Abstract
Objective
The aims of this study were to explore if the ambulatory fludrocortisone suppression test (FST) was safe, accurate and cost‐effective.
Context
The diagnosis of primary aldosteronism (PA) remains time‐consuming and complex. The FST is used to confirm PA, but it is an in‐patient test due to potentially serious complications such as hypokalemia. In Stockholm, FST has been performed since 2005 as an ambulatory procedure.
Design
This is a retrospective study including all patients investigated with FST in four hospitals in Stockholm, Sweden, during 2005–2019.
Patients/Measurements
In total, 156 cases of ambulatory FST (FSTamb) and 15 cases of in‐patient FST (FSTin) were included. FSTamb and FSTin were compared regarding health costs, clinical characteristics and laboratory results.
Results
No difference was found in the outcomes of FSTamb and FSTin. No severe complications were reported in FSTamb patients. No difference was found in the median value for plasma potassium on Day 5 between the two groups. Only three patients (1.9%) in the FSTamb had to repeat the test due to incomplete intake of medications. FSTamb and FSTin were equally accurate. The cost of performing FSTamb was at least 50% lower compared with FSTin ($2400 vs. $5200 per patient). The time needed for FSTamb was 60 min of physician's time and 150 min of nurse's time which were lower than the 5 days in FSTin.
Conclusions
Ambulatory FST is safe and accurate and can be performed with significantly less healthcare costs compared to FSTin.
Keywords: ambulatory, fludrocortisone suppression test, primary aldosteronism
1. INTRODUCTION
Primary aldosteronism (PA) is the most common endocrine cause of secondary hypertension. 1 However, PA is underdiagnosed 2 with reported prevalence rates among patients with hypertension of 4%–14% 3 , 4 , 5 in primary care and 1%–29.8% in referral centres. 2 , 6 , 7 , 8 , 9 The disease is characterised by hypertension, hypokalemia/normokalemia and metabolic alkalosis with diffuse clinical features such as muscle weakness, fatigue, polyuria and polydipsia. 10 , 11 The cause is autonomous aldosterone production which results in sodium and water retention as well as renal potassium excretion. The excess production of aldosterone may occur in one (unilateral) or both (bilateral) adrenal glands. The unilateral disease is usually caused by a solitary adenoma and represents only one‐third of all PA cases. Bilateral disease is caused by bilateral adrenocortical hyperplasia or, rarely, by bilateral adenomas. 12
Patients with PA have an increased risk of heart disease, stroke, diabetes and metabolic syndrome. 13 , 14 , 15 Due to the recognised efficiency of PA treatment (surgery and mineralocorticoid receptor antagonist treatment) and its positive impact on patient outcomes, including health cost gains, screening of risk populations for PA is important and beneficial. 16 , 17 , 18
Even though PA is a common disorder among patients with hypertension, only a relatively small number of patients undergo investigation for PA. For example, recent studies show that only 2.1%–2.7% of patients with hypertension were screened for PA. 19 , 20
The diagnosis of PA requires not only laboratory tests but often also complex confirmatory tests, radiological assessment and adrenal vein sampling for distinguishing between unilateral and bilateral disease. 21 In PA, the increase in aldosterone is independent of renin pathway regulation, causing the suppression of renin secretion and, hence, elevation of the aldosterone to renin ratio (ARR). 21 However, the ARR can be altered by other factors, such as medications interfering with the Renin‐Angiotensin‐Aldosterone System (RAAS), oral contraceptives or chronic kidney disease, why confirmatory tests are most often required to establish the diagnosis of PA. 21 , 22
In PA diagnosis, confirmatory tests aim to demonstrate the inability to suppress the aldosterone production by using various methods. 21 , 22 The confirmatory tests recommended by international guidelines 21 are the oral sodium loading test, the saline infusion test, the captopril challenge test and the fludrocortisone suppression test (FST). Currently, the FST is considered reliable and is used by some centres as a reference test for the evaluation of other confirmatory tests in diagnosing PA. 23 , 24 , 25 The FST traditionally requires that the patient is hospitalised because the risk of hypokalemia has been perceived to be high. 22 Consequently, most centres avoid the FST. 23 , 26 , 27
In the Stockholm area, the FST has been performed ambulatory (FSTamb) starting with the year 2005. The method used is a simplified variant proposed by the European Endocrine Society for the in‐patient FST (FSTin). 21 To our knowledge, no data on the FSTamb has been published to this day. The aim of this study was to evaluate the safety and accuracy of the FSTamb in diagnosing PA. Moreover, we also aimed to explore the cost benefits of using the FSTamb compared to the FSTin.
2. MATERIALS AND METHODS
2.1. Study population
In this retrospective study, we reviewed all medical records for patients who had been screened for PA during 2005–2019 at the Departments of Endocrinology in the four leading hospitals of the Stockholm region: Danderyd Hospital, Karolinska University Hospital Solna, Karolinska University Hospital Huddinge and Södersjukhuset. Only patients examined with FST were included.
The initial screening included measurement of ARR, plasma potassium concentration, blood pressure, body mass index (BMI), ongoing medical treatment, history of other diseases and smoking. If possible, considering the patient's medical history, the antihypertensive treatment was modified by excluding drugs interfering with the ARR and, if needed, replaced by antihypertensive drugs not interfering with the ARR. Mineralocorticoid receptor antagonists, such as spironolactone and eplerenone, were stopped at least 6 weeks before the FST. Other drugs interfering with the RAAS were discontinued at least 2 weeks before the test.
The following data were obtained for all patients examined with FST: ARR, 24‐h urinary aldosterone, 24‐h urinary sodium, serum cortisol concentration, the incidence of hypokalemia during FST, episodes of arrhythmia, need for hospitalisation due to complications during FST, patients' adherence to the FST protocol, blood pressure and intolerance to medications.
The accuracy of FST was assessed by comparing the results of the FST with the combined results of initial laboratory screening, radiological findings, confirmatory tests, adrenal vein sampling and histological findings for those operated, and treatment response.
Before the establishment of the FSTamb as a standard method in the entire Stockholm region, one centre was still performing the FSTin. As a control group, the 15 cases investigated with FSTin were recruited to compare the test safety and accuracy in relation to FSTamb.
The Regional Ethical Review Board approved the study in Stockholm, Sweden, and due to its retrospective nature, consent was waived.
2.2. Local protocol for FSTamb
Patients eligible for the FST were planned to perform the test as an outpatient test in all four centres. Antihypertensive medications were adjusted as required before the test. 10 Fludrocortisone tablets 0.1 mg were administered four times daily (at 08.00, 12.00, 16.00 and 20.00) over 5 days, starting at 12.00 on Day 1 and finishing at 08.00 on Day 5. Sodium chloride capsules 500 mg (three capsules qid) were administered together with fludrocortisone. Slow‐release potassium chloride tablets of 750 mg were distributed according to potassium concentration. All patients received oral and written instructions. If needed, the patients had the opportunity to contact the outpatient clinic during office hours. Plasma potassium concentration was controlled once a day at all sites on Days 1, 2, 5 and at two sites also on Days 3 and 4. The dosage of slow‐release potassium chloride tablets was adjusted and communicated to each patient at the visit to the investigating site on Days 1 and 5, and by telephone contact with an endocrine nurse on Days 2, 3 and 4. Blood pressure and heart rate were registered on Days 1 and 5.
On Days 1 and 5, the plasma aldosterone concentration (PAC), plasma renin concentration (PRC) and serum cortisol concentration were controlled in a recumbent posture after 20min of rest and after 2 h in a seated posture. The patients were instructed to collect urine from the day before Day 1 of the FSTamb and from Day 4 to the morning of Day 5 of the FSTamb for the analysis of 24‐h urinary aldosterone, sodium and potassium.
The FST was considered indicative of PA if PAC in the seated posture was over 220 pmol/L on Day 5 if PRC was inappropriately low and serum cortisol concentration was lower than the value obtained at 08.00 (to exclude a confounding ACTH effect) and/or if the 24‐h urinary aldosterone was over 35 mmol/24 h. This cut‐off was used in consensus with the local guidelines. A 24‐h urinary sodium concentration at the end of the FST was used to determine if salt loading was adequate. A cut‐off of 200 mmol/24 h for urinary sodium was considered acceptable. 22
The protocol for the FSTin included the same laboratory tests on Days 1 and 5. The difference was that the plasma potassium concentration was controlled one or more times daily, permitting more free adjustments of slow‐released potassium tablets.
2.3. Cost
The cost of performing FSTamb and FSTin was calculated and reviewed by the Financial Departments of the hospitals. The costs for the FSTamb included costs for 1 hour of physician's time (30 min on Day 1 and 30 min on Day 5), 30 min per day x 5 days of nurse's time, medications, ambulatory space, the cost for laboratory analyses and other consumables such as equipment to obtain blood and urine samples as well as patients' lunches. For the FSTin the cost was calculated for 5 days of hospitalisation, including physicians' and nurses' time every day, medications, the cost for laboratory analyses and other consumables similar as above.
2.4. Assays
The methods for measuring PAC and PRC changed during the study period. The method for measuring PAC and PRC was changed on June 18, 2014, from Siemens Coat‐A‐Count RIA kit (Siemens Ltd) for PAC and Electrabox CISBIO IRMA kit for PRC to DiaSorin Liaison XL for both. Before mid‐June 2014, the normal range for recumbent PAC was 80–440 and 190–830 pmol/L for seated PAC. The lower limit of detection for PAC was <69 pmol/L. From mid‐June 2014, the normal range for recumbent PAC was <650 and 60–980 pmol/L for seated PAC. The lower limit for detection was <27 pmol/L. The normal range for PRC before mid‐June 2014 was in the recumbent posture 3–16 ng/L and the seated posture 3–33 ng/L. The lower limit was <0.2 ng/L. After that, the normal range in recumbent position was 2.8–40 mIU/L and in seated posture 4.4–46 mIU/L. The lower limit was <0.3 mIU/L. Until mid‐June 2014, the cut‐off for the ARR was 100 pmol/ng and later in connection to the changes in the laboratory methods, it was defined as 60 pmol/mIU.
2.5. Statistical analysis
The data were analysed using the SPSS statistic programme (version 27/2021, IBM Corporation). The results were given as median (range) for numerical data and for categorical data, as number and percentage if not stated otherwise. For comparison between continuous values, the Mann–Whitney U‐test was used. Comparisons of categorical values were made with Fisher's exact test. A p‐value < .05 was considered significant.
3. RESULTS
In total, 156 cases of FSTamb and 15 cases of FSTin were included. The main indications for PA investigation are shown in Table 1. The baseline characteristics of the FSTamb and FSTin patients are summarised in Table 2. The median (range) age was 52 (22–75) years and 50.9% were females. The patients had median blood pressure of 150 (120–239)/90 (57–120) mmHg, and the number of antihypertensive drugs was 2 (0–4). The initial screening showed an ARR for the entire group of 139.8 (1.65–2620.0) pmol/ng and 205.1 (37.09–1172.5) pmol/mIU, respectively, depending on the laboratory methods. The initial potassium concentration in the FSTamb group, registered at the first visit to the centre was 3.2 (2.0–4.0) mmol/L. A history of cardiovascular disease was more common in the FSTin patient group. In the FSTin patient group, there were several more active smokers compared to in the FSTamb group. The 24‐h urinary aldosterone measured during FSTin was higher than that measured in FSTamb. No differences in other clinical data, such as gender, age, BMI, or blood pressure, were found between these two patient groups (Table 2).
Table 1.
Referral causes for patients who had an ambulatory fludrocortisone suppression test in the Stockholm region, Sweden
| Cause for referral | Number | % |
|---|---|---|
| Hypertension and hypokalemia | 81 | 51.9 |
| Hypertension | 46 | 29.4 |
| Adrenal incidentaloma | 13 | 8.3 |
| Hypokalemia | 8 | 5.1 |
| Hypertension and adrenal incidentaloma | 4 | 2.5 |
| Hypertension, hypokalemia and adrenal incidentaloma | 4 | 2.5 |
| Total | 156 | 100 |
Table 2.
Clinical and biochemical variables in patients who had a fludrocortisone suppression test either as an ambulatory or as an in‐patient in the Stockholm region
| Total | FSTamb | FSTin | p‐Value | |
|---|---|---|---|---|
| Number, n (%) | 171 | 156 | 15 | |
| Gender | 0.792 | |||
| Female, n (%) | 87 (50.9) | 80 (51.3) | 7 (46.7) | |
| Male, n (%) | 84 (49.1) | 76 (48.7) | 8 (53.3) | |
| Age median (range) | 52 (22–75) | 51 (22–75) | 54 (33–73) | 0.348 |
| Body mass index (BMI) (kg/m2) | 27.3 (18–47) | 27.4 (18–47) | 26.4 (21–34) | 0.754 |
| Duration of HT (years) | 4 (0–40)40 | 4 (0–39) | 4 (1–40) | 0.764 |
| History of CVD, n (%) | 26 (15.2) | 18 (11.5) | 8 (53.3) | <0.001 |
| History of diabetes, n (%) | 28 (16.4) | 26 (16.7) | 2 (13.3) | 1.000 |
| History of dyslipidemia, n (%) | 11 (6.4) | 9 (5.8) | 2 (13.3) | 0.249 |
| History of cancer, n (%) | 18 (10.5) | 16 (10.3) | 2 (13.3) | 0.661 |
| Creatinine, (μmol/L) median (range) | 75 (4.8–119) | 74 (5–119) | 77 (65–87) | 0.429 |
| SBP (mmHg) | 150 (120–239) | 152 (120–239) | 150 (130–170) | 0.462 |
| DBP (mmHg) | 90 (57–120) | 90 (57–120) | 90 (70–100) | 0.237 |
| BP medications screening, n | 2 (0–4) | 2 (0–4) | 2 (1–4) | 0.270 |
| ARR screening (pmol/ng) | 139.8 (1.65–2620) | 133.1(1.7–2620) | 277.5 (47–698) | 0.074 |
| Plasma potassium concentration at screening (mmol/L) | 3.2 (2.0–4.0) | 3.2 (2.0–4.0) | 2.9 (2.0–4.0) | 0.475 |
| 24‐h urinary aldosterone (nmol/24 h) | 65 (3–680) | 63 (3–680) | 106.5 (63–170) | 0.028 |
Abbreviations: ARR, aldosterone–renin ratio; BMI, body mass index; BP medications, blood pressure medication; CVD, cardiovascular disease; DBP, diastolic blood pressure; n, number; If not n, then median (range); SBP, systolic blood pressure.
Results of the FSTamb and FSTin are presented in Table 3. For the FSTamb group, the median plasma potassium concentration during the FST was stable and varied from 3.5 to 3.6 mmol/L (Table 4, Figure 1A). The lowest plasma potassium concentration in the FSTamb patient group was 2.8 mmol/L, measured on Day 3, and 2.8 mmol/L on Day 5 in the FSTin group. None of the patients required hospitalisation or reported any arrhythmias during the FST.
Table 3.
Results of the fludrocortisone suppression test performed either as an ambulatory or as an in‐patient in the Stockholm region
| FSTamb | FSTin | p‐Value | |
|---|---|---|---|
| Plasma potassium concentration (mmol/L), Day 1 offludrocortisone suppression test (FST) median (range) | 3.6 (2.9–4.3) | 3.8 (3.5–4.1) | .019 |
| Plasma potassium concentration (mmol/L), Day 2 of FST median (range) | 3.6 (2.9–4.1) | 3.6 (3.2–3.9) | .691 |
| Plasma potassium concentration (mmol/L), Day 3 of FST median (range) | 3.5 (2.8–4.4) | 3.6 (3.0–3.9) | .659 |
| Plasma potassium concentration (mmol/L), Day 4 of FST median (range) | 3.6 (3.0–4.2) | 3.5 (3.4–4.1) | .836 |
| Plasma potassium concentration (mmol/L), Day 5 of FST median (range) | 3.6 (2.9–4.8) | 3.5 (2.8–4.2) | .075 |
| Increase potassium tablets n (%) | 113 (74.8) | 9 (60) | .086 |
| Increase potassium tablets, n median (range) | 4 (0–28) | 10.5 (2–25) | .001 |
| Potassium tablets, n, Day 1 median (range) | 6 (0–24) | 9.5 (2–33) | .019 |
| Potassium tablets, n, Day 2 median (range) | 8 (0–24) | 14.5 (3–36) | .030 |
| Potassium tablets, n, Day 3 median (range) | 8 (0–26) | 15.5 (3–36) | .015 |
| Potassium tablets, n, Day 4 median (range) | 8 (0–40) | 18 (3–36) | .004 |
| Potassium tablets, n, Day 5 median (range) | 8 (0–35) | 8.5 (2–34) | .274 |
| 24 h urinary sodium, Day 5 FST (mmol/24 h) | 244.0 (49–741) | 248.0 (123–351) | .784 |
| S‐cortisol recumbent posture, Day 5 FST (nmol/L) | 298.0 (132–1750) | 356.0 (249–667) | .031 |
| S‐cortisol seated posture, Day 5 FST (nmol/L) | 230 (102–866) | 334.0 (121–556)4 | .094 |
| PAC recumbent posture Day 5 FST (pmol/L) | 268.5 (69–1580) | 512.0 (69–1560) | .024 |
| PAC seated posture, Day 5 FST (pmol/L) | 333.5 (69–1320) | 538.0 (117–1980) | .018 |
| 24 h urinary aldosterone, Day 5 FST (nmol/24 h) | 53 (1.9–310) | 107.5 (20–290) | .015 |
| SBP (mmHg), recumbent posture Day 1 | 148 (113–210) | 160 (140–160) | .618 |
| DBP (mmHg), recumbent posture, Day 1 | 90 (65–128) | 100 (90–100) | .705 |
| SBP (mmHg), recumbent posture Day 5 | 150 (104–210) | 165 (145–166) | .339 |
| DBP (mmHg), recumbent posture, Day 5 | 90 (56–125) | 90 (88–100) | .515 |
| PA, n (%) | 109 (69.8) | 12 (80) | .387 |
Abbreviations: DBP, diastolic blood pressure; n, number. If not n, then median (range); PA, primary aldosteronism; PAC, plasma aldosterone concentration; SBP, systolic blood pressure.
Table 4.
Clinical and biochemical variables in patients who had an ambulatory fludrocortisone suppression test in the Stockholm region, divided into those later confirmed to have primary aldosteronism or not
| Total | PA | Non‐PA | p‐Value | |
|---|---|---|---|---|
| Number, n (%) | 156 | 109 (69.9) | 47 (30.1) | |
| Gender | .037 | |||
| Female n (%) | 80 (51.3) | 49 (45.4) | 31 (64.6) | |
| Male n (%) | 76 (48.7) | 59 (54.6) | 17 (35.4) | |
| Age (years) | 54 (33–73) | 52 (22–75) | 51 (26–74) | .997 |
| BMI (kg/m2) | 22.4 (21–34) | 27.3 (18–45) | 27.9 (18–35) | .827 |
| Duration of HT (years) | 4 (0–39) | 5 (0–39) | 4 (0–27) | .221 |
| History of CVD, n (%) | 18 (11.5) | 16 (14.6) | 2 (4.2) | .061 |
| History of diabetes, n (%) | 26 (16.6) | 23 (21.1) | 3 (6.3) | .020 |
| History of dyslipidemia, n (%) | 9 (5.8) | 8 (7.4) | 1 (2.1) | .277 |
| History of cancer, n (%) | 16 (10.3) | 8 (7.3) | 8 (17.0) | .091 |
| Active smoking, n (%) | 17 (13.2) | 8 (8.7) | 9 (24.3) | .024 |
| SBP (mmHg) | 152 (120–239) | 156 (120–239) | 149 (120–200) | .07 |
| DBP (mmHg) | 90 (57–120) | 90 (64–120) | 90 (57–120) | .572 |
| Creatinine (μmol/L) | 74 (4.8–119) | 76 (37–119) | 70 (4.8–105) | .264 |
| BP medications screening, n | 2 (0–4) | 2 (0–4) | 2 (0–4) | .176 |
| ARR screening (pmol/ng) | 133.1 (1.65–2620) | 179.1 (1.65–2620) | 107.6 (12–316.2) | .02 |
| ARR (pmol/mIU) | 205.1 (37.09–1172.5) | 208 (37.0–1172.5) | 194.5 (101.1–820) | .136 |
| 24 h urinary aldosterone, screening (nmol/24 h) | 72 (25–680) | 72 (25–680) | 53 (3–100) | .01 |
| Initial potassium concentration (mmol/L) | 3.2 (2–4) | 3.1 (2–4) | 3.35 (3–4) | <.01 |
| Increase potassium tablets n (%) | 113 (72.4) | 81 (79.4) | 32 (71.1) | .518 |
| Increase potassium, n tablets | 4.0 (0–28) | 4.0 (0–28) | 2.0 (0–10) | .005 |
| SBP follow‐up (mmHg) | 134 (105–210) | 132 (105–210) | 137.5 (110–180) | .120 |
| DBP follow‐up (mmHg) | 80 (50–120) | 80 (50–1110) | 85 (55–120) | .015 |
| BP medication follow‐up, n | 2 (0–6) | 2 (0–6) | 2 (0–5) | .630 |
| Potassium concentration follow‐up(mmol/L) | 4.0 (3.0–5.6) | 4.0 (3.0–5.6) | 3.9 (3.2–4.8) | .005 |
| MRA follow‐up, n | 69 | 69 | 0 | |
| Spironolactone dosage (mg) | 22.0 (0–150) | 22.0 (0–150) | 0 | |
| Eplerenon dosage (mg) | 6.59 (0–50) | 6.59 (0–50) | 0 |
Abbreviations: ARR, aldosterone‐renin ratio; BMI, body mass index; BP, medication, blood pressure medication; CVD, cardiovascular disease; DBP, diastolic blood pressure; n, number. If not n, then median (range); non‐PA, patients without primary aldosteronism; PA, patients with primary aldosteronism; SBP, systolic blood pressure.
Figure 1.
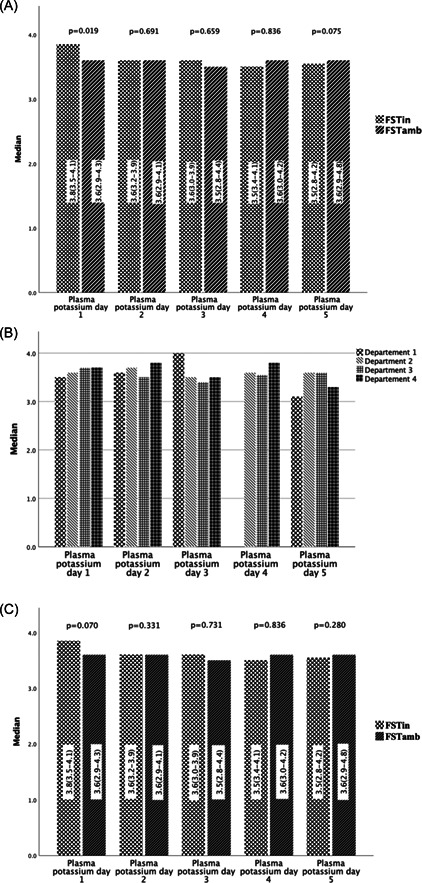
(A) Plasma potassium variation during fludrocortisone suppression test (FST) in‐patient FST (FST)in and ambulatory FST (FSTamb). (B) Plasma potassium variation during FSTamb in the four centres in Stockholm. (C) Plasma potassium variation FSTamb and FSTin in Stockholm when excluding the centre without daily control of plasma potassium.
In centre 1 (Figure 1B), the plasma potassium concentration was measured on Days 1, 2 and 5 but only in a very few cases on Days 3 and 4. This centre performed the FSTamb mostly from Thursday till Monday, that is, they did not check potassium during the weekend. When the plasma potassium concentration on Day 5 in this centre was compared with the other centres, a significant difference was found (3.1 [2.9–3.8] vs. 3.5 [2.9–4.8] mmol/L, p < .001) (Figure 1B). When excluding centre 1, no difference was found in plasma potassium concentration on Day 5 between FSTamb and FSTin (p = .280) (Figure 1C).
In the FSTamb group, the dose of slow‐release potassium chloride tablets was increased in 113 cases (74.8%) with 4 (0–28) tablets, while in the FSTin group, the dose was increased in nine patients (60%) with 10.5 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 tablets (p = .001) (Table 3). The dosage of slow‐release potassium chloride tablets was usually increased on Days 1 or 2 (51 cases [29.8%] and 59 cases [34.5%], respectively). When dividing the FSTamb group into those later diagnosed with PA and those without confirmed PA, the need for slow‐release potassium chloride tablets was higher in the PA group (4.0 [0–28] vs. 2.0 [0–10] tablets, p = .005) (Table 4).
In 112 cases (71.8%) in the FSTamb group and 10 cases (67%) in the FSTin group, the 24‐h urinary sodium concentration on Day 5 was ≥200 mmol/24 h. The 24‐h urinary sodium concentration on Day 5 in the FSTamb group was similar to that found in the FSTin group (244.0 [49–741] vs. 248 [123–351] mmol/24 h, p = .784).
Serum cortisol concentrations at the end of the FST were lower within the FSTamb group than in the FSTin group (Table 3) and displayed a decrease from 08 AM to 12 AM in both groups consistent with the expected circadian rhythm.
PAC in a recumbent and seated posture and 24‐h urinary aldosterone at Day 5 was lower within the FSTamb group then in FSTin group (Table 3).
All patients were questioned about adherence, and three patients (1.9%) in the FST amb group self‐reported issues with medicines intake. Those had to repeat the test due to incomplete intake of medications during the test. When the FST was repeated, the patients received even more careful and detailed information, and the second test was performed adequately.
Out of 156 FSTamb patients, five (3.2%) reported mild adverse events, which did not result in discontinuation of the test, and the patients did not require additional medical assistance. These adverse events were a mild and short episode of abdominal pain (n = 1), diffuse sense of chest discomfort (n = 1), nausea after taking the fludrocortisone tablets (n = 1), light headache (n = 1) and a short episode of palpitations (n = 1). In the FSTin group there were no reports of adverse events during the test. In all patients reporting adverse events, normal plasma potassium concentration was found at that time.
Patients with confirmed PA in the FSTamb group were more often active smokers and more had diabetes compared with the patients in the non‐PA group. A tendency to higher systolic blood pressure and higher rates of cardiovascular disease was also noted in this group (Table 4).
Adrenal venous sampling (AVS) was performed in 59 patients (54.1%) of the confirmed PA cases. All patients in which AVS indicated unilateral disease (n = 40) underwent unilateral adrenalectomy. In the FSTamb group, adrenalectomy was performed in 34 patients (31.2%), and treatment with a mineralocorticoid receptor antagonist was offered to the other 75 patients (68.8%). In the FSTin group, 12 patients (80%) were diagnosed with PA, and out of those, six cases (50%) underwent adrenalectomy. Patients with confirmed PA and bilateral aldosterone secretion according to AVS, as well as those who were not deemed suitable for, or did not accept surgery, were treated with a mineralocorticoid receptor antagonist. Outcomes of the patients in the PA group are described in Table 5. To our knowledge, none of the patients deemed not having PA were later referred for re‐evaluation to our centres.
Table 5.
Follow‐up outcomes of the cases of treated patients with primary aldosteronism
| Operated PA | MRA treated PA | p‐Value | |
|---|---|---|---|
| Number | 40 | 69 | |
| FSTamb | 23 | 13 | |
| PAC pmol/L | 202 (93–702) | NA | |
| PRC ng/L | 14 (3–220) | NA | |
| PRC mIU/L | 16 (6–87) | NA | |
| ARR pmol/ng | 19.54 (2–48) | NA | |
| ARR pmol/mIU | 9.1 (0.4–10) | NA | |
| Systolic blood pressure (mmHg) | 130 (108–170) | 135 (105–210) | .140 |
| Diastolic blood pressure (mmHg) | 80 (60–100) | 80 (50–110) | .095 |
| BP medication, n | 1 (0–6) | 2 (0–5) | <.001 |
| Plasma potassium concentration (mmol/L) | 4.1 (3.6–5.6) | 4.0 (3.0–4.8) | .083 |
| Patients treated with, n | 1 | 69 | <.001 |
| Spironolactone dosage (mg) | 50 | 25 (0–150) | <.001 |
| Eplerenone dosage (mg) | 0 | 10.81 (0–25) | <.001 |
Abbreviations: ARR, aldosterone–renin ratio; BP medications, blood pressure medication; BP, blood pressure; MRA, mineralocorticoid receptor antagonist; n, number; NA, not available; PAC, plasma aldosterone concentration; PRC, plasma renin concentration.
After treatment, a reduction in blood pressure was obtained in the PA group (pretreatment vs. posttreatment: systolic blood pressure was 156 [120–239] vs. 132 [105–145] mmHg, p = .007; diastolic blood pressure 90 [64–120] vs. 80 [60–100] mmHg, respectively, p = .013).
In patients with PA confirmed by FSTamb, the serum potassium concentration after treatment was 4.1 (3.7–5.6) mmol/L.
The costs for FST were much lower when performed ambulatory than in an in‐patient setting. The cost for FSTamb was 20,330 SEK per patient (about $2400) compared to 44,928 SEK (about $5200) per patient for the FSTin.
4. DISCUSSION
To the best of our knowledge, this is the first study to explore the safety and accuracy of FST performed in an ambulatory setting. In Stockholm, there is a long tradition of performing FSTamb, but this practice had not been evaluated systematically. We found that the FSTamb is safe, accurate, and can be performed with about 50% reduction in health costs compared to the FSTin.
The FST is considered to be reliable. Still, it is often avoided because of its complexity and high costs. 27 , 28 The major concerns about performing FSTamb are associated with undiscovered and untreated hypokalemia. The fludrocortisone administered in the FST is associated with a risk of hypokalemia and hence, risk of cardiac arrhythmia. To avoid this, patients selected for FST are admitted to hospital. 22 A normal plasma potassium concentration is desired in both the initial investigation with ARR as well as in the following confirmatory test. A low plasma potassium concentration will decrease the PAC as well as ARR and could lead to false‐negative results. 21 In our study, mild hypokalemia, defined as plasma potassium concentration under the lower normal range (<3.4 mmol/L), was found during one or several occasions during the FSTamb, but it did not require hospitalisation. It is of concern that some patients did not have a stable potassium level during the FSTamb. When we studied the data from the different centres, we found that in centres where the plasma potassium concentration was closely followed day by day, and the slow‐releasing potassium tablets were adjusted daily, the plasma potassium concentration was normal during the entire test (Figure 1). The patients who had a low plasma potassium concentration on Day 5 had nevertheless a high PAC which was indicative of PA. Based on the presented data, our recommendation is that the plasma potassium concentration should be controlled daily during the FSTamb. It could be speculated that using a higher dosage of slow‐release potassium tablets on Day 1 may reduce the number of controls required.
According to the Endocrine Society Clinical Practice Guideline, 21 during the FST, the measurement of plasma potassium concentration is needed four times a day requiring that the patients are carefully controlled in the hospital. Our findings indicate that the FSTamb is safe and can be performed with only one daily measurement of plasma potassium concentration. This is an improvement for the patients as they will be able to continue normal daily activities without the need for hospital care or sick leave.
Another concern with performing FSTamb is the risk of failing to achieve correct results. The patients are required to take a large number of tablets four times a day. Hence, it is essential to rigorously inform the patient and achieve controlled patient compliance during the procedure. In our centres, the patients received both written and oral information before starting FSTamb. Only 1.9% of the patients had to repeat the test due to inappropriate intake of medicines. Furthermore, only a few minor adverse events were reported without causing discontinuation of the ongoing test.
The hospital environment is associated with increased stress levels and thereby with an activation of the ACTH‐cortisol axis when compared with the home environment. 29 This increase in ACTH might interfere with aldosterone suppression during FST resulting in false‐positive results. 21 Although not statistically significant, FSTamb patients exhibited a lower serum cortisol concentration compared to FSTin patients. These findings indicate that the FSTamb patient feels more comfortable, suggesting that performing FST in ambulatory settings might achieve a more reliable result compared to FSTin.
In the present study, we investigated the costs of the FST performed as an ambulatory test. We found that the time needed for health professionals to complete the FSTamb was only 2.5 h of nurse time and 1 h of physician time, respectively. This is a considerable reduction compared with the FSTin. Further, the total health care costs with the FSTamb compared to the FSTin was significantly reduced by more than 50%. We are aware that the health costs will vary depending on the hospital and the country where FST is performed. Nevertheless, we believe that our calculation gives a fair estimation of the cost reduction.
The 24‐h urinary sodium concentration on Day 5 was similar in both the FSTamb and FSTin groups which suggest that both tests were equally accurate. The outcomes of the FSTamb were reliable and could confirm the diagnosis of PA. The PA diagnosis was confirmed by pathology reports after adrenalectomy in 40 cases (33%), and by the correct response to treatment with mineralocorticoid receptor antagonist in the non‐operated PA group of 69 cases (66.9%). After PA treatment, the blood pressure was considerably improved in all patients.
The complexity and concerns regarding the potentially serious complications of the FST have resulted in the use of other confirmatory tests that do not require hospitalisation, such as the oral sodium loading test, the saline infusion test, and the captopril challenge test. However, several studies have reported that the sensitivity and specificity of these confirmatory tests are less compared to FST 23 , 30 and FST is regarded as a reliable confirmatory test. 22 , 27 Hence, by demonstrating the FSTamb as a feasible, reliable and safe test, we believe that the FST will continue to be used as a routine confirmatory test for PA in clinical practice.
There are limitations to the present study. This is a retrospective study with the inherent risk of selection and information bias. The FSTin group was 10 times smaller than the FSTamb. Moreover, the reported data were not homogeneous due to variation in the local FST protocols regarding plasma potassium controls between the four centres. The lack of homogeneous follow‐up is also a limitation.
5. CONCLUSIONS
The FSTin is considered the most reliable confirmatory test in PA but is not used frequently due to its cost and complexity. In this study, we report that the FSTamb is a safe and accurate test with a significant reduction in health costs compared to the FSTin. Hence, we believe that the FSTamb can be used as a safe confirmatory test for PA, both in clinical and research contexts. To our knowledge, this is the first study that systematically evaluates FSTamb and compares it to FSTin. Confirmation of our findings in other patient populations and other centres is needed.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Carasel A, Calissendorff J, Avander K, Shabo I, Volpe C, Falhammar H. Ambulatory fludrocortisone suppression test in the diagnosis of primary aldosteronism: safety, accuracy and cost‐effectiveness. Clin Endocrinol (Oxf). 2022;97:730‐739. 10.1111/cen.14793
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Galati SJ. Primary aldosteronism: challenges in diagnosis and management. Endocrinol Metab Clin North Am. 2015;44(2):355‐369. [DOI] [PubMed] [Google Scholar]
- 2. Gkaniatsa E, Ekerstad E, Gavric M, et al. Increasing incidence of primary aldosteronism in Western Sweden during 3 decades—yet an underdiagnosed disorder. J Clin Endocrinol Metab. 2021;106(9):e3603‐e3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu Z, Yang J, Hu J, et al. Primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol. 2020;75(16):1913‐1922. [DOI] [PubMed] [Google Scholar]
- 4. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811‐1820. [DOI] [PubMed] [Google Scholar]
- 5. Libianto R, Russell GM, Stowasser M, et al. Detecting primary aldosteronism in Australian primary care: a prospective study. Med J Aust. 2022;216:408‐412. [DOI] [PubMed] [Google Scholar]
- 6. Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta‐regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826‐2835. [DOI] [PubMed] [Google Scholar]
- 7. Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient's cohorts and in population‐based studies—a review of the current literature. Horm Metab Res. 2012;44(3):157‐162. [DOI] [PubMed] [Google Scholar]
- 8. Fagugli RM, Taglioni C. Changes in the perceived epidemiology of primary hyperaldosteronism. Int J Hypertens. 2011;2011:162804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet. 2008;371(9628):1921‐1926. [DOI] [PubMed] [Google Scholar]
- 10. Funder JW, Carey RM, Fardella C, et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93(9):3266‐3281. [DOI] [PubMed] [Google Scholar]
- 11. Young WF. Primary aldosteronism: renaissance of a syndrome. Clin Endocrinol (Oxf). 2007;66(5):607‐618. [DOI] [PubMed] [Google Scholar]
- 12. Melby JC. Diagnosis of hyperaldosteronism. Endocrinol Metab Clin North Am. 1991;20(2):247‐255. [PubMed] [Google Scholar]
- 13. Monticone S, D'Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2018;6(1):41‐50. [DOI] [PubMed] [Google Scholar]
- 14. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Funder JW. Primary aldosteronism and cardiovascular risk, before and after treatment. Lancet Diabetes Endocrinol. 2018;6(1):5‐7. [DOI] [PubMed] [Google Scholar]
- 16. Lubitz CC, Economopoulos KP, Sy S, et al. Cost‐effectiveness of screening for primary aldosteronism and subtype diagnosis in the resistant hypertensive patients. Circ Cardiovasc Qual Outcomes. 2015;8(6):621‐630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwartz GL. Screening for adrenal‐endocrine hypertension: overview of accuracy and cost‐effectiveness. Endocrinol Metab Clin North Am. 2011;40(2):279‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Velasco A, Chung O, Raza F, et al. Cost‐effectiveness of therapeutic drug monitoring in diagnosing primary aldosteronism in patients with resistant hypertension. J Clin Hypertens (Greenwich). 2015;17(9):713‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jaffe G, Gray Z, Krishnan G, et al. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension. 2020;75(3):650‐659. [DOI] [PubMed] [Google Scholar]
- 20. Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: deficiencies in screening at‐risk hypertensives. Surgery. 2019;165(1):221‐227. [DOI] [PubMed] [Google Scholar]
- 21. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889‐1916. [DOI] [PubMed] [Google Scholar]
- 22. Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96(4):1327‐1384. [DOI] [PubMed] [Google Scholar]
- 23. Ahmed AH, Cowley D, Wolley M, et al. Seated saline suppression testing for the diagnosis of primary aldosteronism: a preliminary study. J Clin Endocrinol Metab. 2014;99(8):2745‐2753. [DOI] [PubMed] [Google Scholar]
- 24. Liu B, Hu J, Song Y, et al. Seated saline suppression test is comparable with captopril challenge test for the diagnosis of primary aldosteronism: a prospective study. Endocr Pract. 2021;27(4):326‐333. [DOI] [PubMed] [Google Scholar]
- 25. Song Y, Yang S, He W, et al. Confirmatory tests for the diagnosis of primary aldosteronism: a prospective diagnostic accuracy study. Hypertension. 2018;71(1):118‐124. [DOI] [PubMed] [Google Scholar]
- 26. Giacchetti G, Mulatero P, Mantero F, Veglio F, Boscaro M, Fallo F. Primary aldosteronism, a major form of low renin hypertension: from screening to diagnosis. Trends Endocrinol Metab. 2008;19(3):104‐108. [DOI] [PubMed] [Google Scholar]
- 27. Williams TA, Reincke M. Management of endocrine disease: diagnosis and management of primary aldosteronism: the endocrine society guideline 2016 revisited. Eur J Endocrinol. 2018;179(1):R19‐R29. [DOI] [PubMed] [Google Scholar]
- 28. Young WF, Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285(2):126‐148. [DOI] [PubMed] [Google Scholar]
- 29. Scheer FA, Van Paassen B, Van Montfrans GA, et al. Human basal cortisol levels are increased in hospital compared to home setting. Neurosci Lett. 2002;333(2):79‐82. [DOI] [PubMed] [Google Scholar]
- 30. Wu S, Yang J, Hu J, et al. Confirmatory tests for the diagnosis of primary aldosteronism: a systematic review and meta‐analysis. Clin Endocrinol (Oxf). 2019;90(5):641‐648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


