Abstract
Adipose tissue macrophages (ATMs) play key roles in metabolic inflammation, insulin resistance, adipose tissue fibrosis, and immune disorders associated with obesity. Research on ATM biology has mostly been conducted in the setting of adult obesity, since adipocyte hypertrophy is associated with a significant increase in ATM number. Signals that control ATM activation toward a proinflammatory or a proresolving phenotype also determine the developmental program and lipid metabolism of adipocytes after birth. ATMs are present at birth and actively participate in the synthesis of mediators, which induce lipolysis, mitobiogenesis, and mitochondrial uncoupling in adipocytes. ATMs in the newborn and the infant promote a lipolytic and fatty acid oxidizing adipocyte phenotype, which is essential to support the lipid‐fueled metabolism, to maintain nonshivering thermogenesis and counteract an excessive adipose tissue expansion. Since adipose tissue metabolism in the early postnatal life determines obesity status in adulthood, early‐life ATM functions may have a life‐long impact.
Keywords: inflammation, macrophage, obesity, pediatric adiposity
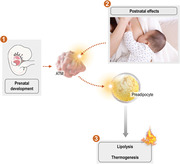
Graphical Abstract
Macrophages in the adipose tissue affect adipocyte functioning in the early life, thereby may have a long‐term impact on obesity status.
Abbreviations
- AA
arachidonic acid
- AKGs
alkylglycerols
- ATMs
adipose tissue macrophages
- LPCAT2
lysophosphatidylcholine acyltransferase 2
- NRs
nuclear receptors
- PAF
platelet activating factor
- PRRs
pathogen recognition receptors
- Rnf128
E3 ubiquitin‐protein ligase ring finger protein 128
- STAT6
signal transducer and activator of transcription 6
- TLRs
Toll‐like receptors
- UCP1
uncoupling protein 1
- VDR
vitamin D receptor
1. INTRODUCTION
Adipose tissue macrophages (ATMs) are resident immune cells of the adipose tissue and are responsible for the development of metabolic inflammation, insulin resistance, adipose tissue fibrosis, and immune disorders associated with obesity, such as diabetes and self‐immunity. 1 , 2 , 3 , 4 , 5 , 6 , 7 ATMs were first identified in the fat depots of obese mice in the 1960s; however, their presence in human adipose tissue and the central role of ATMs in obesity‐associated immune pathologies remained unnoticed until the 2000s. 8 , 9 , 10 , 11 ATMs appear in the adipose tissues of all mammalian species tested—rodents, ruminants, carnivore, and primates. 12 , 13 Adipocyte–ATM interactions have evolved in parallel with the emergence of the adipose tissue in vertebrates, suggested by the presence of ATMs in amphibia. 14 Research on ATM biology has mostly been conducted in the setting of obesity, since adipose tissue hypertrophy is associated with a significant increase in ATM number. 7 Prevalence of ATMs in the obese adipose tissue increases as a result of monocyte infiltration and local proliferation of ATMs. 14 , 15 , 16 Hypertrophic adipocytes release chemotactic and proinflammatory signals, which increase monocyte development in the bone marrow, promote monocyte and macrophage chemotaxis toward the obese fat depots, and eventually increase proinflammatory ATM activation. 8 , 17 ATMs engulf lipid overloaded and apoptotic fat cells, by forming multinucleated syncytia, so‐called crown‐like structures around the dying fat cells. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 16 , 18 , 19 Albeit apoptotic cell uptake promotes anti‐inflammatory macrophage activation in most tissues, removal of apoptotic adipocytes triggers a proinflammatory ATM activation. 20 Since ATMs are situated within a complex adipose tissue immune cell niche, built up by mast cells, T cells, and B cells, a proinflammatory ATM activation may initiate a cascade of intercellular signaling events, leading to an uncontrolled inflammation (Figure 1). The mechanisms leading to metabolic inflammation and the role of ATMs in this process have been extensively reviewed previously. 7 , 21
FIGURE 1.
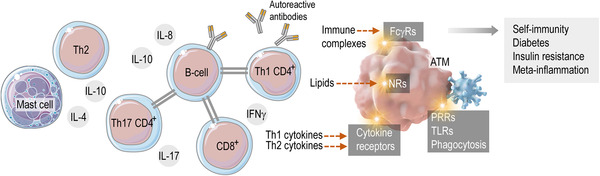
The adipose tissue‐associated immune cell niche
In adult adipose tissue, a set of immune cells build a niche through complex mutual interactions. ATMs respond with specific immune activation to various signals, such as Th1 and Th2 cytokines, lipid mediators, immune complexes, or pathogen‐derived molecules. These signals may evoke a metabolically harmful adipose tissue inflammation, leading to the production of autoreactive antibodies, Th1 cytokines or reactive oxygen species. FcγRs: Fc gamma receptors, NRs: nuclear receptors, PRRs: pathogen recognition receptors, TLRs: Toll‐like receptors. Modified from Refs. 20 and 22.
ATMs are however not only triggers of metabolic inflammation. Indeed, ATMs and several proinflammatory signal mechanisms are required for physiologic adipose tissue development. 23 , 24 There is evidence that ATMs stimulate thermogenic and fat catabolizing adipocyte activities after birth, and ablation of ATMs in newborn mice leads to the loss of thermogenic fat cells in the subcutaneous fat depot. 13 Importantly, ATMs are already present in the fat depots after birth, 13 since the first wave of ATMs develops from embryonic macrophage progenitors. 14 Adipose tissue development in the first year of life is key to determine obesity as an adult. 25 Increased body weight at 3–6 months of age, moreover an increased rate of body weight gain or overweight at the first year of life increase the probability of obesity as a young adult. 26 , 27 , 28 , 29 Similarly, increased adiposity before 5.5 years of age is a predictor of obesity and obesity‐associated diseases in adulthood. 30 , 31 , 32 , 33 , 34 , 35 Mechanisms that control adipose tissue mass in the newborn and in infancy are hence key determinants of obesity and obesity associated diseases. In adult‐onset obesity, the role of ATMs in triggering obesity‐associated diseases has already been established. However, the role of ATMs in the early postnatal adipose tissue development is still largely unexplored. This review provides an update on the possible metabolic roles of ATMs in the early postnatal life.
2. ADIPOSE TISSUE DEVELOPMENT AND FAT METABOLISM IN THE EARLY POSTNATAL LIFE
Carbohydrates are the key fuels of the fetal metabolism during the intrauterine life. Progenitors of the lipid storing, so‐called white adipocytes develop from the lateral plate mesoderm, while lipid oxidizing, so‐called brown adipocytes are descendants of paraxial mesodermal progenitors and in lesser extent of cells derived from the neural crest (Figure 2). 36 , 37 , 38 Fetal ATMs develop from hematopoietic cells of the yolk sac and persist in the newborn 14 (Table 1). Despite the early emergence of the adipocyte progenitors and the ATMs, the adipose tissue begins to expand relatively late: in humans, fat depots develop in the last trimester, using maternal ketone bodies and glucose as lipogenic substrates. 39 , 40 , 41 , 42 , 43 , 44 , 45 In rodents—the most studied animal models of human obesity—the expansion of the fat depots begins after birth, with the exception of the interscapular brown adipose tissue, which is already present at birth. 43 At birth, there is a rapid lipolysis with the release of glycerol and free fatty acid from the subcutaneous adipose tissue depot. 39 , 46 This is concomitant with a metabolic shift from carbohydrate‐dependent energy production to a lipid‐rich nutrition provided by breastfeeding. Breast milk is rich in lipids, of which 85–90% are absorbed by a term infant, and lipid digestion begins in the buccal cavity in the newborn. 41 , 47 The plasma lipid profile of a breastfed infant or a suckling rodent reflects the lipid composition of the breast milk, 48 , 49 and also the maternal adipose tissue and plasma lipids. 50 , 51
FIGURE 2.
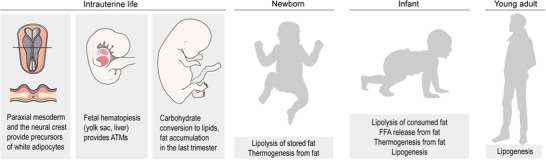
Lipid metabolism in the intrauterine and in the postnatal life
TABLE 1.
Key traits of adipose tissue macrophages in the newborn mouse
| Expression of hematopoietic lineage markers 14 | CD45+, Kitlow, CX3CR1bright, CD115+, F4/80bright |
|---|---|
| Expression of cell cycle associated proteins 19 | Ki67+, MafBlow |
| Expression of lipid metabolizing enzymes 13 | AGMO–, LPCAT2+ |
| Expression of macrophage activation markers 13 , 19 | MHC‐IIlow / high, prone to release PAF and IL‐6 |
The fetus develops in the thermally stable womb; however, it enters a hypothermic environment at birth. Therefore, there is a large energy demand of the newborn to sustain its core body temperature. 52 Nonshivering thermogenesis is important to maintain the core body temperature of the newborn, utilizing the uncoupling of mitochondrial oxidative respiration to generate heat. 52 The substrate of heat produced in a human term infant is mostly fat, 46 , 53 giving importance to the thermogenic potential of the adipose tissue.
Metabolic performance of the adipose tissue of the newborn and the infant reflects distinct physiologic demands (Figure 2). At birth, the adipose tissue serves as an energy reserve, and a rapid lipolysis provides free fatty acids for energy and heat production. 46 This is followed by an increasing fatty acid synthesis and lipogenesis from stored glycogen to avoid the depletion of fat reserves. 54 , 55 During infancy, glycerol is converted to glucose, and free fatty acids are oxidized or re‐esterified in the adipose tissue, 56 with an ongoing fat catabolism to generate energy and heat. 55 ATMs are present at birth, and they retain the ability of self‐replenishment. 14 , 19 Later in infancy, these fetal ATMs are accompanied—and plausibly gradually replaced—by monocyte‐derived ATMs (reviewed in Ref. 57). Interestingly, the interscapular brown adipose tissue, the largest thermogenic fat depot in rodents, is scarce in macrophages. 14 , 58
Adipocyte progenitors develop from the paraxial mesoderm and in a lesser extent from the neural crest. ATM precursors originate from the yolk sac hematopoietic tissue. The last trimester is associated with the expansion of the fat depots—especially of the subcutaneous depot. After birth, the fat reserves are used to generate energy and heat by a rapid lipolysis and uncoupled mitochondrial respiration. In infancy, the fat depots are expanding further, using nutritional lipids as main lipogenic substances. The infant adipose tissue maintains an active oxidative metabolism and generates heat. In adults, these functions are lacking, and the adipose tissue accumulates lipids as an energy reserve and thermal insulator.
3. Th2 CYTOKINE SIGNALING IN THE INFANT ADIPOSE TISSUE
One important signal, which appears in the immune cell niche of the adipose tissue, is IL‐4. It is a Th2 cytokine, with proresolving properties, and it triggers an anti‐inflammatory (often called as M2) macrophage polarization by stimulating STAT6 signaling. Inflammatory milieu in the obese adipose tissue, along with the proinflammatory activation of ATMs, is a key driver of insulin resistance. 7 Therefore, a proresolving IL‐4 signal may help to restore insulin sensitivity by mitigating adipose tissue inflammation. 59 IL‐4 stimulates the accumulation of anti‐inflammatory ATMs in the adipose tissue, as a result of an increased proliferation of ATMs and a polarization of ATMs toward a proresolving activation state. 19 , 60 While an expanding M2 ATM pool may resolve adipose tissue inflammation, it also promotes fibrosis and worsens adipose tissue function. 61 Moreover, an excessive increase of M2 ATM number is impeded by innate lymphoid cells, 62 and by various negative feedback mechanisms, 63 including IL‐4/STAT6 signaling itself. 64
In addition to its immunomodulatory role, IL‐4 has direct effects on adipocyte differentiation and lipid handling. IL‐4 inhibits adipogenesis and activates lipolysis by hormone sensitive lipase, eventually decreasing lipid deposits. 65 The signal transducer responsible for this effect is the cyclic AMP/protein kinase A pathway, the major route leading to lipolysis. Moreover, IL‐4 stimulates uncoupling protein 1 (UCP1) expression in adipocytes. UCP1+ adipocytes have thermogenic potential and dissipate energy as heat by uncoupled mitochondrial respiration. Unlike primates, rodents—due to their need of excess heat production—have large interscapular thermogenic fat depots, often described as classical brown adipose tissue. In cold‐adapted adult animals, the fat storing subcutaneous white adipose tissue depots are enriched in thermogenic fat cells, resembling cells of the classical brown adipose tissue. 66 The development of these thermogenic fat cells is hence often termed as adipose tissue browning. Thermogenic fat within a white adipose tissue depot are described as beige adipocytes, to distinguish them from the classical brown adipocytes. 66 Of note, the ontogeny of beige and brown adipocytes is distinct in mouse, and it is still a subject of debate whether humans have brown or beige adipocytes in their thermogenic fat depots. These aspects of thermogenic adipose tissue development are discussed elsewhere. 55 Nevertheless, the classical brown adipose tissue and the beige adipocyte‐containing adipose tissue have lipolytic and fatty acid oxidizing activity, along with the ability of producing heat in uncoupled mitochondrial respiration. These metabolic traits of the beige adipocytes allow burning off stored lipids as heat, hence supporting both adaptive thermogenesis and reducing fat mass. The latter effect is considered as a possible tool to reduce excessive fat accumulation and obesity. 67
Mast cells are resident immune cells in the adipose tissue. 68 They release IL‐4 in response to cold stress, which in turn stimulates UCP1 expression in adipocytes, promoting adipose tissue browning and ultimately reduces fat mass. 5 , 69 IL‐4 also increases the prevalence of M2 ATMs in the adipose tissue, and M2 macrophages are thought to increase adipose tissue browning. 5 , 70 For instance, the MRL/lpr mouse, which is a genetic model of a generalized autoimmune disease, displays increased adipose tissue browning. 71 This is plausibly due to their increased systemic IL‐4 levels. 71 In addition to M2 polarization, cold stress also increases M2 macrophage content in the adipose tissue via macrophage proliferation. 70 Some inflammatory signals inhibit beige adipogenesis; hence, an anti‐inflammatory IL‐4 effect may favor beige adipogenesis secondarily. 5 , 72 , 73 , 74
Since cold stress induces IL‐4 synthesis in the adipose tissue and IL‐4 triggers M2 macrophage activation, it was initially thought that M2 macrophage activation was key for beige adipocyte development. It is however plausible that M2 ATMs are not crucial for beige adipocyte development (reviewed in Ref. 59). For instance, a recent study suggests that IL‐10, a Th2 cytokine associated with M2 macrophages, acts against beige adipogenesis, and accordingly, mice deficient in IL‐10 signaling have increased adipose tissue thermogenesis. 75 In human adipose tissue, IL‐10 gene expression and protein secretion correlate positively with body mass index and insulin resistance. 76 , 77 The expression of IL‐10 and IL‐10 receptor alpha is significantly enriched in proinflammatory M1 macrophages. Recombinant IL‐10 has no effect on human adipocyte phenotype in vitro, albeit it induces an anti‐inflammatory profile in ATMs and fat‐derived leukocytes. 77 On the other hand, beige adipocyte development in the MRL/lpr mouse is thought to be mediated by IL‐10, and IL‐10 deficiency leads to newborn cold intolerance and impaired UCP1‐dependent brown adipose tissue mitochondrial respiration in mice. 78 It is plausible that IL‐10 plays distinct—actually opposite—roles in classical brown adipocytes and in beige adipocytes of the infant adipose tissue.
In newborns, there are prevalent thermogenic—plausibly beige—adipocyte pools within the subcutaneous fat depots, allowing nonshivering thermogenesis and supporting the core body temperature of the infant in a hypothermic environment. 55 In newborns, a Th2 immune response is more dominant than in adults. For instance, the newborn thymus is abundant in IL‐4+ thymocytes. These cells are IL‐4+/CD4+ T cells and are most likely originate from CD31+/CD4+ thymic naïve T cells. 79 These cells are far more abundant in neonates than in adults; however, IL‐4 secretion from neonatal T cells requires a so far unidentified trigger. 79
A short and transient IL‐4 exposure in neonate rats up‐regulates Ucp1 mRNA expression and decreases fat cell size in the subcutaneous white adipose tissue. 80 Animals treated with IL‐4 in their neonate life have decreased adiponectin (Adipoq) expression in the adipose tissue. Thus, neonatal IL‐4 induces lipolysis and decreases adipogenic differentiation capacity and may induce beige adipocyte development. 80 However, mRNA transcription profiling of the infant (postnatal day 6) subcutaneous adipose tissue in mouse shows the suppression of IL‐4 signaling. 43 The mRNA level of Il4ra is lower in the infant adipose tissue than in its adult counterpart. Conversely, the infant fat expresses high levels of Rnf128, encoding a ubiquitin ligase that inactivates STAT6 signaling. 43 Moreover, a study shows that abdominal circumference of human newborns positively correlates with the plasma levels of IL‐10 and IL‐4. 76 Moreover, monocyte‐derived macrophages from obese newborns show a basal anti‐inflammatory phenotype. 81 Macrophages from obese newborns had increased levels of Tnfa, Il4ra, and Il10 mRNA levels and failed to express IL‐10 properly in response to an M2 stimulus. 81
ATM progenitors are established before birth; therefore, intrauterine signals may affect ATM number or ATM activation at birth (①). In the early postnatal life, ATMs receive signals from the breast milk—and from immune cells and apoptotic cell contents—and transmit these signals to the developing preadipocytes (②). In the newborn and in the infant, the key metabolic traits of adipocytes are lipolysis, fatty acid oxidation, and thermogenesis: all of which have been shown to be stimulated by ATM‐derived signals, such as IL‐4, IL‐6 and various lipid metabolites (③).
4. IL‐6 AND STAT3 SIGNALING IN THE INFANT ADIPOSE TISSUE
Both adult and pediatric obesity is associated with an increased plasma level of IL‐6 82 and there is an association between increased fetal adiposity and maternal systemic IL‐6 levels. 83 Albeit it is not studied, it is plausible that intrauterine cytokine signals may affect prenatal ATM development, hence might determine the ATM‐dependent control of adipocyte functions after birth (Figure 3). In the obese adipose tissue, IL‐6 is associated with a proinflammatory state, which deteriorates insulin sensitivity and provokes obesity‐associated morbidities. 7 However, there is a lack of correlation between obesity parameters and IL6 polymorphisms in human, 84 and the lack of Il6 increases obesity development in mouse. 85 Moreover, IL‐6 stimulates adipocyte lipolysis, fatty acid oxidation, and mitobiogenesis, hence promotes beige adipocyte development. 13 , 86 , 87
FIGURE 3.
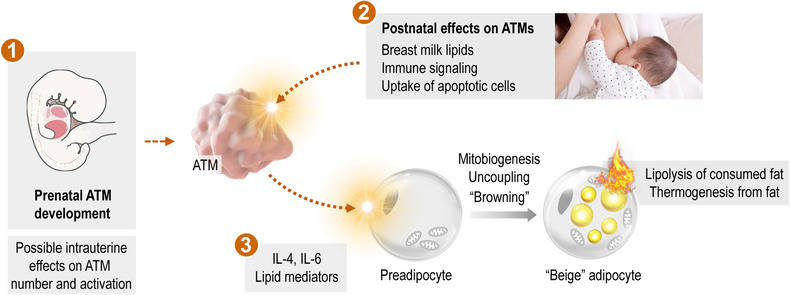
Signals potentially affecting ATM functions in the early postnatal life
The prevailing view that anti‐inflammatory ATM activation is beneficial for adipose tissue function and supports thermogenic fat differentiation is challenged by the role of inflammatory signals in the early postnatal fat development. 23 , 24 In the newborn, IL‐6 and further Th1‐associated cytokines and proinflammatory IFNs are required for physiologic adipose tissue development. 23 , 24 , 88 Both immune cells and preadipocytes are possible sources of IL‐6 in the adipose tissue, and obesity is associated with an increasing IL‐6 secretion from the adipose tissue. Notably however, preadipocytes secrete more IL‐6 in vitro than the fully differentiated adipocytes. 89 This may be an indication of a role of autocrine IL‐6 signaling in the early adipocyte differentiation. However, expression of Il6 mRNA is similar in the subcutaneous adipose tissue of infant and adult mice, 43 and proinflammatory cytokine secretion is a trait of mature and obese adipocytes. 90 This makes plausible that the reduced IL‐6 secretion from in vitro differentiated adipocytes may be due to the inhibition of IL‐6 production by rosiglitazone 91 and dexamethasone, 92 which are routinely used compounds to trigger adipocyte differentiation in vitro. Since these ligands activate peroxisome proliferator activated receptor gamma (PPARγ)‐ and glucocorticoid receptor‐controlled gene expression, they potently inhibit inflammatory cytokine expression. This makes challenging to discern changes in cytokine expression associated specifically with adipocyte maturation.
IL‐6 signaling promotes thermogenic adipocyte development through JAK2/STAT3 pathway, 13 , 93 which is especially relevant in the subcutaneous fat depots of infants. 13 Breast milk‐derived metabolites stimulate IL‐6 production by ATMs, which eventually activates beige adipocyte development through STAT3 signaling. 13 Gene expression of the signal pathway necessary for the IL‐6‐mediated beige adipocyte development is higher in the adipose tissue of newborn than in adult mice. 43 Human neonate monocytes are more prone to release IL‐6 in response to stimuli than their adult counterparts. 92 Spontaneous IL‐6 release is more potently inhibited in adult monocytes by glucocorticoids than in adult monocytes. 92 The human neonatal cord blood immune cells respond to multiple TLR agonists with a prominent IL‐6 and TNFα burst. Similarly, serum collected from newborns during the first week of life have IL‐6 and TNFα ratios higher than does cord blood, associated with increased levels of IL‐6‐inducible acute phase molecules in the first days of life. 94 This makes plausible that IL‐6 is a stimulus of lipolysis and thermogenic fat differentiation in the early postnatal life (Figure 3).
5. LIPID SIGNALS AFFECTING ATMs IN THE INFANT ADIPOSE TISSUE
Lipid species, which are mostly supplied by diet, effectively control lipid metabolism and the immune functioning of ATMs. 95 , 96 , 97 Lipid metabolites, lipid mediators, and lipid soluble vitamins activate nuclear receptors and may trigger inflammation or in turn, may help to resolve inflammation. 7 , 98 , 99 , 100 Apoptotic adipocytes contain various metabolites, including lipid species, which shape macrophage activation (reviewed in Ref. 20). Some immune regulator lipid species are accumulated in the last trimester within the subcutaneous adipose tissue depots. Subcutaneous fat is sensitive to gestational age, 101 hence preterm infants have deficient development of these fat depots, and eventually, have altered bioavailability of some lipid species. 102 For instance, arachidonic acid (AA) and docosahexaenoic acid (DHA) are relevant determinants of adipocyte development, fat‐derived thermogenesis, and adipose tissue inflammation. 102 , 103 Adipose tissue pools of AA and DHA are built up in term infants during the third trimester, stored as adipose tissue triglycerides and predominantly distributed via plasma phosphatidylcholine. 102 After birth, there is an increased lipolysis, accompanied by free fatty acid release and a concomitant re‐esterification of fatty acids into triacylglycerols. Lipolysis increases macrophage recruitment to the adipose tissue 104 and fasting increases cyclooxygenase 1 (COX1) expression, and eventually stimulates prostaglandin E2 (PGE2) biosynthesis from AA. 104 Dietary supplementation of AA during the suckling period increases prostaglandin levels in adipose tissue in guinea pigs. 105 While AA blocks macrophage proliferation by inducing an S‐phase blockage, 106 PGE2 stimulates macrophage migration, and hence may be responsible for lipolysis‐associated enrichment of macrophages in the adipose tissue. 104 AA exerts proinflammatory effects, while PGE2 has an inflammation suppressive effect in the adipose tissue. 104 , 107 Accordingly, plasma level of AA is an important determinant of metabolic diseases associated with childhood obesity. Mean plasma levels of AA, dihomo‐gamma‐linolenic acid and DHA are higher in overweight and obese children, 108 and AA level positively correlates with indicators of insulin resistance and loss of bone mass. 109 However, AA has sex‐dependent metabolic effects, 110 and AA supplementation does not influence early fat mass development in the guinea pig. 105
Similarly, vitamin D, which is a ligand of the immune regulator vitamin D receptor (VDR), is accumulated in the fat depots before birth. 111 Vitamin D deficiency is prevalent among obese children and adolescents and is a risk factor for metabolic diseases, 112 albeit overexpression of VDR promotes weight gain in mouse. 113 Insufficient vitamin D supply in early postnatal life is associated with increased risk of diabetes development in adulthood. 111 Vitamin A, retinoids, and carotenoids also accumulate in the adipose tissue of the infant, 114 and vitamin A metabolites are important immune regulators, which shape macrophage functions and mitigate obesity (reviewed in 114 , 115 ). Breast milk is a natural source of retinoids, and preparation for lactation is associated with a temporal increase of maternal vitamin A pools. 116 A 6‐month long breastfeeding is estimated to transfer the amount of vitamin A that is in the range of causing acute vitamin A toxicity in an adult. 116 Breastfeeding thus provides sufficient vitamin A to the infant and also reduces potentially toxic concentrations of retinoid pools in the lactating mother. 116 However, breast milk from obese mothers have decreased concentrations of carotenoids along with a proinflammatory fatty acid profile. 117 In suckling rats, vitamin A supplementation supports the development of thermogenic fat mass and protects from excess adipose tissue expansion, 118 and carotenoids have protective effects against obesity and increase energy dissipation by adipocytes. 114
Moreover, early postnatal life is the peak of dietary fat intake, and breast milk‐derived lipid species accumulate in the adipose tissue of the newborn. For instance, fatty acid composition of the brown adipose tissue in suckling newborn rats correlates with the fatty acid composition of the rat milk. 49 There is a change from mainly saturated to a greater proportion of unsaturated fatty acids in the brown adipose tissue in newborn rats, which occurs just after the first suckling. 49 Similarly, maternal plasma lipid composition is mirrored by the adipose tissue lipid species in the human neonate. 50 , 51 Effects of dietary lipids are mostly studied in the context of obesity in adulthood, and we know much less about the signaling role of dietary lipids after birth. 55 Breast milk is rich in lipids, and beyond supplying energy rich nutrients, breast milk lipids also function as mediators and immune modulators. 119 We have shown recently that breast milk‐specific alkylglycerol (AKG)‐type ether lipids are metabolized by ATMs in the infant adipose tissue to platelet‐activating factor (PAF). 13 ATMs in the newborn mouse express lysophosphatidylcholine acyltransferase 2 (LPCAT2), which converts AKGs into PAF and lacks the AKG degrading enzyme, AKG‐monooxygenase (AGMO) (Table 1). Accordingly, alkyldiacylglcyerols and alkenylphosphatidylethanolamine are enriched in the adipose tissue of breastfed infants. 120 After the first year of age, the adipose tissue level of the AKG‐related lipid species does not correlate with the length of breastfeeding, 120 and the adult adipose tissue expresses AKG monooxygenase, which breaks down AKGs to free fatty acids. 13 AKGs are lacking from cow milk‐based infant formula 13 , 121 and the lack of AKG intake in the early postnatal life may increase the risk obesity. 13 PAF stimulates IL‐6 release from adipocytes, and PAF is nonenzymatic converted into a PPARγ activating ether lipid—both signals stimulate thermogenic fat differentiation. 13 Similarly, further breast milk‐derived lipids, such as the 12,13‐dihydroxy‐9Z‐octadecenoic acid have the potential to control early adipocyte development, albeit the underlying mechanisms are still to be understood. 122 Further metabolites—other than lipids—may induce ATM activation, as reviewed before. 20 For instance, hyperglycemia sustains a proinflammatory macrophage activation, 123 increases sensitivity of macrophages to proinflammatory signals, and reduces their phagocytic capacity. 124 Intriguingly, newborn infants may develop hyperglycemia without having diabetes or insulin resistance, and hyperglycemic events may have their impact on ATMs as well. Moreover, intrauterine hyperglycemia increases the development of pediatric obesity, 125 and obese children have an increased risk of hyperglycemia. 126 Nutritional status and whole‐body metabolism have their specific impact on immune cell functions (reviewed in Ref. 127). It has been extensively studied how bioactive molecules of diet determine macrophage functions 128 , 129 and eventually, diet may induce epigenetic modifications, which affect metabolism in the offspring. 130 In turn, macrophage breakdown and synthesis of lipids determines inflammation. 131 , 132 Early‐life metabolic imprinting by lipid metabolites and glucose thereby potentially affects ATM phenotype and may account for the immune component of childhood obesity.
6. CONCLUDING REMARKS
Development of the adipose tissue in infancy has late‐acting impact on obesity status and metabolic health. This makes important to understand signals that determine adipocyte development in the early postnatal life, and the number and activation state of ATMs may serve as early diagnostic or prognostic marker for pediatric obesity. Molecular characteristics of ATMs in the newborn are however still incompletely explored (Table 1). A more detailed characterization of ATM activation state in infancy and childhood may help to understand better the association of genetics, nutrition, and comorbidities with pediatric obesity.
ATMs play key roles in obesity‐associated immune disorders in adults; however, the impact of ATMs in the early life determination of adiposity is still largely unexplored. For instance, we lack studies on the transcriptional landscape, expression of M1/M2 markers and lipidomic profile of ATMs during infancy and childhood. We lack information on the impact of prenatal factors (i.e., maternal obesity or diabetes) on ATM number and activation state in the offspring. What is an upcoming challenge in the field of pediatrics is to define early diagnostic and prognostic markers for childhood obesity. Isolation and flow cytometry or single cell sequencing of ATMs are established techniques today. However, analysis of ATMs is still not a routine diagnostic approach, despite the access to adipose tissue specimens is relatively simple during a wide range of elective surgeries in pediatric patients. For instance, approximately 2–8% of male infants are affected by cryptorchidism, 133 1–6% of infants and children may develop inguinal hernia, 134 and infections in the first year of life often lead to the development of anal abscesses and fistulas. 135 When these conditions require surgical repair, there is an inevitable removal of small volumes of adipose tissue from the inguinal canal, the subcutaneous fat layer of the groin region, or the fat pad of the ischiorectal fossa, respectively. Since these fat depots are present at birth and remain persistent throughout life, they offer the possibility of studying ATM ontogeny and describing changes in ATM number or phenotype in course of postnatal development. In infants and preschool children, these fat specimens may be used for quantifying ATM number (routine histology), measuring mRNA levels (single cell sequencing) or by assessing ATM activation state (flow cytometry). Such analyses may catalyze basic research in ATM biology, and eventually might emerge as diagnostic tools for the early identification of obesity risk factors, and hence, increase obesity prevention among children.
Signals that control ATM activation toward a proinflammatory or a proresolving phenotype also determine the developmental program and lipid metabolism of adipocytes. ATMs in the newborn express mediators that promote a lipolytic and fatty acid oxidizing adipocyte functioning. These effects of ATMs support the proper utilization of stored lipids after birth and the catabolism of a lipid‐rich diet to provide energy and heat. Eventually, ATMs counteract the excessive adipose tissue expansion in the early postnatal life. ATM functions in the newborn adipose tissue thus may have a life‐long impact by setting adiposity status and metabolic health. Moreover, signals that control ATMs in the newborn may be exploited as novel targets in the therapy of obesity and support fat catabolism and energy expenditure.
AUTHORSHIP
Conceptualization and writing: T. R.
ACKNOWLEDGMENTS
Figures have been created by using and modifying artworks from Servier Medical Art and Dreamstime Stock Images.
Röszer T. Metabolic impact of adipose tissue macrophages in the early postnatal life. J Leukoc Biol. 2022;112:1515–1524. 10.1002/JLB.3MR0722-201R
Summary Sentence: Macrophages in the adipose tissue affect adipocyte functioning in the early life, which may have a long‐term impact on obesity status.
REFERENCES
- 1. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Osborn O, Olefsky JM. The cellular and signaling networks linking the immune system and metabolism in disease. Nat Med. 2012;18:363‐74. [DOI] [PubMed] [Google Scholar]
- 3. Lee BC, Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity‐induced insulin resistance. Biochim Biophys Acta. 2014;1842:446‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qiu Y, Nguyen KD, Odegaard JI, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li P, Spann NJ, Kaikkonen MU, et al. NCoR repression of LXRs restricts macrophage biosynthesis of insulin‐sensitizing omega 3 fatty acids. Cell. 2013;155:200‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boutens L, Stienstra R. Adipose tissue macrophages: going off track during obesity. Diabetologia. 2016;59:879‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity‐related insulin resistance. J Clin Invest. 2003;112:1821‐1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ampem G, Azegrouz H, Bacsadi A, et al. Adipose tissue macrophages in non‐rodent mammals: a comparative study. Cell Tissue Res. 2016;363:461‐78. [DOI] [PubMed] [Google Scholar]
- 13. Yu H, Dilbaz S, Coßmann J, et al. Breast milk alkylglycerols sustain beige adipocytes through adipose tissue macrophages. J Clin Invest. 2019;129:2485‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waqas SFH, Noble A, Hoang A, et al. Adipose tissue macrophages develop from bone marrow‐independent progenitors in Xenopus laevis and mouse. J Leukocyte Biol. 2017;102:845‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haase J, Weyer U, Immig K, et al. Local proliferation of macrophages in adipose tissue during obesity‐induced inflammation. Diabetologia. 2014;57:562‐71. [DOI] [PubMed] [Google Scholar]
- 16. Amano SU, Cohen JL, Vangala P, et al. Local proliferation of macrophages contributes to obesity‐associated adipose tissue inflammation. Cell Metab. 2014;19:162‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagareddy PR, Kraakman M, Masters SL, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19:821‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuentes L, Röszer T, Ricote M. Inflammatory mediators and insulin resistance in obesity: role of nuclear receptor signaling in macrophages. Mediators Inflamm. 2010;2010:219583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waqas SFH, Hoang A, Lin Y, Ampem G, et al. Neuropeptide FF increases M2 activation and self‐renewal of adipose tissue macrophages. J Clin Invest. 2017;127:2842‐2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Röszer T. Adipose Tissue Immunometabolism and Apoptotic Cell Clearance. Cells. 2021;10:2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coats BR, Schoenfelt KQ, Barbosa‐Lorenzi VC, et al. Metabolically activated adipose tissue macrophages perform detrimental and beneficial functions during diet‐induced obesity. Cell Rep. 2017;20:3149‐3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Winer D, Winer S, Ch'ng M, Shen L, Engleman E, (2013) B Lymphocytes in obesity‐related adipose tissue inflammation and insulin resistance. Cellular and molecular life sciences. CMLS. 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Babaei R, Schuster M, Meln I, et al. Jak‐TGFβ cross‐talk links transient adipose tissue inflammation to beige adipogenesis. Sci Signal. 2018;11. [DOI] [PubMed] [Google Scholar]
- 24. Sun K, Gao Z, Kolonin MG. Transient inflammatory signaling promotes beige adipogenesis. Sci Signal. 2018;11:eaat3192. [DOI] [PubMed] [Google Scholar]
- 25. Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59:955‐9. [DOI] [PubMed] [Google Scholar]
- 26. Landgraf K, Rockstroh D, Wagner IV, et al. Evidence of early alterations in adipose tissue biology and function and its association with obesity‐related inflammation and insulin resistance in children. Diabetes. 2015;64:1249‐1261. [DOI] [PubMed] [Google Scholar]
- 27. Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379:1303‐1312. [DOI] [PubMed] [Google Scholar]
- 28. Fall C. Developmental origins of cardiovascular disease, type 2 diabetes and obesity in humans. In, Wintour‐Coghlan Marelyn and Owens, Julie (eds.) Advances in Experimental Medicine and Biology. Vol. 573. 2006:8‐28; Springer. [Google Scholar]
- 29. Charney E, Goodman HC, McBride M, Lyon B, Pratt R. Childhood antecedents of adult obesity. Do chubby infants become obese adults? N Engl J Med. 1976;295:6‐9. [DOI] [PubMed] [Google Scholar]
- 30. Rolland‐Cachera MF, Deheeger M, Bellisle F, Sempé M, Guilloud‐Bataille M, Patois E. Adiposity rebound in children: a simple indicator for predicting obesity. Am J Clin Nutr. 1984;39:129‐35. [DOI] [PubMed] [Google Scholar]
- 31. Eriksson JG, Forsén T, Tuomilehto J, Osmond C, Barker DJ. Early adiposity rebound in childhood and risk of Type 2 diabetes in adult life. Diabetologia. 2003;46:190‐4. [DOI] [PubMed] [Google Scholar]
- 32. Siervogel RM, Roche AF, Guo SM, Mukherjee D, Chumlea WC. Patterns of change in weight/stature2 from 2 to 18 years: findings from long‐term serial data for children in the Fels longitudinal growth study. Int J Obes. 1991;15:479‐85. [PubMed] [Google Scholar]
- 33. Pietrobelli A, Agosti M, the MeNu G. Nutrition in the First 1000 Days: ten Practices to Minimize Obesity Emerging from Published Science. Int J Environ Res Public Health. 2017;14:1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carolan‐Olah M, Duarte‐Gardea M, Lechuga J. A critical review: early life nutrition and prenatal programming for adult disease. J Clin Nurs. 2015;24:3716‐3729. [DOI] [PubMed] [Google Scholar]
- 35. Geserick M, Vogel M, Gausche R, et al. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N Engl J Med. 2018;379:1303‐1312. [DOI] [PubMed] [Google Scholar]
- 36. Sanchez‐Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta. 2014;1842:340‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Billon N, Iannarelli P, Monteiro MC, et al. The generation of adipocytes by the neural crest. Development. 2007;134:2283‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fu M, Xu L, Chen X, et al. Neural crest cells differentiate into brown adipocytes and contribute to periaortic arch adipose tissue formation. Arterioscler Thromb Vasc Biol. 2019;39:1629‐1644. [DOI] [PubMed] [Google Scholar]
- 39. Herrera E, Amusquivar E. Lipid metabolism in the fetus and the newborn. Diabetes Metab Res Rev. 2000;16:202‐10. [DOI] [PubMed] [Google Scholar]
- 40. Van Aerde JE, Wilke MS, Feldman M, Clandinin MT. Chapter 40 ‐ Accretion of lipid in the fetus and newborn. In: Polin RA, Fox WW, and Abman SH, eds. Fetal and Neonatal Physiology. 3rd ed. W.B. Saunders; 2004:388‐404. [Google Scholar]
- 41. Whyte RK, Bayley HS. Energy Metabolism of the Newborn Infant. In: Draper HH, ed. Advances in nutritional research. Springer US; 1990:79‐108. [DOI] [PubMed] [Google Scholar]
- 42. Farkas V, Kelenyi G, Sandor A. A dramatic accumulation of glycogen in the brown adipose tissue of rats following recovery from cold exposure. Arch Biochem Biophys. 1999;365:54‐61. [DOI] [PubMed] [Google Scholar]
- 43. Hoang AC, Yu H, Röszer T. Transcriptional landscaping identifies a beige adipocyte depot in the newborn mouse. Cells. 2021;10:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Novak M, Monkus E, Pardo V. Human neonatal subcutaneous adipose tissue. Function and ultrastructure. Biol Neonate. 1971;19:306‐21. [DOI] [PubMed] [Google Scholar]
- 45. Mayeuf‐Louchart A, Lancel S, Sebti Y, et al. Glycogen dynamics drives lipid droplet biogenesis during brown adipocyte differentiation. Cell Rep. 2019;29:1410‐1418.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Persson B. Carbohydrate and lipid metabolism in the newborn infant. Acta Anaesthesiol Scand. 1974;18. [DOI] [PubMed] [Google Scholar]
- 47. Hamosh M. Lingual lipase and fat digestion in the neonatal period. J Pediatr Gastroenterol Nutr. 1983;2:S236‐41. [DOI] [PubMed] [Google Scholar]
- 48. Furse S, Billing G, Snowden S, Smith J, Goldberg G, Koulman A. Relationship between the lipid composition of maternal plasma and infant plasma through breast milk. Metabolomics. 2019;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cogneville AM, Cividino N, Tordet‐Caridroit C. Lipid composition of brown adipose tissue as related to nutrition during the neonatal period in hypotrophic rats. J Nutr. 1975;105:982‐8. [DOI] [PubMed] [Google Scholar]
- 50. Yin J, Quinn S, Dwyer T, Ponsonby AL, Jones G. Maternal diet, breastfeeding and adolescent body composition: a 16‐year prospective study. Eur J Clin Nutr. 2012;66:1329‐34. [DOI] [PubMed] [Google Scholar]
- 51. Insull W Jr, Hirsch J, James T. The fatty acids of human milk. II. Alterations produced by manipulation of caloric balance and exchange of dietary fats. J Clin Invest. 1959;38:443‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sinclair JC. Temperature regulation and energy metabolism in the newborn (monographs in neonatalogy). Grune & Stratton, Florence, Kentucky, U.S.A. 1978. [Google Scholar]
- 53. Stave U. Perinatal physiology. Plenum Medical Company, New York, London. 1970. [Google Scholar]
- 54. Hahn P, Greenberg R, Dobiášová M, Drahota Z. Triglyceride synthesis from various precursors in adipose tissue of the rat during development. Can J Biochem. 1968;46:735‐741. [DOI] [PubMed] [Google Scholar]
- 55. Röszer T. Co‐evolution of breast milk lipid signaling and thermogenic adipose tissue. Biomolecules. 2021;11:1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Patel D, Kalhan S. Glycerol metabolism and triglyceride‐fatty acid cycling in the human newborn: effect of maternal diabetes and intrauterine growth retardation. Pediatr Res. 1992;31:52‐58. [DOI] [PubMed] [Google Scholar]
- 57. Röszer T. Mechanisms which control the size of M2 macrophage‐dominated tissue macrophage niches. In: Röszer T, ed. The M2 macrophage. Springer International Publishing; 2020:99‐111. [Google Scholar]
- 58. Alcalá M, Calderon‐Dominguez M, Bustos E, et al. Increased inflammation, oxidative stress and mitochondrial respiration in brown adipose tissue from obese mice. Sci Rep. 2017;7:16082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Röszer T. M2 macrophages in the metabolic organs and in the neuroendocrine system. In: Röszer T, ed. The M2 macrophage. Springer International Publishing; 2020:171‐187. [Google Scholar]
- 60. Zheng C, Yang Q, Cao J, et al. Local proliferation initiates macrophage accumulation in adipose tissue during obesity. Cell Death Dis. 2016;7:e2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Spencer M, Yao‐Borengasser A, Unal R, et al. Adipose tissue macrophages in insulin‐resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016‐E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Boulenouar S, Michelet X, Duquette D, et al. Adipose type one innate lymphoid cells regulate macrophage homeostasis through targeted cytotoxicity. Immunity. 2017;46:273‐286. [DOI] [PubMed] [Google Scholar]
- 63. Zheng C, Yang Q, Xu C, et al. CD11b regulates obesity‐induced insulin resistance via limiting alternative activation and proliferation of adipose tissue macrophages. Proc Natl Acad Sci USA. 2015;112:E7239‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arpa L, Valledor AF, Lloberas J, Celada A. IL‐4 blocks M‐CSF‐dependent macrophage proliferation by inducing p21Waf1 in a STAT6‐dependent way. Eur J Immunol. 2009;39:514‐26. [DOI] [PubMed] [Google Scholar]
- 65. Shiau M‐Y, Chuang P‐H, Yang C‐P, et al. Mechanism of interleukin‐4 reducing lipid deposit by regulating hormone‐sensitive lipase. Sci Rep. 2019;9:11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sidossis L, Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J Clin Invest. 2015;125:478‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Verduci E, Calcaterra V, Di Profio E, et al. Brown adipose tissue: new challenges for prevention of childhood obesity. A narrative review. Nutrients. 2021;13:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Divoux A, Moutel S, Poitou C, et al. Mast cells in human adipose tissue: link with morbid obesity, inflammatory status, and diabetes. J Clin Endocrinol Metab. 2012;97:E1677‐85. [DOI] [PubMed] [Google Scholar]
- 69. Finlin BS, Zhu B, Confides AL, et al. Mast cells promote seasonal white adipose beiging in humans. Diabetes. 2017;66:1237‐1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hui X, Gu P, Zhang J, et al. Adiponectin enhances cold‐induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 2015;22:279‐90. [DOI] [PubMed] [Google Scholar]
- 71. Choi EW, Lee M, Song JW, et al. Fas mutation reduces obesity by increasing IL‐4 and IL‐10 expression and promoting white adipose tissue browning. Sci Rep. 2020;10:12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jun H, Yu H, Gong J, et al. An immune‐beige adipocyte communication via nicotinic acetylcholine receptor signaling. Nat Med. 2018;24:814‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bolus WR, Hasty AH. Contributions of innate type 2 inflammation to adipose function. J Lipid Res. 2019;60:1698‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kumari M, Wang X, Lantier L, et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J Clin Invest. 2016;126:2839‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rajbhandari P, Thomas BJ, Feng A‐C, et al. IL‐10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell. 2018;172:218‐233.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hernandez‐Trejo M, Sámano R, Chico‐Barba G, Pizano‐Zarate ML, Herrera‐González NE. Neonatal adiposity may increase plasmatic cytokines. PLoS One. 2020;15:e0238370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Acosta JR, Tavira B, Douagi I, et al. Human‐specific function of IL‐10 in adipose tissue linked to insulin resistance. J Clin Endocrinol Metab. 2019;104:4552‐4562. [DOI] [PubMed] [Google Scholar]
- 78. de‐Lima‐Júnior JC, Souza GF, Moura‐Assis A, et al, (2019) Abnormal brown adipose tissue mitochondrial structure and function in IL10 deficiency. EBioMedicine 39, 436‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hebel K, Weinert S, Kuropka B, et al. CD4+ T cells from human neonates and infants are poised spontaneously to run a nonclassical IL‐4 program. J Immunol. 2014;192:5160‐70. [DOI] [PubMed] [Google Scholar]
- 80. Ying T, Golden, T . Neonatal IL‐4 exposure decreases adipogenesis of male rats into adulthood. 2021;320:E1148‐e1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cifuentes‐Zúñiga F, Arroyo‐Jousse V, Soto‐Carrasco G, et al. IL‐10 expression in macrophages from neonates born from obese mothers is suppressed by IL‐4 and LPS/INFγ. J Cell Physiol. 2017;232:3693‐3701. [DOI] [PubMed] [Google Scholar]
- 82. Stolzman S, Bement MH. Inflammatory markers in pediatric obesity: health and physical activity implications. ICAN. 2012;4:297‐302. [Google Scholar]
- 83. Radaelli T, Uvena‐Celebrezze J, Minium J, Huston‐Presley L, Catalano P, Hauguel‐de Mouzon S. Maternal interleukin‐6: marker of fetal growth and adiposity. J Soc Gynecol Investig. 2006;13:53‐7. [DOI] [PubMed] [Google Scholar]
- 84. Maculewicz E, Antkowiak B, Antkowiak O, et al. IL‐6 polymorphisms are not related to obesity parameters in physically active young men. Genes. 2021;12:1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wallenius V, Wallenius K, Ahrén B, et al. Interleukin‐6‐deficient mice develop mature‐onset obesity. Nat Med. 2002;8:75‐9. [DOI] [PubMed] [Google Scholar]
- 86. van Hall G, Steensberg A, Sacchetti M, et al. Interleukin‐6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab. 2003;88:3005‐3010. [DOI] [PubMed] [Google Scholar]
- 87. Yang Y, Yang G. Rosiglitazone regulates IL‐6‐stimulated lipolysis in porcine adipocytes. Biochem Cell Biol. 2010;88:853‐60. [DOI] [PubMed] [Google Scholar]
- 88. Alsaggar M, Mills M, Liu D. Interferon beta overexpression attenuates adipose tissue inflammation and high‐fat diet‐induced obesity and maintains glucose homeostasis. Gene Ther. 2017;24:60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Harkins JM, Moustaid‐Moussa N, Chung Y‐J, et al. Expression of interleukin‐6 is greater in preadipocytes than in adipocytes of 3T3‐L1 cells and C57BL/6J and ob/ob mice. J Nutr. 2004;134:2673‐2677. [DOI] [PubMed] [Google Scholar]
- 90. Berg AH, Lin Y, Lisanti MP, Scherer PE. Adipocyte differentiation induces dynamic changes in NF‐κB expression and activity. Am J Physiol Endocrinol Metab. 2004;287:E1178‐E1188. [DOI] [PubMed] [Google Scholar]
- 91. Li J, Shen X. Effect of rosiglitazone on inflammatory cytokines and oxidative stress after intensive insulin therapy in patients with newly diagnosed type 2 diabetes. Diabetol Metab Syndr. 2019;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bessler H, Mendel C, Straussberg R, Gurary N, Aloni D, Sirota L. Effects of dexamethasone on IL‐1beta, IL‐6, and TNF‐alpha production by mononuclear cells of newborns and adults. Biol Neonate. 1999;75:225‐33. [DOI] [PubMed] [Google Scholar]
- 93. Chen Q, Shi P, Wang D, et al. Epidermis‐activated gasdermin‐A3 enhances thermogenesis of brown adipose tissue through IL‐6/Stat3 signaling. Am J Pathol. 2019;189:1041‐1052. [DOI] [PubMed] [Google Scholar]
- 94. Angelone DF, Wessels MR, Coughlin M, et al. Innate immunity of the human newborn is polarized toward a high ratio of IL‐6/TNF‐α production in vitro and in vivo. Pediatr Res. 2006;60:205‐209. [DOI] [PubMed] [Google Scholar]
- 95. Clària J, Nguyen BT, Madenci AL, Ozaki CK, Serhan CN. Diversity of lipid mediators in human adipose tissue depots. Am J Physiol Cell Physiol. 2013;304:C1141‐C1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sullivan EM, Pennington ER, Green WD, Beck MA, Brown DA, Shaikh SR. Mechanisms by which dietary fatty acids regulate mitochondrial structure‐function in health and disease. Adv Nutr. 2018;9:247‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid‐activated nuclear receptors. Nature. 2008;454:470‐477. [DOI] [PubMed] [Google Scholar]
- 98. Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306‐321. [DOI] [PubMed] [Google Scholar]
- 99. Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Odegaard JustinI, Ganeshan K, Chawla A. Adipose tissue macrophages: “Amicus adipem.” Cell Metab. 2013;18:767‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Harrington TAM, Thomas EL, Frost G, Modi N, Bell JD. Distribution of adipose tissue in the newborn. Pediatr Res. 2004;55:437‐441. [DOI] [PubMed] [Google Scholar]
- 102. Böckmann KA, von Stumpff A, Bernhard W, et al. Fatty acid composition of adipose tissue at term indicates deficiency of arachidonic and docosahexaenoic acid and excessive linoleic acid supply in preterm infants. Eur J Nutr. 2021;60:861‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Félix‐Soriano E, Sáinz N, Gil‐Iturbe E, et al. Changes in brown adipose tissue lipid mediator signatures with aging, obesity, and DHA supplementation in female mice. FASEB J. 2021;35:e21592. [DOI] [PubMed] [Google Scholar]
- 104. Hu X, Cifarelli V, Sun S, Kuda O, Abumrad NA, Su X. Major role of adipocyte prostaglandin E2 in lipolysis‐induced macrophage recruitment[S]. J Lipid Res. 2016;57:663‐673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Aprikian O, Reynaud D, Pace‐Asciak C, et al. Neonatal dietary supplementation of arachidonic acid increases prostaglandin levels in adipose tissue but does not promote fat mass development in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2006‐R2012. [DOI] [PubMed] [Google Scholar]
- 106. Shen Z, Ma Y, Ji Z, et al. Arachidonic acid induces macrophage cell cycle arrest through the JNK signaling pathway. Lipids Health Dis. 2018;17:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Xu M, Wang X, Li Y, et al. Arachidonic acid metabolism controls macrophage alternative activation through regulating oxidative phosphorylation in PPARγ dependent manner. Front Immunol. 2021;12:618501‐618501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Savva SC, Chadjigeorgiou C, Hatzis C, et al. Association of adipose tissue arachidonic acid content with BMI and overweight status in children from Cyprus and Crete. Br J Nutr. 2004;91:643‐9. [DOI] [PubMed] [Google Scholar]
- 109. Mak IL, Cohen TR, Vanstone CA, Weiler HA. Arachidonic acid status negatively associates with forearm bone outcomes and glucose homeostasis in children with an overweight condition or obesity. Appl Physiol Nutr Metab. 2020;45:146‐154. [DOI] [PubMed] [Google Scholar]
- 110. Zhuang P, Shou Q, Lu Y, et al. Arachidonic acid sex‐dependently affects obesity through linking gut microbiota‐driven inflammation to hypothalamus‐adipose‐liver axis. Biochim Biophys Acta Mol Basis Dis. 2017;1863:2715‐2726. [DOI] [PubMed] [Google Scholar]
- 111. Tare M, Parkington HC, Morley R. Vitamin D in pregnancy and offspring health. In: Wintour EM and Owens JA, eds. Early life origin of health and disease. Springer; 2006. [Google Scholar]
- 112. Roth CL, Elfers C, Kratz M, Hoofnagle AN. Vitamin d deficiency in obese children and its relationship to insulin resistance and adipokines. J Obes. 2011;2011:495101‐495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Xu Y, Lou Y, Kong J. VDR regulates energy metabolism by modulating remodeling in adipose tissue. Eur J Pharmacol. 2019;865:172761. [DOI] [PubMed] [Google Scholar]
- 114. Bonet Luisa M, Canas, AJ , Ribot J, Palou A. Carotenoids and their conversion products in the control of adipocyte function, adiposity and obesity. Arch Biochem Biophys. 2015;572:112‐125. [DOI] [PubMed] [Google Scholar]
- 115. Mounien L, Tourniaire F, Landrier J‐F. Anti‐obesity effect of carotenoids: direct impact on adipose tissue and adipose tissue‐driven indirect effects. Nutrients. 2019;11:1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mawson AR, Xueyuan W. Breastfeeding, retinoids, and postpartum depression: a new theory. J Affect Disord. 2013;150:1129‐35. [DOI] [PubMed] [Google Scholar]
- 117. Panagos PG, Vishwanathan R, Penfield‐Cyr A, et al. Breastmilk from obese mothers has pro‐inflammatory properties and decreased neuroprotective factors. J Perinatol. 2016;36:284‐290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Tan L, Zhang Y, Crowe‐White KM, Senkus KE, Erwin ME, Wang H. Vitamin A supplementation during suckling and postweaning periods attenuates the adverse metabolic effects of maternal high‐fat diet consumption in Sprague‐Dawley rats. Curr Dev Nutr. 2020;4:nzaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Martin CR, Ling PR, Blackburn GL. Review of infant feeding: key features of breast milk and infant formula. Nutrients. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Burugupalli S, Smith AAT, Oshlensky G, et al., Ontogeny of circulating lipid metabolism in pregnancy and early childhood – a longitudinal population study. eLife. 2022;11:e72779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Hewelt‐Belka W, Garwolinska D, Mlynarczyk M, Kot‐Wasik A. Comparative lipidomic study of human milk from different lactation stages and milk formulas. Nutrients. 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Wolfs D, Lynes MD, Tseng YH, et al., Brown fat‐activating lipokine 12,13‐diHOME in human milk is associated with infant adiposity. 2020;106(2):e943‐e956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Morey M, O'Gaora P, Pandit A. Hyperglycemia acts in synergy with hypoxia to maintain the pro‐inflammatory phenotype of macrophages. 2019;14:e0220577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pavlou S, Lindsay J, Ingram R, Xu H, Chen M. Sustained high glucose exposure sensitizes macrophage responses to cytokine stimuli but reduces their phagocytic activity. BMC Immunol. 2018;19:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287‐92. [DOI] [PubMed] [Google Scholar]
- 126. Lu J, Gu Y, Wang L, et al. Glucose metabolism among obese and non‐obese children of mothers with gestational diabetes. BMJ Open Diabetes Res Care. 2020;8:e000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Alwarawrah Y, Kiernan K, MacIver NJ. Changes in nutritional status impact immune cell metabolism and function. Front Immunol. 2018;9:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Lovaszi M, Mattii M, Eyerich K, et al. Sebum lipids influence macrophage polarization and activation. Br J Dermatol. 2017;177:1671‐1682. [DOI] [PubMed] [Google Scholar]
- 129. Leussink S, Aranda‐Pardos I, A‐Gonzalez N. Lipid metabolism as a mechanism of immunomodulation in macrophages: the role of liver X receptors. Curr Opin Pharmacol. 2020;53:18‐26. [DOI] [PubMed] [Google Scholar]
- 130. Kaspar D, Hastreiter S, Irmler M, Hrabé de Angelis M, Beckers J. Nutrition and its role in epigenetic inheritance of obesity and diabetes across generations. Mamm Genome. 2020;31:119‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dierendonck XAMHv, Vrieling F, Smeehuijzen L, et al. Triglyceride breakdown from lipid droplets regulates the inflammatory response in macrophages. Proc Natl Acad Sci USA. 2022;119:e2114739119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Röszer T. Signal mechanisms of M2 macrophage activation. In: The M2 macrophage. Springer International Publishing; 2020:73‐97. [Google Scholar]
- 133. Virtanen HE, Toppari J. Epidemiology and pathogenesis of cryptorchidism. Hum Reprod Update. 2007;14:49‐58. [DOI] [PubMed] [Google Scholar]
- 134. Chang SJ, Chen JYC, Hsu CK, Chuang FC, Yang SSD. The incidence of inguinal hernia and associated risk factors of incarceration in pediatric inguinal hernia: a nation‐wide longitudinal population‐based study. Hernia. 2016;20:559‐563. [DOI] [PubMed] [Google Scholar]
- 135. Park J. Management of perianal abscess and fistula‐in‐ano in infants and children. Clin Exp Pediatr. 2020;63:261‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]


