SUMMARY
Background
Prolonged systemic antibiotic treatment is often a part of management of hidradenitis suppurativa (HS). Although biologic therapies are now available, the patient’s treatment journey leading to biologic therapy is unclear.
Objectives
To examine treatment patterns and duration of systemic treatment use in patients with HS preceding biologic therapy.
Methods
We identified all patients with HS receiving treatment with biologics in the Danish National Patient Registry from 2010 to 2018 and extracted their entire prescription history of specific systemic treatments from the Danish National Prescription Registry since its inception in 1995. The patients’ treatment journeys are graphically displayed through Sankey diagrams and box plots generated to show temporal distributions. Descriptive patient characteristics were presented as frequencies with percentages for categorical variables and as means with SDs or medians with interquartile ranges (IQRs) for continuous variables.
Results
A total of 225 patients with HS were included. Patients had most frequently been treated with penicillin (n = 214; 95·1%), dicloxacillin (n = 194; 86·2%), tetracycline (n = 145; 64·4%) and rifampicin/clindamycin (n = 111; 49·3%), as well as the retinoids isotretinoin and acitretin, and dapsone. Prior to biologic therapy, patients received a mean of 4·0 (SD 1·3) different systemic therapies, across a mean of 16·9 (SD 11·3) different treatment series. The mean time from first systemic therapy until biologic therapy was initiated was 15·3 (SD 5·1) years [8·2 (SD 5·9) years when excluding penicillin and dicloxacillin].
Conclusions
Patients with HS who receive biologic therapy have long preceding treatment histories with multiple drug classes and treatment series, many of which are supported by relatively weak evidence in HS. Delay in the initiation of biologic therapy may represent a missed opportunity to prevent disease progression.
What is already known about this topic?
The treatment journey leading to biologic therapy in patients with HS has not previously been investigated.
What does this study add?
Our data from 225 patients with HS illustrate that patients who receive biologic therapy have long preceding treatment histories with multiple drug classes and treatment series, many of which are supported by relatively weak evidence in HS.
In this study we found that 225 HS patients on average were treated with systemic therapies for eight years before starting biologic therapy. The high number of systemic treatment series used prior to initiation of biologics may reflect referral delays or difficulties in obtaining disease control in patients with moderate‐to‐severe HS.

Linked Comment: T. Tzellos. Br J Dermatol 2022; 187:462–463.
Plain language summary available online
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disease of the hair follicle defined by recurrent nodules, draining tunnels and scarring in the intertriginous regions. 1 In European countries, the estimated prevalence of HS is 1–4%. 2 , 3 HS can be challenging to treat, and patients often undergo several prolonged treatment courses of systemic antibiotics. 4 , 5 In patients with moderate‐to‐severe HS, guidelines suggest that systemic antibiotics (e.g. clindamycin and rifampicin, dosage: 300 mg twice daily or tetracycline, dosage: 500 mg twice daily) should be administered for at least 3 months. 6 , 7 Moreover, HS guidelines recommend biologic therapy should pose an alternative if the above‐mentioned conventional therapies fail. 7
With the introduction of biologics in HS a significant reduction in disease flares has been reported in patients with moderate‐to‐severe HS. 8 In particular, the monoclonal antibody adalimumab, which targets tumour necrosis factor‐alpha, has shown efficacy compared with placebo in two phase III trials 8 , 9 and is considered as a first‐choice biologic agent after failure of conventional therapy. 7 However, patients may go for several years, and have frequent visits to emergency departments and inpatient care units, before they initiate biologic therapy. 10 , 11 Moreover, patients have symptoms for a mean of 7·2 (SD 8·7) years before they are adequately diagnosed with HS. 12 Such delay may expose patients to multiple systemic therapies, including potentially inappropriate treatments with drugs that are not considered effective in HS, until patients are started on targeted HS therapies.
So far, data on real‐world systemic HS therapies prior to use of biologics are relatively lacking. We therefore examined the patterns and quantity of systemic treatment regimens in patients with HS prior to use of biologic therapy in the Danish national healthcare system, and whether observed real‐world clinical practice follows international recommendations for HS management.
Patients and methods
Data sources
At birth or immigration, all Danish residents are given a unique personal identification number, 13 which enables unambiguous individual‐level linkage of nationwide administrative registry‐data. The Danish tax‐supported healthcare system provides equal and unrestricted access to general practitioners and specialists without co‐pay. Biologics for HS are given directly from tertiary dermatology clinics, and dispensing of these drugs is recorded in the Danish National Patient Registry, 14 which records data on diagnoses and interventions from inpatient and outpatient (ambulatory) contacts from all hospitals in Denmark, as well as from a number of private clinics. Data on dispensed medication from all community pharmacies in Denmark are recorded in the Danish National Prescription Registry using the Anatomical Therapeutic Classification (ATC) system. 15
Study design
We included all patients who initiated treatment with biologics for HS (International Classification of Diseases, tenth revision, code L73.2) in Denmark between 1 January, 2005 and 31 December 2018. The date of first‐ever biologic prescription served as the index date, from which the patients’ historical treatment journeys were mapped back to the inception of the Danish National Prescription Registry (1995). The following systemic treatments were identified: tetracycline‐class drugs (henceforth ‘tetracycline’; ATC code group J01AA), rifampicin and clindamycin (ATC code J04AB02 together with J01FF0), acitretin (ATC code D05BB02), isotretinoin (ATC code D10BA01), dapsone (ATC code J04BA02), phenoxymethylpenicillin (henceforth ‘penicillin’, ATC code J01CE02) and dicloxacillin (ATC code J01CF01). These drugs, their role in HS, and potential competing indications are summarized in Table S1 (see Supporting Information). We generated treatment series (i.e. sequences of continuous treatment with the same drug) and a treatment was considered valid for 90 days following a filled prescription (except for isotretinoin, which was valid for 30 days, as this is only dispensed as 30‐day dosing in Denmark, and penicillin and/or dicloxacillin, which was valid for 14 days). Two treatment sequences were merged if the same drug was used in two consecutive series and the discontinuation was less than 30 days, with the exception of penicillin and/or dicloxacillin, which were always regarded as a new treatment series whenever a new prescription was filled.
Statistical analysis
We presented descriptive patient characteristics as frequencies with percentages for categorical variables and as means with SDs or medians with interquartile ranges (IQRs) for continuous variables. We displayed patients’ treatment journeys graphically through Sankey diagrams and generated box plots to show temporal distributions. For practical purposes, only the five most recent treatment sequences were displayed in the Sankey diagram but all treatment sequences were included in calculations of number of prior treatments. To avoid loss of anonymity, groups containing data on only one or two patients are presented as ‘< 3’. Analyses were performed using SAS v. 9.4 (SAS Institute Inc. Cary, NC, USA), R v. 4.1.0 (R core Team, Vienna, Austria) and Python v. 3.7.4 (Python Software Foundation, Wilmington, Delaware, USA). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations were used for conduct and reporting of this study. 16
Results
Patient characteristics
A total of 225 patients with HS (63·1% women) were included. The mean age at first biologic treatment was 41·2 (SD 12·4) years (Table 1). Before biologic therapy, patients had most frequently been treated with penicillin (n = 214; 95·1%), dicloxacillin (n = 194; 86·2%), tetracycline (n = 145; 64·4%) and rifampicin/clindamycin (n = 111; 49·3%), respectively. Ninety‐six (42·7%) patients had been treated with isotretinoin, whereas doxycycline (n = 65; 28·9%), dapsone (n = 28; 12·4%) and acitretin (n = 17; 7·6 %) were used less frequently (Table 1). Forty‐eight (21·3%) patients received five different treatments prior to biologic therapy, and 24 (10·7%) patients received more than five different treatments. The mean number of different treatments prior to biologic therapy was 4·0 (SD 1·3) (Figure 1).
Table 1.
Patient characteristics at initiation of first biologic therapy (n = 225) a
| Characteristic | Value |
|---|---|
| Sex, n (%) | |
| Women | 142 (63·1) |
| Men | 83 (36·9) |
| Age at first biologic therapy, mean (SD) | 41·2 (12·4) |
| Systemic treatment prior to biologic therapy, n (%) | |
| Penicillin | 214 (95·1) |
| Dicloxacillin | 194 (86·2) |
| Tetracycline | 145 (64·4) |
| Doxycycline | 65 (28·9) |
| Rifampicin/clindamycin | 111 (49·3) |
| Dapsone | 28 (12·4) |
| Isotretinoin | 96 (42·7) |
| Acitretin | 17 (7·6) |
Two drugs given in combination (e.g. rifampicin/clindamycin) is counted as one treatment modality.
Figure 1.

(a) Number of systemic treatment series and (b) number of different systemic treatments. The mean number of different systemic treatments prior to biologic therapy was 4·0 (SD 1·3). The mean number of systemic treatment series prior to biologics was 16·9 (SD 11·3). [Colour figure can be viewed at wileyonlinelibrary.com]
In 95 (42·2%) patients, the number of different treatment series prior to biologic therapy was between 11 and 20, and in 49 (21·8%) patients between 21 and 40. The mean number of systemic treatment series prior to biologics was 16·9 (SD 11·3) (Figure 1).
Among patients treated with penicillin and dicloxacillin, these groups received a mean of 0·6 (SD 0·6) and 0·8 (SD 0·8) treatment series with these drugs each year from first such prescription until initiation of biologics, respectively. This totalled a mean of 7·3 (SD 5·6) and 6·7 (SD 6·9) treatment series of penicillin and dicloxacillin, respectively. Overall, patients started a mean of 1·2 (SD 0·7) new treatment series annually before starting biologic therapy.
Time to biologic therapy
During the study, the mean time from first systemic therapy until initiation of biologic therapy was 15·3 (SD 5·1) years [8·2 (SD 5·9) years when excluding penicillin or dicloxacillin]. As seen in Figure 2, there was no significant trend in the time to initiation of biologics over the study period (Mann–Kendall P = 0·755). A considerable temporal variation was evident from 2010 until 2015, with fluctuations from 4·5 (SD 3·7) years to 10·2 (SD 5·2) years. Hereafter, a slightly more uniform pattern was observed, ranging from 6·9 (SD 4·2) years in 2015 to 8·9 (SD 7·3) years in 2017 (Table S2; see Supporting Information). Similar findings, albeit somewhat longer durations were seen when penicillin and dicloxacillin were included (Table S3; see Supporting Information).
Figure 2.
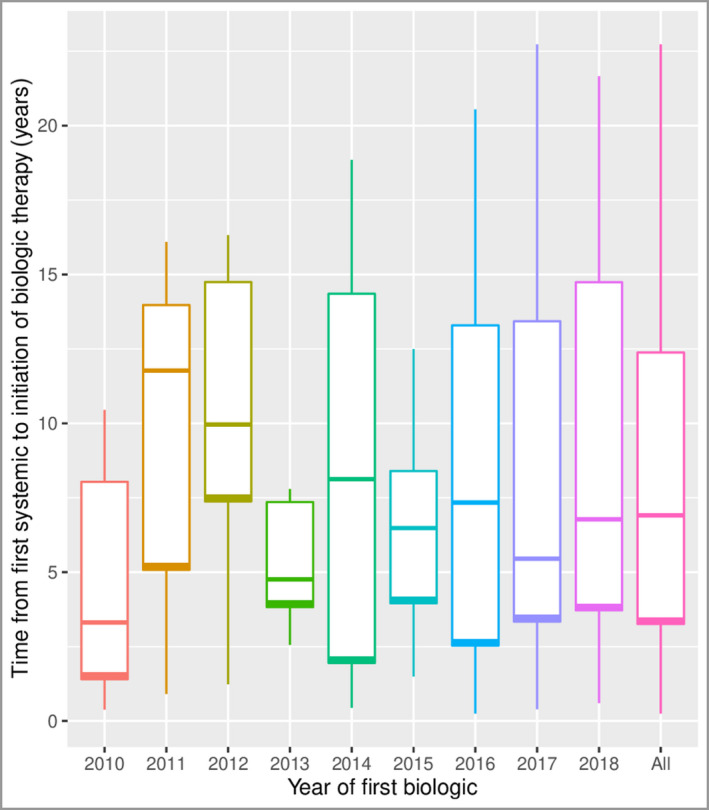
Box plot of time from first systemic therapy to initiation of biologic therapy. The systemic treatments include dapsone, isotretinoin, rifampicin/clindamycin, tetracycline, doxycycline and acitretin. Penicillin and/or dicloxacillin are not included in this graph. The figure shows no significant progression in the time to initiation of biologics over the study period (Mann–Kendall P = 0·755). The horizontal lines in the boxes represent the median, the box represents the interquartile range and the whiskers the range of the data. The overall mean is 8·2 (SD 5·9) years. The overall median is 6·9 (IQR 3·3–12·4) years. [Colour figure can be viewed at wileyonlinelibrary.com]
Systemic treatment patterns prior to biologics
We observed no overall pattern in the progression of prescribed drug classes in the treatment series preceding initiation of biologic treatment (Figure 3). A significant proportion of the patients, 36·9%, did not receive any systemic treatment immediately prior to biologics (i.e. last 90 days). Additionally, 16·4% and 21·3% of the patients were treated with tetracycline or rifampicin/clindamycin, respectively, directly prior to the initiation of biologic therapy. Several single courses of penicillin and dicloxacillin constituted the majority of the treatment path with several patients switching directly from penicillin (6·7%) and dicloxacillin (7·6%) to biologics (Figure 3). In contrast, combination therapy of penicillin and dicloxacillin only constituted a minor part of the road towards biologics. A smaller fraction of the patients (7·6%) received isotretinoin prior to biologic therapy.
Figure 3.

Visualization of treatment patterns prior to initiation of biologic therapy. Only the five most recent treatment sequences are visualized. The figure illustrates no significant tendency of the various drug classes towards initiation of biologic therapy. ‘Tetracyclines’ represents the drug class, and therefore also includes, for example, doxycycline. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
In this nationwide drug utilization study, we found a large variation in the treatment journey towards biologics in patients with HS, and patients waited a long time from first systemic treatment until they were started on biologic therapy. There was no apparent algorithm for treatments that were chosen immediately prior to biologic therapy.
The treatment journeys for Danish patients prior to initiation of biologics were dominated by systemic therapies that have low evidence of efficacy (i.e. dicloxacillin, penicillin, dapsone or isotretinoin). These observations may reflect potential unawareness of HS guidelines 6 , 7 which recommend tetracyclines and rifampicin/clindamycin as the cornerstone of first‐line systemic therapy for HS. Furthermore, the tendency to prescribe penicillin and dicloxacillin for HS may indicate that physicians misdiagnose HS as a skin infection associated with common infectious pathogens such as staphylococci or streptococci. In Denmark, general practitioners are responsible for referrals to dermatologists. This may partly contribute to the potential incorrect use of antibiotics as well as misdiagnosis of HS. Alternatively, the frequent treatment series with β‐lactam antibiotics may also indicate the intractable nature of HS with intermittent fluctuations in HS disease activity. The data highlight that lack of antibiotic stewardship continues to be an issue in the HS care pathway for many patients.
Our data show that isotretinoin plays a considerable part in the HS treatment journey in Denmark. Although conflicting results have been demonstrated, 17 , 18 , 19 isotretinoin is generally not considered an efficacious treatment in HS, 6 unless there is concomitant moderate‐to‐severe acne. Interestingly our data indicate that several patients with HS switch directly from isotretinoin to biologics (7·6%). Although competing indications, such as concomitant acne (Table S1), may partly explain this pattern, the limited treatment options in HS may also at least in theory contribute to this tendency.
The two major treatment regimens in patients’ treatment journeys were tetracyclines and rifampicin/clindamycin, which are also the recommended first‐line treatment modalities in HS. 6 Although these therapies may be associated with severe adverse effects (particularly gastrointestinal symptoms such diarrhoea and nausea for rifampicin/clindamycin), both treatments have demonstrated efficacy in several retrospective studies. 20 , 21 , 22 , 23 Increasing bacterial resistance, however, is reported in large HS populations. Recently, among purulent material from 137 skin lesions, the prevalence of resistance was: clindamycin 65·7%, rifampicin 69·3%, penicillin 70·0%, ciprofloxacin 74·0%, tetracycline 84·7% and erythromycin 89·0%. 24 From this perspective a more targeted antibiotic therapy that also covers the recently reported cutaneous core microbiome in HS may yield higher efficacy. 25 , 26
It is possible that initiation of biologics at an earlier stage in the HS disease course might halt disease progression, prevent severe clinical consequences (e.g. inflamed draining tunnels and excessive scarring), and reduce the need for emergency departments visits and inpatient care. 10 Although our data did not demonstrate a significant trend in the time to initiation of biologics over the study period, the potential future introduction of more targeted biologics may result in earlier initiation of biologics.
Currently adalimumab is the only US Food and Drug Administration‐approved biologic for HS; however, a number of other biologics have demonstrated efficacy for HS in smaller clinical trials. Indeed, biologics such as infliximab, ustekinumab, anakinra and secukinumab have shown promising results in the treatment of moderate‐to‐severe HS 27 , 28 , 29 , 30 , 31 and secukinumab and bimekizumab are currently undergoing phase III trials for the treatment of HS (NCT03713632, NCT03713619, NCT04179175, NCT04242446).
Certain limitations apply to the interpretation of the current study results. Although tertiary centre therapies (i.e. biologics) are linked to specific diagnoses such as HS, we lacked data on the specific indications of pre‐biologic systemic therapies as well as Hurley staging. It is well known that patients with HS have higher risk of other concomitant skin conditions 32 and some treatments may have been prescribed for these conditions instead. Penicillin and dicloxacillin may in some cases have been prescribed for other indications, albeit we addressed this issue through sensitivity analyses with these drugs excluded. Our study focused on systemic treatments and therefore did not include topical treatments such as topical clindamycin and azelaic acid. Finally, as the Danish National Prescription Registry was established in 1995 our data may potentially be left‐censored for some patients with long treatments histories, albeit that our histogram suggests that this was a minor issue.
In conclusion, in this nationwide drug utilization study, we found that patients with HS on average were treated with systemic therapies for 8 years before starting biologic therapy (when excluding penicillin and/or dicloxacillin). The large number of systemic treatment series used prior to initiation of biologics may reflect referral delays or difficulties in achieving disease control in patients with moderate‐to‐severe HS. Our findings emphasize the need for optimized implementation of evidence‐based guidelines to harmonize treatment strategies, as well as the need to develop and license additional effective therapies for treatment of HS.
Funding sources
No funding was received for this study. With no relation to the work in the present manuscript, J.P.T. is supported by a grant from the Lundbeck Foundation.
Conflicts of interest
H.C.R. has received research funding from the Kgl Hofbundtmager Aage Bang Foundation and honoraria as speaker from LEO Pharma. J.‐T.M has served as advisor and/or received speaking fees and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, BMS, Celgene, Eli Lilly, LEO Pharma, Janssen‐Cilag, MSD, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi and UCB. J.W.F. has conducted advisory work for Janssen, Boehringer Ingelheim, Pfizer, Kyowa Kirin, LEO Pharma, Regeneron, Chemocentryx, AbbVie and UCB, participated in trials for Pfizer, UCB, Boehringer Ingelheim, Eli Lilly, CSL and Janssen, and received research support from Ortho Dermatologics and Sun Pharma. J.R.I. is a consultant and/or advisory board member for Novartis, UCB, ChemoCentryx, Boehringer Ingelheim, Viela Bio and Kymera Therapeutics and receives, as Editor‐in‐Chief, an editorial stipend from the British Journal of Dermatology and an author honorarium from UpToDate. He is co‐copyright holder of the Hidradenitis Suppurativa Quality Of Life scale and Investigator and Patient Global Assessment instruments for hidradenitis suppurativa. J.J.W. is or has been an investigator, consultant or speaker for AbbVie, Almirall, Amgen, Arcutis, Aristea Therapeutics, Bausch Health (Ortho Dermatologics), Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Dr. Reddy's Laboratories, Eli Lilly, Galderma, Janssen, LEO Pharma, Mindera, Novartis, Regeneron, Sanofi‐Genzyme, Solius, Sun Pharmaceutical, UCB and Zerigo Health. J.P.T. is an advisor for AbbVie, Almirall, Arena Pharmaceuticals, Coloplast, OM Pharma, Aslan Pharmaceuticals, Union Therapeutics, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron and Sanofi‐Genzyme, a speaker for AbbVie, Almirall, Eli Lilly & Co, LEO Pharma, Pfizer, Regeneron and Sanofi‐Genzyme and received research grants from Pfizer, Regeneron and Sanofi‐Genzyme. S.F.T. has been a speaker and/or served on advisory boards for AbbVie, Almirall, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi and UCB and has received research support from AbbVie, Janssen, LEO Pharma, Novartis, Sanofi and UCB. A.E. has received research funding from Pfizer, Eli Lilly, Novartis, Bristol‐Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker from AbbVie, Almirall, LEO Pharma, Zuellig Pharma, Galápagos NV, Sun Pharmaceuticals, Samsung Bioepis, Pfizer, Eli Lilly, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol‐Myers Squibb and Janssen Pharmaceuticals. The other authors declare they have no conflicts of interest.
Data availability
The data that support the findings of this study are available in the Danish National Patient Registry
Ethics statement
Approval from an ethics committee is not required for register studies in Denmark (Danish law: Lov om videnskabsetisk behandling af sundhedsvidenskabelige forskningsprojekter, § 14, stk. 2).
Author contributions
Hans Christian Ring: Conceptualization (equal); formal analysis (equal); writing – original draft (lead); writing – review and editing (equal). Yiqiu Yao: Formal analysis (equal); writing – review and editing (equal). Julia‐Tatjana Maul: Formal analysis (equal); writing – review and editing (equal). John R Ingram: Formal analysis (equal); writing – review and editing (equal). John Walter Frew: Formal analysis (equal); writing – review and editing (equal). Jonathan Thorsen: Formal analysis (equal); writing – review and editing (equal). Mia‐Louise Nielsen: Formal analysis (equal); writing – review and editing (equal). Jashin J. Wu: Formal analysis (equal); writing – review and editing (equal). Jacob Pontoppidan Thyssen: Formal analysis (equal); writing – review and editing (equal). Simon Francis Thomsen: Formal analysis (equal); writing – review and editing (equal). Alexander Egeberg: Conceptualization (lead); formal analysis (equal); supervision (lead); writing – original draft (lead); writing – review and editing (equal).
Supporting information
Table S1 Systemic treatments and associated competing indications and suggested roles in the treatment of hidradenitis suppurativa.
Table S2 Time from first systemic therapy (excluding penicillin and/or dicloxacillin) to initiation of biologic therapy.
Table S3 Time from first systemic therapy (including penicillin and/or dicloxacillin) to initiation of biologic therapy.
Acknowledgment
Open access funding enabled and organized by ProjektDEAL.
Plain language summary available online
References
- 1. Jemec GB. Clinical practice. Hidradenitis suppurativa. N Engl J Med 2012; 366:158–64. [DOI] [PubMed] [Google Scholar]
- 2. Revuz JE, Canoui‐Poitrine F, Wolkenstein P et al. Prevalence and factors associated with hidradenitis suppurativa: results from two case‐control studies. J Am Acad Dermatol 2008; 59:596–601. [DOI] [PubMed] [Google Scholar]
- 3. Jemec GBE, Heidenheim M, Nielsen NH. The prevalence of hidradenitis suppurativa and its potential precursor lesions. J Am Acad Dermatol 1996; 35:191–4. [DOI] [PubMed] [Google Scholar]
- 4. van Straalen KR, Tzellos T, Guillem P et al. The efficacy and tolerability of tetracyclines and clindamycin plus rifampicin for the treatment of hidradenitis suppurativa: results of a prospective European cohort study. J Am Acad Dermatol 2021; 85:369–78. [DOI] [PubMed] [Google Scholar]
- 5. Kitts S, Govea R, Maczuga S, Kirby J. Long‐term antibiotic use for the treatment of hidradenitis suppurativa consistent with guideline recommendations. Clin Exp Dermatol 2021; 46:582–3. [DOI] [PubMed] [Google Scholar]
- 6. Zouboulis CC, Desai N, Emtestam L et al. European S1 guideline for the treatment of hidradenitis suppurativa/acne inversa. J Eur Acad Dermatol Venereol 2015; 29:619–44. [DOI] [PubMed] [Google Scholar]
- 7. Zouboulis CC, Bechara FG, Dickinson‐Blok JL et al. Hidradenitis suppurativa/acne inversa: a practical framework for treatment optimization – systematic review and recommendations from the HS ALLIANCE working group. J Eur Acad Dermatol Venereol 2019; 33:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimball AB, Okun MM, Williams DA et al. Two Phase 3 trials of adalimumab for hidradenitis suppurativa. N Engl J Med 2016; 375:422–34. [DOI] [PubMed] [Google Scholar]
- 9. Kimball AB, Kerdel F, Adams D et al. Adalimumab for the treatment of moderate to severe hidradenitis suppurativa: a parallel randomized trial. Ann Intern Med 2012; 157:846–55. [DOI] [PubMed] [Google Scholar]
- 10. Kirby JS, Miller JJ, Adams DR, Leslie D. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatology 2014; 150:937–44. [DOI] [PubMed] [Google Scholar]
- 11. Khalsa A, Liu G, Kirby JS. Increased utilization of emergency department and inpatient care by patients with hidradenitis suppurativa. J Am Acad Dermatol 2015; 73:609–14. [DOI] [PubMed] [Google Scholar]
- 12. Saunte DM, Boer J, Stratigos A et al. Diagnostic delay in hidradenitis suppurativa is a global problem. Br J Dermatol 2015; 173:1546–9. [DOI] [PubMed] [Google Scholar]
- 13. Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol 2014; 29:541–9. [DOI] [PubMed] [Google Scholar]
- 14. Schmidt M, Schmidt SA, Sandegaard JL et al. The Danish National patient registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7:449–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H et al. Data resource profile: the Danish national prescription registry. Int J Epidemiol 2017; 46:798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. von Elm E, Altman DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370:1453–7. [DOI] [PubMed] [Google Scholar]
- 17. Gallagher CG, Kirthi SK, Cotter CC et al. Could isotretinoin flare hidradenitis suppurativa? A case series. Clin Exp Dermatol 2019; 44:777–80. [DOI] [PubMed] [Google Scholar]
- 18. Patel N, McKenzie SA, Harview CL et al. Isotretinoin in the treatment of hidradenitis suppurativa: a retrospective study. J. Dermatolog Treat 2021; 32:473–5. [DOI] [PubMed] [Google Scholar]
- 19. Huang CM, Kirchhof MG. A new perspective on isotretinoin treatment of hidradenitis suppurativa: a retrospective chart review of patient outcomes. Dermatology 2017; 233:120–5. [DOI] [PubMed] [Google Scholar]
- 20. Bettoli V, Join‐Lambert O, Nassif A. Antibiotic treatment of hidradenitis suppurativa. Dermatol Clin 2016; 34:81–9. [DOI] [PubMed] [Google Scholar]
- 21. Marasca C, Masarà A, Annunziata MC et al. Long‐term clinical safety of clindamycin and rifampicin combination for the treatment of hidradenitis suppurativa: a strategy to reduce side‐effects, improving patients’ compliance. Br J Dermatol 2019; 180:949. [DOI] [PubMed] [Google Scholar]
- 22. Jørgensen AR, Yao Y, Thomsen SF, Ring HC. Treatment of hidradenitis suppurativa with tetracycline, doxycycline, or lymecycline: a prospective study. Int J Dermatol 2021; 60:785–91. [DOI] [PubMed] [Google Scholar]
- 23. Jemec GBE, Wendelboe P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J Am Acad Dermatol 1998; 39:971–4. [DOI] [PubMed] [Google Scholar]
- 24. Bettoli V, Manfredini M, Massoli L et al. Rates of antibiotic resistance/sensitivity in bacterial cultures of hidradenitis suppurativa patients. J Eur Acad Dermatol Venereol 2019; 33:930–6. [DOI] [PubMed] [Google Scholar]
- 25. Join‐Lambert O, Coignard‐Biehler H, Jais JP et al. Efficacy of ertapenem in severe hidradenitis suppurativa: a pilot study in a cohort of 30 consecutive patients. J Antimicrob Chemother 2016; 71:513–20. [DOI] [PubMed] [Google Scholar]
- 26. Wark KJL, Cains GD. The microbiome in hidradenitis suppurativa: a review. Dermatol Ther (Heidelb) 2021; 11:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee RA, Dommasch E, Treat J et al. A prospective clinical trial of open‐label etanercept for the treatment of hidradenitis suppurativa. J Am Acad Dermatol 2009; 60:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prussick L, Rothstein B, Joshipura D et al. Open‐label, investigator‐initiated, single‐site exploratory trial evaluating secukinumab, an anti‐interleukin‐17A monoclonal antibody, for patients with moderate‐to‐severe hidradenitis suppurativa. Br J Dermatol 2019; 181:609–11. [DOI] [PubMed] [Google Scholar]
- 29. Blok JL, Li K, Brodmerkel C, Horvátovich P et al. Ustekinumab in hidradenitis suppurativa: clinical results and a search for potential biomarkers in serum. Br J Dermatol 2016; 174:839–46. [DOI] [PubMed] [Google Scholar]
- 30. Leslie KS, Tripathi SV, Nguyen TV, Pauli M, Rosenblum MD. An open‐label study of anakinra for the treatment of moderate to severe hidradenitis suppurativa. J Am Acad Dermatol 2014; 70:243–51. [DOI] [PubMed] [Google Scholar]
- 31. Grant A, Gonzalez T, Montgomery MO et al. A. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: a randomized, double‐blind, placebo‐controlled crossover trial. J Am Acad Dermatol 2010; 62:205–17. [DOI] [PubMed] [Google Scholar]
- 32. Reddy S, Strunk A, Garg A. Comparative overall comorbidity burden among patients with hidradenitis suppurativa. JAMA Dermatol 2019; 155:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Systemic treatments and associated competing indications and suggested roles in the treatment of hidradenitis suppurativa.
Table S2 Time from first systemic therapy (excluding penicillin and/or dicloxacillin) to initiation of biologic therapy.
Table S3 Time from first systemic therapy (including penicillin and/or dicloxacillin) to initiation of biologic therapy.
Data Availability Statement
The data that support the findings of this study are available in the Danish National Patient Registry


