Abstract
Background
Genetic and environmental influences on externalizing problems are often studied separately. Here, we extended prior work by investigating the implications of gene–environment interplay in childhood for early adult externalizing behavior. Genetic nurture would be indicated if parents' genetic predisposition for externalizing behavior operates through the family environment in predicting offspring early adult externalizing behavior. Evocative gene–environment correlation would be indicated if offspring genetic predisposition for externalizing behavior operates through child externalizing behavior in affecting the family environment and later early adult externalizing behavior.
Method
Longitudinal data from seven waves of the TRacking Adolescents' Individual Lives Survey, a prospective cohort study of Dutch adolescents followed from age 11 to age 29 (n at baseline = 2,734) were used. Child externalizing behavior was assessed using self and parent reports. Family dysfunction was assessed by parents. Early adult externalizing behavior was assessed using self‐reports. Genome‐wide polygenic scores for externalizing problems were constructed for mothers, fathers, and offspring.
Results
Offspring polygenic score and child behavior each predicted early adult externalizing problems, as did family dysfunction to a small extent. Parents' polygenic scores were not associated with offspring's early adult externalizing behavior. Indirect effect tests indicated that offspring polygenic score was associated with greater family dysfunction via child externalizing behavior (evocative gene–environment correlation) but the effect was just significant and the effect size was very small. Parents' polygenic scores did not predict family dysfunction, thus the data do not provide support for genetic nurture.
Conclusions
A very small evocative gene–environment correlation was detected but effect sizes were much more pronounced for stability in externalizing behavior from childhood through early adulthood, which highlights the necessity to intervene early to prevent later problems.
Keywords: Externalising disorder, longitudinal studies, molecular genetics, gene‐environment interaction (GxE), family functioning
Introduction
Externalizing behavior refers to difficulties with regulating emotions, exerting self‐control, and inhibiting impulsivity. Examples of externalizing behavior in childhood are defiance and opposition, temper tantrums, and aggressive behavior toward peers. In adolescence and adulthood, externalizing problems continue to include aggressive behavior, but may also be expressed as substance use and sexual risk‐taking. Despite the substantial personal and societal toll resulting from externalizing behavior in early adulthood, our understanding of its origins is much less comprehensive than for externalizing behavior in childhood and adolescence.
Externalizing behavior is influenced by genetic and environmental factors. Genetic influence on externalizing behavior has been established in quantitative genetic (i.e., twin and adoption) studies on antisocial behavior (Rhee & Waldman, 2002), self‐control (Willems, Boesen, Li, Finkenauer, & Bartels, 2019), and substance use (Verhulst, Neale, & Kendler, 2015). More recently, findings of genetic influences have been corroborated by molecular genetic work that uses genome‐wide association studies to identify DNA markers for externalizing problems. The polygenic nature of a complex trait such as externalizing behavior means that single genes explain only trivial amounts of variance. However, cumulative indices comprising many genetic markers (so‐called polygenic scores) which explain greater portions of variance can be constructed. A recent polygenic score derived from a genome‐wide association study on multiple aspects of externalizing behavior including attention‐deficit hyperactivity disorder (ADHD) symptoms, substance use, first sexual intercourse, number of sexual partners, and risk tolerance explained over 10% of the variance in externalizing behavior (Karlsson Linnér et al., 2021). Though this still falls short of estimates from quantitative genetic studies, polygenic scores can be highly valuable in studies that seek to probe genetic influences on externalizing behavior.
Externalizing problems are also linked to environmental factors and the quality of one's social environment plays an important role. Research has mostly focussed on parental behavior, parenting, and the quality of the parent–child relationship (Pinquart, 2017) but broader measures of the general functioning of a family are also linked to offspring adjustment. In that respect, family dysfunction has been linked to attention problems and antisocial behavior (Richards et al., 2019) and substance use (Cox et al., 2021; Hill, Shanahan, Costello, & Copeland, 2017). Importantly, although family functioning is usually conceptualized as an “environmental condition,” it is partly under genetic influence (Vinkhuyzen, Van Der Sluis, De Geus, Boomsma, & Posthuma, 2010) from at least two sources: parental genes and offspring genes. This means that both parental genes and offspring genes might influence the family environment and, by extension, explain elevated risk for early adult externalizing problems.
When parental genes influence offspring outcomes via the family environment over and above the influence of the offspring's own genes, the mechanism is termed genetic nurture. Studies support genetic nurture for offspring's cognitive abilities and educational outcomes (de Zeeuw et al., 2020; Wang et al., 2021; Wertz et al., 2019). It is plausible that parental genetic predisposition for externalizing problems contributes to a dysfunctional family environment, which in turn is associated with offspring negative outcomes (Jaffee, Belsky, Harrington, Caspi, & Moffitt, 2006). However, information on genetic nurture as a process predicting externalizing behavior beyond childhood is scarce and most studies rely on genetic information from one parent only.
When offspring genes influence their own externalizing behavior via the family environment, a possible mechanism is evocative gene–environment correlation, whereby genetically influenced traits and behaviors in offspring elicit specific behaviors and feelings from parents and thereby contribute to family functioning. For instance, heritable child traits such as aggression, ADHD symptoms, and difficult temperament might contribute to family discord and parental stress (Avinun & Knafo, 2014). In this case, the association between the family environment and adult externalizing behavior would thus initially be driven by child factors. There is some support for this possibility from research showing that family functioning changed as a function of offspring externalizing problems (Mastrotheodoros, Canário, Gugliandolo, Merkas, & Keijsers, 2020) and that children's heritable traits predicted reactions from adoptive parents, which in turn were associated with child externalizing problems at age 7 (Shewark et al., 2021). More broadly, however, research into evocative gene–environment correlation is scarce, especially on externalizing behavior beyond middle childhood.
Current study
We used longitudinal data spanning almost two decades and polygenic scores of externalizing behavior available for offspring and both parents to test genetic nurture and evocative gene–environment correlation. With respect to genetic nurture, we hypothesized that parents' genes would predict family dysfunction which in turn would predict the offspring early adult externalizing behavior. We tested whether family dysfunction functions as an intermediate variable in the pathway from parents' genes to offspring externalizing behavior in early adulthood while controlling for offspring polygenic score (Armstrong‐Carter et al., 2020; Willoughby, McGue, Iacono, Rustichini, & Lee, 2021). The availability of both parents' genetic information is particularly valuable in this test of genetic nurture. With respect to evocative gene–environment correlation, we hypothesized that genetically influenced offspring externalizing traits would predict family dysfunction, which in turn would predict early adult externalizing problems. We tested whether offspring externalizing behavior in childhood functions as an intermediate variable in the pathway from offspring genes to family dysfunction (in line with evocative gene–environment correlation), and on to externalizing behavior in early adulthood. Parental polygenic scores were included as a covariate in this model. Offspring sex was included as a covariate in both models.
Methods
Participants
The present study includes data from the first seven waves of the TRacking Adolescents' Individual Lives Survey (TRAILS), a prospective cohort study of Dutch adolescents, with bi‐ or triennial follow‐up assessments. TRAILS consists of a population and high‐risk sample: the population sample was collected in five municipalities in the north of the Netherlands, including urban and rural areas. Initially, 135 primary schools were approached of which 122 agreed to participate. In brief, a total of 2,935 children were invited to participate of whom 2,229 (51% female) did so at T1. Data collection at the first assessment wave (T1) took place in 2001 and 2002 (mean age 11.1 years), the second wave (T2) in 2003 and 2004 (mean age 13.6 years), the third wave (T3) in 2006 and 2007 (mean age 16.3 years), the fourth wave (T4) in 2008 to 2010 (mean age 19.1 years), the fifth wave (T5) was conducted in 2012 and 2013 (average age 22.3 years), the sixth wave (T6) took place in 2016 (average 25.7 years), and the seventh wave (T7) was conducted in 2020 when participants were on average 28.9 years old.
The TRAILS population sample was complemented by a sample selected based on contact with the child and adolescent mental health services before age 11. This “high‐risk sample” was set up in 2004, with the inclusion of 543 children (response rate 43%). Boys were over‐represented (66%), in line with boy/girl ratios for the most common childhood psychopathologies. Comparable to the population sample, follow‐up data collection waves occurred at intervals of 2–3 years but lag behind by approximately one assessment wave, which means that T7 for the high‐risk cohort is not included in the analyses reported here. Details about the study and attrition have been published in several reports (Huisman et al., 2008; Oldehinkel et al., 2015). We use full information maximum likelihood estimation to make use of all available data points, resulting in an analytic sample size of n = 2,693 for the model testing genetic nurture and n = 2,734 for the model testing evocative gene–environment correlation.
Ethical considerations
Ethics approval for TRAILS was obtained from the Dutch national ethics committee CCMO and both parents and children provided informed consent.
Measures
Detailed information including example items, response options, and descriptive statistics for all measures used in the analyses is provided in Appendix S1.
Child externalizing behavior was assessed at T1 using the Youth Self Report (Achenbach & Rescorla, 2001) Aggressive behavior and ADHD symptoms subscales and parent‐reported child temperament, specifically Early Adolescent Temperament Questionnaire (Ellis & Rothbart, 1999) subscales Frustration and Effortful control (reverse coded).
Family dysfunction was assessed using the Family Assessment Device (Epstein, Baldwin, & Bishop, 1983), which was completed by one parent – most often the mother – at T1 to T3.
Early adult externalizing behavior was assessed at T4 to T7 using the Adult Self Report (Achenbach & Rescorla, 2001) Aggressive behavior and ADHD symptoms subscales, as well as an item on Risk tolerance. We assessed Substance use using single items on Daily tobacco and Lifetime cannabis use, and Hazardous alcohol use with the Alcohol Use Disorder Identification Test (Saunders, Aasland, Babor, Fuente, & Grant, 1993). Sexual risk‐taking included Age at first sex and Number of partners. These indicators reflect the measures used in the genome‐wide association study on which the polygenic score used here is based (Karlsson Linnér et al., 2021).
Polygenic scores. Genotyping procedures are described in detail in Appendix S2. Polygenic scores for offspring and parents were generated as the weighted sum of alleles using LDPred (Vilhjálmsson et al., 2015), where weights were the effect sizes taken from the summary statistics of the GWAS on externalizing problems (Karlsson Linnér et al., 2021), multiplied by linkage disequilibrium scores as calculated by LDPred using the respective TRAILS cohort. R package MetaSubtract version 1.60 (Nolte, 2020a) was used to subtract the results of the validation cohort from the meta‐GWAS results analytically and produce meta‐GWAS summary statistics that were independent of the TRAILS sample (Nolte, 2020b). We used the polygenic score with all available variants included, that is, did not apply a p‐threshold for inclusion. Where parents shared >20% of their genes, indicating first‐ or second‐degree relatives, the genetic information of only one of the parents was used in the analyses. Finally, we only included one child per family giving priority to the participant in the population cohort as these were already assessed for T7.
Analytic procedure
Descriptive statistics and correlations between variables were computed in Stata and path models including estimation of indirect effects were computed in Mplus. To aid model stability, we first computed confirmatory factor analyses and extracted factor scores in separate models for child externalizing behavior, family dysfunction, and early adult externalizing behavior (see Appendix S3 for correlations between factor indicators). Details on the model selection are provided in Appendix S4; note that we excluded effortful control as an indicator of child externalizing behavior and age at first sex as an indicator of early adult externalizing behavior due to non‐significant/very low loadings. All other indicators were kept to stay as much as possible in line with the early adult externalizing behavior construct used in the genome‐wide association study on which the polygenic score is based (Karlsson Linnér et al., 2021). Extracted factors were regressed on sex and residuals were used in subsequent analyses. We computed path models for testing genetic nurture and evocative gene–environment correlations using maximum likelihood estimation. We first tested whether parents' genes predicted offspring outcomes after controlling for offspring genes. To evaluate the indirect effects of parents' polygenic scores on offspring early adult externalizing behavior via family dysfunction, we used the Model Indirect command in Mplus and employed bootstrapping with 5,000 draws (Hayes, 2009). The same indirect effects procedure was used to test evocative gene–environment correlation, that is, from offspring polygenic score to family dysfunction via child externalizing behavior, and on to early adult externalizing behavior. We report standardized estimates and 95% confidence intervals for path estimates.
Some studies on the effects of polygenic scores use “midparent” scores (Willoughby et al., 2021), where polygenic scores of mothers and fathers are averaged and the average is used instead of separate scores. We report analyses using the midparent score in Appendix S5.
Results
Preliminary analyses
We validated the externalizing polygenic score in our sample, by testing whether offspring polygenic scores were associated with offspring externalizing problems, as would be expected (Table 1). The results show that offspring externalizing polygenic score was associated with child externalizing behavior (r = .07, p = .003). As young adults, participants with higher externalizing polygenic scores continued to display more externalizing behavior (r = .15, p < .001). Family dysfunction was higher in families where offspring displayed externalizing behavior in childhood (r = .12, p < .001) and in early adulthood (r = .14, p < .001).
Table 1.
Bivariate correlations of variables included in path models
| Offspring polygenic score | Father polygenic score | Mother polygenic score | Child externalizing behavior | Family dysfunction | Early adult externalizing behavior | |
|---|---|---|---|---|---|---|
| Father polygenic score | .48, p < .001, n = 863 | |||||
| Mother polygenic score | .45, p < .001, n = 1,063 | .05, p = .203, n = 756 | ||||
| Child externalizing behavior | .07, p = .003, n = 1,670 | .05, p = .172, n = 863 | .03, p = .302, n = 1,067 | |||
| Family dysfunction | .04, p = .124, n = 1,662 | .01, p = .835, n = 866 | −.01, p = .687, n = 1,071 | .12, p < .001, n = 2,641 | ||
| Early adult externalizing behavior | .15, p < .001, n = 1,540 | .11, p = .001, n = 827 | .11, p < .001, n = 998 | .33, p < .001, n = 2,150 | .14, p < .001, n = 2,118 | |
| Offspring sex | .02, p = .496, n = 1,677 | −.02, p = .583, n = 867 | .01, p = .682, n = 1,071 | .12, p < .001, n = 2,721 | .04, p = .070, n = 2,652 | .003, p = .908, n = 2,156 |
All correlations involving offspring sex are Spearman rho coefficients, all other correlations are Pearson's r coefficients. Sex is coded as 0 = female, 1 = male. Bivariate correlations involving polygenic scores are adjusted for 20 principal components.
Genetic nurture
We first assessed whether parents' polygenic scores predicted offspring's early adult externalizing behavior while taking into account the offspring's polygenic score. This was not the case, effect sizes were β = .03, 95%CI = −.05/.11 for father polygenic score and β = .05, 95%CI = −.02/.13 for mother polygenic score. As indirect effects might be present even in the absence of direct statistical effects, we nonetheless tested the full genetic nurture model (Figure 1). Our analysis shows that both family dysfunction and offspring polygenic score predict offspring early adult externalizing behavior. Indirect effects were established from mother and father polygenic scores to offspring externalizing behavior via offspring polygenic score (mothers: β = .05, 95%CI = .01/.08, fathers: β = .05, 95%CI = .02/.08), reflecting genetic transmission. No indirect effects from parents' polygenic scores to offspring early adult externalizing behavior via family dysfunction were detected.
Figure 1.
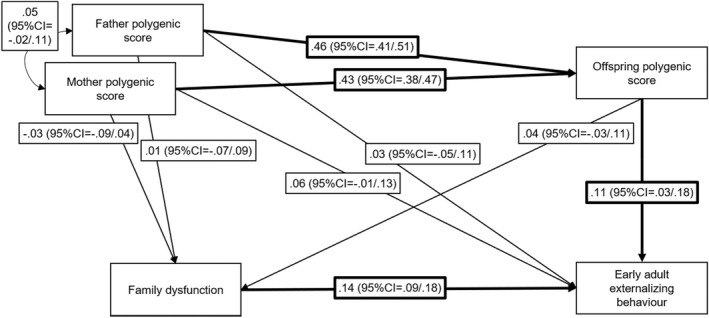
Path model testing genetic nurture. N = 2,693, the model is just‐identified. Reported estimates are standardized with bootstrapped 95% confidence intervals in brackets. Significant estimates are highlighted by bold arrows. Family dysfunction and early adult externalizing behavior were derived as factor scores from factor models (see Appendix S4 for detail) and regressed on sex before computing the path model; included here are therefore residual variances after controlling for sex. Polygenic scores were regressed on 20 principal components; included here are therefore residual variances following this step. R 2 for family dysfunction = .002, p = .546; R 2 for early adult externalizing behavior = .04, p < .001
Evocative gene–environment correlation
Our analysis of gene–environment correlation (Figure 2) shows that offspring polygenic score predicts child externalizing behavior and early adult externalizing behavior but does not directly predict family dysfunction. We did, however, detect a very small indirect effect from offspring polygenic score to family dysfunction via child externalizing behavior (β = .009, 95%CI = .001/.02), reflecting evocative gene–environment correlation. The indirect effect from offspring polygenic score to early adult externalizing behavior via family dysfunction and child externalizing behavior did not reach statistical significance (β = .001, 95%CI = .000/.002).
Figure 2.
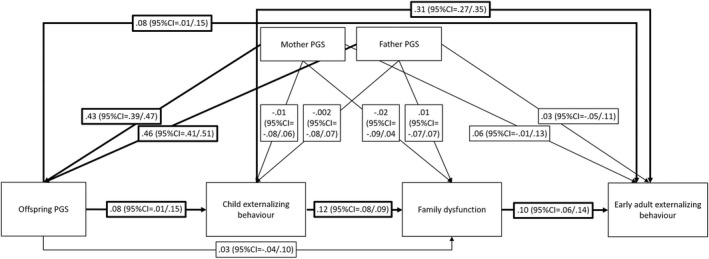
Path model testing evocative gene–environment correlation. N = 2,732, the model is just‐identified. Reported estimates are standardized with bootstrapped 95% confidence intervals in brackets. Significant estimates are highlighted by bold arrows. Childhood externalizing behavior, family dysfunction, and early adult externalizing behavior were derived as factor scores from factor models (see Appendix S4 for detail) and regressed on sex before computing the path model; included here are therefore residual variances after controlling for sex. Polygenic scores were regressed on 20 principal components; included here are therefore residual variances following this step. R 2 for child externalizing behavior = .01, p = .220, R 2 for family dysfunction = .02, p = .003; R 2 for early adult externalizing behavior = .14, p < .001. The correlation between parents' polygenic scores is r = .05 (95%CI = −.02/.11)
Analyses using midparent scores
We computed all analyses also using a midparent polygenic score, that is, averaged across mothers' and fathers' polygenic scores. Results for genetic nurture and evocative gene–environment correlation are presented in Appendix S5. There were no noteworthy differences between the original and these supplementary analyses.
Discussion and conclusion
This study combined longitudinal data spanning two decades with the genetic information of offspring and both parents to elucidate gene–environment mechanisms that might connect individual and environmental precursors to externalizing problems in early adulthood. We tested genetic nurture, whereby parents' genes influence offspring outcomes not only via genetic transmission from parent to child but also via environmental pathways, and gene–environment correlation, whereby children's heritable traits elicit environmental reactions, reflecting children's role in creating their own environment. We explored the role of both of these processes in predicting early adult externalizing problems. Overall, very modest support for evocative gene–environment correlation was detected but we found no support for genetic nurture.
Different explanations are possible for the lack of more consistent support for genetic nurture and evocative gene–environment correlation. Most prominently, the strongest predictor of early adult externalizing behavior was child externalizing behavior, which underlines the substantial developmental stability even though different aspects were captured by the child and early adult measures and assessments were at least 8 years apart. Child externalizing behavior was not included in the genetic nurture model but given its impact on early adult behavior, the question remains as to how much influence the family environment – a component of all indirect effects tested here – would actually have on early adult externalizing behavior.
Next to that, the polygenic score was constructed based on adult samples and predicted early adult externalizing behavior better than child externalizing behavior, even when child externalizing behavior and family dysfunction were accounted for. This might suggest that the polygenic score used here is less well suited to understand behaviors and processes earlier in development. Stability in externalizing behavior is partly influenced by the same genetic factors but new genetic influence across development has been observed as well. It may be that genetic factors that play a role in adult externalizing problems are less important for gene–environment interplay in childhood.
Finally, family dysfunction was used as a global measure of the family environment but might capture parental behaviors that are relevant for externalizing behavior less well than, for instance, measures of warmth, sensitivity, or neglect and harsh parenting. The latter in particular might be more strongly predicted by parents' polygenic scores for externalizing behavior but reflects the family environment less broadly.
Limitations and future directions
Longitudinal data spanning two decades and the availability of genetic information from both parents' are particular strengths of this study and our analyses present a rigorous test of gene–environment interplay. Nonetheless, some limitations need to be noted.
First, genetic data were not available from all TRAILS parents and offspring, which reduced statistical power in bivariate correlations based on complete cases. Large longitudinal datasets with genetic information on both parents are still scarce but a comprehensive understanding of genetic nurture effects necessitates their use.
Second, we constructed the early adult externalizing behavior factor based on the genome‐wide association on which the polygenic score used here was based and aligned the selection of indicators for child externalizing behavior. We also used parent‐ and self‐reports as indicators for child externalizing behavior to represent a wider spectrum of perspectives but different methodological choices would have been possible. This also applies to family dysfunction as a global assessment of relationships in the family. Analyses including other indicators or more specific representations of parent–child relationship quality are important to better understand whether gene–environment interplay effects are distinct to some aspects of the family environment or generalizable.
Third, parent‐based assessments of child externalizing behavior and family dysfunction were mostly completed by mothers. This extends to other measures in TRAILS, as only one caregiver participated in the study. To elucidate whether the genetic predisposition of one parent influences behavior or perceptions of the other over and above their own genetic predisposition, both parents' reports and genetic information needs to be included in a study. TRAILS NEXT, the next generation spin‐off study to TRAILS in which the current offspring generation, their partners, and offspring are followed into the first years of family life will provide such data (Hartman et al., 2022).
Fourth, we tested theory‐driven models: the genetic nurture model has been tested in a similar form for other aspects of the home environment and other outcomes (e.g., Wertz et al., 2019). The evocative gene–environment correlation model has recently been tested using adoption data (Shewark et al., 2021). Nonetheless, the directions of effects might be different. For instance, the home environment – here conceptualized as family dysfunction – might affect a child's externalizing behavior rather than vice versa.
Finally, we tested genetic nurture and evocative gene–environment correlation using a bootstrap‐based method and indirect effects calculation. This method directly quantifies the hypothesized indirect relationship that can exist even in the absence of a direct effect (Hayes, 2009). Direct effects were not significant in our genetic nurture model (from parental polygenic scores to offspring early adult externalizing problems) and also not for all hypothesized paths in the evocative gene–environment correlation model (from offspring polygenic score to family dysfunction). Nonetheless, an indirect effect from offspring polygenic score to family dysfunction via child externalizing behavior was detected, supporting the idea that indirect effects might be present in the absence of direct effects. That said, and also considering earlier points with respect to the selection of measures, analyses based on alternative methodological decisions would be beneficial attempts at conceptual replications of our work.
Conclusion
Externalizing behavior in early adulthood can exert a substantive toll on individual adjustment and society and our analyses show that own genes and child behavior, in particular, are implicated in its development. Genetic nurture plays less of a role in externalizing psychopathology than for other outcomes, and the quality of the family environment does not seem to function as an important pathway between genetically influenced child behavior and early adult externalizing behavior. In contrast, stability in externalizing behavior from childhood through early adulthood was strong which highlights the necessity to intervene early.
Supporting information
Appendix S1 Items, answer options, and descriptive statistics for all measures.
Appendix S2. Genotyping and polygenic score construction.
Appendix S3. Pairwise correlations between study variables.
Appendix S4. Model selection and derivation of factors.
Appendix S5. Results using midparent score.
Acknowledgements
This research is part of the TRAILS. Participating centers of TRAILS include various departments of the University Medical Center and the University of Groningen, the University of Utrecht, the Radboud Medical Center Nijmegen, and the Parnassia Group, all in the Netherlands. TRAILS has been supported by the Netherlands Organization for Scientific Research, ZonMW, GB‐MaGW, the Dutch Ministry of Justice, European Science Foundation, European Research Council, BBMRI‐NL, and participating universities. The authors are grateful to everyone who participated in this research or worked on this project to make it possible. The authors would like to thank the University of Groningen Center for Information Technology for access to the Peregrine high‐performance computing cluster. The polygenic score used here is based on work by the Externalizing Consortium (PIs: Danielle M. Dick, Philipp Koellinger, K. Paige Harden, Abraham A. Palmer. Lead Analysts: Richard Karlsson Linnér, Travis T. Mallard, Peter B. Barr, Sandra Sanchez‐Roige. Significant Contributor: Irwin Waldman) which has been supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA015416 – administrative supplement), and the National Institute on Drug Abuse (R01DA050721). Additional funding for investigator effort has been provided by K02AA018755, U10AA008401, P50AA022537, as well as a European Research Council Consolidator Grant (647648 EdGe to Koellinger). The content is solely the responsibility of the authors and does not necessarily represent the official views of the above funding bodies. The Externalizing Consortium would like to thank the following groups for making the research possible: 23andMe, Add Health, Vanderbilt University Medical Center's BioVU, Collaborative Study on the Genetics of Alcoholism (COGA), the Psychiatric Genomics Consortium's Substance Use Disorders working group, UK10K Consortium, UK Biobank, and Philadelphia Neurodevelopmental Cohort. Tina Kretschmer and Charlotte Vrijen are supported by a European Research Council Starting Grant (Grant Agreement Number 757364). Jasmin Wertz was supported by a Postdoctoral Fellowship from the AXA Research Fund at time of writing. The authors have declared that they have no competing or potential conflicts of interest.
Key points.
Genetic and environmental influences are both implicated in externalizing behavior but are often studied separately. Here, we investigated genetic nurture and evocative gene–environment correlation.
The strongest predictor of early adult externalizing behavior is externalizing problems in childhood. Offspring's genetic predisposition for externalizing problems was associated with a greater risk for externalizing problems in childhood, which in turn predicted greater family dysfunction. This indirect effect, which is suggestive of evocative gene–environment correlation, was small and did not extend to early adult externalizing behavior.
Results underline the importance of a longitudinal approach that considers child and family factors when studying early adult externalizing behavior and highlight the need for early intervention.
Conflict of interest statement: No conflicts declared.
References
- Achenbach, T.M. , & Rescorla, L.A. (2001). Manual for the ASEBA school‐age forms & profiles: Child behavior checklist for ages 6–18, teacher's report form, youth self‐report: An integrated system of multi‐informant assessment. Burlington, Vermont: University of Vermont, Research Center for Children Youth & Families. [Google Scholar]
- Armstrong‐Carter, E. , Trejo, S. , Hill, L.J.B. , Crossley, K.L. , Mason, D. , & Domingue, B.W. (2020). The earliest origins of genetic nurture: The prenatal environment mediates the association between maternal genetics and child development. Psychological Science, 31, 781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avinun, R. , & Knafo, A. (2014). Parenting as a reaction evoked by children's genotype: A meta‐analysis of children‐as‐twins studies. Personality and Social Psychology Review, 18, 87–102. [DOI] [PubMed] [Google Scholar]
- Cox, S.M.L. , Castellanos‐Ryan, N. , Parent, S. , Benkelfat, C. , Vitaro, F. , Pihl, R.O. , … & Séguin, J.R. (2021). Externalizing risk pathways for adolescent substance use and its developmental onset: A Canadian birth cohort study: The. Canadian Journal of Psychiatry, 66, 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zeeuw, E.L. , Hottenga, J.‐J. , Ouwens, K.G. , Dolan, C.V. , Ehli, E.A. , Davies, G.E. , … & van Bergen, E. (2020). Intergenerational transmission of education and ADHD: Effects of parental genotypes. Behavior Genetics, 50, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, L. K. , & Rothbart, M. K. (1999). Early adolescent temperament questionnaire‐revised . Available from Mary Rothbart, University of Oregon, Maryroth@ OREGON. UOREGON. EDU.
- Epstein, N.B. , Baldwin, L.M. , & Bishop, D.S. (1983). The McMaster family assessment device. Journal of Marital and Family Therapy, 9, 171–180. [Google Scholar]
- Hartman, C.A. , Richards, J.S. , Vrijen, C. , Oldehinkel, A.J. , Oerlemans, A.M. , & Kretschmer, T. (2022). Cohort profile update: The TRacking Adolescents' individual lives survey – The next generation (TRAILS NEXT). International Journal of Epidemiology. 10.1093/ije/dyac066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A.F. (2009). Beyond baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs, 76, 408–420. [Google Scholar]
- Hill, S. , Shanahan, L. , Costello, E.J. , & Copeland, W. (2017). Predicting persistent, limited, and delayed problematic cannabis use in early adulthood: Findings from a longitudinal study. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman, M. , Oldehinkel, A.J. , Winter, A.D. , Minderaa, R.B. , Bildt, A.D. , Huizink, A.C. , … & Ormel, J. (2008). Cohort profile: The Dutch ‘TRacking adolescents’ individual lives' survey’ TRAILS. International Journal of Epidemiology, 37, 1227–1235. [DOI] [PubMed] [Google Scholar]
- Jaffee, S.R. , Belsky, J. , Harrington, H. , Caspi, A. , & Moffitt, T.E. (2006). When parents have a history of conduct disorder: How is the caregiving environment affected? Journal of Abnormal Psychology, 115, 309–319. [DOI] [PubMed] [Google Scholar]
- Karlsson Linnér, R. , Mallard, T.T. , Barr, P.B. , Sanchez‐Roige, S. , Madole, J.W. , Driver, M.N. , … & Dick, D.M. (2021). Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self‐regulation and addiction. Nature Neuroscience, 24, 1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrotheodoros, S. , Canário, C. , Gugliandolo, M.C. , Merkas, M. , & Keijsers, L. (2020). Family functioning and adolescent internalizing and externalizing problems: Disentangling between‐, and within‐family associations. Journal of Youth and Adolescence, 49, 804–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte, I. M. (2020a). MetaSubtract: Subtracting Summary Statistics of One or more Cohorts from Meta‐GWAS Results. https://CRAN.R‐project.org/package=MetaSubtract
- Nolte, I.M. (2020b). Metasubtract: An R‐package to analytically produce leave‐one‐out meta‐analysis GWAS summary statistics. Bioinformatics, 36, 4521–4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel, A.J. , Rosmalen, J.G. , Buitelaar, J.K. , Hoek, H.W. , Ormel, J. , Raven, D. , … & Hartman, C.A. (2015). Cohort profile update: The TRacking Adolescents' individual lives survey (TRAILS). International Journal of Epidemiology, 44, 76–76n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart, M. (2017). Associations of parenting dimensions and styles with externalizing problems of children and adolescents: An updated meta‐analysis. Developmental Psychology, 53, 873–932. [DOI] [PubMed] [Google Scholar]
- Rhee, S.H. , & Waldman, I.D. (2002). Genetic and environmental influences on antisocial behavior: A meta‐analysis of twin and adoption studies. Psychological Bulletin, 128, 490–529. [PubMed] [Google Scholar]
- Richards, J. , Hartman, C. , Jeronimus, B. , Ormel, J. , Reijneveld, S. , Veenstra, R. , … & Oldehinkel, A. (2019). Beyond not bad or just okay: Social predictors of young adults' wellbeing and functioning (a TRAILS study). Psychological Medicine, 49, 1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders, J.B. , Aasland, O.G. , Babor, T.F. , Fuente, J.R.D.L. , & Grant, M. (1993). Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption‐II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Shewark, E.A. , Ramos, A.M. , Liu, C. , Ganiban, J.M. , Fosco, G. , Shaw, D.S. , … & Neiderhiser, J.M. (2021). The role of child negative emotionality in parenting and child adjustment: Gene–environment interplay. Journal of Child Psychology and Psychiatry., 62, 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst, B. , Neale, M. , & Kendler, K. (2015). The heritability of alcohol use disorders: A meta‐analysis of twin and adoption studies. Psychological Medicine, 45, 1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhjálmsson, B.J. , Yang, J. , Finucane, H.K. , Gusev, A. , Lindström, S. , Ripke, S. , … & Price, A.L. (2015). Modeling linkage disequilibrium increases accuracy of polygenic risk scores. American Journal of Human Genetics, 97, 576–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkhuyzen, A.A. , Van Der Sluis, S. , De Geus, E.J. , Boomsma, D.I. , & Posthuma, D. (2010). Genetic influences on ‘environmental’ factors. Genes, Brain and Behavior, 9, 276–287. [DOI] [PubMed] [Google Scholar]
- Wang, B. , Baldwin, J.R. , Schoeler, T. , Cheesman, R. , Barkhuizen, W. , Dudbridge, F. , … & Pingault, J.‐B. (2021). Robust genetic nurture effects on education: A systematic review and meta‐analysis based on 38,654 families across 8 cohorts. The American Journal of Human Genetics, 108, 1780–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz, J. , Belsky, J. , Moffitt, T.E. , Belsky, D.W. , Harrington, H. , Avinun, R. , … & Caspi, A. (2019). Genetics of nurture: A test of the hypothesis that parents' genetics predict their observed caregiving. Developmental Psychology, 55, 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems, Y. , Boesen, N. , Li, J. , Finkenauer, C. , & Bartels, M. (2019). The heritability of self‐control: A meta‐analysis. Neuroscience & Biobehavioral Reviews, 100, 324–334. [DOI] [PubMed] [Google Scholar]
- Willoughby, E.A. , McGue, M. , Iacono, W.G. , Rustichini, A. , & Lee, J.J. (2021). The role of parental genotype in predicting offspring years of education: Evidence for genetic nurture. Molecular Psychiatry, 26, 3896–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Items, answer options, and descriptive statistics for all measures.
Appendix S2. Genotyping and polygenic score construction.
Appendix S3. Pairwise correlations between study variables.
Appendix S4. Model selection and derivation of factors.
Appendix S5. Results using midparent score.


