Abstract
Background and aims
The United Kingdom (UK) Psychoactive Substances Act (PSA), implemented on the 26th May 2016, made the production, supply and sale of all non‐exempted psychoactive substances illegal. The aim of this study was to measure trends in hospital presentations for severe toxicity following analytically confirmed synthetic cannabinoid receptor agonist (SCRA) exposure before and after implementation of the PSA.
Design
Observational study.
Setting
Thirty‐four hospitals across the UK participating in the Identification of Novel Psychoactive Substances (IONA) study.
Participants
A total of 627 (79.9% male) consenting individuals who presented to participating hospitals between July 2015 and December 2019 with severe acute toxicity and suspected novel psychoactive substances exposure.
Measurements
Toxicological analyses of patient samples were conducted using liquid‐chromatography tandem mass‐spectrometry. Time‐series analysis was conducted on the monthly number of patients with and without analytically confirmed SCRA exposure using Poisson segmented regression.
Findings
SCRAs were detected in 35.7% (n = 224) of patients. After adjusting for seasonality and the number of active sites, models showed no clear evidence of an upward or downward trend in the number of SCRA exposure cases in the period before (incidence rate ratio [IRR], 1.12; 95% CI, 0.99–1.26; P = 0.068) or after (IRR, 0.97; 95% CI, 0.94–1.01; P = 0.202) the implementation of the PSA. There was also no clear evidence of an upward or downward trend in non‐SCRA exposure cases before (IRR, 1.12; 95% CI, 0.98–1.27; P = 0.105) or after (IRR, 1.01; 95% CI, 0.98–1.04; P = 0.478) implementation of the PSA.
Conclusions
There is no clear evidence of an upward or downward trend in the number of patients presenting to UK hospitals with severe acute toxicity following analytically confirmed synthetic cannabinoid receptor agonist exposure since the implementation of the Psychoactive Substances Act.
Keywords: NPS, PSA, Psychoactive Substances Act, SCRA, synthetic cannabinoid receptor agonists, time series analysis, toxicity
INTRODUCTION
Novel psychoactive substances (NPS) are a broad range of drugs that are not controlled by the United Nations international drug conventions, but may pose a threat to public health [1]. Previously marketed as ‘legal highs’ or ‘designer drugs’, NPS are often structural analogues of conventional illicit drugs or medicinal products designed to mimic their effects while evading legal control. Their prevalence in the general population is low, with ~0.5% of those ages 16 to 59 reporting past year use in the United Kingdom (UK) in 2019 [2]. However, NPS are often considerably more harmful than the drugs they were designed to mimic and pose a significant challenge to health‐care services [3]. In 2020, the Office for National Statistics (ONS) recorded 137 deaths involving NPS use in the United Kingdom [4]. In 2017, data from the three UK hospitals reporting to the European Drug Emergencies Network (EURO‐DEN) show that patients reported NPS use in 15% of all drug‐related hospital emergency presentations [5]. Definitions and classifications of NPS vary between data collection systems, but those most commonly encountered are benzodiazepines, cathinones, and synthetic cannabinoid receptor agonists (SCRAs).
With over 200 different compounds currently being monitored by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [6], SCRAs are the largest group of NPS, and in recent years they have been involved in the greatest proportion of deaths and emergency hospital presentations [4, 5]. Although structurally diverse, SCRAs elicit their effects via interaction with the endocannabinoid system, typically acting as full agonists at CB1 and CB2 receptors [7]. The main psychoactive compound of cannabis, delta‐9‐tetrahydrocannabinol (THC), is a partial CB1 and CB2 receptor agonist, and comparatively SCRAs show considerably greater potency and binding affinity at these receptors [8, 9, 10].
The individual SCRAs most commonly encountered have changed with time, presumably to evade national and international legislative changes. In the United Kingdom, following classification under consecutive amendments to the Misuse of Drugs Act (MDA)—first in 2009 and then in 2013—novel groups of compounds with structural modifications that circumvented these controls quickly emerged with a general increasing trend in potency [10, 11, 12]. In response, and in attempt to end the open sale of SCRAs and other ‘legal highs’ in high‐street retailers, the United Kingdom introduced the Psychoactive Substances Act (PSA) on the 26th May 2016 to be used alongside the MDA [13]. Whereas the MDA controls individual or small groups of compounds according to chemical structure, the PSA made illegal the importation, production, and supply (but not possession, except in custodial settings) of all psychoactive substances (not already controlled under the MDA), although with several named exemptions including alcohol, nicotine and licensed medications. Alongside this, a third amendment to the MDA in December 2016 (although based on recommendations made by the Advisory Council for the Misuse of Drugs [ACMD] in 2014) [14] increased the scope of the generic definition of SCRAs to include the then emerging third generation compounds that were controlled as Class B substances in line with the compounds already scheduled, thereby extending sanctions beyond those covered by the PSA (including penalties for possession).
In 2018, the UK Home Office conducted a review into the effectiveness of the PSA. The report concluded that although ending the open sale and reducing use of NPS within the general population, SCRAs (and other NPS) had been integrated into the illicit market and use had increased within vulnerable populations, such as the homeless or those in prison [15]. More recently, a multidisciplinary study interviewing SCRA users and various stakeholders (e.g. emergency services, outreach engagement workers etc.) outlined several detrimental impacts of the PSA on the UK SCRA market, which the authors conclude have increased the risk of individual and societal harm [16]. This is supported by mortality data from the ONS and National Program on Substance Abuse Deaths (NPSAD), which show that since the PSA, the number of deaths where SCRAs are implicated and/or detected at post‐mortem have increased [4, 17, 18].
Data from hospital presentations can also provide important insight into the nature and epidemiology of SCRA related harm; however, there is currently a lack of routinely collected, systematic data of this kind in the UKor elsewhere [19]. Data from the National Poisons Information Service, a service for clinicians seeking advice for the diagnosis/management of individual cases of poisoning, has demonstrated that telephone enquiries citing NPS exposure have decreased since the enactment of the PSA, however, the impact on the number of enquiries citing SCRA exposure specifically was not reported [20]. Poison centre data have several limitations and are not representative of the true number of clinical exposures or hospital admissions, see Wood et al. [21]. Previous studies analysing data from individual hospitals in Edinburgh [22] and London [23] have reported that the number of NPS‐related toxicity presentations did not significantly change following the implementation of the PSA, although in London, there were changes in the types of NPS involved and the number of SCRA‐related presentations had increased in the year after its implementation [23]. However, confirmatory toxicological analysis is not typically available in acute clinical settings, and previous studies have relied on details of drug exposure reported by the patient (or witness). This is unreliable because there may be substantial variability in the composition of NPS products that may not be known to the user, and patients may report SCRAs when they have not been used or fail to report them when they have [24, 25]. Therefore, without analytical confirmation, clinical data may not accurately reflect trends in SCRA related harm.
The Identification of Novel Psychoactive Substance (IONA) study has been collecting clinical data and biological samples from patients attending emergency departments across the UK with severe toxicity associated with suspected NPS use since 2015. By conducting time series analysis using IONA data, we aimed to examine trends in the number of hospital presentations for severe acute toxicity involving analytically confirmed SCRA exposure before and after the implementation of the PSA between July 2015 and December 2019. For comparison, we also examined trends in the number of non‐SCRA drug toxicity presentations during the same period.
METHODS
The IONA study is an ongoing multi‐centre observational study that has been taking place in participating hospitals across mainland UK since 2015. The IONA study has ethical and research governance approval and all participants provided immediate or retrospective consent [26, 27].
Toxicological data were collected from consenting patients presenting to participating hospitals with at least one feature of severe acute toxicity suspected to have resulted from NPS use (for a more detailed description of inclusion criteria see Supporting information). Based on toxicological analyses, patients were categorised as either SCRA or non‐SCRA exposure cases. SCRA exposure cases were those in whom at least one SCRA (regardless of its legal status under the MDA) was detected; non‐SCRA exposure cases were those in whom no SCRAs, but at least one other drug was detected. Although inclusion criteria necessitated suspected NPS use, the majority of drugs detected among the non‐SCRA exposure cohort were conventional illicit drugs already controlled under the MDA, often in the absence of any NPS previously uncontrolled before the implementation of the PSA. Therefore, we excluded any non‐SCRA exposure cases in whom only previously uncontrolled NPS were detected (i.e. any patient not using at least one drug controlled under the MDA before July 2015 or a prescribed/non‐prescribed medication; n = 5) such that this cohort provided a reference for trends in hospital presentations involving drugs not directly affected by the PSA.
Data reported here were collected between July 2015 and December 2019 (inclusive). During the study, the numbers of participating hospitals changed as additional sites were recruited, whereas others became inactive. The number of participating sites recruiting at least one patient in any month over this period ranged from 3 to 21 (median, 15.5; interquartile range [IQR], 9) with the number of sites generally increasing over time. In total, data are taken from 34 different sites including 7 in London, 7 in the South East, 4 in the South West, 2 in the Midlands, 6 in the North West, 5 in the North East and Yorkshire, 2 in Scotland, and 1 in Wales. The number of active sites within each month is displayed in Supporting information Figure 1.
Toxicological analysis of biological samples
Biological samples (blood, urine and/or saliva) were qualitatively analysed for all psychoactive substances (excluding alcohol) by liquid‐chromatography tandem mass‐spectrometry (see Supporting information).
Statistical analysis
Monthly trends in the number of hospital presentations for severe acute toxicity involving and not involving SCRA exposure before and after the implementation of the PSA (and the y‐intercept for each period) were estimated using segmented Poisson regression. A detailed description of the model specification is provided in the statistical analyses section of the Supporting information. Models were fitted separately for SCRA and non‐SCRA exposure cases and the change point for the implementation of the PSA was specified at June 2016. Therefore, the pre intervention period included data collected between July 2015 and May 2016 (inclusive), whereas the post intervention period included data collected between June 2016 and December 2019 (inclusive).
Because the third SCRA‐related amendment to the MDA was also enacted on the 14th December 2016, we fitted an additional model (separately for SCRA and non‐SCRA exposure cases) estimating the monthly trend (and y‐intercept) for the period before and after the MDA amendment (for which the change point was specified at January 2017). All models were also adjusted for the number of active sites during each month. To account for seasonal and long‐term secular trends in the data, a series of Fourier terms (i.e. pairs of sine and cosine functions) [28] were sequentially added to each model and the optimal number of pairs were retained in the final models if they improved model fit (as determined by likelihood ratio tests). To account for over‐dispersion, a scaling adjustment was used based on the Pearson's χ2 statistic, which allows the variance to be proportional rather than equal to the mean.
Finally, to examine whether the varying number of active sites in each month was influencing model results, we conducted sensitivity analyses in which the above models (for SCRA exposure cases only) were replicated, but with the total number of hospital presentations within each month (i.e. SCRA and non‐SCRA exposures combined) included as an offset variable. Therefore, in these models, SCRA exposure cases were analysed as a rate of all suspected NPS toxicity cases (see Supporting information Table S1).
For all models, IRRs and 95% CIs are reported. The statistical analysis plans were not pre‐registered.
RESULTS
Between July 2015 and December 2019, a total of 627 consenting individuals presented to participating hospitals and met inclusion criteria. At least one SCRA was detected in 224 of these (35.7%; SCRA exposure cases). The mean (± standard deviation; SD) age of those with SCRA exposure was 34.4 (±10.7) and 82.1% were male. The mean (±SD) age of the non‐SCRA exposure cohort was 32.1 (±10.6) and 78.7% were male. The age of SCRA and non‐SCRA cases within each year of the study period are displayed in Supporting information Figures 2a and 2b.
SCRA and other drug use characteristics
Among SCRA exposure cases, SCRA use (or a named SCRA product, e.g. Spice, Pandora's Box) was self‐reported or suspected by clinicians in 63.0% of cases. Conversely, SCRAs were not detected in 34.0% of patients who reported or were suspected of using them. The total number of patients reporting or were suspected of using SCRAs and the number of patients where SCRAs were reported/suspected, but not detected in each year of the study period are displayed in Supporting information Figure 3.
Overall, 31 different SCRAs (parent compounds, not including metabolites) were detected. The most common were 5F‐MDMB‐PINACA (n = 101, 45.1% of SCRA exposure cases), AMB‐FUBINACA (n = 66, 29.5%); MDMB‐CHMICA (n = 57, 25.5%), 5F‐NPB‐22 (n = 27, 12.1%) and 5F‐PB‐22 (n = 25, 11.2%). Figure 1 depicts the prevalence of SCRA compounds detected within each year of the study period.
FIGURE 1.
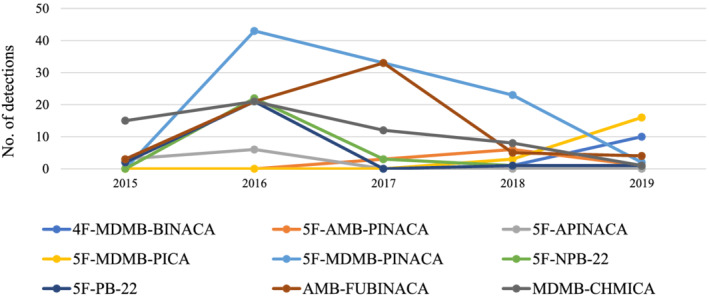
Prevalence of individual SCRA compounds detected among SCRA exposure cases within each year between July 2015 and December 2019. Note: Only compounds detected in ≥9 cases are displayed. SCRA, synthetic cannabinoid receptor agonist
A single SCRA was detected in 48.7% of SCRA exposure cases, two were detected in 29.9% of cases, three in 16.1%, four in 4.9% and in one case (0.5%) five different SCRAs were detected. Regarding other, non‐SCRA drugs, co‐use was detected in the majority of SCRA exposure cases (78.6%) and the mean (±SD) number of additional non‐SCRA drugs detected (among all SCRA exposure cases) was 2.6 (±2.7). Most commonly detected were opioids (48.7% of all SCRA exposure cases), benzodiazepines (48.2%) and cocaine (29.0%) (see Figure 2 for the prevalence of drugs detected among SCRA and non‐SCRA exposure cases).
FIGURE 2.
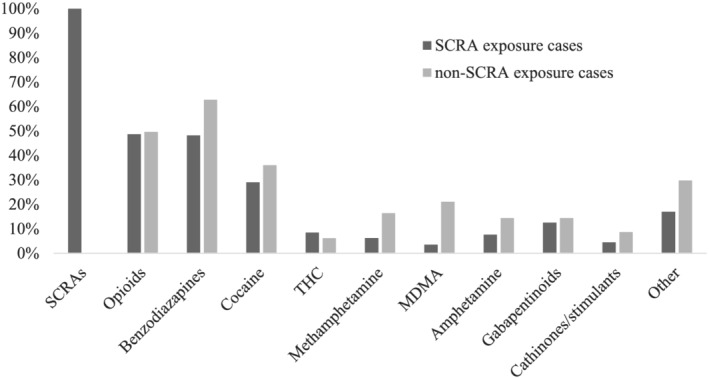
Prevalence of drugs detected among SCRA and non‐SCRA exposure cases. Note: SCRA exposure cases are those where at least one SCRA was detected, non‐SCRA exposure cases are those where no SCRAs, but at least one drug (previously controlled under the MDA before July 2015) was detected. SCRA, synthetic cannabinoid receptor agonist; THC, delta‐9‐tetrahydrocannabinol; MDA, Misuse of Drugs Act; MDMA, 3,4‐methylenedioxymethamphetamine
Among non‐SCRA exposure cases, the drugs most commonly detected were benzodiazepines (62.8%); opioids (49.6%) and cocaine (36.0%) (Figure 2). The detection of multiple drugs was also common among these patients (77.4%), and the mean (±SD) number of drugs detected was 3.5 (±2.4).
Time series analysis
Across the whole period, there were an average of 4.1 (±3.3) and 7.5 (±4.1) cases per month involving SCRA and non‐SCRA exposure, respectively. The final segmented Poisson regression models estimating trends in the monthly number of SCRA and non‐SCRA toxicity cases before and after the implementation of the PSA and the amendment to the MDA are reported in Table 1. The observed and predicted (i.e. model fitted) number of monthly SCRA and non‐SCRA exposure cases before and after the implementation of the PSA are displayed in Figure 3a,b respectively, whilst the observed and predicted number of monthly SCRA and non‐SCRA exposure cases before and after the amendment to the MDA are displayed in Supporting information Figure 4a and 4b respectively. In all models, model fit was improved by adding a single Fourier term to account for seasonal trends.
TABLE 1.
Segmented Poisson regression models estimating trends in the number of hospital presentations for severe acute toxicity involving analytically confirmed SCRA and non‐SCRA exposure before and after implementation of the PSA (and the amendment to the MDA)
| SCRA exposure cases | Non‐SCRA exposure cases | |||||
|---|---|---|---|---|---|---|
| IRR | P‐value | 95% CI | IRR | P‐value | 95% CI | |
| PSA 26th May 2016 | ||||||
| Pre‐intervention y‐intercept | 9.33 | 0.003 | 2.08–41.74 | 11.64 | 0.001 | 2.90–46.71 |
| Pre‐intervention trend | 1.12 | 0.068 | 0.99–1.26 | 1.12 | 0.105 | 0.98–1.27 |
| Post‐intervention y‐intercept | 3.67 | 0.084 | 0.84–15.98 | 9.59 | 0.001 | 2.67–34.41 |
| Post‐intervention trend | 0.97 | 0.202 | 0.94–1.01 | 1.01 | 0.478 | 0.98–1.04 |
| MDA 14th December 2016 | ||||||
| Pre‐intervention y‐intercept | 2.97 | 0.321 | 0.35–25.32 | 16.24 | 0.001 | 3.08–85.53 |
| Pre‐intervention trend | 0.98 | 0.706 | 0.90–1.08 | 1.06 | 0.128 | 0.98–1.15 |
| Post‐intervention y‐intercept | 2.48 | 0.410 | 0.29–21.46 | 21.52 | <0.001 | 3.98–116.45 |
| Post‐intervention trend | 0.96 | 0.102 | 0.92–1.01 | 1.01 | 0.490 | 0.98–1.04 |
All models are adjusted for the number of active sites within each month and include a single pair of Fourier terms to account for seasonal trends.
Abbreviations: 95% CI, 95% confidence interval for IRR; IRR, incidence rate ratio; MDA, Misuse of Drugs Act; PSA, Psychoactive Substances Act; SCRA, synthetic cannabinoid receptor agonist.
FIGURE 3.
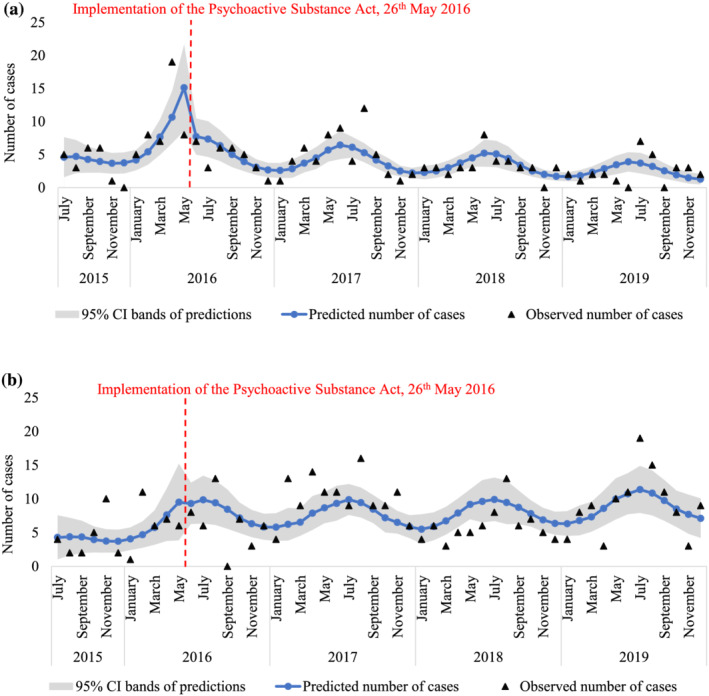
(a) Observed and predicted number of SCRA exposes cases within each month between July 2015 to December 2019 based on segmented Poisson regression estimating trends before and after implementation of the PSA (see Table 1). (b) Observed and predicted number of non‐SCRA exposes cases within each month between July 2015 to December 2019 based on segmented Poisson regression estimating trends before and after implementation of the PSA (see Table 1). Note: Predicted number of cases refer to fitted values (and the 95% CI bands) of the final segmented Poison regression models reported in Table 1, whereas the observed cases are the true number of hospital presentations across the study period. SCRA, synthetic cannabinoid receptor agonist; PSA, Psychoactive Substances Act
After adjusting for seasonality and the number of active sites, data were inconclusive and these models showed no clear evidence of an upward or downward trend in the number of SCRA exposure cases in the period before (IRR, 1.12; 95% CI, 0.99–1.26; P = 0.068) or after (IRR, 0.97; 95% CI, 0.94–1.01; P = 0.202) the implementation of the PSA, or in the period before (IRR, 0.98; 95% CI, 0.90–1.08; P = 0.706) or after (IRR, 0.96; 95% CI, 0.92–1.01; P = 0.102) the amendment to the MDA. Similarly, there was no clear evidence of an upward or downward trend in non‐SCRA exposure cases before (IRR, 1.12; 95% CI, 0.98–1.27; P = 0.105) or after (IRR, 1.01; 95% CI, 0.98–1.04; P = 0.478) implementation of the PSA, or before (IRR, 1.06; 95% CI, 0.98–1.15; P = 0.128) or after (IRR, 1.01; 95% CI, 0.98–1.04; P = 0.490) the amendment to the MDA. These results are also corroborated by sensitivity analyses, in which SCRA exposure cases are analysed as a rate of all NPS suspected toxicity cases (Supporting information Table S1).
DISCUSSION
Using time series analysis, this is the first study to examine trends in hospital presentations for severe acute toxicity involving SCRA exposure before and after the implementation of the PSA to (i) use analytical confirmation of SCRA exposure and (ii) collect data from several hospitals from different regions across the UK.
Overall, SCRAs were detected in ~36% of patients presenting to participating IONA hospitals with severe acute toxicity suspected to involve NPS between July 2015 and December 2019. Across this period, we found no clear evidence of an upward or downward trend in the number of hospital presentations involving SCRA exposure before or after the implementation of the PSA or before or after the amendment to the MDA. There was also no clear evidence of a significant trend in the number of hospital presentations involving exposure to non‐SCRA drugs before or after either of these two policy changes. Interestingly, there was evidence of seasonal trends in the number of hospital presentations involving both SCRA and non‐SCRA exposures, with presentations increasing over summer months (i.e., May to September). Although not explored further, this is likely to reflect seasonal variability in the prevalence of drug use [29] and/or time available for participant recruitment at participating sites.
Our findings contrast with previous studies reporting increasing indicators of SCRA related mortality and morbidity since the PSA [4, 16, 17, 18, 23]. Increasing trends in SCRA related mortality reported by the ONS and NPSAD [4, 17, 18] may be a reflection of more detailed forensic analysis of drug‐related deaths and improvements in methods for detecting SCRA over time. However, in the current study, analytical methods for detecting SCRA in patient samples were consistent throughout the study period, but with reference libraries updated to include new compounds as they emerge. Our findings also contrast with data from an individual (London) hospital that showed that the number of presentations for acute toxicity involving patient‐reported SCRA exposure had increased in the year after the implementation of the PSA [23]. Those data are confined to a single region and are only inclusive of the year after the PSA; however, this may reflect the lack of consistency between patient‐reported and analytically confirmed exposure details. Previously, Abouchedid et al. [24] reported that SCRAs were reported by only 50% of patients in whom they were detected, whereas Tebo et al. [25] reported that SCRAs were reported (or suspected by clinicians), but not detected in ~45% of patients. In the current study, SCRA use was reported (or suspected by clinicians) in ~65% of patients with confirmed exposure and ~34% of those without. Although there is a possibility that novel SCRAs not detectable by current analytical methods may be present in patient samples, the likelihood of this is small because analytical methods used here can account for the emergence of new compounds and indicate the presence of previously unidentified ones. It is more likely that discrepancies between drugs detected and those reported by patients or suspected by clinicians reflect the mis‐sale or contamination of drug products, the prolonged time between exposure and hospital presentation/sample collection and/or the misattribution of other drugs' effects to SCRA use. It might also reflect potential limitations of self‐report measures to correctly identify SCRAs using suitable terminology understood by clinicians and the full drug using population [30]. In any case, these findings highlight the importance of analytical confirmation when investigating SCRA exposure.
The prevalence of SCRA compounds detected here—with 5F‐MDMB‐PINACA, AMB‐FUBINACA and MDMB‐CHMICA being predominant—are similar to previous reports in the UK and elsewhere [18, 24, 31, 32]. There was notable variability in the prevalence of these compounds across the study period, and consistent with a previous analysis of compounds presents in seized SCRA products [33] this appears to coincide with changes to legislative controls of SCRA compounds in China in 2018, as well scheduling under the MDA in the UK in 2016. In the majority of SCRA exposure cases, use of other substances were also detected; most common were opioids, benzodiazepines and cocaine. Also consistent with previous reports [18, 24, 25, 32], in the majority of cases, multiple different SCRAs were detected. Although this may be because of the use of separate products, SCRA products often contain multiple compounds in varying concentrations [16, 33], and it is possible that co‐administration with SCRA and/or other drugs may influence toxicity. Because this appears to be common among people who use SCRAs, enhanced toxicovigilance within clinical settings is necessary to help understand the interaction effects between different SCRAs and other commonly co‐administered drugs and improve the treatment response.
This study has important limitations. First, with a total of 224 confirmed SCRA cases across a 54‐month period, these data are only likely to represent a small number of SCRA related hospital presentations, and accurately estimating trends in data of this size can be problematic. Additionally, data were only collected for 11‐months before the implementation of the PSA, and we were not able to estimate potential anticipatory effects occurring in the period between the PSA being announced and implemented. It has been reported that many outlets held ‘fire sales’, in which remaining stocks of SCRAs and other NPS were sold at heavily discounted prices before the ban took effect [15], and this may have resulted in increased hospital presentations. Second, there were changes in the hospitals collecting data during the study period. Although analyses were adjusted for the changing number of hospitals over time it is possible that regional variability in patterns of drug use may have influenced the trends in SCRA (and non‐SCRA) detections as sites became active and inactive. Third, because of these inconsistencies in the data collection periods and difficulties in appropriately modelling potential anticipatory effects, we only examined changes in the trends within and not between the pre‐intervention and post‐intervention periods. For the same reasons, and because of high month‐to‐month variability in hospital admissions, we also did not attempt to interpret changes in the y‐intercept between these two periods, which may have provided an indication of any immediate changes in the number of hospital presentations occurring immediately after the PSA was implemented. Therefore, although these data can provide insight into the monthly trend in SCRA related hospital admissions since the implementation of PSA, it is not possible to comment on the policy's effect on pre‐policy hospital presentation levels. Fourth, although data were collected from 34 hospitals throughout the UK, hospital participation was voluntary and not determined by probability‐based sampling methods, therefore, these data cannot be considered nationally representative. Fifth, although we also examined trends in SCRA related hospital admissions before and after the amendment to MDA we did not explore potential impacts of international policy changes occurring within the study period (e.g. national control of several SCRA compounds in 2015 and 2018 in China) [11]. Finally, our analysis of patient samples could only determine whether or not SCRAs (and other drugs) were present, and we could not quantify concentrations or their clinical significance. It is possible that SCRAs present in analysed samples, especially those with prolonged elimination (including active metabolites), were used in earlier episodes of intoxication and may not have contributed to the toxic effects for which patients were seeking treatment. Nonetheless, these data provide an important indication of trends in the number of SCRA clinical exposures before and after implementation of the PSA.
CONCLUSIONS
In this time series, analysis of data collected across hospitals throughout the mainland UK between 2015 and 2019, data were inconclusive with no clear evidence of an upward or downward trend in the number of patients presenting to hospital with analytically confirmed SCRA exposure before or after the implementation of the PSA (or before or after the amendment to the MDA). Accurate monitoring of epidemiological trends and toxicovigilance of SCRAs within clinical settings are necessary to better inform evidence‐based policy decisions and guide clinical practice.
DECLARATION OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Sam Craft: Conceptualization; formal analysis. Michael Dunn: Data curation; investigation. Dan Vidler: Data curation; investigation. Jane Officer: Data curation; investigation. Ian Blagbrough: Formal analysis; supervision. Christopher Pudney: Supervision. Graeme Henderson: Supervision. Ahmed Abouzeid: Data curation; investigation; project administration. Paul Dargan: Data curation; investigation; project administration. Michael Eddleston: Data curation; project administration. Jamie Cooper: Data curation; investigation; project administration. Simon Hill: Data curation; investigation; project administration. Clair Roper: Data curation; investigation; project administration. Tom Freeman: Conceptualization; formal analysis; supervision. Simon Thomas: Data curation; formal analysis; funding acquisition; investigation; methodology; project administration; supervision.
Supporting information
Table S1 Replicated segmented Poisson regression models with SCRA exposure cases analysed as a rate of all suspected NPS toxicity cases.
Figure S1 Total number of hospital presentations and active sites recruiting participants within each month between July 2015 and December 2019.
Figure S2a Percentage of SCRA exposure cases by age range within each year of the study period.
Figure S2b Percentage of non‐SCRA exposure cases by age range within each year of the study period.
Figure S3 Total number of patients where SCRA exposure was reported or suspected and total number of patients where SCRA exposure was reported or suspected but not detected within each year of the study period.
Figure S4a Observed and predicted number of SCRA exposes cases within each month between July 2015 and December 2019 based on segmented Poisson regression estimating trends before and after the amendment to the MDA (see Table 1).
Figure S4b Observed and predicted number of non‐SCRA exposes cases within each month between July 2015 and December 2019 based on segmented Poisson regression estimating trends before and after the amendment to the MDA (see Table 1).
ACKNOWLEDGEMENTS
We acknowledge with gratitude the work done by the research staff in all IONA sites and thank all the participants for allowing their data and samples to be used for this study.
Craft S, Dunn M, Vidler D, Officer J, Blagbrough IS, Pudney CR, et al. Trends in hospital presentations following analytically confirmed synthetic cannabinoid receptor agonist exposure before and after implementation of the 2016 UK Psychoactive Substances Act. Addiction. 2022;117(11):2899–2906. 10.1111/add.15967
Funding informationThe IONA study was funded by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) for Chemical and Radiation Threats and Hazards at Newcastle University. This work was supported in part by MR/N0137941/1 for the GW4 BIOMED MRC DTP, awarded to the Universities of Bath, Bristol, Cardiff and Exeter from the Medical Research Council (MRC)/UKRI. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, the Department of Health, Public Health England or the MRC.
Tom P. Freeman and Simon H. L. Thomas are joint senior authors.
REFERENCES
- 1. United Nations Office on Drugs and Crime . Regional diversity and the impact of scheduling on NPS trends, Global SMART Update, vol. 25; 2021.
- 2. Office for National Statistics . Drugs Misuse: Findings from the 2018/19 Crime Survey for England and Wales, 2019.
- 3. Wood DM, Ceronie B, Dargan PI. Healthcare professionals are less confident in managing acute toxicity related to the use of new psychoactive substances (NPS) compared with classical recreational drugs. QJM. 2016;109:527–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Office for National Statistics . Deaths related to drug poisoning in England and Wales: 2020 registrations; 2021.
- 5. European Monitoring Centre for Drugs and Drug Addiction . Drug‐related hospital emergency presentations in Europe: update from the Euro‐DEN Plus expert network, Technical report Luxembourg: Publications Office of the European Union; 2020. [Google Scholar]
- 6. European Monitoring Centre for Drugs and Drug Addiction . European Drug Report 2021: Trends and Developments Luxembourg: Publications Office of the European Union; 2021. [Google Scholar]
- 7. Banister SD, Connor M. The Chemistry and Pharmacology of Synthetic Cannabinoid Receptor Agonist New Psychoactive Substances: Evolution Springer International Publishing; 2018. p. 191–226. [DOI] [PubMed] [Google Scholar]
- 8. Fantegrossi WE, Moran JH, Radominska‐Pandya A, Prather PL. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ9‐THC: Mechanism underlying greater toxicity? Life Sci. 2014;97:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wiley JL, Lefever TW, Marusich JA, Grabenauer M, Moore KN, Huffman JW, et al. Evaluation of first generation synthetic cannabinoids on binding at non‐cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacology. 2016;110:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sachdev S, Vemuri K, Banister SD, Longworth M, Kassiou M, Santiago M, et al. In vitro determination of the efficacy of illicit synthetic cannabinoids at CB1 receptors. Br J Pharmacol. 2019;176:4653–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Advisory Council for the Misuse of Drugs . Synthetic cannabinoid receptor agonists (SCRA) an updated harms assessment and a review of classification and scheduling under the misuse of drugs act 1971 and its regulations; 2020.
- 12. Hermanns‐Clausen M, Kneisel S, Szabo B, Auwärter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: Clinical and laboratory findings. Addiction. 2013;108:534–44. [DOI] [PubMed] [Google Scholar]
- 13. UK Government . The Psychoactive Substances Act 2016; 2016.
- 14. Advisory Council for the Misuse of Drugs . 'Third generation' synthetic cannabinoids London: Home Office; 2014. [Google Scholar]
- 15. Home Office . Review of the Psychoactive Substances Act 2016; 2018.
- 16. Ralphs R, Gray P, Sutcliffe OB. The impact of the 2016 psychoactive substances act on synthetic cannabinoid use within the homeless population: Markets, content and user harms. Int J Drug Policy. 2021;97:103305. [DOI] [PubMed] [Google Scholar]
- 17. Deen AA, Claridge H, Treble RD, Hamnett HJ, Copeland CS. Deaths from novel psychoactive substances in England, Wales and Northern Ireland: Evaluating the impact of the UK psychoactive substances act 2016. J Psychopharmacol. 2021;35:1315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoganathan P, Claridge H, Chester L, Englund A, Kalk NJ, Copeland CS. Synthetic Cannabinoid‐Related Deaths in England, 2012–2019, Cannabis and Cannabinoid Research; 2021. [DOI] [PMC free article] [PubMed]
- 19. Heyerdahl F, Hovda KE, Giraudon I, Yates C, Dines AM, Sedefov R, et al. Current European data collection on emergency department presentations with acute recreational drug toxicity: Gaps and national variations. Clin Toxicol. 2014;52:1005–12. [DOI] [PubMed] [Google Scholar]
- 20. Al‐Banaa I, Hawkins L, Hill SL, Lupton DJ, Jackson G, Sandilands EA, et al. Effect of the UK psychoactive substances act 2016 on episodes of toxicity related to new psychoactive substances as reported to the National Poisons Information Service. A time series analysis. Int J Drug Policy. 2020;77:102672. [DOI] [PubMed] [Google Scholar]
- 21. Wood DM, Hill SL, Thomas SHL, Dargan PI. Using poisons information service data to assess the acute harms associated with novel psychoactive substances. Drug Test Anal. 2014;6:850–60. [DOI] [PubMed] [Google Scholar]
- 22. Pettie J, Burt A, Knipe DW, Torrance H, Dow M, Osinski K, et al. New drug controls and reduced hospital presentations due to novel psychoactive substances in Edinburgh. Br J Clin Pharmacol. 2018;84:2303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Webb NE, Wood DM, Greene SL, Hunter LJ, Archer JRH, Dines AM, et al. Change in the new psychoactive substances associated with emergency department acute toxicity presentations associated with the introduction of the UK 2016 psychoactive substances act. Clin Toxicol (Phila). 2019;57:36–41. [DOI] [PubMed] [Google Scholar]
- 24. Abouchedid R, Hudson S, Thurtle N, Yamamoto T, Ho JH, Bailey G, et al. Analytical confirmation of synthetic cannabinoids in a cohort of 179 presentations with acute recreational drug toxicity to an emergency Department in London, UK in the first half of 2015. Clin Toxicol. 2017;55:338–45. [DOI] [PubMed] [Google Scholar]
- 25. Tebo C, Mazer‐Amirshahi M, DeGeorge L, Gelfand B, Leak C, Tolliver S, et al. Suspected synthetic cannabinoid receptor agonist intoxication: Does analysis of samples reflect the presence of suspected agents? Am J Emerg Med. 2019;37:1846–9. [DOI] [PubMed] [Google Scholar]
- 26. Hill SL, Najafi J, Dunn M, Acheampong P, Kamour A, Grundlingh J, et al. Clinical toxicity following analytically confirmed use of the synthetic cannabinoid receptor agonist MDMB‐CHMICA. A report from the identification of novel psychoActive substances (IONA) study. Clin Toxicol. 2016;54:638–43. [DOI] [PubMed] [Google Scholar]
- 27. White JC, Wood DM, Hill SL, Eddleston M, Officer J, Dargan PI, et al. Acute toxicity following analytically confirmed use of the novel psychoactive substance (NPS) methiopropamine. A report from the identification of novel psychoActive substances (IONA) study. Clin Toxicol. 2019;57:663–7. [DOI] [PubMed] [Google Scholar]
- 28. Bhaskaran K, Gasparrini A, Hajat S, Smeeth L, Armstrong B. Time series regression studies in environmental epidemiology. Int J Epidemiol. 2013;42:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palamar JJ, Rutherford C, Keyes KM. Summer as a risk factor for drug initiation. J Gen Intern Med. 2020;35:947–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Palamar JJ. When accurate drug terminology reduces reporting and readership: The need for a happy medium regarding “synthetic cannabis” terminology. Int J Drug Policy. 2021;98:103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bäckberg M, Tworek L, Beck O, Helander A. Analytically confirmed intoxications involving MDMB‐CHMICA from the STRIDA project. J Med Toxicol. 2017;13:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hermanns‐Clausen M, Müller D, Kithinji J, Angerer V, Franz F, Eyer F, et al. Acute side effects after consumption of the new synthetic cannabinoids AB‐CHMINACA and MDMB‐CHMICA. Clin Toxicol. 2018;56:404–11. [DOI] [PubMed] [Google Scholar]
- 33. Norman C, Walker G, McKirdy B, McDonald C, Fletcher D, Antonides LH, et al. Detection and quantitation of synthetic cannabinoid receptor agonists in infused papers from prisons in a constantly evolving illicit market. Drug Test Anal. 2020;12:538–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Replicated segmented Poisson regression models with SCRA exposure cases analysed as a rate of all suspected NPS toxicity cases.
Figure S1 Total number of hospital presentations and active sites recruiting participants within each month between July 2015 and December 2019.
Figure S2a Percentage of SCRA exposure cases by age range within each year of the study period.
Figure S2b Percentage of non‐SCRA exposure cases by age range within each year of the study period.
Figure S3 Total number of patients where SCRA exposure was reported or suspected and total number of patients where SCRA exposure was reported or suspected but not detected within each year of the study period.
Figure S4a Observed and predicted number of SCRA exposes cases within each month between July 2015 and December 2019 based on segmented Poisson regression estimating trends before and after the amendment to the MDA (see Table 1).
Figure S4b Observed and predicted number of non‐SCRA exposes cases within each month between July 2015 and December 2019 based on segmented Poisson regression estimating trends before and after the amendment to the MDA (see Table 1).


