Abstract
BACKGROUND
Table olives are a food with a high content of bioactive compounds with cardioprotective properties, such as oleic acid, polyphenols, and pentacyclic triterpenes. Here, we investigate the effect of the intake of table olives on blood pressure (BP) and body weight in spontaneously hypertensive rats (SHR) and their normotensive controls, Wistar Kyoto (WKY) rats. ‘Arbequina’ table olives (3.85 g kg−1) were administered by gavage to SHR and WKY rats in short‐term (1 day) and long‐term (7 weeks) experiments. BP was measured by the tail‐cuff method, and polyphenols and triterpenes were determined in olives and plasma by liquid chromatography–mass spectrometry.
RESULTS
Administration of ‘Arbequina’ olives to WKY rats did not exert any change in BP in any of the experiments. However, in SHR, the single dose induced a transient reduction in BP of approximately 15 mmHg, from the second to the tenth hour after the administration. In the long‐term assay, a similar decrease was established in the second week and was maintained throughout the experiment. Moreover, the daily administration of olives to rats did not affect their body weight when compared with controls in either the WKY rats or SHR. The determination of polyphenols and triterpenes in plasma indicated that, at the end of the experiment, only maslinic acid, oleanolic acid, hydroxytyrosol, and luteolin were found, all of them being compounds with already described capacity to decrease BP.
CONCLUSION
The results suggest that the daily intake of table olives could decrease BP in hypertension without affecting body weight, indicating that table olives could contribute to improving cardiovascular health. © 2022 The Authors. Journal of The Science of Food and Agriculture published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Olea europaea L., blood pressure, body weight, spontaneously hypertensive rats
INTRODUCTION
Hypertension is considered one of the main preventable factors of cardiovascular disease burden and mortality worldwide. 1 The adoption of a healthy lifestyle and high‐quality dietary patterns, such as those provided by the Mediterranean diet, could afford considerable benefit in the prevention and to help treat hypertension and cardiovascular diseases. 2 , 3 , 4 This eating pattern is characterized among others, by a high consumption of vegetables, fruits, and legumes, along with the ingestion of the products of Olea europaea L., namely olive oil and table olives. 5 Among the foods recognized as being heart‐healthy, extra virgin olive oil (EVOO) stands out, being deemed to exert a key role in the primary prevention of cardiovascular diseases in nutritional interventions 6 and in preclinical studies. 7 , 8 In this context, the fruit of Olea europaea L., from which olive oil is obtained, could exert a relevant contribution to health. Table olives have been explicitly included in the second level of the Mediterranean diet pyramid with a recommended daily consumption of one or two portions (15–30 g). 5 The recommendation of the daily intake of table olives as a healthy snack choice arises from the high content of monounsaturated lipids that yields a balanced profile of monounsaturated/saturated fatty acids. 5 Furthermore, this food could contribute to the general well‐being not only for its elevated content in oleic acid but also in minerals, vitamins, and fiber, along with other minor components, such as polyphenols and pentacyclic triterpenes. 9 Regarding phytochemicals, hydroxytyrosol is the main polyphenol in all types of table olives, 9 and holds a claim on cardiovascular health stated by the European Food Safety Authority. 10 The claim acknowledges that the daily consumption of 5 mg of hydroxytyrosol and its derivatives (e.g. oleuropein complex and tyrosol) per 20 g of olive oil contributes to the protection of blood lipids from oxidative stress. 10 Studies performed in our group with different varieties of table olives have indicated that the cv. Arbequina contains important concentrations of polyphenols, and the consumption of nine small‐sized olives could provide the amount of hydroxytyrosol and derivatives stated in the health claim. 11 In addition, this number of olives would supply 25 mg of maslinic acid and 7 mg of oleanolic acid, 11 which are pentacyclic triterpenes that also exert multiple biological activities. 12
Notwithstanding the undeniable nutritional value of table olives and their relevant content of phytochemicals, there is a lack of knowledge of the effect of the regular consumption on health. Consequently, the aim of this study was to investigate the consequence of the ingestion of table olives on blood pressure (BP) and body weight of spontaneously hypertensive rats (SHR) and their normotensive control Wistar Kyoto (WKY) rats. The experimental model requires no intervention to induce hypertension, and its pathophysiology is similar to essential hypertension in humans. 13 The dose administered to the rats was the equivalent to the human consumption of 30 ‘Arbequina’ table olives that were given as a single dose or as a daily administration throughout 7 weeks to establish the effect in the short and long term of the intake of the fruit of Olea europaea L.
MATERIALS AND METHODS
Animals
Male SHR (n = 15) and their normotensive genetic control WKY rats (n = 13) were purchased from Envigo Laboratories (Huntingdon, UK) at 9 weeks old and were quarantined for 1 week. Animals were kept in groups of two rats per cage under controlled conditions of room temperature (22 ± 2 °C), humidity (50 ± 10%), and light–dark cycle of 12 h. Rats were fed a standard diet (2014 Teklad Global 14%; Harlan, Barcelona, Spain) and water ad libitum. All experimental procedures strictly adhered to the European Community guidelines for the care and management of laboratory animals. The study was approved by the Ethic Committee of Animal Experimentation of the Universitat de Barcelona (Ref. 105/17) and by the Generalitat de Catalunya (Ref. 9468).
Experimental design and dose preparation
After the quarantine, WKY rats and SHR were orally administered by gavage with water, daily for 2 weeks, and BP was measured daily during the same time frame, as an adaptation period. Afterwards, animals were randomly distributed into a control group (WKY control, n = 6; SHR control, n = 7) and olives group (WKY olives, n = 7; SHR olives, n = 8). In all groups, the oral dosing was carried out by gavage using a stainless‐steel animal feeding tube (18 gauge × 76 mm, ref. FTSS‐18S‐76; Instech Laboratories, Inc., Plymouth Meeting, PA, USA) at a volume of administration of 10 mL kg−1. Control animals only received water, whereas those in the olives group were treated with ‘Arbequina’ table olives at a dose of 3.85 g of destoned olives (wet weight) per kilogram of body weight.
Dose preparation
‘Arbequina’ table olives (Cooperativa del Camp, Maials, Lleida, Spain) from the 2016–2017 harvest were processed following the Greek style, which consisted of a natural fermentation in brine. The composition of ‘Arbequina’ table olives expressed as grams per kilogram of destoned olives (wet weight), consisted of 210 g kg−1 lipids, 16 g kg−1 proteins, 72 g kg−1 fiber, and 413 g kg−1 salt, yielding a total metabolizable energy content of 211 kcal. The dose of 3.85 g kg−1 is equivalent to the intake of 30 ‘Arbequina’ table olives by a person according to the body surface area normalization method. 14 The olives were administered as a finely ground homogeneous suspension. Therefore, destoned olives were mixed with Milli‐Q water and were carefully ground with a Polytron homogenizer (Kinematica AG, Lucerne, Switzerland) using a 20 TS arm by means of six cycles of 30 s at a speed set at 5. The olives suspension was prepared every 2 days and was kept at 4 °C in a 50 mL conical tube tightly closed and protected from light by aluminium foil. An aliquot of each homogeneous suspension of olives was stored at −20 °C protected from light until the analysis of polyphenols and pentacyclic triterpenes by liquid chromatography (LC)–mass spectrometry (MS).
Experiment 1: effect of a single dose of table olives on BP
The acute effect of ‘Arbequina’ table olives was evaluated in WKY rats and SHR aged 12 weeks. BP was measured between 8:00 and 10:00 a.m. Then, animals received by gavage a single oral dose of 3.85 g kg−1 of Arbequina table olives or water, and BP was determined at 2, 4, 6, 8, 10, and 24 h post‐administration.
Experiment 2: effect of repeated ingestion of table olives for 49 days
A washout period of 2 weeks was allowed after the completion of the acute experiment. BP was recorded in WKY rats and SHR prior to the first oral administration. Then, BP was measured once a week for 7 weeks between 08:00 and 10:00 a.m. in order to minimize the effects of any circadian rhythm. The oral administration of animals with 3.85 g kg−1 of ‘Arbequina’ table olives or water was performed daily between 5:00 and 6:00 p.m. Hence, BP was assessed 15–17 h after the last oral administration. Body weight was monitored daily, whereas water and food intake were assessed once a week.
BP measurements
BP was measured in conscious rats by the tail‐cuff method using the non‐invasive BP system for rodents (LE5001 Harvard Apparatus; Panlab, Barcelona, Spain). This instrument measures systolic, diastolic, and mean BP. Mean BP is the average of BP throughout one cardiac cycle and is calculated by adding one‐third of the difference between systolic and diastolic BP to the diastolic BP. Animals were restrained in a holder and were kept at 30 °C for 10 min to make the pulsations of the tail artery detectable. To avoid stress‐induced variations, all the BP determinations were taken by the same person in a peaceful environment. Once the cuffs were attached to the tail, a stabilization period of 5–10 min was followed prior to the acquisition of systolic, diastolic, and mean BP. The mean of at least seven measurements within a range of 5 mmHg was considered as the final reading. ΔBP was defined as the difference between BP pre‑ and post‐administration of table olives or water at the different time points.
Gross necropsy and sample collection
At the end of experiment 2, overnight‐fasted rats were anesthetized by intramuscular injection of ketamine (Imalgene®, Merial, Lyon, France) and xylazine (Rompun®, Bayer Hispania SL, Sant Joan Despí, Spain) at concentrations of 90 mg kg−1 and 10 mg kg−1 respectively. Blood was extracted from the rats in the WKY control (n = 6) and olives (n = 7) and SHR control (n = 7) and olives (n = 8) groups by cardiac puncture. Specimens were transferred to tubes with tripotassium ethylenediaminetetraacetic acid (EDTA K3) and were kept on ice until centrifugation at 1500 × g for 15 min at 4 °C (Centrifuge Megafuge 1.0; Heraeus, Boadilla, Spain). Then, plasma was separated from the blood cells. Individual plasma from each rat was immediately stored protected from light at −20 °C until the analysis of polyphenols and pentacyclic triterpenes by LC–MS.
Subsequently, liver, kidneys, heart, spleen, and lungs from the rats in the WKY control (n = 6) and olives (n = 7) groups and in the SHR control (n = 7) and olives (n = 8) groups were excised and trimmed of any adherent tissue. The adipose depots (omental, left epididymal pad, and left retroperitoneal pad) were collected as previously described. 15 The dissected tissues were washed in saline solution, slightly blotted on filter paper, and immediately weighed to obtain the wet weight. Results were expressed as milligrams of organ per gram of body weight.
Determination of pentacyclic triterpenes and phenolic compounds in animal feed and ‘Arbequina’ table olives
Pentacyclic triterpenes and phenolic compounds were determined in six independent olives suspensions prepared on different days along the 7 weeks of treatment as well as in three independent samples of animal feed. Therefore, 1 g of the homogenate of olives or 1 g of animal feed was submitted to liquid extraction with methanol–ethanol prior to the analysis of pentacyclic triterpenes by LC–atmospheric pressure chemical ionization (APCI)‐MS and phenolic compounds by LC–electrospray ionization (ESI)‐MS/MS as previously reported. 11 Results were expressed as milligrams of compound per kilogram of destoned olives (wet weight) or milligrams of compound per kilogram of animal fed.
Analysis of pentacyclic triterpenes and polyphenols in plasma
Pentacyclic triterpenes and phenolic compounds were extracted simultaneously from each plasma of the rats in the WKY control (n = 6) and olives (n = 7) groups and SHR control (n = 7) and olives (n = 8) groups as previously described. 16 Once the plasma was extracted using ethyl acetate as a solvent, pentacyclic triterpenes were analyzed by LC–APCI‐MS 11 and phenolic compounds by LC–ESI‐MS/MS. 16 The method was validated following the European Medicines Agency guidelines. 17
Statistical analysis
Results are expressed as mean ± standard error of the mean. Chauvenet's criterion was applied to identify and reject outliers. Statistical analysis and elaboration of graphs were performed in Prism version 6 (GraphPad Software, Inc., San Diego, CA, USA). The normality of the data was evaluated by the Kolmogorov–Smirnov test. To evaluate the main effects and interactions in experiments 1 and 2, systolic, diastolic, and mean BP were the dependent variables and time and treatment (water or table olives) were the independent variables. Moreover, the statistical analysis of ΔBP, body weight, food intake, water consumption, the relative weight of the organs, and fat depots was performed considering treatment (water or table olives) and strain as independent variables. The statistical analysis was carried out by two‐way analysis of variance followed by Fisher least significant difference (LSD) post hoc test. Values of P < 0.05 were considered statistically significant.
RESULTS
Pentacyclic triterpenes and polyphenols in ‘Arbequina’ table olives
The analysis of ‘Arbequina’ table olives enabled the identification and quantification of three pentacyclic triterpenes and 15 phenolic compounds. Pentacyclic triterpenes were the most abundant, with a total content of 3214 ± 123 mg kg−1 (n = 6), ahead of 1043 ± 46.9 mg kg−1 (n = 6) for polyphenols (Table 1). The main pentacyclic triterpene was maslinic acid, which accounted for 72.9%. Concerning polyphenols, the highest concentrations corresponded to hydroxytyrosol, verbascoside, and luteolin, which accounted for 86% of this group of bioactive compounds (Table 1).
Table 1.
Determination of pentacyclic triterpenes by liquid chromatography (LC)–atmospheric pressure chemical ionization mass spectrometry (MS) and polyphenols by LC–electrospray ionization MS/MS
| Compound | Animal feed | ‘Arbequina’ table olives | Plasma after treatment | |||
|---|---|---|---|---|---|---|
| (mg kg−1) | (mg kg−1) | (μg) | WKY olives (nmol L−1) | SHR olives (nmol L−1) | ||
| Pentacyclic triterpenes | ||||||
| Triterpenic acids | Maslinic acid | ND | 2341 ± 82.2 | 2.708 | 3.13 ± 0.85 | 3.51 ± 1.10 |
| Oleanolic acid | ND | 862 ± 44.0 | 0.997 | 1.08 ± 0.19 | 1.34 ± 0.40 | |
| Ursolic acid | ND | <LOD | — | <LOD | <LOD | |
| Triterpenic alcohols | Erythrodiol | ND | 10.4 ± 0.1 | 0.012 | <LOD | <LOD |
| Uvaol | ND | <LOD | — | <LOD | <LOD | |
| Polyphenols | ||||||
| Phenolic alcohols | Hydroxytyrosol | ND | 475 ± 11.8 | 0.549 | 0.50 ± 0.20 | 0.48 ± 0.14 |
| Hydroxytyrosol acetate | ND | 26.9 ± 0.7 | 0.031 | <LOD | <LOD | |
| Tyrosol | ND | 23.1 ± 0.6 | 0.027 | <LOD | <LOD | |
| Salidroside | ND | 17.4 ± 1.0 | 0.020 | <LOD | <LOD | |
| Catechol | ND | <LOD | — | <LOD | <LOD | |
| Phenolic acids | Vanillic acid | 0.54 ± 0.004 | 3.56 ± 0.1 | 0.004 | <LOQ | <LOQ |
| p‐Coumaric acid | 1.69 ± 0.02 | 5.65 ± 0.1 | 0.007 | <LOQ | <LOQ | |
| Caffeic acid | 0.26 ± 0.001 | 4.64 ± 0.1 | 0.005 | <LOD | <LOD | |
| Verbascoside | ND | 334 ± 30.8 | 0.387 | <LOQ | <LOQ | |
| Flavonoids | Luteolin | ND | 89.6 ± 3.0 | 0.104 | 0.42 ± 0.02 | 0.12 ± 0.02 |
| Luteolin‐7‐O‐glucoside | ND | 11.1 ± 1.7 | 0.013 | <LOQ | <LOQ | |
| Quercetin | ND | 6.49 ± 0.2 | 0.008 | <LOD | <LOD | |
| Rutin | ND | 26.0 ± 3.1 | 0.030 | <LOQ | <LOQ | |
| Apigenin | ND | 4.52 ± 0.2 | 0.005 | <LOQ | <LOQ | |
| Secoiridoids | Oleuropein | ND | 12.6 ± 0.2 | 0.015 | <LOQ | <LOQ |
| Lignans | (+)‐Pinoresinol | ND | 3.08 ± 0.2 | 0.004 | <LOD | <LOD |
Concentrations in animal feed, ‘Arbequina’ table olives, dose administered to the animals in the WKY olives and SHR olives groups, and the plasmatic concentrations obtained 15–17 h after the oral administration of 3.85 g kg−1 of body weight for 49 days.
Results are expressed as mean ± standard error of the mean in the analysis of animal fed (n = 3), ‘Arbequina’ table olives (n = 6), and plasma samples from WKY (n = 7) and SHR (n = 8) olive groups. WKY, Wistar Kyoto (rats); SHR, spontaneously hypertensive rats; ND, not detected; LOD, limit of detection; LOQ, limit of quantification.
Pentacyclic triterpenes and polyphenols in the commercial diet
No pentacyclic triterpenes were detected in the animal feed (n = 3). Most of the polyphenols were also absent and only small amounts of p‐coumaric acid, vanillic acid, and caffeic acid were found (Table 1).
Experiment 1: effect of a single dose of table olives on blood pressure
The acute experiment was performed in 12‐week‐old animals. The WKY rats weighed 252 ± 3.31 g (n = 13) and the SHR were 261 ± 2.49 g (n = 15).
BP in normotensive WKY rats
BP at time 0 h yielded normotensive values for systolic (140.8 ± 1.7 mmHg; n = 13) and diastolic measurements (97.1 ± 1.9 mmHg; n = 13) in all the animals (Fig. 1(A)). The oral administration of Arbequina table olives yielded non‐significant differences in systolic, diastolic, mean BP, and ΔBP (Fig. 1).
Figure 1.
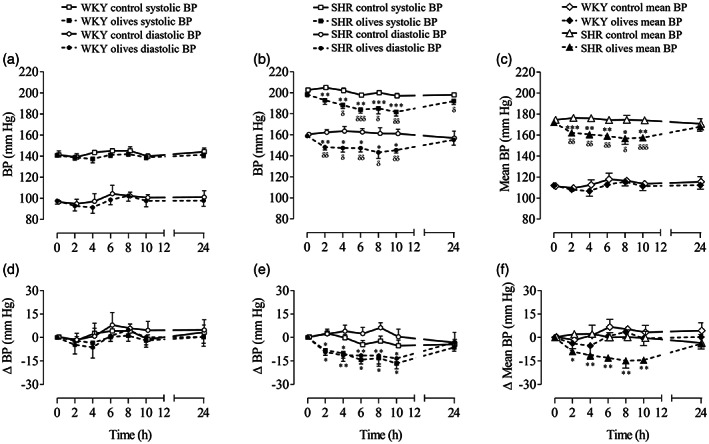
Blood pressure (BP) in normotensive (WKY) rats and spontaneously hypertensive rats (SHR) after the single oral administration of ‘Arbequina’ table olives at a dose of 3.85 g kg−1 or water. The graphs depict the time course over 24 h of the systolic, diastolic, mean, and ΔBP in (A, C, D, F) WKY rats and (B, C, E, F) SHR. Values are presented as mean + standard error of the mean (SEM) in the WKY (n = 6) and SHR (n = 7) control groups and as mean − SEM in the WKY (n = 7) and SHR (n = 8) olives groups. Only the SEMs that exceed the size of the symbol are shown. Data were analyzed by two‐way analysis of variance followed by a Fischer multiple comparison test. Different from control rats: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Different from time 0 h: δ, P < 0.05; δδ, P < 0.01; δδδ, P < 0.001.
BP in hypertensive SHR
The mean initial values of systolic BP (200.3 ± 1.4 mmHg; n = 15) and diastolic BP (159.4 ± 1.1 mmHg; n = 14) indicated that hypertension was established in SHR. Throughout the 24 h of the acute study, BP remained similar in the animals that were orally administered with water (Fig. 1(B)). In contrast, the oral intake of ‘Arbequina’ table olives induced a statistically significant decline of 6.95 ± 0.32% (n = 8; P < 0.05) in systolic BP and 9.66 ± 0.43% (n = 8; P < 0.05) in diastolic BP from 2 to 10 h when compared with the control group (n = 7) (Fig. 1(B)), which can also be observed when delta systolic and diastolic BP were calculated (Fig. 1(E)). The mean BP showed the same pattern as described for systolic and diastolic BP in the control and olives groups (Fig. 1(C), (F)).
Experiment 2: effect of the repeated ingestion of table olives for 49 days
Body weight and food and water consumption
The chronic study started when animals were 14 weeks old after a washout period of 2 weeks. In the WKY control group, the initial body weight was 274 ± 7.48 g (n = 6) and after 7 weeks increased to 346 ± 11.83 g (n = 6) (Fig. 2). Similar growth was observed in the WKY olives group, since the body weight changed from 272 ± 4.95 g (n = 7) to 347 ± 6.87 g (n = 7) after 49 days of treatment. Concerning the hypertensive strain, animals in the control group grew from 270 ± 5.40 g (n = 7) to 322 ± 6.14 g (n = 7) after 7 weeks whereas the body weight in the SHR receiving olives varied from 274 ± 6.44 g (n = 8) to 326 ± 7.98 g (n = 8) at the end of the experiment (Fig. 2).
Figure 2.
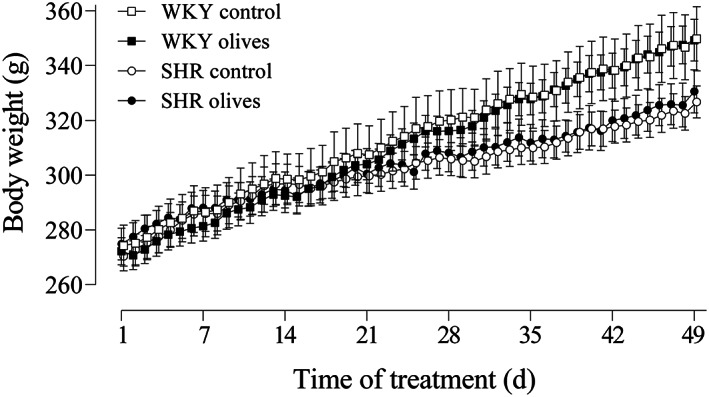
Body weight in normotensive (WKY) rats and spontaneously hypertensive rats (SHR). The graph represents the time course of the WKY rats and SHR treated with ‘Arbequina’ table olives at a dose of 3.85 g kg−1 or water. Values are presented as mean ± standard error of the mean in the WKY control (n = 6), WKY olives (n = 7), SHR control (n = 7) and SHR olives (n = 8) groups. Body weight was analyzed by two‐way analysis of variance followed by the Fischer multiple comparison test; P > 0.05 control versus treated animals.
The weight gain was 72 ± 6 g in the control WKY group (1.47 g day−1), 75 ± 3 g in the WKY olives group (1.53 g day−1), 52 ± 4 g in the SHR control group (1.06 g day−1), and 51 ± 6 g in the SHR olives group (1.04 g day−1). The total weight gain of the control SHR was 30% lower (P < 0.05) than that found in the WKY control group. Within the same strain, no differences were found between groups.
The mean daily food consumption per rat was 18.1 ± 0.1 g day−1 in the WKY control (n = 6), 17.9 ± 0.1 g day−1 in the WKY olives (n = 7), 17.8 ± 0.1 g day−1 in the SHR control (n = 7), and 17.7 ± 0.1 g day−1 in the SHR olives (n = 8). No statistically significant differences in food consumption were observed between the control and treated groups.
Finally, the mean daily water consumption per rat was 24.6 ± 0.3 mL day−1 in the WKY control (n = 6) and 25.5 ± 0.4 mL day−1 in the WKY olives (n = 7), whereas in the SHR control (n = 7) and SHR olives (n = 8) were 36.5 ± 0.6 mL day−1 and 37.5 ± 0.6 mL day−1, respectively. The water intake in the hypertensive rats was around 32% higher than in the normotensive animals (P < 0.05). Within each strain, no statistically significant difference was found in water consumption between the control and the treated animals.
BP in normotensive WKY rats
At day 1, WKY rats hold similar systolic BP (148.1 ± 2.1 mmHg; n = 13) and diastolic BP (100.3 ± 5.1 mmHg; n = 13). The chronic intake of table olives did not modify BP with respect to the control (Fig. 3(A), (D)). Mean BP measured during the 49 days of the experiment followed the same pattern as described for systolic and diastolic BP (Fig. 3(C)). No differences were observed when ΔBP was calculated (Fig. 3(D), (F)).
Figure 3.
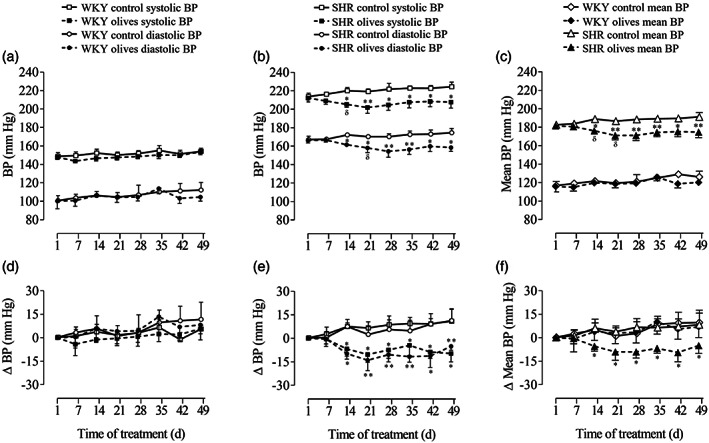
Blood pressure (BP) in normotensive (WKY) rats and spontaneously hypertensive rats (SHR) after the daily oral administration of ‘Arbequina’ table olives at a dose of 3.85 g kg−1 or water. The graphs represent the time course over 7 weeks of the systolic, diastolic, mean, and ΔBP in (A, C, D, F) WKY rats and (B, C, E, F) SHR. Values are presented as mean + standard error of the mean (SEM) in the WKY (n = 6) and SHR (n = 7) control groups and as mean − SEM in the WKY (n = 7) and SHR (n = 8) olives groups. Only SEMs that exceed the size of the symbol are shown. Data were analyzed by two‐way analysis of variance followed by a Fischer multiple comparison test. Different from control rats: *, P < 0.05; **, P < 0.01. Different from day 1: δ, P < 0.05.
BP in hypertensive SHR
In control SHR (n = 7), systolic BP was 213.5 ± 3.5 mmHg on day 1 and 224.1 ± 5.0 mmHg after 7 weeks (P > 0.05). In the same group, diastolic BP presented a similar pattern, ranging from 166.9 ± 3.7 mmHg on day 1 to 174.2 ± 4.7 mmHg on day 49 (Fig. 3(B), (E)).
The oral administration of ‘Arbequina’ table olives induced a statistically significant reduction of 7.1 ± 0.2% (n = 8; P < 0.05) in systolic BP and 8.01 ± 0.56% (n = 8; P < 0.05) in diastolic BP from day 14 to day 49 when compared with the control animals (Fig. 3(B)). The ΔBP of SHR supplemented with table olives for 49 days showed a similar decrease for both systolic and diastolic BP (Fig. 3(E)). Finally, the mean BP in hypertensive rats reflects the changes described for the systolic and diastolic BP in both the control and olives groups (Fig. 3(C), (F)).
Concentrations of pentacyclic triterpenes and polyphenols in plasma
Plasma samples from rats in the WKY (n = 7) and SHR (n = 8) olives groups contained four main bioactive compounds from olives, namely, maslinic acid, oleanolic acid, hydroxytyrosol, and luteolin (Table 2). The higher plasmatic concentrations corresponded to pentacyclic triterpenes. Maslinic acid was found at 3.13 ± 0.85 nmol L−1 and 3.51 ± 1.10 nmol L−1 in the rats from the groups WKY olives and SHR olives respectively. Oleanolic acid was 1.08 ± 0.19 nmol L−1 and 1.34 ± 0.40 nmol L−1 respectively in the normotensive and hypertensive rats that were orally administered with olives. Regarding polyphenols, only hydroxytyrosol and luteolin were determined in plasma at concentrations below 1 nmol L−1 (Table 1).
Table 2.
Relative organ weight and fat depots of Wistar Kyoto (WKY) rats and spontaneously hypertensive rats (SHR) after the oral administration of ‘Arbequina’ table olives at a dose of 3.85 g kg−1 for 49 days compared with control animals
| Relative weight (mg g−1 body weight) | ||||
|---|---|---|---|---|
| Organ | WKY control | WKY olives | SHR control | SHR olives |
| Kidneys | 5.79 ± 0.06a | 5.51 ± 0.09a | 5.53 ± 0.08a | 5.70 ± 0.14a |
| Liver | 27.21 ± 0.46a | 26.19 ± 0.38a | 33.87 ± 0.38b | 32.94 ± 1.07b |
| Heart | 3.69 ± 0.12a | 3.63 ± 0.13a | 4.65 ± 0.03b | 4.70 ± 0.25b |
| Spleen | 1.71 ± 0.02a | 1.67 ± 0.02a | 1.95 ± 0.04b | 1.91 ± 0.04b |
| Lungs | 3.57 ± 0.04a | 3.65 ± 0.14a | 3.78 ± 0.17a | 3.93 ± 0.19a |
| Omental depot | 3.70 ± 0.28a | 4.08 ± 0.18a | 4.39 ± 0.25a | 4.30 ± 0.27a |
| Left retroperitoneal pad | 8.52 ± 0.51a | 9.06 ± 0.53a | 8.25 ± 0.21a | 7.94 ± 0.53a |
| Left epididymal pad | 4.86 ± 0.54a | 5.49 ± 0.39a | 3.34 ± 0.15b | 3.60 ± 0.14b |
Results are expressed as mean ± standard error of the mean in the WKY control (n = 6), WKY olive (n = 7), SHR control (n = 7), and SHR olive (n = 8) groups. Data were analyzed by two‐way analysis of variance followed by a Fischer multiple comparison test. No differences between control and olives groups were found within each strain (P > 0.05). Means without a common letter differ (P < 0.01).
Abbreviations: SHR, spontaneously hypertensive rats; WKY, Wistar‐Kyoto rats.
Gross necropsy
The repeated oral administration of ‘Arbequina’ table olives at a dose of 3.85 g kg−1 for 49 days did not induce any adverse effects or mortality during the experimental period, either in normotensive or in hypertensive rats. A thorough post‐mortem assessment of the internal organs was performed at the end of the chronic study. No macroscopic differences in color, size, or texture were observed in the different groups.
The final relative weights (mg g−1 body weight) of kidney, liver, heart, spleen, lungs, and fat depots were not affected by the daily oral administration of table olives compared with the control group within each strain (Table 2). However, differences between strains were found. The relative weights of the liver, heart, and spleen of the SHR control (n = 7) were respectively 24.5%, 26.0%, and 14.0% higher (P < 0.01) than in the WKY control (n = 6). Conversely, the epididymal pad showed a lower relative weight in the SHR control than in the WKY control (P < 0.01) animals.
DISCUSSION
The regular intake of table olives could ameliorate human health not only for their important nutritional content but also for the high presence of different bioactive compounds. 5 , 9 , 18 Recently, table olives have been regarded as a healthy appetizer and their consumption continues to grow worldwide. 9 However, despite the unquestionable nutritional value of this food, there are relevant aspects for the consumer's health that are not yet well known, such as the effect of table olives intake on BP and in body weight. In this study, we administered a dose of 3.85 g kg−1 of destoned ‘Arbequina’ olives to rats, which is equivalent to a human consumption of 30 of them and double the recommended intake. 5 WKY rats were used to assess the effect of the olives on normotensive animals, whereas SHR were employed as a suitable animal model of primary hypertension. 19 This model holds similarities to the development of human essential hypertension that represents 90–95% of human adult cases. 13 According to the literature, 20 the SHR rats used were hypertensive at 12 weeks old, whereas WKY rats displayed normotensive systolic and diastolic values. Then, we performed a first study using a single dose of ‘Arbequina’ olives that showed no changes either in systolic or in diastolic BP on normotensive animals during 24 h. However, in hypertensive rats, a decrease of approximately 15 mmHg in both systolic and diastolic PB was found from the second to the tenth hour after the consumption of this food when compared with SHR controls. Although there are no data on the acute effect of Olea europaea L. in an animal model of hypertension, a single dose of 2 g kg−1 of virgin olive oil in normotensive Sprague–Dawley rats has been reported to induce a transient reduction in BP between 2 and 4 h post‐administration. 7 The authors attributed this rather quick action to the high concentration of oleic acid that induced changes in the cell signaling cascade favoring a vasodilator effect and a reduction in BP. 7
Our findings of the short‐term assay were substantiated by the results obtained in the chronic study involving the daily oral administration of table olives for 7 weeks. No changes in BP were found in normotensive rats, whereas a decline of systolic and diastolic BP of approximately 15 mmHg was observed in hypertensive animals that were administered with table olives. These results were established from the second week up to the end of the experiment when compared with the SHR control. This hypotensive effect could be relevant, since it has been described that a relatively small reduction in systolic BP (10 mmHg) in humans significantly lowered the risk of major cardiovascular disease events, coronary heart disease, stroke, and heart failure. 21 However, the complex composition of table olives hinders the elucidation of the mechanisms involved in the improvement of BP observed in SHR as well as to ascribe the activity to a particular component. Other studies carried out in the same experimental model have also shown an antihypertensive effect using different derivatives from Olea europaea L. 7 , 8 , 22 , 23 The administration to SHR of 2 g kg−1 of virgin olive oil every 12 h for 14 days induced a significant and progressive reduction of systolic BP as early as 4 days after the start of the treatment. 7 Oleic acid was considered to be responsible for the decrease of BP through an adaptive process that led to an enhancement in the production of vasodilator mediators. 7 However, the hypotensive effect of oleic acid in olive oil could barely be extrapolated to table olives due to the different content of fat between both foods. 9 , 24 In our study, the dose of oleic acid administered to rats was 0.65 g kg−1, which is 80% lower than that given daily by Terés et al. 7 which corresponded to 3.17 g kg−1. Therefore, the content of oleic acid in table olives could hardly explain the reduction in BP observed in SHR after the ingestion of this food, and other minor compounds could possibly also be involved. In this respect, Vazquez et al. 8 were not able to find a reduction in BP in SHR after the administration for 8 weeks of 1 mL day−1 of an EVOO containing oleic acid at a dose of 1.98 mg kg−1 and a low content of polyphenols (17.6 mg kg−1), although a decrease in vasoconstrictor biomarkers and an increase of vasodilatory nitric oxide were reported. However, when the same EVOO was enriched with 750 mg kg−1 of phenolic compounds (mainly hydroxytyrosol, 3,4‐dihydroxyphenylglycol, and oleuropein) a progressive reduction in systolic BP of SHR was observed from the fifth week until the eighth week. 8 The authors attributed the effect to an improvement of the endothelial dysfunction and a decreased oxidative status. 8 Considering the content of polyphenols, the enriched EVOO yielded a dose of approximately 1.88 mg kg−1, whereas the table olives used in our study provided around double this (4.01 mg kg−1). Hence, the high content of polyphenols supplied by the cv. Arbequina could be involved in the reduction of BP in SHR animals that was already observed in the second week. Noteworthy is the fact that, in addition to polyphenols, table olives are an important source of pentacyclic triterpenes with vasorelaxant activity. 25 Our results showed that ‘Arbequina’ olives contained a total of 3214 mg kg−1of pentacyclic triterpenes. Hence, SHR received a dose of 12.4 mg kg−1 of these bioactive compounds that had also been deemed to play an antihypertensive activity in the same animal model. 22 In this regard, the administration for 8 weeks to SHR animals of a pomace oil concentrated in triterpenic acids that were given at a dose of 100 mg kg−1 (56.8% oleanolic acid and 38% maslinic acid) not only reduced BP but also improved the expression of endothelial nitric oxide synthase, ameliorated endothelial dysfunction, and diminished the expression of inflammatory and fibrotic mediators in the aorta. 22 Although this study is relevant in demonstrating the potential cardiovascular benefits of a pomace olive oil enriched in pentacyclic triterpenes, the supplementation in maslinic and oleanolic acids is closer to a pharmacological regime than to a nutritional pattern. Finally, the involvement of bioactive compounds from Olea europaea L. in lowering BP has been supported by Romero et al. 23 The administration to SHR for 5 weeks of a dose of 30 mg kg−1 of olive leaf extract containing oleuropein (4.5 mg kg−1), triterpenic acids (3 mg kg−1), and hydroxytyrosol (0.3 mg kg−1) decreased systolic BP from the third to the fifth weeks of treatment, compared with the control that only received water. 23 The attenuation of high BP in SHR animals was ascribed to an enhancement of vascular function related to a decline in inflammation and oxidative markers.
Given the key role that pentacyclic triterpenes and polyphenols may play in lowering BP, these compounds were determined in ‘Arbequina’ table olives, animal feed, and in plasma withdrawn 15–17 h after the last administration. At this sampling point, which corresponds to the time interval in which BP was measured, only maslinic acid, oleanolic acid, hydroxytyrosol, and luteolin were found in plasma. None of these compounds were detected in the animal feed; thus, their presence in plasma must come from the supplementation of WKY rats and SHR with ‘Arbequina’ table olives. Noteworthy is the fact that all of them have been described to exert cardioprotective activities associated with an improvement of high BP. 25 , 26 , 27 In this sense, maslinic and oleanolic acids have been reported to induce a concentration‐dependent vasorelaxation in isolated aorta of SHR by increasing the bioavailability of nitric oxide from the vascular endothelium. 25 The treatment with hydroxytyrosol improved endothelial function with lower systolic BP in a diet‐induced rat model of metabolic syndrome. 26 Luteolin was able to decrease BP in SHR rats and was described to ameliorate a hypertensive complication such as vascular remodeling. 27 Furthermore, the consumption of table olives supplies other polyphenols that can be absorbed. This was demonstrated in Sprague–Dawley rats that received the same dose of ‘Arbequina’ table olives where, at 30 min post‐administration, salidroside, p‐coumaric acid, hydroxytyrosol, verbascoside, tyrosol, luteolin, and luteolin‐7‐O‐glucoside were found in plasma. 16
In summary, the effects of a regular intake of table olives equivalent to human consumption of 30 ‘Arbequina’ olives did not affect systolic, diastolic, and mean BP in normotensive rats and induced a decrease in hypertensive animals. It is noteworthy that the salt intake provided by 30 ‘Arbequina’ olives supplies 624 mg of sodium. This value is approximately one‐fourth the daily intake recommended by the US Food and Drug Administration. However, despite this supply of salt, the BP did not increase in either normotensive or hypertensive rats.
Finally, the possible impact of the regular eating of table olives on body weight was also considered. The energy content of ‘Arbequina’ table olives used was 211 kcal, and the consumption of these olives increased the calories by approximately 5% with respect to the control groups. However, it did not affect the final body weight or the mean growth rate, either in WKY rats or in SHR. There are no data in the literature about the effects of table olives on body weight, but there are data on Olea europaea L. by‐products. In this sense, in the SHR strain, no differences in the body weight of treated animals with respect to the control group were observed after the oral administration of pomace olive oil concentrated in triterpenic acids for 8 weeks 22 or oleuropein‐enriched olive leaf extract for 5 weeks. 23 The oral administration for 8 weeks of virgin olive oil (17.6 mg kg−1 of polyphenols) to SHR did not induce any change in body weight with respect to the SHR control, whereas the oil enriched with 750 mg kg−1 of polyphenols produced a slight decrease in body weight. 8
CONCLUSIONS
Our study demonstrates in a rat model of hypertension that the daily intake of table olives ameliorates high BP without increasing the body weight of the animals. The results shed light on the beneficial properties of table olives on cardiovascular health, which have been disregarded despite the well‐known effects described for the Mediterranean diet and EVOO. The chemical profile of table olives is similar to that of olive oil in the composition of fatty acids; however, interesting differences can be found at the level of minor components. Thus, the proportion of pentacyclic triterpenes and polyphenols in table olives is higher than in olive oil, highlighting its high content of maslinic and oleanolic acids to which antihypertensive effects have been attributed. Hence, table olives arise as a wholesome food and their rational consumption can improve human health and, in particular, could be considered as a useful tool in the protection against cardiovascular disease as proposed by olive oil. Therefore, the present results contribute to raising awareness about table olives as a valuable functional food with cardiovascular properties.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by the Ministerio de Economía y Competitividad (AGL2013‐41188) and the Generalitat de Catalunya (2014SGR1221 and 2017SGR945). TF‐A was a recipient of a fellowship of Consejo Nacional de Ciencia y Tecnología, CONACYT (México). The authors thank Dr Juan Carlos Laguna for providing the equipment to measure the BP, and Dr Isidre Casals, Dr Alberto Adeva, and Dr Olga Jáuregui from CCiTUB for technical assistance.
Contributor Information
M. Emília Juan, Email: mejuan@ub.edu.
Joana M. Planas, Email: jmplanas@ub.edu.
REFERENCES
- 1. Zhou B, Perel P, Mensah GA and Ezzati M, Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol 28:785–802 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ozemek C, Laddu DR, Arena R and Lavie CJ, The role of diet for prevention and management of hypertension. Curr Opin Cardiol 33:388–393 (2018). [DOI] [PubMed] [Google Scholar]
- 3. De Pergola G and D'Alessandro A, Influence of Mediterranean diet on blood pressure. Nutrients 10:1700 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Islam FMA, Lambert EA, Islam SMS, Islam MA, Biswas D, McDonald R et al., Lowering blood pressure by changing lifestyle through a motivational education program: a cluster randomized controlled trial study protocol. Trials 22:438 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Serra‐Majem L, Tomaino L, Dernini S, Berry EM, Lairon D, Ngo de la Cruz J et al., Updating the Mediterranean diet pyramid towards sustainability: focus on environmental concerns. Int J Environ Res Public Health 17:8758 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Estruch R, Ros E, Salas‐Salvadó J, Covas MI, Corella D, Arós F et al., Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra‐virgin olive oil or nuts. N Engl J Med 378:e34 (2018). [DOI] [PubMed] [Google Scholar]
- 7. Terés S, Barceló‐Coblijn G, Benet M, Alvarez R, Bressani R, Halver JE et al., Oleic acid content is responsible for the reduction in blood pressure induced by olive oil. Pro Natl Acad Sci U S A 105:13811–13816 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vazquez A, Sanchez‐Rodriguez E, Vargas F, Montoro‐Molina S, Romero M, Espejo‐Calvo JA et al., Cardioprotective effect of a virgin olive oil enriched with bioactive compounds in spontaneously hypertensive rats. Nutrients 11:1728 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rocha J, Borges N and Pinho O, Table olives and health: a review. J Nutr Sci 9:e57 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. European Commission , Commission regulation (EU) No 432/2012 of 16 May 2012 establishing a list of permitted health claims made on foods, other than those referring to the reduction of disease risk and to children's development and health. Off J Eur Union Legis 136:1–40 (2012). [Google Scholar]
- 11. Moreno‐González R, Juan ME and Planas JM, Profiling of pentacyclic triterpenes and polyphenols by LC–MS in Arbequina and Empeltre table olives. LWT Food Sci Technol 126:109310 (2020). [Google Scholar]
- 12. Sánchez‐Quesada C, López‐Biedma A, Warleta F, Campos M, Beltrán G and Gaforio JJ, Bioactive properties of the main triterpenes found in olives, virgin olive oil, and leaves of Olea europaea . J Agric Food Chem 61:12173–12182 (2013). [DOI] [PubMed] [Google Scholar]
- 13. Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD et al., Animal models of hypertension: a scientific statement from the American Heart Association. Hypertension 73:e87–e120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reagan‐Shaw S, Nihal M and Ahmad N, Dose translation from animal to human studies revisited. FASEB J 22:659–661 (2008). [DOI] [PubMed] [Google Scholar]
- 15. Johnson PR and Hirsch J, Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res 13:2–11 (1972). [PubMed] [Google Scholar]
- 16. Kundisová I, Juan ME and Planas JM, Simultaneous determination of phenolic compounds in plasma by LC‐ESI‐MS/MS and their bioavailability after the ingestion of table olives. J Agric Food Chem 68:10213–10222 (2020). [DOI] [PubMed] [Google Scholar]
- 17. European Medicines Agency , Guideline on bioanalytical method validation EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2. European Medicines Agency, London, UK: (2011). [Google Scholar]
- 18. Boskou D, Table olives: a vehicle for the delivery of bioactive compounds. J Exp Food Chem 3:123–129 (2017). [Google Scholar]
- 19. Lin HY, Lee YT, Chan YW and Tse G, Animal models for the study of primary and secondary hypertension in humans. Biomed Rep 5:653–659 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maris ME, Melchert RB, Joseph J and Kennedy RH, Gender differences in blood pressure and heart rate in spontaneously hypertensive and Wistar‐Kyoto rats. Clin Exp Pharmacol Physiol 32:35–39 (2005). [DOI] [PubMed] [Google Scholar]
- 21. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J et al., Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta‐analysis. Lancet 387:957–967 (2016). [DOI] [PubMed] [Google Scholar]
- 22. Valero‐Muñoz M, Martín‐Fernández B, Ballesteros S, de la Fuente E, Quintela JC, Lahera V et al., Protective effect of a pomace olive oil concentrated in triterpenic acids in alterations related to hypertension in rats: mechanisms involved. Mol Nutr Food Res 58:376–383 (2014). [DOI] [PubMed] [Google Scholar]
- 23. Romero M, Toral M, Gómez‐Guzmán M, Jiménez R, Galindo P, Sánchez M et al., Antihypertensive effects of oleuropein‐enriched olive leaf extract in spontaneously hypertensive rats. Food Funct 7:584–593 (2016). [DOI] [PubMed] [Google Scholar]
- 24. Jimenez‐Lopez C, Carpena M, Lourenço‐Lopes C, Gallardo‐Gomez M, Lorenzo JM, Barba FJ et al., Bioactive compounds and quality of extra virgin olive oil. Foods 9:1014 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodríguez‐Rodríguez R, Perona JS, Herrera MD and Ruiz‐Gutierrez V, Triterpenic compounds from ‘orujo’ olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J Agric Food Chem 54:2096–2102 (2006). [DOI] [PubMed] [Google Scholar]
- 26. Poudyal H, Lemonakis K, Efentakis P, Gikas K, Halabalaki M, Andreadou I et al., Hydroxytyrosol ameliorates metabolic, cardiovascular and liver changes in a rat model of diet‐induced metabolic syndrome: pharmacological and metabolism‐based investigation. Pharmacol Res 117:32–45 (2017). [DOI] [PubMed] [Google Scholar]
- 27. Su J, Xu HT, Yu JJ, Gao JL, Lei J, Yin QS et al., Luteolin ameliorates hypertensive vascular remodeling through inhibiting the proliferation and migration of vascular smooth muscle cells. Evid Based Complement Alternat Med 2015:364876 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


