Abstract
The pyrogenic exotoxins of group A streptococci and staphylococcal enterotoxins are a family of structurally related superantigens with similar biological activity. Two distinct areas have been identified which have a highly conserved amino acid homology in all of the toxin families. A number of peptides were constructed from these regions, some of which were concatenated and polymerized to enhance their immunogenicity in animals. Antibodies prepared against these polymerized peptides were used to serologically identify the majority of the superantigen toxins, block the biological activities of the superantigens, and protect an experimental animal model against shock. In addition certain peptides were able per se to block up to 90% of the proliferative responses induced by the toxins. The peptide also proved protective in a septic shock model in mice. Binding experiments indicate that the peptide binds tightly to the major histocompatibility complex class II molecule, thus preventing binding and hence activation of the superantigen. The selective and rapid binding of the peptide to the major histocompatibility complex class II molecule may lead to a novel therapeutic modality in treatment of superantigen-mediated diseases.
Toxic shock syndrome (TSS) is still among the most-life-threatening syndromes affecting humans. In the United States approximately 20,000 cases of TSS occur each year with a 10% mortality rate (7). Present therapy is primarily supportive, with administration of fluids, antibiotics, vasopressor agents, and occasional steroids (20). There is no single immunotherapy available for TSS since all of the superantigens are antigenically distinct.
Septic shock is another disease of medical importance, with 500,000 cases each year in the United States alone of which 200,000 develop shock with a 40% mortality rate (23). While the disease is multifactorial, we offer a “two-hit” hypothesis (3) in which the individual, sensitized by lipopolysaccharide (LPS) released during the initial gram-negative sepsis, becomes highly susceptible to gram-positive toxins released during a second infection, and we believe this hypothesis is quite plausible. Support for this concept can be seen in the well-known observation that LPS and toxins, when administered together to animals, are 1,000-fold more lethal compared to the same molecules given separately (4).
Bacterial superantigens are toxins, mostly from gram-positive bacteria, that cause the stimulation of large populations of T cells. These stimulated T cells produce toxic concentrations of cytokines that have major effects on the host. In order to have their effect, superantigens bridge T-cell-antigen receptors (TCR) and major histocompatibility complex (MHC) class II (MHC-II) antigens binding at concentrations as low as 10−9 mol/liter. In contrast to normal antigens, superantigens are large molecules which are not processed into small peptides and are usually not MHC restricted. The binding of staphylococcal and streptococcal superantigens typically involves (i) a binding site on the MHC-II α-chain (α1 domain) and/or (ii) a zinc-dependent high-affinity site on the MHC-II β-chain (β1 domain) (5, 16). Bacterial superantigens are directly responsible for a number of important clinical syndromes including TSS.
We have developed peptides and antipeptide antibodies constructed from the most conserved regions of a number of bacterial superantigens. These proteins demonstrate an ability to markedly inhibit the T-cell proliferative response of all the bacterial superantigens tested in vitro. The present study was undertaken to determine the extent, in vivo functional importance, specificity, and nature of this unique inhibition.
MATERIALS AND METHODS
Superantigens.
All superantigens were purchased from Toxin Technology (Sarasota, Fla.), except for the newly characterized streptococcal pyrogenic exotoxins (SPEs) SPEG, SPEH, and SPEZ (19), which were kindly supplied by J. Fraser (Auckland, New Zealand).
Peptide construction.
All peptides were constructed by solid-phase synthesis according to standard methods (15, 18), and high-performance liquid chromatographic analysis revealed that all peptides had greater than 95% purity. These peptides were kindly provided by M. Patarroyo, Institut Immologica, Bogota, Columbia.
Antibody production.
WNZ female rabbits weighing approximately 3 kg each were used for the injections. The initial injection was 500 mg of polymerized peptide 6348 (both peptide regions) in complete Freund's adjuvant. Two boosters in incomplete adjuvant were then given 21 days apart. Antibody titers of 106/ml were routinely obtained using enzyme-linked immunosorbent assay (ELISA) plates coated with peptide 6348. The larger polymerized peptides are known to be more immunogenic (17).
Immunoblots.
Each of the staphylococcal exotoxins (SEs) and SPEs were electropheresed through a sodium dodecyl sulfate (SDS)-15% polyacrylamide gel electrophoresis gels and transferred to nitrocellulose for Western blotting. The Western blots were developed using the rabbit anti-peptide 6348 serum diluted at 1:5,000, followed by goat anti-rabbit immunoglobulin G (IgG) alkaline phosphatase conjugate (Sigma). A similar gel was stained with Coomassie blue for molecular weight locations and proteins.
Protein A Enrichment of peptide 6348 antibody.
Some of the peptide 6348 antibody raised in rabbits was further enriched on a column as described below. In brief, the column of protein A-Sepharose (15 ml) (Sigma) beads was prepared at room temperature. Ten milliliters of immune rabbit serum, diluted 1:1 in phosphate-buffered saline (PBS), was run through the column. The column was then washed with four column volumes of PBS and eluted with 10 ml of 0.1 M NaAc (pH 2.5), and 10 1-ml fractions were collected. One hundred microliters of 1 M sodium bicarbonate was added to each fraction to neutralize the acidic elution step. Protein elution and concentration were then determined via spectrophotometer, and the protein A-enriched antibody preparation was readjusted appropriately to be equal to the anti peptide titers of the original serum. Similar experiments were performed with the streptococcal group A carbohydrate antibody.
Proliferation assays.
Human peripheral blood mononuclear cells (PBMCs) were isolated by standard Ficoll-Hypaque techniques and adjusted to 2 × 106 cells/ml. PBMCs (2 × 105) in 200 μl of complete medium (RPMI medium 1640 + 10% human type AB serum) were placed in 96-well titer plates and stimulated with various doses of superantigen or with a combination of each toxin and various doses of peptide. The cells were incubated for 6 days, and the results were measured via tritiated thymidine incorporation. The data presented are averages of the results of four different experiments. All tests were done in triplicate.
Viability studies.
Human PBMCs were isolated as described above, and 2 × 105 cells were placed in 96-well titer plates. Phytohemagglutinin (PHA) was added at a concentration of 5 μg/well. All peptides were added to PHA at a concentration of 200 μg/well. The plates were incubated at 37°C in a CO2 incubator for 72 h at which time tritated thymidine was added to the cells. The cells were harvested 18 h later, and the counts per minute of tritated thymidine were measured. All experiments were carried out in triplicate.
A second viability test was performed as follows: 2 × 105 PBMCs were plated in 96-well titer plates to which various concentrations of peptide 6343 were added. Aliquots of cells with and without peptide were stained with trypan blue each day for 5 days to observe viability of the cells in the presence or absence of peptide.
Rabbit experiments.
Female NZW rabbits >1 year of age were obtained from Hazelton Dutchland Labs, Inc. (Denver, Pa.). Older animals are used in these experiments because they are more sensitive to the effects of LPS and toxins (9). The lethal shock experiments were performed as follows: Animals were given 50 μg of either SEB or SPEA toxins per kg of body weight, mixed with 0.5 ml of either preimmune normal rabbit serum or anti-peptide antibody. Four hours later, all rabbits received 5 μg of LPS (List Biological Labs, Campbell, Calif.) per kg of body weight. Animals were monitored for 72 h for clinical signs of toxicity. Moribund animals were sacrificed with an overdose of nembutal. All toxins and LPS were delivered intravenously.
Murine experiments.
Eight-week-old Female BALB/c mice were used for all experiments. Animals were housed at the Rockefeller Laboratory Animal Research Facility, and experiments were undertaken after approval by institutional animal care and ethics boards. All mice were sensitized with 0.001 mg of LPS and 20 mg of d-galactosamine via intraperitoneal injection (4). Eight hours later, mice were injected with various doses of superantigen that had been shown to cause 100% lethality. In protection experiments, at 6 h postinoculation saline or 1.5 mg of the peptide was administered to the experimental mice by subcutaneous injection. One hour later, the mice were injected again with either saline or 1.5 mg of peptide (3.0 mg total). One hour after the second injection, all mice were challenged with the appropriate dose of toxin (via intraperitoneal injection), and the mice were observed for 24 to 48 h.
Endotoxin determination.
Assays were performed with a stimulus a mebocyte lysate chromogenic ELISA (Associates of Cape Cod). Control standards and solutions were provided with the kit. Control standard endotoxin was reconstituted and was used to construct a standard curve. The negative control, control standard, test samples, and a positive control were plated on a Linbro 96-well ELISA plate (ICN Biomedicals Inc., Aurora, Ohio). Pyrochrome was placed in each well to yield a 1:1 solution. The plate was agitated for 30 s to ensure incorporation. The plate was then incubated for 30 min in a 37°C water bath. After 30 min, the plate was read at an optical density of 405 nm. Using the standard curve, endotoxin concentrations of the toxin solutions were determined (14).
MHC class II binding assay.
The plates were coated with immunoaffinity-purified soluble human DR-1 (kindly provided by J. Strominger, Harvard University) overnight at 4°C in 0.1 M Tris (pH 8.0) at a concentration of 1 μg per well. A solution of 1% bovine serum albumin in PBS was used to block the coated plates for 1 h. The primary antibody (anti-6348) was diluted in RPMI 1640, added to the wells of the plate, and incubated for 1 h. Horseradish peroxidase-conjugated antibodies of appropriate affinity were used at a dilution of 1:1,000. One hundred microliters of a 1:1 mixture of hydrogen peroxide and TMB substrate (Kirkegaard and Perry Inc.) was applied in the dark for 20 min after which the plate was read. All incubation steps were carried out at room temperature. Plates were washed three times with ELISA wash buffer between every incubation step. The pH of the binding medium was adjusted to ensure that all assays were at pH 7.0. Care was taken to ensure that the ionic strength was adjusted for in each assay. Apparent Kd, the dissociation constant at equilibrium, was calculated using the Lineweaver-Burk equation (21) as previously described by Fridkis-Hareli and Strominger (6).
RESULTS
Peptide and antibody design.
Two distinct regions of the SE/SPE toxins have been identified which share highly conserved amino acid homology, and consensus patterns have been identified as common to all members of this family of toxins (3). The first consensus region has the amino acid sequence Y-G-G-(LIV)-T-x(4)-N. This pattern has been identified in all of the SEs and SPEs pyrogenic exotoxins, but not in TSS toxin 1 (TSST-1). The sequence begins immediately at the COOH-terminal side of the cysteine loop. In selected cases we have given the amino acid sequence without the leader sequence for more appropriate orientation. The second consensus region has the following amino acid sequence: K-x(2)-(LIV)-x(4)-(LIV)-d-x(3)-r-x(2)-l-x(5)-(LIV)-Y. This pattern had been identified in all of the SEs and SPEs, including TSST-1 (Fig. 1A).
FIG. 1.
(A) Alignment of the two highly conserved regions of the SE/SPE family of toxins and TSST-1. Amino acid residue positions of the regions flank the sequences. The asterisks refer to SEB amino acid numbers without the leader sequences. (B) The sequences of the various peptides constructed.
Considering the high degree of conservation of the SE/SPE toxins, and the immunologic cross-reactivity previously mentioned for these toxins, the possibility that antibodies raised against the two conserved regions might block the biological activities of all toxins was considered (2). A number of peptides were constructed based on the consensus sequences described above. These were made and purified using high-performance liquid chromatography according to previous protocols (17). As illustrated in Fig. 1B, peptides 6343 and 6344 were derived from the first consensus sequence, peptides 6345 and 6346 were constructed from the second consensus sequence, and peptides 6347 and 6348 were combinations of both sequences attached in the proper order. Peptides 6344, 6346, and 6348 were polymerized with the addition of cysteine residues at the N and C terminals with glycine molecules used as spacers. This process was used to increase the immunogenicity of these peptides for vaccination (18).
Anti-peptide antibody activity in Western blotting.
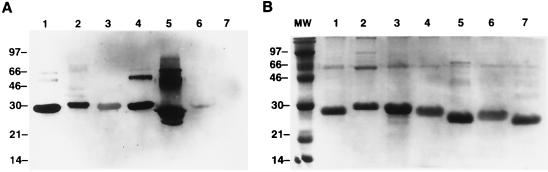
Peptides constructed of the combined consensus regions of the toxins (6348) were used to raise antibodies in rabbits. Sera from all rabbits showed similar antibody production. Western blots indicated that the peptide antiserum was able not only to recognize the conserved regions of SEB but also those of other streptococcal and staphylococcal toxins including SEA, SEB, SEC, SED, SPEA, and SPEC, but not TSST-1 (Fig. 2A). Figure 2B is an SDS-15% gel with the same toxins stained with Coomassie blue. As seen in both the Western blot and in the stained gel, the upper bands most likely represent either dimers of the toxins or partially digested larger-molecular-size fractions of the toxins. The major bands correspond to the known molecular size of the toxins. Similar results were obtained using sera from three other rabbits (data not shown).
FIG. 2.
(A) SDS-15% gel of various toxins transferred to and developed by Western blot. The blot demonstrates the fact that the 6348 anti-peptide antibody (raised in rabbit) immunoprecipitates with SEA (lane 1), SEB (lane 2), SEC (lane 3), SED (lane 4), and SPEA (lane 5), and a faint band is seen with SPEC (lane 6). No band was seen with TSST-1 (lane 7). (B) SDS-15% gel of the same toxins stained with Coomassie blue. All lanes were loaded with 5 μg of each toxin. Upper bands most likely represent dimers or partially digested forms of the toxins. The major bands are seen between 28 and 25 kDa, corresponding to their known molecular sizes.
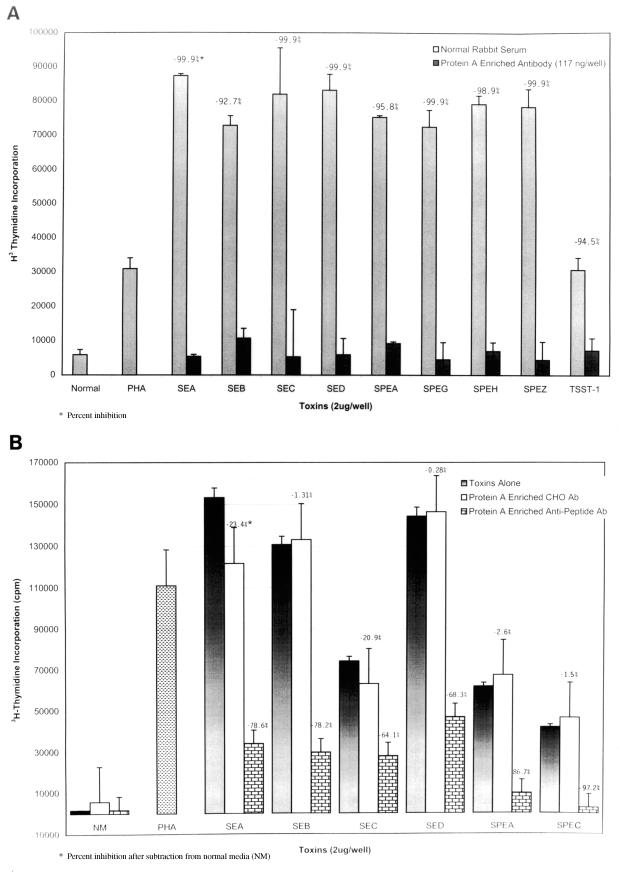
The effect of protein A-enriched anti-peptide antibody on blastogenesis.
To further enrich our serum anti-peptide antibodies, we isolated the IgG fraction of our anti-peptide antibody using a protein A column and used these antibodies in experiments similar to that described above. The antibodies showed strong inhibition of blastogenesis to all of the staphylococcal and streptococcal superantigens tested (Fig. 3A). In addition we determined that 117 ng of total IgG was enough to achieve 93 to 100% inhibition of all the superantigens. Figure 3B demonstrates that a high-titer anti-group A streptococcal carbohydrate antibody enriched in a similar manner was unable to block the biological properties of the toxins.
FIG. 3.
(A) Inhibition of toxin blastogenesis of PBMCs by protein A-enriched anti-peptide antibody. PBMCs (2 × 105) were stimulated with either 2 μg of each toxin or a combination of 2 μg of each toxin with anti-peptide antibody. These cells were incubated for 6 days, and the results were measured via tritiated thymidine incorporation. cpm, counts per minute. (B) Note the lack of inhibition of blastogenesis when a high-titered group A carbohydrate antibody (Ab) was used in the assay.
Rabbit challenge experiments.
To determine whether our anti-peptide antibody could block the lethal effects of the toxins, animals were given either toxins plus preimmune normal rabbit serum or anti-peptide antibody. As seen in Table 1, all rabbits who received anti-peptide antibody survived while rabbits that received toxin plus pre immune rabbit serum did not.
TABLE 1.
Passive protection of rabbits with anti-peptide antibodya
| Toxin (dosage) | Anti-peptide antibody | Live/total |
|---|---|---|
| Control | 2/2 | |
| SEB (50–100 μg/kg) | − | 0/3 |
| SEB (50–100 μg/kg) | + | 2/2 |
| SPEA (50–100 μg/kg) | − | 0/2 |
| SPEA (50–100 μg/kg) | + | 2/2 |
Rabbits were given 5 μg of LPS/Kg of body weight alone (control) or in combination with the indicated toxin with (+) or without (−) the anti-peptide antibody.
Direct peptide inhibition of blastogenesis.
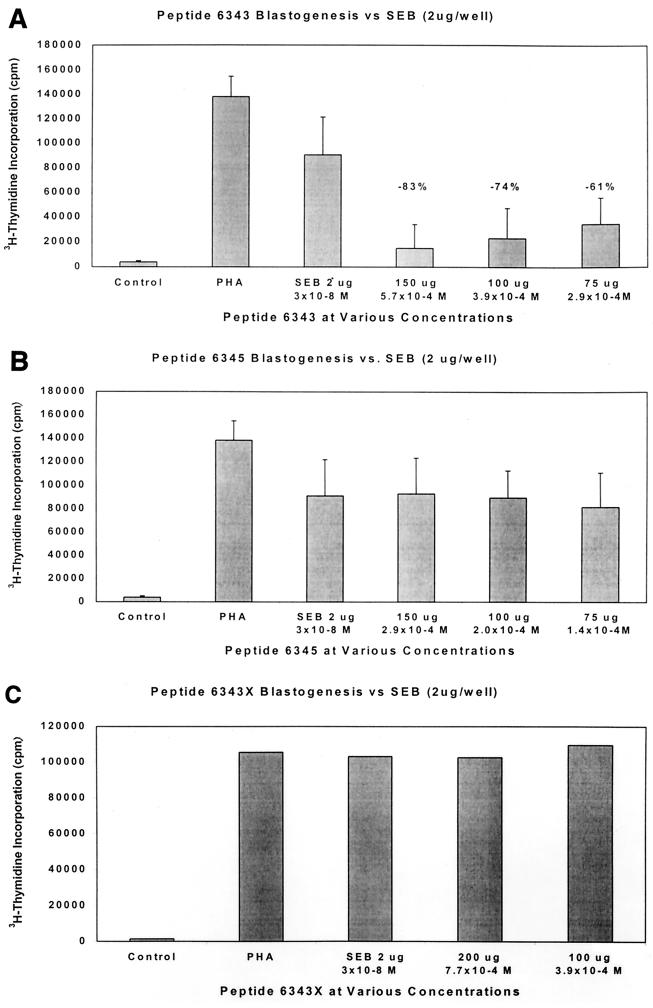
Theoretically, the peptides we designed could act as peptidomimetics and directly inhibit superantigen stimulation of T cells. Using various concentrations of peptide 6343, up to 83% inhibition of blastogenesis was seen (Fig. 4A). This inhibition was dose responsive in nature and indicated probable competitive inhibition. Other peptides tested, including 6346 and 6348, also demonstrated some inhibition but less than that induced by 6343 (data not shown), whereas other peptides including 6345 (Fig. 4B) showed no inhibition by the peptide. Most important was our observation that a single-amino-acid substitution of a lysine for cysteine in the original 6343 peptide at the N-terminal-end-labeled 6343X resulted in complete loss of the original blocking activity (Fig. 4C). These experiments indicate the specificity of the 6343 peptide for the inhibition of blastogenesis.
FIG. 4.
(A) Inhibition of SEB toxin blastogenesis of PBMCs by the 6343 peptide PBMCs (2 × 105) were stimulated with either 2 μg of SEB or a combination of 2 μg of SEB with various doses of the peptide. These were incubated for 6 days, and the results were measured via tritiated thymidine incorporation. cpm, counts per minute. Note the dose-related inhibition of SEB tested by the 6343 peptide. Data are the averages of four experiments, and error bars are the standard errors of the mean. ∗P < 0.05 equals the difference between SEB and all doses of the peptide. The molar concentrations of toxins and peptide are also given. (B) Noninhibition of SEB toxin blastogenesis of PBMCs by the 6345 peptide under the conditions described above. (C) Noninhibition of SEB toxin blastogenesis of PBMCs by the 6343X peptide (lysine substituted for cysteine) under the conditions described above.
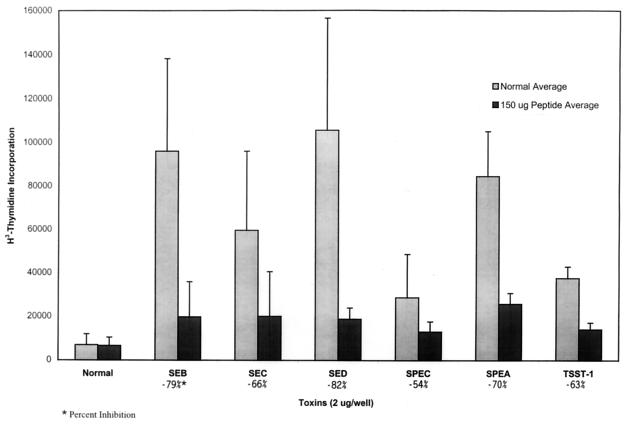
The most striking feature about this inhibition was that it inhibited all of the streptococcal and staphylococcal toxins tested, including TSST-1, which shows no sequence homology to the 6343 peptide (see Fig. 5).
FIG. 5.
Inhibition by the 6343 peptide of PBMC blastogenesis by various toxins. PBMCs (2 × 105) were stimulated with 2 μg of each of the various toxins or a combination of 2 μg of each toxin with 150 μg of peptide. These cells were incubated for 6 days, and the results were measured via tritiated thymidine incorporation. Note that the single peptide (6343) inhibited all of the superantigens tested.
Peptide inhibition of newly described SEs.
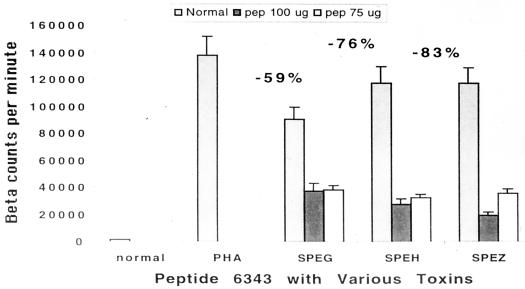
We tested the three new SEs recently described by Proft et al. (19). These were identified and cloned from the Streptococcus pyogenes M1 genomic database at the University of Oklahoma. These toxins have been shown to have the characteristics of superantigens, including specific Vβ stimulation profiles. Peptide 6343 in doses of 75 and 150 μg/well showed between 59 and 83% inhibition of blastogenesis of PBMCs (Fig. 6).
FIG. 6.
Inhibition of PBMC blastogenesis of three newly described SEs by peptide 6343. PBMCs (2 × 105) were stimulated with either 2 μg of each toxin or a combination of 2 μg of each toxin with various doses of peptide. These were incubated for 6 days, and the results were measured via tritiated thymidine incorporation. Note the strong inhibition of all three toxins by 6343 peptide. Addition of 100 μg of peptide did not inhibit the PHA stimulation.
Viability studies.
While the studies described above indicated that the peptide was quite specific in its inhibition of the toxins, we wanted to be sure that the peptide was not interfering with cell function in some other manner. We approached this question in two different ways.
First, 200 μg each of different lots of 6343 peptide including a dimer form of 6343 was added to 2 × 105 cells per well in complete medium in a 96-well microtiter plate. Controls were 2 × 105 cells/well without peptide. Aliquots were removed from the wells each day. The cells were centrifuged at 250 × g and diluted 1:1 with trypan blue, and the numbers of viable cells were counted each day. Daily inspection of the cells revealed that the same number of cells remained viable throughout the 5 days, with or without added peptides.
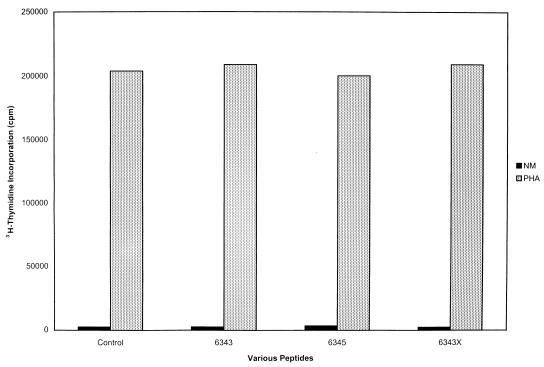
The second approach was to test the PHA stimulation of cell with and without different peptides in a 72-h blastogenesis assay. Peptides 6343, 6345, and 6343X, as described in Fig. 4, were added at concentrations of 200 μg of each peptide to PHA-stimulated PBMCs. As seen in Fig. 7, the addition of each peptide to PHA-stimulated cells did not inhibit PHA blastogenesis, attesting to the viability of the PHA-stimulated cells in the presence of the peptide.
FIG. 7.
Stimulation of human PBMCs with PHA in the presence or absence of peptides 6343, 6345, and 6343X. All peptides were added at a concentration of 200 μg/well, and all experiments were carried out in triplicate. PHA was added at a concentration of 5 μg/well. Note that the addition of each peptide did not decrease the PHA stimulation of the cells, attesting to the viability of the cells in the presence of the peptide.
“Two-hit” animal model of septic shock.
Based on a model of the synergistic effects of LPS and the toxins when administered together (3, 4), called the “two hit”model of septic shock, we established an animal model of septic shock. After priming with d-galactosamine (20 mg/mouse), BALB/c mice (Jackson Laboratory) were challenged intraperitoneally with LPS followed by SEB. The results demonstrated that extremely small amounts of LPS and SEB were needed to effect lethality (4, 13). The synergy between these two mediators of shock was extremely impressive and extended for at least an 18-h period. We chose an 8-h delay between the two toxins for our model to mimic the clinical picture of septic shock. We established and optimized doses of toxins that would lead to 100% lethality (see Table 2).
TABLE 2.
Minimum doses of LPS and/or toxins needed to induce lethality either alone or in the two-hit shock modela
| Experimental groupb | Dosage (μg) of
|
Response (live mice/total mice) | ||
|---|---|---|---|---|
| LPSc | SEB | SPEA | ||
| Group I (single inoculum) | ||||
| LPS alone | 100 | 0 | 0 | 0/3 |
| 10 | 0 | 0 | 0/3 | |
| 5 | 0 | 0 | 2/3 | |
| SEB alone | 0 | 10 | 0 | 0/3 |
| 0 | 2.5 | 0 | 0/3 | |
| 0 | 1.0 | 0 | 0/3 | |
| SPEA alone | 0 | 0 | 10 | 0/3 |
| 0 | 0 | 5 | 0/3 | |
| 0 | 0 | 2.5 | 2/3 | |
| Group II (“two-hit” inoculum) | ||||
| LPS + SEB | 0.01 | 0.02 | 0 | 0/3 |
| 0.001 | 0.02 | 0 | 0/3 | |
| 0.0005 | 0.02 | 0 | 3/3 | |
| LPS + SPEA | 0.01 | 0 | 0.1 | 0/3 |
| 0.001 | 0 | 0.1 | 0/3 | |
| 0.0005 | 0 | 0.1 | 2/3 | |
Note the marked synergism of up to 1,000-fold when the toxin is used in conjunction with LPS.
Three mice were used in each phase of the experiment.
Animals receiving LPS also received 20 mg of d-galactosamine each.
Peptide inhibition in animal model of sepsis.
Our preliminary experiments (Table 2) indicated that at a concentration of LPS of 0.001 μg in d-galactosamine-primed mice, only small amounts of toxins were needed to cause 100% lethality in the mice. This LPS dosage combined with 0.2 μg of SPEA, 0.02 μg of SEB, or 0.2 μg of TSST-1 resulted in approximately 100% lethality in three control groups of six mice each.
Introduction of peptide 6343 at a total of 3 mg per mouse injected subcutaneously at 2 and 1 h before administration of the above toxins completely protected most of the mice for each toxin tested (ratio of surviving mice per experimental group: SPEA + 6343, 6/6 (100%), SEB + 6343, 5/6 (83%); TSST-1 + 6343, 6/6 (100%). Studies in which the peptide is given at specified intervals after the administration of toxin are currently under investigation.
Direct binding of peptide to MHC-II molecules.
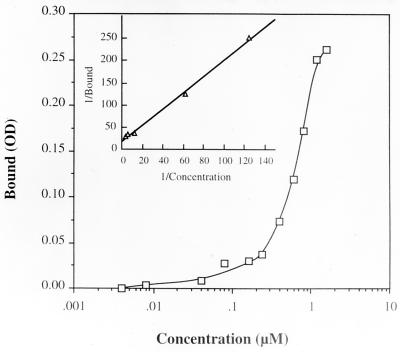
Using an ELISA technique and purified human DR-1 molecules (with and without peptides in the groove) supplied by J. Strominger (Harvard University), we were able to demonstrate strong binding of the 6343 peptide to the DR-1 molecule in the nanomolar range. The results are shown in Fig. 8.
FIG. 8.
Binding of 6343 peptide to soluble human DR-1. The plates were coated with immunoaffinity purified soluble human DR-1 (kindly provided by J. Strominger, Harvard University) overnight at 4°C in 0.1 M Tris (pH 8.0) at a concentration of 1 μg per well. A solution of 1% bovine serum albumin in PBS was used to block the coated plates for 1 h. Peptide 6343 was added to the wells at various concentrations and allowed to incubate for 1 h. After washing in ELISA wash buffer three times, the rabbit anti-peptide (6348) at a dilution of 1:500 was added and incubated for another hour. After washing, horseradish peroxidase-conjugated signal antibody was used at a dilution of 1:1,000. One hundred microliters of a 1:1 mixture of H2O2 and TMB substrate were applied in the dark for 20 min, after which the plate was read. All incubation steps were carried out at room temperature. Inset is the Lineweaver-Burk plot of the binding data. The apparent Kd was calculated to be 1.1 × 10−9 M.
DISCUSSION
In this report we have clearly demonstrated that both the 6343 peptide and the antibody to the 6348 peptide can independently block the proliferative effects of all of the staphylococcal and streptococcal superantigens and that this inhibition is specific for the 6343 peptide. We have also demonstrated that the anti-peptide antibody can provide passive protection against toxic shock in a rabbit model. In addition the peptide itself is protective in a two-hit model of septic shock described above.
This is not the first time synthesized peptides have been used to study the structure-function relationship of the various toxins. A number of synthetic peptides corresponding to different regions of various superantigens including SEA and TSST-1 have been studied by other researchers (5, 8, 11, 12). Important sites for cytokine production and other functions were found including inhibiting peptides. However, we are the first to describe a single peptide that inhibits all of the known bacterial superantigens.
The amount of peptide required to achieve the blocking effects (i.e., 1,000-fold higher than the amount of toxin administered) against the toxins is puzzling. This is especially true because the peptide has such high-affinity binding to MHC-II molecules. However, the fact that unrelated peptides or the lysine-substituted peptide 6343X did not inhibit the proliferative activity of the toxins (Fig. 4) strengthens our belief that the inhibition is specific for peptide 6343. Whether the peptide is degraded by serum enzymes or needs to be in excess to cover the MHC receptor sites for the toxins is unknown and is currently under investigation.
As the various superantigen toxins bind and stimulate different Vβ regions of the TCR, we believe the more likely site of binding of the 6343 peptide is to the MHC-II molecule. This hypothesis is strengthened by the fact that TSST-1, which does not have any sequence similarity to the first consensus region (Fig. 1), is nevertheless inhibited (50%) by the peptide in the proliferation assay. While this concept is attractive, it should be emphasized that the peptide does not appear to bind to the residues of SEB recognized to bind to the MHC molecule (10). However, it is true that a cysteine is involved in the interaction between the superantigens and the MHC. The observation that substitution of a lysine for the N-terminal cysteine in our peptide abolished its biological activity suggests that the cysteine might play an important role in the inhibition of the binding of superantigens to the MHC.
Using SEA as a template, the two regions of the superantigens from which the peptides are made are the β5 region and part of the α4 helix. These areas, although they are highly conserved, are not known binding regions of the superantigen to the MHC molecule. Also, neither seems to represent surface epitopes. However, it is clear from the evidence presented that the peptide (especially the one that is derived from the β5 region) binds very strongly to the intact MHC-II molecule. We believe that the 6343 peptide itself in its 12-amino-acid configuration (with its lack of tertiary structure) is able to fit into the MHC superantigen-binding area and thus compete with the natural superantigens. This effect is very specific because either a single amino acid variation of the peptide sequence or an unrelated peptide leads to poor binding and lack of functional activity. Experiments in which alanine has been substituted for each amino acid of the 12-amino-acid peptide are currently underway to determine whether other amino acids are important for the biological activity of this peptide.
The antibody to the same core regions of the superantigens described above also has substantial activity against the various superantigens. As demonstrated in the Western blot (Fig. 2), the antibody clearly binds to the majority of the superantigens with the exception of TSST-1. The binding of the antibody to the superantigens, even though the residues are not normally exposed, indicates that either the antibody binding involves some aspect of the tertiary structure of the antibody or buried residues are variably exposed. The disparity between the lack of serological binding of the antibody to TSST-1 and the inhibition of TSST-1 proliferation by the peptide emphasizes the fact that serological recognition or lack thereof to a given toxin does not always predict the biological activities of these toxins with respect to the peptides.
Functionally we have shown that our peptide is able to effectively block the clinical effects of superantigens in a two-hit mouse model of septic shock. Using this model, we have shown that the peptide can block the effects of the toxins even when the interaction between the superantigens and LPS enhances the lethal potency of both antigens by 1,000-fold. This may account for the fact that endotoxin-mediated events are, in many circumstances, insufficient to explain the deleterious effects of septic shock in clinical practice. Hence, at least two independent pathways of lethal shock can occur. LPS and peptidoglycan interact with macrophages whereas superantigens interact with T cells. In both cases target cells are induced to release large amounts of cytokines. Since both gram-negative and gram-positive organisms frequently can be recovered from patients with sepsis, we believe a two-hit hypothesis is operative and that the interaction between LPS and the superantigens markedly enhances the lethal properties of both molecules. The interruption of the toxin pathway by peptides may prevent the onset of lethal shock induced by the combination of the two molecules.
Repeated injections of the 6343 superantigen peptide (nonpolymerized) into normal rabbits even with the addition of Freund's adjuvant failed to produce any antibodies. This suggests that the smallest peptide (6343) is poorly immunogenic (data not shown) and thus can be used for repeated administration as a therapeutic agent.
All peptides and reagents were tested for endotoxin contamination using the Limulus amebocyte lysate assay and were shown to have less than 0.1 ng of endotoxin per ml (9). These results indicate that endotoxin itself did not play an important role in these experiments. Our preliminary evidence confirms the specific binding of the peptide directly to MHC-II molecules and suggests that it does not affect the normal immune function of this molecule.
During the drafting of this manuscript, Arad et al. (1) published findings similar to but not identical to ours. Using a slightly different and shorter segment of region II (see Fig. 1), YNKKKATVQELD, which is a variant of SEB (150-TNKKKVTAQELD-161), they were able to inhibit expression of interleukin 2 RNA by 18- to 40-fold after stimulation with the native toxin. Arad et al. also noted that their peptide was not close to the domains of SEB known to participate in binding to the TCR or MHC-II molecules. They tested only a limited number of toxins while our inhibition was present in a large number of toxins tested. Their peptide was also able to rescue mice from toxic shock in a mouse model similar to ours. No binding studies were carried out by these authors. An interesting and as yet unexplained feature of their studies was that mice protected by the peptide and toxin challenge were then resistant to a second challenge of the same toxin 2 weeks later, even though no peptide was administered.
In summary, these experiments indicate that both peptides and antibodies thereto directly block the interaction of staphylococcal and streptococcal superantigens, with their binding site thus preventing the proliferative and massive inflammatory responses these antigens usually generate. Vaccination with either the combination peptide (6348) or potentially the smaller peptide (6344) conjugated to an appropriate carrier may be protective in humans against a large number of bacterial superantigen toxins. The peptide may prove to be useful directly or indirectly as an antigen in a vaccine for the treatment of both TSS and septic shock as well as other diseases that are due to superantigen activation.
REFERENCES
- 1.Arad G, Levy R, Hillman D, Kaempfer R. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat Med. 2000;6:414–420. doi: 10.1038/74672. [DOI] [PubMed] [Google Scholar]
- 2.Bannan J D, Mingo F, Viteri A, Zabriskie J B. Neutralization of streptococcal pyrogenic exotoxins and staphylococcal enterotoxins by antisera to synthetic peptides representing conserved amino acid motifs. Adv Exp Med Biol. 1997;418:903–907. doi: 10.1007/978-1-4899-1825-3_211. [DOI] [PubMed] [Google Scholar]
- 3.Bannan J, Visvanathan K, Zabriskie J B. Structure and function of streptococcal and staphylococcal superantigens in septic shock. Infect Dis Clin North Am. 1999;13(2):387–96. doi: 10.1016/s0891-5520(05)70081-7. [DOI] [PubMed] [Google Scholar]
- 4.Blank C, Luz A, Bendigs S, Erdmann A, Wagner H, Heeg K. Superantigens and endotoxin synergize in the induction of lethal shock. Eur J Immunol. 1997;27(4):825–833. doi: 10.1002/eji.1830270405. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson A, Holm S E, Norgren M. Identification of domains involved in superantigenicity of streptococcal pyrogenic exotoxin F (SpeF) Microb Pathog. 1998;25:279–290. doi: 10.1006/mpat.1998.0234. [DOI] [PubMed] [Google Scholar]
- 6.Fridkis-Hareli M, Strominger J L. Promiscuous binding of synthetic copolymer 1 to purified HLA-DR molecules. J Immunol. 1998;160(9):4386–4397. [PubMed] [Google Scholar]
- 7.Howe L M. Treatment of endotoxic shock: glucocorticoids, lazaroids, nonsteroidals, others. Vet Clin N Am Small Anim Pract. 1998;28:249–267. doi: 10.1016/s0195-5616(98)82004-4. [DOI] [PubMed] [Google Scholar]
- 8.Hu D L, Omoe K, Nakane A, Sugii S, Ono K, Sasaki S, Shinagawa K. Studies on the functional site on staphylococcal enterotoxin A responsible for production of murine gamma interferon. FEMS Immunol Med Microbiol. 1999;25:237–244. doi: 10.1111/j.1574-695X.1999.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 9.Hull D, McIntyre J, Vinter J. Age-related changes in endotoxin sensitivity and the febrile response of newborn rabbits. Biol Neonate. 1993;63:370–379. doi: 10.1159/000243957. [DOI] [PubMed] [Google Scholar]
- 10.Jardetzky T, Brown J, Gorga J, Stern L, Urban R, Chi Y, Stauffacher C, Strominger J, Wiley D. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994;368:711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- 11.Jett M, Neill R, Welch C, Boyle T, Bernton E, Hoover D, Lowell G, Hunt R E, Chatterjee S, Gemski P. Identification of staphylococcal enterotoxin B sequences important for induction of lymphocyte proliferation by using synthetic peptide fragments of the toxin. Infect Immun. 1994;62:3408–3415. doi: 10.1128/iai.62.8.3408-3415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kum W W, Laupland K B, Chow A W. Defining a novel domain of staphylococcal toxic shock syndrome toxin-1 critical for major histocompatibility complex class II binding, superantigenic activity, and lethality. Can J Microbiol. 2000;46:171–179. [PubMed] [Google Scholar]
- 13.Leonard B A, Schlievert P M. Immune cell lethality induced by streptococcal pyrogenic exotoxin A and endotoxin. Infect Immun. 1992;60:3747–3755. doi: 10.1128/iai.60.9.3747-3755.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay G K, Roslansky P F, Novitsky T J. Single-step, chromogenic Limulus amebocyte lysate assay for endotoxin. J Clin Microbiol. 1989;27:947–951. doi: 10.1128/jcm.27.5.947-951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrifield B. Solid phase synthesis. Science. 1986;232:341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- 16.Olson M A, Cuff L. Molecular docking of superantigens with class II major histocompatibility complex proteins. J Mol Recognit. 1997;10:277–289. doi: 10.1002/(SICI)1099-1352(199711/12)10:6<277::AID-JMR376>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.Patarroyo M E, Romero P, Torres M L, Clavijo P, Moreno A, Martinez A, Rodriquez R, Guzman F, Cabezas E. Induction of protective immunity against experimental infection with malaria using synthetic peptides. Nature. 1987;328:629–632. doi: 10.1038/328629a0. [DOI] [PubMed] [Google Scholar]
- 18.Patarroyo M E, Amador R, Clavijo P, Moreno A, Guzman F, Romero P, Tascon R, Franco A, Murillo L A, Ponton G, et al. A synthetic vaccine protects humans against challenge with asexual blood stages of Plasmodium falciparum malaria. Nature. 1988;332:158–161. doi: 10.1038/332158a0. [DOI] [PubMed] [Google Scholar]
- 19.Proft T, Moffatt S L, Berkahn C J, Fraser J D. Identification and characterization of novel superantigens from Streptococcus pyogenes. J Exp Med. 1999;189:89–102. doi: 10.1084/jem.189.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoenberg M H, Weiss M, Radermacher P. Outcome of patients with sepsis and septic shock after ICU treatment. Langenbecks Arch Surg. 1998;383(1):44–48. doi: 10.1007/s004230050090. [DOI] [PubMed] [Google Scholar]
- 21.Segel I H. Behavior and analysis of rapid equilibrium and steady-state enzyme systems. New York, NY: John Wiley & Sons, Inc.; 1975. Enzyme kinetics; pp. 107–108. [Google Scholar]
- 22.Soos J M, Johnson H M. Multiple binding sites on the superantigen, staphylococcal enterotoxin B, imparts versatility in binding to MHC class II molecules. Biochem Biophys Res Commun. 1994;201:596–602. doi: 10.1006/bbrc.1994.1743. [DOI] [PubMed] [Google Scholar]
- 23.Weiss K A, Laverdiere M. Group A Streptococcus invasive infections: a review. Can J Surg. 1997;40(1):18–25. [PMC free article] [PubMed] [Google Scholar]