Abstract
Hepatitis E virus (HEV) is an emerging zoonotic pathogen in Europe. In the Iberian Peninsula, wild boar (Sus scrofa) is considered the main wildlife reservoir of HEV. This wild ungulate shares habitat and resources with other potential HEV carriers in Iberian Mediterranean ecosystems, although information about the role of such sympatric species in the HEV epidemiological cycle is still very limited. The aims of the present large‐scale, long‐term study were: (1) to determine the seroprevalence and prevalence of HEV in both free‐living and captive populations of the Iberian lynx (Lynx pardinus), the most endangered felid in the world; (2) to determine potential risk factors associated with HEV exposure in this species and (3) to evaluate the dynamics of seropositivity in longitudinally sampled animals during the study period. Between 2010 and 2021, serum samples from 275 Iberian lynxes were collected in free‐ranging and captive populations across the Iberian Peninsula. Forty‐four of the 275 lynxes were also longitudinally sampled during the study period. A double‐antigen sandwich ELISA was used to test for the presence of antibodies against HEV. A subset of seropositive samples was analysed by Western blot (WB) assay to confirm exposure to HEV. In addition, serum, liver and/or faecal samples from 367 individuals were tested for orthohepevirus RNA by RT‐PCR. A total of 50 (18.2%; 95% CI: 14.1–23.2) of the 275 animals analysed had anti‐HEV antibodies by ELISA. Exposure to HEV was confirmed by WB in most of the ELISA‐positive Iberian lynxes analysed. Significantly higher seroprevalence was found in captive (33.6%) compared to free‐ranging (7.4%) individuals. Within captive population, the GEE model identified ‘age’ (senile, adult and subadult) as risk a factor potentially associated with HEV exposure in the Iberian lynx. Thirteen (29.5%) of 44 longitudinally surveyed individuals seroconverted against HEV during the study period. HEV RNA was detected in the faeces of one (1/364; 0.3%; 95% CI: 0.0–0.8) free‐ranging adult animal sampled in 2021. Phylogenetic analysis showed that the sequenced strain belongs to HEV‐3f subtype and shared a high nucleotide sequence identity (97–99.6%) with human HEV‐3f sequences from Spain and France. To the best of the authors’ knowledge, this is the first survey study on HEV in the Iberian lynx and the first molecular report of HEV‐A infection in free‐ranging felines. Our results indicate high exposure to HEV‐3 in Iberian lynx populations, particularly those kept in captivity. The serological results suggest widespread but not homogeneous circulation of HEV in Iberian lynx populations. Further studies are required to assess the epidemiological role of this endangered species as a potential spillover host of HEV.
Keywords: epidemiology, hepatitis E, HEV‐3f, Iberian lynx, risk factors
1. INTRODUCTION
Hepatitis E virus (HEV; family Hepeviridae; genus Orthohepevirus) is an important emerging and zoonotic pathogen currently considered the main cause of acute viral human hepatitis worldwide (Lhomme et al., 2019). At present, four species of Orthohepevirus (henceforth HEV‐A to HEV‐D) have been confirmed, with HEV‐A, particularly genotypes HEV‐1 to HEV‐4 of this species, being the most important in terms of public health concern. Although the domestic pig and wild boar (Sus scrofa) are the primary reservoirs (Pavio et al., 2017), susceptibility to HEV‐A infection has been confirmed in an expanding range of mammal species (Meng, 2016; Sarchese et al., 2021), whose role in the epidemiology of this virus is still poorly understood.
The Iberian lynx (Lynx pardinus) is the most endangered felid species in the world (Nowell & Jackson, 1996) and one of the most endangered carnivores in Europe (International Union for Conservation of Nature, 2021). Iberian lynx populations declined drastically during the last decades of the 20th century, and it was estimated that there were only around 100 individuals in 2002, distributed in two isolated areas of Andalusia (southern Spain) (Simón et al., 2012). The decline was associated with a reduction in the numbers of its staple prey, the European wild rabbit (Oryctolagus cuniculus), habitat destruction, illegal trapping and hunting, road kills and infectious diseases (Ferreras et al., 1992, 2001; López et al., 2014; Rodríguez & Delibes, 2004). Over the last two decades, clinical cases and mortalities in this endangered species have been reported due to feline leukaemia virus, Suid alphaherpesvirus 1 and Mycobacterium bovis infections, among others (Briones et al., 2000; Masot et al., 2016; Meli et al., 2010). Since 2000, a number of projects, including EU LIFE‐Nature projects, have been launched to save the Iberian lynx from extinction, focusing on in situ and also ex situ conservation programs (Vargas, 2009). As a result, the Iberian lynx census has soared during the last decade, reaching more than 1,100 free‐ranging individuals in 2020 ( Ministerio para la Transición Ecológica y Reto Demográfico, 2021). The monitoring of pathogens that could affect the Iberian lynx in the two different epidemiological habitats in which it is found (in captivity and in the wild) is a key component of the conservation programs of this endangered species, and health surveillance programs are being conducted in both free‐living and captive populations (Nájera et al., 2021; Rivas et al., 2016). Nevertheless, information about the susceptibility of the Iberian lynx to pathogens that are not monitored but highly prevalent in their habitat, which may be important in terms of conservation and animal and public health, is still very limited.
In the Iberian Mediterranean ecosystems where the Iberian lynx is distributed, different studies have confirmed HEV‐3 infection in extensively raised Iberian pig, a breed of domestic pig native to the Iberian Peninsula, wild boar (seroprevalence ranging from 5.2% to 57.6%), red deer (Cervus elaphus) (seroprevalence ranging from 10.2 to 12.9%) and horses (Caballero‐Gómez et al., 2019; García‐Bocanegra et al., 2019; Kukielka et al., 2015; López‐López et al., 2018; Risalde et al., 2017). In addition, HEV exposure has been reported in other sympatric species that could act as potential reservoirs or spillover hosts, including wild rabbits (4.1%) and extensively raised goats (8.9%) and sheep (2.2%) (Boadella et al., 2010; Caballero‐Gómez et al., 2022; Lopes & Abrantes, 2020; Kukielka et al., 2015). However, there is no information about the susceptibility of the Iberian lynx to HEV infection and its possible role in the transmission of the virus in these ecosystems. HEV infection or exposure has so far been detected in several felid species, including the Eurasian lynx (Lynx lynx), and the captive clouded (Neofelis nebulosa), Persian (Panthera pardus saxicolor) and snow leopards (Uncia uncia), as well as in domestic pet and stray cats (Caballero‐Gómez et al., 2022; Song et al., 2013; Spahr, Ryll, et al., 2017; Zhang et al., 2008). Our hypothesis is that free‐living and captive Iberian lynx populations may be exposed to HEV, in which case, this species could play a role in the epidemiology of this emerging virus. The objectives of the present large‐scale, long‐term study were therefore: (1) to determine the seroprevalence and prevalence of HEV in Iberian lynx populations, (2) to determine potential risk factors associated with HEV exposure in this species and (3) to evaluate the dynamics of seropositivity in longitudinally sampled animals during the study period.
2. MATERIAL AND METHODS
2.1. Sampling
Blood samples were collected from 275 Iberian lynxes across the Iberian Peninsula between 2010 and 2021. Thus, a total of 162 were free‐ranging animals in the three areas where the Iberian lynx population is already distributed (central, south and southwest Spain). In addition, 113 lynxes kept in captivity, including 106 from the four captive breeding centres (BC1–BC4) belonging to the Iberian lynx ex situ conservation program and seven from four zoological parks/conservation centres (ZC1–ZC4), were sampled. A total of 44 (including 10 free‐ranging, 26 captive and eight animals translocated from captivity to free‐range areas or vice versa) of the 275 sampled individuals were also longitudinally surveyed (between two and four samplings per animal) during the study period. During follow‐up, the median (Q1–Q3) interval between consecutive samplings was 48 months (24–60). Sera were obtained by blood centrifugation at 400 × g for 15 min and stored at −80°C until laboratory analysis. Between 2017 and 2021, liver and/or faecal samples from 176 Iberian lynxes (including 131 free‐ranging and 45 captive individuals) were also collected and stored at −20°C until molecular analysis. Of these, both liver and faecal samples were collected from 51 animals (46 free‐ranging and five captive Iberian lynxes).
All available samples were used in the present study. Serum, liver and faecal samples collected from individuals subjected to health programs, medical check‐ups or necropsy during the study period were obtained from serum/tissue banks at the Center for Analysis and Diagnosis of Wildlife (CAD, Andalusia, southern Spain). This study did not involve the intentional killing of animals. Iberian lynxes were sampled by authorized veterinarians and animal keepers following routine procedures on live and dead individuals before the design of this study, in compliance with Ethical Principles in Animal Research. Samples from a representative number of free‐ranging individuals and almost all the captive population were analysed in the present study.
Whenever possible, epidemiological information about each individual animal was recorded, including age (yearlings: < 1 year old; subadults: 1 to 3 years old; adults: 3 to 10 years old; senile: > 10 years old), sex, habitat status (free‐ranging vs. captivity), origin (free‐range area, breeding centre, zoological park/conservation centre), sampling date and georeferenced location.
2.2. Serological analysis
The presence of total antibodies against HEV was assessed using a commercial double‐antigen sandwich multi‐species ELISA (HEV 4.0v; MP Diagnostics, Illkirch, France) following the manufacturer's instructions. This assay is based on the highly conserved recombinant protein ET2.1 of the HEV capsid (Hu et al., 2008) and detects anti‐HEV antibodies in serum or plasma in a wide range of animal species, including felids such as cats (Caballero‐Gómez et al., 2022), the Eurasian lynx and the serval (Leptailurus serval) (unpublished data), in which the presence of anti‐HEV antibodies in ELISA‐positive animals was also confirmed by Western Blot assay (WB).
Serum samples from 34 Iberian lynx were randomly selected for WB analyses in order to confirm exposure to either HEV‐A and/or HEV‐C in Iberian lynx populations (Kubickova et al., 2021). Carboxy‐terminal segments of the capsid proteins of HEV‐3 and rat HEV‐C1 and a nucleocapsid protein derivative (amino acid residues 1–39/213–433) of the Puumala orthohantavirus strain Vranica/Hällnäs, as negative control, were produced as His‐tagged recombinant proteins in Escherichia coli and purified by nickel‐chelate affinity chromatography (Dremsek et al., 2012; Lundkvist et al., 2002). Purified proteins were run in 12% SDS‐PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane and analysed for control by anti‐His tag and HEV capsid protein cross‐reactive monoclonal antibodies (Merck, Darmstadt, Germany; Kubickova et al., 2021, Supplementary Figure 1). Serum samples were diluted 1:100 in 5% skimmed milk in phosphate‐buffered saline (PBS)‐0.1% Tween 20 (PBS‐T), and the antigen–antibody reaction was detected by adding purified recombinant protein A/G conjugated with horse‐radish peroxidase (HRP) (Thermo Scientific, Schwerte, Germany), diluted 1:50,000 in 5% PBS‐T. The immunoreaction was detected using Clarity Western ECL Substrate (Biorad, Feldkirchen, Germany) and documented in a VersaDoc 4000MP system (Bio‐Rad) with an exposure time between 1 s and 60 s.
2.3. Molecular analysis
For the molecular analysis, RNA from serum (number of samples analysed (n) = 248 from 220 individuals), liver (n = 158) and faecal (n = 73) samples was extracted using the QIAmp MinElute Virus Spin, RNeasy Mini, and QIAamp cador Pathogen Mini Kits (QIAGEN, Hilden, Germany), respectively, whenever possible. Seropositive sera as well as liver and stool samples were individually extracted, whereas seronegative serum samples were randomly subjected to a pool testing approach (pools of eight samples; total volume: 400 μl) and extracted. The sensitivity of RT‐PCR in pools was set at 670 IU/ml (Rivero‐Juárez et al., 2019).
The presence of HEV RNA was assessed using two RT‐PCR assays in parallel. A real‐time RT‐PCR (CFX Connect Real Time PCR System) capable of detecting all HEV‐A genotypes was performed using 10 μl of RNA template and the QIAGEN One‐Step RT‐PCR kit as previously described (Frias et al., 2021). Briefly, the primers employed were FWD 5′‐RGTRGTTTCTGGGGTGAC‐3′ and RVS 5′‐AKGGRTTGGTTGGRTGA‐3′, and the probe was 5′‐FAM‐TGAYTCYCARCCCTTCGC‐TAMRA‐3′. In addition, a nested broad‐spectrum RT‐PCR (Fisher Scientific Applied Biosystems SimpliAmp™) able to detect HEV‐A, HEV‐B and HEV‐C strains was performed using the QIAGEN One‐Step RT‐PCR kit and HEV‐cs and HEV‐cas primers for the first round, and the premixed 2× solution containing Taq DNA Polymerase, dNTPs and Reaction Buffer (Promega, Madison, WI, USA) and HEV‐csn and HEV‐casn primers for the second round (Johne et al., 2010). The amplicons of the nested RT‐PCR were examined on 1.5% agarose gels stained with RedSafe™ Nucleic Acid Staining solution (iNtRON Biotechnology, Seongnam, Korea). Positive samples using this assay were sequenced with the BigDye Terminator Cycle Sequencing Ready Reaction Kit on an ABI PRISM 3100 Genetic Analyser (Applied Biosystems, Foster City, CA, USA). The consensus sequence was obtained using SeqMan Software NGen® Version 12.0 (DNASTAR. Madison, WI, USA). Subtype assignment and phylogenetic analyses were performed using the HEVnet genotyping too1 (Mulder et al., 2019) and confirmed by Basic Local Alignment Search Tool (BLASTn). Sequence alignments were generated by the MAFFT online service (https://mafft.cbrc.jp/alignment/server/): multiple sequence alignment, interactive sequence choice and visualization. Phylogenetic trees were reconstructed with the maximum likelihood method using the proposed HEV‐A genotype/subtype standard reference strains (Smith et al., 2016, 2020). The final tree was obtained with MEGA Software (Version 10) using the bootstrap method (with 1,000 replicates).
2.4. Statistical analyses
Seroprevalence and prevalence were estimated by dividing the number of positive or seropositive animals by the total number of animals tested, using two‐sided exact binomial 95% confidence intervals (95% CI). Associations between the presence of anti‐HEV antibodies and the variable ‘habitat status’ were analysed using Pearson's chi‐square test. To avoid a possible collinearity bias, free‐ranging and captive populations were tested separately. Associations between the presence of anti‐HEV antibodies and explanatory variables (age, sex and sampling period [categorized by terciles in each population]) were analysed using Pearson's chi‐square test or Fisher's exact test, as appropriate. Variables with p < .10 in bivariate analyses were selected for inclusion in the multivariate analyses. Collinearity between pairs of variables was tested using Spearman's Rho test. Finally, a generalized estimating equation model (GEE) was used to assess the effect of the variables selected in bivariate analysis. ‘Origin’ was included as a random factor, and the number of seropositive animals was assumed to follow a binomial distribution. A manual forward stepwise approach was used, starting with the variable with the lowest p‐value in bivariate analysis. At each step, the confounding effect of the included variable was assessed by computing change in odds ratios (OR) greater than 30%. Variables with p < .05 were considered statistically significant. Statistical analyses were performed using SPSS 25.0 software (Statistical Package for Social Sciences, Inc., Chicago, IL, USA).
3. RESULTS
A total of 50 (18.2%; 95% CI: 14.1–23.2) of 275 Iberian lynxes sampled had antibodies against HEV. Seroprevalence was significantly higher in captive (38/113; 33.6%; CI 95%: 25.6–42.8) compared to free‐ranging (12/162; 7.4%; CI 95%: 4.3‐12.5) individuals (p < .001; Relative Risk: 6.3; CI 95%: 3.1–12.8). The frequency of anti‐HEV antibodies in free‐ranging and captive populations by age, sex and sampling periods is shown in Table 1. The GEE model identified ‘age’ as a risk factor potentially associated with HEV exposure in captive Iberian lynxes (Table 2). The seroprevalence was significantly higher in captive senile, adult and subadult individuals compared to captive yearling animals (50.0%, 42.9% and 22.7% vs. 5.6%; p = .005, p = .001 and p = .049). Exposure to HEV was detected in two yearling animals sampled in 2017 and 2021: one captive 3 months‐old and one free‐ranging 10 months‐old lynxes, respectively. Antibodies against HEV‐3 were confirmed by WB in 29 (85.3%) of 34 ELISA‐positive animals, and 21 of these also reacted against rat HEV‐C1 antigen (Supplementary Figure 1).
TABLE 1.
Distribution of HEV seroprevalence in free‐ranging and captive Iberian lynx populations and results of bivariate analysis
| Free‐ranging | Captive | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Categories | No. Positives/no. analysed a | Seroprevalence (%) (95% CI) | p | Categories | No. Positives/No. analysed a | Seroprevalence (%) (95% CI) | p |
| Age | Yearling | 1/27 | 3.7 (0.0–10.8) | .269 | Yearling | 1/18 | 5.6 (0.0–16.1) | .011 |
| Subadult | 3/61 | 4.9 (0.0–10.3) | Subadult | 5/22 | 22.7 (5.2–40.2) | |||
| Adult | 6/59 | 10.2 (2.5–17.9) | Adult | 27/63 | 42.9 (30.6–55.1) | |||
| Senile | 2/10 | 20.0 (0.0–44.8) | Senile | 5/10 | 50.0 (19.0–81.0) | |||
| Sex | Female | 7/81 | 8.6 (2.5–14.8) | .313 | Female | 22/61 | 36.1 (24.0–48.1) | .294 |
| Male | 4/75 | 5.3 (0.3–10.4) | Male | 15/51 | 29.4 (16.9–41.9) | |||
| Sampling period | 2010–2016 b | 6/63 | 9.5 (2.3–16.8) | .705 | 2010–2014 b | 15/30 | 50.0 (32.1–67.9) | .067 |
| 2017–2018 | 3/53 | 5.7 (0.0–11.9) | 2015–2016 | 9/38 | 23.7 (10.2‐37.2) | |||
| 2019–2021 | 3/46 | 6.5 (0.0––13.7) | 2017–2020 | 14/45 | 31.1 (17.5–44.6) | |||
Missing values excluded.
No animals were sampled in 2011.
TABLE 2.
Results of the generalized estimating equation analysis of potential risk factors associated with HEV exposure in the Iberian lynx
| Variable | Categories | β | p | OR (95%CI) |
|---|---|---|---|---|
| Age | Senile | 2.693 | .005 | 14.8 (2.2–98.5) |
| Adult | 2.348 | .001 | 10.5 (2.5–43.0) | |
| Subadult | 1.499 | .049 | 4.5 (1.0–19.9) | |
| Yearling | a | a | a |
Reference category; OR, odds ratio; CI, confidence interval.
The distribution of seroprevalences by origin is shown in Table 3 and Figure 1. Seropositive Iberian lynx were detected in the three free‐ranging areas sampled, with frequencies ranging from 5.5% in the south to 11.6% in southwest Spain. Animals with anti‐HEV antibodies were also found in the four captive breeding centres, with within‐centre rates ranging between 11.1% in BC2 and 51.4% in BC4. Statistically significant differences were observed between captive breeding centres (p = .019) but not between free‐living areas (p = .466) (Table 3). Two Iberian lynxes from zoological parks/conservation centres, both from the same zoo (ZC2), were also found to be seropositive to HEV.
TABLE 3.
Distribution of HEV seroprevalence in Iberian lynx by origin and results of bivariate analysis
| Variable | Categories | No. positives/no. analysed | Seroprevalence (%) (95%CI) | p |
|---|---|---|---|---|
| Free‐range areas | Central Spain | 3/53 | 5.7 (0.0–11.9) | .466 |
| South Spain | 4/66 | 6.1 (0.3–11.8) | ||
| Southwest Spain | 5/43 | 11.6 (2.1–21.2) | ||
| Breeding centres | BC1 | 10/31 | 32.3 (15.8–48.7) | .019 |
| BC2 | 2/18 | 11.1 (0.0–25.6) | ||
| BC3 | 5/20 | 25.0 (6.0–44.0) | ||
| BC4 | 19/37 | 51.4 (35.3–67.5) | ||
| Zoological parks/conservation centres | ZC1 | 0/2 | 0.0 (0.0–84.2) | NA |
| ZC2 | 2/2 | 100.0 (15.8–100.0) | ||
| ZC3 | 0/1 | 0.0 (0.0–97.5) | ||
| ZC4 | 0/2 | 0.0 (0.0–84.2) |
Abbreviation: NA, Not analysed because of the low number of individuals sampled.
FIGURE 1.
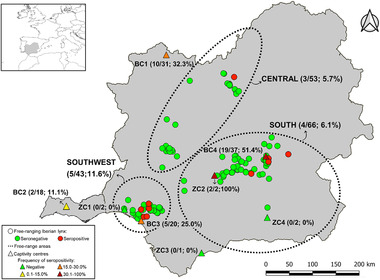
Spatial distribution and serological results of Iberian lynxes sampled in the Iberian Peninsula. The HEV seroprevalence, numbers of seropositives and total number of animals analysed in each free‐range area and captivity centre are shown in brackets. The abbreviations ‘BC’ and ‘ZC’ refer to breeding centres and zoological parks/conservation centres, respectively
Seropositive Iberian lynxes were observed in all years of the study period except for 2011, since no animals were sampled. Similar seroprevalence was found among sampling periods in free‐ranging Iberian lynxes (9.5% in 2010–2016, 5.7% in 2017–2018 and 6.5% in 2019–2021), whereas fluctuations were observed in captive animals (50.0% in 2010–2014, 23.7% in 2015–2016 and 31.1% in 2017–2020) (Table 1). ‘Sampling period’ was selected from bivariate analysis in the captive population but was identified as a confounding variable of ‘age’ and removed from the final GEE model. Of the 44 longitudinally surveyed animals, 15 (34.1%) tested positive by ELISA at each sampling in the study period (Table 4) and 15 animals also remained seronegative at the different samplings. Of note, 13 (29.5%) of the 44 individuals seroconverted against HEV during the study period. In addition, seroreversion was detected in two lynxes; the sampling intervals between the seropositive and first seronegative sampling in these animals ranged between 6 and 7 years.
TABLE 4.
Exposure to HEV in longitudinally sampled animals determined by ELISA
| ID | Life condition | Origin | 2010 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 157 | Free‐ranging | South Spain | – | – | – | – | – | Pos | – | Pos | – | – |
| 158 | Free‐ranging | South Spain | Pos | – | – | – | – | Neg | – | Neg | – | – |
| 319 | Free‐ranging | South Spain | Neg | – | – | – | Neg | – | – | – | – | – |
| 307 | Free‐ranging | South Spain/Southwest Spain | – | – | – | – | Neg | Neg | – | – | – | – |
| 113 | Free‐ranging | South Spain | – | – | – | – | – | – | – | – | Neg | Neg |
| 142 | Free‐ranging | South Spain | – | – | – | – | – | – | – | Neg | – | Neg |
| 270 | Free‐ranging | South Spain | – | – | – | – | – | – | Neg | – | – | Neg |
| 70 | Free‐ranging | Central Spain | – | – | – | – | – | – | – | Neg | Neg | – |
| 239 | Free‐ranging | Central Spain | – | – | – | – | – | – | – | Neg | Neg | – |
| 228 | Free‐ranging | Central Spain | – | – | – | – | – | – | Neg | – | Neg | – |
| 145 | Free‐ranging/captivity | South Spain/BC1 | – | – | – | – | – | – | – | Neg | – | Pos |
| 337 | Free‐ranging/captivity | South Spain/BC4 | – | Neg | – | – | – | Pos | Pos | – | – | – |
| 171 | Free‐ranging/captivity | Central Spain/BC3 | Pos | – | – | – | – | Pos | – | – | – | – |
| 180 | Captivity/free‐ranging | BC3/Central Spain | – | – | – | – | Neg | – | – | Pos | – | – |
| 458 | Captivity/free‐ranging | BC3/Central Spain | – | – | – | – | Neg | – | Neg | – | – | – |
| 308 | Captivity/free‐ranging | BC4/South Spain | – | – | – | – | – | Neg | – | – | – | Neg |
| 267 | Captivity/free‐ranging | BC2/South Spain | – | – | Neg | – | – | – | Neg | – | – | – |
| 475 | Captivity/free‐ranging | BC2/South Spain | – | – | Neg | – | – | – | Pos | – | – | – |
| 432 | Captivity | ZC2 | Pos | – | – | – | – | – | Neg | – | – | Pos; Pos 7 |
| 496 | Captivity | ZC2 | Pos | – | Pos | – | Pos | – | – | – | – | – |
| 264 | Captivity | BC3 | – | – | – | – | – | Pos | Pos | – | – | – |
| 254 | Captivity | BC3/BC4 | – | Neg | – | – | – | – | Pos | – | – | – |
| 34 | Captivity | BC4 | – | Neg | – | – | – | – | Neg | – | – | – |
| 369 | Captivity | BC4 | Pos | – | – | – | Pos | – | – | – | – | – |
| 335 | Captivity | BC4 | Pos | – | – | – | – | – | Pos | – | – | – |
| 568 | Captivity | BC4 | Pos | – | – | – | Pos | – | – | – | – | – |
| 431 | Captivity | BC4 | Pos | – | – | – | Pos | – | Pos | – | – | – |
| 259 | Captivity | BC4 | Neg | – | – | – | Pos | – | Pos | – | – | – |
| 392 | Captivity | BC4 | Pos | Pos | – | – | – | – | Pos | – | – | – |
| 465 | Captivity | BC4 | Pos | – | – | – | – | – | Pos | – | – | – |
| 470 | Captivity | BC4 | Pos | – | – | – | Pos | – | – | – | – | – |
| 487 | Captivity | BC4 | – | – | Neg | – | Pos | – | – | – | – | – |
| 476 | Captivity | BC4 | – | – | – | – | Neg | – | Pos | – | – | – |
| 265 | Captivity | BC4 | Pos | – | – | – | – | Pos | – | – | – | – |
| 168 | Captivity | BC4/BC1 | Neg | – | – | – | – | Pos | – | – | – | – |
| 724 | Captivity | BC4/BC3 | – | – | Neg | – | – | – | Pos | – | – | – |
| 770 | Captivity | BC2 | Neg | Pos | – | – | – | – | – | – | – | – |
| 388 | Captivity | BC1 | – | – | – | Pos | – | – | Pos | – | – | – |
| 838 | Captivity | BC1 | – | Pos | – | – | – | Pos | – | – | – | – |
| 285 | Captivity | BC1 | – | – | – | – | – | Neg; Neg 5 | Pos | – | – | – |
| 339 | Captivity | BC1 | – | – | – | – | – | Pos | – | – | – | Pos |
| 403 | Captivity | BC1 | – | – | – | – | – | – | Neg | – | – | Neg |
| 387 | Captivity | BC1 | – | – | – | – | – | – | Neg | – | – | Neg |
| 732 | Captivity | BC1 | – | – | – | – | – | – | Neg | – | – | Neg |
Note: When two samplings were carried out in the same year, superscript ‘a’ indicates the number of interval months.
HEV RNA was detected in one (0.3%; 95% CI: 0.0–0.8) of the 364 individuals tested by RT‐PCR. One of 73 stool (individual prevalence: 1/73; 1.4%; 95% CI: 0.0–4.0) samples tested positive for HEV RNA. None of the 248 serum (individual prevalence: 0/220; 0.0%; 95% CI: 0.0–1.5) or 158 liver (0.0%; 95% CI: 0.0–1.9) samples were positive for active HEV infection (Figure 2). The infected Iberian lynx, in which both liver (negative) and faeces (positive) were analysed, was an adult male sampled in the free‐range area of southwestern Spain in 2021 (Figure 2). The sequenced ORF2 fragment of 285 base pairs belongs to genotype 3, subtype 3f (GenBank Accession Number: OL840902). BLAST analysis showed high nucleotide sequence identity (97–99.6%) with human HEV‐3f sequences obtained from the same Spanish study area (GenBank Accession Numbers: OL741651, OL741653 and OL746155) and France (Figure 3).
FIGURE 2.
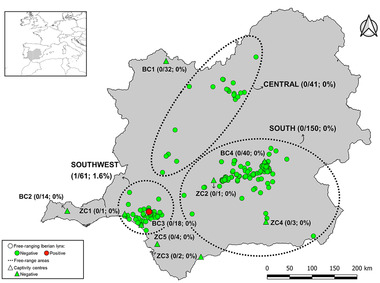
Spatial distribution and molecular results of Iberian lynx sampled in the Iberian Peninsula. Prevalence of HEV infection, number of RNA positive and total number of animals analysed in each free‐range area and captivity centre are shown in brackets. The abbreviations ‘BC’ and ‘ZC’ refer to Breeding centres and zoological parks/conservation centres, respectively
FIGURE 3.
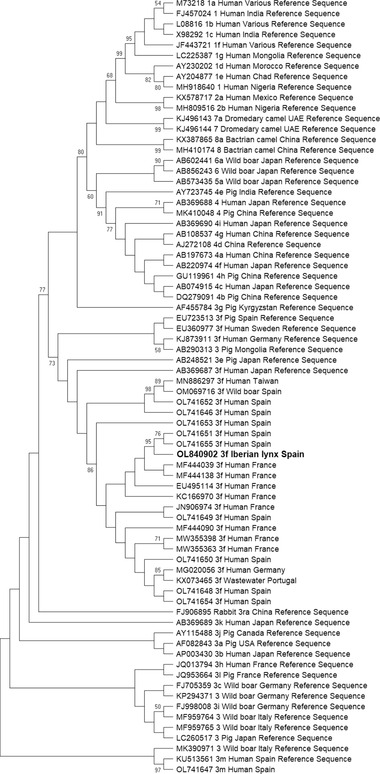
Phylogenetic tree constructed by the maximum likelihood method and Kimura 2‐parameter model. The bootstrap consensus tree was inferred from 1,000 replicates and used to represent the evolutionary history of the taxa analysed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches when values were larger than 70. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor‐Joining and BioNJ algorithms to a matrix of pairwise distances estimated using the Maximum Composite Likelihood (MCL) approach, and then selecting the topology with superior log likelihood value. The analysis involved 74 nucleotide sequences. Sequences proposed by Smith et al. (2020) as reference sequences are shown as ‘Reference Sequence’ in the tree. There was a total of 270 positions in the final dataset. Evolutionary analyses were conducted in MEGA X. The sequence retrieved from the positive Iberian lynx is given in bold type (accession number OL840902)
4. DISCUSSION
This is the first survey study on HEV in the Iberian lynx. The seroprevalences detected in the present study in free‐ranging (7.4%) and captive (33.6%) populations indicate moderate and high circulation, respectively, of this emerging virus. In most ELISA‐positive animals, antibodies against HEV‐3 were confirmed by WB, showing circulation of this genotype in Iberian lynx populations. Some of these individuals also reacted against rat HEV‐C1. Although cross‐reactivity among hepeviruses has previously been observed (Sridhar et al., 2021; Wang et al., 2020), exposure to both genotypes or to a hitherto unknown virus of a putative novel genotype in the Iberian lynx population cannot be ruled out. In line with this, rat HEV‐C1 exposure and infection have previously been reported in stray cats and humans, respectively, in the study area, and infection with this genotype has also been found in other carnivores, such as the Syrian brown bear (Ursus arctos syriacus), in a zoo in Germany (Caballero‐Gómez et al., 2022; Rivero‐Juárez et al., 2022; Spahr, Ryll, et al., 2017).
The detection of seropositive lynxes in all free‐range areas and captive breeding centres, with seroprevalence rates ranging from 5.7% to 51.4%, suggests widespread but not homogeneous HEV circulation among their populations. Indeed, animals kept in captivity had a more than six times higher risk of being exposed to the virus than free‐ranging individuals. Statistically significant differences between the four captive breeding centres were also found. This finding may be associated with differences in food suppliers or management measures between centres. Consumption of products derived from infected animals is considered to be the main route of HEV transmission, not only for humans but also for other mammal species. The Iberian lynx is a trophic specialist that depends mainly on rabbits, which are the main reservoir of the HEV‐3ra subtype but also susceptible to HEV‐4 and other strains of HEV‐3 (Spahr, Knauf‐Witzens, et al., 2017). Limited circulation of HEV has been demonstrated in free‐ranging wild rabbits in Iberian Mediterranean ecosystems (Caballero‐Gómez et al., 2020; Lopes & Abrantes, 2020), which is consistent with the low seroprevalence found in free‐ranging (6.5%) Iberian lynx populations in the present study. On the other hand, it should be noted that Iberian lynxes in captive breeding centres feed mainly on domestic farmed rabbits (Rivas et al., 2016) and that HEV circulation has been confirmed in farmed rabbits in other European countries (Bigoraj et al., 2020; Di Bartolo et al., 2016), although further studies are warranted to assess the role of wild and domestic rabbits in the epidemiology of HEV in Spain. In the event that its role as an HEV reservoir is confirmed, using domestic rabbits from HEV‐free farms to feed Iberian lynxes kept in captive breeding centres could be a useful tool to limit HEV transmission in this species. Captive Iberian lynxes can also be fed sporadically with partridge, quail and veal (Rivas et al., 2016). However, orthohepevirus infection in partridge and quail has not been reported so far, and the susceptibility of cattle to this virus remains under debate (Yugo et al., 2018).
Interestingly, seroconversions were detected in a high percentage (29.5%) of the longitudinally sampled individuals. This, coupled with the detection of seropositive yearlings, indicates HEV circulation during the study period. In addition, 15 animals were seropositive at all samplings, which could be related to repeated exposure to the virus or more likely to the lifelong persistence of antibodies in the Iberian lynx, as has previously been shown in other species (Pavio et al., 2017; Seminati et al., 2008). The significantly higher seroprevalence detected in older captive animals in multivariate analysis supports this hypothesis. There is no known information about the persistence of anti‐HEV antibodies in the Iberian lynx, although experimental studies conducted on nonhuman primates have confirmed that the persistence of antibodies against this virus can range from 100 days to more than 7 years post‐infection (Arankalle et al., 1999; Li et al., 1994). In our study, the sampling intervals for the two lynxes that seroreverted were 6 and 7 years, and while some individuals remained seropositive with similar sampling periods, this finding suggest that, in the absence of re‐exposure, anti‐HEV antibodies may decline 6 or 7 years after infection in this felid species. In any case, additional studies are needed to determine the duration of persistence of anti‐HEV antibodies in the Iberian lynx.
None of the 250 serum and 161 liver samples were positive for HEV‐A HEV‐B or HEV‐C infection. However, one of the 76 stool samples tested positive for HEV RNA, which confirm the susceptibility of the Iberian lynx to virus infection and increase the host range of HEV‐A. To the best of the authors’ knowledge, this is the first molecular report of HEV‐A infection in free‐ranging felines. Interestingly, HEV RNA was detected in the faeces, but not the liver, of the infected Iberian lynx. This could be related to an early or even late stage of acute infection as has previously been suggested for suid species (de Deus et al., 2008; Kozyra et al., 2020). Unfortunately, serum from this animal could not be obtained. The sequenced subgenotype in the positive lynx belonging to HEV‐3f (GenBank Accession Number: OL840902) has previously been detected in different wild and domestic ungulate species in the study area, including domestic pigs, horses, red deer and wild boar (García‐Bocanegra et al., 2019; Kukielka et al., 2015; López‐López et al., 2018; Rivero‐Juárez et al., 2018). Besides lagomorphs, free‐ranging Iberian lynxes may sporadically consume different ungulate species, such as red deer or wild boar (Masot et al., 2016; Rivas et al., 2016). There may also be direct or indirect contact between the Iberian lynx, and these as well as other susceptible wild and domestic ungulates, including fallow deer, pigs, goats and sheep, can occur in the Iberian Mediterranean ecosystems, which may increase the risk of HEV transmission among these sympatric species. In connection with this, the HEV‐positive lynx was sampled in a region characterized by high densities of wild boar, Iberian pig and red deer populations (Gortázar et al., 2011; Jiménez‐Ruiz et al., 2022) and where HEV‐3r has previously been found in wild boar (Caballero‐Gómez et al., 2019). This, coupled with the detection of HEV‐3f in the present study, confirms the circulation of both subtypes in wildlife in southwest Spain. HEV‐3f is also the main HEV subtype detected in humans in this country (Izopet et al., 2019). Detection of this subtype in both humans and the Iberian lynx and the strong similarity of HEV‐3f sequences retrieved from them (Figure 3) points to HEV circulation in different epidemiological contexts and suggest a common epidemiological cycle. In this context, cross‐species foodborne transmission of HEV‐3 from suids to humans or carnivores, such as the wolf (Canis lupus), has been demonstrated and recently suggested, respectively, in European countries, such as Spain and Italy (Riveiro‐Barciela et al., 2015; Rivero‐Juárez et al., 2017; Sarchese et al., 2021). This finding may also suggest that there is a possible risk of zoonotic transmission of HEV from the Iberian lynx, although further studies are warranted to support this hypothesis.
In conclusion, the serological and molecular results obtained in the present study provide evidence of HEV infection in free‐ranging and captive Iberian lynx populations and suggest a possible role for this species as spillover hosts of this virus in Iberian Mediterranean ecosystems. We also confirmed infection with the zoonotic genotype HEV‐3f in the Iberian lynx, which may of public health concern. The serological results indicate the widespread but not homogeneous circulation of HEV in Iberian lynx populations. Additional studies are needed to determine the source of HEV infection in the Iberian lynx, particularly in animals kept in captivity, in order to implement control measures to limit exposure to this virus.
CONFLICT OF INTEREST
None of the authors of this study has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
ETHICAL APPROVAL
This study did not involve killing of animals for the purpose of the investigations. Samples from Iberian lynx were collected by authorised veterinarians and animal keepers following routine procedures with alive and dead individuals before the design of this study, in compliance with the Ethical Principles in Animal Research. Thus, ethical approval by an Institutional Animal Care and Use Committee was not deemed necessary.
Supporting information
Supplementary Figure 1. Detection of anti‐HEV antibodies in three lynx sera by western blot assay illustrating the reactivity against HEV‐3 (C) or against both HEV‐3 and HEV‐C1 (D and E). For control, the recombinant antigens and a total lysate of M15pREP4 cells were tested by anti‐His tag antibody (A) and HEV capsid protein cross‐reactive monoclonal antibody (B). Nickel‐chelate affinity chromatography purified carboxy‐terminal segments of the capsid proteins of HEV‐3 and rat HEV‐C1 (Dremsek et al., 2012) and a nucleocapsid protein derivative (amino acid residues 1–39/213‐433) of Puumala orthohantavirus strain Vranica/Hällnäs, as negative control, were run in a 12% SDS‐PAGE and transferred to a PVDF membrane. Membranes were incubated with serum samples diluted 1:100, the antigen–antibody reaction was detected by incubation with purified recombinant protein A/G conjugated with horse‐radish peroxidase and visualized using Clarity Western ECL Substrate.
ACKNOWLEDGEMENTS
This work was supported by the Ministerio de Sanidad (RD12/0017/0012) integrated in the Plan Nacional de I+D+I and cofinanced by the ISCIII‐Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER), Fundación para la Investigación en Salud (FIS) del Instituto Carlos III (PI19/00864 and PI21/00793). J. Caballero‐Gómez is supported by an FPU grant of the Spanish Ministry of Science, Innovation and Universities (FPU17/01319). A. Rivero‐Juarez is the recipient of a Miguel Servet Research Contract by the Spanish Ministry of Science, Innovation and Universities (CP18/00111). R.G. Ulrich acknowledges support by the German Centre for Infection Research (DZIF), TTU ‘Emerging infections’ (TTU 01.808_00). We thank all the veterinarians and animal keepers, including Tere del Rey and those from the sampled breeding and captivity centres, involved in the sampling of animals as well as P., S. Castro Scholten, I. Zafra, G. Dolores and all the members of the CAD for their help in the collection of samples and laboratory analyses. We would also like to thank Barbara Kubickova for providing recombinant HEV antigens. We gratefully acknowledge to Castilla‐La Mancha veterinary team (especially Crespo, Grande‐Gomez, Apruzzese, Garcia‐Talens and Ramiro) for their assistance with lynx sampling. We also thank the Dirección General de Medio Natural y Biodiversidad of Junta de Comunidades de Castilla‐La Mancha (especially Antonio Aranda, Marino López de Carrión and Rafael Cubero). We are indebted to all the wildlife rangers (Agentes Medioambientales) of Castilla‐La Mancha and GEACAM technicians (especially Paco Sanchez, Javier Herrera‐Sanchez, Manuel Mata and Javier Caceres) for their assistance during trapping lynxes. The funders did not play any role in the design, conclusions or interpretation of the study. Funding for open access charge: Universidad de Córdoba / CBUA.
Caballero‐Gómez, J. , Rivero‐Juarez, A. , Zorrilla, I. , López, G. , Nájera, F. , Ulrich, R. G. , Ruiz‐Rubio, C. , Salcedo, J. , Rivero, A. , Paniagua, J. , & García‐Bocanegra, I. (2022). Hepatitis E virus in the endangered Iberian lynx (Lynx pardinus). Transboundary and Emerging Diseases, 69, e2745–e2756. 10.1111/tbed.14624
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the authors upon reasonable request.
REFERENCES
- Arankalle, V. A. , Chadha, M. S. , & Chobe, L. P. (1999). Long‐term serological follow up and cross‐challenge studies in rhesus monkeys experimentally infected with hepatitis E virus. Journal of Hepatology, 30(2), 199–204. 10.1016/S0168-8278(99)80062-2 [DOI] [PubMed] [Google Scholar]
- Bigoraj, E. , Kozyra, I. , Kwit, E. , & Rzeżutka, A. (2020). Detection of hepatitis E virus (rabbit genotype) in farmed rabbits entering the food chain. International Journal of Food Microbiology, 319, 108507. 10.1016/j.ijfoodmicro.2020.108507 [DOI] [PubMed] [Google Scholar]
- Boadella, M. , Casas, M. , Martín, M. , Vicente, J. , Segalés, J. , De la Fuente, J. , & Gortázar, C. (2010). Increasing contact with hepatitis E virus in red deer, Spain. Emerging infectious diseases, 16(12), 1994–1996. 10.3201/eid1612.100557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones, V. , De Juan, L. , Sánchez, C. , Vela, A. I. , & Galka, M. (2000). Bovine tuberculosis and the endangered Iberian lynx. Emerging Infectious Diseases, 6(2), 189. 10.3201/eid0602.000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero‐Gómez, J. , García‐Bocanegra, I. , Gómez‐Guillamón, F. , Camacho‐Sillero, L. , Zorrilla, I. , Lopez‐Lopez, P. , Cano‐Terriza, D. , Jiménez‐Ruiz, S. , Frias, M. , & Rivero‐Juarez, A. (2020). Absence of Hepatitis E virus circulation in wild rabbits (Oryctolagus cuniculus) and Iberian hares (Lepus granatensis) in Mediterranean ecosystems in Spain. Transboundary and Emerging Diseases, 67(4), 1422–1427. 10.1111/tbed.13478 [DOI] [PubMed] [Google Scholar]
- Caballero‐Gómez, J. , García‐Bocanegra, I. , Jiménez‐Martín, D. , Cano‐Terriza, D. , Risalde, M. A. , López‐López, P. , Jiménez‐Ruiz, S. , Rivero, A. , & Rivero‐Juarez, A. (2022). Epidemiological survey and risk factors associated with hepatitis E virus in small ruminants in southern Spain. Zoonoses and Public Health, 69(4), 387–393. 10.1111/zph.12935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero‐Gómez, J. , Jiménez‐Ruiz, S. , Lopez‐Lopez, P. , Vicente, J. , Risalde, M. A. , Cano‐Terriza, D. , Frias, M. , Barasona, J. A. , Rivero, A. , García‐Bocanegra, I. , & Rivero‐Juarez, A. (2019). Emergent subtype of hepatitis E virus genotype 3 in wild boar in Spain. Transboundary and Emerging Diseases, 66(5), 1803–1808. 10.1111/tbed.13251 [DOI] [PubMed] [Google Scholar]
- Caballero‐Gómez, J. , Rivero‐Juarez, A. , Jurado‐Tarifa, E. , Jiménez‐Martín, D. , Jiménez‐Ruiz, E. , Castro‐Scholten, S. , Ulrich, R. G. , López‐López, P. , Rivero, A. , & García‐Bocanegra, I. (2022). Serological and molecular survey of hepatitis E virus in cats and dogs in Spain. Transboundary and Emerging Diseases, 69(2), 240–248. 10.1111/tbed.14437 [DOI] [PubMed] [Google Scholar]
- de Deus, N. , Casas, M. , Peralta, B. , Nofrarías, M. , Pina, S. , Martín, M. , & Segalés, J. (2008). Hepatitis E virus infection dynamics and organic distribution in naturally infected pigs in a farrow‐to‐finish farm. Veterinary Microbiology, 132(1–2), 19–28. 10.1016/j.vetmic.2008.04.036 [DOI] [PubMed] [Google Scholar]
- Di Bartolo, I. , De Sabato, L. , Marata, A. , Martinelli, N. , Magistrali, C. F. , Monini, M. , Ponterio, E. , Ostanello, F. , & Ruggeri, F. M. (2016). Serological survey of hepatitis E virus infection in farmed and pet rabbits in Italy. Archives of Virology, 161(5), 1343–1346. https://10.1007/s00705‐016‐2778‐y [DOI] [PubMed] [Google Scholar]
- Dremsek, P. , Wenzel, J. J. , Johne, R. , Ziller, M. , Hofmann, J. , Groschup, M. H. , Werdermann, S. , Mohn, U. , Dorn, S. , Motz, M. , Mertens, M. , Jilg, W. , & Ulrich, R. G. (2012). Seroprevalence study in forestry workers from eastern Germany using novel genotype 3‐and rat hepatitis E virus‐specific immunoglobulin G ELISAs. Medical Microbiology and Immunology, 201(2), 189–200. 10.1007/s00430-011-0221-2 [DOI] [PubMed] [Google Scholar]
- Ferreras, P. , Aldama, J. J. , Beltrán, J. F. , & Delibes, M. (1992). Rates and causes of mortality in a fragmented population of Iberian lynx Felis pardina Temminck, 1824. Biological conservation, 61(3), 197–202. 10.1016/0006-3207(92)91116-A [DOI] [Google Scholar]
- Ferreras, P. , Gaona, P. , Palomares, F. , & Delibes, M. (2001). Restore habitat or reduce mortality? Implications from a population viability analysis of the Iberian lynx. In Animal conservation forum (Vol. 4, pp. 265–274). Cambridge University Press. 10.1017/S1367943001001317 [DOI] [Google Scholar]
- Frías, M. , López‐López, P. , Zafra, I. , Caballero‐Gómez, J. , Machuca, I. , Camacho, Á. , Risalde, M. A. , Rivero‐Juárez, A. , & Rivero, A. (2021). Development and clinical validation of a pangenotypic PCR‐based assay for the detection and quantification of hepatitis E virus (Orthohepevirus A genus). Journal of Clinical Microbiology, 59(2), e02075–20. 10.1128/JCM.02075-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Bocanegra, I. , Rivero, A. , Caballero‐Gómez, J. , López‐López, P. , Cano‐Terriza, D. , Frías, M. , Jiménez‐Ruiz, S. , Risalde, M. A. , Gómez‐Villamandos, J. C. , & Rivero‐Juarez, A. (2019). Hepatitis E virus infection in equines in Spain. Transboundary and Emerging Diseases, 66(1), 66–71. 10.1111/tbed.12962 [DOI] [PubMed] [Google Scholar]
- Gortazar, C. , Vicente, J. , Boadella, M. , Ballesteros, C. , Galindo, R. C. , Garrido, J. , Aranaz, A. , & de la Fuente, J. (2011). Progress in the control of bovine tuberculosis in Spanish wildlife. Veterinary Microbiology, 151(1–2), 170–178. 10.1016/j.vetmic.2011.02.041 [DOI] [PubMed] [Google Scholar]
- Hu, W. P. , Lu, Y. , Precioso, N. A. , Chen, H. Y. , Howard, T. , Anderson, D. , & Guan, M. (2008). Double‐antigen enzyme‐linked immunosorbent assay for detection of hepatitis E virus‐specific antibodies in human or swine sera. Clinical and Vaccine Immunology, 15(8), 1151–1157. 10.1128/CVI.00186-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Union for Conservation of Nature . (2021). The IUCN red list of threatened species. https://www.iucnredlist.org/
- Izopet, J. , Tremeaux, P. , Marion, O. , Migueres, M. , Capelli, N. , Chapuy‐Regaud, S. , Mansuy, J. M. , Abravanel, F. , Kamar, N. , & Lhomme, S. (2019). Hepatitis E virus infections in Europe. Journal of Clinical Virology, 120, 20–26. 10.1016/j.jcv.2019.09.004 [DOI] [PubMed] [Google Scholar]
- Jiménez‐Ruiz, S. , Laguna, E. , Vicente, J. , García‐Bocanegra, I. , Martínez‐Guijosa, J. , Cano‐Terriza, D. , Risalde, M. A. , & Acevedo, P. (2022). Characterization and management of interaction risks between livestock and wild ungulates on outdoor pig farms in Spain. Porcine Health Management, 8(1), 1–14. 10.1186/s40813-021-00246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johne, R. , Plenge‐Bönig, A. , Hess, M. , Ulrich, R. G. , Reetz, J. , & Schielke, A. (2010). Detection of a novel hepatitis E‐like virus in faeces of wild rats using a nested broad‐spectrum RT‐PCR. Journal of General Virology, 91(3), 750–758. 10.1099/vir.0.016584-0 [DOI] [PubMed] [Google Scholar]
- Kozyra, I. , Jabłoński, A. , Bigoraj, E. , & Rzeżutka, A. (2020). Wild boar as a sylvatic reservoir of hepatitis E virus in Poland: A cross‐sectional population study. Viruses, 12(10), 1113. 10.3390/v12101113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubickova, B. , Schenk, J. A. , Ramm, F. , Markuškienė, K. , Reetz, J. , Dremsek, P. , Tamosiunas, P. L. , Cepulyte, L. , Trinh, H. A. , Scholz, J. , Memczak, H. , Hovestädt, M. , Ryll, R. , Petraityte‐Burneikiene, R. , Corman, V. M. , Andersson, A. , Becher, D. , Groschup, M. H. , Kubick, S. , … Ulrich, R. G. (2021). A broadly cross‐reactive monoclonal antibody against hepatitis E virus capsid antigen. Applied Microbiology and Biotechnology, 105, 4957–4973. 10.1007/s00253-021-11342-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukielka, D. , Rodriguez‐Prieto, V. , Vicente, J. , & Sánchez‐Vizcaíno, J. M. (2015). Constant hepatitis E virus (HEV) circulation in wild boar and red deer in Spain: An increasing concern source of HEV zoonotic transmission. Transboundary and Emerging Diseases, 63(5), e360–e368. 10.1111/tbed.12311 [DOI] [PubMed] [Google Scholar]
- Lhomme, S. , Legrand‐Abravanel, F. , Kamar, N. , & Izopet, J. (2019). Screening, diagnosis and risks associated with Hepatitis E virus infection. Expert Review of Anti‐infective Therapy, 17(6), 403–418. 10.1080/14787210.2019.1613889 [DOI] [PubMed] [Google Scholar]
- Li, F. , Zhuang, H. , Kolivas, S. , Locarnini, S. A. , & Anderson, D. A. (1994). Persistent and transient antibody responses to hepatitis E virus detected by Western immunoblot using open reading frame 2 and 3 and glutathione S‐transferase fusion proteins. Journal of Clinical Microbiology, 32(9), 2060–2066. 10.1128/jcm.32.9.2060-2066.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, A. M. , & Abrantes, J. (2020). Hepatitis E virus is likely circulating in wild rabbits from Iberian Peninsula. Transboundary and Emerging Diseases, 67(5), 1761–1763. 10.1111/tbed.13702 [DOI] [PubMed] [Google Scholar]
- López, G. , López‐Parra, M. , Garrote, G. , Fernández, L. , del Rey‐Wamba, T. , Arenas‐Rojas, R. , Garcia‐Tardio, M. , Ruiz, G. , Zorilla, I. , Moral, M. , & Simón, M. A. (2014). Evaluating mortality rates and causalities in a critically endangered felid across its whole distribution range. European Journal of Wildlife Research, 60(2), 359–366. 10.1007/s10344-013-0794-8 [DOI] [Google Scholar]
- Lopez‐Lopez, P. , de los Angeles Risalde, M. , Frias, M. , García‐Bocanegra, I. , Brieva, T. , Caballero‐Gomez, J. , Camacho, A. , Fernández‐Molera, V. , Machuca, I. , Gomez‐Villamandos, J. C. , Rivero, A. , & Rivero‐Juarez, A. (2018). Risk factors associated with hepatitis E virus in pigs from different production systems. Veterinary Microbiology, 224, 88–92. 10.1016/j.vetmic.2018.08.020 [DOI] [PubMed] [Google Scholar]
- Lundkvist, Å. , Meisel, H. , Koletzki, D. , Lankinen, H. , Cifire, F. , Geldmacher, A. , Sibold, C. , Gött, P. , Vaheri, A. , Krüger, D. H. , & Ulrich, R. (2002). Mapping of B‐cell epitopes in the nucleocapsid protein of Puumala hantavirus. Viral Immunology, 15(1), 177–192. 10.1089/088282402317340323 [DOI] [PubMed] [Google Scholar]
- Masot, A. J. , Gil, M. , Risco, D. , Jiménez, O. M. , Núñez, J. I. , & Redondo, E. (2016). Pseudorabies virus infection (Aujeszky's disease) in an Iberian lynx (Lynx pardinus) in Spain: A case report. BMC Veterinary Research, 13(1), 1–8. 10.1186/s12917-016-0938-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meli, M. L. , Cattori, V. , Martínez, F. , López, G. , Vargas, A. , Palomares, F. , López‐Bao, J. V. , Hofmann‐Lehmann, R. , & Lutz, H. (2010). Feline leukemia virus infection: A threat for the survival of the critically endangered Iberian lynx (Lynx pardinus). Veterinary Immunology and Immunopathology, 134(1–2), 61–67. 10.1016/j.vetimm.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, X. J. (2016). Expanding host range and cross‐species infection of hepatitis E virus. PLoS Pathogens, 12(8), e1005695. 10.1371/journal.ppat.1005695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministerio para la Transición Ecológica y Reto Demográfico. (2021). La población de linces ibéricos alcanza su máximo histórico con más de un millar de ejemplares censados en 2020. https://www.miteco.gob.es/es/prensa/ultimas‐noticias/la‐poblaci%C3%B3n‐de‐linces‐ib%C3%A9ricos‐supera‐el‐millar‐de‐ejemplares‐en‐2020/tcm:30‐526
- Mulder, A. C. , Kroneman, A. , Franz, E. , Vennema, H. , Tulen, A. D. , Takkinen, J. , Hofhuis, A. , & Adlhoch, C. (2019). HEVnet: A One Health, collaborative, interdisciplinary network and sequence data repository for enhanced hepatitis E virus molecular typing, characterisation and epidemiological investigations. Eurosurveillance, 24(10), 1800407. 10.2807/1560-7917.ES.2019.24.10.1800407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nájera, F. , Grande‐Gómez, R. , Peña, J. , Vázquez, A. , Palacios, M. J. , Rueda, C. , Corona‐Bravo, A. I. , Zorrilla, I. , Revuelta, L. , Gil‐Molino, M. , & Jiménez, J. (2021). Disease Surveillance during the reintroduction of the Iberian Lynx (Lynx pardinus) in Southwestern Spain. Animals, 11(2), 547. 10.3390/ani11020547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell, K. , & Jackson, P. (1996). Wild Cats. Status survey and conservation action plan. IUCN/SSC Cat Specialist Group. IUCN. [Google Scholar]
- Pavio, N. , Doceul, V. , Bagdassarian, E. , & Johne, R. (2017). Recent knowledge on hepatitis E virus in Suidae reservoirs and transmission routes to human. Veterinary Research, 48(1), 1–14. 10.1186/s13567-017-0483-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risalde, M. A. , Rivero‐Juarez, A. , Romero‐Palomo, F. , Frias, M. , Lopez‐Lopez, P. , Cano‐Terriza, D. , García‐Bocanegra, I. , Jiménez‐Ruíz, S. , Camacho, Á. , Machuca, I. , Gomez‐Villamandos, J. C. , & Rivero, A. (2017). Persistence of hepatitis E virus in the liver of non‐viremic naturally infected wild boar. Plos One, 12(11), e0186858. 10.1371/journal.pone.0186858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas, A. (2016). Manual del manejo del lince ibérico en cautividad. Programa de Conservación ex‐situ del Lince Ibérico. https://www.lynxexsitu.es/ficheros/documentos_pdf/84/Manual_Manejo_Lince_Iberico_2016.pdf
- Riveiro‐Barciela, M. , Mínguez, B. , Gironés, R. , Rodriguez‐Frías, F. , Quer, J. , & Buti, M. (2015). Phylogenetic demonstration of hepatitis E infection transmitted by pork meat ingestion. Journal of Clinical Gastroenterology, 49(2), 165–168. 10.1097/MCG.0000000000000113 [DOI] [PubMed] [Google Scholar]
- Rivero‐Juarez, A. , Frias, M. , Martinez‐Peinado, A. , Risalde, M. A. , Rodriguez‐Cano, D. , Camacho, A. , García‐Bocanegra, I. , Cuenca‐Lopez, F. , Gomez‐Villamandos, J. C. , & Rivero, A. (2017). Familial hepatitis E outbreak linked to wild boar meat consumption. Zoonoses and Public Health, 64(7), 561–565. 10.1111/zph.12343 [DOI] [PubMed] [Google Scholar]
- Rivero‐Juarez, A. , Frias, M. , Perez, A. B. , Pineda, J. A. , Reina, G. , Fuentes‐Lopez, A. , Gómez‐Ayerbe, C. , Rivero‐Juárez, A. , Domínguez, C. , Santos, M. , Viñuela, L. , Palacios, R. , Real, L. M. , Rivero, A. , Macías, J. , Pineda, J. A. , & Rivero, A. (2022). Orthohepevirus C infection as an emerging cause of acute hepatitis in Spain: First report in Europe. Journal of Hepatology, . Advance online publication. 10.1016/j.jhep.2022.01.028 [DOI] [PubMed] [Google Scholar]
- Rivero‐Juarez, A. , Jarilla‐Fernandez, M. , Frias, M. , Madrigal‐Sanchez, E. , López‐López, P. , Andújar‐Troncoso, G. , Machuca, I. , Camacho, A. , Muñoz‐Valbuena, P. , & Rivero, A. (2019). Hepatitis E virus in Spanish donors and the necessity for screening. Journal of Viral Hepatitis, 26(5), 603–608. 10.1111/jvh.13064 [DOI] [PubMed] [Google Scholar]
- Rivero‐Juarez, A. , Risalde, M. A. , Frias, M. , García‐Bocanegra, I. , Lopez‐Lopez, P. , Cano‐Terriza, D. , Camacho, A. , Jimenez‐Ruiz, S. , Gomez‐Villamandos, J. C. , & Rivero, A. (2018). Prevalence of hepatitis E virus infection in wild boars from Spain: A possible seasonal pattern? BMC Veterinary Research, 14(1), 1–6. 10.1186/s12917-018-1377-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez, A. , & Delibes, M. (2004). Patterns and causes of non‐natural mortality in the Iberian lynx during a 40‐year period of range contraction. Biological Conservation, 118(2), 151–161. 10.1016/j.biocon.2003.07.018 [DOI] [Google Scholar]
- Sarchese, V. , Fruci, P. , Palombieri, A. , Di Profio, F. , Robetto, S. , Ercolini, C. , Orusa, R. , Marsilio, F. , Martella, V. , & Di Martino, B. (2021). Molecular identification and characterization of a genotype 3 hepatitis E virus (HEV) strain detected in a wolf faecal sample, Italy. Animals, 11(12), 3465. 10.3390/ani11123465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminati, C. , Mateu, E. , Peralta, B. , De Deus, N. , & Martin, M. (2008). Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. The Veterinary Journal, 175(1), 130–132. 10.1016/j.tvjl.2006.11.018 [DOI] [PubMed] [Google Scholar]
- Simon, M. A. , Gil‐Sánchez, J. M. , Ruiz, G. , Garrote, G. , Mccain, E. B. , Fernandez, L. , López‐Parra, M. , Rojas, E. , Arenas‐Rojas, R. , Rey, T. D. , García‐Tardío, M. , & Lopez, G. (2012). Reverse of the decline of the endangered Iberian lynx. Conservation Biology, 26(4), 731–736. 10.1111/j.1523-1739.2012.01871.x [DOI] [PubMed] [Google Scholar]
- Smith, D. B. , Simmonds, P. , Izopet, J. , Oliveira‐Filho, E. F. , Ulrich, R. G. , Johne, R. , Koenig, M. , Jameel, S. , Harrison, T. J. , Meng, X. J. , Okamoto, H. , Van der Poel, W. H. M. , & Purdy, M. A. (2016). Proposed reference sequences for hepatitis E virus subtypes. The Journal of General Virology, 97(3), 537. 10.1099/jgv.0.000393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, D. B. , Izopet, J. , Nicot, F. , Simmonds, P. , Jameel, S. , Meng, X. J. , Norder, H. , Okamoto, H. , van der Poel, W. H. M. , Reuter, G. , & Purdy, M. A. (2020). Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). The Journal of General Virology, 101(7), 692. 10.1099/jgv.0.001435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y.‐J. , Kim, B.‐S. , Park, W.‐J. , Park, B.‐J. , Lee, S.‐K. , Shin, J.‐I. , Lee, N.‐H. , Lee, J.‐B. , Park, S.‐Y. , Song, C.‐S. , Seo, K.‐H. , & Choi, I.‐S. (2013). Seroprevalence of hepatitis E virus in zoo animal species in Korea. Korean Journal of Veterinary Research, 53(1), 65–68. 10.14405/kjvr.2013.53.1.065 [DOI] [Google Scholar]
- Spahr, C. , Knauf‐Witzens, T. , Vahlenkamp, T. , Ulrich, R. G. , & Johne, R. (2017). Hepatitis E virus and related viruses in wild, domestic and zoo animals: A review. Zoonoses and Public Health, 65(1), 11–29. 10.1111/zph.12405 [DOI] [PubMed] [Google Scholar]
- Spahr, C. , Ryll, R. , Knauf‐Witzens, T. , Vahlenkamp, T. W. , Ulrich, R. G. , & Johne, R. (2017). Serological evidence of hepatitis E virus infection in zoo animals and identification of a rodent‐borne strain in a Syrian brown bear. Veterinary Microbiology, 212, 87–92. 10.1016/j.vetmic.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Sridhar, S. , Situ, J. , Cai, J. P. , Yip, C. C. Y. , Wu, S. , Zhang, A. J. , Wen, L. , Chew, N. F. , Chan, W. M. , Poon, R. W. , Chan, J. F. , Tsang, D. N. , Chen, H. , Xia, N. S. , & Yuen, K. Y. (2021). Multimodal investigation of rat hepatitis E virus antigenicity: Implications for infection, diagnostics, and vaccine efficacy. Journal of Hepatology, 74(6), 1315–1324. 10.1016/j.jhep.2020.12.028 [DOI] [PubMed] [Google Scholar]
- Vargas, A. (2009). Iberian Lynx ex situ conservation: An interdisciplinary approach. Fundación Biodiversidad. [Google Scholar]
- Wang, B. , Harms, D. , Yang, X. L. , & Bock, C. (2020). Orthohepevirus C: An expanding species of emerging hepatitis e virus variants. Pathogens, 9(3), 154. 10.3390/pathogens9030154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugo, D. M. , Cossaboom, C. M. , Heffron, C. L. , Huang, Y. W. , Kenney, S. P. , Woolums, A. R. , Hurley, D. J. , Opriessnig, T. , Li, L. , Delwart, E. , Kanevsky, I. , & Meng, X. J. (2018). Evidence for an unknown agent antigenically related to the hepatitis E virus in dairy cows in the United States. Journal of Medical Virology, 91(4), 677–686. 10.1002/jmv.25339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W. , Shen, Q. , Mou, J. , Yang, Z. B. , Yuan, C. L. , Cui, L. , Zhu, J. G. , Hua, X. G. , Xu, C. M. , & Hu, J. (2008). Cross‐species infection of hepatitis E virus in a zoo‐like location, including birds. Epidemiology & Infection, 136(8), 1020–1026. 10.1017/S095026880700965X [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Detection of anti‐HEV antibodies in three lynx sera by western blot assay illustrating the reactivity against HEV‐3 (C) or against both HEV‐3 and HEV‐C1 (D and E). For control, the recombinant antigens and a total lysate of M15pREP4 cells were tested by anti‐His tag antibody (A) and HEV capsid protein cross‐reactive monoclonal antibody (B). Nickel‐chelate affinity chromatography purified carboxy‐terminal segments of the capsid proteins of HEV‐3 and rat HEV‐C1 (Dremsek et al., 2012) and a nucleocapsid protein derivative (amino acid residues 1–39/213‐433) of Puumala orthohantavirus strain Vranica/Hällnäs, as negative control, were run in a 12% SDS‐PAGE and transferred to a PVDF membrane. Membranes were incubated with serum samples diluted 1:100, the antigen–antibody reaction was detected by incubation with purified recombinant protein A/G conjugated with horse‐radish peroxidase and visualized using Clarity Western ECL Substrate.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.


