Abstract
Background
Climate warming presents physiological challenges to insects, manifesting as loss of key life‐history fitness traits and survival. For interacting host–parasitoid species, physiological responses to heat stress may vary, thereby potentially uncoupling trophic ecological relationships. Here, we assessed heat tolerance traits and sensitivity to prevailing and future maximum temperatures for the cereal stemborer pests, Chilo partellus, Busseola fusca and Sesamia calamistis and their endo‐parasitoids, Cotesia sesamiae and Cotesia flavipes. We further used the machine learning algorithm, Maximum Entropy (MaxEnt), to model current and potential distribution of these species.
Results
The mean critical thermal maxima (CT max) ranged from 39.5 ± 0.9°C to 44.6 ± 0.6°C and from 46.8 ± 0.7°C to 48.5 ± 0.9°C for parasitoids and stemborers, with C. sesamiae and Ch. partellus exhibiting the lowest and highest CT max respectively. From the current climate to the 2050s scenario, parasitoids recorded a significant reduction in warming tolerance compared with their hosts. Habitat suitability for all stemborer–parasitoid species was spatially heterogeneous under current and future climatic scenarios. Cotesia sesamiae C. flavipes and B. fusca exhibited significant habitat loss, whereas Ch. partellus and S. calamistis showed a significant habitat gain under future 2050s predictions. Model metrics based on mean area under the curve ranged from 0.72 to 0.84 for all species, indicating a good predictive performance of the models.
Conclusion
These results suggest C. sesamiae and C. flavipes may face survival constraints or extirpation compared with their pest hosts when environmental temperature reaches their upper thermal limits earlier, likely reducing pest regulation through density‐mediated effects. The results demonstrate potential destabilization of stemborer–parasitoid trophic systems potentially compromising biocontrol efficacy under climate warming. © 2022 The Authors. Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: biogeography, climate change, host–parasitoid interaction, MaxEnt, warming tolerance
Parasitoids recorded significant warming tolerance reduction compared with stemborers under current and future projections, suggesting potential compromise for biological control.
1. INTRODUCTION
Evidence of anthropogenic global climate change is rapidly being experienced, with the increased magnitude and frequency of global climate ‘presses’ and ‘pulses’ such as droughts, floods and heatwaves. 1 , 2 Global carbon dioxide concentrations and mean surface temperatures are projected to increase by 540–970 p.p.m. and 1.4–5.8°C, respectively, by 2100. 3 , 4 Such scenarios will have deleterious effects on biodiversity, community structure, composition and function, species geographic range and ecosystem function. 3 , 4 , 5 Given that approximately 70% of the population in sub‐Saharan Africa (SSA) depends on subsistence agriculture and natural pest suppression (for example, natural substances) for sustainable agricultural livelihoods, climate warming presents a significant threat to crop productivity and natural pest regulation. 6 , 7
Interactions between insects and their natural enemies are controlled by environmental factors. 6 , 8 Among these, temperature and relative humidity (RH) have key roles affecting organismal reproduction, development, abundance, parasitism, functional responses, geographic distribution and overall survival. 3 , 9 , 10 , 11 The geographical range limit of an insect species is dependent on its ecological tolerance to environmental conditions. 12 Hence, determination of ecological tolerance allows for better prediction of pest species biogeographical patterns. 12 Although a poleward shift in species distribution has been predicted with climate warming, 13 , 14 tropical regions nevertheless remain vulnerable to pest insect outbreaks relative to temperate regions owing to optimal temperature and food resources. 15 Thus, predictive models should also extend to tropical areas given their vulnerability to new invasions.
Cereal crops, such as sorghum (Sorghum bicolor L. Moench) and maize (Zea mays L.) are significant food crops in SSA. 16 , 17 However, lepidopteran stemborers, mostly Chilo partellus (Swinhoe), Busseola fusca (Fuller) and Sesamia calamistis (Hampson) represent a significant pest pressure on cereal food crop production. 16 , 18 This is exacerbated by the recent addition of the devastating invasive leaf defoliator, fall armyworm Spodoptera frugiperda (J.E. Smith), a significant biosecurity threat, affecting food security resilience in SSA. 19 The distribution of stemborer species reportedly varies among different agroecological zones and regions. 15 For example, although native to Asia, Ch. partellus has since spread across Africa, mostly in lowland to high altitude, highland tropical and humid transitional areas. 15 , 20 , 21 , 22 , 23 Busseola fusca is, however, indigenous to Africa and predominant in cooler higher altitudes (above 600 m.a.s.l.) in East and southern Africa, at 2000 m.a.s.l. or above in central Africa and in dry lowland savanna in West Africa. 16 , 24 , 25 Sesamia calamistis, also indigenous to Africa, is widely present across 33 SSA countries, albeit at lower populations. 26 , 27 Naturally, these stemborer populations are modulated by natural enemies through density‐dependent factors. For example, the larval parasitoids, Cotesia sesamiae (Cameron) and Cotesia flavipes Cameron (Hymenoptera: Braconidae) exert significant biotic pressure on these stemborers. 28 , 29 , 30 In particular, C. sesamiae, is an endoparasitoid of the whole lepidopteran cereal stemborer complex, 29 and is native to Africa, whereas C. flavipes, which prefers Ch. partellus, is native to Asia but was introduced into African agroecosystems in the 1990s. 30 Both parasitoid species are efficacious, accounting for between 32% and 55% of stemborer reduction in agroecosystems. 16 , 31
The effect of climate warming may manifest in different ways and at different magnitudes with the potential of destabilizing co‐evolved trophic interactions. 6 Similarly, because of the direct effects of temperature on organismal metabolism and activity, differential effects of high temperature across trophic levels may affect phenological synchrony, 8 , 32 or activity time syncs owing to different thermal sensitivity. 3 Thus, the strength and effect of stemborer–parasitoid interactions may be mediated by temperature. 18 , 33 , 34 For example, empirical evidence suggests insects on different trophic levels respond differentially to temperature stress, both in terrestrial 35 and aquatic environments 36 with negative effects on biological control. 37 Furthermore, temperature‐induced changes in the abundance and distribution of herbivore hosts may reduce the success of parasitoids in insect pest management. In this regard, the outcome is partly dependent on the ability of the parasitoids to improve their host‐searching capacity and extension of their geographical range. 38 Nevertheless, overwhelming evidence suggests that parasitoids at higher trophic levels may suffer thermal stress resulting in decreased longevity, fecundity and mobility. 6 Given the economic importance of pest stemborer herbivores (Ch. partellus, B. fusca and S. calamistis) and their natural enemies (C. sesamiae and C. flavipes), predicting the outcomes of their interactions under climate warming is of paramount importance for pest management practitioners. Although previous studies looked into thermal tolerances of stemborers and their larval parasitoids, 35 , 39 , 40 the results have not been incorporated into models to predict their future interactions under climate warming.
Recent predictions of insect pests’ biogeographical patterns have centred on the use of models, 22 , 41 , 42 particularly species distribution models (SDMs). 43 SDMs use spatial occurrence data combined with environmental data to predict spatial patterns of environmental suitability and distribution of a particular species. 43 Occurrence (‘presence’) data usually consist of latitude and longitude coordinates where the species of interest is present. In addition, environmental data consist of environmental descriptors such as abiotic measurements of temperature and rainfall, as well as biotic factors such as the presence or absence of other interacting species (for example, predators, parasitoids, competitors, food sources). 43 Various SDMs have been used in species biogeographical modelling. 44 , 45 Among these models, MaxEnt and GARP have been reported as more robust, providing stronger predictions using presence‐only data. 45 , 46 Whereas GARP predicts a species’ spatial presence, MaxEnt also estimates the spatial probability of occurrence, thus is a key methodology in species distribution modelling. 47
Temperature‐driven phenology models based on species distribution records and phenology data have been used to forecast the potential distribution of Ch. partellus in Africa. 22 In addition, the models have also been used to predict the distribution and abundance of Ch. partellus and C. flavipes in Ethiopia, 48 Ch. partellus and B. fusca and their natural enemies (C. flavipes and C. sesamiae) along Kilimanjaro and Taita Hills gradients in the East African region 15 and recently Ch. partellus, B. fusca, S. calamistis, C. sesamiae and C. flavipes in Kenya and Tanzania. 49 However, no studies have predicted the abundance and distribution of these species with respect to southern Africa given that the region is projected to be drier (10% less rainfall) 50 , 51 , 52 and warmer (+3 to 4°C) by 2100. 1 , 53 , 54 , 55 These abiotic shocks present challenges to pest–natural enemy interaction outcomes and pest management. 36 , 54 Using MaxEnt, we thus predicted the current and future distribution of indigenous stemborer species, B. fusca and S. calamistis, exotic Ch. partellus, their indigenous larval parasitoid, C. sesamiae and exotic C. flavipes in southern Africa. We used warming tolerance (WT) as a key determinant of climate warming sensitivity for all the study species with respect to southern Africa using thermal limits to activity (critical thermal maxima) and environmental mean temperatures (mean maximum) under current and future climatic conditions. We hypothesize that thermal sensitivity between stemborer pests and their natural enemies is dissimilar and that there is asynchrony in abundance and distribution between these two interacting trophic levels. This information is important in conducting pest risk assessments as well as designing, planning and developing improved pest management systems under climate warming.
2. MATERIALS AND METHODS
2.1. Study insects and rearing conditions
The initial colony of C. sesamiae was obtained from the South African Sugarcane Research Institute (SASRI), South Africa, whereas C. flavipes, B. fusca, S. calamistis and Ch. partellus colonies were obtained from the International Centre of Insect Physiology and Ecology (ICIPE), Kenya. These organisms had been in culture for more than 20 generations with regular augmentation with wild populations to minimize inbreeding depression. The C. sesamiae colony was generated from parasitized S. calamistis larvae, whereas the C. flavipes colony was obtained from parasitized Ch. partellus larvae. Both colonies were maintained in climate chambers (HPP 260; Memmert) at 28 ± 1°C, 65% ± 10% RH and a 12:12 h light/dark photoperiod on an artificial diet 56 in 30‐ml plastic vials with perforated screw‐cap lids. Parasitoid cocoons were maintained according to species, under similar optimal conditions in open Petri dishes placed in Bugdorm rearing cages (240 cm3; Bugdorm‐BD43030F; Megaview Science) until eclosion. Eclosed parasitoids had access to food (25% honey and water from a cotton wick) ad libitum until they were used in experiments as 24–48 h old adults. Ch. partellus and S. calamistis pupae were maintained in open Petri dishes in rearing cages under the same optimum conditions as parasitoids (28 ± 1°C, 65% ± 10% RH), whereas B. fusca pupae were maintained at 25 ± 1°C, 75% ± 10% RH and a 12:12 h light/dark photoperiod in climate chambers until adult eclosion. Following emergence, adult stemborers had access to 25% sugar‐water from moistened cotton wads. Wax papers folded into pleats were placed in the rearing cages as an oviposition substrate for gravid females. To maintain uniformity among test insects, eggs were harvested after every 12 h, and transferred to an artificial diet 56 where they were allowed to hatch into larvae that were later used in subsequent experiments. In all cases, sixth instar larvae (last instar developmental stage) were used in critical thermal maxima (CT max) assays for all stemborer species.
2.2. Critical thermal maxima ( CT max ) determination
Ten individual sixth instar larvae of Ch. partellus, B. fusca and S. calamistis and adults (24–48 h old) of C. sesamiae and C. flavipes were randomly placed in a series of test tubes (‘organ pipes’) connected to a programmable water bath (Lauda Eco Gold, Lauda) and subjected to constant heating rates. In all cases, assays started at a set point (optimum temperature: 28°C for all stemborers and parasitoids except for B. fusca at 25°C) for 10 min (to allow insect temperature equilibration) before increasing the temperature at a rate of 0.25°C min−1 until CT max was recorded. CT max was defined as the upper temperature at which each insect lost coordinated muscle function, which was regarded as a lack of response to mild prodding. 57
2.3. Statistical analyses
CT max data analyses were conducted in STATISTICA, version 13.2 (StatSoft). Data were first checked for normality and equality of variance using the Shapiro–Wilk and Hartley–Bartlett tests, respectively. CT max data met assumptions of constant variance and were analysed using one‐way analysis of variance (ANOVA) with CT max as the dependent variable and species as the categorical factor. Tukey–Kramer's post hoc tests were used to separate statistically heterogeneous groups.
2.4. Determination of warming tolerance
Warming tolerance, regarded as a measure of an insect's sensitivity to climate warming was calculated using standardized methods, and derived from our CT max values. 54 , 58 The mean temperature of the warmest (T max) quarter for both current and 2050 climate scenarios was downloaded from WorldClim (https://www.worldclim.org/data/bioclim.html) version 2.1 59 and mapped over the national boundary map for southern Africa in ArcGIS version 10.3 (ESRI). The WorldClim platform comprises a set of data layers derived from interpolations of mean monthly weather data from various global meteorological stations with a 30 arc‐seconds of grid resolution. 60 The raster data sets were clipped to southern Africa using ArcGIS (ESRI). WT was calculated using the raster calculator function from ArcToolbox in ArcGIS using the following formulae:
where WT is the warming tolerance, CT max is the critical thermal maximum and T max is the mean temperature of warmest quarter.
As a result, WT plotted onto the southern African map layer was used to generate current and future scenarios (2050s) maps.
2.5. Presence records for stemborers and their parasitoids in southern Africa
Occurrence records of stemborers (Ch. partellus, B. fusca and S. calamistis) and larval parasitoids (C. sesamiae and C. flavipes) were obtained from previous physical surveys conducted in southern African countries. 61 , 62 , 63 , 64 , 65 , 66 , 67
2.6. Model environmental variables
Predictor climatic variables, based on temperature and precipitation were downloaded from WorldClim version 2.1 (www.worldclim.org) 59 at a spatial resolution of 2.5 arc‐minutes (approximately 5 km at the equator). The current bioclimatic variables were derived from the annual means for the period 1970–2000, whereas future variables were based on annual means for the 20‐years period 2041–2060. 59 All 19 bioclimatic variables may not necessarily determine the potential habitat distribution of the study insect species; therefore, they were first subjected to multicollinearity analysis using Pearson's correlation in R, and further selected based on the variables that best described the ecology of lepidopteran stemborers and their parasitoids. When two variables had a Pearson's coefficient value of (r) ≥ 0.9, only one variable from such pair considering its relative importance in determining stemborers and parasitoids distribution and their predictive power (percentage contribution) was selected for model development. As a result, from the 19 bioclimatic variables, a total of 8 bioclimatic variables and elevation were used in the development and simulation of the MaxEnt model. These were: average annual precipitation, average annual temperature, mean diurnal temperature range, mean temperature of the wettest quarter, temperature annual range, precipitation of the wettest month, precipitation seasonality, temperature seasonality and elevation (Table 1), as well as the Digital Elevation Model (DEM) from the NASA Shuttle Radar Topography Mission provided at 30 m pixel size (SRTM 30). 68 The DEM was resampled to the spatial resolution of the bioclimatic variables to match the pixel size for use in the MaxEnt. These bioclimatic variables were considered as potential predictors of the stemborer and parasitoid habitat distribution based on their biological significance to species distributions, habitat modelling and ability to define eco‐physiological tolerances of the study species. 69 Although most of the variables were hinged on temperature and precipitation, elevation was also taken into consideration because the distribution of stemborers and their parasitoids is dependent on altitude. 15 , 49 , 62 The final selection of predictor variables used for each species was based on the initial jack‐knife test (Table 1). Variables that had the least training gain when used in isolation were dropped from the model.
TABLE 1.
Bioclimatic variables and their contribution used to model areas suitable for Chilo partellus, Busseola fusca, Sesamia calamistis, Cotesia sesamiae and Cotesia flavipes
| Variable | Percentage contribution | ||||
|---|---|---|---|---|---|
| B. fusca | C. partellus | C. sesamiae | C. flavipes | S. calamistis | |
| Average annual precipitation | 36.5 | 10.6 | 0.6 | 24.4 | |
| Average annual temperature | 1.8 | 7.7 | 6 | 3.4 | 9.5 |
| Mean diurnal temperature range | 5.2 | 17.9 | 5.7 | 5.3 | 0.3 |
| Mean temperature of the wettest quarter | 4.8 | 5.3 | 15.6 | 8.2 | 1.6 |
| Temperature annual range | 41.4 | ||||
| Precipitation of the wettest month | 27.6 | 4.8 | 2.3 | 37.3 | |
| Precipitation seasonality | 1.1 | 0.6 | 1.1 | 6.2 | |
| Temperature seasonality | 21.8 | 12.1 | 22.8 | 18.6 | 17.7 |
| Elevation | 1.3 | 10.3 | 37 | 62.8 | 3 |
| Number of significant variables | 8 | 8 | 7 | 7 | 8 |
Note: Selected variables were limited to those with r ≥ 0.9 within the area of model development.
At the same resolution, the aforementioned eight bioclimatic variables for future climatic conditions were also downloaded from WorldClim 2.1 for the Shared Socioeconomic Pathway 2 (SSP2‐4.5) 2041–2060 climate scenario. 59 , 70 SSP2‐4.5 is termed the ‘middle of the road’ scenario characterized by intermediate greenhouse gas emissions, which are expected to start declining by 2050 but will not reach net zero by 2100. Thus, temperatures are expected to rise by approximately 2°C between 2041 and 2060. 71 , 72 The elevation variable was maintained across the current and future climate scenarios because it was assumed that the altitude would not change significantly in the future to influence the results of this study.
2.7. Species distribution model
The MaxEnt model, a tool used to predict the distribution of a species from presence‐only data and environmental variables, and effective with small sample sizes, 73 was used as the principal SDM to determine the potential habitat distribution and climate change impacts on Ch. partellus, B. fusca, S. calamistis, C. sesamiae and C. flavipes in southern Africa. The model was run using presence‐only data. 46 The presence data sets for each species were split into training (75%) and validation (25%), and the number of iterations was set at 5000. The kernel density estimator, that is the ‘kde2d’ function of the MASS package 74 using the ‘block’ sampling approach in R 75 was used to generate the bias file. The ‘kde2d’ function affords the performance of a two‐dimensional kernel density estimate that is based on the spatial ‘X’ and ‘Y’ coordinates of the occurrence points to generate a raster bias file. 74 It is important to correct for sampling bias, particularly where collection of the data may be biased toward settlement areas, roads or easily accessible areas. 76 The MaxEnt modelling approach allows the inclusion of bias files in the model, which facilitates the choice of background data with similar bias. Furthermore, the optimum tuning and parameter settings for the MaxEnt models were derived from the ‘ENMevaluate’ function in the package ENMeval 77 available in R software. 75 This approach calculates multiple metrics to aid in selecting optimum model settings that balance goodness‐of‐fit and model complexity. 77 The following model parameters were derived for each pest and parasitoid using ‘ENMevaluate’ from the models with the lowest change in the Akaike information criterion (delta.AICc = 0): linear (L), quadratic (Q), product (P), threshold (T), hinge (H) and regularized multiplier (RM). In addition, multivariate environmental similarity surface (MESS) analysis together with clamping, extrapolate and fade with clamping were also used for all the models. MESS analysis in MaxEnt quantifies the measure of projection uncertainty by calculating the similarity of each point in the projected region to a set of reference points, 78 in our case in future scenarios for which occurrence reference data are unavailable.
A projection file containing predictor variables for the 2041–2060 climate scenario was uploaded to MaxEnt to perform the future projections using the current models. Model performance was assessed quantitatively using the area under the curve (AUC) statistic. The AUC was derived from threshold‐independent receiver operating characteristic (ROC) analysis, significant in assessing the discriminative power of the model. 69 , 73 The ROC curve is a plot of true positives against false positives with AUC values between 0 and 1. An AUC closer to 1 indicates a high predictive capability of the model. 79 In addition, using the AUC metrics to evaluate the models has the advantage of being threshold‐independent, and as such does not require decisions regarding thresholds of what constitutes a prediction of presence versus a prediction of absence. This was very relevant in this study because the occurrence points of the different pests and parasitoids varied spatially across the study area. 80 , 81
The ASCII outputs from MaxEnt were converted to GEOTIFF binary maps depicting suitable and unsuitable habitats based on the ten‐percentile training presence logistic threshold.
3. RESULTS
3.1. Critical thermal maxima
Heat tolerance (CT max) varied significantly across species (p ˂ 0.001) (Table 2). The mean CT max values for C. sesamiae, C. flavipes, B. fusca, S. calamistis and Chilo partellus were 39.5 ± 0.9, 44.6 ± 0.6, 46.8 ± 0.7, 48.0 ± 0.8 and 48.5 ± 0.9°C respectively (Figure 1). Ch. partellus recorded the highest CT max, although this heat tolerance did not differ significantly from that of S. calamistis (Figure 1). Cotesia sesamiae recorded the lowest CT max (Figure 1), whereas C. flavipes and B. fusca were intermediate, although B. fusca had significantly higher CT max. However, CT max values for both C. flavipes and B. fusca were significantly different from those of C. sesamiae, S. calamistis and Ch. partellus (Figure 1).
Table 2.
Summary statistical results using one‐way ANOVA showing effects of species (Cotesia sesamiae, Cotesia flavipes, Busseola fusca, Sesamia calamistis and Chilo partellus) on critical thermal maxima (CTmax)
| Trait | Effect | SS | DF | MS | F | p‐value |
|---|---|---|---|---|---|---|
| CTmax | Intercept | 86 722.66 | 1 | 86 722.66 | 241 647 | ˂0.001 |
| Species | 150.16 | 1 | 150.16 | 418.4 | ˂0.001 | |
| Error | 13.64 | 38 | 0.36 |
Abbreviations: DF, degrees of freedom; MS, variance; SS, the sum of squares due to the source.
FIGURE 1.
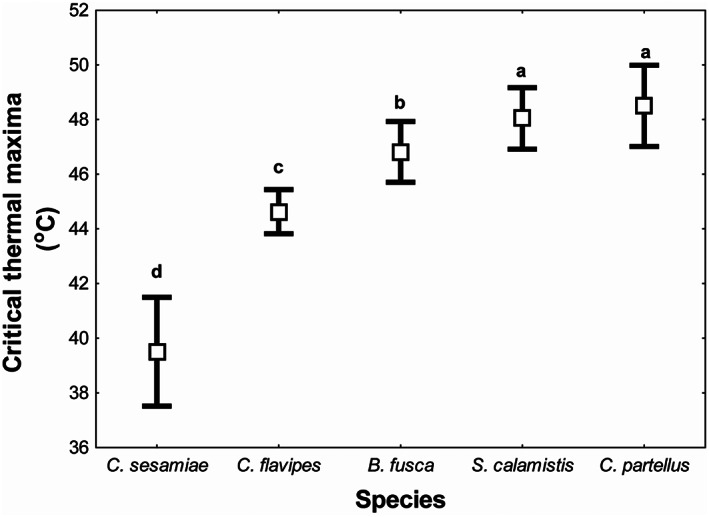
Species differences in critical thermal maxima for maize stemborers and their parasitoids. Error bars represent 95% confidence limits (N = 20). Means with the same letter are not significantly different from each other.
3.2. Warming tolerances for stemborers and parasitoids
WT values under current climatic conditions were 7.65 to 23.94°C (C. sesamiae), 12.77–29.06°C (C. flavipes), 14.96–31.25°C (B. fusca), 16.18–32.47°C (S. calamistis) and 16.64–32.93°C (Ch. partellus), with C. sesamiae and Ch. partellus recording the lowest and highest WT ranges, respectively (Figure 2A,C,E,G,I). Similarly, projected WT values by 2050 were 5.38–23.03°C (C. sesamiae), 10.5–28.15°C (C. flavipes), 12.69–30.34°C (B. fusca), 13.91–31.56°C (S. calamistis) and 14.37–32.02°C (Ch. partellus) (Figure 2B,D,F,H,J). Although all species showed a reduction in projected WT, Ch. partellus exhibited highest projected WT range, whereas the generalist stemborer parasitoid C. sesamiae recorded the lowest WT range.
FIGURE 2.
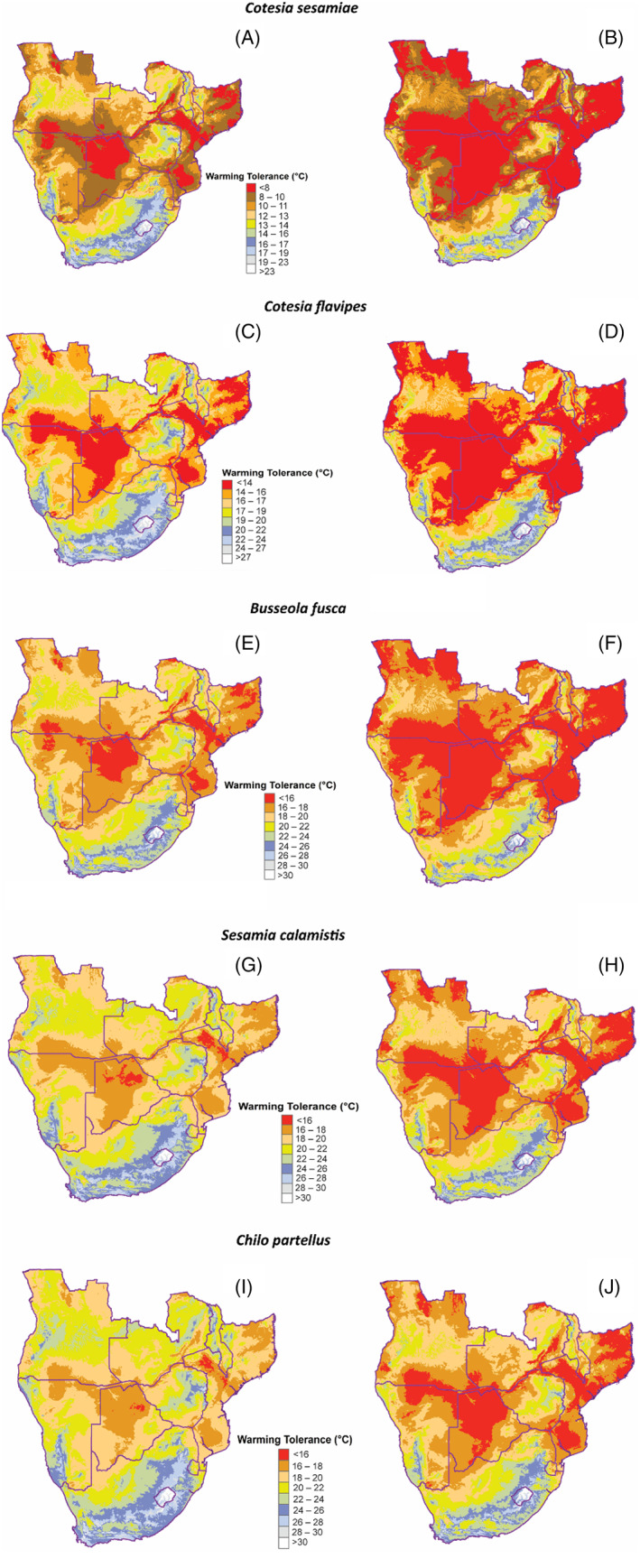
Warming tolerances for Cotesia sesamiae, Cotesia flavipes, Busseola fusca, Sesamia calamistis and Chilo partellus under current (A, C, E, G and I) and future (B, D, F, H and J) thermal conditions.
3.3. Model performance
The ROC is the good measure of model performance with widespread use in species distribution modelling. MaxEnt model outputs for parasitoids and stemborers showed strong goodness‐of‐fit (AUCs 0.72–0.84) (Figure 3), indicating that the model showed good predictive performance for suitable and unsuitable habitat differentiation for stemborer and parasitoid occurrence. 69
FIGURE 3.
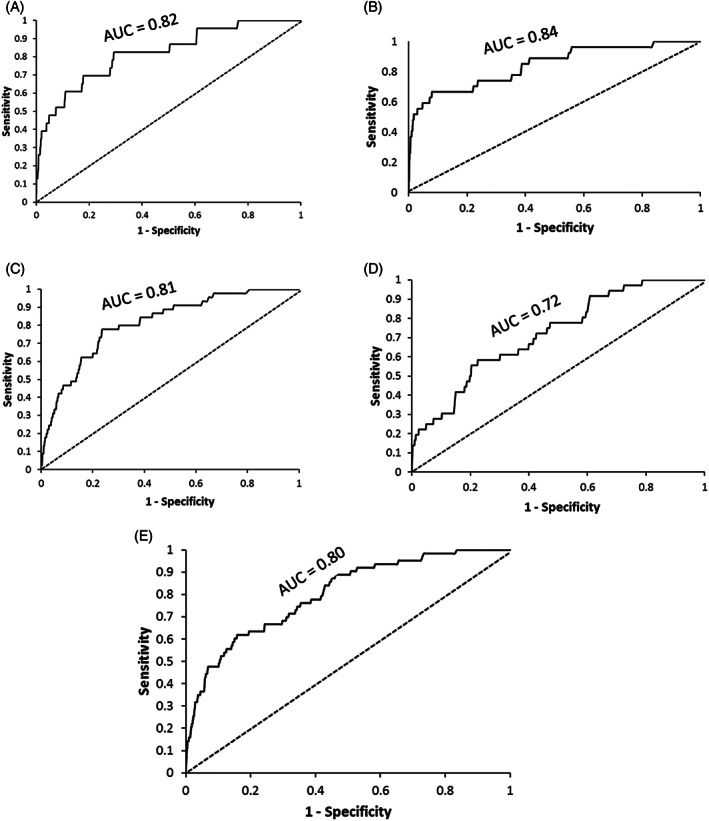
Receiver operating characteristic (ROC) curve and area under the curve (AUC) of the MaxEnt model for (A) Cotesia sesamiae, (B) Cotesia flavipes, (C) Busseola fusca, (D) Sesamia calamistis and (E) Chilo partellus.
3.4. Stemborer and parasitoid habitat suitability
The highly suitable habitats for C. sesamiae and C. flavipes in the present include most parts of Angola, Zambia, Zimbabwe and South Africa, whereas most parts of Namibia and Botswana dominate as least suitable habitats (Figure 4A,C). For stemborers, Botswana, Namibia, southern Angola, southern and western parts of Zimbabwe and South Africa are not suitable for B. fusca at present (Figure 4E). Using current predictions, S. calamistis remains mostly confined to central parts of Angola and Zambia as well as northern parts of Zimbabwe, whereas Ch. partellus is mostly confined to western Angola, eastern Malawi and northern parts of Mozambique (Figure 4G,I). Habitat distributions for both parasitoids and stemborers evaluated under a future climate (2050) were spatially heterogeneous (Figure 4). As such, the greater parts of the terrestrial ecosystems are projected to be significantly suitable for both parasitoids and their herbivore stemborer hosts (Figure 4). Although parasitoids (C. sesamiae and C. flavipes), noctuids (B. fusca and S. calamistis) and crambid (Ch. partellus) showed changes in future distribution, C. sesamiae C. flavipes and B. fusca exhibited a reduction in suitable habitats by 2050 (Figures 4B,D,F and 5; Table 3). By contrast, S. calamistis recorded an increase in suitable habitats by 2050 extending into northern parts of Zambia and eastern parts of South Africa (Figures 4H and 5; Table 3). Likewise, Ch. partellus showed a significant increase in suitable habitats by 2050 with most parts of Mozambique, western Angola, eastern Malawi, northern parts of Botswana and Zimbabwe dominating (Figure 4J; Table 3). Although current climate showed Lesotho being unsuitable for Ch. partellus, 2050's projection showed sporadic occurrences indicating habitat gain for this invasive insect species (Figure 4I,J). Comparison of the parasitoids’ future distribution showed more habitat gain for C. flavipes than for congeneric C. sesamiae (Figure 4B,D; Table 3).
FIGURE 4.
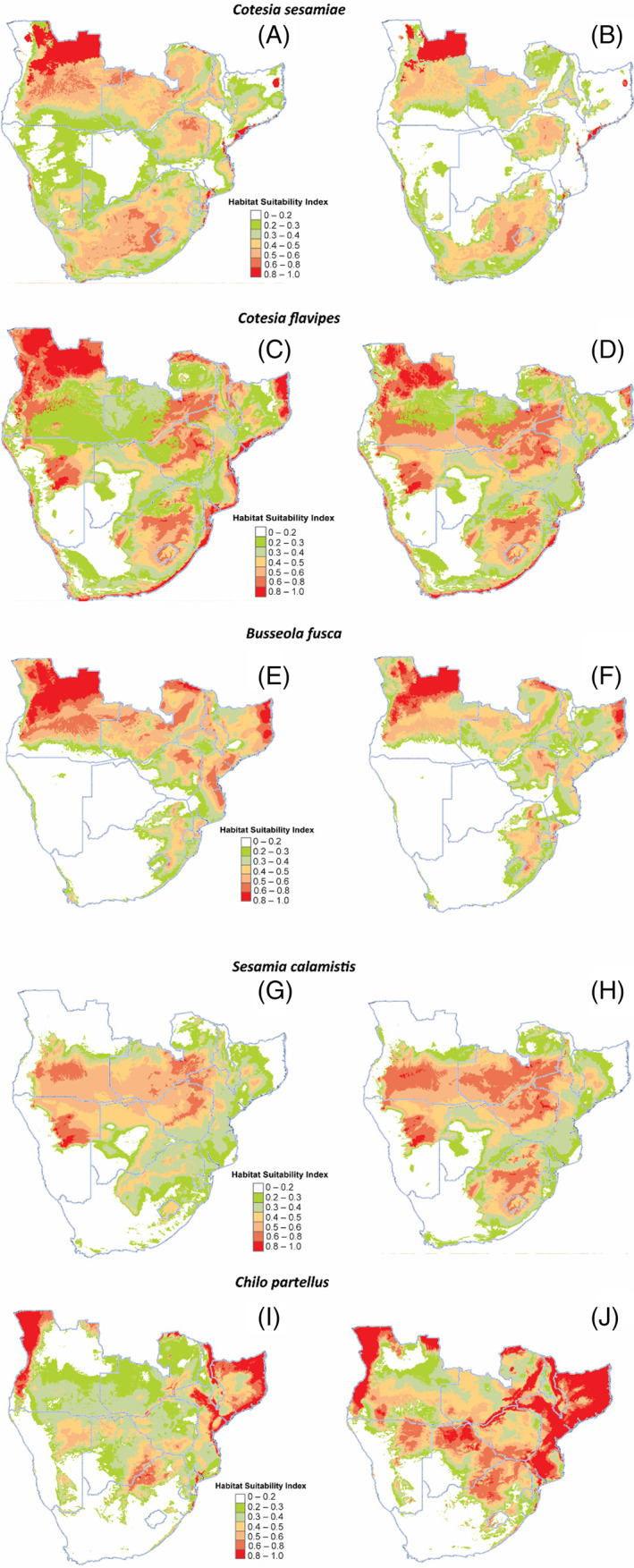
Predicted habitat suitability model for Cotesia sesamiae, Cotesia flavipes, Busseola fusca, Sesamia calamistis and Chilo partellus under current (A, C, E, G and I) and future (B, D, F, H and J) climatic conditions.
FIGURE 5.
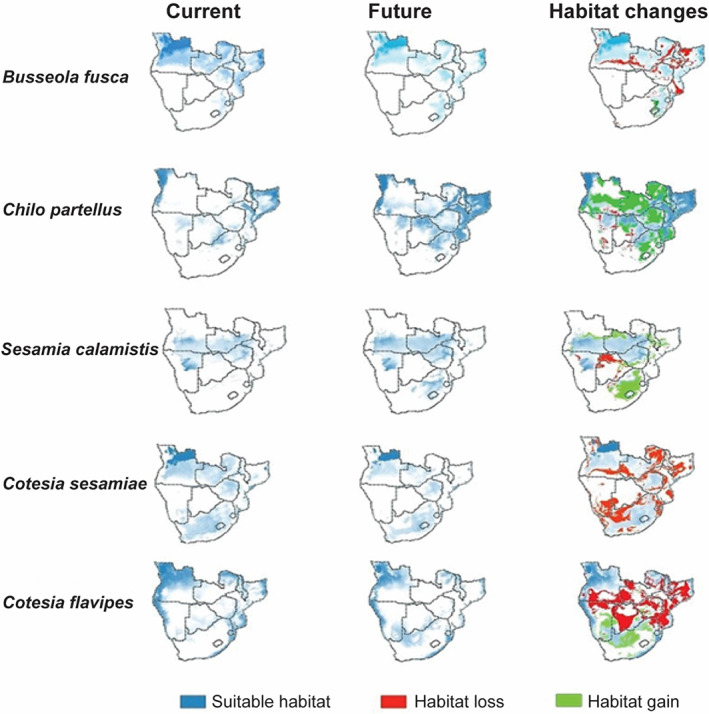
Habitat shifts (loss or gain) for Busseola fusca, Chilo partellus, Sesamia calamistis, Cotesia sesamiae and Cotesia flavipes due to changing climatic conditions.
Table 3.
Changes in suitable habitats for Chilo partellus, Busseola fusca, Sesamia calamistis, Cotesia sesamiae and Cotesia flavipes
| Species | Current (km2) | Future (km2) | Loss (km2) | Gain (km2) | Net change (km2) |
|---|---|---|---|---|---|
| Busseola fusca | 3 281 696.33 | 2 852 341 | 485 767.7 | 56 412.48 | −429 355.13 |
| Chilo partellus | 2 755 862.33 | 4 155 005 | 119 134.6 | 1 518 277 | 1 399 142.61 |
| Sesamia calamistis | 2 193 018.97 | 2 670 806 | 187 833.7 | 665 621.1 | 477 787.39 |
| Cotesia sesamiae | 4 014 634.51 | 2 685 027 | 1 336 515 | 6907.07 | −1 329 608.01 |
| Cotesia flavipes | 4 347 853.28 | 3 223 629 | 1 639 882 | 515 658 | −1 124 224.36 |
4. DISCUSSION
Climate warming is altering ecosystems by shifting species performance, biogeographic ranges and trophic interaction outcomes. 5 Our results demonstrate differential spatiotemporal distribution of host stemborer–natural enemy habitat suitability, and by inference their numbers under current and future climate warming scenarios. In particular, we show that some previously pest‐unsuitable habitats will be more optimal under climate warming. For example, the range of Ch. partellus will increase by 2050. By contrast, habitats for antagonist parasitoids species are expected to decrease under climate warming, probably reducing the biotic pressure on stemborer pests. For example, the range of C. sesamiae is expected to dwindle with warming, probably offsetting stemborer pest management. This study thus provides novel context‐specific southern African insights into the potential effects of climate warming on the spatial distribution of key larval parasitoids and their herbivorous hosts, and may help inform efficacious pest management under changing climates.
Insect species potentially survive projected warmer climate environments through either having higher basal stress tolerance 82 , 83 or remodelling their thermal phenotypes. 54 , 84 However, increasing evidence suggest that insects’ acclimation response ratios are low and as such, phenotypic plasticity may be ecologically insufficient to cushion insects against climate change. 85 , 86 Thus, high basal heat tolerance remains far more ecologically relevant in cushioning climate warming effects than phenotypic plasticity (see discussions in Kelley 82 and Wan and Yang 83 ). Our basal heat tolerance results varied across species with invasive Ch. partellus exhibiting the highest CT max overall. This indicates that Ch. partellus has inherent high basal heat tolerance relative to other stemborer and Cotesia parasitoid species, and this may confer fitness and survival advantage under projected climate warming 82 . However, this differential thermal sensitivity to high temperature may result in disruption of stemborer–parasitoid trophic interactions and biological control efficacy. 5 Similarly, the efficacy of parasitoids is dependent on adult performance vis‐à‐vis host‐searching ability (activity), population establishment, persistence, reproduction and development. 54 The current study demonstrates that both C. sesamiae and C. flavipes stop activity at approximately 39.5 and 44.6°C respectively, showing lower basal heat tolerance than their stemborer hosts. This likely translates into a spatial or temporal activity mismatch between the two levels, and may affect pest parasitism. Furthermore, because the generalist parasitoid C. sesamiae had lower heat tolerance than the more specific C. flavipes, 29 , 30 , 40 there is a higher likelihood of future stemborer biological control programmes being negatively affected, albeit more for S. calamistis and B. fusca than for Ch. partellus.
Stemborer–parasitoid phenological mismatch can arise when ovipositing parasitoid adults and their respective susceptible stemborer developmental stages occur asynchronously. 5 Most parasitoid species rely on plant chemical cues to locate their hosts, but their activity can be altered by temperature stress resulting in reduced parasitoid recruitment and parasitism rates. 5 , 8 , 58 In addition, high temperatures can negatively influence parasitoid efficacy through top‐down effects, for example by impacting behavioural activities such as flying and foraging. 8 , 58 Although all stemborer and parasitoid species tested here showed a decrease in WT values from the current climatic conditions to the 2050s, both Cotesia parasitoids exhibited a greater reduction in WT than their host stemborer species, suggesting increased chances of deleterious effects during extreme weather events. Although southern Africa is projected to be warmer and drier, 50 , 51 , 54 there is a higher likelihood of more stress exposure for parasitoids relative to their hosts. This asymmetrical stress exposure elicits major physiological and ecological constraints to the parasitoids, influencing key activity traits, for example host‐searching capacity, oviposition rates and dispersal. In particular, C. sesamiae and C. flavipes may face greater constraints on survival than B. fusca, S. calamistis and Ch. partellus with warming temperatures, resulting in a stemborer–natural enemy mismatch that may affect pest management.
Selection of the correct climatic variables based on the eco‐biological aspects of a target species is fundamental in precisely modelling its spatiotemporal potential distribution. 69 The key bioclimatic variables used in this study were primarily hinged on temperature and precipitation. Previous studies showed that temperature and RH are key abiotic factors mediating insect physiological fitness, survival, distribution and the population dynamics of stemborers and their natural enemies. 15 , 23 , 40 , 48 , 87 Thus, average annual precipitation, precipitation in the wettest month and temperature annual range were the key driving factors for the distribution of B. fusca, S. calamistis and Ch. partellus respectively, whereas elevation and temperature seasonality showed a significant distribution impact on parasitoids (C. sesamiae and C. flavipes).
Our results add to the current literature detailing shifts in the spatiotemporal distribution of pest insects with climate change. 45 , 49 , 69 , 88 , 89 In particular, Jendritzki et al. 49 projected a significant decrease in parasitoid (C. sesamiae and C. flavipes) populations relative to their hosts (B. fusca, Ch. partellus and S. calamistis) and a decrease in parasitism in East Africa. The current results showed that climate change may have significant species‐dependent impacts on the distribution of parasitoids and their stemborer hosts. For example, parasitoids (C. sesamiae and C. flavipes) and B. fusca showed a significant reduction in future suitable habitats, whereas S. calamistis and Ch. partellus showed a 30% and 55% increase in future habitat suitability respectively. Although C. sesamiae and C. flavipes have been reported to reduce stemborer densities between 32% and 55% in Z. mays and S. bicolor, 16 , 31 the results suggest disruption of biological control programmes as a result of a mismatch in biogeography between the pests and their parasitoids, in agreement with Jendritzki et al. 49 Lepidopteran stemborers reportedly account for 5%–75% of cereal yield losses in SSA. 16 , 17 As a result, this may negatively affect cereal production systems, resilience and food security in SSA. This may be exacerbated by the notion that approximately 70%–80% of African populations rely on subsistence agriculture, and natural enemy antagonists for pest control. 15 , 49 , 90 Previous studies have projected increased numbers of generations and geographic expansion of Ch. partellus from dry lowland to the higher elevated areas of southeastern African countries. 22 , 91 In addition, Mwalusepo et al. 15 predicted an increase in climatic suitability for Ch. partellus with increased extension to moist mid‐altitude and highland transitional areas of the East African region. This is consistent with current results indicating a projected gain in Ch. partellus‐suitable habitats in southern Africa. Specifically, Ch. partellus showed a significant range expansion, potentially including Angola, Zambia, Zimbabwe, Malawi, Mozambique, South Africa and Lesotho. Given that Ch. partellus key host plants (maize and sorghum) are grown in these countries on both small‐ and large‐scale farms, there is a greater likelihood of frequent and severe pest outbreaks. Supported by other non‐crop wild plant hosts, 66 this may support Ch. partellus pest proliferation that may increase pest populations. Although indigenous B. fusca and S. calamistis exhibited projected habitat loss and a lower habitat gain than Ch. partellus respectively, exotic Ch. partellus may competitively outcompete and displace the native species, in keeping with the findings of Mutamiswa et al. 23 As a co‐evolved endoparasitoid of Ch. partellus, C. flavipes may be expected to follow its host for survival. 15 In Ethiopia, model studies predicted that C. flavipes distribution may coincide greatly with Ch. partellus potential distribution with parasitoid density projected to be high in areas where host density is high. 48 However, this may not be the case under projected climate change in southern Africa because C. flavipes exhibited suitable habitat loss, whereas its host showed habitat gain. This implies that the parasitoid may not be able to follow the host in all its projected distribution areas, hence negatively affecting biological control under climate warming. Although C. sesamiae exhibited a greater projected reduction in suitable habitats, as well as being a generalist compared with its congener, this may potentially impact its ecological roles as a stemborer antagonist.
In conclusion, this study documents the projected regional spatiotemporal distribution of lepidopteran cereal stemborers and their natural enemies under climate change. Our model indicates: (1) more significant geographic expansion of exotic Ch. partellus relative to other indigenous stemborer species (B. fusca and S. calamistis) and larval parasitoids (C. sesamiae and C. flavipes); and (2) a more significant reduction in suitable habitats for associated parasitoids relative to their herbivore hosts. This infers potential success of Ch. partellus amidst warming habitats, and coupled with underperforming parasitoids owing to climate warming, Ch. partellus may thus dominate SSA ecosystems in the future (also see Mutamiswa et al. 23 ). In addition, C. sesamiae and C. flavipes parasitoids may not match the geographic expansion and/or activity timing of their host species, and hence is a potential spatiotemporal mismatch under projected climate warming. 92 This will in all likelihood compromise future biological control programmes. Overall, the study underlies the importance of climate warming in influencing trophic interactions outcomes, with implications for biological pest control and potentially food security.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support for this research by the following organizations and agencies: the Norwegian Agency for Development Cooperation (NORAD) project on Combating Arthropod Pest for Better Health, Food and Resilience to Climate Change (CAP‐AFRICA) grant number RAF‐3058 KEN‐18/0005; the Swedish International Development Cooperation Agency (SIDA); the Swiss Agency for Development and Cooperation (SDC); the Federal Democratic Republic of Ethiopia; and the Government of the Republic of Kenya. Valuable institutional support from University of the Free State (UFS) to RM, GC and FC, Botswana International University of Science and Technology (BIUST) and Rhodes University to CN as well as Midlands State University (MSU) to RM is highly appreciated. The views expressed herein do not necessarily reflect the official opinion of the donors.
Funding information
Norwegian Agency for Development Cooperation, Grant/Award Number: RAF‐3058 KEN‐18/0005; Swedish International Development Cooperation Agency; Swiss Agency for Development and Cooperation; Federal Democratic Republic of Ethiopia; Government of the Republic of Kenya
DATA AVAILABILITY STATEMENT
Data largely from public sources of data. The rest is available upon request
REFERENCES
- 1. IPCC , Climate Change 2014: Synthesis Report, in Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, ed. by Core Writing Team , Pachauri RK and Meyer LA. IPCC, Geneva, Switzerland, p. 151 (2014). [Google Scholar]
- 2. Harris RMB, Beaumont LJ, Vance TR, Tozer CR, Remenyi TA, Perkins‐Kirkpatrick SE et al., Biological responses to the press and pulse of climate trends and extreme events. Nat Clim Change 8:579–587 (2018). [Google Scholar]
- 3. Bale JS, Masters GJ, Hodkinson ID, Awmack C, Bezemer TM, Brown VK et al., Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Glob Chang Biol 8:1–16 (2002). [Google Scholar]
- 4. Karuppaiah V and Sujayanad GK, Impacts of climate change on population dynamics of insect pests. World J Agric Sci 8:240–246 (2012). [Google Scholar]
- 5. Abarca M and Spahn R, Direct and indirect effects of altered temperature regimes and phenological mismatches on insect populations. Curr Opin Insect Sci 47:67–74 (2021). [DOI] [PubMed] [Google Scholar]
- 6. Hance T, van Baaren J, Vernon P and Boivin G, Impact of extreme temperatures on parasitoids in a climate change perspective. Annu Rev Entomol 52:107–126 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Hellmuth ME, Moorhead A, Thomson MC and Williams J, Climate Risk Management in Africa: Learning from Practice. International Research Institute for Climate and Society, Columbia University, New York, USA: (2007). [Google Scholar]
- 8. Chidawanyika F, Mudavanhu P and Nyamukondiwa C, Global climate change as a driver of bottom‐up and top‐down factors in agricultural landscapes and the fate of host‐parasitoid interactions. Front Ecol Evol 7:80 (2019). [Google Scholar]
- 9. Ma FZ, Lu ZC, Wang R and Wan FH, Heritability and evolutionary potential in thermal tolerance traits in the invasive Mediterranean cryptic species of Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS ONE 9:e103279 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen C, Bahar MH, Baker G and Andrew NR, Thermal tolerance limits of diamondback moth in ramping and plunging assays. PLOS ONE 9:e87535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gotcha N, Cuthbert RN, Machekano H and Nyamukondiwa C, Density‐dependent ecosystem service delivery under shifting temperatures by dung beetles. Sci Total Environ 807:150575 (2021). [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez‐Castañeda G, MacVean C, Cardona C and Hof AR, What limits the distribution of Liriomyza huidobrensis and its congener Liriomyza sativae in their native niche: when temperature and competition affect species' distribution range in Guatemala. J Insect Sci 17:88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H et al., Pole ward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583 (1999). [Google Scholar]
- 14. Parmesan C, Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst 37:637–669 (2006). [Google Scholar]
- 15. Mwalusepo S, Tonnang HEZ, Massawe ES, Okuku GO, Khadioli N, Johansson T et al., Predicting the impact of temperature change on the future distribution of maize stemborers and their natural enemies along east African mountain gradients using phenology models. PLoS ONE 10:e0130427 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kfir R, Overholt WA, Khan ZR and Polaszek A, Biology and management of economically important lepidopteran cereal stemborers in Africa. Annu Rev Entomol 47:701–731 (2002). [DOI] [PubMed] [Google Scholar]
- 17. Moolman HJ, Van den Berg J, Conlong D and Cugala D, Siebert SJ and Le Ru BP diversity of stemborer parasitoids and their associated wild host plants in South Africa and Mozambique. Phytoparasitica 41:89–104 (2013). [Google Scholar]
- 18. Addo‐Bediako A and Thanguane N, Stemborer distribution in different sorghum cultivars as influenced by soil fertility. Agric Sci Res J 2:189–194 (2012). [Google Scholar]
- 19. Stokstad E, New crop pest takes Africa at lightning speed. Science 356:473–474 (2017). [DOI] [PubMed] [Google Scholar]
- 20. Tams WHT, New species of African Heterocera. Entomologist 65:1241–1249 (1932). [Google Scholar]
- 21. Kfir R, Competitive displacement of Busseola fusca (Lepidoptera: Noctuidae) by Chilo partellus (Lepdoptera: Pyralidae). Ann Entomol Soc Am 90:619–624 (1997). [Google Scholar]
- 22. Khadioli N, Tonnang ZEH, Muchugu E, Ong'amo G, Achia T, Kipchirchir I et al., Effect of temperature on the phenology of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae), simulation and visualization of the potential future distribution of C. partellus in Africa under warmer temperatures through the development of life‐table parameters. Bull Entomol Res 104:809–822 (2014). [DOI] [PubMed] [Google Scholar]
- 23. Mutamiswa R, Chidawanyika F and Nyamukondiwa C, Dominance of spotted stemborer Chilo partellus Swinhoe (Lepidoptera: Crambidae) over indigenous stemborer species in Africa's changing climates: ecological and thermal biology perspectives. Agric For 19:344–356 (2017a). [Google Scholar]
- 24. Ajayi O, Sorghum: West Africa, in African Cereal Stem Borers: Economic Importance, Taxonomy, Natural Enemies and Control, ed. by Polaszek A. CABI, Wallingford, UK, pp. 39–45 (1998). [Google Scholar]
- 25. van Asch M and Visser ME, Phenology of forest caterpillars and their host trees: the importance of synchrony. Annu Rev Entomol 52:37–55 (2007). [DOI] [PubMed] [Google Scholar]
- 26. Ntiri ES, Calatayud PA, Van den Berg J and Le Ru BP, Density dependence and temporal plasticity of competitive interactions during utilisation of resources by a community of lepidopteran stemborer species. Entomol Exp Appl 162:272–283 (2016). [Google Scholar]
- 27. Centre for agriculture and bioscience international (CABI), crop protection. Compendium 42:560–561 (2021). https://www.cabi.org/cpc.35380853 [Google Scholar]
- 28. Assefa Y, Mitchell A, Conlong DE and Muirhead KA, Establishment of Cotesia flavipes (hymenoptera: Braconidae) in sugarcane fields of Ethiopia and origin of founding population. J Econ Entomol 101:686–691 (2008). [DOI] [PubMed] [Google Scholar]
- 29. Mailafiya DM, Le Ru BP, Kairu EW, Calatayud P‐A and Dupas S, Geographic distribution, host range and perennation of Cotesia sesamiae and Cotesia flavipes Cameron in cultivated and natural habitats in Kenya. Biol Control 54:1–8 (2010). [Google Scholar]
- 30. Dejen A, Getu E, Azerefegne F and Ayalew A, Distribution and extent of Cotesia flavipes Cameron (hymenoptera: Braconidae) parasitism in northeastern Ethiopia. Int J Insect Sci 5:9–19 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou G, Baumgartner J and Overholt WA, Impact of an exotic parasitoid on stemborer (Lepidoptera) populations dynamics in Kenya. Ecol Appl 11:1554–1562 (2001). [Google Scholar]
- 32. Singer MC and Parmesan C, Phenological asynchrony between herbivorous insects and their hosts: signal of climate change or pre‐existing adaptive strategy? Phil Trans R Soc B 365:316–3176 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Songa JM, Overholt WA, Mueke JM and Okell RO, Colonization of Cotesia flavipes (hymenoptera: Braconidae) in stem borers in the semi‐arid eastern province of Kenya. Insect Sci Appl 21:289–295 (2001). [Google Scholar]
- 34. Mailafiya DM, Le Ru BP, Kairu EW, Calatayud PA and Dupas S, Species diversity of lepidopteran stem borer parasitoids in cultivated and natural habitats in Kenya. J Appl Entomol 133:416–429 (2009). [Google Scholar]
- 35. Mutamiswa R, Chidawanyika F and Nyamukondiwa C, Comparative assessment of the thermal tolerance of spotted stemborer, Chilo partellus Swinhoe (Lepidoptera: Crambidae) and its larval parasitoid, Cotesia sesamiae Cameron (hymenoptera: Braconidae). Insect Sci 25:847–860 (2017b). [DOI] [PubMed] [Google Scholar]
- 36. Buxton M, Nyamukondiwa C, Dalu T, Cuthbert RN and Wasserman RJ, Implications of increasing temperature stress for predatory biocontrol of vector mosquitoes. Parasites Vectors 13:604 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fand BB, Kamble AL and Kumar M, Will climate change pose serious threat to crop pest management: a critical review? Int J Sci Res Publ 2:2250–3153 (2012). [Google Scholar]
- 38. Thomson LJ, Macfadyen S and Hoffmann AA, Predicting the effects of climate change on natural enemies of agricultural pests. Biol Control 52:296–306 (2010). [Google Scholar]
- 39. Mutamiswa R, Chidawanyika F and Nyamukondiwa C, Superior basal and plastic thermal responses to environmental heterogeneity in invasive exotic stemborer Chilo partellus Swinhoe over indigenous Busseola fusca (fuller) and Sesamia calamistis Hampson. Physiol Entomol 43:108–119 (2018a). [Google Scholar]
- 40. Mutamiswa R, Machekano H, Chidawanyika F and Nyamukondiwa C, Thermal resilience may shape population abundance of two sympatric congeneric Cotesia species (hymenoptera: Braconidae). PLOS ONE 13:e0191840 (2018b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pearson RG and Dawson TP, Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr 12:361–371 (2003). [Google Scholar]
- 42. Estay SA, Lima M and Labra FA, Predicting insect pest status under climate change scenarios: combining experimental data and population dynamics modeling. J Appl Entomol 133:491–499 (2009). [Google Scholar]
- 43. Sofaer HR, Jarnevich CS, Pearse IS, Smyth RL, Auer S, Cook GL et al., Development and delivery of species distribution models to inform decision making. Bioscience 69:544–557 (2019). [Google Scholar]
- 44. Molloy SW, Davis RA and Van Etten EJB, Species distribution modelling using bioclimatic variables to determine the impacts of a changing climate on the western ringtail possum (Pseudocheirus occidentals; Pseudocheiridae). Environ Conserv 41:176–186 (2013). [Google Scholar]
- 45. Srivastava V, Griess VC and Keena MA, Assessing the potential distribution of Asian gypsy moth in Canada: a comparison of two methodological approaches. Sci Rep 10:22 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Phillips SJ and Dudík M, Modeling of species distributions with MaxEnt: new extensions and a comprehensive evaluation. Ecography 31:161–175 (2008). [Google Scholar]
- 47. Padalia H, Srivastava V and Kushwaha SPS, Modeling potential invasion range of alien invasive species, Hyptis suaveolens (L.) Poit. In India: Comparison of MaxEnt and GARP. Ecol Inform 22: 36–43 (2014). [Google Scholar]
- 48. Getu E, Overholt WA, Kairu E, Macopiyo L and Zhou G, Predicting the distribution of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) and Cotesia flavipes (Cameron) (hymenoptera: Braconidae) in Ethiopia using correlation, step‐wise regression and geographic information system. Insect Sci Appl 22:123–129 (2002). [Google Scholar]
- 49. Jendritzki IG, Tonnang HEZ, Calatayud PA, Borgemeister C, Johannson T and Biber‐Freudenberger L, Uncertainties in the effectiveness of biological control of stem borers under different climate change scenarios in eastern Africa. Research Square (2021). 10.21203/rs.3.rs-876884/v1. [DOI] [Google Scholar]
- 50. Lobell DB, Burke MB, Tebaldi C, Mastrandrea MD, Falcon WP and Naylor RI, Prioritising climate change adaptation needs for food security in 2030. Science 319:607–610 (2008). [DOI] [PubMed] [Google Scholar]
- 51. Stathers T, Lamboll R and Mvumi BM, Postharvest agriculture in changing climates: its importance to African smallholder farmers. Food Secur 5:361–392 (2013). [Google Scholar]
- 52. Engelbrecht F, Adegoke J, Bopape MJ, Naidoo M, Garland R, Thatcher M et al., Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ Res Lett 10:085004 (2015). [Google Scholar]
- 53. Stocker T, Dahe Q and Plattner G‐K, Climate Change 2013: The Physical Basis Technical Summary. IPCC Fifth Assessment Report, Geneva, Switzerland: (2013). [Google Scholar]
- 54. Allen JL, Clusella‐Trullas S and Chown SL, Thermal tolerance of Cyrtobagous salviniae: a biocontrol agent in a changing world. BioControl 59:357–366 (2014). [Google Scholar]
- 55. Kruger AC and Sekele SS, Trends in extreme temperature indices in South Africa:1962‐2009. Int J Climatol 33:661–676 (2013). [Google Scholar]
- 56. Ochieng RS, Onyango FO and Bungu MDO, Improvement of techniques for mass culture of Chilo partellus (Swinhoe). Insect Sci Appl 6:425–428 (1985). [Google Scholar]
- 57. Nyamukondiwa C and Terblanche JS, Thermal tolerance in adult Mediterranean and Natal fruit flies (Ceratitis capitata and Ceratitis rosa): effects of age, gender and feeding status. J Therm Biol 34:406–414 (2009). [Google Scholar]
- 58. Chidawanyika F, Chikowore G and Mutamiswa R, Thermal tolerance of the biological control agent Neolema abbreviata and its potential geographic distribution together with its host Tradescantia fluminensis in South Africa. Biol Control 149:104315 (2020). [Google Scholar]
- 59. Fick SE and Hijmans RJ, (2017) WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int J Climatol 37:4302–4315 (2017). [Google Scholar]
- 60. Castro‐Sosa R, Castillo‐Peralta MR, Monterroso‐Rivas AI, Gomez‐Díaz JD, Flores‐González E and Rebollar‐Alviter A, Potential distribution of Drosophila suzukii (Diptera: Drosophilidae) in relation to alternate hosts in Mexico. Fla Entomol 100:787–794 (2017). [Google Scholar]
- 61. Chinwada P and Overholt WA, Natural enemies of maize stemborers on the highveld of Zimbabwe. Afr Entomol 9:67–75 (2001). [Google Scholar]
- 62. Cugala DR and Omwega CO, Cereal stem borer distribution and abundance and the introduction and establishment of Cotesia flavipes Cameron (hymenoptera: Braconidae) in Mozambique. Insect Sci Appl 21:281–287 (2001). [Google Scholar]
- 63. Sohati PH, Musonda EM and Mukanga M, Distribution of cereal stem borers in Zambia and release of Cotesia flavipes Cameron, an exotic natural enemy of Chilo partellus (Swinhoe). Insect Sci Appl 21:311–316 (2001). [Google Scholar]
- 64. Chinwada P, Schulthess AF, Overholt WA, Jowah P and Omwega CO, Release and establishment of Cotesia flavipes for biological control of maize stemborers in Zimbabwe. Phytoparasitica 36:160–167 (2008). [Google Scholar]
- 65. Mutamiswa R, Moeng E, Le Ru BP, Conlong DE, Assefa Y, Goftishu M et al., Diversity and abundance of lepidopteran stem borer natural enemies in natural and cultivated habitats in Botswana. Biol Control 115:1–11 (2017c). [Google Scholar]
- 66. Moeng E, Mutamiswa R, Conlong DE, Assefa Y, Le Ru BP, Goftishu M et al., Diversity and distribution of lepidopteran stemborer species and their host plants in Botswana. Arthropod Plant Interact 12:733–749 (2018). [Google Scholar]
- 67. Mutamiswa R, Machekano H, Nyamukondiwa C and Chidawanyika F, Host plant related responses on the thermal fitness of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae). Arthropod Plant Interact 14:463–471 (2020). [Google Scholar]
- 68. National Aeronautics and Space Administration Shuttle Radar Topography Mission 1 Arc‐Second Global, U.S. Geological Survey (USGS) Earth Resources Observation and Science (EROS) Center (2000).
- 69. Fand BB, Shashank PR, Suroshe SS, Chandrashekar K, Meshram NM and Timmanna HN, Invasion risk of the south American tomato pinworm Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in India: predictions based on MaxEnt ecological niche modelling. Int J Trop Insect Sci 40:561–571 (2020). [Google Scholar]
- 70. Meinshausen M, Nicholls ZRJ, Lewis J, Gidden MJ, Vogel E, Freund M et al., The shared socio‐economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geosci Model Dev 13:3571–3608 (2020). [Google Scholar]
- 71. Girvetz E, Ramirez‐Villegas J, Claessens L, Lamana C, Navarro‐Racines C, Nowak A et al., Future climate projections in Africa: where are we headed? in The Climate‐Smart Agriculture Papers, ed. by Rosenstock T, Nowak A and Girvetz E. Springer, Cham: (2019). [Google Scholar]
- 72. IPCC , Climate Change 2021: The Physical Science Basis, in Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, ed. by Masson‐Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S et al. Cambridge University Press, United Kingdom and New York, NY, USA (2021). [Google Scholar]
- 73. Phillips SJ, Anderson RP and Schapire RE, Maximum entropy modeling of species geographic distributions. Ecol Model 190:231–259 (2006). [Google Scholar]
- 74. Venables WN and Ripley BD, Modern applied statistics with S, 4th edn. Springer, New York, NY: (2002). [Google Scholar]
- 75. R Core Team , R: A language and environment for statistical com‐ puting. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org (2021). [Google Scholar]
- 76. Kramer‐Schadt S, Niedballa J, Pilgrim JD, Schröder B, Lindenborn J, Reinfelder V et al., The importance of correcting for sampling bias in MaxEnt species distribution models. Divers Distrib 19:1366–1379 (2013). [Google Scholar]
- 77. Muscarella R, Galante PJ, Soley‐Guardia M, Boria RA, Kass JM, Uriarte M et al., ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol Evol 5:1198–1205 (2014). 10.1111/2041-210x.12261. [DOI] [Google Scholar]
- 78. Mudereri BT, Kimathi E, Chitata T, Moshobane MC and Abdel‐Rahman EM, Landscape‐scale biogeographic distribution analysis of the whitefly, Bemisia tabaci (Gennadius, 1889) in Kenya. Int J Trop Insect Sci 41:1585–1599 (2021). [Google Scholar]
- 79. Moshobane MC, Mudereri BT, Mukundamago M and Chitata T, Predicting future distribution patterns of Jatropha gossypiifolia L. in South Africa in response to climate change. South African J Bot 146:417–425 (2022). [Google Scholar]
- 80. Marchioro CA and Krechemer FS, Potential global distribution of Diabrotica species and the risks for agricultural production. Pest Manag Sci 74:2100–2109 (2018). [DOI] [PubMed] [Google Scholar]
- 81. Mota JDS, Barbosa LR and Marchioro CA, Suitable areas for invasive insect pests in Brazil and the potential impacts for eucalyptus forestry. Pest Manag Sci 78:2596–2606 (2022). 10.1002/ps.6891. [DOI] [PubMed] [Google Scholar]
- 82. Kelley AL, The role thermal physiology plays in species invasion. Conserv Physiol 2:cou045 (2014). 10.1093/conphys/cou045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wan F and Yang N, Invasion and management of agricultural alien insects in China. Annu Rev Entomol 61:77–98 (2016). [DOI] [PubMed] [Google Scholar]
- 84. Sgrò CM, Terblanche JS and Hoffmann AA, What can plasticity contribute to insect responses to climate change? Annu Rev Entomol 61:433–451 (2016). [DOI] [PubMed] [Google Scholar]
- 85. van Heerwaarden B, Kellermann V and Sgrò CM, Limited scope for plasticity to increase upper thermal limits. Funct Ecol 30:1947–1956 (2016). [Google Scholar]
- 86. Machekano H, Zidana C, Gotcha N and Nyamukondiwa C, Limited thermal plasticity may constrain ecosystem service delivery in a basally heat tolerant tropical telecoprid dung beetle, Allogymnopleurus thalassinus (Klug, 1855). Sci Rep 11:22192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mutamiswa R, Machekano H, Chidawanyika F and Nyamukondiwa C, Life‐stage related responses to combined effects of acclimation temperature and humidity on the thermal tolerance of Chilo partellus (Swinhoe) (Lepidoptera: Crambidae). J Therm Biol 79:85–94 (2019). [DOI] [PubMed] [Google Scholar]
- 88. Liu Y and Shi J, Predicting the potential global geographical distribution of two Icerya species under climate change. Forests 11:684 (2020). [Google Scholar]
- 89. Tepa‐Yotto GT, Tonnang HEZ, Goergen G, Subramanian S, Kimathi E, Abdel‐Rahman EM et al., Global habitat suitability of Spodoptera frugiperda (JE Smith) (Lepidoptera, Noctuidae): key parasitoids considered for its biological control. Insects 12:273 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. FAO , Comprehensive Africa Agriculture Development Programme (CAADP). NEPAD, Rome, Italy: (2002). [Google Scholar]
- 91. Kroschel J, Tonnang HEZ, Le Ru B and Hanna R, Analysing Climate Impacts on Insect Pests Using Phenology Modeling and Geographic Information System Implemented in the Insect Life Cycle Modeling Software. ICIPE, Nairobi, Kenya: (2013). [Google Scholar]
- 92. Taylor RAJ, Herms DA, Cardina J and Moore RH, Climate change and pest management: unanticipated consequences of trophic dislocation. Agronomy 8:7 (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data largely from public sources of data. The rest is available upon request


