Dear Editor,
Hypermobile Ehlers–Danlos syndrome (hEDS) and hypermobility spectrum disorders (HSD) are incapacitating and painful syndromes involving a generalized connective tissue disorder with joint hypermobility and musculoskeletal complications. A neuropathic component seemed clinically likely given frequent burning sensations, hypoesthesia, or allodynia [1]. A small fiber neuropathy (SFN), that is, the dysfunction of A‐δ and C‐fibers relaying thermal, nociceptive, and autonomic information, have been suggested in previous case series of hEDS/HSD [2, 3]. These reports provided early findings of small fiber involvement in hEDS, but their limited sample size (n < 20) and sole reliance on skin biopsies and questionnaires called for further investigations.
In our retrospective study, we report structural and functional evaluations of small nerve fibers in a large cohort of patients suffering from hEDS/HSD (n = 79, 71% hEDS). Patients were referred to the Pain Center of Lausanne University Hospital when a small fiber neuropathy was suspected based on anamnestic complaints (hypoesthesia, burning pain, allodynia, or dysautonomia). The standardized assessment included a clinical examination, questionnaires, quantitative sensory testing (QST, according to the protocol of the German Research Network on Neuropathic Pain) and skin biopsies (declined by 10 patients) (see Supplementary Material).
We observed a small nerve fiber dysfunction based on QST in 55 of 79 patients (70%) as well as a structural deficit based on intraepidermal nerve fiber density (IENFD) in 54 of 69 patients (78%). Considering only the 69 patients who underwent these two assessments (Fig. 1), SFN was definite (both abnormal structure and function) in 40 of 69 patients (58%), possible (either one or the other) in 23 of 69 patients (33%) and excluded (both normal) in only six of 69 patients (9%).
Fig. 1.
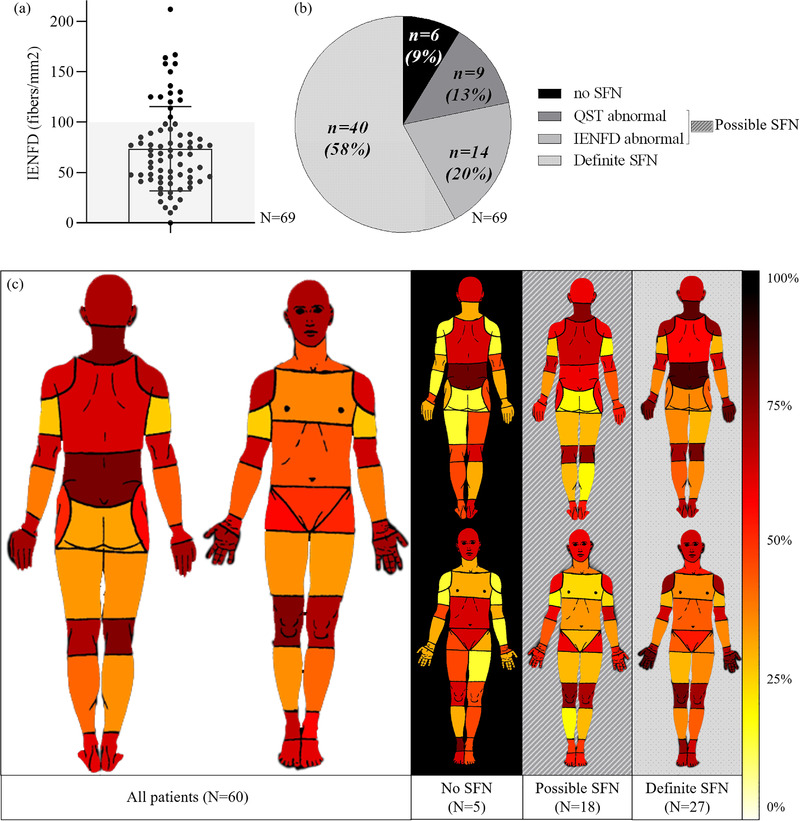
Distribution of the intraepidermal nerve fiber density (IENFD) count in the hypermobile Ehlers–Danlos syndrome (hEDS)/hypermobility spectrum disorders (HSD) population (a); subdivision of the hEDS/HSD population according to the likelihood of small fiber neuropathy based on quantitative sensory testing (QST) and IENFD (b); pain distribution heat map (c). The left part of panel c represents the frequency of pain localization of hEDS/HSD patients as reported on pain drawings; the right panel shows the body map representations in sub‐groups of patients based on their small fiber neuropathy (SFN) likelihood. The color gradient represents the percentage of patients reporting pain in each body area from light yellow (0%) to dark red (100%). Definite SFN = both QST and IENFD abnormal. No SFN = both QST and IENFD normal. Possible SFN = either QST or IENFD abnormal.
Patients in this hEDS/HSD cohort reported at least moderate pain intensity (Brief Pain Inventory [BPI], mean pain intensity, M = 5.7, SD = 1.9) interfering with their daily life (BPI–mean pain interference, M = 5.7, SD = 2.4). The mean score for the Widespread Pain Index was 11.3/19 (SD = 4.1). Sixty of the 79 patients drew their pain on the BPI body map (with the pain distribution reported in Fig. 1c). On average, 58% of the body zones were considered painful. A sub‐group analysis according to the likelihood of SFN is shown on the right panel of Fig. 1c. The amount of patients reporting pain in the hand significantly differed among these categories (SFN, possible SFN, no SFN; H(2) = 8.6, p = 0.01), with a significant difference between patients with and without SFN (adjusted p = 0.03).
The large proportion of patients in this study displaying small nerve fiber abnormalities underscores the possible contribution of the peripheral nervous system to hEDS/HSD symptoms. Interestingly, unmyelinated small fibers contribute to 80% of joint and musculoskeletal innervation [4]. Afferent nerve fibers also affect muscle development, including spindle formation and maintenance [5]. A loss of sensory innervation impairs the healing of bone fractures. Such a deficit negatively affects tissue development and homeostasis and may contribute to chronic musculoskeletal diseases [6]. SFN could therefore participate to hypermobility through an impairment of development, or an impairement of recovery following repetitive trauma.
Nevertheless, the pain experience in hEDS/HSD is likely to be multifactorial, including a nociceptive contribution (joint instabilities resulting in repetitive dislocations, a muscular component or periarticular inflammation) [7] and a likely central sensitization component [8, 9]. In our cohort, there is evidence for neuropathic, nociceptive musculoskeletal, and nociplastic contributions. The reported proportion of each of these factors varies, which is not surprising given the heterogeneity of the hEDS/HSD population and the various recruitment criteria.
The retrospective design entails some significant limitations. Patients were referred to the pain center because of symptoms compatible with SFN. Hence, the described cohort is not reflecting a full population of hEDS/HSD patients (as described in [10]), but only a significant proportion (50%). The assessment of an entire cohort would allow determining more precisely the SFN prevalence in a general population of hEDS/HSD, including those who are asymptomatic.
The presence of small fiber pathology could help a better stratification of the heterogeneous hEDS/HSD population in terms of severity, disease extent and therapeutic options. Furthermore, the co‐occurrence with SFN, a neurological disorder that can be diagnosed by objective measures, might improve the recognition of the complex hEDS/HSD pathology.
Conflict of interest
The authors declare no conflict of interest.
Author contributions
Aurore Fernandez: Conceptualization, data curation, formal analysis, methodology, writing–original draft, and writing–review and editing. Mathieu Vautey: Resources, software, and visualization. Berengère Aubry‐Rozier: Conceptualization. Chantal Berna: Conceptualization, writing‐original draft, writing‐review and editing. Marc Suter: Conceptualization, writing‐original draft, writing‐review and editing.
Supporting information
Supporting Material
Acknowledgments
We are grateful to Prof. Isabelle Décosterd for her support and the nurturing research environment she has created in the Pain Center. We also thank Prof. Charles Benaim, Service of Rheumatology, for his collaboration around the hEDS/HSD cohort. We are grateful to Dr. Nicole Kamber and Mrs. Martina Schobesberger from the Neuromorphology Laboratory, Bern University Hospital, for the harmonious clinical collaboration.
Open access funding provided by Universite de Lausanne.
Chantal Berna and Marc R. Suter contributed equally to this work.
References
- 1. Camerota F, Celletti C, Castori M, Grammatico P, Padua L. Neuropathic pain is a common feature in Ehlers‐Danlos syndrome. J Pain Symptom Manage. 2011;41(1):e2–24. [DOI] [PubMed] [Google Scholar]
- 2. Pascarella A, Provitera V, Lullo F, Stancanelli A, Saltalamacchia AM, Caporaso G, et al. Evidence of small fiber neuropathy in a patient with Ehlers‐Danlos syndrome, hypermobility‐type. Clin Neurophysiol. 2016;127(3):1914–6. [DOI] [PubMed] [Google Scholar]
- 3. Cazzato D, Castori M, Lombardi R, Caravello F, Bella ED, Petrucci A, et al. Small fiber neuropathy is a common feature of Ehlers‐Danlos syndromes. Neurology. 2016;87(2):155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alpantaki K, McLaughlin D, Karagogeos D, Hadjipavlou A, Kontakis G. Sympathetic and sensory neural elements in the tendon of the long head of the biceps. J Bone Joint Surg Am. 2005;87(7):1580–3. [DOI] [PubMed] [Google Scholar]
- 5. Ho BL, Goh Q, Nikolaou S, Hu L, Shay‐Winkler K, Cornwall R. NRG/ErbB signaling regulates neonatal muscle growth but not neuromuscular contractures in neonatal brachial plexus injury. FEBS Lett. 2021;595(5):655–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rajpar I, Tomlinson RE, Function of peripheral nerves in the development and healing of tendon and bone. Semin Cell Dev Biol. 2022;123:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rombaut L, Malfait F, Cools A, De Paepe A, Calders P. Musculoskeletal complaints, physical activity and health‐related quality of life among patients with the Ehlers‐Danlos syndrome hypermobility type. Disabil Rehabil. 2010;32(16):1339–45. [DOI] [PubMed] [Google Scholar]
- 8. Di Stefano G, Celletti C, Baron R, Castori M, Di Franco M, La Cesa S, et al. Central sensitization as the mechanism underlying pain in joint hypermobility syndrome/Ehlers‐Danlos syndrome, hypermobility type. Eur J Pain. 2016;20(8):1319–25. [DOI] [PubMed] [Google Scholar]
- 9. Leone CM, Celletti C, Gaudiano G, Puglisi PA, Fasolino A, Cruccu G, et al. Pain due to Ehlers‐Danlos syndrome is associated with deficit of the endogenous pain inhibitory control. Pain Med. 2020;21(9):1929–35. [DOI] [PubMed] [Google Scholar]
- 10. Aubry‐Rozier B, Schwitzguebel A, Valerio F, Tanniger J, Paquier C, Berna C, et al. Are patients with hypermobile Ehlers‐Danlos syndrome or hypermobility spectrum disorder so different? Rheumatol Int. 2021;41(10):1785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Material


