Summary
Acalabrutinib, a Bruton tyrosine kinase inhibitor, demonstrated greater selectivity and improved safety versus ibrutinib in a head‐to‐head trial in relapsed/refractory (R/R) chronic lymphocytic leukaemia. In the R/R marginal zone lymphoma (MZL) cohort (phase 2) of a phase 1b/2 trial (NCT02180711), 43 patients with MZL and at least one prior therapy received acalabrutinib 100 mg twice daily until disease progression or unacceptable toxicity [median age 69 years (range 42–84); median one (1–4) prior systemic regimens]. Median follow‐up was 13.3 months (range 0.5–45.5). Among 40 patients evaluable for response, investigator‐assessed overall response rate was 53% [95% confidence interval (CI) 36%–69%] with five (13%) complete responses. Tumour reduction occurred in 40 (93%) of the treated patients. Median time to response was 2.9 months (median duration of response not estimable). Estimated median progression‐free survival (PFS) was 27.4 months (12‐month PFS rate, 67%). Five patients died (disease progression, n = 4; septic shock, n = 1). Seventeen patients (40%) had grade 3 or higher adverse events (AEs), most commonly neutropenia (14%), anaemia, dyspnoea (7% each), fatigue and thrombocytopenia (5% each). Hypertension occurred in 5%; atrial fibrillation/flutter and major haemorrhage were not reported. AEs led to treatment discontinuation in three (7%) patients. Acalabrutinib was active and well tolerated in patients with R/R MZL.
Keywords: B‐cell lymphoma, Bruton tyrosine kinase, non‐Hodgkin lymphoma
INTRODUCTION
Marginal zone lymphoma (MZL) is an indolent B‐cell malignancy comprising approximately 7% of B‐cell non‐Hodgkin lymphomas (NHLs). 1 The World Health Organisation categorises MZL into three main subtypes, extranodal, splenic and nodal, each with specific diagnostic criteria, genetic attributes and therapeutic implications. 2 Treatment typically consists of chemoimmunotherapy for first‐line therapy in high‐tumour‐burden disease 3 ; however, less toxic treatment alternatives are needed in relapsed/refractory (R/R) disease, particularly for those who may not be candidates for chemoimmunotherapy.
Similar to other lymphoid malignancies, MZL pathophysiology is driven in part by dysregulation of the NF‐κB signalling pathway. 4 B‐cell receptor signalling, mediated through the activation of the phosphoinositide 3‐kinase (PI3K) pathway, results in downstream activation of NF‐κB, sustaining MZL cells. 4 Bruton tyrosine kinase (BTK) inhibitors, which modulate B‐cell receptor signalling, provide a chemotherapy‐free alternative therapy for use in several B‐cell malignancies, including chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL), mantle cell lymphoma (MCL), and Waldenström macroglobulinaemia. 5 Two BTK inhibitors, ibrutinib and zanubrutinib, are currently approved to treat R/R MZL. 6 , 7 , 8 , 9
Acalabrutinib is a next‐generation BTK inhibitor currently approved to treat CLL/SLL and R/R MCL, having greater selectivity for BTK and an improved safety profile compared with ibrutinib in a head‐to‐head trial. 10 , 11 , 12 Acalabrutinib in combination with a PI3K inhibitor has demonstrated activity in lymphoma cell lines of several aggressive lymphomas and MZL. 13 Clinical activity of acalabrutinib alone or as part of combination therapy was also demonstrated in other aggressive lymphomas in phase 1/2 clinical trials that did not include patients with MZL. 14 , 15 , 16 , 17 In order to explore the efficacy and safety of acalabrutinib in MZL, we report the results of a phase 2 proof‐of‐concept study which examined acalabrutinib monotherapy in patients with R/R MZL.
METHODS
Study design
This analysis focuses on part 2 of an ongoing three‐part, multicentre, open‐label phase 1b/2 trial (NCT02180711). Part 1 evaluated acalabrutinib in combination with rituximab in patients with treatment‐naive and R/R follicular lymphoma (and acalabrutinib alone in R/R follicular lymphoma), and part 3 evaluated acalabrutinib in combination with rituximab and lenalidomide in patients with R/R follicular lymphoma. Part 2 was a phase 2 study evaluating acalabrutinib in patients with R/R MZL. Eligible patients were aged 18 years or older with histologically confirmed splenic, nodal, or extranodal MZL. Patients were required to have radiographically measurable lymphadenopathy or extranodal lymphoid malignancy defined as the presence of one or more lesions that measured at least 2.0 cm in the longest dimension and at least 1.0 cm in the longest perpendicular dimension as assessed by computed tomography (CT) scan. Lesions that were not well visualised by CT could be measured by magnetic resonance imaging (MRI) instead. Patients with spleen‐only disease were considered as not having measurable disease and excluded from the trial. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, and had received at least one prior systemic therapy (including at least one CD20‐directed regimen). Patients were excluded if they had prior exposure to a BTK inhibitor, central nervous system (CNS) involvement of lymphoma, clinically relevant cardiovascular disease, active infection, or required treatment with proton‐pump inhibitors or anticoagulants. Patients received oral acalabrutinib 100 mg twice daily as monotherapy until disease progression or unacceptable toxicity.
The study was designed and implemented in accordance with the protocol, the International Conference on Harmonisation Harmonised Tripartite Guidelines for Good Clinical Practices, applicable local regulations, and the ethical principles as established in the Declaration of Helsinki. An investigational review board/independent ethics committee approved the protocol at each site and all patients provided written informed consent.
Outcomes and assessments
The primary end‐point was overall response rate (ORR) per Lugano criteria 18 as assessed by the investigators. Bone marrow aspirate and/or biopsy was performed at screening or up to 90 days before the first dose of acalabrutinib. For tumour assessments, pre‐treatment CT scan with contrast was required within 30 days before first dose of acalabrutinib, and positron emission tomography (PET)/CT was required within 60 days before first dose of acalabrutinib. Patients with gastric mucosa‐associated lymphoid tissue (MALT) lymphoma also had an endoscopy performed at screening or up to 90 days before the first dose of acalabrutinib. CT scan was then conducted every three cycles (12 weeks, ±7 days) starting at day 1 of cycles 4, 7, 10, and 13, then every 24 weeks thereafter or more frequently at the investigator's discretion. Bone marrow biopsy (if involved by lymphoma at baseline), endoscopy (for patients with gastric MALT), and PET/CT (PET used if a pretreatment PET scan was positive) were required to confirm complete response (CR). Secondary end‐points included duration of response (DOR), progression‐free survival (PFS), overall survival (OS), and safety. Minimal residual disease (MRD) negativity rate was an exploratory end‐point and was assessed centrally using the ImmunoSeq next ‐generation sequencing assay (Adaptive Biotechnologies, Seattle, WA, USA) for patients with paired archival tumour and whole‐blood and/or bone marrow sample available at response. MRD shown in this report was measured in peripheral blood.
Safety assessments consisted of monitoring adverse events (AEs), laboratory assessments (haematology, clinical chemistry and urinalysis), vital signs and other tests. AEs were coded using MedDRA, and the severity of AEs and laboratory abnormalities were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical analysis
Data were summarised using descriptive statistics, including means, standard deviations, and medians for continuous variables and proportions for discrete variables as appropriate. No formal hypothesis testing was planned. Safety and efficacy analyses were conducted in all patients who received one or more doses of acalabrutinib, unless otherwise specified. Analyses for efficacy were conducted in those who were evaluable for response, defined as having one or more response assessment after the first dose of study treatment (i.e., overall response was not ‘unknown’ or missing). A sample size of at least 40 patients was planned to allow characterisation of acalabrutinib treatment in this part of the study, based on feasibility. The study was fully enrolled and ongoing at the time of data cut‐off. Through retrospective calculation, under a binomial distribution and assuming an ORR of 50%, it was determined that 40 patients will provide a 95% confidence interval (CI) half‐width of 15% using normal approximation for the primary end‐point of ORR.
ORR was defined as the proportion of patients who achieved a CR or partial response (PR) according to the Lugano Classification for NHL 18 and calculated along with its corresponding two‐sided CI. Subgroup analyses included evaluation of ORR by MZL subtype, those refractory to previous rituximab therapy, and other baseline disease characteristics. DOR was defined as the time from first documentation of CR or PR to first documentation of definitive disease progression or death from any cause, whichever came first. PFS was defined as the time from start of acalabrutinib therapy to first documentation of objective disease progression or death from any cause, whichever came first. Patients who did not have disease progression or death were censored for DOR and PFS. OS duration was measured from the start of acalabrutinib therapy until the date of death from any cause. Surviving patients were censored at their date of last contact. Kaplan–Meier methodology was used to estimate DOR, PFS, and OS curves.
RESULTS
Patients
In total, 43 patients with R/R MZL received acalabrutinib monotherapy as of the 4 January 2022 data cut‐off. Patient accrual occurred over an approximately 3.5‐year period with the first patient's first dose on 22 March 2018, and the last patient's first dose on 4 October 2021. The median age was 69 years (range 42–84). The median number of prior systemic regimens was one (range 1–4). Prior systemic cancer therapies included anti‐CD20 therapies (98%), chemotherapy (58%), targeted therapies (9%), and other therapies (19%) (Table 1). Rituximab was the only prior therapy in 30.2% of patients and 19 (44.2%) were considered refractory to any prior rituximab‐containing therapy. Overall, 44% of patients were refractory to their last treatment, an additional 44% relapsed after their last treatment, and the response status or duration of response for the remaining 12% was unknown. Nineteen (44%) patients had extranodal subtype, 13 patients (30%) had nodal subtype, and 11 patients (26%) had splenic subtype.
TABLE 1.
Demographics and baseline disease characteristics
| Characteristic | Acalabrutinib monotherapy N = 43 |
|---|---|
| Age, median (range), years | 69 (42–84) |
| Age group, n (%) | |
| ≥70 years | 20 (46.5) |
| Sex, male, n (%) | 26 (60.5) |
| Race, n (%) | |
| White | 38 (88.4) |
| Black or African American | 4 (9.3) |
| Not reported | 1 (2.3) |
| Refractory vs relapsed to last treatment, n (%) | |
| Refractory a | 19 (44.2) |
| Relapsed b | 19 (44.2) |
| Unknown | 5 (11.6) |
| ECOG performance status, n (%) | |
| 0 | 23 (53.5) |
| 1 | 19 (44.2) |
| 2 | 1 (2.3) |
| Tumour bulk, n (%) c | |
| ≥5 cm | 15 (34.9) |
| ≥10 cm | 3 (7.0) |
| Bone marrow involvement, n (%) | 19 (44.2) |
| MZL subtype, n (%) | |
| Extranodal | 19 (44.2) |
| Gastric MALT | 2 (4.7) |
| Non‐gastric MALT | 17 (39.5) |
| Nodal | 13 (30.2) |
| Splenic | 11 (25.6) |
| LDH >1× to 3× ULN, n (%) | 11 (25.6) |
| Number of patients with prior systemic regimens, n (%) d | |
| 1–2 prior therapies | 38 (88.4) |
| ≥3 prior therapies | 5 (11.6) |
| Number of prior systemic regimens, median (range) | 1 (1–4) |
| Prior systemic therapy, n (%) e | |
| Anti‐CD20 mAb monotherapy | 42 (97.7) |
| Rituximab | 42 (97.7) |
| Obinutuzumab | 1 (2.3) |
| Chemotherapy f | 25 (58.1) |
| Targeted therapy | 4 (9.3) |
| Lenalidomide | 2 (4.7) |
| PI3K inhibitor | 2 (4.7) |
| Other therapy | 4 (9.3) g |
| Bortezomib | 1 (2.3) |
| Prednisone | 1 (2.3) |
| Radioimmunotherapy | 1 (2.3) |
| Vorinostat | 1 (2.3) |
Abbreviations: BR, bendamustine + rituximab; ECOG, Eastern Cooperative Oncology Group; FR, fludarabine + rituximab; LDH, lactate dehydrogenase; mAb, monoclonal antibody; MALT, mucosa‐associated lymphoid tissue; MZL, marginal zone lymphoma; PI3K, phosphoinositide 3‐kinase; R, rituximab; R‐CHOP, rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone; R‐CODBLAM, rituximab + cyclophosphamide + vincristine + dexamethasone + bleomycin + doxorubicin + procarbazine; R‐CVP, rituximab + cyclophosphamide + vincristine + prednisone; R‐EPOCH, rituximab + etoposide + prednisolone + vincristine + cyclophosphamide + doxorubicin; R‐TNOP, rituximab + thiotepa + mitoxantrone + vincristine + prednisone/dexamethasone; RiBVD, rituximab + bendamustine + bortezomib + dexamethasone; ULN, upper limit of normal.
Refractory is defined as best response of stable disease, or progressive disease, or a partial response/complete response with duration of response ≤6 months.
Relapsed is defined as best response of partial response/complete response with duration of response >6 months.
Defined as the longest diameter of any target lesion at baseline.
Maintenance therapies were not counted separately.
Patients could have been treated with more than one of these therapies.
Regimens included BR, FR, R‐CHOP, R‐CVP, R‐EPOCH followed by R‐CODBLAM followed by R‐CHOP, R‐TNOP, single‐agent alkylators + R, and RiBVD.
Three additional patients received cholecalciferol/placebo, and one additional patient received clarithromycin.
Median time on study was 13.3 months (range 0.5–45.5), and median follow‐up by reverse Kaplan–Meier method was 10.8 months (95% CI 8.1–17.0). Eighteen (42%) patients discontinued acalabrutinib; reasons for acalabrutinib discontinuation were disease progression in 12 patients (28%), AEs and investigator's decision in two patients (5%) each, and patient decision and death in one patient (2%) each. Twenty‐five patients (58%) continued treatment with acalabrutinib and 37 patients (86%) remained in the study.
Efficacy
Among 43 treated patients, 40 were considered evaluable for response. Two patients were not evaluable because they exited the study prior to completing the first scheduled response assessment. Among these two patients were one patient who withdrew consent after discontinuing treatment due to an AE and another patient who died due to septic shock. A third patient had one response assessment, but the overall response was ‘unknown’ at the time of data cut‐off; this patient, however, will be evaluable at a later date. Regarding the patient with septic shock, the investigator chose to not subject the patient to excessive radiation at the time of the initial response assessment (cycle 4) but planned to complete the next scheduled assessment. The patient appeared well at the cycle 6 visit (ECOG 0, normal/baseline laboratory measures and vital signs), but 12 days later, the patient presented to the emergency room at a different hospital with signs of sepsis and leukopenia and died while on maximum therapy in the intensive‐care unit. Overall, of the 40 patients with baseline PET assessment, eight patients did not have F‐18 fluorodeoxyglucose (FDG)‐avid disease (baseline Deauville score of 1, 2, or 3), and responses were assessed by CT alone in these patients. The ORR among evaluable patients was 52.5% (95% CI 36%–69%) including five patients (12.5%) with CR, and 16 patients (40%) with PR. Among the five patients with CRs, four had a Deauville score of 5 at screening, and two had bone marrow involvement at screening. One of the two patients with bone marrow involvement at screening had PET/CT‐assessed CR demonstrated by a Deauville score of 1 without bone marrow biopsy confirmation. The other patient had CT‐assessed CR confirmed with bone marrow biopsy. For the three patients without bone marrow involvement at screening, one had PET/CT‐assessed CR with a Deauville score of 1. The other two patients had CT‐assessed CRs. Among the 16 patients with PRs, two patients met CR criteria based on CT; however, both had bone marrow involvement at screening and did not have bone marrow biopsy to confirm CR at the time of response assessments; therefore, they were considered PRs. Nineteen (47.5%) patients had stable disease as best overall response (Table 2; Figure S1A). When analysed by MZL subtype, ORRs were 65% (CR rate 12%), 42% (CR rate 17%) and 46% (CR rate 9%) in extranodal, nodal and splenic subtypes respectively (Table 2; Figure S1B). Subgroup analysis evaluating ORR is presented in Table 3. There was no observable difference in ORR between patients refractory to previous rituximab therapy compared with the overall group (Table 3). Median time to initial response was 2.9 months, median time to best response was 3.0 months, and median DOR was not estimable (95% CI 8.4 months–not estimable; Table 2; Figure 1). A swimmer plot of response timeline for each patient is provided in Figure S2.
TABLE 2.
Treatment response in evaluable patients
| Overall N = 40 | MZL subtype | |||
|---|---|---|---|---|
| Extranodal | Nodal | Splenic | ||
| n = 17 | n = 12 | n = 11 | ||
| Best response | ||||
| ORR a , % (95% CI) | 52.5 (36.1–68.5) | 64.7 (38.3–85.8) | 41.7 (15.2–72.3) | 45.5 (16.8–76.6) |
| CR, n (%) | 5 (12.5) | 2 (11.8) | 2 (16.7) | 1 (9.1) |
| PR, n (%) | 16 (40.0) | 9 (52.9) | 3 (25.0) | 4 (36.4) |
| SD, n (%) | 19 (47.5) | 6 (35.3) | 7 (58.3) | 6 (54.5) |
| Responders | ||||
| N = 21 | ||||
| Time to initial response, median (range), months | 2.9 (2.5–22.0) | |||
| Time to best response, median (range), months | 3.0 (2.5–22.0) | |||
| Duration of response | ||||
| Number of progression events, n (%) | 5 (23.8) | |||
| Censored after response, n (%) | 16 (76.2) | |||
| 12‐month DOR, % (95% CI) | 75.8 (46.2–90.5) | |||
Abbreviations: CI, confidence interval; CR, complete response; DOR, duration of response; MZL, marginal zone lymphoma; ORR, overall response rate; PR, partial response; SD, stable disease.
Proportion of patients who achieved a CR or PR per Lugano criteria 18 as assessed by the investigator.
TABLE 3.
Overall response rate subgroup analysis among evaluable patients
| Subgroup | Number of responders | ORR (95% CI) |
|---|---|---|
| (n/N) | ||
| Age group | ||
| <65 years | 7/15 | 46.7 (21.3–73.4) |
| ≥65 years | 14/25 | 56.0 (34.9–75.6) |
| <75 years | 19/37 | 51.4 (34.4–68.1) |
| ≥75 years | 2/3 | 66.7 (9.4–99.2) |
| ECOG performance status | ||
| 0 | 11/20 | 55.0 (31.5–76.9) |
| ≥1 | 10/20 | 50.0 (27.2–72.8) |
| Bulky disease | ||
| <5 cm | 10/26 | 38.5 (20.2–59.4) |
| ≥5 cm | 11/14 | 78.6 (49.2–95.3) |
| Baseline extranodal disease | ||
| Yes | 11/17 | 64.7 (38.3–85.8) |
| No | 10/23 | 43.5 (23.2–65.5) |
| Bone marrow involvement | ||
| Yes | 10/19 | 52.6 (28.9–75.6) |
| No | 11/21 | 52.4 (29.8–74.3) |
| Prior lines of systemic therapy | ||
| <3 | 19/35 | 54.3 (36.6–71.2) |
| ≥3 | 2/5 | 40.0 (5.3–85.3) |
| Prior treatment | ||
| Anti‐CD20 | 21/40 | 52.5 (36.1–68.5) |
| Chemotherapy | 16/25 | 64.0 (42.5–82.0) |
| Other therapy | 4/7 | 57.1 (18.4–90.1) |
| Targeted therapy | 4/4 | 100.0 (39.8–100.0) |
| Refractory to rituximab | ||
| Any rituximab therapy | 8/17 | 47.1 (23.0–72.2) |
| Rituximab combination | 6/10 | 60.0 (26.2–87.8) |
| Rituximab monotherapy | 7/13 | 53.8 (25.1–80.8) |
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; ORR, overall response rate.
FIGURE 1.
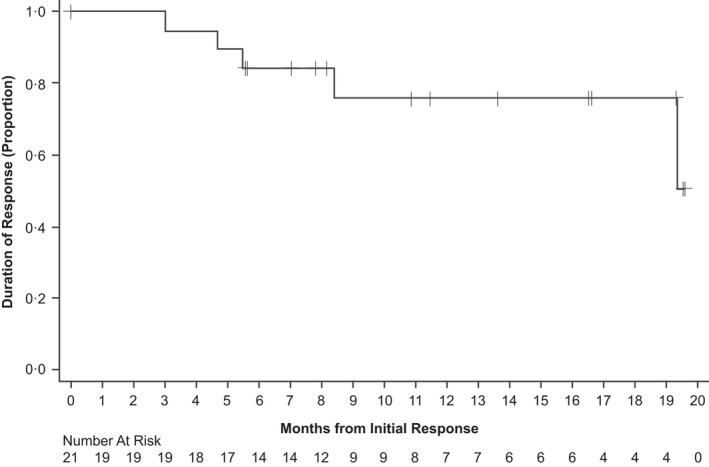
Kaplan–Meier plot of duration of response. Duration of response defined as the months from first documented response to disease progression, death, or date of censoring.
Out of 21 responders, six (28.6%) were evaluable for MRD in blood, including two patients with CR and four with PR. Of these six patients, two became MRD‐negative (defined as less than 1 × 10−4) during treatment, and both had PR. A third patient with PR who was tested for MRD after disease progression and cessation of treatment had converted to MRD‐negative since the previous test (Figure S3).
After a median follow‐up of 13.3 months, estimated median PFS was 27.4 months (95% CI 11.1 months–not estimable) with two patients at risk; the 12‐month PFS rate was 67.0% (95% CI 46.4%–81.1%; Figure 2A). Five patients died [disease progression, n = 3; transformation to diffuse large B‐cell lymphoma after stopping treatment, n = 1; AE, n = 1 (septic shock occurring after approximately 5.2 months of acalabrutinib, considered unrelated to treatment)]. Median OS was not reached (Figure 2B) and the 12‐month OS rate was 91.4% (95% CI 75.6%–97.1%).
FIGURE 2.
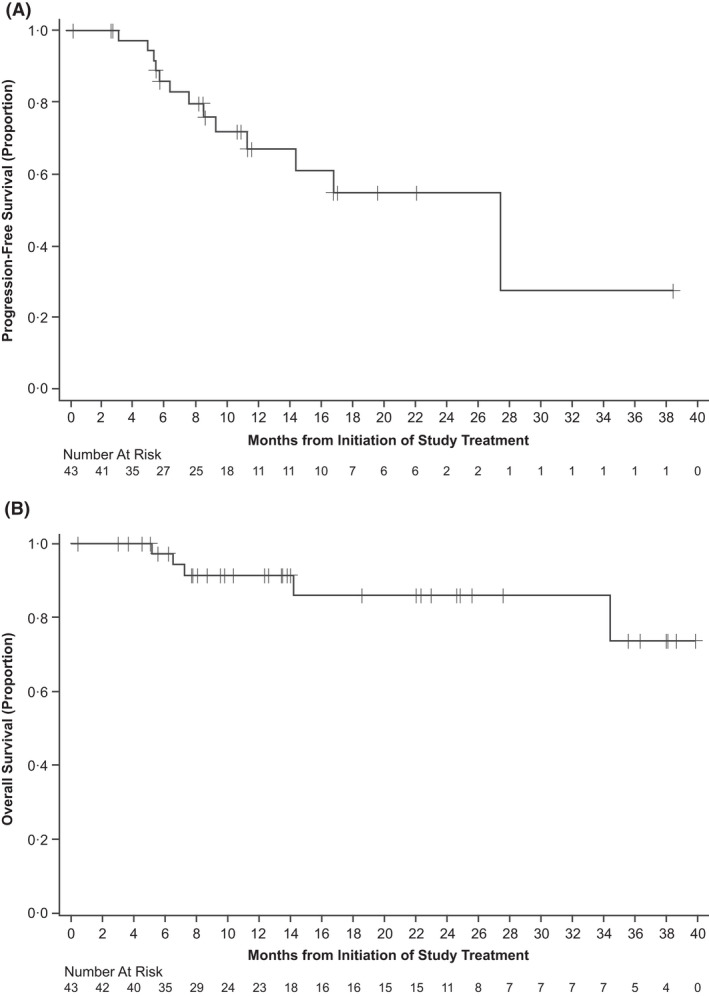
Kaplan–Meier plots of (A) progression‐free survival and (B) overall survival.
Safety
At the time of data cut‐off, median duration of acalabrutinib exposure was 10 months (range 0.3–38.6). Most patients (95%; n = 41) experienced at least one treatment‐emergent AE. Most AEs were grade 1 or 2. The most common any‐grade AEs are presented in Table 4. Seventeen patients (40%) had AEs of grade 3 or higher. Grade 3 or higher AEs occurring in two or more patients are included in Table 4. Serious AEs occurred in seven patients (16%) and included acute myocardial infarction, cellulitis, chronic obstructive pulmonary disease exacerbation, dyspnoea, hypotension, pneumonia, pneumonitis and septic shock in one patient each. Thirteen patients (30%) required treatment withholding due to AEs; the most common AEs leading to dose withholdings were pneumonia in three patients (7%) and fatigue in two patients (5%). Two patients (5%) required acalabrutinib dose reduction due to fatigue. AEs led to acalabrutinib discontinuation in three patients (7%), including one event of grade 3 hypotension, one event of grade 1 myalgia, and one event of grade 3 pneumonitis.
TABLE 4.
Treatment‐emergent adverse events
| Acalabrutinib monotherapy N = 43 | ||
|---|---|---|
| Common TEAEs (any grade in >10% of patients or grade ≥3 in ≥2 patients), n (%) | Any grade | Grade ≥3 |
| Any TEAE | 41 (95.3) | 17 (39.5) |
| Headache | 14 (32.6) | 0 |
| Diarrhoea | 11 (25.6) | 0 |
| Fatigue | 12 (27.9) | 2 (4.7) |
| Nausea | 12 (27.9) | 0 |
| Blood creatinine increased | 8 (18.6) | 0 |
| Cough | 8 (18.6) | 0 |
| Constipation | 7 (16.3) | 0 |
| Neutropenia a | 6 (14.0) | 6 (14.0) |
| Anaemia | 6 (14.0) | 3 (7.0) |
| Arthralgia | 6 (14.0) | 0 |
| Contusion | 6 (14.0) | 0 |
| Myalgia | 6 (14.0) | 0 |
| Back pain | 5 (11.6) | 0 |
| Dyspnoea | 5 (11.6) | 3 (7.0) |
| Insomnia | 5 (11.6) | 0 |
| Muscle spasms | 5 (11.6) | 0 |
| Pain in extremity | 5 (11.6) | 1 (2.3) |
| Rash | 5 (11.6) | 1 (2.3) |
| Thrombocytopenia | 3 (7.0) | 2 (4.7) |
| Events of clinical interest, n (%) | ||
| Cardiac events | 6 (14.0) b | 1 (2.3) |
| Atrial fibrillation c | 0 | 0 |
| Ventricular tachyarrhythmias d | 0 | 0 |
| Anaemia | 6 (14.0) | 3 (7.0) |
| Neutropenia a | 6 (14.0) | 6 (14.0) |
| Thrombocytopenia | 5 (11.6) | 3 (7.0) |
| Bleeding | 10 (23.3) | 0 |
| Major bleeding e | 0 | 0 |
| Hepatotoxicity | 5 (11.6) | 1 (2.3) |
| Hypertension | 2 (4.7) | 0 |
| Infections | 15 (34.9) | 3 (7.0) |
| Interstitial lung disease/pneumonitis | 1 (2.3) | 1 (2.3) |
| Second primary malignancies | 2 (4.7) | 0 |
| Second primary malignancies, excluding non‐melanoma skin cancer | 1 (2.3) | 0 |
Note: Treatment‐emergent AEs were defined as those events with onset or worsening on or after the date of the first dose of acalabrutinib, through the treatment phase to 30 days after the last dose of acalabrutinib, or the first date of subsequent anticancer therapy, whichever is earlier.
Abbreviations: AE, adverse event; CNS, central nervous system; TEAEs, treatment‐emergent adverse events.
Includes preferred terms neutropenia and neutrophil count decreased.
Cardiac events included tachycardia (n = 2), palpitations (n = 2), sinus bradycardia (n = 1), and acute myocardial infarction (n = 1).
Includes preferred terms atrial fibrillation and atrial flutter.
Includes preferred terms torsade de pointes, ventricular arrhythmia, ventricular extrasystoles, ventricular fibrillation, ventricular flutter, ventricular tachyarrhythmia, and ventricular tachycardia.
Defined as any haemorrhagic event that is serious, or grade ≥3 in severity, or that is a CNS haemorrhage.
Among AEs of clinical interest (Table 4), grade 2 hypertension was reported in two patients (5%). One of these patients had a history of hypertension which worsened while on acalabrutinib. The other patient had no history of hypertension. Infections occurred in 15 patients (35%); the most common infections were pneumonia (7%, n = 3), cellulitis, bronchitis, COVID‐19, sinusitis, tooth infection, upper respiratory tract infection and urinary tract infection (5%, n = 2 each). Both cases of COVID‐19 were grade 2 and resolved. Any‐grade neutropenia occurred in six patients (14%), one of whom developed a concurrent infection (grade 2 urinary tract infection during grade 3 decreased neutrophil count, both of which resolved without acalabrutinib dose modification). Neutropenia resulted in dose modification (withholding) in one patient. No febrile neutropenia was reported. Infections of grade 3 or higher included pneumonia, cellulitis and septic shock (the only grade 5 AE in the trial) in one patient each. A second primary malignancy occurred in two patients (unspecified squamous cell carcinoma and squamous cell carcinoma of the skin). One patient had grade 3 drug‐induced pneumonitis which resolved approximately 40 days after acalabrutinib discontinuation. No cases of atrial fibrillation/flutter, ventricular arrythmias, tumour lysis syndrome or major haemorrhage were reported.
DISCUSSION
BTK inhibitors modulate the B‐cell receptor pathway to inhibit downstream activation of NF‐κB signalling, which is critical in the pathogenesis of MZL. 4 As a consequence, these therapies provide targeted treatment that is less toxic than chemotherapy. Chimaeric antigen receptor T‐cell (CAR‐T) therapy, a promising treatment for certain R/R NHLs, including MZL (ORR 85%, CR 55%), is under investigation for the treatment of MZL. 19 , 20 Should it gain approval for treating MZL, more therapies in R/R diseases are still needed, particularly those without the limitations of CAR‐T therapies, such as CAR‐T‐associated toxicities, cost, and dependency on T cell and patient fitness. 21 , 22 , 23
This is the first clinical trial evaluating the BTK inhibitor acalabrutinib for treatment of patients with R/R MZL. Acalabrutinib was found to have activity in R/R MZL with an ORR of 53% among evaluable patients after a median follow‐up of 13.3 months. In similar studies, the BTK inhibitors ibrutinib and zanubrutinib demonstrated ORRs of 48% (95% CI 35%–62%) and 68% (95% CI 56%–79%) respectively, with median follow‐up times of 19.4 months and 15.7 months respectively, in patients with R/R MZL. 6 , 8 Responses with ibrutinib were durable and deepened with longer follow‐up (ORR of 58% and median DOR of 27.6 months after a median 33.1 months of follow‐up). 24 It is possible that with longer follow‐up, acalabrutinib may produce higher rates of response that remain durable. Other targeted therapies, such as lenalidomide (in combination with rituximab) and the PI3Kδ inhibitor umbralisib, have also demonstrated comparable response rates in patients with R/R MZL (65% and 49% respectively) with longer follow‐up times (28.3 months and 27.8 months respectively) 25 , 26 ; lenalidomide plus rituximab has also demonstrated a response rate of 80% in MALT lymphoma after a median follow‐up of 27.0 months 27 compared with the ORR of 53% after a median follow‐up of 13.3 months for acalabrutinib in the current study.
In our study, acalabrutinib was well tolerated, with safety results consistent with its known safety profile. Most AEs were grade 1/2 in severity and treatment discontinuations due to AEs were relatively low (7%), comparing favourably to the rates reported in trials evaluating ibrutinib (17%) and umbralisib (15%), and similar to that of lenalidomide plus rituximab (9%) and zanubrutinib (6%), albeit with a shorter follow‐up in the current study. 6 , 8 , 25 , 26 Some events of clinical interest observed with ibrutinib were of lower incidence with acalabrutinib in the current study, such as atrial fibrillation (which did not occur in this study), compared with 6% in a similar ibrutinib trial. 6 This lower rate of atrial fibrillation with acalabrutinib compared with ibrutinib was also demonstrated in the head‐to‐head ELEVATE‐RR trial (9.4% vs. 16.0%) in CLL. Similarly, hypertension of grade 3 or higher was lower in the current study (0%) compared with the ibrutinib trial (5%). 6 The potential for lower cardiac toxicity makes acalabrutinib a more suitable targeted treatment as a component of combination therapy and, based partly on this rationale along with enhanced clinical activity, acalabrutinib is also being evaluated in combination with chemoimmunotherapy in ongoing phase 1–3 trials (NCT03571308, NCT04002947, NCT04529772, NCT02972840) for aggressive lymphomas such as DLBCL and MCL, and in combination with rituximab with or without lenalidomide for follicular lymphoma in parts 1 and 3 of the current phase 1b/2 trial (NCT02180711). However, the observations about atrial fibrillation in the current trial should be interpreted with caution due to the shorter follow‐up.
A limitation of our study is the relatively small sample size, particularly in subgroup analyses by MZL subtype, the open‐label design, and the lack of a control group. In addition, all efficacy measures, including response, were evaluated by an investigator and not by an independent review committee. Lastly, median follow‐up was shorter in this study compared with studies evaluating similar agents in R/R MZL, and longer‐term follow‐up is needed to assess the overall balance between efficacy and safety.
In conclusion, acalabrutinib was active and well tolerated in this initial report of a phase 2 trial of patients with R/R MZL. The results of this study support acalabrutinib as a safe alternative therapy and a feasible chemotherapy‐free option for patients with R/R MZL. Longer follow‐up with the current study and future studies comparing acalabrutinib alone or in combination to standard of care R/R MZL therapies are needed to add further context to the potential role of acalabrutinib for the treatment of R/R MZL, similar to other BTK inhibitors.
AUTHOR CONTRIBUTIONS
Study design: Paolo Strati, Shuo Ma, Izidore S. Lossos, Praveen Ramakrishnan Geethakumari, Lihua E. Budde. Study investigator: Paolo Strati, Morton Coleman, Shuo Ma, Caterina Patti, Izidore S. Lossos, Praveen Ramakrishnan Geethakumari, Selay Lam, Lihua E. Budde. Provided patients or study materials: Paolo Strati, Morton Coleman, Rebecca Champion, Shuo Ma, Caterina Patti, Izidore S. Lossos, Praveen Ramakrishnan Geethakumari, Selay Lam, Lihua E. Budde. Collection and assembly of data: Paolo Strati, Shuo Ma, Moshe Y. Levy, Izidore S. Lossos, Praveen Ramakrishnan Geethakumari, Selay Lam, Lihua E. Budde. Data analysis: Paolo Strati, Morton Coleman, Shuo Ma, Moshe Y. Levy, Izidore S. Lossos, Praveen Ramakrishnan Geethakumari, Selay Lam, Kara Higgins, Lihua E. Budde. Data interpretation: Paolo Strati, Morton Coleman, Shuo Ma, Moshe Y. Levy, Rebecca Champion, Kara Higgins, Lihua E. Budde. Manuscript preparation: Paolo Strati, Morton Coleman, Moshe Y. Levy, Izidore S. Lossos, Praveen Ramakrishnan Geethakumari, Rebecca Champion, Kara Higgins, Selay Lam, Lihua E. Budde. All authors participated in the critical review and revision of this manuscript and provided approval of the manuscript for submission.
CONFLICT OF INTEREST
Paolo Strati has consulted and served on an advisory board for Roche‐Genentech, ADC Therapeutics, TG Therapeutics and Hutchinson‐MediPharma, and has received research support from AstraZeneca, Acerta and ALX Oncology. Morton Coleman has served on advisory boards and received research support from AbbVie, AstraZeneca, BeiGene, Loxo Oncology, Janssen Pharmaceuticals, Pharmacyclics, Bristol Meyers Squibb and Gilead Sciences. Rebecca Champion has served on the speakers' bureau for Bristol Myers Squibb and AbbVie and has received honoraria for advisory boards for BeiGene. Shuo Ma has received research grants from AbbVie, AstraZeneca, BeiGene, Janssen, Juno, Loxo, Pharmacyclics and TG Therapeutics; received honoraria for advisory board or consulting from AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Pharmacyclics and TG Therapeutics, and served on speakers' bureaus for AstraZeneca, BeiGene, Janssen and Pharmacyclics. Caterina Patti has received honoraria for advisory board or consulting from AbbVie, AstraZeneca, Janssen and Takeda. Moshe Y. Levy has served as a consultant/promotional speaker for AbbVie, Amgen Inc., Bristol Myers Squibb, Janssen Pharmaceuticals, Karyopharm, Morphosys, Seattle Genetics, Takeda, AstraZeneca, BeiGene, Gilead Sciences, Jazz Pharmaceuticals, TG Therapeutics, Epizyme, GSK, Novartis and Dova. Izidore S. Lossos has served on advisory boards for Seattle Genetics, Janssen Scientific, Adaptive Biotechnologies, Verastem and Pharmacyclics. Praveen Ramakrishnan Geethakumari has provided consultancy for Kite Pharma, Pharmacyclics and Rafael Pharmaceuticals. Selay Lam has received honoraria from AbbVie, Amgen, AstraZeneca, BeiGene, BMS, Gilead Sciences, Roche, Janssen, Novartis, Sanofi and Seattle Genetics, and has served in a consulting or advisory role for AbbVie, Amgen, AstraZeneca, BeiGene, BMS, Gilead Sciences, Roche, Janssen, Novartis, Sanofi and Seattle Genetics. Rebecca Champion is a paid employee of AstraZeneca and reports stock ownership in the company. Kara Higgins is a paid employee of AstraZeneca. Lihua E. Budde has served on advisory boards for Genentech/Roche, ADC Therapeutics, BeiGene and Gilead Sciences and has received research grants from Mustang Therapeutics, Merck, Amgen and AstraZeneca.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
The authors would like to thank the investigators, study/site/data coordinators, regulatory personnel, patients who participated in the current study and their families and Robin Lesley, PhD, of AstraZeneca, for data analysis support. The study was funded by AstraZeneca. Medical writing assistance, funded by AstraZeneca, was provided by Robert J. Schoen, PharmD, of Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors.
Strati P, Coleman M, Champion R, Ma S, Patti C, Levy MY, et al. A phase 2, multicentre, open‐label trial (ACE‐LY‐003) of acalabrutinib in patients with relapsed or refractory marginal zone lymphoma. Br J Haematol. 2022;199(1):76–85. 10.1111/bjh.18368
Contributor Information
Paolo Strati, Email: pstrati@mdanderson.org.
Lihua E. Budde, Email: ebudde@coh.org.
DATA AVAILABILITY STATEMENT
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
REFERENCES
- 1. National Cancer Institute SEER incidence rates and annual percent change by age at diagnosis. Bethesda, MD: National Cancer Institute. 2020 [cited April 2020]. Available from: https://seer.cancer.gov/archive/csr/1975_2017/
- 2. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, et al. Marginal zone lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2020;31:17–29. [DOI] [PubMed] [Google Scholar]
- 4. Lue JK, O'Connor OA, Bertoni F. Targeting pathogenic mechanisms in marginal zone lymphoma: from concepts and beyond. Ann Lymphoma. 2020;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wen T, Wang J, Shi Y, Qian H, Liu P. Inhibitors targeting Bruton's tyrosine kinase in cancers: drug development advances. Leukemia. 2021;35:312–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noy A, de Vos S, Thieblemont C, Martin P, Flowers CR, Morschhauser F, et al. Targeting Bruton tyrosine kinase with ibrutinib in relapsed/refractory marginal zone lymphoma. Blood. 2017;129:2224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Imbruvica [package insert]. Sunnyvale, CA, Horsham, PA: Pharmacyclics, Janssen Biotech, Inc.; May 2022.
- 8. Opat S, Tedeschi A, Linton K, McKay P, Hu B, Chan H, et al. The MAGNOLIA trial: zanubrutinib, a next‐generation bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res. 2021;27:6323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brukinsa [package insert]. San Mateo, CA: BeiGene USA, Inc; September 2021.
- 10. Barf T, Covey T, Izumi R, van de Kar B, Gulrajani M, van Lith B, et al. Acalabrutinib (ACP‐196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240–52. [DOI] [PubMed] [Google Scholar]
- 11. Calquence [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; November 2019.
- 12. Byrd JC, Hillmen P, Ghia P, Kater AP, Chanan‐Khan A, Furman RR, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase 3 trial. J Clin Oncol. 2021;39:3441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Spriano F, Tarantelli C, Gaudio E, Gerlach MM, Priebe V, Cascione L, et al. Single and combined BTK and PI3Kδ inhibition with acalabrutinib and ACP‐319 in pre‐clinical models of aggressive lymphomas. Br J Haematol. 2019;187:595–601. [DOI] [PubMed] [Google Scholar]
- 14. Barr PM, Smith SD, Roschewski MJ, O'Brien SM, Sharman JP, Melear JM, et al. Phase 1/2 study of acalabrutinib and the PI3K delta inhibitor ACP‐319 in relapsed/refractory B‐cell Non‐Hodgkin lymphoma. Leuk Lymphoma. 2022;63(7):1728–32. [DOI] [PubMed] [Google Scholar]
- 15. Strati P, De Vos S, Ruan J, Maddocks KJ, Flowers CR, Rule S, et al. Acalabrutinib for treatment of diffuse large B‐cell lymphoma: results from a phase Ib study. Haematologica. 2021;106:2774–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang M, Robak T, Maddocks KJ, Phillips T, Smith SD, Gallinson D, Calvo R, Wun CC, Munugalavadla V, Jurczak W Safety and efficacy of acalabrutinib plus venetoclax and rituximab in patients with treatment‐naive mantle cell lymphoma [poster 2416]. Annual meeting and exposition of the American Society of Hematology; 11‐14 December 2021; Atlanta, GA, 138, 2416 [Google Scholar]
- 17. Phillips T, Smith SD, Jurczak W, Robak T, Stevens DA, Farber CM, Pagel JM, Maddocks K, Flinn I, Jedrzejczak WW, Goy A, Zinzani PL, Zaucha J, Coleman M, Chen T, Lee SK, Liang W, Seto A, Wang M Safety and efficacy of acalabrutinib plus bendamustine and rituximab in patients with treatment‐naive or relapsed/refractory mantle cell lymphoma [poster MCL‐194]. Annual meeting of the Society of Hematological Oncology; 11‐14 September 2019; Houston, TX. 19, S317 [Google Scholar]
- 18. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leslie LA. Novel therapies for follicular lymphoma and other indolent non‐Hodgkin lymphomas. Curr Treat Options Oncol. 2021;22:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non‐Hodgkin lymphoma (ZUMA‐5): a single‐arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23:91–103. [DOI] [PubMed] [Google Scholar]
- 21. Mehta PH, Fiorenza S, Koldej RM, Jaworowski A, Ritchie DS, Quinn KM. T cell fitness and autologous CAR T cell therapy in haematologic malignancy. Front Immunol. 2021;12:780442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borgert R. Improving outcomes and mitigating costs associated with CAR T‐cell therapy. Am J Manag Care. 2021;27:S253–s61. [DOI] [PubMed] [Google Scholar]
- 23. Sterner RC, Sterner RM. CAR‐T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noy A, de Vos S, Coleman M, Martin P, Flowers CR, Thieblemont C, et al. Durable ibrutinib responses in relapsed/refractory marginal zone lymphoma: long‐term follow‐up and biomarker analysis. Blood Adv. 2020;4:5773–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37:1188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fowler NH, Samaniego F, Jurczak W, Ghosh N, Derenzini E, Reeves JA, et al. Umbralisib, a dual PI3Kδ/CK1ε inhibitor in patients with relapsed or refractory indolent lymphoma. J Clin Oncol. 2021;39:1609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kiesewetter B, Willenbacher E, Willenbacher W, Egle A, Neumeister P, Voskova D, et al. A phase 2 study of rituximab plus lenalidomide for mucosa‐associated lymphoid tissue lymphoma. Blood. 2017;129:383–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


