Abstract
Graves’ orbitopathy (GO) is an orbital autoimmune disorder and the main extrathyroidal manifestation of Graves’ disease, the most common cause of hyperthyroidism. GO affects about 30% of Graves’ patients, although fewer than 10% have severe forms requiring immunosuppressive treatments. Management of GO requires a multidisciplinary approach. Medical therapies for active moderate‐to‐severe forms of GO (traditionally, high‐dose glucocorticoids) often provide unsatisfactory results, and subsequently surgeries are often needed to cure residual manifestations. The aim of this review is to provide an updated overview of current concepts regarding the epidemiology, pathogenesis, assessment, and treatment of GO, and to present emerging targeted therapies and therapeutic perspectives. Original articles, clinical trials, systematic reviews, and meta‐analyses from 1980 to 2021 were searched using the following terms: Graves’ disease, Graves’ orbitopathy, thyroid eye disease, glucocorticoids, orbital radiotherapy, rituximab, cyclosporine, azathioprine, teprotumumab, TSH‐receptor antibody, smoking, hyperthyroidism, hypothyroidism, thyroidectomy, radioactive iodine, and antithyroid drugs. Recent studies suggest a secular trend toward a milder phenotype of GO. Standardized assessment at a thyroid eye clinic allows for a better general management plan. Treatment of active moderate‐to‐severe forms of GO still relies in most cases on high‐dose systemic—mainly intravenous—glucocorticoids as monotherapy or in combination with other therapies—such as mycophenolate, cyclosporine, azathioprine, or orbital radiotherapy—but novel biological agents—including teprotumumab, rituximab, and tocilizumab—have achieved encouraging results.
Keywords: glucocorticoids, Graves’ orbitopathy, iscalimab, rituximab, teprotumumab, thyrotropin receptor, tocilizumab, TSH receptor
Abbreviations
- ACE
angiotensin‐converting enzyme
- ATD
antithyroid drug
- CAS
clinical activity score
- DON
dysthyroid optic neuropathy
- ETA
European Thyroid Association
- EUGOGO
European Group on Graves’ Orbitopathy
- GCs
glucocorticoids
- GO
Graves’ orbitopathy
- IGF‐1
insulin‐like growth factor‐1
- IGF‐1R‐Ab
IGF‐1 receptor antibodies
- IL‐6
interleukin‐6
- ivGCs
intravenous glucocorticoids
- OR
orbital radiotherapy
- RAI
radioactive iodine
- RCT
randomized clinical trial
- TSHR
TSH receptor
- TSHR‐Ab
TSHR antibodies
Introduction
Graves’ orbitopathy (GO) is an orbital autoimmune disease constituting the most frequent extrathyroidal expression of Graves’ disease [1]. Full‐blown disease is associated with disfiguring features (exophthalmos, stare), inflammatory signs and symptoms, ocular dysfunction (diplopia), and, rarely, visual loss due to compressive dysthyroid optic neuropathy (DON) [1] (Table 1). GO heavily affects quality of life and bears relevant public health consequences, including sick leave, work disability, and costs of therapies [2, 3, 4].
Table 1.
Main symptoms and signs of Graves’ orbitopathy
| Symptoms | |
| Lacrimation | |
| Grittiness (sandy sensation) | |
| Photophobia | |
| Ocular pain (spontaneous or with eye movements) | |
| Diplopia (intermittent, when tired or awakening; inconstant, at extremes of gaze; constant, in all positions of gaze) | |
| Decreased color sensitivity | |
| Visual loss | |
| Signs | |
| Lid retraction (stare) | |
| Periocular soft tissue swelling and redness | |
| Conjunctival hyperemia and edema (chemosis) | |
| Exophthalmos (bulging eyes) | |
| Lagophthalmos (incomplete eye closure at night) | |
| Decreased ocular motility/strabismus | |
| Decreased visual acuity (due to dysthyroid optic neuropathy) |
Relevant progress has been made in the last 20 years in the understanding of GO epidemiology and pathogenesis, the identification of risk factors, the standardized assessment of patients, and general treatment plan. Novel biological agents have been introduced for GO targeted treatment, with promising results. These recent achievements are presented in this review.
Epidemiology
The estimated incidence of GO—based on registry studies [5, 6]—is 3.3–8.0/100,000/year in women and 0.9–1.6/100,000/year in men [7], lower than previously reported [8]. The estimated prevalence of GO in the general population is around 9/10,000 population [9]. Thus, although uncommon, GO does not fulfill—with the exception of the variant euthyroid GO—the major criterion of prevalence of less than 5/10,000 population to be designated as a rare disease [9].
The prevalence of GO among Graves’ patients is around 30% [10, 11]. In a single‐center study of more than 300 consecutive patients with recent onset Graves’ hyperthyroidism, 74% had no signs/symptoms of GO, 20% had mild GO, and only 6% had moderate‐to‐severe or, rarely, sight‐threatening GO [12, 13] (Fig. 1). Interestingly, consecutive patients referred to tertiary centers of the European Group on Graves’ Orbitopathy (EUGOGO) in 2012 had less severe and active GO than in 2000 [14]. Lower prevalence and severity of GO may reflect a secular trend toward a milder phenotype of Graves’ disease [11, 13]. Earlier diagnosis and treatment of both hyperthyroidism and GO, and other factors—including decreased smoking and micronutrient supplementation—likely play a fundamental role [11].
Fig. 1.
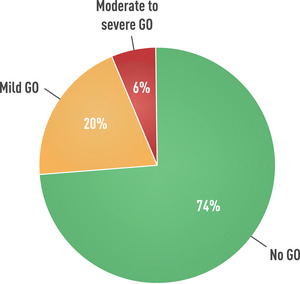
Prevalence of Graves’ orbitopathy in patients with newly diagnosed, recent‐onset Graves’ hyperthyroidism. Derived from Tanda et al. [12].
Pathogenesis
GO is mostly associated with Graves’ hyperthyroidism, although in 7%–8% of cases it may occur with euthyroid/hypothyroid chronic autoimmune thyroiditis [1]. As Graves’ disease is due to loss of tolerance to TSH receptor (TSHR), GO may be triggered by autoimmune reactions against TSHR [15], as supported by experimental and clinical evidence [16]. After initial demonstration of TSHR expression in orbital tissue [17, 18], immunohistochemical studies have shown TSHR overexpression in GO orbital tissues [19, 20, 21]. TSHR activation enhances the differentiation of orbital preadipocytes into adipocytes, favoring the expansion of orbital adipose tissue [22, 23]. The key role of TSHR is supported by recent animal models obtained in rodents [24, 25, 26, 27]. From a clinical standpoint, circulating TSHR antibodies (TSHR‐Ab) correlate with GO activity [28, 29, 30]. In a prospective study of consecutive GO patients, novel cell‐based TSHR‐Ab bioassays measuring stimulatory TSHR‐Ab showed higher sensitivity and specificity than commonly used immunoassays in differentiating clinically active from inactive, and mild from moderate‐to‐severe, GO [31]. Finally, TSHR‐Ab is an independent risk factor for GO and may predict GO severity and outcome [32]. Therefore, solid evidence links TSHR and stimulatory TSHR‐Ab to GO development. TSHR‐Ab can be considered a biomarker of GO [33, 34].
Insulin‐like growth factor‐1 (IGF‐1) receptor (IGF‐1R) is another important player [35, 36, 37]. B cells [38] and T cells [39] from Graves’ patients overexpress IGF‐1R; Graves’ immunoglobulins stimulate hyaluronan synthesis by orbital fibroblasts through IGF‐1R [40] and induce orbital fibroblast proliferation and cytokine secretion [41]. TSHR and IGF‐1R colocalize on orbital fibroblasts and thyrocytes [42]. IGF‐1R activation on orbital fibroblasts may follow the binding of stimulatory IGF‐1R antibodies (IGF‐1R‐Ab) to IGF‐1R. Alternatively, IGF‐1R activation may result from synergistic cross‐talk between TSHR and IGF‐1R after the binding of stimulatory TSHR‐Ab to TSHR, causing activation of IGF‐1R‐dependent intracellular signaling pathways [43]. Consistent with this hypothesis, teprotumumab—an IGF‐1R‐blocking monoclonal antibody that does not bind to TSHR—inhibited IGF‐1R‐dependent production of hyaluronan induced by a monoclonal stimulatory TSHR‐Ab (M22), but not IGF‐1R‐independent M22 stimulation [44]. There is no animal model of GO employing IGF‐1R for immunization. From a clinical standpoint, after initial demonstration of Graves’ immunoglobulins displacing IGF‐1 bound to GO orbital fibroblasts [45], subsequent studies have provided conflicting results, showing that a subset of patients with GO may have circulating IGF‐1R‐Ab with either inhibitory [46] or stimulatory IGF‐1R functional properties [47]. More recently, a pilot study using a novel IGF‐1R‐Ab assay showed that Graves’ patients without GO had higher serum IGF‐1R‐Ab concentrations than those with GO [48], suggesting a putative “protective” role of IGF‐1R‐Ab for GO. For the time being, the role of IGF‐1R‐Ab in GO pathogenesis is speculative.
Fibroblasts are the primary victims of the autoimmune attack [49]. Orbital fibroblasts also include a subset of CD34+ bone‐marrow‐derived fibrocytes expressing thyroid antigens, including TSHR and thyroglobulin [50]. Serum thyroglobulin levels are higher in Graves’ patients with GO than in those without [51], but whether thyroglobulin is another thyroid antigen involved in GO pathogenesis is not settled. B cells, dendritic cells, and T cells—activated after presentation of thyroid autoantigen(s)—infiltrate the orbit and produce proinflammatory cytokines, growth factors, and chemoattractants [20, 49]. In immunohistochemical studies, both T cells (CD3+ and CD4+) and B cells (CD20+) are often organized into distinct foci and associated with GO activity [52, 53]. B cells work as antigen‐producing cells and autoantibody‐producing cells [49]. Interaction of T cells with orbital fibroblasts, as well as the binding of TSHR‐Ab—and possibly IGF‐1R‐Ab—produced by B cells, activates the TSHR‐IGF‐1R complex. As a consequence, orbital fibroblasts proliferate—further releasing inflammatory molecules—but also differentiate into adipocytes, thereby eventually causing an increase in the orbital fibro‐adipose tissue, while extraocular muscles are infiltrated by inflammatory cells [49]. Orbital thyroid tissue from GO patients under‐ and overexpress numerous genes [54, 55, 56]. Genetic profiling of GO and control fibroblasts confirmed that some of the underexpressed genes encode proteins involved in the downregulation of cell growth, apoptosis, and antioxidant activity, while some of the overexpressed genes encode proteins enhancing cell growth and inhibiting proptosis [57]. In addition, after autoimmune reactions have been triggered, orbital fibroblasts produce glycosaminoglycans—mainly hyaluronan—which are hydrophilic and attract water, causing edema in orbital tissue and extraocular muscle. The increase in orbital volume and edema in a limited space such as the orbit mechanically explain manifestations of GO including exophthalmos, periocular soft tissue swelling, extraocular muscle enlargement/dysfunction, and optic nerve compression [58].
Risk factors and prevention
GO results from a complex interplay between endogenous (nonmodifiable) and exogenous (modifiable) risk factors [7] (Fig. 2).
Fig. 2.
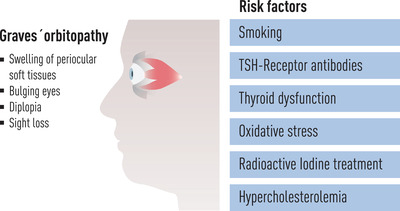
Modifiable risk factors for de novo occurrence or progression of Graves’ orbitopathy.
GO is rare in childhood [59]. Moderate‐to‐severe GO is less frequent in patients younger than 40 years, with the mean age of patients with severe forms of GO being 50–56 years [6, 12]. Although more frequent in women, gender difference attenuates in severe GO. In a study of more than 2000 Graves’ patients, clinically relevant GO was found in about half of women and men, but patients with moderate‐to‐severe GO were more commonly men (30% vs. 21%) and older [60]. Thus, men tend to have more severe GO at an older age [7, 61, 62].
The role of genetic factors conferring predisposition to Graves’ disease has long been proposed, based on twin and family studies, and family clustering of thyroid autoimmune disorders [63, 64, 65]. Several candidate genes have been indicated, but the differentiation of the genetic profile of Graves’ patients with or without GO is uncertain [66, 67]. Additionally, in twin studies the concordance rate is only about 30%, suggesting a low penetrance of involved genes [63, 68]. Epigenetic factors—including abnormal DNA methylation, histone modification, and noncoding RNAs—may contribute to GO pathogenesis [69], but their role is not clear. Preliminary studies reporting differences in gut microbiota composition in Graves’ patients with or without GO [70]—as well as animal studies suggesting a pivotal role of gut microbiota in TSHR‐induced disease [71]—opened an interesting field of research [72].
Cigarette smoking is an important modifiable risk factor [73] (Table 2). The incidence of clinically relevant GO is lower in European countries where tobacco consumption has decreased [7]. Smoking is associated with a lower [76] and slower [77] response to immunosuppressive treatment, and smoking withdrawal decreases the risk of GO development [78] (Table 2). Passive smoking might also be an important risk factor [79]. Mechanisms whereby smoking affects GO likely include increased generation of oxygen free radicals and hypoxia in the orbit, increased cytokine secretion, enhanced adipogenesis, and changes in the gut microbiome affecting the autoimmune process [7]. EUGOGO guidelines strongly recommend that physicians urge Graves’ patients to quit smoking [80].
Table 2.
Modifiable risk factors for Graves’ orbitopathy (GO) and evidence supporting their role
| Risk factor | Evidence | Author |
|---|---|---|
| Smoking | Higher prevalence of smokers in patients with GO than in patients without GO | Bartalena et al. [74] |
| Greater chance of Graves’ smoker patients to develop severe forms of GO | Prummel and Wiersinga [75] | |
| Lower and slower response to treatments for GO in smokers | Bartalena et al. [76] | |
| Eckstein et al. [77] | ||
| Decreased risk of developing GO in former smokers than in current smokers | Pfeilschifter and Ziegler [78] | |
| Thyroid dysfunction | Amelioration of GO following restoration of euthyroidism | Prummel et al. [85] |
| Higher proportion of uncontrolled hyperthyroidism in patients with moderate‐to‐severe GO than in those with mild GO | Prummel et al. [86] | |
| GO occurrence during a period of uncontrolled hypothyroidism | Karlsson et al. [87] | |
| Decreased progression of GO after RAI treatment if hypothyroidism is controlled promptly |
Perros et al. [94] Tallstedt et al. [96] |
|
| RAI treatment | RAI‐associated progression of GO in 15%–20% of cases |
Tallstedt et al. [90] Bartalena et al. [91] |
| RAI‐associated progression of GO more likely in smokers |
Bartalena et al. [91] Traisk et al. [92] |
|
| TSH‐R‐Ab | TSHR‐Ab are higher in patients with GO than in those without GO and correlate with GO activity and severity | Lytton et al. [29] |
| Increased risk of GO if TSHR‐Ab are high | Khoo et al. [101] | |
| Oxidative stress | Increased oxidative stress in patients with Graves’ disease and GO | Bartalena et al. [103] |
| Increased concentrations of reactive oxygen species in blood, tears, and urine of GO patients | Hou et al. [104] | |
| Hypercholesterolemia | High total and LDL cholesterol associated with the presence of GO | Sabini et al. [109] |
| Reduced risk of GO in patients receiving statin treatment |
Stein et al. [111] Nilsson et al. [112] |
|
| Increased effectiveness of ivGCs in patients treated with atorvastatin |
Lanzolla et al. [114] |
Abbreviations: ivGCs, intravenous glucocorticoids; LDL, low‐density lipoprotein; RAI, radioactive iodine; TSHR‐Ab, TSH receptor antibodies.
Thyroid dysfunction is another major risk factor (Table 2). Although most GO patients have hyperthyroidism [81, 82], some of them have euthyroidism or hypothyroidism [83, 84]. In a prospective study, GO remained stable in 54 patients with euthyroidism when first seen, while it progressively improved in 33 patients with hyperthyroidism following restoration of euthyroidism [85]. Uncontrolled hyperthyroidism is more frequent in patients with moderate‐to‐severe GO than in those with mild GO [86]. Hypothyroidism is also relevant, as GO developed in 15 of 30 referred patients during a period of uncontrolled hypothyroidism [87]. In a cohort of 700 patients with chronic autoimmune thyroiditis, overt GO was present in 6% of patients, 68% of whom had circulating stimulatory TSHR‐Ab [88]. Both hyper‐ and hypothyroidism activate TSHR through TSHR‐Ab and TSH, respectively, thereby inducing an increased expression of thyroid/orbital antigens and worsening of autoimmune reactions directed against them. Therefore, EUGOGO guidelines [80] and European Thyroid Association (ETA) guidelines [89] recommend that both hyper‐ and hypothyroidism should be promptly corrected, and euthyroidism stably maintained [80].
Radioactive iodine (RAI) treatment may exacerbate GO (Table 2). Two randomized clinical trials (RCTs) showed that RAI treatment—at variance with antithyroid drug (ATD) treatment or thyroidectomy—bears a small but definite risk of GO progression [90, 91], particularly in smokers and in patients with preexisting mild GO [91]. A third RCT found that not only progression of preexisting GO, but also de novo development of GO may occur after RAI treatment [92]. This occurs in 15%–20% of cases [93] and is permanent in 5% [91]. This risk is minimal if GO is inactive [94] or longstanding (>5 years) [95]. Late correction of post‐RAI hypothyroidism likely plays a role in GO progression [94, 96]. Several studies [97, 98, 99] showed that steroid prophylaxis using low doses of prednisone can almost always prevent RAI‐associated GO progression. A starting dose of 0.3–0.5 mg prednisone/kg bodyweight, tapered and withdrawn after 3 months [91], was originally used, but lower doses (0.1–0.2 mg prednisone/kg bodyweight, tapered and withdrawn after 6 weeks) are similarly effective [98]. RAI likely causes progression of GO through the exacerbation of orbital autoimmune reactions following RAI‐associated cytolytic effect. Indeed, RAI treatment is followed by a long‐lasting increase in circulating TSHR‐Ab [100]. Based on strong evidence of its effectiveness and safety, and in agreement with current guidelines [80, 89], we believe that low‐dose oral prednisone prophylaxis should be used after RAI treatment in most patients, with the possible exception of those with longstanding and inactive GO [80, 89].
Using sensitive bioassays, stimulatory TSHR‐Ab are found in almost all patients with Graves’ disease, but circulating levels are threefold higher in GO patients, with a strong correlation with GO activity and severity [29]. In a series of 100 consecutive Graves’ patients, the odds ratio of GO was markedly increased when serum TSHR‐Ab levels were above median, and a phenotype of high TSHR‐Ab and absent thyroid peroxidase antibodies identified a group at very high risk of developing GO [101, 102]. There are no tools to reduce or block TSHR‐Ab, but ATD treatment and thyroidectomy—contrary to RAI treatment—are associated with a progressive decline in serum TSHR‐Ab levels [100].
Increased oxidative stress is found in patients with Graves’ disease and GO [103]. Reactive oxygen species stimulate orbital fibroblast proliferation, glycosaminoglycan synthesis, and upregulation of inflammatory and noninflammatory mediators [104] (Table 2). Increased concentrations of reactive oxygen species have been reported in the blood, tears, and urine of GO patients [104]. Selenium is a trace element relevant for the thyroid because selenocysteine is incorporated into selenoproteins such as iodothyronine deiodinases—involved in thyroid hormone deiodination—or thioredoxin reductase and glutathione peroxidase [105], which exert antioxidant effects [106]. In addition, selenium has immunomodulating properties [107]. In a EUGOGO multicenter RCT, selenium supplementation effectively improved mild and active GO and prevented progression to more severe forms [108], as is detailed further on.
Hypercholesterolemia is a novel risk factor (Table 2). High total and low‐density lipoprotein cholesterol levels have been associated with the presence of GO [109, 110]. Two large retrospective studies—one based on an insurance database from the United States [111] and the other on a Swedish national register [112]—showed that Graves’ patients receiving statin treatment for hypercholesterolemia had a decreased risk of GO occurrence. Finally, a single‐center, retrospective study of consecutive patients with active moderate‐to‐severe GO found that high low‐density lipoprotein cholesterol levels increased the likelihood of a poor response to intravenous glucocorticoid (ivGC) treatment [113]. More recently, a phase II, open label, single‐center RCT of 88 patients with active moderate‐to‐severe GO and increased low‐density lipoprotein cholesterol levels demonstrated that adding atorvastatin to ivGCs led to a better treatment outcome compared to ivGC monotherapy [114]. There are two relevant open questions: (i) Might statin treatment be an add‐on treatment in patients with active moderate‐to‐severe GO and normal cholesterol levels? And (ii) might statins be given to Graves’ patients with absent/mild GO, independently of cholesterol levels, to prevent GO progression [115]? Further studies are needed to address these issues. For the time being, it seems reasonable to follow the suggestion of guidelines [80] and correct hypercholesterolemia in all patients with newly diagnosed Graves’ disease, independently of the absence, presence, or degree of GO.
Triggering events
The postpartum period is a triggering event for Graves’ hyperthyroidism [116]. Postpartum‐associated rebound immune phenomena may also favor relapse of hyperthyroidism [117, 118]. Among women in remission after ATD treatment, relapse occurred more frequently in women who then had a successful pregnancy than in those who had no pregnancy [119], although women with hyperthyroidism relapsing in the postpartum period had a greater decrease in TSHR‐Ab levels and a higher rate of remission compared to women whose relapse of hyperthyroidism was independent of pregnancy/being postpartum [120]. As far as GO is concerned, no differences in GO prevalence and degree of severity were found in women whose hyperthyroidism relapsed in the postpartum period [120]. Thus, the postpartum period is a triggering event for Graves’ disease, but not specifically for GO.
Graves’ disease and GO might be activated by infections through various mechanisms, including molecular mimicry, aberrant induction of HLA antigens, and interaction with autoantigens [121, 122]. During SARS‐CoV‐2 infection, thyrotoxicosis has been reported, mainly due to subacute/atypical (painless) thyroiditis [123, 124, 125, 126, 127, 128, 129, 130, 131]. SARS‐CoV‐2 may enter cells through the angiotensin‐converting enzyme (ACE‐2) receptor. ACE‐2 receptor mRNA has been detected both in thyroid tissue samples and in primary thyroid cell cultures [132]. In addition, the SARS‐CoV‐2 genome was reported in 36% of thyroid samples from autopsies of patients who died of COVID‐19 [133] and in one case of primary thyroid sarcoma [134]. Thus, thyrotoxicosis might be related to direct thyroid infection. Alternatively, it might result from cytokine‐induced thyroid damage [131, 132, 133, 134, 135]. Graves’ disease has been reported more rarely in patients with SARS‐CoV‐2 infection [136, 137, 138]. Three of the five patients described thus far were in long‐term remission of hyperthyroidism after ATD treatment, while two patients received a new diagnosis of Graves’ disease [136, 137, 138]. Longstanding inactive GO—previously treated with glucocorticoids (GCs)—was described in one patient, with no reactivation following SARS‐CoV‐2 infection [137]; one patient developed hyperthyroidism with mild GO [138]; and the remaining three patients presumably had no GO. Cases of thyrotoxicosis apparently induced by SARS‐CoV‐2 vaccination have also been described. In most instances, thyrotoxicosis was due to subacute/atypical thyroiditis [139]. For the time being, 25 cases of Graves’ disease have been reported after vaccination [140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153]. Interestingly, most cases occurred shortly after the first or second vaccination using mRNA‐based vaccines, were generally mild‐to‐moderate in severity, and were easily controlled with ATDs. Reactivation of Graves’ disease after long‐term remission (7 and 17 years) following ATD treatment was reported in two cases after vaccination [149, 153]. More pertinent to this review, only one patient developed moderate‐to‐severe GO [144], while two additional patients had minimal‐to‐mild ocular involvement [149, 152]. Possible mechanisms for Graves’ disease occurrence after vaccination might include autoimmune/inflammatory syndrome induced by adjuvants [154, 155], or molecular mimicry with cross‐talk between antibodies to SARS‐CoV‐2 and thyroid—and perhaps orbital—antigens. Most of the above studies are single case reports or small series, and the occurrence of Graves’ disease might very well be only by chance. In any case, the risk of GO occurrence seems extremely low.
Graves’ disease and GO may also be triggered by thyroid damage, including subacute thyroiditis [156, 157, 158, 159], RAI treatment for toxic adenoma/multinodular goiter [160, 161], ethanol percutaneous injection for toxic adenoma [162], fine needle aspiration biopsy of thyroid nodules [163], and external radiotherapy in the head and neck region [164, 165]— although a recent, large retrospective study of patients who had received neck, craniospinal, or total body irradiation for various tumors found no cases of radiation‐induced hyperthyroidism [166]. As for RAI‐associated GO progression, the underpinning mechanism is likely the release of thyroid antigens triggering or exacerbating autoimmunity.
Graves’ disease may be triggered by immune reconstitution therapy, in particular by alemtuzumab, a monoclonal antibody that targets the CD52 antigen expressed on T cells and monocytes, thereby causing marked lymphopenia, followed by a progressive recovery of lymphocyte counts [167]. Alemtuzumab has been employed with very good results in relapsing‐remitting multiple sclerosis (RRMS), but may cause thyroid dysfunction, particularly Graves’ disease. This is not surprising, because reconstitution autoimmunity is more commonly represented by autoantibody‐mediated disorders, and Graves’ hyperthyroidism and GO are driven by TSHR‐Ab [168]. Graves’ disease was reported in 22%–33% of RRMS patients treated with alemtuzumab [169, 170, 171], and was by far the most common thyroid disorder, accounting for 63% of all cases of thyroid dysfunction in a recent systematic review and meta‐analysis of seven studies (1362 patients) [172]. In the two major original studies, GO occurred in 13 out of 136 patients—accounting for less than 10% of cases of alemtuzumab‐associated Graves’ disease—and was moderate to severe in five (4%) [169, 170]. Thus, GO in patients with alemtuzumab‐associated Graves’ disease is neither more frequent nor more severe than in the general population. However, an important feature of alemtuzumab‐associated Graves’ disease is that it often fluctuates from hyperthyroidism to hypothyroidism and vice versa, in relation to the prevailing activities of stimulatory and blocking TSHR‐Ab [170]. Instability of thyroid status is a well‐known risk factor for GO [7]. Accordingly, ETA guidelines suggest that in patients with fluctuating hyperthyroidism, consideration may be given to a definitive treatment by either RAI or thyroidectomy [173].
Natural history
Mild GO involves an initial inflammatory period underpinned by ongoing orbital activation of autoimmune reactions (active phase). GO then stabilizes when inflammation starts to subside (plateau or static phase) and progressively remits following burning out of inflammation (inactive phase), possibly associated with fibrotic changes [4]. Extraocular muscle enlargement seems to occur earlier than orbital fat expansion [174]. Studies have shown that mild/minimal GO rarely progresses to more severe forms, whereas stabilization and remission are frequent [12, 13, 175, 176] (Fig. 3). In a retrospective study of 226 patients with initially active moderate‐to‐severe GO, reevaluated 4 years after GO onset and various nonsurgical and surgical treatments, further improvement was reported in 60% of responders to treatment at the last visit [177]. In a placebo group of the second teprotumumab study, at 6‐month assessment a reduction in exophthalmos ≥2 mm was observed in 10% of patients, an amelioration in diplopia in 29%, and a clinical activity score (CAS) 0–1 (inactive GO) in 21% [178]. Therefore, patients with moderate‐to‐severe GO may have the same natural history as patients with mild GO. This by no means implies that management of patients with moderate‐to‐severe GO can be delayed, because immunosuppressive treatments are largely ineffective in patients with longstanding disease.
Fig. 3.
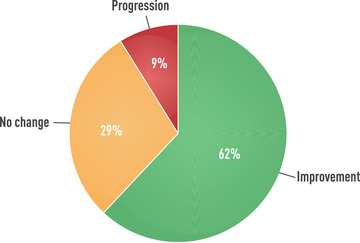
Natural history of Graves’ orbitopathy in patients with mild ocular involvement at baseline. Derived from Tanda et al. [12] and Perros et al. [175].
Early referral to specialists and thyroid eye disease clinics
Early diagnosis and management of Graves’ disease and GO is fundamental because: (i) Prompt correction of thyroid dysfunction is associated with a lower risk of progression of initially mild or absent GO [12, 85] and (ii) a favorable outcome of immunosuppressive treatment for more severe forms of GO is inversely correlated with disease duration [4, 179]. In a nationwide UK survey, correct diagnosis of GO was delayed by up to 1 year in one fourth of patients, because mild ocular inflammatory symptoms were misdiagnosed as conjunctivitis or allergic phenomena [180]. For many years, a major goal of EUGOGO has been to improve patient care through the establishment of thyroid eye disease outpatient clinics run by endocrinologists and ophthalmologists/orbital surgeons, with interdisciplinary collaboration of other specialists such as orthoptists, thyroid surgeons, radiotherapists, and radiologists [181]. Satisfaction regarding the management of GO is higher among patients who attend such specialized centers [180]. Propagation of this interdisciplinary approach introduced by EUGOGO contributed to a decrease in patient referral time and reduced GO severity of patients referred to EUGOGO tertiary centers over a 12‐year period [14]. Helpful indications on the management of GO outside specialized centers and referral pathways have been published [182]. According to EUGOGO guidelines, primary‐care physicians, general practitioners, general internists, and specialists should promptly refer all patients with overt GO or at risk of progression (e.g., smokers and those with unstable hyperthyroidism or high TSHR‐Ab levels) to thyroid eye clinics or specialized centers [80].
Assessment
The main features of GO to be assessed are activity and severity because both contribute to identifying and prescribing appropriate treatments according to the phase and degree of orbital involvement.
CAS—a scoring system that substantially evaluates inflammation [183]—is the best validated and most used tool to assess GO activity. CAS includes seven items, with one point given to each item present. GO is defined as active if the sum is ≥3/7 (Fig. 4). A 10‐item version of the CAS includes three additional features—increase in exophthalmos of ≥2 mm, decrease in eye motility, and decrease in visual acuity—that are useful in defining recent (1–3 months) GO progression. CAS is not perfectly objective because two items—spontaneous and gaze‐evoked pain—are subjective. In addition, CAS is binary—indicating presence or absence, without grading—but an image atlas may help to improve the consistency of CAS assessment [184]. Thus, CAS is a useful and quick tool, easily applicable in daily clinical practice to assess the activity of GO and to monitor the effects of treatment on inflammation. Other scores, such as the VISA (Vision, Inflammation, Strabismus, Appearance/exposure) score [185], have been proposed, but they need more thorough validation. Magnetic resonance imaging may also be useful, but it is expensive and not always available.
Fig. 4.
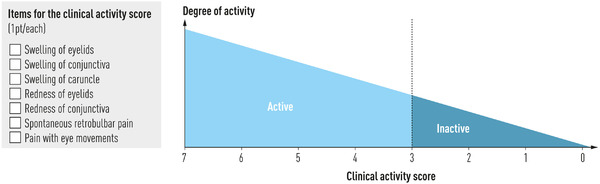
Assessment of activity of Graves’ orbitopathy by the clinical activity score.
Measures of severity include assessment of lid aperture by a ruler, exophthalmos by a Hertel exophthalmometer, ocular motility by ductions, corneal status by slit lamp [181], and assessment of optic nerve status by visual acuity, visual fields, color vision, afferent pupil defects, fundus oculi evaluation, and optical coherence tomography [186]. A precise definition of GO severity is somehow elusive, but EUGOGO has proposed a classification into mild, moderate‐to‐severe, and sight‐threatening—very severe—GO, based on the combination of different features [80] (Table 3).
Table 3.
Classification of severity of Graves’ orbitopathy
| Degree of severity | Features |
|---|---|
| Mild | One or more of: |
| Lid retraction <2 mm | |
| Mild soft tissue involvement | |
| Exophthalmos <3 mm above normal | |
| No/intermittent diplopia | |
| Moderate to severe | Two or more of: |
| Lid retraction ≥2 mm | |
| Moderate‐to‐severe soft tissue involvement | |
| Exophthalmos ≥3 mm above normal | |
| Inconstant/constant diplopia | |
| Sight threatening | Presence of DON and/or corneal breakdown |
Note: Adapted from Bartalena et al. [80].
Abbreviation: DON, dysthyroid optic neuropathy.
Standardization of assessment should be implemented to allow homogeneous reporting of treatment outcomes and, thereby, the comparison of effects of different therapies for GO [187].
Management of mild GO
General measures include control of modifiable risk factors for GO progression—for example, refraining from smoking, tight control of thyroid dysfunction, steroid prophylaxis after RAI treatment, and correction of hypercholesterolemia [80]. Artificial tears during the day and ocular gels/ointments at night are useful to reduce symptoms due to inflammation or dry eye [188].
In an RCT of 159 patients with active mild GO, selenium administration (selenium selenite 100 µg twice a day, equivalent to 91.2 µg of selenium) for 6 months induced an improvement in quality of life and overall ocular involvement significantly better than placebo [108]. Furthermore, selenium treatment effectively prevented progression of GO to more severe forms 6 months after intervention [108]. No cases of selenium toxicity were observed. Most patients came from marginally selenium‐deficient European areas; accordingly, it is not clear whether selenium supplementation is also helpful in patients living in selenium‐sufficient regions. An Italian questionnaire‐based survey revealed a wide use of selenium supplementation for conditions lacking evidence of benefit, including autoimmune thyroiditis and Graves’ hyperthyroidism without GO [189]. This misuse should be avoided [107, 190, 191]. Likewise, there is no evidence that selenium may be considered an adjuvant therapy for active moderate‐to‐severe GO treated with immunosuppressive/targeted therapies. Accordingly, current guidelines recommend a 6‐month course of selenium only for active mild GO [80]. If selenium selenite is unavailable, it can be substituted with seleniomethionine (100 µg daily).
In patients whose quality of life is markedly impaired despite mild features of active GO, a course of low‐dose ivGCs can be considered within a shared decision‐making process [80]. Likewise, in patients with residual manifestations of mild and inactive GO (lid malposition, minimal exophthalmos), rehabilitative surgery can also be considered, as needed or requested by the informed patient [80].
Management of active moderate‐to‐severe GO
Active moderate‐to‐severe forms of GO affect a minority of patients but are a major challenge and dilemma because established medical treatments often achieve incomplete results [192, 193]. GCs still constitute the mainstay in the management of active moderate‐to‐severe and sight‐threatening GO [80], but recent advances using either old drugs with new applications or novel biological agents have expanded the armamentarium available for treatment [194, 195].
GCs as monotherapy or in combination with other therapies
GCs have been used for decades for their potent anti‐inflammatory and immunosuppressive effects achieved through interference with T‐ and B‐cell functions, decreased recruitment of monocytes and macrophages, reduced secretion of proinflammatory cytokines and increased secretion of anti‐inflammatory cytokines, and decreased synthesis of glycosaminoglycans [195, 196]. High doses of GCs are required, and both the oral route and intravenous (iv) route can be used, while locally administered GCs are poorly effective [1]. RCTs have demonstrated that the iv route is more effective and better tolerated than the oral route [197, 198]. A proof‐of‐principle demonstration of ivGC efficacy was provided by an RCT showing favorable responses in 83% of patients treated with ivGCs versus 11% of placebo‐treated patients [199]. The small‐sized study was stopped before the end of recruitment because the difference between treatment and placebo groups was so striking that it was deemed unethical to leave patients untreated [199]. Higher efficacy of the iv route was confirmed by a systematic review and meta‐analysis [200] and an overall response rate of about 80% was reported in another systematic review [201]. In a large RCT from EUGOGO centers, the overall response rate was lower than that above [202], possibly in relation to the relatively long duration of GO and not very severe clinical features of enrolled patients. A careful selection of patients is needed, because the activity of GO—indicated by CAS—is predictive of a good response to immunosuppressive treatment, whereas a duration of GO longer than 16 months is associated with a poor response [203]. Contraindications to high‐dose systemic GC treatment include recent viral hepatitis, psychiatric disorders, and severe cardiovascular morbidity, while hypertension and diabetes are not absolute contraindications, but should be kept under control before treatment [80]. Oral GC therapy is typically instituted using 50–100 mg/day prednisone and the dose is gradually tapered and withdrawn after 6 months [4]. Most commonly, ivGCs are given as 12 weekly infusions of methylprednisolone (six using 500 mg and six using 250 mg, cumulative dose: 4.5 g) [198]. Higher doses can be used for more severe forms within the spectrum of moderate‐to‐severe GO, but the cumulative dose should not exceed 8 g per cycle, and a single dose should not be >750 mg—with possible exception of DON—to minimize risks of major adverse events [204]. Infusion should be slow (1–2 h) under close surveillance [80]. If facilities are not available, oral GCs represent an acceptable alternative [205]. Response to treatment usually occurs early but may become evident in the second half of the course [206, 207]. Inflammatory signs and symptoms are highly responsive, improving in 59%–83% of cases in a large RCT [202]. In a systematic review of seven RCTs, ocular motility improved in 57% of cases and diplopia improved/disappeared in 64% [201]. Exophthalmos is the least responsive feature, with a mean decrease of 1.14 mm in RCTs and 1.58 mm in non‐RCTs [201]. A limitation of GC treatment is relapse after drug discontinuation, occurring in about 10% of cases [194]. Adverse events are relatively common [208], although usually minor to moderate [201] in severity. The most threatening adverse event is severe hepatotoxicity, more frequent in the past when much higher single dose and cumulative doses (10–24 g) of methylprednisolone were used [209, 210]. In a comprehensive review published more than 10 years ago, overall morbidity was 6.5% and mortality 0.6% (six patients out of 1045, all treated with doses of GCs higher than currently used) [201].
Mycophenolate inhibits proliferation of both T and B cells and antibody and adhesion molecule formation, thereby modulating tissue inflammation. For these actions, mycophenolate mofetil is used to prevent transplant rejection [211] and for autoimmune disorders. Most relevant adverse events of mycophenolate mofetil are gastrointestinal, but enteric‐coated tablets of mycophenolate sodium are better tolerated [212]. One gram of mycophenolate mofetil is equivalent to 720 mg of mycophenolate sodium [212]. Mycophenolate mofetil was used for active moderate‐to‐severe GO in a single‐center RCT from China in which 80 patients were treated with mycophenolate mofetil monotherapy (500 mg twice a day for 24 weeks) and 78 patients with high‐dose GC monotherapy (an unusual regimen of 500 mg iv methylprednisolone for three consecutive days for 2 weeks, followed by oral prednisone, 60 mg starting dose, gradually tapered down and withdrawn at week 24) [213]. Both treatments were effective, but mycophenolate mofetil provided better results than GC monotherapy in terms of improvement of CAS, diplopia, and exophthalmos (mean reduction at 24 weeks: 3.2–3.4 mm vs. 1.9–2.2 mm after GCs). In a retrospective study of 20 patients with moderate‐to‐severe to sight‐threatening GO, mycophenolate mofetil was successfully used as a second‐line treatment in patients previously treated with ivGCs [214]. In a subsequent multicenter European RCT, 81 patients were allocated to receive ivGC monotherapy (cumulative dose: 4.5 g) and 83 patients to receive combined therapy with the same ivGC dose plus mycophenolate sodium (720 mg daily orally for 24 weeks) [215]. Although variation of a composite index of ocular assessment at 12 weeks (end of GC treatment)—indicated as primary outcome—improved significantly in both groups, with no significant difference between groups, analysis at 24 weeks (end of mycophenolate sodium treatment) and 36 weeks (3‐month follow‐up) clearly showed that combination therapy was more effective in terms of overall response and changes of individual features [215]. At variance with the Chinese study, response of exophthalmos was less impressive. Treatment was generally well tolerated, and neither dose reduction nor drug withdrawal because of drug‐related side effects was needed.
Orbital radiotherapy (OR) is a well‐established treatment for GO due to anti‐inflammatory effects and radiosensitivity of lymphocytes infiltrating the orbit [216]. In a double‐blind RCT of 60 patients with active moderate‐to‐severe GO, 30 patients were treated with OR (20 Gy subdivided into 10 doses of 2 Gy) and 29 were sham irradiated [217]. Favorable responses were achieved in 60% of irradiated patients and in only 31% of sham‐irradiated patients [217]. The difference between groups was particularly evident for ocular motility and related diplopia [217]. Another RCT of patients with mild GO confirmed OR effectiveness, particularly in patients whose disease duration was ≤18 months, and, again, especially in the presence of ocular dysmotility and diplopia [218]. A third RCT provided negative results, but this study was limited by a long duration of GO and previous unresponsiveness to GCs, which introduced a serious selection bias [219]. OR effectiveness was also demonstrated in an RCT of 56 patients treated either with oral GCs and sham irradiation or with OR and placebo [220]. Two RCTs investigating a combination of OR and oral GCs versus either oral GCs or OR monotherapy [179, 221] showed that combined treatment was more effective than either treatment alone. A study from the UK reported that adding OR to oral prednisolone did not provide substantial benefits [222], but a recent systematic review and meta‐analysis of published literature showed that OR is effective in selected patients, particularly those with disturbances of ocular motility and in combination with GCs [223]. Combination of OR with ivGCs is more effective than combination of OR with oral GCs [197]. In addition, two retrospective studies have shown that ivGCs combined with OR are more effective than ivGC monotherapy, particularly in improving ocular motility and reducing GO severity [224, 225]. The most common schedule of OR is 10 daily doses of 2 Gy over a 2‐week period, but a weekly dose of 1 Gy for 20 weeks may be equally effective [226]. OR is safe [227, 228] but it should be avoided in patients with hypertensive or diabetic retinopathy because of related microvascular lesions, and it is usually not used in patients younger than 35 years for a remote carcinogenic potential [216].
Cyclosporine is a potent immunosuppressive drug acting on both humoral and cellular immunity. In a single‐center RCT of 40 patients with active moderate‐to‐severe GO, cyclosporine (starting dose: 5–7.5 mg/kg body weight; duration of treatment: 6 months) used in combination with oral prednisone was more effective than prednisone monotherapy and associated with a lower relapse rate [229]. Several cases of a reversible rise in blood pressure and liver enzymes and one case of Klebsiella pneumoniae pneumonia were observed [229]. A second, single‐blind single‐center RCT of 36 patients compared oral prednisone monotherapy and cyclosporine monotherapy (dose: 7.5 mg/kg body weight) over a period of 3 months [230]. Responders were 61% among prednisone‐treated patients and only 22% among cyclosporine‐treated patients [230]. However, when nonresponders of either group were given a second treatment with a combination of the two drugs, around 60% of initial nonresponders significantly improved [230]. Cyclosporine was better tolerated than prednisone, with six cases of reversible hypertension and one case of irreversible rise in the plasma creatinine concentration [230]. No RCTs have investigated the combination of ivGCs and cyclosporine.
Azathioprine is used for GO but its role is uncertain. In an old prospective study (10 patients treated with azathioprine and 10 untreated), azathioprine had no beneficial effects on several ophthalmic parameters [231]. Conversely, in a recent RCT, the addition of azathioprine to oral prednisolone was in post‐hoc analysis associated with an improved outcome compared to oral prednisolone alone, but results were affected by the large number of patients who withdrew from the study because of drug‐related adverse events [222].
Methotrexate has been reported for the management of GO in small and retrospective studies [232, 233, 234], and no sound conclusions can be drawn on its role.
Combination of GCs with other immunosuppressive drugs or therapies is interesting because combined therapy may potentiate the effects of GC monotherapy and have a steroid‐sparing effect [234, 235, 236].
Biological agents
Targeted therapies aimed at intervening on pivotal steps of GO pathogenesis have recently been investigated [194] with promising results, triggering a debate on whether they may replace GCs as first‐line treatment for active moderate‐to‐severe GO [237] (Table 4).
Table 4.
Biological agents investigated in randomized trials for the treatment of active moderate‐to‐severe Graves’ orbitopathy
| Agent | Target | Effectiveness | Safety | Durability | Cost |
|---|---|---|---|---|---|
| Teprotumumab | IGF‐1 receptor | Yes | Long‐term safety unknown | Frequent relapses | Very high |
| Rituximab | CD20‐positive B cells | Conflicting a | Long‐term safety unknown | Yes | High |
| Tocilizumab | IL‐6 receptor | Yes b | Long‐term safety unknown | Unknown | High |
Abbreviations: IGF‐1, insulin‐like growth factor‐1; IL‐6, interleukin‐6.
Two small randomized clinical trials, one with negative results (vs. placebo) and one with positive results (same effectiveness as intravenous glucocorticoids, better durability).
In glucocorticoid‐resistant Graves’ orbitopathy.
Teprotumumab is a fully humanized monoclonal antibody blocking IGF‐1R. Blocking IGF‐1R and its cross‐talk with TSHR may be relevant to arresting GO development/progression [36]. In a first double‐masked, multicenter RCT, 87 patients were enrolled, of whom 76 (39 in the placebo group, 37 in the teprotumumab group) completed the 24‐week intervention [238]. Teprotumumab treatment consisted of eight iv infusions at 3‐week intervals (starting dose: 10 mg/kg bodyweight, then increased to 20 mg/kg bodyweight); the primary composite end point (response) included a CAS reduction ≥2 points and decrease in exophthalmos ≥2 mm [238]. At 24 weeks, 69% of teprotumumab‐treated patients and 20% of placebo‐treated patients had a positive response [238]. The most striking effect was on exophthalmos, with a mean reduction of about 2.5 mm (compared to 0.15 mm in the placebo group), and 40% of patients showing a decrease of ≥4 mm [238]. Positive results in the teprotumumab group included CAS reduction averaging 3.4 points, with 69% of patients showing a CAS of 0–1 point at week 24 (vs. 21% in the placebo group) [238]. The most severe grades of diplopia (inconstant and constant) were present at baseline in 52% of patients but persisted at week 24 in 31% of patients. Likewise, improvement in the quality of life occurred, less impressively, in both visual functioning and appearance subscales [238]. A subsequent double‐masked, placebo‐controlled phase 3 multicenter RCT from the same investigators (OPTIC trial, 41 patients treated with teprotumumab, 42 with placebo) had as the primary outcome a decrease in exophthalmos ≥2 mm [178]. Response to teprotumumab was observed in 83% versus 10% of placebo‐treated patients and secondary outcomes (CAS, diplopia score, quality of life) were significantly better in the teprotumumab group [178]. These results in patients with active moderate‐to‐severe GO convinced the US Food and Drug Administration to approve teprotumumab in January 2020 for GO treatment, irrespective of grade (mild, moderate, sight threatening) or state (active, inactive). To assess the durability of therapeutic response, the same investigators performed a pooled data analysis of the two studies and an off‐treatment follow‐up [239]. At a longer follow‐up (72 weeks), exophthalmos relapsed in 33% of patients, diplopia in 31%, and using a composite overall evaluation, 17% could no longer be considered responders [239]. In a recent study (OPTIC‐X), OPTIC nonresponders or those who flared (37 in placebo group and 14 in the teprotumumab group) were treated or retreated with teprotumumab, and a response was achieved in the majority of cases [240]. It should be noted that the cost of a teprotumumab course is exceedingly high, in the order of a few hundred thousand US dollars, compared to a few hundred US dollars for ivGCs. In addition, teprotumumab has not been approved by the European Medicines Agency. Another relevant issue, as with any novel drug, is safety. In the two registration studies, teprotumumab was relatively safe, although several adverse events, usually mild to moderate—including muscle spasms, diarrhea, and hyperglycemia—were reported [178, 238]. In the post‐marketing period, one case of amyloid encephalopathy [241] and two cases of new inflammatory bowel disease (ulcerative colitis) [242, 243] have been described. Even more worrisome is the report of permanent sensorineural hearing loss, so far described in 13 of 190 patients from five series [244]. This warrants a careful identification of risk factors and careful on‐treatment and post‐treatment monitoring of patients treated with teprotumumab. In addition, for the time being it seems advisable to limit the use of teprotumumab to active moderate‐to‐severe GO, where it is evidence‐based, and the risk–benefit ratio is reasonable [244]. Finally, head‐to‐head comparison of ivGCs and teprotumumab in a large multicenter RCT in the same clinical setting is warranted and cannot be bypassed.
Rituximab is a chimeric mouse–human monoclonal antibody targeting CD20‐positive B cells; its immunosuppressive action is related to B‐cell depletion [245], although it also causes a decrease of IGF‐1R+ T cells [246]. Rituximab was initially reported in small and uncontrolled studies to be beneficial for active moderate‐to‐severe GO, especially when resistant to GC treatment [247, 248, 249]. Then, two small single‐center RCTs were published—from the Mayo Clinic in the United States and from Italy [249, 250]. In the Mayo Clinic RCT, 25 patients were randomized to receive either rituximab (two 1000‐mg infusions at a 2‐week interval) or saline. The primary outcome was CAS change at 24 weeks, and secondary outcomes were changes in several ocular parameters and quality of life. Eleven patients in the rituximab group and 10 in the placebo group completed the study [250]. This study failed to find significant differences in treatment outcomes between groups [250]. In the Italian RCT, 32 patients were randomized to receive either ivGCs (cumulative dose, 7.5 g) or rituximab (two 1000‐mg infusions at a 2‐week interval for the first 12 patients, then a single 500‐mg infusion for the last four patients). The primary outcome was a decrease in CAS ≥2 points or disease inactivation (final CAS <3) [251]. At 24 weeks, 100% of rituximab‐treated patients versus 69% of GC‐treated patients improved, and no patient in the rituximab group had subsequent GO reactivation, observed in one third of GC‐treated patients [251]. An attempt was made to reconcile these conflicting results, which might somehow be explained by longer GO duration in the Mayo Clinic study, making patients less responsive [252]. In the above studies, DON occurred in two patients and vasculitis in one patient treated with rituximab [250], and severe cytokine release syndrome (severe periorbital swelling and decreased vision)—reverted by ivGCs—in two patients [251]. A retrospective multicenter nationwide French study reported an efficacy of rituximab as intermediate between the two RCTs [253]. A meta‐analysis of 12 studies (152 patients) concluded that rituximab has a beneficial effect on CAS (inflammation) and limited efficacy on exophthalmos [254]. Recently, a small and uncontrolled study of 17 GC‐resistant patients reported that, after a single 100‐mg rituximab infusion, GO inactivation occurred in more than 90% of patients, and severity improved in about 60%, with no flares [255]. To summarize, evidence from the small RCTs is conflicting, and larger multicenter RCTs are needed. For the time being, rituximab may represent a valid option as a second‐line treatment for active moderate‐to‐severe GO.
Tocilizumab is a humanized monoclonal antibody targeting interleukin‐6 (IL‐6) receptor, approved for treatment of rheumatoid arthritis. IL‐6 is a proinflammatory cytokine overexpressed in orbital fibroblasts [256, 257] and capable, among other actions, of stimulating TSHR expression in human orbital preadipocyte GO fibroblasts [258]. Following a few small uncontrolled studies [259, 260, 261], in a small placebo‐controlled RCT, 32 GC‐resistant GO patients were given either iv tocilizumab (8 mg/kg bodyweight, four doses at a 4‐week interval) or placebo [262]. Tocilizumab‐treated patients had a significantly higher rate of GO inactivation, but a surprisingly high rate of inactivation was also observed in placebo‐treated patients [262], suggesting that improvement may partially reflect a late effect of previous GC treatment or GO natural history. Tocilizumab had limited efficacy on exophthalmos and diplopia [262]; it was well tolerated, but there was a higher rate of infections and headache [262]. Other small uncontrolled or retrospective studies seem to confirm tocilizumab efficacy, particularly in GC‐resistant GO [263, 264, 265], but larger multicenter RCTs are warranted to better define its role. For the time being, tocilizumab may be considered as a second‐line treatment for active moderate‐to‐severe GO, particularly if refractory to ivGCs.
Approach to the management of active moderate‐to‐severe GO
High‐dose GCs, preferably given intravenously, currently represent the mainstay of treatment for active moderate‐to‐severe GO. Recently published EUGOGO guidelines recommend using iv methylprednisolone (cumulative dose: 4.5 g) in combination with mycophenolate sodium (720 mg daily for 24 weeks), while for most severe forms of GO (with inconstant/constant diplopia) within the spectrum of moderate‐to‐severe GO, a higher dose of ivGC monotherapy (cumulative dose: 7.5 g) is recommended, because evidence on using the highest dose of methylprednisolone in combination with mycophenolate is lacking [80] (Fig. 5). For the time being, the guidelines do not consider teprotumumab as an alternative first‐line treatment—despite its efficacy—because of the current lack of data on its long‐term efficacy and safety, its exceedingly high cost, and its unavailability outside the United States [80].
Fig. 5.
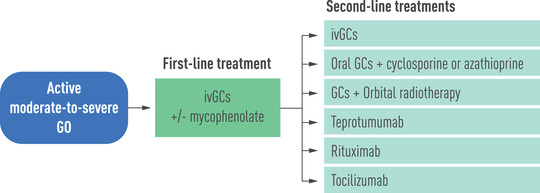
First‐line and second‐line treatments for active moderate‐to‐severe Graves’ orbitopathy, according to the European Group on Graves’ Orbitopathy clinical practice guidelines [80]. GCs, glucocorticoids; ivGCs, intravenous glucocorticoids.
If response to first‐line treatment is partial or absent, second‐line treatments include: (i) a second course of ivGCs, using the highest cumulative dose (7.5 g), (ii) oral GCs combined with either cyclosporine or azathioprine, (iii) OR combined with either oral or iv GCs, (iv) teprotumumab, (v) rituximab, and (vi) tocilizumab [80] (Fig. 5). Clearly, due to rapid progress in this field, these recommendations might need to be revised in a few years.
When GO has been inactive at least 6–12 months, rehabilitative surgeries—such as orbital decompression, squint surgery, and eyelid surgery—can be performed, if needed or required by the patient, following the above order. Patient choice, within a thorough shared decision‐making process, is of the utmost importance at this stage as well as in the choice of first‐ and second‐line medical treatments.
Management of sight‐threatening GO
Sight‐threatening GO, due to corneal breakdown or DON, is an emergency condition warranting immediate treatment. The corneal breakdown may be related to severe corneal exposure in the case of severe exophthalmos and/or lagophthalmos (incomplete eyelid closure at night). This condition requires intensive local care, using gluing, antibiotics, blepharorrhaphy, tarsorrhaphy, and lid lengthening.
In a small RCT of DON, 15 patients were randomized to immediate orbital decompression or ivGC treatment [266]. Five of nine patients treated medically did not need subsequent decompression, whereas all six patients submitted to an initial surgical treatment then required immunosuppressive treatment [266]. There was no better outcome in patients treated with immediate surgery [266]. In a retrospective study, 40% of patients initially treated with ivGCs experienced a permanent normalization of visual acuity [267]. Accordingly, current guidelines recommend the following treatment schedule: (i) administer iv methylprednisolone 500–1000 mg for three consecutive days or on alternate days, (ii) repeat the cycle the next week, (iii) in the case of a good response, continue with weekly infusion as for active moderate‐to‐severe GO, and (iv) in the case of an absent/poor response, submit the patient to urgent orbital decompression [80].
Treatment of hyperthyroidism in patients with GO
Graves’ hyperthyroidism can be treated by ATDs, RAI, or thyroidectomy [268]. Neither ATDs nor thyroidectomy directly modify GO natural history [90, 91, 269]. Long‐term ATD treatment‐associated gradual decrease in serum TSHR‐Ab levels may be beneficial for GO. A small RCT [270] and a retrospective case study [271] reported that early thyroidectomy may result in a more favorable outcome of immunosuppressive therapy. As discussed, RAI bears a small but definite risk for GO [90, 91, 97], preventable by steroid prophylaxis [80]. Total thyroid ablation (thyroidectomy followed by RAI treatment) may be beneficial in the short run, but not in the long [272, 273, 274].
Given these premises, the optimal treatment for hyperthyroidism in patients with GO remains a dilemma. If GO—either mild or moderate to severe—is stable and longstanding as inactive, any treatment for hyperthyroidism can be chosen, as it is unlikely to cause GO recurrence/progression [80]. In patients with residual but longstanding inactive moderate‐to‐severe GO, steroid prophylaxis can be considered if RAI treatment is selected and risk factors are still present [80]. If GO is active and mild, ATD treatment (or thyroidectomy) is preferred, but RAI treatment can also be selected, with steroid prophylaxis [80]. In the presence of active moderate‐to‐severe GO, treatment of GO should be prioritized, as delayed treatment is associated with a worse response to immunosuppressive treatment [80]. While GO is being treated, hyperthyroidism is controlled by ATD treatment, which can be continued for several years until GO is cured [275, 276]. If GO is sight threatening, its emergency treatment—either medical and/or surgical—is an absolute priority. Hyperthyroidism is stabilized by ATDs until treatment of GO is completed [80].
Conclusions and perspectives
Recent years have witnessed the development of novel therapies targeting different crucial steps/sites for GO development: B cells as antigen‐presenting cells and autoantibody producers (rituximab), IGF‐1R (teprotumumab), and IL‐6 receptor (tocilizumab). The results are certainly promising, for some drugs more than for others, but they need to be corroborated by larger studies. And, given the complexity of the autoimmune orbital process, other biological agents—such as tumor necrosis factor α inhibitors (etanercept, infliximab, adalimumab, belimumab)—interfering with a cytokine cascade might prove to be effective.
Iscalimab—a monoclonal antibody targeting CD40 (found on the surface of antigen‐presenting cells and thyrocytes), thus interrupting CD40 interaction with its ligand (CD154) and, thereby, abating autoimmune response—was used in a phase II study of 15 Graves’ patients with normalization of thyroid status and decrease in serum TSHR‐Ab levels in 47% [277]. In view of its mechanism of action, this agent might in the future prove beneficial for GO as well.
As loss of tolerance to TSHR is central in the pathogenesis of both Graves’ hyperthyroidism and GO, antigen‐specific immunotherapy is a fascinating goal. Retolerization by repeated injections of small and increasing amounts of synthetic peptides based on a sequence of TSHR (ATX‐GD‐59) restored euthyroidism in five of 10 patients in a phase I study [278]. If these preliminary results are confirmed in larger studies, it is conceivable that this approach might also be useful for GO.
Small molecule/peptides binding to transmembrane portions of TSHR and, thereby, antagonizing signaling induced by TSHR‐Ab may represent a future treatment for both hyperthyroidism and GO, but studies are thus far preclinical [279, 280, 281].
A blocking TSHR‐Ab, K1‐70, effectively blocked a stimulating monoclonal TSHR‐Ab, M22, in an animal model [282]. K1‐70 was administered to a 51‐year‐old woman affected by Graves’ disease associated with high stimulatory TSHR‐Ab levels, GO, and metastatic differentiated thyroid cancer [283]. Following K1‐70 administration, serum‐blocking TSHR activity increased, and this was associated with an improvement of GO and stabilization of metastatic lesions (during an interval of lenvatinib therapy) [283]. More recently, in an open‐label phase I study, 18 Graves’ patients—euthyroid or hyperthyroid under ATD treatment—were treated with a single shot of either intramuscular or iv K1‐70 [284]. Patients were allocated to six cohorts of three patients each and given increasing doses of K1‐70 (0.25–150 mg) and followed for 100 days [284]. On day 28, most patients—especially in the high‐dose cohorts—progressed to a hypothyroid state, with improvement of symptoms that persisted in many instances at the end of follow‐up [284]. Interestingly, two patients with associated GO experienced improvement of GO symptoms and signs, including a marked reduction in exophthalmometer readings [284]. These results, although preliminary, suggest that K1‐70 might represent a TSHR‐specific drug with a potential for managing both Graves’ hyperthyroidism and GO.
Conflict of interest
The authors declare there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. This paper was not funded.
Acknowledgments
Open Access Funding provided by Universita degli Studi dell'Insubria within the CRUI‐CARE Agreement.
[Correction added on 30 June 2022, after first online publication: Projekt CRUI‐CARE funding statement has been added.]
Bartalena L, Tanda ML. Current concepts regarding Graves’ orbitopathy. J Intern Med. 2022;292:692–716.
References
- 1. Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest. 2014;37:691–700. [DOI] [PubMed] [Google Scholar]
- 2. Wiersinga WM. Quality of life in Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:359–70. [DOI] [PubMed] [Google Scholar]
- 3. Ponto KA, Merkesdal S, Hommel G, Pitz S, Pfeiffer N, Kahaly GJ. Public health relevance of Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98:145–52. [DOI] [PubMed] [Google Scholar]
- 4. Bartalena L, Pinchera A, Marcocci C. Management of Graves’ ophthalmopathy: reality and perspectives. Endocr Rev. 2002;21:168–99. [DOI] [PubMed] [Google Scholar]
- 5. Abraham‐Nordling M, Bystrom K, Torring O, Lantz M, Berg G, Calissendorff J, et al. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011;165:899–905. [DOI] [PubMed] [Google Scholar]
- 6. Laurberg P, Berman BC, Bulow Pedersen I, Andersen S, Carlé A. Incidence and presentation of moderate to severe Graves’ orbitopathy in a Danish population before and after iodine fortification of salt. J Clin Endocrinol Metab. 2012;97:2325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartalena L, Piantanida E, Gallo D, Lai A, Tanda ML. Epidemiology, natural history, risk factors, and prevention of Graves’ orbitopathy. Front Endocrinol. 2020;11:615993. 10.3389/fendo.2020.615993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Trans Am Ophthalmol Soc. 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 9. Perros P, Hegedus L, Bartalena L, Marcocci C, Kahaly GJ, Baldeschi L, et al. Graves’ orbitopathy as a rare disease in Europe: a European Group on Graves’ Orbitopathy (EUGOGO) position statement. Orphanet J Rare Dis. 2017;12:72. 10.1186/s13023-017-0625-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chin YH, Ng CH, Lee MH, Koh JWH, Kiew J, Yang SP, et al. Prevalence of thyroid eye disease in Graves’ disease: a meta‐analysis and systematic review. Clin Endocrinol. 2020;93:363–74. [DOI] [PubMed] [Google Scholar]
- 11. Ippolito S, Cusini C, Lasalvia P, Gianfagna F, Veronesi G, Gallo D, et al. Change in newly diagnosed Graves’ disease phenotype between the twentieth and the twenty‐first centuries: meta‐analysis and meta‐regression. J Endocrinol Invest. 2021;44:1707–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanda ML, Piantanida E, Liparulo L, Veronesi G, Lai A, Sassi L, et al. Prevalence and natural history of Graves’ orbitopathy in a large series of newly diagnosed Graves’ hyperthyroidism seen at a single center. J Clin Endocrinol Metab. 2013;98:1443–9. [DOI] [PubMed] [Google Scholar]
- 13. Piantanida E, Tanda ML, Sassi L, Lai A, Bartalena L. Prevalence and natural history of Graves’ orbitopathy in the XXI century. J Endocrinol Invest. 2013;36;444–9. [DOI] [PubMed] [Google Scholar]
- 14. Perros P, Zarkovic M, Azzolini C, Ayvaz G, Baldeschi L, Bartalena L, et al. PREGO (presentation of Graves’ orbitopathy) study: changes in referral patterns to European Group on Graves’ Orbitopathy (EUGOGO) centres over the period from 2000 to 2012. Br J Ophthalmol. 2015;99:1531–5. [DOI] [PubMed] [Google Scholar]
- 15. Burch HB, Wartofsky L. Graves’ ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev. 1993;14:747–93. [DOI] [PubMed] [Google Scholar]
- 16. Wiersinga WM. Autoimmunity in Graves’ ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF‐1 receptors? J Clin Endocrinol Metab. 2011;96:2386–94. [DOI] [PubMed] [Google Scholar]
- 17. Feliciello A, Porcellini A, Ciullo I, Bonavolontà G, Avvedimento EV, Fenzi G. Expression of thyrotropin‐receptor mRNA in healthy and Graves’ disease retro‐orbital tissue. Lancet. 1993;342:337–8. [DOI] [PubMed] [Google Scholar]
- 18. Crisp MS, Lane C, Halliwell M, Wynford‐Thomas D, Ludgate M. Thyrotropin receptor transcripts in human adipose tissue. J Clin Endocrinol Metab. 1997;82:2003–5. [PubMed] [Google Scholar]
- 19. Boschi A, Daumerie C, Spiritus M, Beguion C, Senous M, Yuksel D, et al. Quantification of cells expressing the thyrotropin receptor in extraocular muscles in thyroid associated orbitopathy. Br J Ophthalmol. 2005;89:724–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hai YP, Lee ACH, Frommer L, Diana T, Kahaly GJ. Immunohistochemical analysis of human orbital tissue in Graves’ orbitopathy. J Endocrinol Invest. 2020;43:123–37. [DOI] [PubMed] [Google Scholar]
- 21. Wakelkamp IM, Bakker O, Baldeschi L, Wiersinga WM, Prummel MF. TSH‐receptor expression and cytokine profile in orbital tissue of active vs. inactive Graves’ ophthalmopathy patients. Clin Endocrinol. 2003;58:280–7. [DOI] [PubMed] [Google Scholar]
- 22. Zhang L, Baker G, Janus D, Paddon CA, Fuhrer D, Ludgate M. Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest Ophthalmol Vis Sci. 2006;47:5197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kumar S, Nadeem S, Stan MN, Coenen M, Ludgate M. A stimulatory TSH receptor antibody enhances adipogenesis via phosphoinositide 3‐kinase activation in orbital preadipocytes from patients with Graves’ ophthalmopathy. J Mol Endocrinol. 2011;46:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moshkelgosha S, So PW, Deasy N, Diaz‐Cano S, Banga JP. Cutting edge: retrobulbar inflammation, adipogenesis, and acute orbital congestion in a preclinical female mouse model of Graves’ orbitopathy induced by thyrotropin receptor plasmid‐in vivo electroporation. Endocrinology. 2013;154:3008–15. [DOI] [PubMed] [Google Scholar]
- 25. Holthoff HP, Goebel S, Li Z, Fassbender J, Reimann A, Zeibig S, et al. Prolonged TSH receptor A subunit immunization of female mice leads to a long‐term model of Graves’ disease, tachycardia, and cardiac hypertrophy. Endocrinology. 2015;156:1577–89. [DOI] [PubMed] [Google Scholar]
- 26. Zhang M, Ding X, Wu LP, He MQ, Zy Chen, Shi BY, et al. A promising mouse model of Graves’ orbitopathy induced by adenovirus expressing thyrotropin receptor a subunit. Thyroid. 2021;31:638–48. [DOI] [PubMed] [Google Scholar]
- 27. Nakahara M, Johnson K, Eckstein A, Taguchi R, Yamada M, Abiru N, et al. Adoptive transfer of antithyrotropin receptor (TSHR) autoimmunity from TSHR knockout mice to athymic nude mice. Endocrinology. 2012;153:2034–42. [DOI] [PubMed] [Google Scholar]
- 28. Gerding MN, Van der Meer JWC, Broenink M, Bakker O, Wiersinga WM, Prummel MF, et al. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol. 2000;52:267–71. [DOI] [PubMed] [Google Scholar]
- 29. Lytton SD, Ponto KA, Kanitz M, Matheis N, Kohn LD, Kahaly GJ. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab. 2010;95:2123–31. [DOI] [PubMed] [Google Scholar]
- 30. Nicolì F, Lanzolla G, Mantuano M, Ionni I, Mazzi B, Leo M, et al. Correlation between serum anti‐TSH receptor autoantibodies (TRAbs) and the clinical feature of Graves’ orbitopathy. J Endocrinol Invest. 2021;44:581–5. [DOI] [PubMed] [Google Scholar]
- 31. Saric Matutinovic M, Diana T, Nedelikovic Beleslin B, Ciric J, Zarkovic M, Kahaly GJ, et al. Clinical value of functional receptor antibodies in Serbian patients with Graves’ orbitopathy. J Endocrinol Invest. 2022;45:189–97. [DOI] [PubMed] [Google Scholar]
- 32. Eckstein AK, Plicht M, Lax H, Neuhauser M, Mann K, Lederbogen S, et al. Thyrotropin receptor autoantibodies are indepedent risk factors for Graves’ ophthalmopathy and help to predict severity and outcome of the disease. J Clin Endocrinol Metab. 2006;91:3464–70. [DOI] [PubMed] [Google Scholar]
- 33. Diana T, Ponto KA, Kahaly GJ. Thyrotropin receptor antibodies and Graves’ orbitopathy. J Endocrinol Invest. 2021;44:703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. George A, Diana T, Langericht J, Kahaly GJ. Stimulatory thyrotropin receptor antibodies are a biomarker for Graves’ orbitopathy. Front Endocrinol. 2021;11:629925. 10.3389/fendo.2020.629925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith TJ. Insulin‐like growth factor pathway and the thyroid. Front Endocrinol. 2021;12:653627. 10.3389/fendo.2021.653627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neag EJ, Smith TJ. 2021 update on thyroid‐associated ophthalmopathy. J Endocrinol Invest. 2022;45:235–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the insulin‐like growth factor‐1 receptor pathway. J Immunol. 2003;170:6348–54. [DOI] [PubMed] [Google Scholar]
- 38. Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, et al. B cells from patients with Graves’ disease aberrantly express the IGF‐1 receptor: implications for disease pathogenesis. J Immunol. 2008;181:5768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin‐like growth factor‐1 receptor by T cells from patients with Graves’ disease may carry functional consequences for disease pathogenesis. J Immunol. 2007;178:3281–7. [DOI] [PubMed] [Google Scholar]
- 40. Smith TJ, Hoa N. Immunoglobulin from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self‐antigen, insulin‐like growth factor‐1 receptor. J Clin Endocrinol Metab. 2004;89:5076–80. [DOI] [PubMed] [Google Scholar]
- 41. Smith TJ, Janssen AJ. Insulin‐like growth factor‐1 receptor and thyroid‐associated ophthalmopathy. Endocr Rev. 2016;40:236–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Hikim AS, et al. Evidence for an association between thyroid‐stimulating hormone and insulin‐like growth factor‐1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181:4397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krieger CC, Morgan SJ, Neumann S, Gershengorn MC. Thyroid stimulating hormone (TSH)/insulin‐like growth factor 1 (IGF1) receptor cross‐talk in human cells. Curr Opin Endocr Metab Res. 2018;2:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Krieger CC, Sui X, Kahaly GJ, Neumann S, Gershengorn MC. Inhibition of TSH/IGF‐1 receptor crosstalk by teprotumumab as a treatment modality of thyroid eye disease. J Clin Endocrinol Metab. 2021. 10.1210/clinem/dgab824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weightman DR, Perros P, Sherif IH, Kendall‐Taylor P. Autoantibodies to IGF‐1 binding sites in thyroid‐associated ophthalmopathy. Autoimmunity. 1993;16:251–7. [DOI] [PubMed] [Google Scholar]
- 46. Minich WB, Dehina N, Welsink T, Schwiebert C, Morgenthaler NG, Kohrle J, et al. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98:752–60. [DOI] [PubMed] [Google Scholar]
- 47. Varewijck AJ, Boelen A, Lamberts SW, Fliers E, Hofland LJ, Wiersinga WM, et al. Circulating IgGs may modulate IGF‐1 receptor stimulating activity in a subset of patients with Graves’ ophthalmopahy. J Clin Endocrinol Metab. 2013;98:769–76. [DOI] [PubMed] [Google Scholar]
- 48. Lanzolla G, Ricci D, Nicolì F, Sabini E, Sframeli A, Brancatella A, et al. Putative role of autoantibodies against the insulin‐like growth factor‐1 receptor in Graves’ disease: results of a pilot study. J Endocrinol Invest. 2020;43:1759–68. [DOI] [PubMed] [Google Scholar]
- 49. Smith TJ, Hegedus L. Graves’ disease. N Engl J Med. 2016;375:1552–65. [DOI] [PubMed] [Google Scholar]
- 50. Fernando R, Atkins S, Raychauduri N, Lu Y, Douglas RS, Smith TJ. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109:7427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khamisi S, Lundqvist M, Emadi P, Almby K, Ljunggren O, Karlsson FA. Serum thyroglobulin is associated with orbitopathy in Graves’ disease. J Endocrinol Invest. 2021;44:1905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rotondo Dottore G, Torregrossa L, Caturegli P, Ionni I, Sframeli A, Sabini E, et al. Association of T and B cells infiltrating orbital tissues with clinical features of Graves orbitopathy. JAMA Ophthalmol. 2018;136:613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rotondo Dottore G, Torregrossa L, Lanzolla G, Mariotti S, Menconi F, Piaggi P, et al. Role of the mononuclear cell infiltrate in Graves’ orbitopathy (GO): results of a large cohort study. J Endocrinol Invest. 2021. 10.1007/s40618-021-01692-4 [DOI] [PubMed] [Google Scholar]
- 54. Lantz M, Vondrichova D, Parikh H, Frenander C, Ridderstrale M, Asman P, et al. Overexpression of immediate early genes in active Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2005;90:4784–91. [DOI] [PubMed] [Google Scholar]
- 55. Ezra DG, Krell J, G Rose, Bailly M, Stebbing J, Castellano M. Transcriptome‐level microarray expression profiling implicates IGF‐1 and Wnt signalling dysregulation in the pathogenesis of thyroid‐associated orbitopathy. J Clin Pathol. 2012;65:608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Matheis N, Lantz M, Grus FH, Ponto KA, Wolters D, Brorson H, et al. Proteomics of orbital tissue in thyroid‐associated orbitopathy. J Clin Endocrinol Metab. 2015;100:e1523–30. [DOI] [PubMed] [Google Scholar]
- 57. Rotondo Dottore G, Bucci I, Lanzolla G, Dallan I, Sframeli A, Torregrossa L, et al. Genetic profiling of orbital fibroblasts from patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2021;106:e2176–90. [DOI] [PubMed] [Google Scholar]
- 58. Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362:726–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rivkees SA. Controversies in the management of Graves’ disease in children. J Endocrinol Invest. 2016;39:1247–57. [DOI] [PubMed] [Google Scholar]
- 60. Manji N, Carr Smith JD, Boelaert K, Allahabadia A, Armitage M, Chatterjee VK, et al. Influence of age, gender, smoking, and family history on autoimmune thyroid disease phenotype. J Clin Endocrinol Metab. 2006;91:4873–80. [DOI] [PubMed] [Google Scholar]
- 61. Perros P, Crombie AL, Matthews JN, Kendall‐Taylor P. Age and gender influence the severity of thyroid‐associated ophthalmopathy: a study of 101 patients attending a thyroid‐eye clinic. Clin Endocrinol. 1993;38:367–72. [DOI] [PubMed] [Google Scholar]
- 62. Lee MH, Chin YH, Ng CH, Nistala KRY, Ow ZGW, Sundar G, et al. Risk factors of thyroid eye disease. Endocr Pract. 2021;27:245–53. [DOI] [PubMed] [Google Scholar]
- 63. Brix TH, Hegedus L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol. 2012;76:457–64. [DOI] [PubMed] [Google Scholar]
- 64. Villanueva R, Greenberg DA, Davies TF, Tomer Y. Sibling recurrence risk in autoimmune thyroid disease. Thyroid. 2003;13:761–4. [DOI] [PubMed] [Google Scholar]
- 65. Marinò M, Latrofa F, Menconi F, Chiovato L, Vitti P. Role of genetic and non‐genetic factors in the etiology of Graves’ disease. J Endocrinol Invest. 2015;38:283–94. [DOI] [PubMed] [Google Scholar]
- 66. Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yin X, Latif R, Bahn R, Davies TF. Genetic profiling in Graves’ disease: further evidence for lack of a distinct genetic contribution to Graves’ orbitopathy. Thyroid. 2012;22:730–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves’ disease: a population‐based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86:930–4. [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, Ma X‐M, Wang X, Sun X, Wang L‐J, Li X‐Q, et al. Emerging insights into the role of epigenetics and gut microbiome in the pathogenesis of Graves’ ophthalmopathy. Front Endocrinol. 2022;12:788535. 10.3389/fendo.2021.788535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shi T‐T, Xin Z, Hua L, Wang H, Zhao R‐X, Yang Y‐L, et al. Comparative assessment of gut microbial composition and function in patients with Graves’ disease and Graves’ orbitopathy. J Endocrinol Invest. 2021;44:297–310. [DOI] [PubMed] [Google Scholar]
- 71. Moshkelgosha S, Verhasselt HD, Masetti G, Covelli D, Biscarini F, Hortsmann M, et al. Modulating gut microbiota in a mouse model of Graves’ orbitopathy and its impact on induced disease. Microbiome. 2021;9:45. 10.1186/s40168-020-00952-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Masetti G, Ludgate M. Microbiome and Graves’ orbitopathy. Eur Thyroid J. 2020;9(suppl 1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bartalena L. Prevention of Graves’ ophthalmopathy. Best Pract Res Clin Endocrinol Metab. 2012;26:371–9. [DOI] [PubMed] [Google Scholar]
- 74. Bartalena L, Martino E, Marcocci C, Bogazzi F, Panicucci M, Velluzzi F, et al. More on smoking habits and Graves’ ophthalmopathy. J Endocrinol Invest. 1989;12:733–7. [DOI] [PubMed] [Google Scholar]
- 75. Prummel MF, Wiersinga WM. Smoking and risk of Graves’ disease. JAMA. 1993;269:479–82. [PubMed] [Google Scholar]
- 76. Bartalena L, Marcocci C, Tanda ML, Manetti L, Dell'Unto E, Bartolomei MP, et al. Cigarette smoking and treatment outcomes in Graves’ ophthalmopathy. Ann Intern Med. 1998;129:632–5. [DOI] [PubMed] [Google Scholar]
- 77. Eckstein A, Quadbeck P, Mueller G, Rettenmeier AW, Hoermann R, Mann K, et al. Impact of smoking on the response to treatment of thyroid associated ophthalmopathy. Br J Ophthalmol. 2003;87:773–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pfeilschifter J, Ziegler R. Smoking and endocrine ophthalmopathy: impact of smoking severity and current versus lifetime cigarette consumption. Clin Endocrinol. 1996;45:477–81. [DOI] [PubMed] [Google Scholar]
- 79. Krassas GE, Segni M, Wiersinga WM. Childhood Graves’ ophthalmopathy: results of a European questionnaire study. Eur J Endocrinol. 2005;153:515–21. [DOI] [PubMed] [Google Scholar]
- 80. Bartalena L, Kahaly GJ, Baldeschi L, Dayan CM, Eckstein A, Marcocci C, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur J Endocrinol. 2021;185:G43–67. [DOI] [PubMed] [Google Scholar]
- 81. Bartalena L, Tanda ML. Graves’ ophthalmopathy. N Engl J Med. 2009;360:994–1001. [DOI] [PubMed] [Google Scholar]
- 82. Kahaly GJ. Management of Graves thyroidal and extrathyroidal disease: an update. J Clin Endocrinol Metab. 2020;105:3704–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eckstein AK, Losch C, Glowacka D, Schott M, Mann K, Esser J, et al. Euthyroid and primarily hypothyroid patients develop milder and significantly more asymmetrical Graves ophthalmopathy. Br J Ophthalmol. 2009;93:1052–6. [DOI] [PubMed] [Google Scholar]
- 84. Ponto KA, Binder H, Diana T, Matheis N, Otto AF, Pitz S, et al. Prevalence, phenotype, and psychological well being in euthyroid/hypothyroid thyroid associated orbitopathy. Thyroid. 2015;25:942–8. [DOI] [PubMed] [Google Scholar]
- 85. Prummel MF, Wiersinga WM, Mourits MP, Koornneef L, Berghout A, van der Gaag R. Amelioration of eye changes of Graves’ ophthalmopathy by achieving euthyroidism. Acta Endocrinol (Copenh). 1989;121(Suppl 2):185–9.2549753 [Google Scholar]
- 86. Prummel MF, Wiersinga WM, Mourits MP, Koornneef L, Berghout A, van der Gaag R. Effect of abnormal thyroid function on the severity of Graves’ ophthalmopathy. Arch Intern Med. 1990;150:1098–101. [PubMed] [Google Scholar]
- 87. Karlsson F, Westermark K, Dahlberg PA, Jansson R, Enoksson P. Ophthalmopathy and thyroid stimulation. Lancet. 1989;2(8664):691. 10.1016/s0140-6736(89)90945-8 [DOI] [PubMed] [Google Scholar]
- 88. Kahaly GJ, Diana T, Glang J, Kanitz M, Pitz S, Konig J. Thyroid stimulating antibodies are highly prevalent in Hashimoto's thyroiditis and associated orbitopathy. J Clin Endocrinol Metab. 2016;101:1998–2004. [DOI] [PubMed] [Google Scholar]
- 89. Kahaly GJ, Bartalena L, Hegedus L, Leenhardt L, Poppe K, Pearce SH. 2018 European Thyroid Association guideline for the management of Graves’ hyperthyroidism. Eur Thyroid J. 2018;7:167–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tallstedt L, Lundell G, Torring O, Wallin G, Ljunggren JG, Blomgren H, et al. Occurrence of ophthalmopathy after treatment for Graves’ hyperthyroidism. N Engl J Med. 1992;326:1733–8. [DOI] [PubMed] [Google Scholar]
- 91. Bartalena L, Marcocci C, Bogazzi F, Manetti L, Tanda ML, Dell'Unto E, et al. Relation between therapy for hyperthyroidism and the course of Graves’ ophthalmopathy. N Engl J Med. 1998;338:73–8. [DOI] [PubMed] [Google Scholar]
- 92. Traisk F, Tallstedt L, Abraham‐Nordling M, Andersson T, Berg G, Calissendorff J, et al. Thyroid‐associated ophthalmopathy after treatment for Graves’ hyperthyroidism with antithyroid drugs or iodine‐131. J Clin Endocrinol Metab. 2009;94:3700–7. [DOI] [PubMed] [Google Scholar]
- 93. Acharya SH, Avenell A, Philip S, Burr J, Bevan JS, Abraham P. Radioiodine therapy (RAI) for Graves’ disease (GD) and the effect on ophthalmopathy: a systematic review. Clin Endocrinol. 2008;69:943–50. [DOI] [PubMed] [Google Scholar]
- 94. Perros P, Kendall‐Taylor P, Neoh C, Frewin S, Dickinson J. A prospective study of the effects of radioiodine therapy for hyperthyroidism in patients with minimally active Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2005;90:5321–3. [DOI] [PubMed] [Google Scholar]
- 95. Vannucchi G, Covelli D, Campi I, Currò N, Dazzi D, Rodari N, et al. Prevention of orbitopathy by oral or intravenous steroid prophylaxis in short duration Graves’ disease patients undergoing radioiodine ablation: a prospective randomized control study. Thyroid. 2019;29:1828–33. [DOI] [PubMed] [Google Scholar]
- 96. Tallstedt L, Lundell G, Blomgren H, Bring J. Does early administration of thyroxine reduce the development of Graves’ ophthalmopathy after radioiodine treatment? Eur J Endocrinol. 2008;130:494–7. [DOI] [PubMed] [Google Scholar]
- 97. Bartalena L, Marcocci C, Bogazzi F, Panicucci M, Lepri A, Pinchera A. Use of corticosteroids to prevent progression of Graves’ ophthalmopathy after radioiodine treatment for hyperthyroidism. N Engl J Med. 1989;321:1349–52. [DOI] [PubMed] [Google Scholar]
- 98. Lai A, Sassi L, Compri E, Marino F, Sivelli P, Piantanida E, et al. Lower dose prednisone prevents radioiodine‐associated exacerbation of initially mild or absent Graves’ orbitopathy: a retrospective cohort study. J Clin Endocrinol Metab. 2010;95:1333–7. [DOI] [PubMed] [Google Scholar]
- 99. Shiber S, Stiebel‐Kalish H, Shimon I, Grossman A, Robenshtok E. Glucocorticoid regimens for prevention of Graves’ ophthalmopathy progression following radioiodine treatment: systematic review and meta‐analysis. Thyroid. 2014;24:1515–23. [DOI] [PubMed] [Google Scholar]
- 100. Laurberg P, Wallin G, Tallstedt L, Abraham‐Nordling M, Lundell G, Tørring O. TSH‐receptor autoimmunity in Graves’ disease after therapy with anti‐thyroid drugs, surgery, or radioiodine: a 5‐year prospective randomized study. Eur J Endocrinol. 2008;158:69–75. [DOI] [PubMed] [Google Scholar]
- 101. Khoo DHC, Ho SC, Seah LL, Fong KS, Tal ES, Chee SP, et al. The combination of absent thyroid peroxidase antibodies and high thyroid‐stimulating immunoglobulin levels in Graves’ disease identifies a group at markedly increased risk of ophthalmopathy. Thyroid. 1999;9:1775–80. [DOI] [PubMed] [Google Scholar]
- 102. Goh SH, Ho SC, Seah LL, Fong KS, Khoo DHC. Thyroid autoantibody profile in ophthalmic dominant and thyroid dominant Graves’ disease differ and suggest ophthalmopathy is a multiantigenic disease. Clin Endocrinol. 2004;60:600–7. [DOI] [PubMed] [Google Scholar]
- 103. Bartalena L, Tanda ML, Piantanida E, Lai A. Oxidative stress and Graves’ ophthalmopathy: in vitro studies and therapeutic implications. Biofactors. 2003;19:155–63. [DOI] [PubMed] [Google Scholar]
- 104. Hou T‐Y, Wu S‐B, Kau H‐C, Tsai C‐C. The role of oxidative stress and therapeutic potential of antioxidants in Graves’ ophthalmopathy. Biomedicines. 2021;9:1871. 10.3390/biomedicines9121871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kohrle J. Selenium in endocrinology—selenoproteins‐related diseases, population studies, and epidemiological evidence. Endocrinology. 2021;162:1–14. [DOI] [PubMed] [Google Scholar]
- 106. Rayman MP. Selenium and human health. Lancet. 2012;379:1256–68. [DOI] [PubMed] [Google Scholar]
- 107. Lanzolla G, Marinò M, Marcocci C. Selenium in the treatment of Graves’ hyperthyroidism and eye disease. Front Endocrinol. 2021;11:608428. 10.3389/fendo.2020.608428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Marcocci C, Kahaly GJ, Krassas GE, Bartalena L, Prummel MF, Stahl M, et al. Selenium and the course of mild Graves orbitopathy. N Engl J Med. 2011;364:1920–31. [DOI] [PubMed] [Google Scholar]
- 109. Sabini E, Mazzi B, Profilo MA, Mautone T, Casini G, Rocchi R, et al. High serum cholesterol is a novel risk factor for Graves’ orbitopathy: results of a cross‐sectional study. Thyroid. 2018;28:386–94. [DOI] [PubMed] [Google Scholar]
- 110. Lanzolla G, Sabini E, Profilo MA, Mazzi B, Sframeli A, Rocchi R, et al. Relationship between serum cholesterol and Graves’ orbitopathy (GO): a confirmatory study. J Endocrinol Invest. 2018;41:1417–23. [DOI] [PubMed] [Google Scholar]
- 111. Stein JD, Childers D, Gupta S, Talwar N, Nan B, Lee BJ, et al. Risk factors for developing thyroid‐associated ophthalmopathy among individuals with Graves disease. JAMA Ophthalmol. 2015;133:290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nilsson A, Tsoumani K, Planck T. Statins decrease the risk of orbitopathy in newly diagnosed patients with Graves’ disease. J Clin Endocrinol Metab. 2021;106:1325–32. [DOI] [PubMed] [Google Scholar]
- 113. Naselli A, Moretti D, Regalbuto G, Arpi ML, Lo Giudice F, Frasca F, et al. Evidence that baseline levels of low‐density lipoproteins cholesterol affect the clinical response of Graves’ ophthalmopathy to parenteral corticosteroids. Front Endocrinol. 2020;11:609895. 10.3389/fendo.2020.609895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lanzolla G, Sabini E, Leo M, Menconi F, Rocchi R, Sframeli A, et al. Statins for Graves’ Orbitopathy (STAGO): a phase II, open label, adaptive, single centre, randomized clinical trial. Lancet Diabetes Endocrinol. 2021;9:733–42. [DOI] [PubMed] [Google Scholar]
- 115. Bartalena L, Piantanida E, Tanda ML. Statins for Graves’ orbitopathy: a new tool for prevention and treatment? Lancet Diabetes Endocrinol. 2021;9:726–7. [DOI] [PubMed] [Google Scholar]
- 116. Peng CC‐H, Pearce EN. An update on thyroid disorders in the postpartum period. J Endocrinol Invest. 2022. 10.1007/s40618-022-01762-1 [DOI] [PubMed] [Google Scholar]
- 117. Andersen SL, Olsen J, Carlé A, Laurberg P. Hyperthyroidism incidence fluctuates widely in and around pregnancy and is at variance with some other autoimmune diseases: a Danish population‐based study. J Clin Endocrinol Metab. 2015;100:1164–71. [DOI] [PubMed] [Google Scholar]
- 118. Croce L, Di Dalmazi G, Orsolini F, Virili C, Brigante G, Gianetti E, et al. Graves’ disease and the post‐partum period: an intriguing relationship. Front Endocrinol. 2019;10:853. 10.3389/fendo.2019.00853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rotondi M, Cappelli C, Pirali B, Pirola I, Magri F, Fonte R, et al. The effect of pregnancy on subsequent relapse from Graves’ disease after a successful course of antithyroid drug therapy. J Clin Endocrinol Metab. 2008;93:3985–8. [DOI] [PubMed] [Google Scholar]
- 120. Rotondi M, Capelli V, Coperchini F, Pinto S, Croce L, Tonacchera M, et al. Post‐partum and non‐post‐partum relapsing Graves’ hyperthyroidism display different response to anti‐thyroid drugs. Eur J Endocrinol. 2018;178:589–94. [DOI] [PubMed] [Google Scholar]
- 121. Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves’ disease and ophthalmopathy. Endocr Rev. 2003;24:802–35. [DOI] [PubMed] [Google Scholar]
- 122. Davies TF. Infection and autoimmune thyroid disease. J Clin Endocrinol Metab. 2008;93:674–6. [DOI] [PubMed] [Google Scholar]
- 123. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars‐CoV‐2 infection. J Clin Endocrinol Metab. 2020;105:2367–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ippolito S, Dentali F, Tanda ML. SARS‐CoV‐2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest. 2020;43:1171–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID‐19: the THYRCOV study. Eur J Endocrinol. 2020;183:381–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Asfuroglu Karkan E, Ates I. A case of subacute thyroiditis associated with Covid‐19 infection. J Endocrinol Invest. 2020;43:1173–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Muller I, Cannavaro D, Dazzi D, Covelli D, Mantovani G, Muscatello A, et al. SARS‐CoV‐2‐related atypical thyroiditis. Lancet Diabetes Endocrinol. 2020;8:739–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS‐CoV‐2: an endocrine complication linked to the COVID‐19 pandemic. Hormones (Athens). 2021;20:219–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Brancatella A, Viola N, Rutigliano G, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis during the SARS‐CoV‐2 pandemic. J Endocr Soc. 2021;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Trimboli P, Cappelli C, Croce L, Scappaticcio L, Chiovato L, Rotondi M. COVID‐19‐associated subacute thyroiditis: evidence‐based data from a systematic review. Front Endocrinol. 2021;12:707726. 10.3389/fendo.2021.707726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Duntas LH, Jonklaas J. COVID‐19 and thyroid diseases: a bidirectional impact. J Endocr Soc. 2021;5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, et al. Detection of SARS‐CoV‐2 receptor ACE‐2 mRNA in thyroid cells: a clue for COVID‐19‐related subacute thyroiditis. J Endocrinol Invest. 2021;44:1085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Poma AM, Bonuccelli D, Giannini R, Macerola E, Vignali P, Ugolini C, et al. COVID‐19 autopsy cases: detection of virus in endocrine tissues. J Endocrinol Invest. 2022;45:209–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tanda ML, Ippolito S, Gallo D, Baj A, Novazzi F, Genoni A, et al. SARS‐CoV‐2 detection in primary thyroid sarcoma: coincidence or interaction? J Endocrinol Invest. 2022;45:1059–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Croce L, Gangemi D, Ancona G, Liboà F, Bendotti G, Minelli L, et al. The cytokine storm and thyroid hormone changes in COVID‐19. J Endocrinol Invest. 2021;44:891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Mateu‐Salat M, Urgell E, Chico A. SARS‐Cov‐2 as a trigger for autoimmune disease: report of two cases of Graves’ disease after COVID‐19. J Endocrinol Invest. 2020;43:1527–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jiménez‐Blanco S, Bla‐Peris B, Marazuela M. COVID‐19: a cause of recurrent Graves’ hyperthyroidism? J Endocrinol Invest. 2021;44:387–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lanzolla G, Marcocci C, Marinò M. Graves’ disease and Graves’ orbitopathy following COVID‐19. J Endocrinol Invest. 2021;44:2011–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ippolito S, Gallo D, Rossini A, Patera B, Lanzo N, Fazzino GFM, et al. SARS‐CoV‐2 vaccine associated subacute thyroiditis: insights from a systematic review. J Endocrinol Invest. 2022. 10.1007/s40618-022-01747-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Vera‐Lastra O, Ordinola Navarro A, Cruz Dominguez MP, Medina G, Sanchez Valadez TI, Jara LJ. Two cases of Graves’ disease following SARS‐CoV‐2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;31:1436–9. [DOI] [PubMed] [Google Scholar]
- 141. Pujol A, Gomez L‐A, Gallegos C, Nicolau J, Sanchis P, Gonzalez‐Freire M, et al. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA‐based SARS‐CoV‐2 vaccination: from Graves’ disease to silent thyroiditis. J Endocrinol Invest. 2022;45:875–82. 10.1007/s40618-021-01707-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yamamoto K, Mashiba T, Takano K, Suzuki N, Kami M, Takita M, et al. A case of exacerbation of subclinical hyperthyroidism after first administration of BNT162b2 mRNA COVID‐19 vaccine. Vaccines. 2021;9:1108. 10.3390/vaccines9101108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pla Peris B, Merchante Alfaro AA, Maravall Rojo FJ, Abellan Galiana P, Perez Naranjo S, Gonzalez Boillos M. Thyrotoxicosis following SARS‐COV‐2 vaccination: a case series and discussion. J Endocrinol Invest. 2022;45:1071–7. 10.1007/s40618-022-01739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Golbrisch TJ, Paulson AE, Tashko G, Mekonnen AJ. Graves’ disease following administration of second dose of SARS‐CoV‐2 vaccine. BMJ Case Rep. 2021;14:e246432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of Graves’ disease and type 1 diabetes mellitus following SARS‐CoV‐2 vaccination. J Autoimmun. 2021;125:102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Lui DTW, Lee KK, Lee CH, Lee ACH, Hung IFN, Tan KCB. Development of Graves’ disease after SARS‐CoV‐2 mRNA vaccination: a case report and literaure review. Front Public Health. 2021;9:778964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sriphrapradang C, Shantavasinkul PC. Graves’ disease following SARS‐CoV‐2 vaccination. Endocrine. 2021;74:473–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Weintraub MA, Ameer B, Sinha Gregory N. Graves’ disease following the SARS‐CoV‐2 vaccine: case series. J Invest Med High Impac Case Rep. 2021;9:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pierman G, Delgrange E, Jonas C. Recurrence of Graves’ disease (a Th1‐type cytokine disease) following SARS‐CoV‐2 mRNA vaccine administration: a simple coincidence? Eur J Case Rep Intern Med. 2021. 10.12890/2021_002807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Raven LM, McCormack AI, Greenfield JR. Three cases of subacute thyroiditis following SARS‐CoV‐2 vaccine. J Clin Endocrinol Metab. 2021. 10.1210/clinem/dgab822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Oguz SH, Sendur NH, Iremli BG, Gurlek A, Erbas T, Unluturk U. SARS‐CoV‐2 vaccine‐induced thyroiditis: safety of revaccinations and clinical follow‐up. J Clin Endocrinol Metab. 2022;107:e1823–34. 10.1210/clinem/dgac049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Di Filippo L, Castellino L, Giustina A. Occurrence and response to treatment of Graves’ disease after COVID vaccination in two male patients. Endocrine. 2022;75:19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zettinig G, Krebs M. Two further cases of Graves’ disease following SARS‐CoV‐2 vaccination. J Endocrinol Invest. 2022;45:227–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metabol. 2020;34:101412. [DOI] [PubMed] [Google Scholar]
- 155. Das L, Bhadada SK, Sood A. Post‐COVID‐vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest. 2022;45:465–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Werner SC. Graves’ disease following acute (subacute) thyroiditis. Arch Intern Med. 1979;139:1313–5. [PubMed] [Google Scholar]
- 157. Bartalena L, Bogazzi F, Pecori F, Martino E. Graves’ disease occurring after subacute thyroiditis: report of a case and review of the literature. Thyroid. 1996;6:345–8. [DOI] [PubMed] [Google Scholar]
- 158. Nagai Y, Toya T, Fukuoka K, Tanaka N, Yanagi S, Kobayashi K. Occurrence and spontaneous remission of Graves’ hyperthyroidism preceded by painless thyroiditis. Endocr J. 1997;44:881–5. [DOI] [PubMed] [Google Scholar]
- 159. Hallengren B, Planck T, Asman P, Lantz M. Presence of thyroid‐stimulating hormone receptor antibodies in a patient with subacute thyroiditis followed by hypothyroidism and later Graves’ disease with ophthalmopathy: a case report. Eur Thyroid J. 2015;4:197–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chiovato L, Santini F, Vitti P, Bendinelli G, Pinchera A. Appearnce of thyroid stimulating antibody and Graves’ disease after radioiodine therapy for toxic nodular goitre. Clin Endocrinol. 1994;40:803–6. [DOI] [PubMed] [Google Scholar]
- 161. Tahrani AA, Rangan S, Moulik P. Grave's eye disease developing after radioiodine treatment for toxic nodular goitre. Exp Clin Endocrinol Diabetes. 2007;115:471–3. [DOI] [PubMed] [Google Scholar]
- 162. Monzani F, Del Guerra P, Caraccio N, Casolaro A, Lippolis PV, Goletti O. Appearance of Graves’ disease after percutaneous ethanol injection for the treatment of hyperfunctioning thyroid adenoma. J Endocrinol Invest. 1997;20:294–8. [DOI] [PubMed] [Google Scholar]
- 163. Lanzolla G, Marcocci C, Marinò M. Occurrence of Graves’ hyperthyroidism and Graves’ orbitopathy after fine‐needle aspiration biopsy of thyroid nodules. J Endocrinol Invest. 2020;43:1033–4. [DOI] [PubMed] [Google Scholar]
- 164. Jackson R, Rosenberg C, Kleinmann R, Vagenakis AE, Braverman LE. Ophthalmopathy after neck irradiation therapy for Hodgkin's disease. Cancer Treat Rep. 1979;63:1393–5. [PubMed] [Google Scholar]
- 165. Jaber JJ, Thomas FJ, Carfrae MJ, Galati LT. Radiotherapy‐associated euthyroid Graves’ ophthalmopathy following floor‐of‐mouth surgery: a case report. Ear Nose Throat J. 2008;87:533–6. [PubMed] [Google Scholar]
- 166. Duarte V, Maciel J, Cavaco D, Donato S, Damasio I, Pinheiro S, et al. Predictive factors for thyroid complications after radiation therapy – data from a cohort of cancer patients closely followed since they were irradiated. Clin Endocrinol. 2021. 10.1111/cen.14665 [DOI] [PubMed] [Google Scholar]
- 167. Weetman AP. Graves’ disease following immune reconstitution or immunomodulatory treatment: should we manage it any differently? Clin Endocrinol. 2014;80:629–32. [DOI] [PubMed] [Google Scholar]
- 168. Rotondi M, Molteni M, Leporati P, Capelli V, Marinò M, Chiovato L. Autoimmune thyroid diseases in patients treated with alemtuzumab for multiple sclerosis: an example of selective anti‐TSH‐receptor immune response. Front Endocrinol. 2017;8:254. 10.3389/fendo-2017.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, et al. Alemtuzumab‐related thyroid dysfunction in a phase 2 trial of patients with relapsing‐remitting multiple sclerosis. J Clin Endocrinol Metab. 2014;99:80–9. [DOI] [PubMed] [Google Scholar]
- 170. Pariani N, Willis M, Muller I, Healy S, Nasser T, McGowan A, et al. Alemtuzumab‐induced thyroid dysfunction exhibits distinctive clinical and immunological features. J Clin Endocrinol Metab. 2018;103:3010–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tsourdi E, Gruber M, Rauner M, Blankenburg J, Ziemssen T, Hofbauer LC. Graves’ disease after treatment with alemtuzumab for multiple sclerosis. Hormones (Athens). 2015;14:148–53. [DOI] [PubMed] [Google Scholar]
- 172. Scappaticcio L, Castellana M, Virili C, Bellastella G, Centanni M, Cannavò S, et al. Alemtuzumab‐induced thyroid events in multiple sclerosis: a systematic review and meta‐analysis. J Endocrinol Invest. 2020;43:219–29. [DOI] [PubMed] [Google Scholar]
- 173. Muller I, Moran C, Lecumberri B, Decallonne B, Robertson N, Jones J, et al. 2019 European Thyroid Association guidelines on the management of thyroid dysfunction following immune reconstitution therapy. Eur Thyroid J. 2019;8:173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Potgieser PW, Wiersinga WM, Regensburg NI, Mourits MP. Some studies on the natural history of Graves’ orbitopathy: increase in orbital fat is a rather late phenomenon. Eur J Endocrinol. 2015;173:149–53. [DOI] [PubMed] [Google Scholar]
- 175. Perros P, Crombie AL, Kendall‐Taylor P. Natural history of thyroid associated ophthalmopathy. Clin Endocrinol. 1995;42:45–50. [DOI] [PubMed] [Google Scholar]
- 176. Menconi F, Profilo MA, Leo M, Sisti E, Altea MA, Rocchi R, et al. Spontaneous improvement of untreated mild Graves’ ophthalmopathy: Rundle's curve revisited. Thyroid. 2014;24:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Menconi F, Leo M, Sabini E, Mautone T, Nardi M, Sainato A, et al. Natural history of Graves’ orbitopathy after treatment. Endocrine. 2017;57:226–33. [DOI] [PubMed] [Google Scholar]
- 178. Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R¸ et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382:341–52. [DOI] [PubMed] [Google Scholar]
- 179. Bartalena L, Marcocci C, Chiovato L, Laddaga M, Lepri G, Andreani D, et al. Orbital cobal irradiation combined with systemic corticosteroids for Graves’ ophthalmopathy: comparison with systemic corticosteroids alone. J Clin Endocrinol Metab. 1983;56:1139–44. [DOI] [PubMed] [Google Scholar]
- 180. Estcourt S, Hickey J, Perros P, Dayan C, Vaidya B. The patient experience of services for thyroid eye disease in the United Kingdom: results of a nationwide survey. Eur J Endocrinol. 2009;161:483–7. [DOI] [PubMed] [Google Scholar]
- 181. Wiersinga WM, Perros P, Kahaly GJ, Mourits P, Baldeschi L, Boboridis K, et al. Clinical assessment of patients with Graves’ orbitopathy: the European Group on Graves’ orbitopathy recommendations to generalists, specialists and clinical researchers. Eur J Endocrinol. 2006;155:387–9. [DOI] [PubMed] [Google Scholar]
- 182. Perros P, Dayan C, Dickinson AJ, Ezra D, Estcourt S, Foley P, et al. Management of patients with Graves’ orbitopathy: initial assessment, management outside specialized centres and referral pathways. Clin Med. 2015;15:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Mourits MP, Koornneef L, Wiersinga M, Prummel MF, Berghout A, van der Gaag R. Clinical criteria for the assessment of disease activity in Graves’ ophthalmopathy: a novel approach. Br J Ophthalmol. 1989;73:639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Dickinson AJ, Perros P. Controversies in the clinical evaluation of active thyroid‐associated orbitopathy: use of a detailed protocol with comparative photographs for objective assessment. Clin Endocrinol. 2001;55:283–303. [DOI] [PubMed] [Google Scholar]
- 185. Dolman PJ, Rootman J. VISA classification for Graves orbitopathy. Ophthalm Plast Reconstr Surg. 2006;22:319–24. [DOI] [PubMed] [Google Scholar]
- 186. Dolman PJ. Dysthyroid optic neuropathy: evaluation and management. J Endocrinol Invest. 2021;44:421–9. [DOI] [PubMed] [Google Scholar]
- 187. Bartalena L, Wiersinga WM. Proposal for standardization of primary and secondary outcomes in patients with active, moderate‐to‐severe Graves’ orbitopathy. Eur Thyroid J. 2020;9(Suppl 1):3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Gurdal C, Sarac O, Genc I, Kirimlioglu H, Takmaz T, Can I. Ocular surface and dry eye in Graves’ disease. Curr Eye Res. 2011;36:8–13. [DOI] [PubMed] [Google Scholar]
- 189. Negro R, Attanasio R, Grimaldi F, Marcocci C, Guglielmi R, Papini E. A 2016 Italian survey about the clinical use of selenium in thyroid disease. Eur Thyroid J. 2016;5:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Hegedus L, Bonnema SJ, Winther KH. Selenium in the treatment of thyroid diseases: an element in search of the relevant indications? Eur Thyroid J. 2016;5:149–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Marinò M, Marcocci C, Vitti P, Chiovato L, Bartalena L. Selenium in the treatment of thyroid diseases. Eur Thyroid J. 2017;6:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Bartalena L. Graves’ orbitopathy: imperfect treatments for a rare disease. Eur Thyroid J. 2013;2:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Campi I, Vannucchi G, Salvi M. Endocrine dilemma: management of Graves’ orbitopathy. Eur J Endocrinol. 2016;175:R117–33. [DOI] [PubMed] [Google Scholar]
- 194. Wiersinga WM. Advances in treatment of active, moderate‐to‐severe Graves’ ophthalmopathy: Lancet Diabetes Endocrinol. 2017;5:134–42. [DOI] [PubMed] [Google Scholar]
- 195. Lee ACH, Kahaly GJ. Novel approaches for immunosuppression in Graves’ hyperthyroidism and associated orbitopathy. Eur Thyroid J. 2020;9(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Langericht J, Kramer I, Kahaly GJ. Glucocorticoids in Graves’ orbitopathy: mechanisms of action and clinical application. Ther Adv Endocrinol Metab. 2020;11:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Marcocci C, Bartalena L, Tanda ML, Manetti L, Dell'Unto E, Rocchi R, et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: results of a prospective, single‐blind, randomized study. J Clin Endocrinol Metab. 2001;86:3562–7. [DOI] [PubMed] [Google Scholar]
- 198. Kahaly GJ, Pitz S, Hommel G, Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J Clin Endocrinol Metab. 2005;90:5234–40. [DOI] [PubMed] [Google Scholar]
- 199. Van Geest RJ, Sasim IV, Koppeschaar HPF, Kalmann R, Stravers SN, Bijlsma WR, et al. Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: a prospective, randomized, placebo‐controlled study. Eur J Endocrinol. 2008;158:229–37. [DOI] [PubMed] [Google Scholar]
- 200. Stiebel‐Kalish H, Robenshtok E, Hasanreisoglu M, Ezrachi D, Shimon I, Leibovici L. Treatment modalities for Graves’ ophthalmopathy: systematic review and metaanalysis. J Clin Endocrinol Metab. 2009;94:2708–16. [DOI] [PubMed] [Google Scholar]
- 201. Zang S, Ponto KA, Kahaly GJ. Intravenous glucocorticoids for Graves’ orbitopathy: efficacy and morbidity. J Clin Endocrinol Metab. 2011;96:320–32. [DOI] [PubMed] [Google Scholar]
- 202. Bartalena L, Krassas GE, Wiersinga W, Marcocci C, Salvi M, Daumerie C, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97:4454–63. [DOI] [PubMed] [Google Scholar]
- 203. Terwee CB, Prummel MF, Gerding MN, Kahaly GJ, Dekker F, Wiersinga WM. Measuring disease activity to predict therapeutic outcome in Graves’ ophthalmopathy. Clin Endocrinol. 2005;62:145–55. [DOI] [PubMed] [Google Scholar]
- 204. Zang S, Ponto KA, Pitz S, Kahaly GJ. Dose of intravenous steroids and therapy outcome in Graves’ orbitopathy. J Endocrinol Invest. 2011;34:876–80. [DOI] [PubMed] [Google Scholar]
- 205. Nedelljkovic Beleslin B, Ciric J, Stojkovic M, Miletic M, Knezevic M, Stankovic B, et al. Comparison of efficacy and safety of parenteral versus parenteral and oral glucocorticoid therapy in Graves’ orbitopathy. Int J Clin Pract. 2020;74:e13608. [DOI] [PubMed] [Google Scholar]
- 206. Vannucchi G, Covelli D, Campi I, Origo D, Currò N, Cirello V, et al. The therapeutic outcome of intravenous steroid therapy for active Graves’ orbitopathy is influenced by the time of response but not polymorphisms of the glucocorticoid receptor. Eur J Endocrinol. 2014;170:55–61. [DOI] [PubMed] [Google Scholar]
- 207. Bartalena L, Veronesi G, Krassas GE, Wiersinga WM, Marcocci C, Marinò M, et al. Does early response to intravenous glucocorticoids predict the final outcome in patients with moderate‐to‐severe and active Graves’ orbitopathy? J Endocrinol Invest. 2017;40:547–53. [DOI] [PubMed] [Google Scholar]
- 208. Marcocci C, Watt T, Altea MA, Rasmussen AK, Feldt‐Rasmussen U, Orgiazzi J, et al. Fatal and non‐fatal adverse events of glucocorticoid therapy for Graves’ ophthalmopathy: a questionnaire survey among members of the European Thyroid Association. Eur J Endocrinol. 2012;166:247–53. [DOI] [PubMed] [Google Scholar]
- 209. Marinò M, Morabito E, Brunetto MR, Bartalena L, Pinchera A, Marcocci C. Acute and severe liver damage associated with intravenous glucocorticoid pulse therapy in patients with Graves’ ophtalmopathy. Thyroid. 2004;14:403–6. [DOI] [PubMed] [Google Scholar]
- 210. Sisti E, Coco B, Menconi F, Leo M, Rocchi R, Latrofa F, et al. Age and dose are major risk factors for liver damage associated with intravenous glucocorticoid pulse therapy for Graves’ orbitopathy. Thyroid. 2015;7:846–50. [DOI] [PubMed] [Google Scholar]
- 211. Allison AC. Mechanisms of action of mycophenolate mofetil in preventing chronic rejection. Trasnplan Proc. 2022;34:2863–6. [DOI] [PubMed] [Google Scholar]
- 212. Lee ACH, Riedl M, Frommer L, Diana T, Kahaly GJ. Systemic safety analysis of mycophenolate in Graves’ orbitopathy. J Endocrinol Invest. 2020;43:767–77. [DOI] [PubMed] [Google Scholar]
- 213. Ye X, Bo X, Hu X, Cui H, Lu B, Shao J, et al. Efficacy and safety of mycophenolate mofetil in patients with active moderate‐to‐severe Graves’ orbitopathy. Clin Endocrinol. 2017;86:247–55. [DOI] [PubMed] [Google Scholar]
- 214. Xian NQQ, Alnahrawy A, Akshikar R, Lee V. Real‐world efficacy and safety of mycophenolate mofetil in active moderate‐to‐sight‐threatening thyroid eye disease. Clin Ophthalmol. 2021;15:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Kahaly GJ, Riedl M, Konig J, Pitz S, Ponto K, Diana T, et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate‐to‐severe Graves’ orbitopathy (MINGO): a randomised, observer‐masked, multicentre trial. Lancet Diabetes Endocrinol. 2018;6:287–98. [DOI] [PubMed] [Google Scholar]
- 216. Tanda ML, Bartalena L. Efficacy and safety of orbital radiotherapy for Graves’ orbitopathy. J Clin Endocrinol Metab. 2012;97:3857–65. [DOI] [PubMed] [Google Scholar]
- 217. Mourits MP, van Kempen‐Hartefeld ML, Garcia MP, Koppeschaar HO, Tick L, Terwee CB. Radiotherapy for Graves’ orbitopathy: randomised placebo‐controlled study: Lancet. 2000;355:1505–9. [DOI] [PubMed] [Google Scholar]
- 218. Prummel MF, Terwee CB, Gerding MN, Baldeschi L, Mourits MP, Blank L, et al. A randomized controlled trial of orbital radiotherapy versus sham irradiation in patients with mild Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2004;89:15–20. [DOI] [PubMed] [Google Scholar]
- 219. Gorman CA, Garrity JA, Fatourechi V, Bahn RS, Petersen IA, Stafford SL, et al. A prospective, randomized, double‐blind placebo‐controlled study of orbital radiotherapy for Graves’ ophthalmopathy. Ophthalmology. 2001;108:1523–4. [DOI] [PubMed] [Google Scholar]
- 220. Prummel MF, Mourits MP, Blank L, Berghout A, Koornneef L, Wiersinga WM. Randomized double‐blind trial of prednisone versus radiotherapy in Graves’ ophthalmopathy. Lancet. 1993;342:949–54. [DOI] [PubMed] [Google Scholar]
- 221. Marcocci C, Bartalena L, Bogazzi F, Bruno‐Bossio G, Lepri A, Pinchera A. Orbital radiotherapy combined with high‐dose systemic glucocorticoids is more effective than radiotherapy alone: results of a prospective randomized study. J Endocrinol Invest. 1991;14:853–60. [DOI] [PubMed] [Google Scholar]
- 222. Rajendram R, Taylor PN, Wilson VJ, Harris N, Morris OC, Tomlinson M, et al. Combined immunosuppression and radiotherapy in thyroid eye disease (CIRTED): a multicentre, 2×2 factorial, double‐blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:299–309. [DOI] [PubMed] [Google Scholar]
- 223. Godfrey KJ, Kazim M. Radiotherapy for active thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018;34(suppl.1):S98–104. [DOI] [PubMed] [Google Scholar]
- 224. Kim JW, Han SH, Son BJ, Rim TH, Keum KC, Yoon JS. Efficacy of combined orbital radiation and systemic steroids in the management of Graves’ orbitopathy. Graefe's Arch Clin Exp Ophthalmol. 2016;254:991–8. [DOI] [PubMed] [Google Scholar]
- 225. Oeverhaus M, Witteler T, Lax H, Esser J, Fuhrer D, Eckstein A. Combination therapy of intravenous steroids and orbital irradiation is more effective than intravenous steroids alone in patients with Graves’ orbitopathy. Horm Metab Res. 2017;49:739–47. [DOI] [PubMed] [Google Scholar]
- 226. Kahaly GJ, Rosler HP, Pitz S, Hommel G. Low‐ versus high‐dose radiotherapy for Graves’ ophthalmopathy: a randomized, single blind trial. J Clin Endocrinol Metab. 2000;85:102–8. [DOI] [PubMed] [Google Scholar]
- 227. Marcocci C, Bartalena L, Rocchi R, Marinò M, Menconi F, Morabito E, et al. Long‐term safety of orbital radiotherapy for Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2003;88:3561–6. [DOI] [PubMed] [Google Scholar]
- 228. Wakelkamp IM, Tan H, Saeed P, Schlingemann RO, Verbraak FD, Blank LE, et al. Orbital irradiation for Graves’ ophthalmopathy: is it safe? A long‐term follow‐up study. Ophthalmology. 2004;111:1557–62. [DOI] [PubMed] [Google Scholar]
- 229. Kahaly GJ, Schrezenmeir J, Krause U, Schweikert B, Meuer S, Muller W, et al. Ciclosporin and prednisone in treatment of Graves’ ophthalmopathy: a controlled, randomized and prospective study. Eur J Clin Invest. 1986;16:415–22. [DOI] [PubMed] [Google Scholar]
- 230. Prummel MF, Mourits MP, Berghout A, Krenning EP, van der Gaar R, Koornneef L, et al. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N Engl J Med. 1989;321:1353–9. [DOI] [PubMed] [Google Scholar]
- 231. Perros P, Weightman DR, Crombie AL, Kendall‐Taylor P. Azathioprine in the treatment of thyroid‐associated ophthalmopathy. Acta Endocrinol (Copenh). 1990;122:8–12. [DOI] [PubMed] [Google Scholar]
- 232. Strianese D, Iuliano A, Ferrara M, Comune C, Baronissi I, Napolitano P, et al. Methotrexate for the treatment of thyroid eye disease. J Ophthalmol. 2014;2014:128903. 10.1155/2014/128903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Rubinov A, Zommer H, Aghazadeh H, Weis E, Role of methotrexate in thyroid‐related orbitopathy. Can J Ophthalmol. 2018;53:34–8. [DOI] [PubMed] [Google Scholar]
- 234. Sipkova Z, Insull EA, David J, Turner HE, Keren S, Norris JH. Early use of steroid‐sparing agents in the inactivation of moderate‐to‐severe active thyroid eye disease: a step‐down approach. Clin Endocrinol. 2018;89:834–9. [DOI] [PubMed] [Google Scholar]
- 235. Salvi M, Covelli D. Combined immunosuppressants and less steroids in active Graves’ orbitopathy? Clin Endocrinol. 2019;90:525–7. [DOI] [PubMed] [Google Scholar]
- 236. Tanda ML, Piantanida E, Masiello E, Cusini C, Bartalena L. Can combination of glucocorticoids with other immunosuppressive drugs reduce the cumulative dose of glucocorticoids for moderate‐to‐severe and active Graves’ orbitopathy? J Endocrinol Invest. 2019;43:351–2. [DOI] [PubMed] [Google Scholar]
- 237. Smith TJ, Bartalena L. Will biological agents supplant systemic glucocorticoids as the first‐line treatment for thyroid‐associated ophthalmopathy? Eur J Endocrinol. 2019;181:D27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, et al. Teprotumumab for thyroid‐associated ophthalmopathy. N Engl J Med. 2017;376:1748–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off‐treatment follow‐up results from two randomised, double‐masked, placebo‐controlled, multicentre trials. Lancet Diabetes Endocrinol. 2021;9:360–72. [DOI] [PubMed] [Google Scholar]
- 240. Douglas RS, Kahaly GJ, Ugradar S, Elflein H, Ponto KA, Fowler BT, et al. Teprotumumab efficacy, safety, and durability in longer‐duration thyroid eye disease and re‐treatment. Ophthalmology. 2022;129:438–49. [DOI] [PubMed] [Google Scholar]
- 241. Hoang TD, Nguyen NT, Chou E, Skakir MKM. Rapidly progressive cognitive decline associated with teprotumumab in thyroid eye disease. BMJ Case Rep. 2021;14:e242153. 10.1136/bcr-2021-242153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Safo MB, Silkiss RZ. A case of ulcerative colitis associated with teprotumumab treatment for thyroid eye disease. Am J Ophthalmol Case Rep. 2021;22:101069. 10.1016/j.ajoc.2021.101069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Ashraf DC, Jankovic I, El‐Nachef N, Winn BJ, Kim GE, Kersten RC. New‐onset of inflammatory bowel disease in a patient treated with teprotumumab for thyroid associated ophthalmopathy. Ophthalmic Plast Reconstr Surg. 2021;37:e160–4. 10.1097/IOP.0000000000001943 [DOI] [PubMed] [Google Scholar]
- 244. Bartalena L, Marinò M, Marcocci C, Tanda ML. Teprotumumab for Graves’ orbitopathy and ototoxicity: moving problems for eyes to ears? J Endocrinol Invest. 2022. 10.1007/s40618-022-01791-w [DOI] [PubMed] [Google Scholar]
- 245. Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 246. McCoy AN, Kim DS, Gillespie EF, Atkins SJ, Smith TJ, Douglas RS. Rituximab (Rituxan) therapy for severe thyroid‐associated ophthalmopathy diminishes IGF‐1R+ T cells. J Clin Endocrinol Metab. 2014;99:E1294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Salvi M, Vannucchi G, Campi I, Currò N, Dazzi D, Simonetta S, et al. Treatment of Graves’ disease and associated ophthalmopathy with the anti‐CD20 monoclonal antibody rituximab: an open study. Eur J Endocrinol. 2007;156:33–40. [DOI] [PubMed] [Google Scholar]
- 248. Khanna D, Chong KK, Afifiyan NF, Hwang CJ, Lee DK, Garneau HC, et al. Rituximab treatment of patients with severe, corticosteroid‐resistant thyroid‐associated ophthalmopathy. Ophthalmology. 2010;117:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Mitchell AL, Gan EH, Morris M, Johnson K, Neoh C, Dickinson AJ, et al. The effect of B cell depletion therapy on anti‐TSH receptor antibodies and clinical outcome in glucocorticoid‐refractory Graves’ orbitopathy. Clin Endocrinol. 2013;79:437–42. [DOI] [PubMed] [Google Scholar]
- 250. Stan MN, Garrity JA, Carranza Leon BG, Prabin T, Bradley EA, Bahn RS. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab. 2015;100:432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Salvi M, Vannucchi G, Currò N, Campi I, Covelli D, Dazzi D, et al. Efficacy of B‐cell targeted therapy with rituximab in patients with active moderate‐to‐severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab. 2015;100:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Stan MN, Salvi M. Rituximab therapy for Graves’ orbitopathy—lessons from randomized controlled trials. Eur J Endocrinol. 2017;176:R101–9. [DOI] [PubMed] [Google Scholar]
- 253. Deltour J‐B, d'Assigny Flamen M, Ladsous M, Giovansili L, Cariou B, Caron P, et al. Efficacy of rituximab in patients with Graves’ orbitopathy: a retrospective multicenter nationwide study. Graefe's Arch Clin Exp Ophthalmol. 2021;258:2013–21. [DOI] [PubMed] [Google Scholar]
- 254. Chen J, Chen G, Sun H. Intravenous rituximab therapy for active Graves’ ophthalmopathy: a meta‐analysis. Hormones (Athens). 2021;20:279–86. [DOI] [PubMed] [Google Scholar]
- 255. Vannucchi G, Campi I, Covelli D, Currò N, Lazzaroni E, Palomba A, et al. Efficacy profile and safety of very low‐dose rituximab in patients with Graves’ orbitopathy. Thyroid. 2021;31:821–8. [DOI] [PubMed] [Google Scholar]
- 256. Chen B, Tsui S, Smith TJ. IL‐1 beta induced IL‐6 expression in human orbital fibroblasts: identification of an anatomic‐site specific phenotypic attribute relevant to thyroid‐associated ophthalmopathy. J Immunol. 2005;175:1310–9. [DOI] [PubMed] [Google Scholar]
- 257. Kumar S, Schiefer R, Coenen MJ, Bahn RS. A stimulatory thyrotropin receptor antibody (M22) and thyrotropin increase interleukin‐6 expression and secretion in Graves’ orbital preadipocyte fibroblasts. Thyroid. 2010;20:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Jyonouchi SC, Valyasevi RW, Harteneck DA, Dutton CM, Bahn RS. Interleukin‐6 stimulates thyrotropin receptor expression in human orbital preadipocyte fibroblasts from patients with Graves’ ophthalmopathy. Thyroid. 2001;11:929–34. [DOI] [PubMed] [Google Scholar]
- 259. Pérez‐Moreiras JV, Alvarez‐Lopez A, Gomez EC. Treatment of active corticosteroid‐resistant Graves’ orbitopathy. Ophthalmic Plast Reconstr Surg. 2014;30:162–7. [DOI] [PubMed] [Google Scholar]
- 260. Sy A, Eliaseh K, Silkiss RZ. Clinical response to tocilizumab in severe thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2017;33:e55–7. [DOI] [PubMed] [Google Scholar]
- 261. Russell DJ, Wagner LH, Seiff SR. Tocilizumab as a steroid sparing agent for the treatment of Graves’ orbitopathy. Am J Ophthalmol Case Rep. 2017;7:146–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Pérez‐Moreiras JV, Gomez‐Reino JJ, Maneiro JR, Perez‐Pampin E, Romo Lopez A, Rodriguez Alvarez EM, et al. Efficacy of tocilizumab in patients with moderate‐to‐severe corticosteroid‐resistant Graves’ orbitopathy: a randomized clinical trial. Am J Ophthalmol. 2018;195:181–90. [DOI] [PubMed] [Google Scholar]
- 263. Pérez‐Moreiras JV, Varela‐Agra M, Prada‐Sanchez MC, Prada‐Ramalla G. Steroid‐resistant Graves’ orbitopathy treated with tocilizumab in real‐world clinical practice: a 9‐year single‐center experience. J Clin Med. 2021;10:706. 10.3390/jcm10040706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Sanchez‐Bilbao L, Martinez‐Lopez D, Revenga M, Lopez‐Vazquez A, Valls‐Pascual E, Atienza‐Mateo B, et al. Anti‐IL‐6 receptor tocilizumab in refractory Graves’ orbitopathy: national multicenter observational study of 48 patients. J Clin Med. 2020;9:2816. 10.3390/jcm9092816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Jose C‐MJ, Rivera‐Moscoso R, Jorge F‐RA, Vargas‐Sanchez J, Ortega‐Gutierrez G, Madriz‐Prado R, et al. Tocilizumab in glucocorticoid‐resistant Graves’ orbitopathy. A case series report of a Mexican population. Ann Endocrinol. 2020;81:78–82. [DOI] [PubMed] [Google Scholar]
- 266. Wakelkamp IM, Baldeschi L, Saeed P, Mourits MP, Prummel MF, Wiersinga WM. Surgical or medical decompression as a first‐line treatment of optic neuropathy in Graves’ ophthalmopathy? A randomized controlled trial. Clin Endocrinol. 2005;63:323–8. [DOI] [PubMed] [Google Scholar]
- 267. Currò N, Covelli D, Vannucchi G, Campi I, Pirola G, Simonetta S, et al. Therapeutic outcomes of high‐dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014;24:897–905. [DOI] [PubMed] [Google Scholar]
- 268. Bartalena L, Piantanida E, Gallo D, Ippolito S, Tanda ML. Management of Graves’ hyperthyroidism: present and future. Exp Rev Endocrinol Metab. 2022. 10.1080/17446651.2022.2052044 [DOI] [PubMed] [Google Scholar]
- 269. Marcocci C, Bruno‐Bossio G, Manetti L, Tanda ML, Miccoli P, Iacconi P, et al. The course of Graves’ ophthalmopathy is not influenced by near‐total thyroidectomy: a case‐control study. Clin Endocrinol. 2011;51:503–8. [DOI] [PubMed] [Google Scholar]
- 270. Erdogan ME, Demir O, Ersoy RU, Gul K, Aydogan BI, Uc ZA, et al. Comparison of early total thyroidectomy with antithyroid treatment in patients with moderate‐severe Graves’ orbitopathy: a randomized prospective trial. Eur Thyroid J. 2016;5:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Meyer Zu Horste M, Pateronis K, Walz MK, Alesina P, Mann K, Schott M, et al. The effect of early thyroidectomy on the course of active Graves’ orbitopathy (GO): a retrospective case study. Horm Metab Res. 2016;48:433–9. [DOI] [PubMed] [Google Scholar]
- 272. Menconi F, Marinò M, Pinchera A, Rocchi R, Mazzi B, Nardi M, et al. Effect of total thyroid ablation versus near‐total thyroidectomy alone on mild to moderate Graves’ orbitopathy treated with intravenous glucocorticoids. J Clin Endocrinol Metab. 2007;92:1653–8. [DOI] [PubMed] [Google Scholar]
- 273. Leo M, Marcocci C, Pinchera A, Nardi M, Megna L, Rocchi R, et al. Outcome of Graves’ orbitopathy after total thyroid ablation and glucocorticoid treatment: follow‐up of a randomized clinical trial. J Clin Endocrinol Metab. 2012;97:E44–8. [DOI] [PubMed] [Google Scholar]
- 274. Moleti M, Violi MA, Montanini D, Trombetta C, Di Bella B, Sturniolo G, et al. Radioiodine ablation of postsurgical thyroid remnants after treatment with recombinant human TSH (rhTSH) in patients with moderate‐to‐severe Graves’ orbitopathy (GO): a prospective, randomized, single‐blind clinical trial. J Clin Endocrinol Metab. 2014;99:1783–9. [DOI] [PubMed] [Google Scholar]
- 275. Laurberg P, Berman DC, Andersen S, Bulow Pedersen I. Sustained control of Graves’ hyperthyroidism during long‐term low‐dose antithyroid drug therapy of patients with Graves’ orbitopathy. Thyroid. 2011;21:951–6. [DOI] [PubMed] [Google Scholar]
- 276. Elbers L, Mourits M, Wiersinga W. Outcome of very‐long treatment with antithyroid drugs in Graves’ hyperthyroidism associated with Graves’ orbitopathy. Thyroid. 2011;21:279–83. [DOI] [PubMed] [Google Scholar]
- 277. Kahaly GJ, Stan MN, Frommer L, Gergely P, Colin L, Amer A, et al. A novel anti‐CD40 monoclonal antibody, iscalimab, for control of Graves’ hyperthyroidism—a proof‐of‐concept trial. J Clin Endocrinol Metab. 2020;105:696–704. [DOI] [PubMed] [Google Scholar]
- 278. Pearce SHS, Dayan C, Wraith DC, Barrell K, Olive N, Jansson L, et al. Antigen‐specific immunotherapy with thyrotropin receptor peptides in Graves’ hyperthyroidism: a phase I study. Thyroid. 2019;29:1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Neumann S, Nir EA, Eliseeva E, Huang W, Marugan J, Xiao J, et al. A selective TSH receptor antagonists inhibits stimulation of thyroid function in female mice. Endocrinology. 2014;155:310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Latif R, Realubit RB, Karan C, Mezei M, Davies TF. TSH receptor signaling abrogation by a novel small molecule. Front Endocrinol. 2016;7:130. 10.3389/fendo.2016.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Marcinkowski P, Hoyer I, Specker E, Furkert J, Rutz C, Neuenschwander M, et al. A new highly thyrotropin‐receptor‐selective small‐molecule antagonist with potential for the treatment of Graves’ orbitopathy. Thyroid. 2019;29:111–23. [DOI] [PubMed] [Google Scholar]
- 282. Furmaniak J, Sanders J, Young S, Kabelis K, Sanders P, Evans M, et al. In vivo effects of a human thyroid‐stimulating monoclonal autoantibody (M22) and a human thyroid‐blocking monoclonal autoantibody (K1‐70). Autommun Highlights. 2012;3:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ryder M, Wentworth M, Algeciras‐Schimnich A, Morris JC, Garrity J, Sanders J, et al. Blocking the thyrotropin receptor with K1‐70 in a patient with follicular thyroid cancer, Graves’ disease, and Graves’ ophthalmopathy. Thyroid. 2021;31:1597–602. [DOI] [PubMed] [Google Scholar]
- 284. Furmaniak J, Sanders J, Sanders P, Li Y, Rees Smith B. TSH receptor specific monoclonal autoantibody K1‐70TM targeting of the TSH receptor in subjects with Graves’ disease and Graves’ orbitopathy—results from a phase I clinical trial. Clin Endocrinol. 2022. 10.1111/cen.14681 [DOI] [PMC free article] [PubMed] [Google Scholar]


