Abstract
Autoimmune hepatitis (AIH) is a rare disease of unclear etiology characterized by loss of self‐tolerance that can lead to liver injury, cirrhosis, and acute liver failure. First‐line treatment consists of systemic corticosteroids, or budesonide, and azathioprine, to which most patients are initially responsive, although predictors of response are lacking. Relapses are very common, correlate with histological activity despite normal serum transaminases, and increase hepatic fibrosis. Furthermore, current regimens lead to adverse effects and reduced quality of life, whereas medication titration is imprecise. Biomarkers that can predict the clinical course of disease, identify patients at elevated risk for relapse, and improve monitoring and medication dosing beyond current practice would have high clinical value. Herein, we review novel candidate biomarkers in adult and pediatric AIH based on prespecified criteria, including gene expression profiles, proteins, metabolites, and immune cell phenotypes in different stages of AIH. We also discuss biomarkers relevant to AIH from other immune diseases. We conclude with proposed future directions in which biomarker implementation into clinical practice could lead to advances in personalized therapeutic management of AIH.
Potential noninvasive biomarkers and immune cell populations may help predict histological activity versus quiescence in patients with autoimmune hepatitis.
Abbreviations
- AASLD
American Association for the Study of Liver Diseases
- ADA
adenosine deaminase
- AIH
autoimmune hepatitis
- ALT
alanine aminotransferase
- ANA
antinuclear antibody
- ASGPR
anti‐asialoglycoprotein receptor
- AST
aspartate aminotransferase
- BAFF
tumor necrosis factor family B‐cell activating factor
- CCL
chemokine ligand
- CCL11
eotaxin‐1
- CCL26
chemokine eotaxin‐3
- DI‐AIH
drug‐induced AIH
- DILI
drug‐induced liver injury
- FOXP3
forkhead box P3
- hnRNP
heterogeneous nuclear ribonucleoprotein
- IBD
inflammatory bowel disease
- IFN‐ɣ
interferon‐gamma
- IgG
immunoglobulin G
- IST
immunosuppressive therapy
- LKM‐1
liver kidney microsome type 1
- LTR
liver transplant recipients
- MIF
macrophage migration inhibitor factor
- NK
natural killer
- NF‐κß
nuclear factor kappa‐beta
- PBC
primary biliary cholangitis
- PD
programmed cell death
- RA
rheumatoid arthritis
- RNA
ribonucleic acid
- RORɣt
retinoid‐related orphan receptor gamma t
- SLE
Systemic Lupus Erythematosus
- SLA
soluble liver antigen
- SMA
smooth muscle antibodies
- sPD1
soluble PD1
- SPRi
Surface Plasmon Resonance Imaging
- T1D
type 1 diabetes
- T‐bet
t‐box TF expressed in T cells
- TE
transient elastography
- TF
transcription factor
- TGF‐ß
transforming growth factor‐beta
INTRODUCTION
Autoimmune hepatitis (AIH) is a rare disease of unclear etiology thought to be due to a lack of self‐tolerance ultimately leading to liver injury and, in some cases, cirrhosis or acute liver failure.[ 1 , 2 , 3 ] The diagnosis requires exclusion of other etiologies and is multilayered, consisting of specific histological abnormalities with elevated liver enzymes (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)), immunoglobulin G (IgG) levels, and one or more associated autoantibodies including antinuclear antibody (ANA), smooth muscle antibodies (SMA), and rarely antibodies to liver kidney microsome type 1 (anti‐LKM1) and soluble liver antigen (SLA).[ 3 , 4 ] First‐line treatment of AIH consists of corticosteroids with azathioprine, to which a majority of patients respond by achieving remission.[ 4 , 5 , 6 ] Unfortunately, immunosuppressive therapy (IST) has wide‐ranging side effects and is associated with long‐term morbidities (infection, malignancy) that reduce patient quality of life and outcomes.[ 7 , 8 ] Because of this, the recent American Association for the Study of Liver Diseases (AASLD) practice guidelines recommend considering IST withdrawal in patients who have liver enzymes and IgG levels within normal limits for at least 2 years.[ 4 , 9 ] This guideline is made with known hesitancy though, as relapse during or after IST withdrawal is common in AIH (>80% in some studies)[ 8 ] and thus withdrawal needs to be conducted in a stepwise fashion with close monitoring. Histologically active AIH on liver biopsy, despite normal serum transaminases, predicts relapse following attempted drug withdrawal.[ 10 ] Although liver biopsies are specific for active disease on initial presentation, they have risks and are impractical to perform serially, particularly for IST optimization decisions (augmentation, reduction, withdrawal).[ 11 ]
Therefore, there is an unmet need for noninvasive blood‐based biomarkers that could help predict patients at high risk for relapse or serve as an early marker for relapse. A biomarker has been defined according to the US Food and Drug Administration as a characteristic measured as an indicator of normal biological processes, pathogenic processes, or responses to an exposure or intervention, including therapeutic interventions.[ 12 ] Although biomarkers can come from a wide heterogeneity of molecular or physiologic characteristics, broad categories of biomarkers include those that measure susceptibility/risk, diagnosis, monitoring, prognostic, predictive, pharmacodynamic response, and safety.
Some of the various desirable traits of biomarkers in the context of AIH include those that are (1) noninvasive, or readily available from a peripheral source such as blood; (2) easily measured, cost‐effective, and reproducible across a spectrum of patients with AIH (e.g., pediatric and adult patients, varying levels of disease activity); (3) biologically plausible and sufficiently sensitive to serve as a surrogate of liver histologic inflammatory activity; (4) able to prognosticate at diagnosis, predict biochemical remission following IST, or identify those at higher risk of relapse; (5) identify relapse prior to standard clinical signs and symptoms; and (6) provide insight into relevant immune‐based signaling pathways and phenotypes in order to guide personalized therapeutic decisions and promote clinical trials of targeted immunomodulating agents. Although no single biomarker could fulfill each of these diverse characteristics, a determined, systematic approach to biomarker development in AIH is critically important to improve our understanding of the disease, promote the introduction of new targeted therapies, and improve clinical outcomes and quality of life for patients with AIH. The recent 2019 AASLD AIH guidelines cited prognostic and therapeutic biomarkers as a significant unmet need in AIH.[ 4 ]
This paper is a review of the available literature on potential candidate biomarkers in adult and pediatric AIH. These biomarkers, selected based on prespecified criteria (see Methods), include potential indicators of subclinical disease activity, and predictors of clinical relapse, and remission (Table 1). We also delve into relevant biomarker advances that could be borrowed or gleaned from other immune disease states, such as liver transplantation, drug‐induced liver injury, and other nonhepatic autoimmune disorders. We conclude with next steps for novel candidate biomarkers, and how their development and implementation could lead to advances in care and personalization of AIH management.
TABLE 1.
Biomarkers in AIH
| Author | Source | # of participants | Biomarker | Increased risk (+)/protective (‐) | Studied in histological disease: | ||
|---|---|---|---|---|---|---|---|
| Activity | Remission | Relapse | |||||
| Initial Presentation | |||||||
| Bayer et al.,[ 51 ] 1998 | Serum |
18 (AIH) 10 (NASH) 16 (control) |
TGF‐ß1 | + | X | X | |
| Landi et al.,[ 83 ] 2013 | Serum |
40 (AIH) 50 (PBC) 58 (PSC) 54 (HCV) 50 (control) |
TNF‐α | ‐ | X | ||
| Chaouali et al.,[ 143 ] 2020 | Serum |
50 (AIH) 150 (control) |
TNF‐α | + | X | X | |
| Bovensiepen et al.,[ 47 ] 2019 | PBMCs |
49 (AIH) 43 (control) |
TNF‐producing CD4+ T cells | + | X | ||
| Behfarjam et al.,[ 80 ] 2017 | RNA |
18 (AIH) 18 (control) |
T‐bet and IFN‐ɣ mRNA | + | X | ||
| Migita et al.,[ 44 ] 2007 | Serum |
55 (AIH) 14 (acute hepatitis) 33 (HCV) 33 (control) |
BAFF | + | X | X | |
| Nishikawa et al.,[ 43 ] 2016 | Serum | 80 (AIH) | BAFF | + | X | ||
| Efe et al.,[ 55 ] 2014 | Serum |
68 (AIH) 34 (control) |
25(OH)D | ‐ | X | X | X |
| Ebadi et al.,[ 56 ] 2019 | Serum | 209 (AIH) | 25(OH)D | ‐ | X | ||
| Taubert et al.,[ 57 ] 2017 | Serum | 109 (AIH) | Ferritin | ‐ | X | X | |
| Torgutalp et al.,[ 50 ] 2017 | Serum |
52 (AIH) 28 (control) |
ADA | + | X | X | X |
| Remission | |||||||
| Longhi et al.,[ 37 ] 2004 | PBMCs |
41 (AIH) 18 (control) |
CD4+CD25+ Treg cells | ‐ | X | X | |
| Longhi et al.,[ 38 ] 2006 | PBMCs |
25 (AIH) 15 (control) |
CD4+CD25+ Treg cells | ‐ | X | ||
| Ferri et al.,[ 39 ] 2010 | PBMCs |
47 (AIH) 28 (control) |
CD4+CD25hi Treg cells | ‐ | X | X | |
| Peiseler et al.,[ 64 ] 2012 | PBMCs |
77 (AIH) 42 (control) 8 (NASH) |
CD4+CD25+FOXP3+ Treg cells | + | X | X | |
| Grant et al.,[ 40 ] 2014 | PBMCs |
41 (AIH) 25 (control) |
CD39+ Treg cells | ‐ | X | ||
| Liberal et al.,[ 59 ] 2015 | PBMCs |
43 (AIH) 22 (control) |
CD4+CD25+CD127− Treg cells | ‐ | X | X | |
| Liang et al.,[ 49 ] 2018 | PBMCs |
32 (AIH) 20 (control) |
Treg | ‐ | X | X | |
| Chen et al.,[ 60 ] 2019 | PBMCs |
20 (AIH) 20 (viral) 20 (control) |
Foxp3+ Treg cells | ‐ | X | ||
| Gatselis et al.,[ 65 ] 2017 | Serum |
224 (AIH) 249 (PBC) 36 (PSC) 146 (viral hep) 140 (NASH) 114 (control) |
DNAse | ‐ | X | ||
| Drug Withdrawal | |||||||
| Matsumoto et al.,[ 67 ] 2014 | PBMCs |
52 (AIH) 24 (DILI) 30 (viral hep) 11 (PSC) 62 (control) |
anti‐PD‐1 Ab's | + | X | X | X |
| Mitra et al.,[ 66 ] 2015* | RNA |
46 (AILD) 15 (control) |
FOXP3 TF/RORɣt TF ratio | ‐ | X | X | |
| Behfarjam et al.,[ 81 ] 2019 | RNA |
24 (AIH) 24 (control) |
RORɣt TF, IL‐22 mRNA | + | X | ||
| Derben et al.,[ 71 ] 2021 | Plasma | 60 (AIH) | cytokeratin‐18 death marker m65 | + | X | X | |
| Relapse | |||||||
| Treichel et al.,[ 74 ] 1994 | Serum |
79 (AIH) 122 (PBC) 385 (viral hep) 328 (other) |
anti‐ASGPR Abs | + | X | X | |
| Hausdorf et al.,[ 75 ] 2009 | Serum |
45 (AIH) 43 (PBC) 13 (EtOH) 35 (HBV) 53 (HCV) 118 (control) |
anti‐ASGPR Abs | + | X | X | X |
| Assis et al.,[ 76 ] 2013* | Serum |
52 (AIH) 309 (PBC) 71 (control) |
MIF, CD74 | + | X | X | |
| Assis et al.,[ 78 ] 2016 | Serum, DNA |
52 (AIH) 30 (control) |
MIF | + | X | ||
Note: Symbols: *: included patients with PBC.
Abbreviations: Ab: antibody, AIH: autoimmune hepatitis, ADA: adenosine deaminase, ASGPR: asialoglycoprotein receptor, BAFF: tumor necrosis actor family B‐cell activating factor, CD: complementary determining, FOXP3: forkhead box P3, HCV: hepatitis C virus, IFN‐ɣ: interferon‐gamma, MIF: macrophage migration inhibitor factor, mRNA: messenger ribonucleic acid, PBC: primary biliary cholangitis, PBMCs: peripheral blood mononuclear cells, PD: programmed cell death, PSC: primary sclerosing cholangitis, RNA: ribonucleic acid, RORɣt: retinoid‐related orphan receptor gamma t, T‐bet: t‐box TF expressed in T cells, TF: transcription factor, TGF‐ß1: transforming growth factor‐beta, Treg: regulatory T, 25(OH)D: vitamin D.
METHODS
For the purpose of this review of the biomarkers of immune activation and quiescence in different stages of AIH, publications cited in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) were selected in 6/2021–12/2021 using the search words autoimmune hepatitis as well as serum biomarker, cytokine, chemokine, antibodies, receptors, or regulatory T cells (see Figure S1). Citations were chosen based upon their relevance to the aim of this article. Articles excluded included articles with animal data, papers not in English without an English abstract, and review articles. Thirty‐six articles were identified and 28 were chosen to be discussed in further detail (Table 1). The decision to exclude articles was made based upon several factors: expert opinion that these biomarkers were not clinically promising as well as limited quality of data including small cohorts with low power. The eight articles that were omitted are briefly discussed in the review and further details on those biomarkers can be found in Table S1.
CURRENTLY AVAILABLE BIOMARKERS
Current traditional serum biomarkers of liver injury and treatment response in AIH that are used in practice include aminotransferases (AST and ALT), IgG, and, less frequently, 6‐thioguanine (6‐TG). Other clinically available candidate markers such as vitamin D and ferritin will be discussed below. Hartl et al. investigated potential predictors in relapse and found that although ALT, IgG, and overall gamma‐globulin levels were all normal in patients prior to IST withdrawal, there were variations within the normal range that distinguished patients that ultimately relapsed.[ 13 ] Therefore, they advocated for a goal of ALT less than half the upper limit of normal (ULN) and an IgG level less than 1200 mg/dL. When this was sustained for 2 years, they found 46% of patients experienced relapse after cessation of treatment,[ 13 ] which is significantly lower than the current rate of >80%.[ 8 ] This is corroborated by Montano‐Loza et al. who validated that interface hepatitis disappeared when serum AST levels improved to less than two fold the ULN and that high levels of IgG at IST withdrawal correlated significantly with the risk for relapse.[ 14 ]
In contrast, even with biochemical remission, several studies have shown that about 20–50% of patients with AIH still had histological evidence of active disease on liver biopsy which puts them at inevitable risk for relapse after IST withdrawal.[ 10 , 15 , 16 ] Lüth et al. revealed that although elevated ALT and IgG levels have a 97% positive predictive value for relapse, they have only a 33% negative predictive value for remission, suggesting that these markers are not a sufficient surrogate or replacement for liver biopsy.[ 17 ]
In response to these findings, the AASLD changed their guidelines in 2010 to require not only normalization of bilirubin and gamma‐globulin levels but also normal serum aminotransferases for at least 2 years prior to consideration of withdrawal of IST.[ 3 ] One center showed that with the application of the 2010 criteria to their cohort, the number of their patients that met criteria for remission went from 73% with the 2002 criteria to 26% with the 2010 criteria.[ 18 ] Despite evidence that more stringent cutoffs of ALT, IgG, and gamma‐globulin levels may be adequate surrogates for histological remission, the evidence is not compelling enough. Therefore, the AASLD continues to recognize liver biopsy as the gold standard to establish the state of histologic remission and exclude inflammatory activity prior to drug withdrawal. Liver biopsy prior to IST withdrawal remains mandatory in children[ 19 ] however recent studies in adults suggest that liver biopsy may be optional.[ 4 , 20 ] Furthermore, in patients with AIH and cirrhosis, biochemical remission was recently shown to be an inadequate reflection of histological remission.[ 21 ]
Finally, there are limited data on the utility of 6‐TG as a surrogate marker for remission in patients on azathioprine. Dhaliwal et al. conducted a study with 70 patients that showed that higher 6‐TG levels significantly associated with AIH histological remission,[ 22 ] but this was contradicted in other studies.[ 23 , 24 , 25 ] The data on the use of 6‐TG levels in pediatric AIH is also limited.[ 26 , 27 ] It is important to note, though, a recent study by Candels et al. showed that measuring thiopurine metabolite levels helped maintain remission and even allowed for reduction in dosage of both thiopurines and corticosteroids,[ 28 ] providing some promise in its clinical utility. There also may be some practical use of 6‐TG to identify azathioprine nonresponders or as a marker of treatment adherence, but this requires further studies for widespread clinical use.
PATHOPHYSIOLOGY OF AIH
To begin to identify novel candidate biomarkers for exploration, it is important to understand the complex pathophysiology of AIH (Figure 1). Although not completely elucidated, T cell dysregulation plays a primary role in AIH liver injury.[ 29 ] This is supported by the finding that a majority of the pathogenic immune cells in active AIH are T cells, predominately CD4+.[ 30 ] To a lesser extent, there is involvement of other immune cells including B cells, plasma cells, natural killer (NK) cells, and macrophages.[ 30 ] Naïve CD4+ T cells can differentiate into multiple CD4+ T cell subsets based upon the cytokine milieu and the activation of specific transcription factors (TF). For instance, Th1 cells are promoted by IL‐12 and interferon‐gamma (IFN‐γ) [master TF: t‐box TF expressed in T cells (T‐bet)],[ 31 ] Th2 by IL‐4 (master TF: GATA‐3), Th17 cells are dependent upon the presence of transforming growth factor‐beta (TGF‐ß) and IL‐6 [master TF: retinoid‐related orphan receptor gamma t (RORɣt)],[ 32 , 33 ] whereas regulatory cells (Treg) are induced by IL‐2 and TGF‐ß [master TF: forkhead box P3 (FOXP3)].[ 33 , 34 , 35 ]
FIGURE 1.
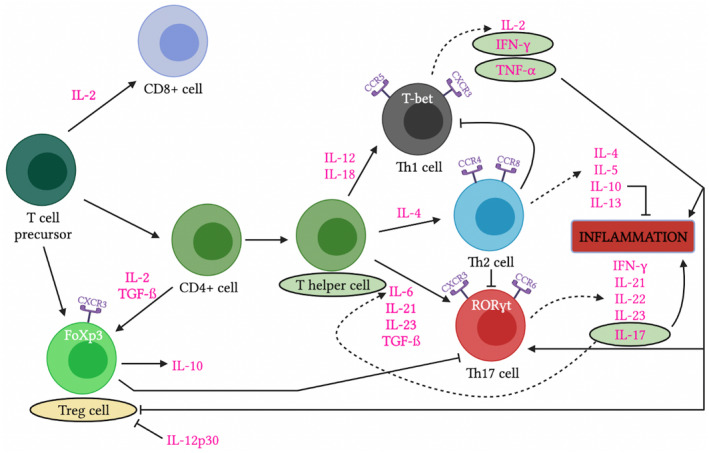
The pathophysiology and candidate biomarkers of autoimmune hepatitis. Depiction of the pathways of inflammation that play a role in that pathophysiology of autoimmune hepatitis. Promising markers of disease activity are circled in green and promising markers of quiescence are circled in yellow. CCR/CXCR, chemokine receptor; FoXp3, forkhead box P3; IFN‐γ, interferon‐gamma; TGF‐ß, transforming growth factor‐beta; Th, helper T; Treg, regulatory T.
Each of the Th cell subsets are, in turn, associated with unique inflammatory and anti‐inflammatory properties that ultimately play a role in AIH pathogenesis and disease activity. Th1 cells release primarily proinflammatory cytokines including interleukin IL‐2 and IFN‐ɣ, Th2 cells produce IL‐4, IL‐10, and IL‐13,[ 29 ] whereas Th17 cells are responsible for producing IL‐17, IL‐22, and TNF‐α which are major contributors to inflammation.[ 32 ] Tregs secrete anti‐inflammatory cytokines such as IL‐10,[ 36 ] and, through multiple mechanisms, inhibit proinflammatory function of effector T cells. Multiple studies have shown that impaired function or reduced numbers of Treg are associated with AIH.[ 37 , 38 , 39 , 40 , 41 ]
A subset of T cells, the follicular helper T cell (Tfh), is responsible for providing help during B‐cell maturation and germinal center generation.[ 42 ] There is a relative paucity of data about the role of B cells in the pathophysiology of AIH, although hypergammaglobulinemia (namely, elevated IgG) as well as defining antibodies are integral to the diagnosis of AIH. However, recent data has emerged regarding the role of TNF family B‐cell activating factor (BAFF), a cytokine essential for the development and maturation of B cells, which is further discussed below as a candidate biomarker.[ 43 , 44 , 45 ]
BIOMARKERS OF IMMUNE ACTIVATION AND QUIESCENCE IN DIFFERENT STAGES OF AIH
We will focus the discussion on the leading candidate biomarkers at different phases of disease states of AIH (Table 1). We selected these biomarkers based upon the number of published studies as well as the number of patients and samples with key clinical stages (activity, remission, relapse) tested in each study. We also took into account the ease of application of each biomarker to the clinical setting (Figure 2).
FIGURE 2.
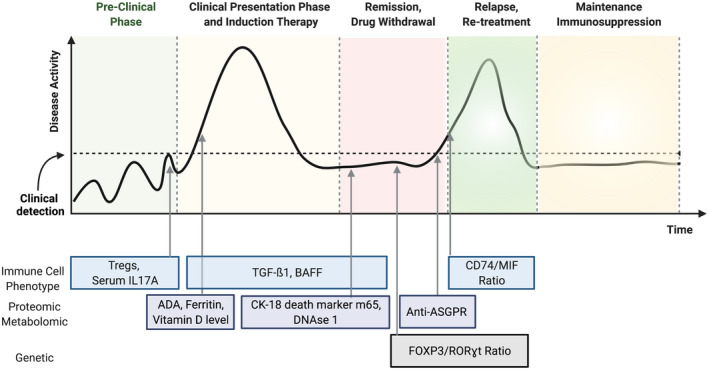
Natural history of autoimmune hepatitis and candidate biomarkers. Depiction of the natural history of autoimmune hepatitis with associated potential biomarkers of activity and quiescence at each stage of disease. ADA, adenosine deaminase; ASGPR, asialoglycoprotein receptor; BAFF, tumor necrosis factor family B‐cell activating factor; CD, complementary determining; CK, cytokeratin; DNAse, deoxyribonuclease; FOXP3, forkhead box P3; MIF, macrophage migration inhibitor factor; RORγt, retinoid‐related orphan receptor gamma t; TGF‐ß, transforming growth factor‐beta; Tregs, regulatory T.
PRECLINICAL PHASE
There are no known preclinical biomarkers capable of identifying signs of AIH before clinical detection or liver damage, but there are certain genetic HLA and non‐HLA based polymorphisms that may be associated with AIH such as DRB1*03:01, SH2B3, and CARD10.[ 4 , 46 ] These genetic polymorphisms are not ideal biomarkers, as defined above, as they are fixed values that have little utility in monitoring over time. We do not recommend screening the general population but recognize they may be helpful for risk stratification in patients with other autoimmune features that may be at higher risk for AIH.
CLINICAL PHASE
Initial presentation
This phase is the initial stage of active disease. These biomarkers may have clinical utility in detecting inflammation in the liver prior to increases in serological markers of liver injury (i.e., aminotransferases), in grading disease severity, and/or in earlier prognostication of the disease course.
As discussed above, AIH is primarily driven by a T cell‐mediated immune process. The frequency of T cells secreting IL‐17 and TNF‐α is significantly increased in patients with AIH.[ 47 , 48 ] Liang et al. found that serum levels of IL‐17A correlated with liver injury and that levels of circulating Th1 and Th17 cells significantly decreased after IST,[ 49 ] which make them a potentially trackable immune marker of disease activity. This study also showed fewer Treg cells in active disease, which will be subsequently discussed as a biomarker of remission.
A recent study found correlations between serum adenosine deaminase (ADA) levels and the severity of interface hepatitis on index liver biopsy.[ 50 ] A cutoff of 24.5 U/L identified patients with severe interface hepatitis, making ADA a candidate biomarker for grading severity of disease at initial presentation.
Similarly, serum TGF‐ß1 is elevated in active AIH, correlates with active histological disease even in the setting of normal aminotransferases, and normalizes upon biochemical remission. Therefore, it may be a proxy for liver biopsy in predicting histologic activity and remission.[ 51 ] BAFF is also elevated in active biochemical and histological AIH disease and reduces with corticosteroid treatment.[ 43 , 44 , 45 ]
The vitamin D receptor and vitamin D resistance have been studied for their role in autoimmunity and inflammation.[ 52 , 53 , 54 ] Efe et al. reported that serum levels of vitamin 25(OH)‐D were associated with liver fibrosis and interface hepatitis in AIH, and nonresponders to IST had significantly lower baseline serum 25(OH)D levels compared to responders.[ 55 ] A recent large study confirmed these findings and showed that patients with AIH and severe vitamin D deficiency (<25 nmol/L) were more likely to have treatment nonresponse and liver‐related mortality,[ 56 ] making it a potential prognosticator at presentation.
Ferritin has also been investigated as a predictive biomarker of treatment response in patients with AIH.[ 57 ] Taubert et al. reported that a baseline ferritin level (>2.09 × ULN) and lower immunoglobulin levels (<1.89 × ULN) were associated with complete biochemical remission. Although counterintuitive as ferritin is an acute phase reactant, this study revealed that the hyperferritinemia was quickly reversible with therapy and seemed dysregulated from hepcidin. This was thought to be secondary to human hepatocyte growth factor, which can have potentially favorable immunomodulatory effects.[ 57 ] The relevance of iron homeostasis to inflammation and immune tolerance has also been previously reported in patients with liver transplant in which hepcidin and ferritin were differentially elevated in patients with operational tolerance.[ 58 ] It is important to note that both these markers are advantageous as they are readily available in clinical practice.
Remission
Remission can be categorized into biochemical (normalization of AST, ALT, and IgG) and histological (lack of inflammation such as interface hepatitis on biopsy) remission.[ 4 ] As discussed above, biochemical remission is likely not a sufficient surrogate for histological remission. Therefore, identification of noninvasive biomarkers of histological remission is an unmet need to prevent the requirement of liver biopsy.
Treg cells have been the focus of many studies in AIH as they control effector responses and are deficient in active disease.[ 37 , 38 , 39 , 59 , 60 ] Studies have reported that patients with AIH have fewer peripheral Treg cells in active disease compared to patients in remission and healthy controls.[ 37 , 60 ] Other studies have revealed that, in active AIH, peripheral Tregs are not only decreased in number but also have impaired suppressive function. In particular, peripheral Tregs from patients with AIH have decreased IL‐10 secretion and they fail to properly regulate CD8+ T cell function and suppress IFN‐ɣ and IL‐17 production.[ 39 , 40 , 59 , 61 ] Intriguingly, immune‐modulating agents such as erythropoietin[ 62 ] and IL‐2[ 63 ] have been shown to increase Treg number and/or function in patients with AIH.
Other studies however reported no difference in Treg cells in active AIH versus remission.[ 41 , 64 ] Peiseler et al. showed that the suppressor function of peripheral Treg cells was not impaired in patients with AIH compared to controls and Treg cells were actually elevated in the liver in active AIH.[ 64 ] The authors suggested that the cytokine microenvironment itself may suppress Treg function in the liver.[ 64 ] Another theory is that Tregs may actually contribute to the inflammation due to their plasticity and ability to convert into effector cells.[ 64 ] Interestingly, Taubert et al. showed a disproportional decrease in intrahepatic Tregs following IST, with the caveat that effector T cells may transiently also express CD25 and FOXP3, making them difficult to distinguish from Tregs.[ 41 ] A potential explanation for these contrasting results may reside in the different approaches and markers that have been used to measure Tregs. Through a comprehensive phenotypic analyses of various Treg subsets, McEachern et al. have been able to show that CD4+CD25+CD127Low Treg expressing HLA‐DR are potentially the ones most impaired in patients with AIH.[ 62 ] Further studies are needed to test this hypothesis and whether the amount of circulating Treg correlates with histological remission.
Another marker, serum DNAse1, is an enzyme related to apoptotic cell degradation, may also represent a protective biomarker and favorable profile for remission in AIH. Gatselis et al. revealed that patients with AIH who experienced sustained remission had higher baseline DNAse1 levels compared to partial responders, nonresponders, and subsequent relapsers.[ 65 ] The role of DNAse1 in the breakdown of self‐DNA and the relationship of self‐DNA to inflammation suggests that regulators of autoantigen breakdown may help determine risk of disease activity versus remission.
Drug withdrawal prediction
This is arguably the most significant disease phase for the development of biomarkers in terms of clinical application. AASLD guidelines recommend consideration of IST cessation if possible, despite a known risk of relapse.[ 4 ] Therefore, it would be significant to develop biomarkers that could risk stratify patients before IST tapering. This would help with the clinical decision‐making for timing of withdrawal of IST, frequency of monitoring after IST withdrawal, and a more educated risk/benefit discussion with the patient about attempt of withdrawal of IST. These markers should also be more sensitive than currently available serologic tests and allow for closer monitoring during the drug withdrawal phase to detect early signs of immune activation. This could promote a practice of reinitiation of IST prior to increases in aminotransferases and liver injury.
In a further evaluation of T cell phenotyping as biomarkers in AIH, a study by Mitra et al. evaluated the ratio of TF gene expression of Treg (FOXP3) over Th17 (RORɣt) cells.[ 66 ] The FOXP3:RORɣt ratio was <1 in active AIH, high in quiescent disease (p < 0.001), and was not significantly different between AIH and primary biliary cholangitis,[ 66 ] suggesting a common TF signature. There was also a correlation between the FOXP3: RORɣt ratio and the histopathological activity score,[ 66 ] indicating a potential surrogate for liver biopsy and patients with more favorable phenotypes for IST withdrawal.
Another potential biomarker is anti‐programmed cell death (PD)‐1 antibody, an inhibitory T cell receptor that plays a role in regulating T cell activation. Blockade of PD‐1 and its ligand PD‐L1 are established strategies in cancer immunotherapy, although activation of T cells can lead to detrimental autoimmune consequences.[ 67 , 68 , 69 ] Matsumoto et al. showed that serum anti‐PD‐1 Abs were higher in patients with acute AIH compared to remission and correlated with liver function tests. Interestingly, the presence of anti‐PD‐1 Abs may be a predictor of poor treatment response and relapse[ 67 ] and so may indicate a patient is at higher risk, requiring more frequent serological monitoring during IST withdrawal.
The anti‐SLA antibody has also been associated with a two‐fold increase in relapse after IST withdrawal,[ 70 ] making it a potential prognostic marker for withdrawal consideration. An additional protein is cytokeratin‐18 death marker m65, found to have an 86% negative predictive value for detection of incomplete histological remission in a recent multicenter study,[ 71 ] which could sway clinicians away from IST withdrawal or obtain a liver biopsy first.
Early relapse detection
Finally, biomarkers during the relapse phase are significant, as relapse is frequent in the disease process of AIH both with and without IST withdrawal.[ 8 ] A helpful biomarker in this phase would be a measure of those with resistant relapses that may need higher doses of IST to achieve remission again. This is clinically significant as it would allow the clinician to be more aggressive with IST to minimize liver injury and fibrosis development.
Although ANA and SMA are part of the diagnostic criteria for type 1 AIH, they are neither disease‐specific nor correlate with AIH activity in adults.[ 9 ] On the contrary, in pediatric populations, LKM‐1 as well as SMA may correlate with disease activity, discussed in a later section.[ 72 ] Anti‐asialoglycoprotein receptor (ASGPR) titers are high in active disease and decrease in response IST.[ 73 , 74 , 75 ] Importantly, they appeared to increase prior to elevation of liver enzymes,[ 75 ] indicating the potential to predict early diagnosis and relapse of AIH.
Macrophage migration inhibitor factor (MIF) is a cytokine originally studied in Th1‐mediated autoimmune disease, including rheumatoid arthritis, systemic sclerosis, and inflammatory bowel disease.[ 76 , 77 ] MIF levels are elevated in patients with AIH despite corticosteroid therapy, and a ‐173C single nucleotide polymorphism in the MIF promoter correlates with steroid resistance in AIH and other disorders.[ 76 , 78 , 79 ] In addition, the ratio between the soluble, neutralizing MIF receptor (CD74) and MIF negatively correlated with ALT in relapsing patients,[ 76 ] showing promise as an immune disease activity biomarker and marker of aggressive disease and steroid resistance.
UNDERSTUDIED BIOMARKERS
We have highlighted the most promising biomarkers according to our literature review, revealing the most studied assays. In addition, it is important to note that there are other potential biomarkers that are understudied but still warrant mention. Gene expression profiles of immune activation, such as IFN‐ɣ, T‐bet, and IL‐22 transcripts, have higher expression in patients with AIH compared to healthy controls.[ 80 , 81 ] There are various cytokines and chemokines that have also been associated with immune activation in AIH including IL‐6, ‐8, ‐21, and ‐23,[ 48 , 82 , 83 , 84 , 85 ] as well as CCL2, CXCL9, and CXCL10[ 86 ]; whereas others are associated with immune quiescence including IL‐2, ‐4, and ‐10,[ 49 , 60 , 87 ] as well as CCL22, CCL13, and eotaxin‐1 (CCL11).[ 83 ] As noted, several of these biomarker changes are not necessarily unique to AIH and seen in other auto/allo‐immune disorders. For instance, Efe et al. reports a potential of angiotensin‐converting enzyme to be a serum biomarker for fibrosis in AIH,[ 88 ] but will require further investigation and validation before it can be used in clinical practice as it may not be a sensitive marker because it is known to be elevated in other conditions, such as sarcoidosis.
Radiographic biomarkers for AIH are limited, but there is some evidence surrounding transient elastography (TE). TE is an established tool to assess liver fibrosis in various liver diseases, but the data in AIH is limited. A study by Hartl et al. revealed that TE had a high utility in separating severe from nonsevere fibrosis after 6 months of IST.[ 89 ] Of note, they found that it was not a reliable tool before the start of IST.[ 89 ] This group also found that remission could potentially be monitored by fibrosis regression via TE,[ 90 ] which would be a valuable addition to the monitoring of serum biomarkers during the remission and potential withdrawal of IST. There has also been investigation into alternative techniques of elastography including ‘ElastPQ’ that which utilizes point shear wave speed measurement,[ 91 ] but requires further investigation for clinical use. Both serum and radiographic markers of histologic fibrosis in AIH warrants further investigation.
SPECIFIC BIOMARKERS OF AUTOIMMUNE HEPATITIS IN THE PEDIATRIC POPULATION
There is a paucity of literature with respect to accurate diagnostic and prognostic biomarkers in children with AIH. The bulk of the studies focus on autoantibodies, specifically on the role of anti‐LKM1 in defining type 2 AIH, a subtype predominantly found in children. Anti‐LKM1 antibodies are present in 1–3% of adults with AIH and in 9–38% of children with AIH, with highest incidences in preteen European and Canadian children.[ 4 ] Compared to type 1 AIH, the presentation of type 2 AIH is more acute and severe, with higher rates of relapse and lower remission following IST withdrawal.[ 92 ] Historically, autoantibody levels are not established biomarkers of AIH disease activity or treatment outcomes.[ 4 ] This dogma was challenged by Couto et al. who identified that the persistence of anti‐SMA and anti‐actin antibodies correlated with biochemical and histological disease activity in adults and children.[ 93 ] After liver transplant, antibodies associated with the diagnosis of de novo AIH include ANA, anti‐SMA/anti‐actin, and donor‐specific anti‐HLA antibodies.[ 94 ]
As in adults, the PD‐1 pathway is associated with pediatric AIH activity. At diagnosis, children with AIH had significantly higher levels of soluble PD‐1 compared to other liver diseases and this positively correlated with liver fibrosis stage and the Child Pugh score.[ 95 ] Furthermore, soluble PD‐1 levels were significantly higher in pediatric patients with AIH with active disease versus remission.[ 96 ]
CANDIDATE BIOMARKERS: LESSONS LEARNED FROM OTHER IMMUNE DISEASE STATES
Liver transplantation
The clinical, biochemical, and histological presentations of AIH can mimic allograft rejection and its plasma‐cell rich variants in liver transplant recipients (LTR).[ 97 , 98 ] In addition, AIH can recur after LT, with similar presentations and responses to therapy. Thus, biomarkers identifying active versus inactive native AIH could parallel similar profiling in transplant graft immune activation versus quiescence/tolerance, particularly those that are not antigen‐specific.
Several LT candidate markers are on the horizon and akin to those developed in nonhepatic organ recipients.[ 11 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 ] Studies using serial samples have demonstrated increasing levels of microRNAs, donor‐specific antibodies, and blood CXCL10 gene expression prior to rejection, mainly during full IST withdrawal in LT tolerance studies.[ 102 , 118 , 119 , 120 ] Additional tolerance assays include blood immunophenotypic assays (Tregs and Vδ1/Vδ2 cell ratios), cytokine gene profiles (NK cells, γδ T cell, Th17 cells, CD8 receptor genes), and genomic microarrays.[ 103 , 121 ]
For the larger LT population not undergoing IST withdrawal, recent studies have reported specific blood gene transcripts that can distinguish rejection from normal graft function and nonrejection causes of graft injury.[ 122 , 123 ] These signatures are detectable in the weeks prior to rejection and resolve with corticosteroid therapy. This is promising as these assays could allow for IST titration guidance during minimization before any biochemical evidence of rejection. Proteoforms may be a more specific marker of immune activation.[ 103 ] In summary, these LT biomarker discovery assays should be investigated in AIH given the similar presentations, IST used and weaning considerations.
Drug‐induced liver injury (DILI)
Another growing area of research in biomarker discovery is in DILI. Three major areas of biomarker utility are (1) diagnosis ‐ confirmation of a specific drug that may be implicated in DILI i.e., APAP‐CYS in predicting APAP‐induced DILI[ 124 ]; (2) prediction ‐ personalized approach to manage risk of developing DILI after exposure to a certain drug i.e., gene variant HLA B*5701 and abacavir toxicity[ 124 , 125 ]; and (3) prognosis ‐ toxicity in early DILI or chance of mortality i.e., miR122 was used to determine likelihood of delayed injury in APAP‐overdose.[ 124 , 125 ]
A smaller subset of patients with DILI display autoimmune features resembling idiopathic AIH.[ 126 , 127 ] Thus, biomarker assessments would be helpful in differentiating between DILI, drug‐induced AIH (DI‐AIH), and idiopathic AIH.[ 126 ] Qu et al. showed significant increases in intrahepatic Tregs in DI‐AIH versus AIH.[ 128 ] Lammert et al. revealed an IgM predominance in patients with DI‐AIH, whereas IgG and IgM autoantibodies characterized idiopathic AIH. Candidate IgGs directed against chromatin, myosin, antimitochondrial antigen, nucleosome antigen, and CENP‐B showed high accuracy in distinguishing idiopathic and DI‐AIH.[ 127 ]
As previously mentioned, immune checkpoint inhibitors targeting PD‐1 and CTLA4 can lead to hepatic injury resembling AIH.[ 129 , 130 , 131 , 132 ] Biomarkers distinguishing checkpoint‐associated hepatitis from idiopathic AIH may aid in predicting predisposition to liver injury and treatment response. Zen et al. showed decreased hepatic CD4+ and CD20+ lymphocytes as well as CD20/CD3 and CD4/CD8 ratios in checkpoint inhibitor‐hepatitis versus AIH. These results may point to the lack of CD4+ T cell interaction with B cells leading to decreased IgG formation in checkpoint‐associated hepatitis.[ 129 ] Hutchinson et al. showed a correlation between effector memory CD4+ T cell expansion due to latent CMV with the development of hepatitis following checkpoint inhibitor therapy.[ 133 ] This could identify those patients who may be susceptible to checkpoint‐associated hepatitis to modify treatment approaches.
Other nonhepatic autoimmune diseases
There is a parallel interest in developing biomarkers across all autoimmune diseases, many of which share characteristics with AIH regarding complex criteria, risk of exacerbations, and lack of therapies to restore tolerance. Lessons and principles from these disciplines may also be helpful in guiding biomarker development in AIH.
Patients with inflammatory bowel disease (IBD) have a growing array of drugs and pathways for therapy, although biomarker development has not been robust or effectively integrated into clinical trials.[ 134 ] A recent systematic review of fibrostenosing Crohn's Disease identified categories of promising biomarkers including serum, genetic, and histologic markers, although they are limited by a lack of standardized disease category definitions and the absence of validation.[ 135 ]
Connective tissue diseases have undergone significant biomarker development in recent years.[ 136 ] This includes interest in biomarkers in the preclinical phase with loss of tolerance (i.e., IFNγ, autoantibodies) and cytokines that are relevant close to the time of diagnosis (i.e., B‐lymphocyte stimulator in lupus), as well as immune cell and cytokine‐based biomarkers of disease onset and progression. An interesting approach, which could be applied to AIH, came from the Biomarkers of Lupus Disease Study in which patients with active non‐organ‐threatening SLE were given a monitored withdrawal of IST.[ 137 ] The study reported the impact of type 1 IFN signatures on the lupus cytokine pathways and the relationship of IL17RA and B‐lymphocyte stimulator levels to different IST regimens. This study also found different gene expression of T cells and IFN, as well as higher frequencies of activated neutrophils, monocytes, and B cells among early versus late flare patients.[ 138 ]
In‐depth characterization of T cell–based biomarkers has been proposed in type 1 diabetes (T1D).[ 139 ] Although the lack of a specific autoantigen in AIH may preclude some of the techniques currently available in T1D, e.g., epitopes from insulin, preproinsulin, and GAD65, antigen‐agnostic biomarker approaches could be emulated in AIH. This includes measuring key CD4+ T cell subpopulations by flow cytometry and Treg signatures by nanostring expression assays, where a gene transcript signature of stimulated Tregs can distinguish between new and longstanding T1D, T2D, and controls.[ 140 ] Importantly, the development of novel T cell biomarkers in T1D parallels the development of targeted therapies such as anti‐CD20 and anti‐CD3 drugs. A consensus‐based process of T cell–based biomarker development in T1D, ranging from discovery to fit‐for‐purpose testing and regulatory qualification, could be adopted in AIH.[ 139 ]
Lastly, candidate biomarkers may be relevant both for AIH and related autoimmune disorders, such as the shared positivity of relevant autoantibodies. Antinuclear autoantibodies against the heterogeneous nuclear ribonucleoprotein (hnRNP) A2 and B1, two splice variants of a protein involved in mRNA processing, were evaluated making use of Surface Plasmon Resonance Imaging (SPRi), a novel technique designed to evaluate stability of immune complexes. Results showed that the peptide 55–70 of the B1 subunit was highly specific for AIH versus SLE and RA.[ 141 ]
FUTURE DIRECTIONS: BENCH TO BEDSIDE AND BEDSIDE BACK TO BENCH, AND THE ROLE OF BIOMARKERS IN CLINICAL TRIALS
The successful introduction of novel AIH biomarkers into clinical care is challenging and requires a thoughtful, longitudinal approach to biomarker development. In this regard, a Roadmap for Discovery and Validation of Candidate Biomarkers can be a useful guide (Table 2). Initial discovery of novel biomarkers should focus on biological plausibility and link histological immune activity with easily measurable peripheral signals such as gene signatures, metabolites, or circulating immune cells. A machine learning approach that incorporates results from multiple different assays may provide distinct signatures that predict diagnosis or remission. Typically, this will involve a small cohort of well‐characterized patients with similar disease phenotypes. The relationship of the marker to AIH disease status (active disease, remission, relapse, on/off treatment) should be defined and closely correlated with standard clinical indicators of disease activity (e.g., ALT and IgG, histological findings). Initial testing at this stage can make use of stored biospecimens and retrospective clinical analyses. Confirmation testing of a candidate biomarker utilizing retrospective or prospective cohorts should focus on optimization of the assay or method, development of standard operating procedures, and testing at additional laboratories and clinical sites. Validation of candidate biomarkers should then focus on determining the assay's reliability across multiple centers using prospective cohorts and may be tested across a range of relevant populations, such as adults and children with AIH. In this phase, critical questions about reliability should be addressed including interassay variability from a larger group of laboratories, with further refinement of protocols as a result.
TABLE 2.
Roadmap for discovery and validation of candidate biomarkers in AIH
| Discovery | Confirmation | Validation | Regulatory approval | |
|---|---|---|---|---|
| Key Properties |
Biologically plausible Noninvasive or from peripheral blood Correlation with clinical markers (ALT, IgG, histology) and disease state |
Assay optimization, standard operating procedure (SOP) | Acceptable interassay variability |
Utility in point‐of‐care testing Cost‐efficient and reproducible at routine clinical labs |
| Testing Setting | Single site/lab |
Two/Three site/lab Transferability of assays |
Multicenter, international sites/labs |
Regulatory Agencies (EMA, FDA) Biomarker qualification process |
| Clinical Dataset for Correlation | Retrospective | Retrospective, Prospective | Prospective |
Prospective Patient Registry |
| Outcome | Sensitivity and Specificity | Reliability | Sensitivity and Specificity | Correlation with relevant clinical outcomes |
| Theoretical Application to AIH | Metabolite of hepatic T cell activity measured in peripheral blood in adults with AIH | Metabolite accurately indicates histological activity despite normal ALT while on IST | Metabolite predicts relapse in adults and children with AIH | Metabolite is a biomarker of disease response to IST and can serve as a measurable datapoint in clinical trials |
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; EMA, European Medicines Agency; FDA, Food and Drug Administration; IgG, immunoglobulin G; IST, immunosuppressive treatment.
Regulatory approval of a biomarker may be considered as a central final goal of biomarker development in the context of AIH, and one that has been similarly considered in related fields of IBD and type 1 DM.[ 134 , 139 ] This phase of evaluation would encompass additional questions of utility in point‐of‐care assays that would be performed widely across commercial laboratories, in cost‐efficiency considerations, and in use of large, prospective patient registries. From the regulatory perspective of the FDA or EMA, biomarker qualification would entail a careful process in coordination with the regulatory agencies and a resulting designation of a biomarker that can be utilized both clinically and as a surrogate endpoint for clinical trials. A theoretical example is an immune cell metabolite or gene signature that highly correlates with histological disease activity and has added accuracy (e.g., C‐statistic) beyond standard laboratory data for determining histological remission in patients with AIH. Such a biomarker could be useful in the context of clinical care and in the context of clinical trials that are designed and powered around remission as an endpoint.
Another example of biomarker utility for clinical use is in the context of development of novel therapies that go beyond standard corticosteroids. This remains an important unmet need in AIH, especially given the meaningful advances in pathway‐based targeted therapies in other autoimmune disorders which have moved beyond corticosteroids over the past decades. Indeed, biomarker development may be considered an essential ingredient to achieve this necessary advance in AIH. Peripheral blood biomarkers that could specify which patients have Th17 or Treg predominant abnormalities could help identify subsets of cohorts best suited to a particular targeted immunomodulatory therapy. Furthermore, there is a more fundamental need to improve the structure of clinical trials in AIH, with better patient selection and identification of trial endpoints with the goal of promoting enrollment. Biomarkers that can serve as predictors of response to therapy over a 1‐ or 2‐year period, or biomarkers that can predict the likelihood of relapse after induction therapy, could aid in this process by permitting a more scientifically driven approach to study design and power analyses, as well as shortening the duration of trials themselves.
Finally, the patient experience must remain at the center of all aspects of care and therapeutic innovations in AIH. In a patient‐centered approach context, novel biomarkers that can avoid unnecessary invasive interventions (e.g., substitute for a liver biopsy when considering withdrawal of IST), inform therapeutic decisions (e.g., identify patients who can receive less initial immunosuppression based on immune‐sensitivity to agents), and improve quality of life (e.g., identify patients at very low risk of relapse not needing indefinite immunosuppression) would add significant value to the management of AIH.
This patient‐centered experience can also be achieved by taking a bedside back to bench approach. Discovery studies where well‐characterized populations of patients with AIH with specific clinical disease states (i.e., active disease, remission both on and off IST) are analyzed for various potential biomarkers would likely be of great promise. This approach would also be beneficial as novel treatments are being developed in AIH. For example, as novel therapies targeting B cells are currently being tested in clinical trials,[ 142 ] this may prompt further translational studies of B‐cell related markers to help clarify the role of B cells in AIH pathogenesis.
CONCLUSIONS
This review highlights promising and emerging candidate biomarkers that may help focus the development of clinically significant biomarkers moving forward, particularly in relation to the different time periods of disease presentation, activity and management (Figure 2). As shown, these include ADA,[ 50 ] cytokeratin‐18 death marker m65,[ 71 ] TGF‐ß1,[ 51 ] BAFF,[ 43 , 44 ] Anti‐ASGPR,[ 75 ]. FOXP3/RORɣt ratio,[ 66 ] DNAse 1,[ 65 ] ferritin,[ 58 ] CD74:MIF ratio[ 76 ] and the vitamin D receptor.[ 55 ] Tregs also hold significant promise as protective markers or therapeutic targets in AIH similar to other disease states including organ rejection and DILI.[ 121 ]
To summarize, biomarkers have significant promise in improving personalized management of AIH (Figure 3). These markers will play various roles, including early identification of AIH, response to therapy, risk of relapse, and safer IST withdrawal. There is a substantial need for larger validation studies and prospective clinical trials, whereby the most promising candidate biomarkers are used in trials and clinical decision‐making.
FIGURE 3.
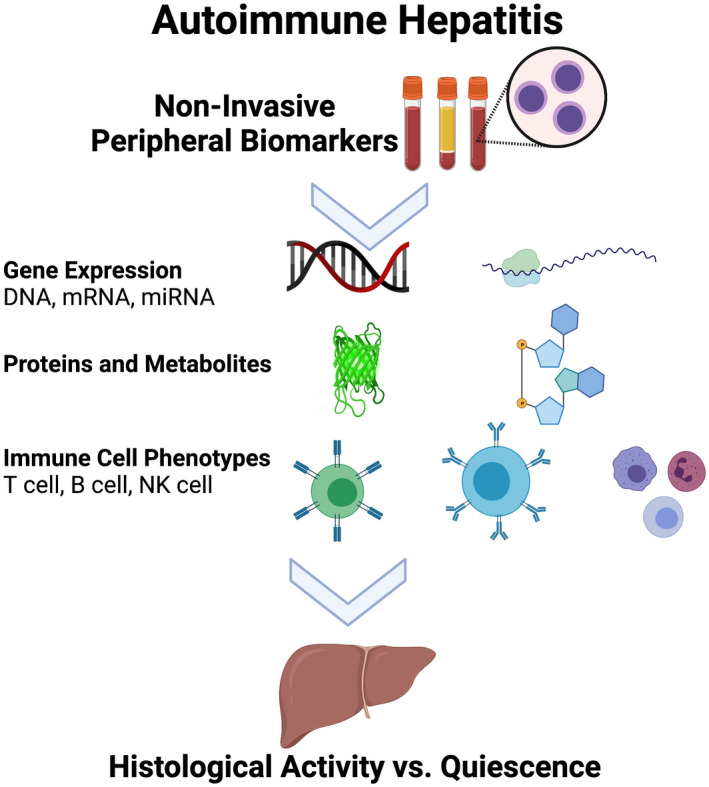
Noninvasive peripheral biomarkers in autoimmune hepatitis. Depiction of potential noninvasive peripheral biomarkers in autoimmune hepatitis that may act as surrogates for histological activity vs quiescence. miRNA, microRNA; mRNA, messenger ribonucleic acid; NK, natural killer.
AUTHOR CONTRIBUTIONS
Claire Harrington: conceptualization, investigation, writing (original draft, review & editing). Swathi Krishnan: writing (original draft, review and editing). Cara Mack: writing (original draft, review and editing). Paolo Cravedi: writing (review and editing). David N. Assis: conceptualization, writing (original draft, review and editing). Josh Levitsky: conceptualization, writing (original draft, review and editing).
FUNDING INFORMATION
None.
CONFLICTs OF INTEREST
Dr. Levitsky consults and received grants from Transplant Genomics Inc. He received grants from Novartis.
Supporting information
Figure S1
Table S1
ACKNOWLEDGMENTS
None.
Harrington C, Krishnan S, Mack CL, Cravedi P, Assis DN, Levitsky J. Noninvasive biomarkers for the diagnosis and management of autoimmune hepatitis. Hepatology. 2022;76:1862–1879. 10.1002/hep.32591
David N. Assis and Josh Levitsky are co‐senior authors.
REFERENCES
- 1. Muri Boberg K. Prevalence and epidemiology of autoimmune hepatitis. Clin Liver Dis. 2002;6:635–47. [DOI] [PubMed] [Google Scholar]
- 2. Floreani A, Restrepo‐Jiménez P, Secchi MF, De Martin S, Leung PSC, Krawitt E, et al. Etiopathogenesis of autoimmune hepatitis. J Autoimmun. 2018;95:133–43. [DOI] [PubMed] [Google Scholar]
- 3. Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli‐Vergani G, Vergani D, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):2193–213. [DOI] [PubMed] [Google Scholar]
- 4. Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72(2):671–722. [DOI] [PubMed] [Google Scholar]
- 5. Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, et al. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63(5):820–33. [PubMed] [Google Scholar]
- 6. Murray‐Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1(7806):735–7. [DOI] [PubMed] [Google Scholar]
- 7. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31(5):929–38. [DOI] [PubMed] [Google Scholar]
- 8. van Gerven NMF, Verwer BJ, Witte BI, van Hoek B, Coenraad MJ, van Erpecum KJ, et al. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58(1):141–7. [DOI] [PubMed] [Google Scholar]
- 9. European Association for the Study of the Liver . EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63(4):971–1004. [DOI] [PubMed] [Google Scholar]
- 10. Czaja AJ, Wolf AM, Baggenstoss AH. Laboratory assessment of severe chronic active liver disease during and after corticosteroid therapy: correlation of serum transaminase and gamma globulin levels with histologic features. Gastroenterology. 1981;80(4):687–92. [PubMed] [Google Scholar]
- 11. Israeli M, Klein T, Brandhorst G, Oellerich M. Confronting the challenge: individualized immune monitoring after organ transplantation using the cellular immune function assay. Clin Chim Acta. 2012;413(17–18):1374–8. [DOI] [PubMed] [Google Scholar]
- 12. FDA‐NIH Biomarker Working Group . BEST (biomarkers, endpoints, and other tools) resource. Silver Spring: Food and Drug Administration (US); 2016. Available from: https://www.ncbi.nlm.nih.gov/books/NBK326791/. Accessed 23 Sept 2021. [PubMed] [Google Scholar]
- 13. Hartl J, Ehlken H, Weiler‐Normann C, Sebode M, Kreuels B, Pannicke N, et al. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis. J Hepatol. 2015;62:642–6. [DOI] [PubMed] [Google Scholar]
- 14. Montano‐Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gasteroenterol. 2007;102(5):1005–12. [DOI] [PubMed] [Google Scholar]
- 15. van den Brand FF, Snijders RJALM, de Boer YS, Verwer BJ, van Nieuwkerk CMJ, Bloemena E, et al. Drug withdrawal in patients with autoimmune hepatitis in long‐term histological remission: a prospective observational study. Eur J Intern Med. 2021;90:30–6. [DOI] [PubMed] [Google Scholar]
- 16. Dhaliwal HK, Hoeroldt BS, Dube AK, McFarlane E, Underwood JCE, Karajeh MA, et al. Long‐term prognostic significance of persisting histological activity despite biochemical remission in autoimmune hepatitis. Am J Gastroenterol. 2015;110(7):993–9. [DOI] [PubMed] [Google Scholar]
- 17. Lüth S, Herkel J, Kanzler S, Frenzel C, Galle PR, Dienes HP, et al. Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. J Clin Gastroenterol. 2008;42(8):926–30. [DOI] [PubMed] [Google Scholar]
- 18. Muratori L, Muratori P, Lanzoni G, Ferri S, Lenzi M. Application of the 2010 American Association for the Study of Liver Diseases criteria of remission to a cohort of Italian patients with autoimmune hepatitis. Hepatology. 2010;52:1857. [DOI] [PubMed] [Google Scholar]
- 19. Deneau M, Book LS, Guthery SL, Jensen MK. Outcome after discontinuation of immunosuppression in children with autoimmune hepatitis: a population‐based study. J Pediatr. 2014;164(4):714–9.e2. [DOI] [PubMed] [Google Scholar]
- 20. Czaja A, Carpenter HA. Histological features associated with relapse after corticosteroid withdrawal in type 1 autoimmune hepatitis. Liver Int. 2003;23(2):116–23. [DOI] [PubMed] [Google Scholar]
- 21. Laschtowitz A, Zachou K, Lygoura V, Pape S, Derben F, Jaeckel E, et al. Histological activity despite normal ALT and IgG serum levels in patients with autoimmune hepatitis and cirrhosis. JHEP Rep. 2021;3(4):100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dhaliwal HK, Anderson R, Thornhill EL, Schneider S, McFarlane E, Gleeson D, et al. Clinical significance of azathioprine metabolites for the maintenance of remission in autoimmune hepatitis. Hepatology. 2012;56(4):1401–8. [DOI] [PubMed] [Google Scholar]
- 23. Heneghan MA, Allan ML, Bornstein JD, Muir AJ, Tendler DA. Utility of thiopurine methyltransferase genotyping and phenotyping, and measurement of azathioprine metabolites in the management of patients with autoimmune hepatitis. J Hepatol. 2006;45(4):584–91. [DOI] [PubMed] [Google Scholar]
- 24. Hindorf U, Jahed K, Bergquist A, Verbaan H, Prytz H, Wallerstedt S, et al. Characterisation and utility of thiopurine methyltransferase and thiopurine metabolite measurements in autoimmune hepatitis. J Hepatol. 2010;52(1):106–11. [DOI] [PubMed] [Google Scholar]
- 25. Ferucci ED, Hurlburt KJ, Mayo MJ, Livingston S, Deubner H, Gove J, et al. Azathioprine metabolite measurements are not useful in following treatment of autoimmune hepatitis in Alaska Native and other non‐Caucasian people. Can J Gastroenterol. 2011;25(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bolia R, Rajanayagam J, Hardikar W. Lower 6‐MMP/6‐TG ratio may be a therapeutic target in pediatric autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2018;67(6):695–700. [DOI] [PubMed] [Google Scholar]
- 27. Sheiko M, Sundaram SS, Capocelli KE, Pan Z, McCoy AM, Mack C. Outcomes in pediatric autoimmune hepatitis and significance of azathioprine metabolites. J Pediatr Gastroenterol Nutr. 2017;65(1):80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Candels LS, Rahim MN, Shah S, Heneghan MA. Towards personalised medicine in autoimmune hepatitis: measurement of thiopurine metabolites results in higher biochemical response rates. J Hepatol. 2021;75(2):324–32. [DOI] [PubMed] [Google Scholar]
- 29. Liberal R, Vergani D, Mieli‐Vergani G. Update on autoimmune hepatitis. J Clin Transl Hepatol. 2015;3(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Senaldi G, Portmann B, Mowat AP, Mieli‐Vergani G, Vergani D. Immuno histochemical features of the portal tract mononuclear cell infiltrate in chronic aggressive hepatitis. Arch Dis Child. 1992;67:1447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun W, Wu HY, Chen S. Influence of TBX21 T‐1993C variant on autoimmune hepatitis development by Yin‐Yang 1 binding. World J Gastroenterol. 2017;23(48):8500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–8. [DOI] [PubMed] [Google Scholar]
- 33. Sofi M, Liu Z, Zhu L, Yu Q, Kaplan MH, Chang CH. Regulation of IL‐17 expression by the developmental pathway of CD4 T cells in the thymus. Mol Immunol. 2010;47(6):1262–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ivanov II, McKenzie BS, Zhou L, Todakoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell. 2006;126(6):1121–33. [DOI] [PubMed] [Google Scholar]
- 35. Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non‐inflammatory conditions. Semin Immunol. 2011;23(6):424–30. [DOI] [PubMed] [Google Scholar]
- 36. Jeffery HC, Braitch MK, Brown S, Oo YH. Clinical potential of regulatory T cell therapy in liver diseases: an overview and current perspectives. Front Immunol. 2016;7:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Longhi MS, Ma Y, Bogdanos DP, Cheeseman P, Mieli‐Vergani G, Vergani D. Impairment of CD4(+)CD25(+) regulatory T‐cells in autoimmune liver disease. J Hepatol. 2004;41(1):31–7. [DOI] [PubMed] [Google Scholar]
- 38. Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli‐Vergani G, Vergani D, et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176(7):4484–91. [DOI] [PubMed] [Google Scholar]
- 39. Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, et al. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52(3):999–1007. [DOI] [PubMed] [Google Scholar]
- 40. Grant C, Liberal R, Holder BS, Cardone J, Ma Y, Robson SC, et al. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T‐helper type 17 cells in autoimmune hepatitis. Hepatology. 2014;59(3):1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Taubert R, Hardtke‐Wolenski M, Noyan F, Wilms A, Baumann AK, Schlue J, et al. Intrahepatic regulatory T cells in autoimmune hepatitis are associated with treatment response and depleted with current therapies. J Hepatol. 2014;61(5):1106–14. [DOI] [PubMed] [Google Scholar]
- 42. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41(4):529–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nishikawa H, Enomoto H, Iwata Y, Kishino K, Shimono Y, Hasegawa K, et al. B‐cell activating factor belonging to tumor necrosis factor family and interferon‐γ‐inducible protein‐10 in autoimmune hepatitis. Medicine (Baltimore). 2016;95(12):e3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Migita K, Abiru S, Maeda Y, Nakamura M, Komori A, Ito M, et al. Elevated serum BAFF levels in patients with autoimmune hepatitis. Hum Immunol. 2007;68(7):586–91. [DOI] [PubMed] [Google Scholar]
- 45. Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285(5425):260–3. [DOI] [PubMed] [Google Scholar]
- 46. de Boer Y, van Gerven NMF, Swiers A, Verwer BJ, van Hoek B, van Erpecum KJ, et al. Genome‐wide association study identifies variants associated with autoimmune hepatitis type 1. Gastroenterology. 2014;147(2):443–52.e5. [DOI] [PubMed] [Google Scholar]
- 47. Bovensiepen C, Schakat M, Sebode M, Zenouzi R, Hartl J, Peiseler M, et al. TNF‐producing Th1 cells are selectively expanded in liver infiltrates of patients with autoimmune hepatitis. J Immunol. 2019;203(12):3148–56. [DOI] [PubMed] [Google Scholar]
- 48. Zhao L, Tang Y, You Z, Wang Q, Liang S, Han X, et al. Interleukin‐17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic interleukin‐6 expression. PLoS One. 2011;6(4):e18909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liang M, Liwen Z, Yun Z, Yanbo D, Jianping C. The imbalance between Foxp3+ Tregs and Th1/Th17/Th22 cells in patients with newly diagnosed autoimmune hepatitis. J Immunol Res. 2018;2018:3753081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Torgutalp M, Efe C, Babaoglu H, Kav T. Relationship between serum adenosine deaminase levels and liver histology in autoimmune hepatitis. World J Gastroenterol. 2017;23(21):3876–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bayer E, Herr W, Kanzler S, Waldmann C, Meyer zum Büschenfelde KH, Dienes HP, et al. Transforming growth factor‐beta1 in autoimmune hepatitis: correlation of liver tissue expression and serum levels with disease activity. J Hepatol. 1998;28(5):803–11. [DOI] [PubMed] [Google Scholar]
- 52. Lemke D, Klement RJ, Schweiger F, Schweiger B, Spitz J. Vitamin D resistance as a possible cause of autoimmune diseases: a hypothesis confirmed by a therapeutic high‐dose vitamin D protocol. Front Immunol. 2021;12:655739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev. 2019;18(9):102350. [DOI] [PubMed] [Google Scholar]
- 54. Ruiz‐Ballesteros AI, Meza‐Meza MR, Vizmanos‐Lamotte B, Parra‐Rojas I, de la Cruz‐Mosso U. Association of vitamin D metabolism gene polymorphisms with autoimmunity: evidence in population genetic studies. Int J Mol Sci. 2020;21(24):9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Efe C, Kav T, Aydin C, Cengiz M, Imga NN, Purnak T, et al. Low serum vitamin D levels are associated with severe histological features and poor response to therapy in patients with autoimmune hepatitis. Dig Dis Sci. 2014;59(12):3035–42. [DOI] [PubMed] [Google Scholar]
- 56. Ebadi M, Bhanji RA, Mazurak VC, Lytvyak E, Mason A, Czaja AJ, et al. Severe vitamin D deficiency is a prognostic biomarker in autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49(2):173–82. [DOI] [PubMed] [Google Scholar]
- 57. Taubert R, Hardtke‐Wolenski M, Noyan F, Lalanne C, Jonigk D, Schlue J, et al. Hyperferritinemia and hypergammaglobulinemia predict the treatment response to standard therapy in autoimmune hepatitis. PLoS One. 2017;12(6):e0179074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bohne F, Martinez‐Llordella M, Lozano JJ, Miquel R, Benitez C, Londono MC, et al. Intra‐graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012;122(1):368–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liberal R, Grant CR, Holder BS, Cardone J, Martinez‐Llordella M, Ma Y, et al. In autoimmune hepatitis type 1 of the autoimmune hepatitis‐sclerosing cholangitis variant defective regulatory T‐cell responsiveness to IL‐2 results in low IL‐10 production and impaired suppression. Hepatology. 2015;62(3):863–75. [DOI] [PubMed] [Google Scholar]
- 60. Chen J, Liu W, Zhu W. Foxp3+ Treg cells are associated with pathological process of autoimmune hepatitis by activating methylation modification in autoimmune hepatitis patients. Med Sci Monit. 2019;25:6204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Longhi MS, Ma Y, Mitry RR, Bogdanos DP, Heneghan M, Cheeseman P, et al. Effect of CD4+CD25+ regulatory T cells on CD8 T cell function in patients with autoimmune hepatitis. J Autoimmun. 2005;25(1):63–71. [DOI] [PubMed] [Google Scholar]
- 62. McEachern E, Carroll AM, Fribourg M, Schiano TD, Hartzell S, Bin S, et al. Erythropoietin administration expands regulatory T cells in patients with autoimmune hepatitis. J Autoimmun. 2021;119:102629. [DOI] [PubMed] [Google Scholar]
- 63. Lim TY, Martinez‐Llordella M, Kodela E, Gray E, Heneghan MA, Sanchez‐Fueyo A. Low‐dose interleukin‐2 for refractory autoimmune hepatitis. Hepatology. 2018;68(4):1649–52. [DOI] [PubMed] [Google Scholar]
- 64. Peiseler M, Sebode M, Franke B, Wortmann F, Schwinge D, Quaas A, et al. FOXP3+ regulatory T cells in autoimmune hepatitis are fully functional and not reduced in frequency. J Hepatol. 2012;57(1):125–32. [DOI] [PubMed] [Google Scholar]
- 65. Gatselis NK, Vakrakou AG, Zachou K, Androutsakos T, Azariadis K, Hatzis G, et al. Decreased serum DNase1‐activity in patients with autoimmune liver diseases. Autoimmunity. 2017;50(2):125–32. [DOI] [PubMed] [Google Scholar]
- 66. Mitra S, Anand S, Das A, Thapa B, Chawla YK, Minz RW. A molecular marker of disease activity in autoimmune liver disease with histopathological correlation; FoXp3/RORɣt ratio. APMIS. 2015;123(11):935–44. [DOI] [PubMed] [Google Scholar]
- 67. Matsumoto K, Miyake Y, Matsushita H, Ohnishi A, Ikeda F, Shiraha H, et al. Anti‐programmed cell death‐1 antibody is a new marker for type 1 autoimmune hepatitis. J Gastroenterol Hepatol. 2014;29(1):110–5. [DOI] [PubMed] [Google Scholar]
- 68. Wafula PO, Teles A, Schumacher A, Pohl K, Yagita H, Volk HD, et al. PD‐1 but not CTLA‐4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am J Reprod Immunol. 2009;62(5):283–92. [DOI] [PubMed] [Google Scholar]
- 69. Ott P, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;21(5):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen ZX, Shao JG, Shen Y, Zhang J, Hua Y, Wang LJ, et al. Prognostic implications of antibodies to soluble liver antigen in autoimmune hepatitis: a PRISMA‐compliant meta‐analysis. Medicine (Baltimore). 2015;94(23):e953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Derben FC, Engel B, Zachou K, Hartl J, Hartleben B, Bantel H, et al. CK‐18 cell death markers improve the prediction of histological remission in autoimmune hepatitis during biochemical remission. Liver Int. 2021;41(1):123–7. [DOI] [PubMed] [Google Scholar]
- 72. Gregorio G, McFarlane B, Bracken P, Vergani D, Mieli‐Vergani G. Organ and non‐organ specific autoantibody titres and IgG levels as markers of disease activity: a longitudinal study in childhood autoimmune liver disease. Autoimmunity. 2002;35(8):515–9. [DOI] [PubMed] [Google Scholar]
- 73. Zachou K, Oikonomou K, Renaudineau Y, Chauveau A, Gatselis N, Youinou P, et al. Anti‐α actinin antibodies as new predictors of response to treatment in autoimmune hepatitis type 1. Aliment Pharmacol Ther. 2012;35(1):116–25. [DOI] [PubMed] [Google Scholar]
- 74. Treichel U, McFarlane BM, Seki T, Krawitt EL, Alessi N, Stickel F, et al. Demographics of anti‐asialoglycoprotein receptor autoantibodies in autoimmune hepatitis. Gastroenterology. 1994;107(3):799–804. [DOI] [PubMed] [Google Scholar]
- 75. Hausdorf G, Roggenbuck D, Feist E, Büttner T, Jungblut PR, Conrad K, et al. Autoantibodies to asialoglycoprotein receptors (ASGPR) measured by a novel ELISA – revival of a disease‐activity marker in autoimmune hepatitis. Clin Chim Acta. 2009;408(1–2):19–24. [DOI] [PubMed] [Google Scholar]
- 76. Assis DN, Leng L, Du X, Zhang CK, Grieb G, Merk M, et al. The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology. 2013;59(2):580–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, et al. An essential regulatory role for macrophage migration inhibitory factor in T‐cell activation. Proc Natl Acad Sci. 1996;93(15):7849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Assis DN, Takahasi H, Leng L, Zeniya M, Boyer JL, Bucala R. A macrophage migration inhibitory factor polymorphism is associated with autoimmune hepatitis severity in US and Japanese patients. Dig Dis Sci. 2016;61(12):3506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Vivarelli M, D'Urbano LE, Stringini G, Ghiggeri GM, Caridi G, Donn R, et al. Association of the macrophage migration inhibitory factor −173*C allele with childhood nephrotic syndrome. Pediatr Nephrol. 2008;23(5):743–8. [DOI] [PubMed] [Google Scholar]
- 80. Behfarjam F, Sanati MH, Nasseri Moghaddam S, Ataei M, Nikfam S, Jadali Z. Role of Th1/Th2 cells and related cytokines in autoimmune hepatitis. Turk J Gastroenterol. 2017;28:110–4. [DOI] [PubMed] [Google Scholar]
- 81. Behfarjam F, Nasseri‐Moghaddam S, Jadali Z. Enhanced Th17 responses in patients with autoimmune hepatitis. Middle East J Dig Dis. 2019;11(2):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kamijo A, Yoshizawa K, Joshita S, Yoneda S, Umemura T, Ichijo T, et al. Cytokine profiles affecting the pathogenesis of autoimmune hepatitis in Japanese patients. Hepatol Res. 2011;41(4):350–7. [DOI] [PubMed] [Google Scholar]
- 83. Landi A, Weismuller TJ, Lankisch TO, Santer DM, Tyrrell DLJ, Manns MP, et al. Differential serum levels of eosinophilic eotaxins in primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis. J Interferon Cytokine Res. 2013;34(3):204–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Abe K, Takahasi A, Imaizumi H, Hayashi M, Okai K, Kanno Y, et al. Interleukin‐21 plays a critical role in the pathogenesis and severity of type I autoimmune hepatitis. Springerplus. 2016;5(1):777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liang M, Liwen Z, Yun Z, Yanbo D, Jianping C. Serum levels of IL‐33 and correlation with IL‐4, IL‐17A, and hypergammaglobulinemia in patients with autoimmune hepatitis. Mediators Inflamm. 2018;2018:7964654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Li YL, Liu N, Zhao DT, Li ZM, Zhang HP, Liu YM, et al. Investigate circulating levels of chemokines and evaluate the correlation between these chemokines and liver function indicators in autoimmune hepatitis. Zhonghua Gan Zang Bing Za Zhi. 2013;21(4):299–303. [DOI] [PubMed] [Google Scholar]
- 87. Czaja A, Sievers C, Zein NN. Nature and behavior of serum cytokines in type 1 autoimmune hepatitis. Dig Dis Sci. 2000;45(5):1028–35. [DOI] [PubMed] [Google Scholar]
- 88. Efe C, Cengiz M, Kahramanoğlu‐Aksoy E, Yilmaz B, Özşeker B, Beyazt Y. Angiotensin‐converting enzyme for noninvasive assessment of liver fibrosis in autoimmune hepatitis. Eur J Gastroenterol Hepatol. 2015;27(6):649–54. [DOI] [PubMed] [Google Scholar]
- 89. Hartl J, Denzer U, Ehlken H, Zenouzi R, Peiseler M, Seboe M, et al. Transient elastography in autoimmune hepatitis: timing determines the impact of inflammation and fibrosis. J Hepatol. 2016;65(4):769–75. [DOI] [PubMed] [Google Scholar]
- 90. Hartl J, Ehlken H, Sebode M, Peiseler M, Krech T, Zenouzi R, et al. Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol. 2018;68(4):754–63. [DOI] [PubMed] [Google Scholar]
- 91. Park DW, Lee YJ, Chang W, Park JH, Lee KH, Kim YH, et al. Diagnostic performance of a point shear wave elastography (pSWE) for hepatic fibrosis in patients with autoimmune liver disease. PLoS One. 2019;14(3):e0212771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sokollik C, McLin VA, Vergani D, Terziroli Beretta‐Piccoli B, Mieli‐Vergani G. Juvenile autoimmune hepatitis: a comprehensive review. J Autoimmun. 2018;95:69–76. [DOI] [PubMed] [Google Scholar]
- 93. Couto CA, Bittencourt PL, Porta G, Abrantes‐Lemos CP, Carrilho FJ, Guardia BD, et al. Antismooth muscle and antiactin antibodies are indirect markers of histological and biochemical activity of autoimmune hepatitis. Hepatology. 2014;59(2):592–600. [DOI] [PubMed] [Google Scholar]
- 94. Wozniak LJ, Hickey MJ, Venick RS, Vargas JH, Farmer DG, Busuttil RW, et al. Donor‐specific HLA antibodies are associated with late allograft dysfunction after pediatric liver transplantation. Transplantation. 2015;99(7):1416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Behairy OG, Behiry EG, El Defrawy MS, El Adly AN. Diagnostic value of soluble programmed cell death protein‐1 in type‐1 autoimmune hepatitis in Egyptian children. Scand J Clin Lab Invest. 2020;80(1):59–65. [DOI] [PubMed] [Google Scholar]
- 96. Hadley T, Gillespie S, Espinoza H, Prince J, Gronbaek H, Chandrakasan S, et al. Soluble PD1 levels are increased with disease activity in paediatric onset autoimmune hepatitis and inflammatory bowel disease. Autoimmunity. 2020;53(5):253–60. [DOI] [PubMed] [Google Scholar]
- 97. Demetris AJ, Sebagh M. Plasma cell hepatitis in liver allografts: variant of rejection or autoimmune hepatitis? Liver Transpl. 2008;14(6):750–5. [DOI] [PubMed] [Google Scholar]
- 98. Demetris AJ, Bellamy C, Hübscher SG, O'Leary J, Randhawa PS, Feng S, et al. 2016 comprehensive update of the Banff Working Group on Liver Allograft Pathology: introduction of antibody‐mediated rejection. Am J Transplant. 2016;16(10):2816–35. [DOI] [PubMed] [Google Scholar]
- 99. Massoud O, Heimbach J, Viker K, Krishnan A, Poterucha J, Sanchez W, et al. Noninvasive diagnosis of acute cellular rejection in liver transplant recipients: a proteomic signature validated by enzyme‐linked immunosorbent assay. Liver Transpl. 2011;17(6):723–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fan H, Li LX, Han DD, Kou JT, Li P, He Q. Increase of peripheral Th17 lymphocytes during acute cellular rejection in liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2012;11(6):606–11. [DOI] [PubMed] [Google Scholar]
- 101. Farid WRR, Pan Q, van der Meer AJ, de Ruiter PE, Ramakrishnaiah V, de Jonge J, et al. Hepatocyte‐derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl. 2012;18(3):290–7. [DOI] [PubMed] [Google Scholar]
- 102. Shaked A, Chang BL, Barnes MR, Sayre P, Li YR, Asare S, et al. An ectopically expressed serum miRNA signature is prognostic, diagnostic, and biologically related to liver allograft rejection. Hepatology. 2017;65(1):269–80. [DOI] [PubMed] [Google Scholar]
- 103. Toby TK, Abecassis M, Kim K, Thomas PM, Fellers RT, LeDuc RD, et al. Proteoforms in peripheral blood mononuclear cells as novel rejection biomarkers in liver transplant recipients. Am J Transplant. 2017;17(9):2458–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sood S, Testro AG. Immune monitoring post liver transplant. World J Transplant. 2014;4(1):30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Modena BD, Kurian SM, Gaber LW, Waalen J, Su AI, Gelbart T, et al. Gene expression in biopsies of acute rejection and interstitial fibrosis/tubular atrophy reveals highly shared mechanisms that correlate with worse long‐term outcomes. Am J Transplant. 2016;16(7):1982–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kurian SM, Williams AN, Gelbart T, Campbell D, Mondala TS, Head SR, et al. Molecular classifiers for acute kidney transplant rejection in peripheral blood by whole genome gene expression profiling. Am J Transplant. 2014;14(5):1164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Mengel M, Sis B, Kim D, Chang J, Famulski KS, Hidalgo LG, et al. The molecular phenotype of heart transplant biopsies: relationship to histopathological and clinical variables. Am J Transplant. 2010;10(9):2105–15. [DOI] [PubMed] [Google Scholar]
- 108. Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, et al. Molecular diagnosis of antibody‐mediated rejection in human kidney transplants. Am J Transplant. 2013;13(4):971–83. [DOI] [PubMed] [Google Scholar]
- 109. Li L, Khatri P, Sigdel TK, Tran T, Ying L, Vitalone MJ, et al. A peripheral blood diagnostic test for acute rejection in renal transplantation. Am J Transplant. 2012;12(10):2710–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Friedewald JJ, Kurian SM, Heilman RL, Whisenant TC, Poggio ED, Marsh C, et al. Development and clinical validity of a novel blood‐based molecular biomarker for subclinical acute rejection following kidney transplant. Am J Transplant. 2019;19(1):98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Suthanthiran M, Schwartz JE, Ding R, Abecassis M, Dadhania D, Samstein B, et al. Urinary‐cell mRNA profile and acute cellular rejection in kidney allografts. N Engl J Med. 2013;369(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Muthukumar T, Dadhania D, Ding R, Snopkowski C, Naqvi R, Lee JB, et al. Messenger RNA for FOXP3 in the urine of renal‐allograft recipients. N Engl J Med. 2005;353(22):2342–51. [DOI] [PubMed] [Google Scholar]
- 113. Li B, Hartono C, Ding R, Sharma VK, Ramaswamy R, Qian B, et al. Noninvasive diagnosis of renal‐allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med. 2001;344(13):947–54. [DOI] [PubMed] [Google Scholar]
- 114. Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, et al. Gene‐expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med. 2010;362(20):1890–900. [DOI] [PubMed] [Google Scholar]
- 115. Rush DN, Gibson IW. Subclinical inflammation in renal transplantation. Transplantation. 2019;103:e139–45. [DOI] [PubMed] [Google Scholar]
- 116. Soma O, Hatakeyama S, Yoneyama T, Saito M, Sasaki H, Tobisawa Y, et al. Serum N‐glycan profiling can predict biopsy‐proven graft rejection after living kidney transplantation. Clin Exp Nephrol. 2019;24:174–84. [DOI] [PubMed] [Google Scholar]
- 117. Zhang W, Yi Z, Keung KL, Shang H, Wei C, Cravedi P, et al. A peripheral blood gene expression signature to diagnose subclinical acute rejection. J Am Soc Nephrol. 2019;30(8):1481–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Shaked A, DesMarais MR, Kopetskie H, Feng S, Punch JD, Levitsky J, et al. Outcomes of immunosuppression minimization and withdrawal early after liver transplantation. Am J Transplant. 2019;19(5):1397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jucaud V, Shaked A, DesMarais M, Sayre P, Feng S, Levitsky J, et al. Prevalence and impact of de novo donor‐specific antibodies during a multicenter immunosuppression withdrawal trial in adult liver transplant recipients. Hepatology. 2019;69(3):1273–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bonaccorsi‐Riani E, Pennycuick A, Londoño MC, Lozano JJ, Benítez C, Sawitzki B, et al. Molecular characterization of acute cellular rejection occurring during intentional immunosuppression withdrawal in liver transplantation. Am J Transplant. 2016;16(2):484–96. [DOI] [PubMed] [Google Scholar]
- 121. Levitsky J. Next level of immunosuppression: drug/immune monitoring. Liver Transpl. 2011;17:S60–5. [DOI] [PubMed] [Google Scholar]
- 122. Levitsky J, Asrani S, Schiano T, Moss A, Chavin K, Miller C, et al. Discovery and validation of a novel blood‐based molecular biomarker of rejection following liver transplantation. Am J Transplant. 2020;20:2173–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Levitsky J, Kandpal M, Guo K, Zhao L, Kurian S, Whisenant T, et al. Prediction of liver transplant rejection with a biologically relevant gene expression signature. Transplantation. 2021;106:1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. McGill MR, Jaeschke H. Biomarkers of drug‐induced liver injury. Adv Pharmacol. 2019;85:221–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. McGill MR, Jaeschke H. Biomarkers of drug‐induced liver injury: progress and utility in research, medicine, and regulation. Expert Rev Mol Diagn. 2018;18(9):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sebode M, Schulz L, Lohse AW. “Autoimmune(‐like)” drug and herb induced liver injury: new insights into molecular pathogenesis. Int J Mol Sci. 2017;18(9):1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lammert C, Zhu C, Lian Y, Raman I, Eckert G, Li QZ, et al. Exploratory study of autoantibody profiling in drug‐induced liver injury with an autoimmune phenotype. Hepatol Commun. 2020;4(11):1651–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Qu LM, Wang SH, Yang K, Brigstock DR, Sun L, Gao RP. CD4(+)Foxp3(+)CD25(+/−) Tregs characterize liver tissue specimens of patients suffering from drug‐induced autoimmune hepatitis: a clinical‐pathological study. Hepatobiliary Pancreat Dis Int. 2018;17(2):133–9. [DOI] [PubMed] [Google Scholar]
- 129. Zen Y, Yeh MM. Hepatotoxicity of immune checkpoint inhibitors: a histology study of seven cases in comparison with autoimmune hepatitis and idiosyncratic drug‐induced liver injury. Mod Pathol. 2018;31(6):965–73. [DOI] [PubMed] [Google Scholar]
- 130. Zen Y, Yeh MM. Checkpoint inhibitor‐induced liver injury: a novel form of liver disease emerging in the era of cancer immunotherapy. Semin Diagn Pathol. 2019;36(6):434–40. [DOI] [PubMed] [Google Scholar]
- 131. Shah P, Sundaram V, Bjornsson E. Biologic and checkpoint inhibitor‐induced liver injury: a systematic literature review. Hepatol Commun. 2020;4(2):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68(6):1181–90. [DOI] [PubMed] [Google Scholar]
- 133. Hutchinson JA, Kronenberg K, Riquelme P, Wenzel JJ, Glehr G, Schilling HL, et al. Virus‐specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis. Nat Commun. 2021;12(1):1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Dulai PS, Peyrin‐Biroulet L, Danese S, Sands BE, Dignass A, Turner D, et al. Approaches to integrating biomarkers into clinical trials and care pathways as targets for the treatment of inflammatory bowel diseases. Gastroenterology. 2019;157(4):1032–43.e1. [DOI] [PubMed] [Google Scholar]
- 135. Steiner CA, Berinstein JA, Louissaint J, Higgins PDR, Spence JR, Shannon C, et al. Biomarkers for the prediction and diagnosis of fibrostenosing Crohn's disease: a systematic review. Clin Gastroenterol Hepatol. 2022;20:817–46.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Jog NR, James JA. Biomarkers in connective tissue diseases. J Allergy Clin Immunol. 2017;140(6):1473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Merrill JT, Immermann F, Whitley M, Zhou T, Hill A, O'Toole M, et al. The biomarkers of lupus disease study: a bold approach may mitigate interference of background immunosuppressants in clinical trials. Arthritis Rheumatol. 2017;69(6):1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Lu R, Guthridge JM, Chen H, Bourn RL, Kamp S, Munroe ME, et al. Immunologic findings precede rapid lupus flare after transient steroid therapy. Sci Rep. 2019;9(1):8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Ahmed S, Cerosaletti K, James E, Long SA, Mannering S, Speake C, et al. Standardizing T‐cell biomarkers in type 1 diabetes: challenges and recent advances. Diabetes. 2019;68(7):1366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Pesenacker AM, Chen V, Gillies J, Speake C, Marwaha AK, Sun A, et al. Treg gene signatures predict and measure type 1 diabetes trajectory. JCI Insight. 2019;4(6):e123879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Beleoken E, Leh H, Arnoux A, Ducot B, Nogues C, De Martin E, et al. SPRi‐based strategy to identify specific biomarkers in systemic lupus erythematosus, rheumatoid arthritis and autoimmune hepatitis. PLoS One. 2013;8(12):e84600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Jones D, Manns MP, Hexham M, Pedrosa MC, Vierling JM. AMBER‐a novel phase 2/3 trial of ianalumab, a human anti‐BAFF receptor antibody, in autoimmune hepatitis, NCT03217422. Hepatology. 2018;68:1120A. [Google Scholar]
- 143. Chaouali M, Azaiez MB, Tezeghdenti A, Yacoubi‐Oueslati B, Ghazouani E, Kochkar R. High levels of proinflammatory cytokines, IL‐6, IL‐8, TNF‐A, IL‐23, and IFN‐A in Tunisian patients with type 1 AIH. Eur Cytokine Netw. 2020. Online ahead of print. 10.1684/ecn.2020.0450 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Table S1


