Abstract
Aims
Heart failure with reduced ejection fraction (HFrEF) remains associated with high morbidity and mortality, poor quality of life (QoL) and significant exercise limitation. Sympatho‐vagal imbalance has been shown to predict adverse prognosis and symptoms in HFrEF, yet it has not been specifically targeted by any guideline‐recommended device therapy to date. Barostim™, which directly addresses this imbalance, is the first Food and Drug Administration approved neuromodulation technology for HFrEF. We aimed to analyse all randomized trial evidence to evaluate the effect of baroreflex activation therapy (BAT) on heart failure symptoms, QoL and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) in HFrEF.
Methods and results
An individual patient data (IPD) meta‐analysis was performed on all eligible trials that randomized HFrEF patients to BAT + guideline‐directed medical therapy (GDMT) or GDMT alone (open label). Endpoints included 6‐month changes in 6‐min hall walk (6MHW) distance, Minnesota Living With Heart Failure (MLWHF) QoL score, NT‐proBNP, and New York Heart Association (NYHA) class in all patients and three subgroups. A total of 554 randomized patients were included. In all patients, BAT provided significant improvement in 6MHW distance of 49 m (95% confidence interval [CI] 33, 64), MLWHF QoL of −13 points (95% CI −17, −10), and 3.4 higher odds of improving at least one NYHA class (95% CI 2.3, 4.9) when comparing from baseline to 6 months. These improvements were similar, or better, in patients who had baseline NT‐proBNP <1600 pg/ml, regardless of the cardiac resynchronization therapy indication status.
Conclusion
An IPD meta‐analysis suggests that BAT improves exercise capacity, NYHA class, and QoL in HFrEF patients receiving GDMT. These clinically meaningful improvements were consistent across the range of patients studies. BAT was also associated with an improvement in NT‐proBNP in subjects with a lower baseline NT‐proBNP.
Keywords: Heart failure, Baroreflex, Autonomic nervous system, Meta‐analysis, Randomized controlled trials
Picture of the Barostim device in‐situ and its mode of action along with summary effects in the meta‐analysis. 6MHW, 6‐min hall walk; CI, confidence interval; CRT, cardiac resynchronization therapy; MLWHF, Minnesota Living With Heart Failure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.

Introduction
There have been significant improvements in the management of heart failure (HF) with reduced ejection fraction (HFrEF). 1 Despite these improvements, many HFrEF patients remain categorized in New York Heart Association (NYHA) functional class II or III and continue to have a reduced life expectancy, frequent HF hospitalizations, poor quality of life (QoL) and limitations on their exercise capacity. 2 , 3 Many HFrEF patients are not considered sick enough for advanced HF treatments, such as left ventricular assist devices or heart transplantation, indicating the need for more effective therapies applicable to moderately symptomatic patients in NYHA class II–III. Activation of the sympathetic nervous system and relative under‐activity of the parasympathetic nervous system are together thought to play a major role in both symptom generation and disease progression in HFrEF patients. 4 The resulting autonomic imbalance exacerbates myocardial remodelling, peripheral vasoconstriction, and renal salt and water retention and increases the risk of mortality and HF hospitalizations. 5 , 6
The CVRx® Barostim NEO™ System is a novel treatment developed to address this unmet need. It is an implantable device capable of producing cardiac autonomic modulation via electrical activation of the baroreflex, the body's natural mechanism that regulates cardiovascular function through reflex inhibition of sympathetic outflow and activation of the parasympathetic nervous system. The Barostim system consists of a carotid sinus lead and an implantable pulse generator (IPG). The carotid sinus lead is surgically placed on the outside of the carotid sinus and tunnelled over the clavicle to the IPG. The IPG is implanted similar to a pacemaker in a standard subcutaneous device pocket. The system contains no hardware in the heart or vasculature.
The effects of baroreflex activation therapy (BAT) in HF have been examined in pre‐clinical trials, 7 , 8 a first‐in‐human single centre trial 9 and two prospective, randomized multicentre controlled trials. 10 , 11 The BAT concept has been reviewed in several publications. 12 , 13 These trials have shown that BAT, which activates afferent signalling to the brain via the baroreceptors in the carotid artery, reduced sympathetic and increased parasympathetic signalling that acted in aggregate to rebalance the autonomic input to the heart. The multicentre randomized trials (HOPE4HF 10 and BeAT‐HF 11 ) demonstrated that BAT is safe to use and significantly improved functional status, QoL scores, exercise capacity, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) levels.
We performed a new individual patient data (IPD) meta‐analysis from all patients enrolled in these two prospective, randomized multicentre controlled trials to systematically review the efficacy of BAT in the full studied patient population. In addition, the meta‐analysis enabled further exploration of data that may be more prevalent in one trial, such as NT‐proBNP and brain natriuretic peptide (BNP). The inclusion of two trials enabled additional analyses to evaluate outcomes from several subsets of this larger patient population.
Methods
A comprehensive list of all trials recruiting HFrEF patients and utilizing the Barostim™ device was ascertained from the sole manufacturer, CVRx, Inc. of Minneapolis, MN, USA.
Selected studies
Individual patient data from the two prospective randomized controlled BAT clinical studies that enrolled patients with NYHA class III or II (NYHA class III within the previous months), a reduced left ventricular ejection fraction (LVEF ≤35%) and on guideline‐directed medical therapy (GDMT) were included in the meta‐analysis. Both studies completed enrolment, complied with the Declaration of Helsinki and were approved by the appropriate regulatory authorities and ethics committees. A total of 554 randomized patients were considered for the meta‐analysis, as shown by trial in Table 1 . Patients were randomized 1:1 to either GDMT (Control) or BAT + GDMT (BAT) (open label).
Table 1.
Comparison of heart failure with reduced ejection fraction randomized studies
| Phase II HOPE4HF10 | Phase III BeAT‐HF11 | |
|---|---|---|
| Overview | ||
| First enrolment | April 2012–April 2014 | April 2016–Oct 2018 |
| Countries | United States, Germany, Italy, France, Canada | United States, United Kingdom |
| Randomized | 146 | 408 |
| Eligibility criteria | ||
| Left ventricular ejection fraction | ≤35% | ≤35% |
| NYHA class | III |
III or II (with recent NYHA class III) |
| 6MHW |
150 m ≤6 MHW ≤400 m (≤450 m in Europe) |
150 m ≤6 MHW ≤400 m |
| Optimal HF guideline‐directed therapy | 4 weeks prior to enrolment | 4 weeks prior to enrolment |
| HF criteria | – |
Prior HF hospitalization or BNP ≥100 pg/ml or NT‐proBNP ≥400 pg/ml |
| CRT | If CRT, >6 months active | Exclude CRT class I indicated |
| Baseline status | ||
| Demographics | ||
| Women | 14% | 20% |
| NYHA class III* | 99% | 94% |
| Age (years), mean ± SD | 65 ± 11 | 63 ± 11 |
| Health status | ||
| Body mass index (kg/m2), mean ± SD | 29 ± 5 | 30 ± 5 |
| Systolic blood pressure (mmHg), mean ± SD | 117 ± 18 | 119 ± 18 |
| Diastolic blood pressure (mmHg), mean ± SD | 72 ± 11 | 73 ± 11 |
| Heart rate (bpm)*, mean ± SD | 74 ± 11 | 75 ± 11 |
| Left ventricular ejection fraction (%), mean ± SD | 25 ± 7 | 26 ± 7 |
| NT‐proBNP (pg/ml), median (IQR) | 1241 (521–4044) | 1058 (594–2424) |
| 6‐min hall walk distance (m) mean ± SD | 303 ± 82 | 295 ± 72 |
| MLWHF QoL*, mean ± SD | 47 ± 22 | 53 ± 25 |
| Comorbidities | ||
| Coronary artery disease | 67% | 66% |
| Atrial fibrillation | 44% | 39% |
| Chronic kidney disease | 29% | 28% |
| Diabetes type 2* | 35% | 45% |
| Heart failure treatments | ||
| ACEi/ARB or ARNI | 80% | 84% |
| Beta‐blocker* | 86% | 95% |
| Diuretics* | 76% | 89% |
| CRT* | 32% | 3% |
| ICD/CRT‐D* | 87% | 78% |
6MHW, 6‐min hall walk; ACEi, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker, ARNI, angiotensin receptor–neprilysin inhibitor; BNP, brain natriuretic peptide; CRT, cardiac resynchronization therapy; CRT‐D, cardiac resynchronization therapy‐defibrillator; HF, heart failure; ICD, implantable cardioverter‐defibrillator; IQ;, interquartile range; MLWHF, Minnesota Living With Heart Failure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; QoL, quality of life; SD, standard deviation.
*p<0.05.
The purpose of the two clinical studies was to describe the safety and performance of BAT using the Barostim NEO System in patients with symptomatic HF. The Phase II HOPE4HF trial was a randomized trial that enrolled patients in the United States (US), Europe and Canada between April 2012 and April 2014. A total of 70 patients were randomized to Control and 76 to BAT in this trial. The Phase III BeAT‐HF trial was also a randomized trial that enrolled almost all its patients in the US. The Barostim device was granted Breakthrough Device Status (formerly Expedited Access) by the Food and Drug Administration (FDA) for the BeAT‐HF trial. 14 Between April 2016 and October 2018, 209 patients were randomized to Control and 199 to BAT. These studies showed that treatment with BAT was safe and demonstrated improvements in 6‐min hall walk (6MHW), NYHA class, QoL, and NT‐proBNP/BNP at 6 months. The trial protocols permitted medication changes for clinical necessity. In the BeAT‐HF trial, during the 6‐month follow‐up there was a significant difference in HF medications between the two randomized arms. For example, the GDMT alone patients were more likely to have a new class of HF drugs added than the BAT + GDMT patients (29% vs. 18%), including a disproportional uptake in sacubitril/valsartan, which was approved during the trial (GDMT patients 16% vs. the BAT + GDMT patients 4%).
A comparison of the demographics, clinical characteristics and eligibility criteria between the studies are shown in Table 1 and combined in online supplementary Table S1 .
Endpoint measures and selected cohorts
At the time of this writing, major clinical outcomes (deaths and hospitalizations) are still being collected in the BeAT‐HF trial, however these will not be unblinded until the pre‐specified number of outcome events are obtained. Thus, these outcomes are not included in this meta‐analysis. The meta‐analysis efficacy endpoints that were evaluated include those consistently collected that are considered valuable markers of clinical efficacy. These included the change from baseline to 6 months for 6MHW distance, Minnesota Living With Heart Failure (MLWHF) QoL, NYHA class, and NT‐proBNP/BNP for BAT and Control patients. As some patients had BNP instead of NT‐proBNP collected, a sensitivity analysis was done in which the diagnostic performance of the relative reduction of BNP and NT‐proBNP were combined, as these two markers are considered comparable with no meaningful difference between the two (online supplementary Tables S2 and S3 ). 15
Results from all patients combined across the two randomized studies were analysed, following which three cohorts from this full patient population were examined. These cohorts were defined to determine the effect of BAT on more homogeneous groups representing different sub‐populations of interest. Given that the FDA indication for this device is for patients without an indication for cardiac resynchronization therapy (CRT) and with NT‐proBNP values <1600 pg/ml, we chose to use these two factors to define specific patient cohorts for further study. The baseline NT‐proBNP value and CRT status, defined as either a presence of a CRT or a class I CRT indication or not, were used to determine the cohorts further described in Table 2 .
Table 2.
Description of patient cohorts
| Cohort | Definition | Trial |
|---|---|---|
| All | All patients randomized |
HOPE4HF, n = 146 BeAT‐HF, n = 408 Total, n = 554 |
| NT‐proBNP <1600 pg/ml | All patients randomized with baseline NT‐proBNP <1600 pg/ml |
HOPE4HF, n = 51 BeAT‐HF, n = 264 Total, n = 315 |
|
No CRT Indication |
All patients randomized excluding:
|
HOPE4HF, n = 100 BeAT‐HF, n = 408 Total, n = 508 |
| No CRT indication and NT‐proBNP <1600 pg/ml | All patients randomized with:
|
HOPE4HF, n = 35 BeAT‐HF, n = 264 Total, n = 299 |
ACC/AHA, American College of Cardiology/American Heart Association; CRT, cardiac resynchronization therapy; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide.
Statistical methods
Endpoints across cohorts were analysed in a consistent manner. For the 6MHW and QoL outcomes, mean differences in the change from baseline to 6 months in the BAT compared to the Control were calculated. For the outcome of NT‐proBNP, the mean change was examined on a log10 scale, and after the appropriate inverse transformation, was interpreted as a comparison of the relative percent change at 6 months. Analyses of covariance were performed using mixed models with adjustment for baseline values. Improvement in NYHA class was defined as a lower class at 6 months. Comparisons between arms were calculated using the odds ratio (OR) and 95% confidence intervals (CI) for each effect. Although improvement is defined as a decrease in NYHA class, a positive OR demonstrates the likelihood that a patient improves at least one NYHA class.
Fixed‐effect models with inverse variance weighting were used to pool primary trial effects across studies, and I 2 statistics calculated to quantify heterogeneity between the two studies. Due to the small number of studies, tau‐squared may be poorly estimated, thus fixed‐effect models with inverse variance weighting were used.
Stata version 16 (16.1; StataCorp 2013, Stata Statistical Software Release 16, College Station, TX, USA) was used for all analyses.
Results
At 6 months, analysis of the full patient population demonstrated that clinically and statistically significant improvements occurred in the BAT arm for 6MHW distance, MLWHF QoL, and NYHA class compared to the Control (Figure 1 and online supplementary Table S2 ). These improvements were consistent across both trials and the I 2 statistics for trial heterogeneity 0% for each of the three endpoints.
Figure 1.

Endpoint results by trial (HOPE4HF, 10 BeAT‐HF 11 ). 6MHW, 6‐min hall walk; CI, confidence interval; MLWHF, Minnesota Living With Heart Failure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
As shown in Figure 2 and online supplementary Table S3 , for 6MHW distance, BAT showed a statistically significant improvement over Control in all patients of 48.5 m (95% CI 32.7, 64.2), with similar improvements shown in all three cohorts. Similarly, for QoL assessed by MLWHF, BAT led to a significant improvement (shown as a decrease) of −13.4 points (95% CI −17.1, −9.6), which was consistent in all studied cohorts. The OR for an improvement in NYHA class was also significantly in favour of BAT versus Control in all patients and in all studied cohorts. NT‐proBNP levels appeared to improve in all patients, but only reached statistical significance in the cohorts that excluded patients with NT‐proBNP >1600 pg/ml. The results from the sensitivity analysis combining NT‐proBNP and BNP were consistent with the results for NT‐proBNP alone (online supplementary Tables S2 and S3 ).
Figure 2.
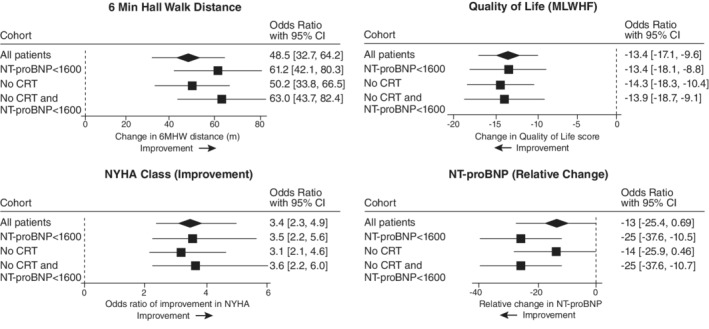
Six‐month improvements in endpoints by cohort. 6MHW, 6‐min hall walk; CI, confidence interval; CRT, cardiac resynchronization therapy; MLWHF, Minnesota Living With Heart Failure; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association. No CRT, excluding patients actively receiving CRT or with a class I indication for CRT (American College of Cardiology/American Heart Association guidelines2).
Figure 3 shows the 6‐month endpoint results for all patients across baseline sex, age and history of atrial fibrillation (AF). BAT demonstrated a statistically significant improvement in 6MHW distance, MLWHF QoL and NYHA class in all patients irrespective of gender, age, or the presence or absence of AF. Of interest, the effects in female patients were notable, with large effects in 6MHW distance, MLWHF QoL, NYHA class and NT‐proBNP (−31% difference [95% CI −50%, −2%]).
Figure 3.

Six‐month improvement in all patients by sex, age and history of atrial fibrillation. 6MHW, 6‐min hall walk; Afb, atrial fibrillation; Hx, history; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; QoL, quality of life as assessed by the Minnesota Living With Heart Failure Questionnaire.
Discussion
In this IPD meta‐analysis, BAT significantly improved exercise capacity, QoL and functional capacity in GDMT‐treated patients with NYHA class II/III HFrEF (Graphical Abstract). The size of the treatment effects was clinically meaningful and seen consistently across the studied patient cohorts. The importance of focusing on improvement in these patient‐centred outcomes has been emphasized by the FDA though the Breakthrough Device Program, industry, and supported by key leaders who treat patients with HF. 16 , 17 , 18 , 19 , 20 , 21 A recent study done in collaboration with the FDA, industry, and the Medical Device Innovation Consortium showed that patients would accept up to a 9% absolute risk of device‐associated mortality for a 1‐year gain in improved functioning capacity. 17 One of the challenges with patient‐centred outcomes is the potential for bias, particularly in device trials where a sham procedure is often problematic and unethical. This potential bias can be mitigated by evaluating supportive objective outcomes, such as NT‐proBNP. A recent editorial emphasized that with the increasing prevalence of HF and the impact of the disease on patients, progressive approaches that focus on endpoints that are impactful to patients must be done to get safe and effective treatments available in a timely manner. 22
When patients with very elevated NT‐proBNP (≥1600 pg/ml) were excluded, NT‐proBNP showed a significant relative reduction. The patients with higher baseline NT‐proBNP levels (≥1600 pg/ml) have demographic profiles consistent with more advanced HF compared to patients with lower NT‐proBNP, as also shown in other studies. 23 , 24 , 25 , 26 This differential therapeutic effect between patients with lower NT‐proBNP versus higher NT‐proBNP, or more advanced HF, has been observed in other studies, and more recently in left ventricular assist devices. 24 , 25 , 26 , 27 , 28 , 29 Thus, the differential therapeutic effect observed may be due to the patients with higher NT‐proBNP having more advanced structural heart disease, as higher levels of natriuretic peptides correlate with greater left ventricular mass, worse left ventricular diastolic dysfunction and higher pulmonary pressure, as well as higher prevalence of comorbidities such as renal dysfunction and AF, and that they may, therefore, be less likely to respond to therapeutic interventions.
Baroreflex activation therapy demonstrated benefit in all the assessed subgroups. Benefits were seen in patients with and without an indication for CRT. In patients who would have satisfied the FDA approved indication, which includes patients with NT‐proBNP <1600 pg/ml and no indication for CRT, the results were the most pronounced. When analysing all patients, there were particularly impressive results in women. Women have often achieved lesser benefits from HF interventions, 30 so it is of clinical interest that the therapeutic effects of BAT were so encouraging for women. Lindenfeld et al. 31 examined the benefits of BAT in women and although no known pathophysiological mechanism was identified as the reason for the benefit in women, they suggested that the findings are consistent with what has been observed in other device therapies such as CRT. One publication suggests that in women of advanced age, the sympathetic baroreflex sensitivity decreases more so than in men of similar age. 32 AF is known to be associated with more marked autonomic dysfunction and lower therapeutic benefit from either beta‐blockade or CRT compared to patients in sinus rhythm, it was therefore reassuring to see similar benefits in this patient cohort. 33
Baroreflex activation therapy is the first neuromodulation device shown to offer symptomatic and functional capacity benefits in patients with HFrEF in NYHA class II and III. It is also the first intervention that directly targets autonomic dysfunction, long known to be an adverse prognostic marker in HFrEF. 34 Effective pharmacological treatments are thought to have some of their benefit via modulation of autonomic nervous system activity in the case of beta‐blockers, mineralocorticoid receptor antagonists and renin–angiotensin–aldosterone system inhibitors, but these effects are indirect and do not target the parasympathetic or reflex systems directly.
Device therapies for autonomic modulation have been tested with a number of different device approaches, including BAT. Direct vagal stimulation has been tried with variable results. Three studies have assessed vagal nerve stimulation by implanted devices in HF. The non‐randomized ANTHEM‐HF 35 trial suggested possible benefits in LVEF, 6MHW distance and NYHA class but this was not confirmed in two subsequent larger randomized trials INOVATE‐HF 36 and NECTAR‐HF. 37 In INOVATE‐HF, 707 class III HFrEF patients were randomized in a 3:2 ratio, but the primary efficacy endpoint of death or worsening HF was not improved (30.3% vs. 25.8%; hazard ratio 1.14; 95% CI 0.86, 1.53; p = 0.37) despite favourable effects on NYHA class and 6MHW distance. In NECTAR‐HF, a total of 87 HFrEF patients were randomized to therapy ‘ON’ versus therapy ‘OFF’ and at 6 months left ventricular end‐systolic diameter was not significantly improved, but quality of life assessed by MLWHF Questionnaire (p = 0.049), NYHA class (p = 0.032), and the Short‐Form‐36 Health Survey Physical Component scores (p = 0.016) were all improved in the ‘ON’ group. Similarly, spinal cord stimulation has not shown consistent benefits in HFrEF. In DEFEAT‐HF, a total of 66 randomized and implanted patients showed no significant change in left ventricular end‐systolic volume index over 6 months of device OFF versus ON, and no significant benefits in secondary endpoints. 38 These results were observed despite an earlier non‐randomized pilot study suggesting the possibility of improvements in NYHA class, MLWHF QoL, peak oxygen consumption and LVEF. 39 Online supplementary Table S4 provides a comparison of 6MHW distance, QoL, NYHA class, and NT‐proBNP data from randomized trials in HF for BAT, vagal nerve stimulation and spinal cord stimulation.
Other approaches have also not been successful to date. Renal denervation can reduce sympathetic predominance, and this was first tested for refractory hypertension, with several smaller trials also testing its possible utility in HF. The REACH pilot study suggested improved functional capacity and symptoms in seven patients, but this was not confirmed in the larger Symplicity‐HF study. 40 , 41 Another small pilot study suggested potential improvements in LVEF and 6MHW distance. 42 Similar to renal denervation, sympathetic denervation of the left side of the heart has been tested in HFrEF patients in a small pilot study with suggestions of improved LVEF and 6MHW distance. 43 This is presently being tested in a prospective trial. 44
One of the difficulties in the field of neurocardiology is that unlike drug interventions, the optimal dose and nature of intervention delivery is inadequately understood. This includes optimization of pacing protocols including pulse amplitude, pulse frequency, and duration, as well as whether therapy should be continuous, day‐time only, or even intermittent. The results of this meta‐analysis of BAT compare favourably with other recent device interventions whose results have been summarized in an IPD meta‐analysis, such as cardiac contractility modulation and secondary mitral regurgitation reduction by indirect mitral annuloplasty. 45 , 46
Limitations
This IPD meta‐analysis included only two randomized trials and with a limited number of patients restricting our ability to see subtle differences in responses between patient cohorts of interest. In addition, both trials were open‐label, which may result in bias in the more subjective endpoints. Nevertheless, the results are encouraging whilst we await longer term clinical outcomes from the second post‐market phase of the BeAT‐HF trial.
Conclusions
Of all the autonomically targeted device therapies, this IPD meta‐analysis has confirmed clinical benefits from BAT as the only device that has been established as a viable option for the treatment of selected HF patients. The results help clarify the proven effects to be expected from the use of BAT in patients that match the selection criteria of the trials. In summary, the results of this IPD meta‐analysis suggest that BAT provides clinically meaningful improvements in exercise capacity, QoL and functional capacity in GDMT‐treated patients with NYHA class II/III HFrEF, across the range of patients studied. BAT was also associated with an improvement in NT‐proBNP in subjects with a lower baseline NT‐proBNP. These benefits were consistent across both trials and across all subgroups evaluated.
Conflict of interest: A.J.S.C. declares having received honoraria and/or lecture fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, Respicardia. M.F. was supported by the National Heart, Lung, and Blood Institute (NHLBI) (K23HL151744), the American Heart Association (20IPA35310955), Mario Family Award, Duke Chair's Award, Translating Duke Health Award, Bayer, Bodyport and BTG Specialty Pharmaceuticals; he receives consulting fees from AxonTherapies, Bodyport, Boston Scientific, CVRx, Daxor, Edwards LifeSciences, Fire1, Inovise, NXT Biomedical, Viscardia, Zoll. All other authors have nothing to disclose. J.B. receives consulting fees from Amgen, Astra Zeneca, Bayer, BMS, Boehringer, Ingelheim, Cardoir, Corvia, CVRX, Novartis, Pfizer and Virfor. M.R.Z., J.A.L, F.A.W. and M.F. declare having received consulting fees from CVRx. EG is an employee of CVRx. F.Z. receives consulting fees from Bayer, Boehringer, CVRx, Merck AG, Novartis, Vifor Fresenius. W.T.A. declares personal fees from Respicardia, Cardionomic, Wite Swell, AquaPass, Cordio, Vectorius, Impulse Dynamics and Edwards Lifesciences and personal equity in V‐Wave Medical.
Supporting information
Appendix S1: Supporting Information.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al.; ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al.; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 4. Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–85. [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Anker SD, Chua TP, Szelemej R, Piepoli M, Adamopoulos S, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1645–50. [DOI] [PubMed] [Google Scholar]
- 6. van Bilsen M, Patel HC, Bauersachs J, Böhm M, Borggrefe M, Brutsaert D, et al. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2017;19:1361–78. [DOI] [PubMed] [Google Scholar]
- 7. Zucker IH, Hackley JF, Cornish KG, Hiser BA, Anderson NR, Kieval R, et al. Chronic baroreceptor activation enhances survival in dogs with pacing‐induced heart failure. Hypertension. 2007;50:904–10. [DOI] [PubMed] [Google Scholar]
- 8. Sabbah HN, Gupta RC, Imai M, Irwin ED, Rastogi S, Rossing MA, et al. Chronic electrical stimulation of the carotid sinus baroreflex improves left ventricular function and promotes reversal of ventricular remodeling in dogs with advanced heart failure. Circ Heart Fail. 2011;4:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gronda E, Seravalle G, Brambilla G, Costantino G, Casini A, Alsheraei A, et al. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof‐of‐concept study. Eur J Heart Fail. 2014;16:977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abraham WT, Zile MR, Weaver FA, Butter C, Ducharme A, Halbach M, et al. Baroreflex activation therapy for the treatment of heart failure with a reduced ejection fraction. JACC Heart Fail. 2015;3:487–96. [DOI] [PubMed] [Google Scholar]
- 11. Zile MR, Lindenfeld J, Weaver FA, Zannad F, Galle E, Rogers T, et al. Baroreflex activation therapy in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol. 2020;76:1–13. [DOI] [PubMed] [Google Scholar]
- 12. Georgakopoulos D, Little WC, Abraham WT, Weaver FA, Zile MR. Chronic baroreflex activation: a potential therapeutic approach to heart failure with preserved ejection fraction. J Card Fail. 2011;17:167–78. [DOI] [PubMed] [Google Scholar]
- 13. Sabbah HN. Baroreflex activation for the treatment of heart failure. Curr Cardiol Rep. 2012;14:326–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration . Available from: https://www.fda.gov/media/108135/download (accessed 19 January 2022).
- 15. Weber M, Hamm C. Role of B‐type natriuretic peptide (BNP) and NT‐proBNP in clinical routine. Heart. 2006;92:843–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. US Food and Drug Administration . April 2022. Available from: Guidance Coversheet ‐ CDRH‐CBER Level 1 DRAFT (https://www.fda.gov/media/108135/download).
- 17. Reed S, Yang J, Rickert T, Johnson F, Gonzalez J, Mentz R, et al. Quantifying benefit‐risk preferences for heart failure devices: a stated‐preference study. Circ Heart Fail. 2022;15:e008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Samman Tahhan A, Vaduganathan M, Greene SJ, Okafor M, Kumar S, Butler J. Evolving landscape of clinical trials in heart failure: patient populations, endpoint selection, and regions of enrollment. Curr Heart Fail Rep. 2018;15:10–6. [DOI] [PubMed] [Google Scholar]
- 19. Hamo CE, Gheorghiade M, Butler J. Novel endpoints for heart failure clinical trials. Curr Heart Fail Rep. 2017;14:210–6. [DOI] [PubMed] [Google Scholar]
- 20. Butler J, Hamo CE, Udelson JE, Pitt B, Yancy C, Shah SJ, et al. Exploring new endpoints for patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9:e003358. [DOI] [PubMed] [Google Scholar]
- 21. Zile MR, Abraham WT, Lindenfeld J, Weaver FA, Zannad F, Graves T, et al. First granted example of novel FDA trial design under expedited access pathway for premarket approval: BeAT‐HF. Am Heart J. 2018;204:139–50. [DOI] [PubMed] [Google Scholar]
- 22. Januzzi JL Jr, Ibrahim NE. The need to innovate and accelerate clinical trial performance: BeAT the clock. J Am Coll Cardiol. 2020;76:14–6. [DOI] [PubMed] [Google Scholar]
- 23. Greene SJ, Maggioni AP, Fonarow GC, Solomon SD, Böhm M, Kandra A, et al.; ASTRONAUT Investigators and Coordinators . Clinical profile and prognostic significance of natriuretic peptide trajectory following hospitalization for worsening chronic heart failure: findings from the ASTRONAUT trial. Eur J Heart Fail. 2015;17:98–108. [DOI] [PubMed] [Google Scholar]
- 24. Cleland JG, McMurray JJ, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, et al.; CORONA Study Group . Plasma concentration of amino‐terminal pro‐brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (controlled rosuvastatin multinational trial in heart failure). J Am Coll Cardiol. 2009;54:1850–9. [DOI] [PubMed] [Google Scholar]
- 25. Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, et al. Prognostic value of baseline plasma amino‐terminal pro‐brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I‐PRESERVE trial. Circ Heart Fail. 2011;4:569–77. [DOI] [PubMed] [Google Scholar]
- 26. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail. 2017;5:241–52. [DOI] [PubMed] [Google Scholar]
- 27. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al.; VICTORIA Study Group . Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883–93. [DOI] [PubMed] [Google Scholar]
- 28. Idzerda NMA, Persson F, Pena MJ, Brenner BM, Brunel P, Chaturvedi N, et al. N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) predicts the cardio‐renal response to aliskiren in patients with type 2 diabetes at high renal and cardiovascular risk. Diabetes Obes Metab. 2018;20:2899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heusser K, Wittkoepper J, Bara C, Haverich A, Diedrich A, Levine BD, et al. Sympathetic vasoconstrictor activity before and after left ventricular assist device implantation in patients with end‐stage heart failure. Eur J Heart Fail. 2021;23:1955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lam CSP, Arnott C, Beale AL, Chandramouli C, Hilfiker‐Kleiner D, Kaye DM, et al. Sex differences in heart failure. Eur Heart J. 2019;40: 3859–68c. [DOI] [PubMed] [Google Scholar]
- 31. Lindenfeld J, Gupta R, Grazette L, Ruddy JM, Tsao L, Galle E, et al. Response by sex in patient‐centered outcomes with baroreflex activation therapy in systolic heart failure. JACC Heart Fail 2021;9:430‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fu Q, Ogoh S. Sex differences in baroreflex function in health and disease. J Physiol Sci. 2019;69:851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, et al.; Beta‐Blockers in Heart Failure Collaborative Group . Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual‐patient data meta‐analysis. Lancet. 2014;384:2235–43. [DOI] [PubMed] [Google Scholar]
- 34. Fudim M, Abraham WT, von Bardeleben RS, Lindenfeld J, Ponikowski PP, Salah HM, et al. Device therapy in chronic heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;78:931–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Premchand RK, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM‐HF trial. J Card Fail. 2014;20:808–16. [DOI] [PubMed] [Google Scholar]
- 36. Gold MR, Van Veldhuisen DJ, Hauptman PJ, Borggrefe M, Kubo SH, Lieberman RA, et al. Vagus nerve stimulation for the treatment of heart failure: the INOVATE‐HF trial. J Am Coll Cardiol. 2016;68:149–58. [DOI] [PubMed] [Google Scholar]
- 37. Zannad F, De Ferrari GM, Tuinenburg AE, Wright D, Brugada J, Butter C, et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural cardiac TherApy foR heart failure (NECTAR‐HF) randomized controlled trial. Eur Heart J. 2015;36:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zipes DP, Neuzil P, Theres H, Caraway D, Mann DL, Mannheimer C, et al.; DEFEAT‐HF Trial Investigators . Determining the feasibility of spinal cord neuromodulation for the treatment of chronic systolic heart failure: the DEFEAT‐HF study. JACC Heart Fail. 2016;4:129–36. [DOI] [PubMed] [Google Scholar]
- 39. Tse HF, Turner S, Sanders P, Okuyama Y, Fujiu K, Cheung CW, et al. Thoracic spinal cord stimulation for heart failure as a restorative treatment (SCS HEART study): first‐in‐man experience. Heart Rhythm. 2015;12:588–95. [DOI] [PubMed] [Google Scholar]
- 40. Davies JE, Manisty CH, Petraco R, Barron AJ, Unsworth B, Mayet J, et al. First‐in‐man safety evaluation of renal denervation for chronic systolic heart failure: primary outcome from REACH‐pilot study. Int J Cardiol. 2013;162:189–92. [DOI] [PubMed] [Google Scholar]
- 41. Hopper I, Gronda E, Hoppe UC, Rundqvist B, Marwick TH, Shetty S, et al. Sympathetic response and outcomes following renal denervation in patients with chronic heart failure: 12‐month outcomes from the Symplicity HF feasibility study. J Card Fail. 2017;23:702–7. [DOI] [PubMed] [Google Scholar]
- 42. Chen W, Ling Z, Xu Y, Liu Z, Su L, Du H, et al. Preliminary effects of renal denervation with saline irrigated catheter on cardiac systolic function in patients with heart failure: a prospective, randomized, controlled, pilot study. Catheter Cardiovasc Interv. 2017;89:E153–61. [DOI] [PubMed] [Google Scholar]
- 43. Conceição‐Souza GE, Pêgo‐Fernandes PM, Cruz F, Guimarães GV, Bacal F, Vieira ML, et al. Left cardiac sympathetic denervation for treatment of symptomatic systolic heart failure patients: a pilot study. Eur J Heart Fail. 2012;14:1366–73. [DOI] [PubMed] [Google Scholar]
- 44. Chin A, Ntsekhe M, Viljoen C, Rossouw J, Pennel T, Schwartz PJ. Rationale and design of a prospective study to assess the effect of left cardiac sympathetic denervation in chronic heart failure. Int J Cardiol. 2017;248:227–31. [DOI] [PubMed] [Google Scholar]
- 45. Giallauria F, Cuomo G, Parlato A, Raval NY, Kuschyk J, Stewart Coats AJ. A comprehensive individual patient data meta‐analysis of the effects of cardiac contractility modulation on functional capacity and heart failure‐related quality of life. ESC Heart Fail. 2020;7:2922–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giallauria F, Di Lorenzo A, Parlato A, Testa C, Bobbio E, Vigorito C, et al. Individual patient data meta‐analysis of the effects of the CARILLON® mitral contour system. ESC Heart Fail. 2020;7:3383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information.


