages exhibit an anti-inflammatory function [58]. We found that the macrophages accumulating in aortas in SAMP1 mice can be effectively attenuated by BMT (Figure 5A), suggesting that they are pro-inflammatory macrophages (M1). Indeed, BMT attenuated senescence-associated increases in inflammatory cytokines/chemokines (Supplemental Fig. S5) which improves arterial stiffening and remodeling in SAMP1 mice. An additional study is warranted for investigating whether BMT regulates macrophage polarization.
Reorganization of the extracellular matrix through protein synthesis and degradation is a key characteristic of arterial stiffness. Vascular accumulation of collagen, which is 100 times stiffer than elastin, was increased in aortas in SAMP1 mice (Supplemental Figs. 2–3). TGF-β1 can stimulate collagen 1 synthesis, and has been shown to be involved in arterial stiffness and vascular remodeling. TGF-β1 expression was increased in aortas in SAMP1 mice (Supplemental Fig. S3). Scleraxis, a basic helix-loop-helix transcription factor, regulates collagen gene transcription and expression and plays a significant role in regulating the development of collagen-rich tissues such as tendons and cardiac valves [34; 60]. Data from the animal and cell study suggested that scleraxis deficiency would decrease collagen 1 expression, and overexpression of scleraxis would increase collagen 1 expression [61]. We found that scleraxis was increased in aortas in SAMP1 mice (Supplemental Fig. S4). Thus, upregulation of TGF-β1 and scleraxis expression would contribute to senescence-associated arterial remodeling and stiffness. BMT from miR-150 KO mice abolished upregulation of TGF-β1 and scleraxis expression which decreases collagen deposition and consequently arterial remodeling and stiffness in SAMP1 mice. Arterial elastin fragmentation impairs arterial compliance. In this study, we found that the elastin levels were decreased while the number of elastin breaks was increased in SAMP1 mice (Fig. 4, Supplemental Figs. S2, S3). The increased aortic elastin fragmentation may be partly attributed to upregulation of MMP2 and MMP9 which fragmentate elastin (Fig. 5). BMT from miR-150 KO mice abolished upregulation of MMP2 and MMP9 expression (Supplemental Fig. S4) which eliminated elastin fragmentation (Fig. 4) contributing to improvements in aortic stiffening in SAMP1 mice (Fig. 3).
Limitations and Perspectives
Accelerated senescence causes arterial stiffness via impairing the humoral immune function. Although microRNAs have been shown to be associated with arterial stiffening [62–64], this study provides the first evidence that transplantation of BMCs from miR-150-KO mice attenuates arterial stiffness and hypertension in SAMP1 mice partly via improving humoral immune function which attenuates vascular inflammation (Fig. S7). It should be mentioned that bone marrow contains several different types of cells including bone marrow stem cells. The limitation of this study is that it cannot determine how transplanted BMCs increase B cell population and promotes IgG generation in SAMP1 mice. Additional studies are warranted for investigating the underlying mechanisms. This study cannot exclude the possibility that other mechanisms in addition to improvement in humoral immune function may also contribute the beneficial effect of BMC transplantation. The data could have been strengthened by increasing the sample size in some assays. We noticed that senescence-associated arterial stiffening and hypertension were decreased to the control level in two weeks following BMT. However, histological and biochemical analysis was not done at two weeks post BMT. Thus, a limitation of this study is that it cannot determine whether, and to what degree, the attenuation in arterial stiffening and hypertension is attributed to improvement in matrix remodeling. The decrease in BP in SAMP1 mice was found as early as one week post BMT. Thus, we cannot exclude the possibility that the transplanted bone marrow cells may decrease vascular tension which leads to a quick drop in BP in SAMP1 mice via stimulating the release of vaso-relaxing factors.
Supplementary Material
Highlights.
Accelerated senescence causes arterial stiffness via impairing the humoral immune function in the senescence-accelerated mouse P1 (SAMP1). Bone marrow cell transplantation from miR-150 knockout mice attenuates arterial matrix remodeling and stiffness and hypertension in SAMP1 mice partly via restoring plasma IgG and IgM levels and improving the humoral immune function which attenuates vascular inflammation.
Acknowledgment
We would like to thank Ms. Mary-Carter Mullins for her critical editing of the manuscript.
Source of Funding
This work was supported by NIH R01 AG062375, AG049780 and HL154147.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests
No.
Disclosures: Nothing to disclose
Financial Disclosure
None.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Oh YS, Berkowitz DE, Cohen RA, Figueroa CA, Harrison DG, Humphrey JD, et al. , 2017. A Special Report on the NHLBI Initiative to Study Cellular and Molecular Mechanisms of Arterial Stiffness and Its Association With Hypertension. Circulation Research 121(11):1216–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sun Z, 2015. Aging, arterial stiffness, and hypertension. Hypertension 65(2):252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, et al. , 2012. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 308(9):875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].AlGhatrif M, Strait JB, Morrell CH, Canepa M, Wright J, Elango P, et al. , 2013. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension 62(5):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, et al. , 2015. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension 66(3):698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laakkonen EK, Karppinen JE, Lehti S, Lee E, Pesonen E, Juppi HK, et al. , 2021. Associations of Sex Hormones and Hormonal Status With Arterial Stiffness in a Female Sample From Reproductive Years to Menopause. Front Endocrinol (Lausanne) 12:765916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chirinos JA, Segers P, Hughes T, Townsend R, 2019. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 74(9):1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jia G, Aroor AR, Sowers JR, 2014. Arterial Stiffness: A Nexus between Cardiac and Renal Disease. Cardiorenal Med 4(1):60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. , 2001. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension 37(5):1236–1241. [DOI] [PubMed] [Google Scholar]
- [10].Shoji T, Emoto M, Shinohara K, Kakiya R, Tsujimoto Y, Kishimoto H, et al. , 2001. Diabetes mellitus, aortic stiffness, and cardiovascular mortality in end-stage renal disease. J Am Soc Nephrol 12(10):2117–2124. [DOI] [PubMed] [Google Scholar]
- [11].Veerasamy M, Ford GA, Neely D, Bagnall A, MacGowan G, Das R, et al. , 2014. Association of aging, arterial stiffness, and cardiovascular disease: a review. Cardiol Rev 22(5):223–232. [DOI] [PubMed] [Google Scholar]
- [12].Mitchell GF, 2009. Clinical achievements of impedance analysis. Med Biol Eng Comput 47(2):153–163. [DOI] [PubMed] [Google Scholar]
- [13].Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. , 2010. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121(4):505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ong KL, Cheung BM, Man YB, Lau CP, Lam KS, 2007. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension 49(1):69–75. [DOI] [PubMed] [Google Scholar]
- [15].Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, et al. , 1995. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 25(3):305–313. [DOI] [PubMed] [Google Scholar]
- [16].Blomberg BB, Frasca D, 2013. Age effects on mouse and human B cells. Immunol Res 57(1–3):354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Castelo-Branco C, Soveral I, 2014. The immune system and aging: a review. Gynecological Endocrinology 30(1):16–22. [DOI] [PubMed] [Google Scholar]
- [18].Elias R, Hartshorn K, Rahma O, Lin N, Snyder-Cappione JE, 2018. Aging, immune senescence, and immunotherapy: A comprehensive review. Seminars in Oncology 45(4):187–200. [DOI] [PubMed] [Google Scholar]
- [19].Sadighi Akha AA, 2018. Aging and the immune system: An overview. Journal of Immunological Methods 463:21–26. [DOI] [PubMed] [Google Scholar]
- [20].Iwai H, Baba S, Omae M, Lee S, Yamashita T, Ikehara S, 2008. Maintenance of systemic immune functions prevents accelerated presbycusis. Brain Res 1208:8–16. [DOI] [PubMed] [Google Scholar]
- [21].Simon AK, Hollander GA, McMichael A, 2015. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282(1821). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lock RJ, Unsworth DJ, 2003. Immunoglobulins and immunoglobulin subclasses in the elderly. Ann Clin Biochem 40(Pt 2):143–148. [DOI] [PubMed] [Google Scholar]
- [23].Buckley CE 3rd, Dorsey FC, 1970. The effect of aging on human serum immunoglobulin concentrations. Journal of Immunology 105(4):964–972. [PubMed] [Google Scholar]
- [24].Han J, Hosokawa M, Umezawa M, Yagi H, Matsushita T, Higuchi K, et al. , 1998. Age-related changes in blood pressure in the senescence-accelerated mouse (SAM): aged SAMP1 mice manifest hypertensive vascular disease. Lab Anim Sci 48(3):256–263. [PubMed] [Google Scholar]
- [25].Hosokawa T, Hosono M, Hanada K, Aoike A, Kawai K, Takeda T, 1987. Immune responses in newly developed short-lived SAM mice. Selectively impaired T-helper cell activity in in vitro antibody response. Immunology 62(3):425–429. [PMC free article] [PubMed] [Google Scholar]
- [26].Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, et al. , 2007. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell 131(1):146–159. [DOI] [PubMed] [Google Scholar]
- [27].Lin YC, Kuo MW, Yu J, Kuo HH, Lin RJ, Lo WL, et al. , 2008. c-Myb is an evolutionary conserved miR-150 target and miR-150/c-Myb interaction is important for embryonic development. Molecular Biology and Evolution 25(10):2189–2198. [DOI] [PubMed] [Google Scholar]
- [28].Bian Z, Li L, Cui J, Zhang H, Liu Y, Zhang CY, et al. , 2011. Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. Journal of Pathology 225(4):544–553. [DOI] [PubMed] [Google Scholar]
- [29].Skrzypczyk P, Zacharzewska A, Szyszka M, Ofiara A, Pańczyk-Tomaszewska M, 2021. Arterial stiffness in children with primary hypertension is related to subclinical inflammation. Cent Eur J Immunol 46(3):336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li H, Bai S, Ao Q, Wang X, Tian X, Li X, et al. , 2018. Modulation of Immune-Inflammatory Responses in Abdominal Aortic Aneurysm: Emerging Molecular Targets. J Immunol Res 2018:7213760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jain S, Khera R, Corrales-Medina VF, Townsend RR, Chirinos JA, 2014. “Inflammation and arterial stiffness in humans”. Atherosclerosis 237(2):381–390. [DOI] [PubMed] [Google Scholar]
- [32].Chen K, Sun Z, 2021. Estrogen inhibits renal Na-Pi Co-transporters and improves klotho deficiency-induced acute heart failure. Redox Biol 47:102173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen K, Wang S, Sun QW, Zhang B, Ullah M, Sun Z, 2021. Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circulation Research 128(4):492–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Espira L, Lamoureux L, Jones SC, Gerard RD, Dixon IM, Czubryt MP, 2009. The basic helix-loop-helix transcription factor scleraxis regulates fibroblast collagen synthesis. J Mol Cell Cardiol 47(2):188–195. [DOI] [PubMed] [Google Scholar]
- [35].Shipley JM, Doyle GA, Fliszar CJ, Ye QZ, Johnson LL, Shapiro SD, et al. , 1996. The structural basis for the elastolytic activity of the 92-kDa and 72-kDa gelatinases. Role of the fibronectin type II-like repeats. J Biol Chem 271(8):4335–4341. [DOI] [PubMed] [Google Scholar]
- [36].Kramer NJ, Wang WL, Reyes EY, Kumar B, Chen CC, Ramakrishna C, et al. , 2015. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood. [DOI] [PubMed] [Google Scholar]
- [37].Tano N, Kim HW, Ashraf M, 2011. microRNA-150 regulates mobilization and migration of bone marrow-derived mononuclear cells by targeting Cxcr4. PLoS One 6(10):e23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li M, Guo K, Ikehara S, 2014. Intractable diseases treated with intra-bone marrow-bone marrow transplantation. Front Cell Dev Biol 2:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ikehara S, 2008. A novel method of bone marrow transplantation (BMT) for intractable autoimmune diseases. J Autoimmun 30(3):108–115. [DOI] [PubMed] [Google Scholar]
- [40].Tha-In T, Bayry J, Metselaar HJ, Kaveri SV, Kwekkeboom J, 2008. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol 29(12):608–615. [DOI] [PubMed] [Google Scholar]
- [41].Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, et al. , 2013. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood 122(8):1419–1427. [DOI] [PubMed] [Google Scholar]
- [42].Galeotti C, Kaveri SV, Bayry J, 2017. IVIG-mediated effector functions in autoimmune and inflammatory diseases. International Immunology 29(11):491–498. [DOI] [PubMed] [Google Scholar]
- [43].Bayry J, Misra N, Latry V, Prost F, Delignat S, Lacroix-Desmazes S, et al. , 2003. Mechanisms of action of intravenous immunoglobulin in autoimmune and inflammatory diseases. Transfusion Clinique et Biologique 10(3):165–169. [DOI] [PubMed] [Google Scholar]
- [44].Kaveri SV, Maddur MS, Hegde P, Lacroix-Desmazes S, Bayry J, 2011. Intravenous immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clinical and Experimental Immunology 164 Suppl 2:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zschuntzsch J, Zhang Y, Klinker F, Makosch G, Klinge L, Malzahn D, et al. , 2015. Treatment with human immunoglobulin G improves the early disease course in a mouse model of Duchenne muscular dystrophy. J Neurochem. [DOI] [PubMed] [Google Scholar]
- [46].Wang X, Wang Q, Sun Z, 2012. Normal IgG downregulates the intracellular superoxide level and attenuates migration and permeability in human aortic endothelial cells isolated from a hypertensive patient. Hypertension 60(3):818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, et al. , 2007. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity 26(1):67–78. [DOI] [PubMed] [Google Scholar]
- [48].Nimmerjahn F, Ravetch JV, 2008. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol 26:513–533. [DOI] [PubMed] [Google Scholar]
- [49].Ebringer A, Doyle AE, 1970. Raised serum IgG levels in hypertension. British Medical Journal 2(5702):146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mikolajczyk TP, Guzik TJ, 2019. Adaptive Immunity in Hypertension. Curr Hypertens Rep 21(9):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chan CT, Sobey CG, Lieu M, Ferens D, Kett MM, Diep H, et al. , 2015. Obligatory Role for B Cells in the Development of Angiotensin II-Dependent Hypertension. Hypertension 66(5):1023–1033. [DOI] [PubMed] [Google Scholar]
- [52].Taylor EB, Barati MT, Powell DW, Turbeville HR, Ryan MJ, 2018. Plasma Cell Depletion Attenuates Hypertension in an Experimental Model of Autoimmune Disease. Hypertension 71(4):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Feng R, Ullah M, Chen K, Ali Q, Lin Y, Sun Z, 2020. Stem cell-derived extracellular vesicles mitigate ageing-associated arterial stiffness and hypertension. J Extracell Vesicles 9(1):1783869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Lin Y, Chen J, Sun Z, 2016. Antiaging Gene Klotho Deficiency Promoted High-Fat Diet-Induced Arterial Stiffening via Inactivation of AMP-Activated Protein Kinase. Hypertension 67(3):564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Song F, Zou J, Song Z, Xu H, Qian Y, Zhu H, et al. , 2020. Association of Adipocytokines With Carotid Intima Media Thickness and Arterial Stiffness in Obstructive Sleep Apnea Patients. Front Endocrinol (Lausanne) 11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Gao D, Zuo Z, Tian J, Ali Q, Lin Y, Lei H, et al. , 2016. Activation of SIRT1 Attenuates Klotho Deficiency-Induced Arterial Stiffness and Hypertension by Enhancing AMP-Activated Protein Kinase Activity. Hypertension 68(5):1191–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lin Y, Kuro-o M, Sun Z, 2013. Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology 154(10):3855–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Koh YC, Yang G, Lai CS, Weerawatanakorn M, Pan MH, 2018. Chemopreventive Effects of Phytochemicals and Medicines on M1/M2 Polarized Macrophage Role in Inflammation-Related Diseases. Int J Mol Sci 19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Arnold CE, Whyte CS, Gordon P, Barker RN, Rees AJ, Wilson HM, 2014. A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo. Immunology 141(1):96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV, 2012. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res 30(4):606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lejard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, et al. , 2007. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem 282(24):17665–17675. [DOI] [PubMed] [Google Scholar]
- [62].Sapp RM, Chesney CA, Eagan LE, Evans WS, Zietowski EM, Prior SJ, et al. , 2020. Changes in circulating microRNA and arterial stiffness following high-intensity interval and moderate intensity continuous exercise. Physiol Rep 8(9):e14431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang C, Wu H, Xing Y, Ye Y, He F, Yin Q, et al. , 2022. Endothelial-derived extracellular microRNA-92a promotes arterial stiffness by regulating phenotype changes of vascular smooth muscle cells. Sci Rep 12(1):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ikonomidis I, Vlastos D, Andreadou I, Gazouli M, Efentakis P, Varoudi M, et al. , 2021. Vascular conditioning prevents adverse left ventricular remodelling after acute myocardial infarction: a randomised remote conditioning study. Basic Research in Cardiology 116(1):9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


