Abstract
Objective
To assess European Association of Urology (EAU) risk groups for biochemical recurrence (BCR) of prostate cancer relative to prostate‐specific membrane antigen‐positron emission tomography (PSMA‐PET) status and oncological outcomes.
Patients and Methods
A retrospective analysis of a study that incorporated PSMA‐PET for men with BCR after radical prostatectomy (RP) was undertaken. EAU risk groups were considered relative to clinical variables, PSMA‐PET findings, and deployment of salvage radiotherapy (SRT). The primary oncological outcome was event‐free survival (EFS) and this was analysed relative to clinical and imaging variables. An ‘event’ occurred if prostate‐specific antigen (PSA) level rose >0.2 ng/mL above nadir or additional therapies were introduced.
Results
A total of 137 patients were included, most of whom had EAU high‐risk disease (76%) and/or low PSA levels (80% <0.5 ng/mL) at the time of PSMA‐PET. EAU risk group was not associated with regional nodal/distant metastasis on PSMA‐PET. Regional nodal/distant metastasis on PSMA PET (compared to negative/local recurrence: hazard ratio [HR] 2.2; P = 0.002) and SRT use (vs no SRT: HR 0.44; P = 0.004) were associated with EFS. EAU high‐risk status was not significantly associated with worse EFS (HR 1.7, P = 0.12) compared to EAU low‐risk status. Among patients who received SRT, both regional/distant metastasis on PSMA‐PET (HR 3.1; P < 0.001) and EAU high‐risk status (HR 2.9; P = 0.04) were independently associated with worse EFS, which was driven by patients in the EAU high‐risk group with regional/distant metastases (38%; HR 3.1, P = 0.001).
Conclusions
In patients with post‐RP BCR, PSMA‐PET findings and receipt of SRT predicted EFS. In patients receiving SRT, PSMA status combined with EAU risk grouping was most predictive of EFS. These findings suggest that the EAU risk groups could be improved with the addition of PSMA‐PET.
Keywords: prostate‐specific membrane antigen, PSMA, PET/CT, biochemical failure, radical prostatectomy, salvage RT
Abbreviations
- ADT
androgen deprivation therapy
- BCR
biochemical recurrence
- EAU
European Association of Urology
- EFS
event‐free survival
- GS
Gleason score
- HR
hazard ratio
- PET
positron emission tomography
- PSA‐DT
PSA doubling time
- PSMA
prostate‐specific membrane antigen
- RP
radical prostatectomy
- SRT
salvage radiotherapy
Introduction
Due to uncertainties regarding the prognostic implications of biochemical recurrence (BCR) after radical prostatectomy (RP) for prostate cancer, the European Association of Urology (EAU) Guidelines panel undertook a scoping systematic review and meta‐analysis in 2018 to determine the prognostic features of BCR to inform management recommendations [1]. Worse survival outcomes were estimated for patients with shorter (<12 months) serum PSA doubling time (PSA‐DT) and high Gleason score (GS; ≥4 + 4) after RP. EAU risk groups were subsequently devised, ‘low’ (PSA‐DT >12 months and pathological GS <8) and ‘high’ (PSA‐DT ≤12 months or pathological GS 8–10), and these were endorsed with recommendations for treatment that included limiting (obviating) salvage radiotherapy (SRT) for ‘selected’ men in the EAU low‐risk group [2]. However, the source data for this recommendation were historical cohorts that used conventional imaging such as CT and/or whole‐body bone scan for assessment of metastatic disease. More accurate molecular imaging techniques, such as prostate‐specific membrane antigen (PSMA) positron emission tomography (PET), were not included in the risk algorithms which we hypothesize may further refine prognostic information.
It has been shown that PSMA‐PET is more accurate than conventional imaging for staging [3] and PSMA‐PET is recommended within the EAU and Australian guidelines for assessment of prostate cancer in the BCR setting [4, 5]. Although the diagnostic accuracy of PSMA with regard to BCR is high, with a positive predictive value of 0.92 [6], there is minimal information regarding its influence on survival outcomes [7]. Novel randomized trials are underway to assess PSMA‐PET as an intervention to aid treatment guidance and improve subsequent oncological outcomes [8], but data regarding the predictive and prognostic properties of PSMA‐PET are limited.
We have previously reported that PSMA‐PET better predicted 3‐year freedom from progression than established clinical predictors in a prospective multicentre study of patients with BCR after RP [9]. The objective of this analysis was to analyse EAU risk groups relative to PSMA‐PET status and subsequent oncological outcomes within our previous study.
Patients and Methods
Study Details
This is a secondary analysis of data collected from 260 patients as part of a prospective, multicentre study performed in Australia across four academic centres (St Vincent's and Royal North Shore, Sydney, Sir Charles Gairdner and Fiona Stanley, Perth) [9]. Institutional Human Research Ethics Committee approval was obtained for each site and all participants provided informed written consent.
Participants with a rising PSA level of between ≥0.05 and <1 ng/mL following RP and eligible to receive SRT were enrolled. After providing written informed consent, participants underwent whole‐body 68Ga‐PSMA‐PET/CT with non‐contrast CT imaging between January 2015 and March 2017 according to a standardized protocol. PET images were prospectively reported by experienced nuclear medicine physicians using a four‐point certainty scoring scale for each site of identified lesions. PSMA‐PET results were provided to the clinician, subsequent treatments were recorded (rather than mandated) and clinical course was followed for at least 3 years.
The SRT treatment after PSMA‐PET with or without short‐term androgen deprivation therapy (ADT; 3–6 months duration according to clinician preference), was performed according to institutional protocols. Subsequent clinical events, including PSA kinetics and use of additional treatments were recorded.
Primary Outcome
The primary outcome of this analysis was event‐free survival (EFS). An event was defined as a PSA >0.2 ng/mL above the nadir PSA following SRT or use of additional hormonal and/or radiotherapy (RT) [10]. When the clinical decision was not to give RT, an event was defined as a rise of ≥0.2 ng/mL in serum PSA from baseline based on two PSA measures 2 weeks apart, or use of additional therapy. This definition was derived to be similar to the post‐SRT definition and to allow for small increases due to insignificant pathology (e.g., residual benign tissue). Published observational data support this definition, as post‐SRT BCR risk increased 3% (or 10% if two or more risk factors were present) per 0.1 ng/mL of PSA level [11], while a pre‐SRT PSA of ≥0.4 ng/mL was most likely to predict worse post‐SRT BCR and progression‐free survival outcomes [12]. Therefore, an increasing PSA of ≥0.2 ng/mL from baseline represents biochemical progression while still allowing for intervention with curative intent. The time to event was determined as the period from the original date of PSMA‐PET to an event or was censored at the last PSA date.
EAU Risk Categorization
The EAU risk categorization was performed post hoc for each patient using PSA‐DT (prior to PSMA‐PET) and GS as reported in RP histopathology [2]. For this analysis, the primary outcome of EFS was analysed according to EAU risk group, PSMA‐PET status and their combination. PSMA‐PET status was dichotomized into negative/locally recurrent (non‐metastatic) vs regional lymph nodal (N1) or distant metastasis (M1). Negative and locally recurrent (to the prostatic fossa alone) PSMA‐PET findings were combined because our previous analysis demonstrated that oncological outcomes were equivalent, while patients with regional nodal (55%) and distant metastases (21–25%) had significantly worse outcomes (hazard ratio [HR] 2.7; P = 0.002) than patients without metastasis so were grouped together for this analysis.
Statistical Analysis
Demographic information was summarized using median with interquartile range or as proportions. The percentage of patients not experiencing an event in each prespecified group was calculated. Cox proportional hazards models were used to calculate the HRs for an event. Analysis was performed using Stata statistical software, v16 (StataCorp, College Station, TX, USA).
Results
Patient Characteristics
A total of 137 patients were included in this analysis (Fig. S1), of whom 80% received SRT. Their demographics are listed in Table S1 according to EAU risk group, PSMA status and SRT. Serum PSA level and GS were both higher among patients with regional/distant metastatic disease reported on PSMA‐PET compared to those with negative/locally recurrent PSMA‐PET findings (median PSA 0.27 vs 0.20 ng/mL, P = 0.005; GS ≥8 46% vs 23%, P < 0.004).
EAU Risk Classification
Most patients (76%) were classified as having EAU high‐risk status and had low PSA levels (<0.5 ng/mL; 109/137, 80%) at the time of PSMA‐PET. Patients in the high‐risk group had markedly earlier BCR after RP (33 vs 76 months; P < 0.001) and a higher GS (GS ≥8 42% vs 0%; P < 0.001), as well as a higher T stage (≥pT3 73% vs 48%; P = 0.02) and a higher incidence of positive surgical margins (25% vs 6%; P = 0.03) than those in the EAU low‐risk group.
High risk (compared to low risk) resulted in worse EFS among patients who received SRT (56% vs 82%; P = 0.04).
PSMA‐PET Findings
The PSMA‐PET findings relative to EAU risk group and PSA level at the time of PSMA‐PET are shown in Table 1. Overall, 61% of patients (83/137) had negative/locally recurrent findings on PSMA‐PET, and 89% of these patients (74/83) had low PSA levels (<0.5 ng/mL). Regional/distant metastatic disease was identified in 39% of patients (54/137; 35/54 regional nodal involvement; 19/54 distant metastasis) despite a low PSA level (<0.5 ng/mL) in 65% (35/54).
Table 1.
Prostate‐specific membrane antigen‐positron emission tomography status according to both European Association of Urology risk group and PSA level.
| PSMA‐PET status | PSA range | Total | |
|---|---|---|---|
| ≤0.5 ng/mL | >0.5 ng/mL | ||
| Overall, n (%) | |||
| Negative/local recurrence | 74 (68) | 9 (32) | 83 (61) |
| Regional/distant metastasis | 35 (32) | 19 (68) | 54 (39) |
| EAU risk group, n (%) | |||
| Low | |||
| Negative/local recurrence | 22 (20) | 1 (4) | 23 (17) |
| Regional/distant metastasis | 7 (6) | 3 (11) | 10 (7) |
| High | |||
| Negative/local recurrence | 52 (48) | 8 (28) | 60 (44) |
| Regional/distant metastasis | 28 (26) | 16 (57) | 44 (32) |
| Total | 109 (100) | 28 (100) | 137 (100) |
Negative PSMA‐PET demonstrated no disease sites; local recurrence showed avidity confined to the prostatic fossa.
EAU, European Association of Urology; PET, positron emission tomography; PSMA, prostate‐specific membrane antigen.
European Association of Urology risk status was not associated with overall PSMA‐PET status (negative/locally recurrent vs regional/distant metastasis; P = 0.2). When PSA groups were considered, regional/distant metastatic disease was more likely when PSA was >0.5 ng/mL for both EAU low‐ (11%) and EAU high‐risk (57%) groups, compared to when PSA was ≤0.5 ng/mL (6% and 26%, respectively; Table 1). Similar regional/distant metastatic disease detection was observed with further PSA subgrouping (Table S2).
Event‐Free Survival
After follow‐up of 28 ± 14 months (mean event‐free period 38 ± 7 months), the EFS rate was 55% (76/137; Table 2). Regional/distant metastatic PSMA‐PET findings were more likely to result in an event (HR 2.2, 95% CI 1.3–3.7; P = 0.002); however, EAU high‐risk status did not confer a statistically significant worse EFS outcome overall (HR 1.7, 95% CI 0.9–3.2; P = 0.12).
Table 2.
Event‐free survival among men who received salvage radiotherapy according to European Association of Urology risk group and PSMA‐PET status.
| EFS | HR (95% CI) | P value | |
|---|---|---|---|
| EAU risk group, n/N (%) | |||
| Low | 18/22 (82) | 2.9 (1.1–8.3) | 0.039 |
| High | 49/88 (56) | ||
| PSMA‐PET status, n/N (%) | |||
| Negative/local recurrence | 47/63 (75) | 3.2 (1.7–5.9) | <0.001 |
| Regional/distant metastasis | 20/47 (43) | ||
EAU, European Association of Urology; EFS, event‐free survival; HR, hazard ratio; PET, positron emission tomography; PSMA, prostate‐specific membrane antigen.
Bold indicates significant value.
The EFS outcomes for patients who received SRT (110/137; 80%) are shown in Table 2. EAU high‐risk status resulted in significantly worse EFS (56% vs 82%; HR 2.9, P = 0.039 [Fig. 1A]). Similarly, significantly worse EFS was observed for patients with regional/distant metastasis than for those with negative/locally recurrent PSMA‐PET findings (43% vs 75%; HR 3.2, P < 0.001 [Fig. 1B]). The majority of patients with regional/distant metastasis underwent RT (n = 47/54; 87%), which incorporated a metastasis‐directed approach (pelvic lymph nodes and/or distant metastasis) for 85% of these patients (40/47).
Fig. 1.
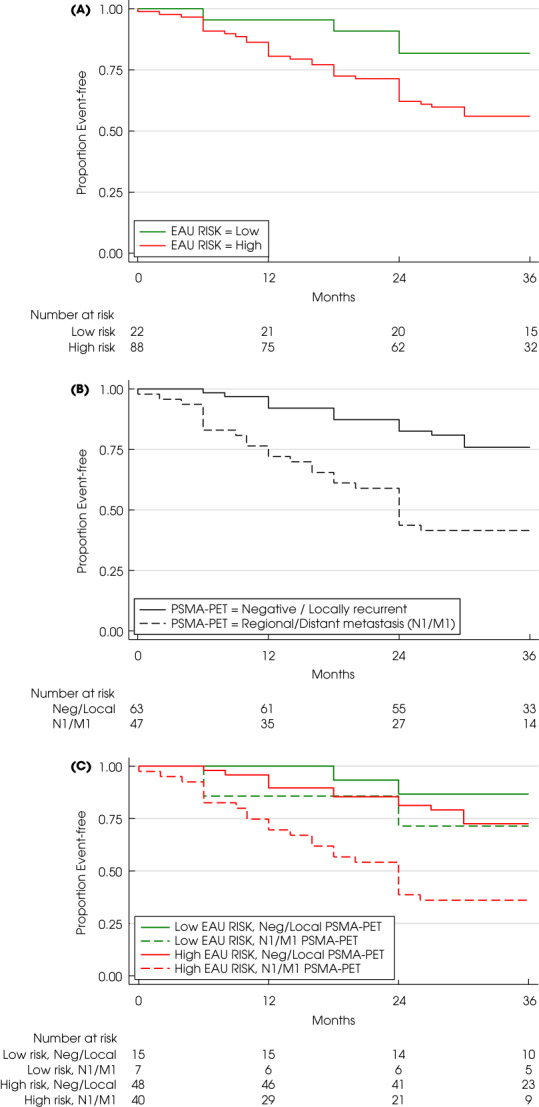
Event‐free survival according to combinations of European Association of Urology (EAU) risk group (low vs high; panels A, B) and prostate‐specific membrane antigen‐positron emission tomography (PSMA‐PET) status (negative/local recurrence vs regional/distant metastasis; panels C, D) in combination with radiotherapy use (panels B, D) or no radiotherapy use (panels A, B) over 36 months of follow‐up.
The combination of EAU risk group and PSMA‐PET status is shown in Table 3 and Fig. 1C. Worse EFS was observed for patients with regional/distant metastasis on PSMA‐PET who received SRT compared to those with negative/locally recurrent disease PSMA‐PET findings in the EAU high‐risk group (37% vs 71%; HR 3.1, 95% CI 1.6–6.1; P = 0.001) but did not reach statistical significance in the EAU low‐risk group (71% vs 87%; HR 2.3, 95% CI 0.3–16; P = 0.84).
Table 3.
Event‐free survival among men who received salvage radiotherapy according to combined European Association of Urology risk group and PSMA‐PET status.
| EAU risk group | HR (95% CI) | P value | ||
|---|---|---|---|---|
| Low (n = 22) | High (n = 88) | |||
| PSMA‐PET status, n/N (%) | ||||
| Negative/local recurrence (n = 63) | 13/15 (87) | 34/48 (71) | 2.4 (0.5–10) | 0.25 |
| Regional/distant metastasis (n = 47) | 5/7 (71) | 15/40 (38) | 2.9 (0.7–12) | 0.15 |
| HR (95% CI) | 2.3 (0.3–16) | 3.1 (1.6–6.1) | ||
| P value | 0.4 | 0.001 | ||
EAU, European Association of Urology; HR, hazard ratio; PET, positron emission tomography; PSMA, prostate‐specific membrane antigen.
Bold indicates significant value.
Adjusted multivariate Cox regression analysis determined that both EAU high‐risk status (HR 2.9, P = 0.04) and regional/distant metastasis on PSMA‐PET (HR 3.1, P < 0.001) were predictive of an event despite SRT.
Special Groups
When regional nodal and distant metastasis on PSMA‐PET were considered as separate groups among patients who received SRT, EFS was 48% and 29%, respectively. Incorporation of regional nodal metastasis into the negative/locally recurrent PSMA‐PET group, to provide a pelvic vs extra‐pelvic comparison, reduced EFS to 66%. Pelvic nodal RT [prostate bed with pelvic nodal RT or nodal RT alone (n = 34, 20 events)] outcomes were compared to prostate bed/fossa RT alone (n = 7, 3 events) and no significant difference in EFS was observed (HR 1.6, 95% CI 0.5–5.5; P = 0.43).
Androgen deprivation therapy was used for 26 patients who underwent SRT (24%), who were mostly in the EAU high‐risk group and had regional/distant metastasis on PSMA‐PET (96%). In these patients, 19 events were observed (EFS 27%). ADT use was associated with worse EFS among patients in the EAU high‐risk group (HR 4.0, 95%CI 2.1–7.5; P < 0.001) and regional/distant metastasis on PSMA‐PET (HR 2.8, 95% CI 1.3–6.0; P = 0.009).
Twenty percent of the cohort (27/137) did not receive SRT and, instead, underwent surveillance (n = 21, 78%), systemic therapy (n = 5, 19%) or surgery (n = 1, 3%). Clinical variables in these patients were similar to those in patients who did receive SRT, except T‐stage was higher among patients receiving SRT (≥pT3 69% vs 59%; P = 0.02). Although use of SRT was predictive of EFS in this group (HR 0.44, 95% CI 0.26, 0.77; P = 0.004), EAU risk status (HR 1.42, P = 0.73) and PSMA‐PET status (HR 1.3, P = 0.47) were not (Table S3). Patients who received SRT had significantly higher EFS in both the EAU low‐risk (82% vs 36%; HR 0.22, P = 0.02) and EAU high‐risk groups (56% vs 31%; HR 0.45, P = 0.02), as well as negative/locally recurrent PSMA‐PET results (75% vs 35%; HR 0.27, P < 0.001), but not when regional/distant metastasis were seen on PSMA‐PET (43% vs 29%; HR 0.61, P = 0.31).
Discussion
The intersection of established clinical variables with potentially additive prognostic and predictive information from novel imaging methods, such as PSMA‐PET, in the setting of BCR following RP is highly relevant for clinicians to guide decisions on appropriate management. Contemporary practice has pivoted to generally favour early SRT rather than adjuvant RT after RP to reduce overtreatment, which was recently supported by three randomized trials and the ARTISTIC meta‐analysis [13]. The EAU risk groups provide a validated framework for risk stratification but are limited to data from patients staged with conventional imaging [14, 15]. In this study, we found that both PSMA‐PET status and EAU risk group were independently predictive of EFS and provided complementary information for patients undergoing SRT. PSMA‐PET status was more predictive of EFS than EAU risk group.
While use of PSMA‐PET in the BCR setting is endorsed by the EAU and Australian guidelines [4, 5], there are limited outcome data for PET‐guided RT. Our previously published data have shown that PSMA‐PET results are predictive of 3‐year outcomes despite recording but not dictating treatment regimens [9]. The EMPIRE‐1 study randomized 165 patients to post‐prostatectomy SRT guided by either 18F‐fluciclovine‐PET/CT or conventional imaging, and reported that PET‐guided RT improved EFS at 3 years (76% vs 63%; P = 0.003) and 4 years (76% vs 51%; P < 0.001) [16]. A similarly designed randomized trial assessing PSMA‐guided SRT is in progress and the results are eagerly awaited [8]. This study demonstrates that both EAU risk group and PSMA‐PET result are important predictors of SRT outcomes and both factors should be included in future trials.
Regional/distant metastasis on PSMA‐PET was significantly predictive of EFS among men who received SRT, as was EAU high‐risk status. Incorporation of PSMA status (HR 3.1, P = 0.001) improved on EAU risk status (HR 2.9, P = 0.039) for prediction of EFS among EAU high‐risk patients who received SRT. Overall, the EFS in this patient cohort was 55%, significantly lower than the EFS observed within randomized trials in the ARTISTIC meta‐analysis (87–94%) [13] which included a higher proportion of EAU low‐risk patients (GS ≤7 82–89%; PSA‐DT unknown). While our EAU low‐risk, negative/locally recurrent group showed similar EFS rates to those observed in the ARTSTIC meta‐analysis (87%), worse EFS was noted for EAU high‐risk status (56%) and especially patients with regional/distant metastasis (43%), with their combination showing worse survival (37%) compared to EAU high‐risk status, negative/locally recurrent patients (71%).
European Association of Urology risk status was shown to predict PSMA‐PET findings in a recent report on 145 patients from two prospective cohorts, in which patients in the EAU high‐risk group were more likely to have a positive scan (82% vs 49%; odds ratio 6.7, P < 0.001) than those in the low‐risk group, and improved prediction of scan positivity on PSA alone (area under the curve 0.83 vs 0.76; P = 0.02) [17]. PSA‐DT, even with PSA values <0.5 ng/mL, has been shown to provide additive benefit for prediction of PSMA‐PET positivity [18], as well as more favourable outcomes being observed after SRT in men with longer PSA‐DT (>10 months) [19]. The EAU risk groups provide an additional composite measure of clinical variables (GS and PSA‐DT), of which PSA‐DT is likely to reflect tumour clonal kinetics and hence cancer biology [20]. Thus, a clinical and imaging‐guided risk stratification framework may further optimize patient selection for imaging and RT use to improve RT outcomes in the BCR setting.
Further trials are needed to investigate biomarker‐guided approaches (clinical, imaging, molecular features) for optimal oncological outcomes. The incorporation of imaging such as PSMA‐PET into clinical practice and the subsequent oncological outcomes can be captured by national registries and provide benchmarking for evidence‐based care [21]. Other considerations include different PSA thresholds to define BCR [22] or other imaging [23, 24] or molecular tests [25]. For instance, molecular tests such as the Decipher® genomic classifier, which is independently prognostic for major oncological outcomes (adverse surgical pathology, progression‐free survival, metastasis‐free survival and overall survival) for patients prior to or after RP may provide further prognostic information [26], and merit inclusion in prospective trials in this setting.
This study has several limitations. One being sample size; in particular, there was a limited proportion of patients with EAU low‐risk status (24%) and high PSA levels (≥0.5 ng/mL; 20%), which may have affected the association of EAU risk with PSMA‐PET findings, as well as a small proportion of patients who did not receive SRT, limiting further exploration of this subgroup. Secondly, the clinically pragmatic but not predetermined intervention was at the discretion of treating urologists or radiation oncologists. Furthermore, variability in clinical practice and censoring in some patients may have overtly influenced observed outcomes. The definition of EFS among men who did not receive SRT is not validated but was defined to be most similar to the well‐accepted post‐SRT definition according to supportive clinical data. The post hoc data collection and variable availability of PSA‐DT values (collected as categorical variables) limited the inclusion of all patients from the original study and further analysis. Use of the EAU risk groups, originally defined and validated for metastasis‐free survival and overall survival, in this study to predict EFS is a deviation from their original intended use, which was necessary given the lack of long‐term follow‐up required to determine metastasis‐free survival and overall survival with PSMA‐PET.
The strengths of this study include the multicentre pragmatic design that allows the findings to be generalizable to clinical practice. Furthermore, the study was conducted by experienced imaging centre staff with high‐quality imaging, and imagers who, as integral members of the urology multidisciplinary team were cognisant of the clinical implications of their reports.
In conclusion, PSMA‐PET was both prognostic and more predictive of outcomes following SRT than was the EAU risk classification. EAU risk grouping and PSMA‐PET findings were complementary variables for prediction of outcomes in patients with BCR after RP receiving SRT. These findings provide early evidence of the value of PSMA‐PET among clinical variables for prediction of oncological outcomes in the BCR setting. Randomized trials assessing the benefit of PSMA‐PET for treatment selection and guidance, including extent of radiation fields and use of ADT, with subsequent long‐term oncological outcomes, are required.
Disclosure of Interests
All authors confirm no relevant conflict of interest.
Supporting information
Appendix S1
Table S1 Demographic data according to EAU risk groups, PSMA‐PET status and radiotherapy (RT) use. RP, radical prostatectomy; IQR, interquartile range.
Table S2 PSMA‐PET status according to both EAU risk and PSA level (further substratified compared to Table 1). Negative PSMA‐PET demonstrated no disease sites; local recurrence showed avidity confined to the prostatic fossa.
Table S3 Event‐free survival (EFS) among the overall population (Table A) and men who did not receive salvage radiotherapy (no SRT; Table B) with Cox regression analysis (Table C) according to EAU risk, PSMA‐PET status and their combination.
Figure S1 Patient inclusion flow chart considering the original patient cohort (n = 260) and the cohort in the present analysis (n = 137); PSAdt, PSA doubling time.
Acknowledgements
This research was supported by the Australian Department of Health and Ageing for its funding of the Australian Prostate Cancer Research Centre NSW, as well as the St Vincent's Prostate Cancer Centre. Matthew Roberts is supported by a Clinician Research Fellowship from the Metro North Office of Research, Queensland Health. Louise Emmett is supported by a clinician research fellowship from the St Vincent's Clinic Foundation. Open access publishing facilitated by The University of Queensland, as part of the Wiley ‐ The University of Queensland agreement via the Council of Australian University Librarians.
References
- 1. Van den Broeck T, van den Bergh RCN, Arfi N et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur Urol 2019; 75: 967–87 [DOI] [PubMed] [Google Scholar]
- 2. Van den Broeck T, van den Bergh RCN, Briers E et al. Biochemical recurrence in prostate cancer: the European Association of Urology prostate cancer guidelines panel recommendations. Eur Urol Focus 2019; 6: 231–4 [DOI] [PubMed] [Google Scholar]
- 3. Hofman MS, Lawrentschuk N, Francis RJ et al. Prostate‐specific membrane antigen PET‐CT in patients with high‐risk prostate cancer before curative‐intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet 2020; 11: 1208–16 [DOI] [PubMed] [Google Scholar]
- 4. Cornford P, van den Bergh RCN, Briers E et al. EAU‐EANM‐ESTRO‐ESUR‐SIOG guidelines on prostate cancer. Part II‐2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol 2021; 79: 263–82 [DOI] [PubMed] [Google Scholar]
- 5. Lieng H, Hayden AJ, Christie DRH et al. Radiotherapy for recurrent prostate cancer: 2018 recommendations of the Australian and New Zealand radiation oncology Genito‐urinary group. Radiotherapy Oncol 2018; 129: 377–86 [DOI] [PubMed] [Google Scholar]
- 6. Fendler WP, Calais J, Eiber M et al. Assessment of 68Ga‐PSMA‐11 PET accuracy in localizing recurrent prostate cancer: a prospective single‐arm clinical trial. JAMA Oncol 2019; 1: 856–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Emmett L, van Leeuwen PJ, Nandurkar R et al. Treatment outcomes from (68)Ga‐PSMA PET/CT‐informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med 2017; 58: 1972–6 [DOI] [PubMed] [Google Scholar]
- 8. Calais J, Armstrong WR, Kishan AU et al. Update from PSMA‐SRT trial NCT03582774: A randomized phase 3 imaging trial of prostate‐specific membrane antigen positron emission tomography for salvage radiation therapy for prostate cancer recurrence powered for clinical outcome. Eur Urol Focus 2021; 7: 238–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emmett L, Tang R, Nandurkar R et al. 3‐year freedom from progression after (68)Ga‐PSMA PET/CT‐triaged Management in men with biochemical recurrence after radical prostatectomy: results of a prospective multicenter trial. J Nucl Med 2020; 61: 866–72 [DOI] [PubMed] [Google Scholar]
- 10. Tendulkar RD, Agrawal S, Gao T et al. Contemporary update of a multi‐institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 2016; 20: 3648–54 [DOI] [PubMed] [Google Scholar]
- 11. Fossati N, Karnes RJ, Cozzarini C et al. Assessing the optimal timing for early salvage radiation therapy in patients with prostate‐specific antigen rise after radical prostatectomy. Eur Urol 2016; 69: 728–33 [DOI] [PubMed] [Google Scholar]
- 12. Bartkowiak D, Thamm R, Siegmann A, Böhmer D, Budach V, Wiegel T. Lead‐time bias does not falsify the efficacy of early salvage radiotherapy for recurrent prostate cancer. Radiother Oncol 2021; 154: 255–9 [DOI] [PubMed] [Google Scholar]
- 13. Vale CL, Fisher D, Kneebone A et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta‐analysis of aggregate data. The Lancet 2020; 396: 1422–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tilki D, Preisser F, Graefen M, Huland H, Pompe RS. External validation of the European Association of Urology biochemical recurrence risk groups to predict metastasis and mortality after radical prostatectomy in a European cohort. Eur Urol 2019; 75: 896–900 [DOI] [PubMed] [Google Scholar]
- 15. Pak S, Lee D‐E, You D et al. Validation of the European association of urology biochemical recurrence risk groups after radical prostatectomy in an Asian cohort and suggestions for refinement. Urol Oncol 2021; 39: 298.e1–e6 [DOI] [PubMed] [Google Scholar]
- 16. Jani AB, Schreibmann E, Goyal S et al. 18F‐fluciclovine‐PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE‐1): a single Centre, open‐label, phase 2/3 randomised controlled trial. The Lancet 2021; 397: 1895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong L, Su Y, Zhu Y et al. The European Association of Urology biochemical recurrence risk groups predict findings on PSMA PET in patients with biochemically recurrent prostate cancer after radical prostatectomy. J Nucl Med 2022; 63: 248–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deandreis D, Guarneri A, Ceci F et al. (68)Ga‐PSMA‐11 PET/CT in recurrent hormone‐sensitive prostate cancer (HSPC): a prospective single‐Centre study in patients eligible for salvage therapy. Eur J Nucl Med Mol Imaging 2020; 47: 2804–15 [DOI] [PubMed] [Google Scholar]
- 19. Stephenson AJ, Shariat SF, Zelefsky MJ et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 2004; 17: 1325–32 [DOI] [PubMed] [Google Scholar]
- 20. Teeter AE, Bañez LL, Presti JC et al. What are the factors associated with short prostate specific antigen doubling time after radical prostatectomy? A report from the SEARCH database group. J Urol 2008; 180: 1980–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Callaghan M, Papa N, Pase M et al. Patterns of care for prostate cancer treatment and improving outcomes – Are national registries the answer? BJU Int 2021; 128: 6–8 [DOI] [PubMed] [Google Scholar]
- 22. Morgan TM, Meng MV, Cooperberg MR et al. A risk‐adjusted definition of biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis 2014; 17: 174–9 [DOI] [PubMed] [Google Scholar]
- 23. Joshi A, Roberts MJ, Perera M et al. The clinical efficacy of PSMA PET/MRI in biochemically recurrent prostate cancer compared with standard of care imaging modalities and confirmatory histopathology: results of a single‐Centre, prospective clinical trial. Clin Exp Metastasis 2020; 37: 551–60 [DOI] [PubMed] [Google Scholar]
- 24. Roberts MJ, Morton A, Papa N et al. Primary tumour PSMA intensity is an independent prognostic biomarker for biochemical recurrence‐free survival following radical prostatectomy. Eur J Nucl Med Mol Imaging 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng FY, Huang HC, Spratt DE et al. Validation of a 22‐gene genomic classifier in patients with recurrent prostate cancer: an ancillary study of the NRG/RTOG 9601 randomized clinical trial. JAMA. Oncologia 2021; 7: 544–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jairath NK, Dal Pra A, Vince R Jr et al. A systematic review of the evidence for the decipher genomic classifier in prostate cancer. Eur Urol 2021; 79: 374–83 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Table S1 Demographic data according to EAU risk groups, PSMA‐PET status and radiotherapy (RT) use. RP, radical prostatectomy; IQR, interquartile range.
Table S2 PSMA‐PET status according to both EAU risk and PSA level (further substratified compared to Table 1). Negative PSMA‐PET demonstrated no disease sites; local recurrence showed avidity confined to the prostatic fossa.
Table S3 Event‐free survival (EFS) among the overall population (Table A) and men who did not receive salvage radiotherapy (no SRT; Table B) with Cox regression analysis (Table C) according to EAU risk, PSMA‐PET status and their combination.
Figure S1 Patient inclusion flow chart considering the original patient cohort (n = 260) and the cohort in the present analysis (n = 137); PSAdt, PSA doubling time.


