Summary
Early palliative care (EPC) significantly improves quality of life, symptoms, and satisfaction with care for patients with advanced cancer. International organizations have recognized and promoted the role of palliative care as a distinct specialty, advocating its involvement throughout the cancer trajectory. Although patients with haematologic malignancies (HMs) have a comparable symptom burden to patients with solid tumours, they face multiple barriers to EPC integration. In this review, we discuss these barriers, present updated evidence from clinical trials of EPC in HMs and propose models to support EPC integration into care for patients with HMs.
Keywords: cancer, delivery of health care, haematologic oncology, haematology, oncology, palliative care
INTRODUCTION
Palliative care (PC) originated from the modern hospice movement in the 1960s, which aimed to provide end‐of‐life (EOL) care for patients with terminal illnesses. In the following decades, PC evolved from providing exclusively EOL care to proactively supporting patients and caregivers facing a life‐threatening disease from the time of diagnosis. 1 Extensive evidence has shown that early palliative intervention significantly improves quality of life, symptoms and satisfaction for patients with advanced cancer and may even prolong survival. 2 , 3 As a result, international cancer organizations have recognized and promoted the role of PC as a distinct specialty, advocating its involvement throughout the cancer trajectory. 4 , 5 , 6 , 7 The World Health Organization now defines PC as “an approach that improves the quality of life of patients and their families facing the problem associated with life‐threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual.” 8 This definition emphasizes a holistic approach to prevent and manage clinical complications from the disease and its treatments, and is equally applicable to patients with solid tumours as well as haematological malignancies (HMs).
Unlike patients with solid tumours, for whom PC is increasingly integrated early in the disease course, referrals to specialized PC for patients with HMs typically occur late in the disease course. 9 , 10 , 11 However, the symptom burden experienced in these diseases is similar to that of solid tumours, and patients and their caregivers experience substantial psychosocial distress, as well as needs related to navigating the complexities of the health care system and advance care planning. 12 , 13 , 14 Thus, patients with HM could benefit greatly from the interdisciplinary approach offered by PC, which is focused on improving symptoms, communication, shared decision making, psychosocial support, community care resources, advance care planning, and caregiver support. 15 , 16 Moreover, there is emerging evidence that supports early specialized PC in HM. 17 , 18 , 19
In this narrative review, we discuss the PC needs of patients with HMs as well as barriers that prevent access to specialized PC for this population. We also present recent evidence from clinical trials demonstrating benefits from the early integration of PC for patients with HM and discuss potential models for early integration.
PC IN HM
Domains of PC and levels of care
PC is provided by interdisciplinary teams, which allows for the delivery of multidimensional care. 20 , 21 PC addresses multiple domains, including not only physical symptoms but psychosocial, spiritual and EOL care as well as ethical, structural and cultural issues. 22 A popular conceptual model classifies PC into primary, secondary and tertiary PC, according to who delivers the care (Figure 1). 23 Primary PC is delivered in the community by primary care clinicians with general knowledge about PC. Accordingly, all clinicians should have basic training in the principles and practice of PC (i.e., basic pain relief, nausea, mood disorders). Secondary PC is provided in hospitals by specialists who have general knowledge about PC for their specialty. Thus, all oncologists, including haemato‐oncologists, should have basic PC training, resulting in a good understanding of symptom management, psychosocial care and advance care planning for patients with HMs. 6 Lastly, tertiary (specialized) PC is provided by PC consultants, who may be consulted to provide specialized PC for more complex situations such as refractory symptoms, severe psychosocial distress or family conflict.
FIGURE 1.

A conceptual model of PC delivery based on setting and provider.[Adapted with permission from Kaasa S, Loge JH, Aapro M, Albreht T, Anderson R, Bruera E, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol. 2018 Nov;19 (11):e588–653]
PC syndromes in HMs
In contrast with HMs, the trajectory of disease in solid tumours is relatively predictable, with metastasis generally heralding incurability and a steady downward trajectory (Figure 2). Previous publications have described the characteristics of various types of HM with respect to PC needs. 24 , 25 We find it helpful to divide HMs into three main groups based on the trajectory of disease, setting of care and challenges to PC delivery. These are described briefly below and are depicted in Figure 2B–D.
FIGURE 2.

(A) Solid malignancy trajectory.[Adapted with permission from Murray SA, Kendall M, Boyd K, Sheikh A. Illness trajectories and Palliative Care. BMJ. 2005 Apr 28;330(7498):1007–11. (B) Aggressive HM trajectory – “The rollercoaster”: acute leukaemia, aggressive lymphoma, stem cell transplantation, acute graft‐vs.‐host disease [developed by the authors]. (C) Indolent HM trajectory – “The war of attrition”: indolent lymphoma, multiple myeloma, chronic lymphocytic lymphoma [developed by the authors]. (D) Bone marrow failure “the transfusion tether”: myelodysplasia, myelofibrosis [developed by the authors]
Aggressive HMs – “The rollercoaster”
The first group of HM diseases includes aggressive life‐threatening diseases with a high risk of harm alongside a realistic possibility for long‐term cure (Figure 2B). This group includes acute leukaemias, aggressive lymphomas, stem cell transplantation and acute graft‐vs.‐host disease. The razor‐edge character of these diseases make prognostication difficult until late in the course of disease. Often cure may still be a reasonable and achievable goal until days before death. For example, life threatening sepsis during nadir of salvage chemotherapy for refractory double‐hit diffuse large B‐cell lymphoma may be seen as a terminal event; however, within days, the reversal of neutropenia may find a patient not only recovering from sepsis but indeed in complete remission. Conversely, the decline before death in such scenarios may be very rapid which may be a barrier to transition to hospice care in a timely manner.
The rollercoaster nature of life in the context of these diseases also presents unique challenges, both physical and psychological. While leukaemias do not commonly present as a compressive mass or nerve infiltration, pain is a prominent symptom due to multiple causes related to neutropenia and infection (e.g., perianal pain) as well as the result of treatment (e.g., mucositis, lumbar puncture). 26 Patients with acute leukaemia report multiple burdensome physical and psychological symptoms, including lack of energy, drowsiness, dry mouth, pain, weight loss, difficulty sleeping, worrying, difficulty concentrating and feeling sad. Uncertainty and psychological distress are also prominent in patients with these diseases. For example, the acute onset of leukaemia coupled with uncertainty regarding prognosis as well as high rates of morbidity and mortality may have a devastating psychological effect. 13 Patients referred to specialist care for acute leukaemia may undergo multiple blood tests, bone marrow biopsy, PICC line insertion, whole body imaging, cardiac imaging and fertility preservation followed by intensive inpatient continuous chemotherapy all within days. Illness understanding in patients with acute leukaemia is severely affected by the sheer volume of information they are asked to process over a short period of time, further compromising their ability to effectively participate in decision making. 27
Weeks of neutropenia are often complicated by infection and mucositis, leading to mortality risk and increasing uncertainty. Mood and hope vary with fluctuations of daily blood counts, while increasing white blood cell counts may either bring the consolation of resolving neutropenia or the harbinger of relapse and increased blast count. It is therefore unsurprising that 32% of acute leukaemia patients suffer from syndromal or subsyndromal acute stress disorder over the course of their disease 14 , 28 , 29 and 17% suffer from depressive symptoms. 30 , 31
Indolent HMs – “The war of attrition”
The second group includes more indolent diseases such as chronic lymphocytic leukaemia (CLL), indolent lymphomas and many cases of multiple myeloma (Figure 2C). Although these diseases are incurable, prognosis may be prolonged, with episodes of decreased function due to multiple relapses entailing multiple and ever‐expanding lines of treatment, alongside prolonged remissions with excellent function. As increasingly more novel drugs have been found to be effective in the treatment of these diseases, clinically significant remissions can be achieved even in advanced disease. Prognostication may therefore be difficult. More importantly, it is difficult to decide whether further treatment may be efficacious or detrimental to quality of life. Even advanced stage disease after multiple lines of therapy may have a significant response to seemingly over‐aggressive treatments. For example, over the past eight years, the FDA has approved five new drugs for the treatment of CLL. Each new approval opens the door for single drug treatment as well as combinations with other novel agents and with chemotherapy, creating an exponential amount of treatment possibilities. Stem cell transplantation, a severely debilitating and dangerous intervention, can attain long term remission and even cure in a significant minority of patients and thus persists as a wild card treatment option in the eyes of both patients and clinicians. CAR‐T cell therapy is an additional treatment modality with activity in indolent as well as aggressive HMs and data as to quality of life at the EOL with these treatments is only beginning to emerge. 32
Physical and psychological challenges are prominent in this group as well. Multiple myeloma is notorious for causing bone pain and spontaneous fractures 33 while lymphomas may behave as solid tumours compressing adjacent organs or nerves causing a variety of symptoms. 34 Moreover, various HMs, as well as the drugs used to treat them, may cause debilitating peripheral neuropathy. 35
Indolent diseases, such as multiple myeloma, are also associated with psychological distress for patients and families, including anxiety and depression associated with fear of inevitable relapse. 36 , 37 Of particular interest are indolent, asymptomatic and premalignant conditions, which need no treatment but may portend future symptomatic disease, such as monoclonal gammopathy of unknown significance. The ‘watch and wait’ approach taken with these patients is in itself a source of psychological distress and decreased quality of life similar to that of multiple myeloma. 38 , 39
Bone marrow failure – the ‘transfusion tether’
The third group is characterized by bone marrow failure resulting in cytopenias (Figure 2D). This group includes the myelodysplastic syndromes, myelofibrosis, aplastic anaemia and end‐stage HM with spent marrow due to infiltration by disease or aplasia from treatment. Profound anaemia causes dependence on blood transfusions for improvement of symptoms such as fatigue, dyspnea, chest pain and more. Severe thrombocytopenia may result in haemorrhage ranging from mild but unsettling mucocutaneous bleeding, which may also exacerbate anaemia, to life‐threatening gastrointestinal or intracranial bleeding. These may be partially avoided by regular platelet transfusions. Most importantly, severe neutropenia in the setting of chronic progressive bone marrow failure results in recurrent bacterial and invasive fungal infections. These infections are both life threatening and debilitating, with no ready solution in the form of transfusion. Treating these infections effectively may entail both identification of the cause of infection using cultures, imaging and invasive procedures, as well as the use of broad‐spectrum antibiotics. Thus, the commonly used term, the ‘transfusion tether’, is but one of many tethers tying these patients to the hospital bed. 40 Despite the low risk of transfusion in a home setting 41 , 42 and many patients' wish to receive transfusions at home, 43 , 44 transfusions are not readily available in home or hospice settings, mostly due to cost and regulation. In this group, prognostication is again a challenge, as death is the result of acute complications of cytopenias rather than disease progression.
Although the three groups mentioned provide a useful framework to understand the complexities of these diseases, the behaviour of various HMs is heterogeneous and does not adhere rigidly to this categorization. HMs can also transform from one group to another; for example, low grade lymphoma or CLL can transform to aggressive lymphoma and MDS can transform to acute leukaemia. Even without overt morphological transformation, HMs can shift their behaviour in surprising ways whether due to treatment or intrinsic biology, thus aggressive diseases may behave as indolent ones and vice versa, further complicating prognostication.
CURRENT STATE OF PC NEEDS IN HMS
Patients suffering from HMs have an overwhelming symptom burden, with a burden of pain, dyspnea, nausea and anorexia similar to that of solid tumour malignancies. 12 , 45 However, the overall spectrum of symptoms and their causes may differ from solid tumours, and a deeper understanding of these nuances (which may also be disease‐related) may be beneficial for the care of these patients. For example, some symptoms, such as drowsiness, delirium, fatigue and loss of appetite, may be more prevalent in HM patients. 12 , 45 , 46
Despite extensive physical and psychological symptoms, referral to PC remains inadequate in HM patients, 9 , 10 , 11 , 47 and despite the high incidence of traumatic stress, only a small proportion of these patients are referred to psychology or psychiatry. 48 In one study, 30% of haemato‐oncologists reported never referring to PC while all solid tumour oncologists had previously referred to PC. 49 Patients with HM have consistently been shown to have decreased referral rates to PC services as well as hospice care compared to patients with solid tumours. 9 , 50 , 51 Patients with HM are less likely to complete advance directives or DNR orders. 11 In comparison with solid tumour malignancies, patients with HM have an increased incidence of emergency room visits, hospital admissions and ICU admissions as well as use of chemotherapy and targeted therapy at the EOL. 10 , 52 , 53 , 54 , 55 In the opinion of most haemato‐oncologists, EOL discussions occur too late, 56 and PC is often discussed only when death is imminent. Further, patients with HM are less likely to have a preexisting opioid prescription upon admission to hospice indicating that symptom management may not be adequate. 11
Haemato‐oncologists are more likely than solid tumour oncologists to equate PC with EOL care. 31 Not only is the EOL more complex to predict in haemato‐oncology; even when death becomes a reasonable scenario, haematologists often feel less comfortable discussing death and dying or referring to hospice and are more likely to feel a sense of failure when disease progresses. 57 A previous review reported a large difference in the number of published studies regarding palliative care in solid tumours compared with haematological malignancies. 24 We performed a focused search for the terms ‘palliate’, ‘palliation’ or ‘palliative’ in article titles of the five leading journals in haemato‐oncology and medical oncology, as ranked by journal citation reports, is illustrative of this point (Figure 3). Although the number of publications on PC in leading oncology journals has fluctuated, the percentage of PC publications in these journals has consistently been more than ten‐fold higher than in leading haematology journals.
FIGURE 3.
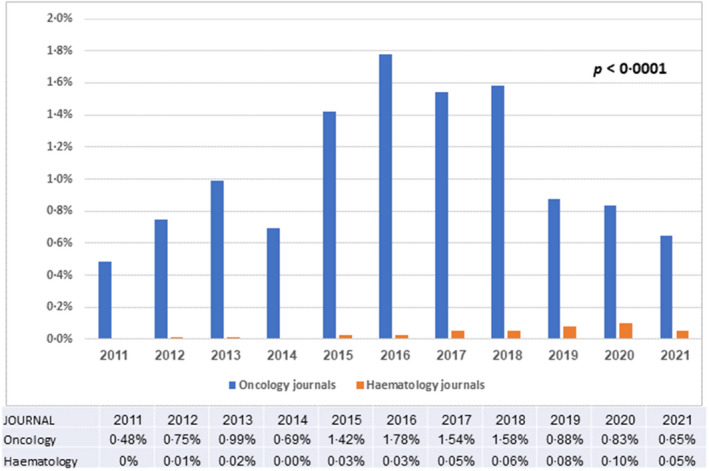
Comparison of the percentage of publications including the keywords ‘palliative’, ‘palliate’ or ‘palliation’ in the article title, in five leading haematology journals versus oncology journals over the years 2011–2021.The leading journals in heamatology and medical oncology were selected by the yearly impact factor designated by Journal Citation Reports. Haematology journals without an oncology focus were not included (i.e., Circulation, Circulation Research). Within each journal, a search for the terms ‘palliate’ ‘palliation’ or ‘palliative’ within the article title, including meeting abstracts, was conducted using the Web of Science database. Oncology journals included were: CA: A Cancer Journal for Clinicians, Nature Reviews Clinical Oncology, Nature Reviews Cancer, Journal of Clinical Oncology and Lancet Oncology. Haematology journals included were: Blood, Lancet Haematology (from 2014), Journal of Haematology and Oncology, Blood Cancer Journal and Leukaemia. Mann–Whitney–Wilcoxon test was used to compare the two groups (p < 0.0001)
BARRIERS TO THE INTEGRATION OF PC IN HM
Difficulties with prognostication
The relative predictability of prognosis in advanced solid tumour malignancies, particularly when combined with prognostic markers such as performance status, 58 has made it possible to routinely implement models of early PC, including involving PC teams in the outpatient setting at the diagnosis of advanced cancer. This steady decline is in contrast to the highly unpredictable course of most HMs, as described above.
A systematic review of the literature failed to find effective prognostic factors in HM beyond imminently life‐threatening events such as multiorgan failure. 59 Factors associated with prognosis in solid tumour malignancies such as level of performance status, symptom burden, cachexia and comorbidities are not as strongly correlated with prognosis in HM patients. 60 Nonetheless, consensus may be found among haemato‐oncologists as to potential signals for transition to the EOL phase of disease which may indicate the need for integration of PC. These include refractory disease, CNS involvement, and worsening performance status. 61 It is clear, however, that prognostication is not the sole barrier to quality EOL care in HM patients. Further barriers will be described in the following sections.
Attitudinal barriers
Haemato‐oncologists are more likely than oncologists to prescribe systemic therapy with moderate toxicity and no survival benefit for patients with a severely impaired performance status and an expected survival of 1 month. 57 PC providers may see this as resistance of haemato‐oncologists to the core tenets of PC while haemato‐oncologists may see the benefit in these therapies in the palliation of symptoms and complications. This attitude is best expressed in the saying by haemato‐oncologists that ‘the best palliation for HM is chemotherapy’. For example, the presence of severe neutropenia would pose a contraindication for chemotherapy in solid tumour patients, however in the setting of bone marrow infiltrated by HM, chemotherapy may be fully indicated. While some have described the approach of haemato‐oncologists as technical rather than holistic, 62 haemato‐oncologists may see this as evidence of the inability of other specialties to comprehend the intricacies of HM. Similarly, accepted EOL quality measures such as admission to hospice or time from last chemotherapy to death are often incompatible with the needs of HM patients and may not be appropriate in the haematology setting. 63 , 64
The differing characteristics of disease may also have a varying effect on the doctor‐patient relationship. While oncologists may see their patients every 3 months, the dynamic character of many HM necessitate visits at shorter time intervals as well as treatment of multiple acute life‐threatening events, commonly with prolonged periods of inpatient treatment. It may be argued that this intense and intimate relationship may result in difficulty for both patient and haemato‐oncologist in referral to PC. 24 , 49 Therefore, haemato‐oncologists may be more inclined to refer to PC if they can maintain clinic visits with their patients. 65
Policy barriers and efforts to overcome them
Hospice criteria may exclude patients with HM by limiting access to therapies that may improve their quality of life. 66 PC services have not been traditionally formed to accommodate the more intensive needs of haematology patients at EOL. Real world experience shows that even within a PC unit the needs of HM patients far exceed that expected including the use of broad spectrum antibiotics and blood products in most patients as well as parenteral nutrition and intravascular devices in many. 67 Home PC services are not generally able to administer wide spectrum antibiotics and blood products, and inpatient hospice rarely have the financial ability or philosophy of care to allow for such treatment. 61 , 68 Many have advocated for policy change including the American Society of Haematology 69 and legislation has recently been introduced to enable administration of blood products in the hospice setting in the US. 70
EVIDENCE FOR BENEFITS OF PC
Evidence in patients with solid tumours
The benefits of early specialized PC were originally demonstrated in randomized controlled trials conducted in patients with advanced solid tumours (Table 1). 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 Most of these trials were conducted in the outpatient setting, with interventions of either free‐standing 71 or embedded 72 , 73 PC clinics. A study in Canada demonstrated that compared to patients who received standard oncologic care alone, those referred early to a free‐standing specialized PC clinic experienced better quality of life, symptom control and satisfaction with care. 71 Other trials in the USA and Italy showed that early specialized PC provided by a PC physician or advanced practice nurse embedded in an outpatient cancer clinic improved quality of life, mood, aggressiveness of EOL care, and survival. 72 , 73 , 74
TABLE 1.
Early palliative care clinical trials including only patients with advanced solid tumours
| Setting | Study/year | Cancer diagnosis | Intervention | Control group | Favoured PC arm | Did not differ | |
|---|---|---|---|---|---|---|---|
| Clinician providing palliative care | Timing of early palliative care | ||||||
| Outpatient: free‐standing |
Zimmermann 2014 |
Advanced lung, GI, GU, Gyn, breast. | Specialized PC physician and nurse | Estimated survival 6–24 months | Standard care with referral on request | QOL, symptoms, patient and caregiver satisfaction with care | Caregiver QOL |
| Maltoni 2016, Maltoni 2016 74 , 96 | Nonresectable or metastatic pancreatic cancer | PC physician | Within 8 weeks of diagnosis and no prior chemotherapy | Standard care with referral on request | QOL, aggressiveness at the EOL | Survival, anxiety, depression | |
| Scarpi 2019 76 | Locally advanced or metastatic gastric cancer | PC physician | Within 8 weeks of diagnosis and no prior chemotherapy | Standard care with referral on request | QOL at 3 months, survival, aggressiveness at the EOL | ||
| Outpatient: embedded |
Temel 2010, Greer 2012 |
Metastatic NSCLC | Palliative care physician and APN | Within 8–12 weeks of diagnosis | Standard care with referral on request | QOL, mood, communication, less aggressive EOLc, survival | |
|
Temel 2016 |
Incurable lung or non‐colorectal GI cancer | Palliative care physician and APN | Within 8 weeks of diagnosis | Standard care with referral on request | QOL, mood, decision making, coping, EOLc discussions, caregiver distress | Illness understanding, caregiver anxiety, QOL at week 12 | |
| Mixed inpatient and outpatient |
Vanbutsele 2018 |
Incurable solid tumour | Specialized PC nurse with PC physician referral if required | Prognosis ≤1 year, and within 12 weeks of diagnosis or progression | Multidisciplinary care with nurse specialist, psychologist and dietitian. PC referral on request |
QOL at 3 months |
Survival, anxiety, depression, symptoms, health care utilization, aggressiveness at the EOL |
| Franciosi 2019 78 | NSCLC (no EGFR), pancreatic, gastric, or biliary tract cancer | Oncologist specialized in PC and PC nurse | Within 8 weeks of diagnosis | Standard care with referral on request | QOL at 3 months, health care utilization | ||
| Mixed face to face and telephone | Groenvold 2017 79 | Stage IV lung, GI, breast, other with at least one PC need and 4 symptoms on EORTC‐QLQ‐C30 | Multidisciplinary team (doctor, nurse, psychologist, among others) | Timing not specified | Standard care with referral on request | Nausea/vomiting | QOL, depression, survival |
| Telehealth | Bakitas 2009 75 | Lung, GI,GU, breast | APN with PC specialty training | Prognosis ≤1 year and within 8 weeks of diagnosis | Standard oncology and supportive care | QOL, mood |
Symptom intensity, Resource use |
Abbreviations: EOLc, end‐of‐life care; EOL, end‐of‐life; QOL, quality of life; GI, gastrointestinal; GU, genitourinary; APN, advance practice nurse; NSCLC, non‐small cell lung cancer; PC, palliative care.
Evidence in patients with HMs
Although the symptom burden of patients with HMs is equivalent or higher than that for patients with solid tumours, the evidence from randomized trials studying the effect of early PC interventions for HM patients is only currently emerging. 17 , 18 , 19 , 75 , 80 , 81 Unlike the trials in patients with solid tumours, most trials in patients with HM have been conducted entirely or almost entirely in the inpatient setting. Some early trials in the emergency and outpatient setting that included mainly patients with solid tumours also included patients with HMs, 75 , 82 but results are difficult to interpret given the heterogeneity of the patient population and the very small percentage of patients with HMs (less than 5%) in these trials.
More recently, PC interventions including exclusively patients with HM have provided conclusive evidence for this population in the inpatient setting (Table 2). 17 , 18 , 19 , 32 , 80 , 81 In a randomized controlled trial in patients with HM who underwent allogeneic or autologous stem cell transplantation, patients assigned to the intervention arm received specialized PC within 72 hours of hospital admission and twice weekly as inpatients. 17 Anxiety was reduced and quality of life improved for patients in the intervention arm after 2 weeks of enrolment. Moreover, both patients and caregivers had reduced levels of depression. The benefit in patient quality of life was sustained after 3 months, and at 6 months, there was a positive impact on post‐traumatic stress disorder (PTSD) and depression for patients receiving the intervention. 80 It is noteworthy that the PC intervention in this study included mostly rapport building, symptom management and assistance with coping while illness understanding, decision‐making and advance care planning received significantly less focus.
TABLE 2.
Early palliative care clinical trials including patients with haematological malignancies
| Type according to setting | Study/year | Cancer diagnosis | Intervention | Comparison group | Favours PC arm | Did not differ | |
|---|---|---|---|---|---|---|---|
| Clinician providing multidisciplinary PC and SC | Timing of early palliative care | ||||||
| Trials that included only patients with haematologic malignancies | |||||||
| Inpatient | El‐Jawahri 2021 18 | High risk AML receiving intensive chemotherapy | PC physician, APN or physician assistant | Within 72 h of receiving chemotherapy | Standard care with referral upon request | QOL, depression, anxiety, PTSD. EOLc preference discussions, less chemotherapy near EOL | Symptom burden, hospice use and length of stay, hospitalization the last week of life |
|
El‐Jawahri 2016, |
HM receiving allogenic or autologous stem cell transplantation | PC physician or APN | Within 72 h of admission for transplantation | Standard care with referral upon request |
‐ At week 2: QOL, depression, anxiety, symptom burden, caregiver depression ‐ At month 3: QOL, depression ‐ At month 6: depression, PTSD |
‐ At week 2: fatigue, caregiver QOL and anxiety ‐ At month 3: anxiety, fatigue, symptom burden ‐ At month 6: QOL, anxiety |
|
| Inpatient, outpatient and telephone |
Rodin 2020 (phase II) 19 |
Newly diagnosed or recently relapsed AML/ ALL within one month of admission |
‐ Specialized PC physician and nurse and ‐Therapist trained in EASE‐Psy plus psychiatrist/psychologist at request |
‐Triggered by ESAS‐AL symptoms ≥4 or upon request −12 EASE‐psy sessions over 8 weeks |
Standard care with referral upon request |
‐ Pain intensity and pain interference at 12 weeks with daily activities ‐ Traumatic stress symptoms at weeks 4 and 12 |
Symptom severity, symptom related distress, depressive symptoms, QOL, satisfaction with care |
| Outpatient | Loggers 2016 81 | HM planned for allogenic or autologous stem cell transplantation | APN or RN trained in PC |
During the 2‐week evaluation period immediately before HCT |
N/A Acceptability of EPC |
‐ 69% provided consent to participate in EPC consult. Comfort with EPC was high (82%) |
No control arm |
| Trials that included patients with solid tumours and haematologic malignancies | |||||||
| ED |
Grudzen 2016 (phase II) 82 |
Advanced solid tumour; relapsed stage III/IV lymphoma or myeloma, non‐transplant or chemotherapy candidate (5.1%) | Palliative care physician, nurse practitioner, social worker and chaplain | Same or following day of ED admission | Standard ED care | QOL | ‐ Survival, ICU admission, hospice discharge |
| Mixed in person (outpatient) and telephone | Bakitas 2015 101 | Advanced solid or HM (5%), within 1 to 2 months of diagnosis, prognosis 6 to 24 months | PC board certified physician (in person), and an APN (telephone) | Estimated survival 6–24 months | Delayed PC intervention after 3 months | Survival | QOL, health care utilization, chemotherapy in the last 14 days, home death |
Abbreviations: QOL, quality of life; EOLc, end‐of‐life care; PTSD, posttraumatic stress disorder; PC, palliative care; ED, emergency department; ICU, intensive care unit; EPC, early palliative care; APN, advance practice nurse; RN, registered nurse; N/A, not applicable; AML/ALL, acute myeloid leukaemia and acute lymphocytic leukaemia; HM, haematologic malignancy.
In Canada, a phase II trial of a combined psychosocial and PC intervention (EASE – Emotion And Symptom Engagement) delivered mainly in the inpatient setting in patients with newly diagnosed or relapsed acute leukaemia demonstrated feasibility of this model as well as improved pain control and decreased traumatic stress symptoms. 19 The positive impact of early specialized PC for patients with acute myeloid leukaemia was affirmed by a randomized controlled trial demonstrating better quality of life and reduced levels of anxiety, depression and PTSD in high‐risk acute myeloid leukaemia patients randomized to early inpatient PC compared to usual care. 18
Further new intervention studies are underway, including a multi‐centre randomized phase III trial of EASE for patients with newly‐diagnosed acute leukaemia and several other trials of early PC in patients with leukaemia, multiple myeloma, lymphoma, myelodysplastic syndrome and mixed haematologic malignancies (Table 3) (clinicaltrials.gov). These include a trial of funding to receive palliative blood transfusions while enrolled on hospice for patients with HMs at the EOL 83 and a trial of outpatient PC for patients with multiple myeloma. 84
TABLE 3.
Ongoing early palliative care clinical trials in patients with haematologic malignancies b
|
[Principal Investigator]; status; phase; setting |
Country; year registered |
Patients; Cancer diagnosis |
Comparison group | Intervention | Outcomes a | |
|---|---|---|---|---|---|---|
| Clinician providing PC | Timing of early PC | |||||
|
[El‐Jawahri] Active, not recruiting N/A inpatient |
USA 2016 |
>60 years old: ‐High risk AML |
Standard leukaemia care | Collaborative PC and leukaemia specialist | Newly diagnosed, relapsed, primary refractory | QOL, psychological distress, symptom burden, PTSD, EOL discussion preference, chemotherapy within 30 days of death, admission within 7 days of death, hospice utilization |
|
[El Chaer] Recruiting Phase II; Inpatient and outpatient |
USA 2020 |
≥18 years old: ‐AML, ALL, MDS, CMML |
Standard care with referral upon request | PC specialist | Newly diagnosed, relapsed, primary refractory | Place of death, survival, duration, admissions duration/type/frequency, ER visits, hospice service use, transfusions, QOL, code status change, GOC discussions |
|
[Samala] Recruiting Phase II Outpatient |
USA 2020 |
≥18 years old: ‐MM |
No comparison group | Specialized physician, APN, care coordinators | Within 8 weeks of diagnosis | QOL, anxiety, depression, health care utilization |
|
[Tanzi] 102 Unknown Phase II Outpatient |
Italy 2020 |
≥18 years old: ‐Incurable haematological tumour and last line of therapy |
Standard care with referral upon request | Integrated PC team | Soon after decision of last active treatment | Adherence to palliative care program, QOL, anxiety, depression, PPS. |
|
[Rodin & Zimmermann] Recruiting Inpatient & outpatient & telephone |
Canada 2020 |
≥18 years old: Newly diagnosed AML and ALL |
Standard care with referral upon request |
‐Supportive psychotherapy: trained therapist ‐Symptom triggered referral: PC specialists |
Within 2 weeks of admission for treatment of acute leukaemia | Traumatic stress symptoms, physical symptom severity, QOL, ASD, depression, satisfaction with care, pain, survival, quality adjusted life years |
|
[Booker] Not yet recruiting N/A Virtually (phone or zoom) |
Canada 2022 |
≥18 years old: ‐Haematologic malignancy ‐Family caregiver |
Standard care with referral upon request | PC nurse practitioner or physician | Scheduled for SCT | QOL, symptom burden, patient and caregiver prognostic understanding, caregiver QOL |
|
[Scarfò] 103 Recruiting Open label Virtually (App) |
Italy 2020 |
≥ years old: ‐CLL/SLL or MDS ‐Users of internet connected device |
Standard care with PC if needed |
MyPal ePRO: PROs at baseline, monthly times 6, and at 12 months |
Scheduled to receive any line of therapy for CLL/SLL or MDS | QOL |
|
[Guastella] Recruiting Phase III Not specified |
France 2019 |
≥70 years old: ‐AML, MDS, diffuse large B cell lymphoma |
Standard care with referral upon request | Palliative and supportive care team | At diagnosis in AML, MDS; after 3rd line therapy in lymphoma | QOL, symptoms, survival, satisfaction with care, cost effectiveness |
|
[Bénite] Completed Phase II Not specified |
France 2021 |
≥18 years old: ‐First relapse on AML or ALL, transplant ineligible |
Standard care with referral upon request | Multidisciplinary palliative care team | Within 8 weeks of diagnosis | QOL, symptom intensity, depression, anxiety, quality of EOL, survival |
Abbreviations: QOL, quality of life; PPS, palliative performance scale; EOL, end of life; GOC, goals of care; PTSD, post‐traumatic stress disorder; ER, emergency room; PC, palliative care; FACT, functional assessment of cancer questionnaire; N/A, not applicable; ESAS, Edmonton System assessment system; GDS, Geriatric Depression Scale; AML, acute myeloid leukaemia; ALL, acute lymphocytic leukaemia; MQOL‐E, McGill Quality of Life Questionnaire; MDS, myelodysplastic syndrome; MM, multiple myeloma; SCT, stem cell transplantation; CLL/SLL, chronic lymphocytic leukaemia and small lymphocytic leukaemia; ASD, acute stress disorder; e‐PRO, electronic patient reported outcomes; APN, advance practice nurse.
Primary outcomes appear in bold.
All trials listed are registered in clinicaltrials.gov.
MODELS FOR PC IN HAEMATOLOGY
PC is provided by interdisciplinary teams, which allows delivery of multidimensional care addressing the complex supportive care needs of people facing life threatening diseases. 20 , 21 However, there is no single model of PC that is appropriate for all settings. Instead, the model of PC provision will depend on national health policies, access to training and education of specialized PC providers, availability of resources, societal and health professional attitudes towards PC, and the setting where PC is delivered. As well, models of care will depend on the disease trajectory, such that models developed for patients with solid tumours may not be appropriate for patients with HMs. Broadly, models of early PC delivery be categorized according to who delivers PC (i.e., primary, secondary and tertiary PC, discussed above); where PC is delivered; and how it is determined who receives PC (i.e., the referral process).
Who delivers care: primary, secondary and tertiary PC
Family physicians, haemato‐oncologists and palliative care physicians all have important roles in providing primary, secondary and tertiary care, respectively. The domains of palliative care and roles of each level of provider in delivering this care are outlined in Table 4, with the recognition that the boundaries between providers' roles are ill‐defined and are based on patient need as well as provider skill. As most HM patients are treated in cancer centres, most PC for HM patients is secondary, delivered by haemato‐oncology teams. Haemato‐oncologists should therefore have better PC training; specifically, HM residents should complete at least a one‐month rotation in PC, including information on symptom management, communication skills, cultural competence, multidisciplinary care, and ensuring timely referrals to PC specialists. 6 Haemato‐oncology team meetings with a palliative focus have been shown to foster a culture change allowing for more goal‐concordant care and should be implemented by haemato‐oncology teams. 85 Tertiary (specialized) PC provided by PC consultants should be available to provide specialized PC for more complex situations such as refractory symptoms, severe psychosocial distress, or family conflict. The referral process to tertiary PC will be discussed below.
TABLE 4.
Domains and providers of palliative care for patients with haematological malignancies
| Domain | Primary PC – Primary care providers | Secondary PC – Haemato‐oncology care teams | Tertiary PC – Specialty PC teams |
|---|---|---|---|
| Domain 1: Structure and processes of care |
|
|
|
| Domain 2: Physical aspects of care |
|
|
|
| Domain 3: Psychological and psychiatric aspects of care |
|
|
|
| Domain 4: Social aspects of care |
|
|
|
| Domain 5: Spiritual, religious, and existential aspects of care |
|
|
|
| Domain 6: Cultural aspects of care |
|
|
|
| Domain 7: Care of the patient nearing the EOL |
|
|
|
| Domain 8: Ethical and legal aspects of care |
|
|
|
Note: Adapted from Clinical Practice Guidelines for Quality Palliative Care, 4th edition 2018, National Coalition for Hospice and Palliative Care. 22
Abbreviations: PC, palliative care; HM, haematological malignancies, EOL, End of life.
Where care is delivered: outpatient, inpatient, home and virtual models
As mentioned above, most models of early PC with proven benefit for patients with advanced solid tumours involve routine involvement of specialized PC delivered in the outpatient setting, either through freestanding PC clinics or in an embedded within the oncology clinic (Table 1). The principle for these clinics is that the PC team provides support with symptom control, coping with illness, decision‐making and future planning, in a model that emphasizes care that is flexible, attentive, patient‐led and family‐centred. 86 This outpatient model works well for patients with solid tumours, who have an illness course that is relatively predictable, and for whom referral ideally occurs at the diagnosis of advanced illness. This model may be of benefit for patients with indolent HMs or for relatively fit patients with bone marrow failure. For these groups of patients, longer term outpatient involvement of PC may improve symptom control, provide psychosocial support, and assist with advance care planning. However, to date, there have been no randomized controlled trials of such an outpatient model for HMs.
For patients with aggressive HMs, inpatient treatment of their disease is necessary, and a model of concurrent early involvement of PC in the inpatient setting is appropriate. As mentioned above, there have been several trials that have demonstrated the feasibility and benefit of involving inpatient PC teams upon admission for treatment of acute leukaemia or stem cell transplantation, with benefits demonstrated long after discharge. 17 , 18 , 80 Providing further ad hoc telephone PC or psychosocial support after discharge may also help to ease the often‐challenging transition from hospital to home. 31
Home‐based PC is usually provided later in the course of advanced disease, when it becomes difficult for patients to travel to and from the hospital, and when the focus is exclusively on EOL care. The further expansion of home‐based PC earlier into the disease trajectory, particularly in patients with bone marrow failure, would be much enabled by the capacity to provide transfusions of blood products in the home setting. This has been implemented in some countries with negligible risk. 41 , 42 , 87 However, in most countries home blood transfusions are impossible due to regulatory and financial constraints, presenting a substantial barrier for early PC in the home setting for HM patients. 88
A novel mode for PC delivery is telemedicine, in which PC is delivered via telephone or video conference to the patient. 89 Telemedicine in PC has become increasingly common during the COVID‐19 pandemic, due to the requirement for social distancing and reluctance of many patients to come to the hospital. 90 , 91 This model of care is particularly promising for patients who are receiving care in outpatient or home settings, to avoid the necessity for travel on the part of the patient or clinician and provide more efficient care. Randomized controlled trials of virtual PC are needed to determine its effectiveness compared to in‐person models.
How specialized PC referral is determined: clinician‐initiated and automated systems for aggressive HMs
Referral to specialized PC can either be based on judgement of the referring clinician or automated in some way. Referral initiated on clinical judgement by oncologists or haemato‐oncologists is currently the most popular method and remains synonymous with usual care, both in practice and for clinical trials. 20 , 92 Oncologists are increasingly making early referrals to PC services at their centres for patients with solid tumours, thanks to the solid evidence favouring multidisciplinary care and the policies and advocacy supporting this approach. 93 On the other hand, haemato‐oncologists face several barriers to effectively integrating PC into their practice under this model, as described above. 64 , 92 Therefore, despite the high symptom burden and needs, clinician‐initiated referral results in most patients diagnosed with HMs being referred very late or never to PC. 26 , 48
Interventions in most existing randomized controlled trials both in solid tumours and for HMs have consisted of routine referral for all patients shortly after the diagnosis of advanced disease (in solid tumours), 72 or shortly after admission for autologous or allogeneic haematopoietic stem cell transplantation or for acute leukaemia treatment. 17 , 18 , 19 Automatic referral to PC upon admission to hospital for treatment led to improved outcomes in two randomized controlled trials: one in HMs requiring stem cell transplantation and the other in acute leukaemia (Table 2). 17 , 18 , 80 Compared to usual care, patients receiving automatically specialized PC experienced better quality of life and reduced psychological symptoms, including depression, anxiety, and post‐traumatic stress disorder. Therefore, automatic referral upon admission for the group of HMs that includes aggressive life‐threatening diseases with a high risk of harm (alongside a realistic possibility for long‐term cure) would be ideal in settings with sufficient PC resources.
An alternate model to automatic referral entails referral to specialized PC according to specific triggers (e.g., moderate symptoms, severe psychological distress). As yet, there is no conclusive evidence for this model in solid tumours or HMs. However, a phase II trial of an intervention entitled Symptom screening with Targeted Early PC (STEP) demonstrated the feasibility of such a model in patients with solid malignancies. 94 Here, patients attending outpatient oncology clinics were screened at each visit for symptoms using the Edmonton Symptom Assessment System; patients with moderate physical (e.g., pain, dyspnea, nausea) or psychological (e.g., depression, anxiety) symptoms, or with severe constitutional symptoms (e.g., fatigue, appetite), received a phone call from a PC nurse who discussed their symptoms and offered a formal PC appointment.
Lastly, the EASE intervention described above involved both automated and triggered components. 19 In this intervention, patients with newly diagnosed or relapsed acute leukaemia who were randomized to the EASE intervention received an automated referral to a psychosocial clinician who provided a tailored psychotherapy delivered over 8 weeks, and weekly physical symptom screening over 8 weeks, with a triggered referral to an inpatient PC team if symptoms were scored above a certain threshold. The intervention was found to be feasible, with promising results for several patient‐reported outcomes: significant treatment‐group differences favouring EASE were observed in traumatic stress symptoms at 4 and 12 weeks, and in pain intensity and interference at 12 weeks.
Possible models for patients with bone marrow failure and indolent HMs
To date, all RCTs have included patient with aggressive disease: either patients with acute leukaemia or those undergoing stem cell transplantation. The trajectory as well as the aggressive, high‐risk, high‐gain nature of these diseases are mirrored in the models of triggered inpatient PC that have been proven to be of benefit. For other disease trajectories, different models may be needed. In indolent relapsing–remitting diseases, there are often challenges of illness understanding and advance care planning due to prognostic uncertainty, and symptoms such as pain and fatigue may also be prominent. For this patient population, the early outpatient integration of specialized PC, with its expertise in symptom management as well as complex decision making may be most beneficial. This could occur using preapproved triggers of symptom severity and may also result from improved referrals due to increased awareness of PC among haemato‐oncologists. As well, bone marrow failure diseases may demand a rethinking and adaptation of home‐based PC and hospice care. The low risk of home transfusion may be vastly outweighed by cultural, bureaucratic, and financial barriers that need to be removed in order to allow for proper care at home. For many of these patients, home care may not be feasible as the need for complex inpatient interventions may be needed to achieve goals of care. In these cases, hospice or PC units may need to adapt to the patients’ complex needs.
CONCLUSIONS AND FUTURE DIRECTIONS
As evidence of the benefits of PC in HM continues to evolve, our understanding of the unique needs of this population continues to grow. Existing models of early tertiary PC for HM are based on specialist PC intervention triggered by admission for inpatient treatment for aggressive HMs, and have demonstrated benefit in randomized controlled trials. 17 , 18 , 19 Future trials of outpatient PC for patients with multiple myeloma and of enabling blood transfusions in hospice settings are underway and will pave the way to enabling better integration of PC for HMs with indolent disease and with bone marrow failure.
In addition to further research on models of tertiary PC, secondary PC services by haemato‐oncologists need to be improved, both through better training for haemato‐oncologists in PC and by expansion of HM research into the PC needs of this population. A PC rotation incorporated into residency training for haemato‐oncological oncologists would provide insight into the possible benefits of integrating PC earlier in the disease trajectory and provide basic education in symptom management, communication skills and advance care planning. Importantly, progress in the integration of PC for patients with HM will necessitate a mutual understanding and collaboration between haemato‐oncologists and PC providers across all levels of care.
As the understanding of the biology of HM and targeted treatments continue to progress, HM patients will continue to face challenges in maintaining quality of life and in making treatment decisions in the context of prognostic uncertainty. With its interdisciplinary and holistic approach, PC has much to offer these patients. Increased understanding of PC by haemato‐oncologists as well as improved models for integration of tertiary PC will open the door to improved quality of care for this underserved population.
AUTHOR CONTRIBUTIONS
Conception and design: AS, AA, CZ. Study implementation, acquisition and/or assembly of data: All authors. Analysis and interpretation of data: All authors. Manuscript writing: All authors. Final approval of manuscript to be published: All authors.
CONFLICT OF INTEREST
The authors report no conflict of interests.
COMPLIANCE WITH ETHICAL STANDARDS
Not applicable (review paper).
PRIOR PRESENTATION
None.
ACKNOWLEDGEMENTS
CZ holds the Harold and Shirley Lederman Chair in Palliative Care and Psychosocial Oncology, a joint chair at the Princess Margaret Cancer Centre, University Health Network, and University of Toronto. The funders of the study had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Shaulov A, Aviv A, Alcalde J, Zimmermann C. Early integration of palliative care for patients with haematological malignancies. Br J Haematol. 2022;199(1):14–30. 10.1111/bjh.18286
REFERENCES
- 1. Ryan S, Wong J, Chow R, Zimmermann C. Evolving definitions of palliative care: upstream migration or confusion? Curr Treat Options in Oncol. 2020;21(3):20. [DOI] [PubMed] [Google Scholar]
- 2. Hui D, De La Rosa A, Chen J, Dibaj S, Delgado Guay M, Heung Y, et al. State of palliative care services at US cancer centers: an updated national survey. Cancer. 2020;126(9):2013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathews J, Zimmermann C. Palliative care services at cancer centres — room for improvement. Nat Rev Clin Oncol. 2020;17(6):339–40. [DOI] [PubMed] [Google Scholar]
- 4. Ferrell BR, Temel JS, Temin S, Alesi ER, Balboni TA, Basch EM, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(1):96–112. [DOI] [PubMed] [Google Scholar]
- 5. Jordan K, Aapro M, Kaasa S, Ripamonti CI, Scotté F, Strasser F, et al. European Society for Medical Oncology (ESMO) position paper on supportive and palliative care. Ann Oncol. 2018;29(1):36–43. [DOI] [PubMed] [Google Scholar]
- 6. Kaasa S, Loge JH, Aapro M, Albreht T, Anderson R, Bruera E, et al. Integration of oncology and palliative care: a Lancet Oncology Commission. Lancet Oncol. 2018;19(11):e588–653. [DOI] [PubMed] [Google Scholar]
- 7. Osman H, Shrestha S, Temin S, Ali ZV, Corvera RA, Ddungu HD, et al. Palliative care in the global setting: ASCO Resource‐Stratified Practice Guideline. J Glob Oncol. 2018;4:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO|WHO Definition of Palliative Care [Internet]. WHO. [cited 2018 Jan 27]. Available from: http://www.who.int/cancer/palliative/definition/en/
- 9. Howell DA, Shellens R, Roman E, Garry AC, Patmore R, Howard MR. Haematological malignancy: are patients appropriately referred for specialist palliative and hospice care? A systematic review and meta‐analysis of published data. Palliat Med. 2011;25(6):630–41. [DOI] [PubMed] [Google Scholar]
- 10. Hui D, Didwaniya N, Vidal M, Shin SH, Chisholm G, Roquemore J, et al. Quality of end‐of‐life care in patients with hematologic malignancies: a retrospective cohort study. Cancer. 2014;120(10):1572–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. LeBlanc TW, Abernethy AP, Casarett DJ. What is different about patients with hematologic malignancies? A retrospective cohort study of cancer patients referred to a hospice research network. J Pain Symptom Manag. 2015;49(3):505–12. [DOI] [PubMed] [Google Scholar]
- 12. LeBlanc TW, Smith JM, Currow DC. Symptom burden of haematological malignancies as death approaches in a community palliative care service: a retrospective cohort study of a consecutive case series. Lancet Haematol. 2015;2(8):e334–8. [DOI] [PubMed] [Google Scholar]
- 13. Boucher NA, Johnson KS, LeBlanc TW. Acute leukemia patients' needs: qualitative findings and opportunities for early palliative care. J Pain Symptom Manag. 2018;55(2):433–9. [DOI] [PubMed] [Google Scholar]
- 14. Rodin G, Yuen D, Mischitelle A, Minden MD, Brandwein J, Schimmer A, et al. Traumatic stress in acute leukemia. Psychooncology. 2013;22(2):299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hannon B, Swami N, Pope A, Leighl N, Rodin G, Krzyzanowska M, et al. Early palliative care and its role in oncology: a qualitative study. Oncologist. 2016;21(11):1387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hannon B, Swami N, Rodin G, Pope A, Zimmermann C. Experiences of patients and caregivers with early palliative care: a qualitative study. Palliat Med. 2017;31(1):72–81. [DOI] [PubMed] [Google Scholar]
- 17. El‐Jawahri A, LeBlanc T, VanDusen H, Traeger L, Greer JA, Pirl WF, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA. 2016;316(20):2094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. El‐Jawahri A, LeBlanc TW, Kavanaugh A, Webb JA, Jackson VA, Campbell TC, et al. Effectiveness of integrated palliative and oncology care for patients with acute myeloid leukemia: a randomized clinical trial. JAMA Oncol. 2021;7(2):238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodin G, Malfitano C, Rydall A, Schimmer A, Marmar CM, Mah K, et al. Emotion and Symptom‐focused Engagement (EASE): a randomized phase II trial of an integrated psychological and palliative care intervention for patients with acute leukemia. Support Care Cancer. 2020;28(1):163–76. [DOI] [PubMed] [Google Scholar]
- 20. Mathews J, Hannon B, Zimmermann C. Models of integration of specialized palliative care with oncology. Curr Treat Options in Oncol. 2021;22(5):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hui D, Hannon BL, Zimmermann C, Bruera E. Improving patient and caregiver outcomes in oncology: team‐based, timely, and targeted palliative care. CA Cancer J Clin. 2018;68(5):356–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahluwalia SC, Chen C, Raaen L, Motala A, Walling AM, Chamberlin M, et al. A systematic review in support of the National Consensus Project Clinical Practice Guidelines for quality palliative care, fourth edition. J Pain Symptom Manag. 2018;56(6):831–70. [DOI] [PubMed] [Google Scholar]
- 23. Hannon B, Kaasa S, Zimmermann C. Specialist palliative care along the trajectory of illness: Issues in the early integration of palliative care. Oxford textbook of palliative medicine. 6th ed. Oxford (United Kingdom): Oxford University Press; 2022. [Google Scholar]
- 24. Wedding U. Palliative care of patients with haematological malignancies: strategies to overcome difficulties via integrated care. Lancet Healthy Longev. 2021;2(11):e746–53. [DOI] [PubMed] [Google Scholar]
- 25. El‐Jawahri A, Nelson AM, Gray TF, Lee SJ, LeBlanc TW. Palliative and end‐of‐life care for patients with hematologic malignancies. J Clin Oncol. 2020;38(9):944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shaulov A, Rodin G, Popovic G, Caraiscos VB, Le LW, Rydall A, et al. Pain in patients with newly diagnosed or relapsed acute leukemia. Support Care Cancer. 2019;27(8):2789–97. [DOI] [PubMed] [Google Scholar]
- 27. LeBlanc TW, Fish LJ, Bloom CT, El‐Jawahri A, Davis DM, Locke SC, et al. Patient experiences of acute myeloid leukemia: a qualitative study about diagnosis, illness understanding, and treatment decision‐making. Psychooncology. 2017;26(12):2063–8. [DOI] [PubMed] [Google Scholar]
- 28. Rodin G, Deckert A, Tong E, Le LW, Rydall A, Schimmer A, et al. Traumatic stress in patients with acute leukemia: a prospective cohort study. Psychooncology. 2017;27:515–23. [DOI] [PubMed] [Google Scholar]
- 29. Nissim R, Zimmermann C, Minden M, Rydall A, Yuen D, Mischitelle A, et al. Abducted by the illness: a qualitative study of traumatic stress in individuals with acute leukemia. Leuk Res. 2013;37(5):496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gheihman G, Zimmermann C, Deckert A, Fitzgerald P, Mischitelle A, Rydall A, et al. Depression and hopelessness in patients with acute leukemia: the psychological impact of an acute and life‐threatening disorder. Psychooncology. 2016;25(8):979–89. [DOI] [PubMed] [Google Scholar]
- 31. Nissim R, Rodin G, Schimmer A, Minden M, Rydall A, Yuen D, et al. Finding new bearings: a qualitative study on the transition from inpatient to ambulatory care of patients with acute myeloid leukemia. Support Care Cancer. 2014;22(9):2435–43. [DOI] [PubMed] [Google Scholar]
- 32. Johnson PC, Jacobson C, Yi A, Saucier A, Dhawale TM, Nelson A, et al. Healthcare utilization and end‐of‐life outcomes in patients receiving CAR T‐cell therapy. J Natl Compr Cancer Netw. 2021;19(8):928–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niscola P, Scaramucci L, Romani C, Giovannini M, Tendas A, Brunetti G, et al. Pain management in multiple myeloma. Expert Rev Anticancer Ther. 2010;10(3):415–25. [DOI] [PubMed] [Google Scholar]
- 34. Niscola P, Tendas A, Scaramucci L, Giovaninni M, Cupelli L, De Sanctis V, et al. Pain in malignant hematology. Expert Rev Hematol. 2011;4(1):81–93. [DOI] [PubMed] [Google Scholar]
- 35. Li T, Timmins HC, Lazarus HM, Park SB. Peripheral neuropathy in hematologic malignancies – Past, present and future. Blood Rev. 2020;43:100653. [DOI] [PubMed] [Google Scholar]
- 36. Ramsenthaler C, Osborne TR, Gao W, Siegert RJ, Edmonds PM, Schey SA, et al. The impact of disease‐related symptoms and palliative care concerns on health‐related quality of life in multiple myeloma: a multi‐centre study. BMC Cancer. 2016;16(1):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Molassiotis A, Wilson B, Blair S, Howe T, Cavet J. Unmet supportive care needs, psychological well‐being and quality of life in patients living with multiple myeloma and their partners. Psychooncology. 2011;20(1):88–97. [DOI] [PubMed] [Google Scholar]
- 38. Maatouk I, He S, Hummel M, Hemmer S, Hillengass M, Goldschmidt H, et al. Patients with precursor disease exhibit similar psychological distress and mental HRQOL as patients with active myeloma. Blood Cancer J. 2019;9(2):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hildebrandt MAT, Callender RA, Belachew AA, Classen C, Lee HC, Patel KK, et al. Quality of life and cancer worry in a follow‐up cohort of patients with asymptomatic monoclonal gammopathies. J Clin Oncol. 2018;36(15_suppl):8049. [Google Scholar]
- 40. Mannis GN, McNey LM, Gupta NK, Gross DM. The transfusion tether: bridging the gap between end‐stage hematologic malignancies and optimal end‐of‐life care. Am J Hematol. 2016;91(4):364–5. [DOI] [PubMed] [Google Scholar]
- 41. Ademokun A, Kaznica S, Deas S. Home blood transfusion: a necessary service development. Transfus Med. 2005;15(3):219–22. [DOI] [PubMed] [Google Scholar]
- 42. García D, Aguilera A, Antolín F, Arroyo JL, Lozano M, Sanroma P, et al. Home transfusion: three decades of practice at a tertiary care hospital. Transfusion. 2018;58(10):2309–19. [DOI] [PubMed] [Google Scholar]
- 43. Benson K, Balducci L, Milo KM, Heckel L, Lyman GH. Patients' attitudes regarding out‐of‐hospital blood transfusion. Transfusion. 1996;36(2):140–3. [DOI] [PubMed] [Google Scholar]
- 44. Barki‐Harrington L, Baron‐Epel O, Shaulov A, Akria L, Barshay Y, Dally N, et al. Willingness and concerns of transfusion‐dependent hematological patients toward the option of home transfusion therapy. Palliat Med. 2021;35(5):927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hochman MJ, Yu Y, Wolf SP, Samsa GP, Kamal AH, LeBlanc TW. Comparing the palliative care needs of patients with hematologic and solid malignancies. J Pain Symptom Manag. 2018;55(1):82–88.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fadul NA, El Osta B, Dalal S, Poulter VA, Bruera E. Comparison of symptom burden among patients referred to palliative care with hematologic malignancies versus those with solid tumors. J Palliat Med. 2008;11(3):422–7. [DOI] [PubMed] [Google Scholar]
- 47. Cheng WW, Willey J, Palmer JL, Zhang T, Bruera E. Interval between palliative care referral and death among patients treated at a comprehensive cancer center. J Palliat Med. 2005;8(5):1025–32. [DOI] [PubMed] [Google Scholar]
- 48. Zimmermann C, Yuen D, Mischitelle A, Minden MD, Brandwein JM, Schimmer A, et al. Symptom burden and supportive care in patients with acute leukemia. Leuk Res. 2013;37(7):731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. LeBlanc TW, O'Donnell JD, Crowley‐Matoka M, Rabow MW, Smith CB, White DB, et al. Perceptions of palliative care among hematologic malignancy specialists: a mixed‐methods study. J Oncol Pract. 2015;11(2):e230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. LeBlanc TW, El‐Jawahri A. When and why should patients with hematologic malignancies see a palliative care specialist? Hematology Am Soc Hematol Educ Program. 2015;2015:471–8. [DOI] [PubMed] [Google Scholar]
- 51. Selvaggi KJ, Vick JB, Jessell SA, Lister J, Abrahm JL, Bernacki R. Bridging the gap: a palliative care consultation service in a hematological malignancy‐bone marrow transplant unit. J Community Support Oncol. 2014;12(2):50–5. [DOI] [PubMed] [Google Scholar]
- 52. Howell DA, Roman E, Cox H, Smith AG, Patmore R, Garry AC, et al. Destined to die in hospital? Systematic review and meta‐analysis of place of death in haematological malignancy. BMC Palliat Care. 2010;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davis MP, Bruera E, Morganstern D. Early integration of palliative and supportive care in the cancer continuum: challenges and opportunities. Am Soc Clin Oncol Educ Book. 2013;33:144–50. [DOI] [PubMed] [Google Scholar]
- 54. Beaussant Y, Daguindau E, Chauchet A, Rochigneux P, Tournigand C, Aubry R, et al. Hospital end‐of‐life care in haematological malignancies. BMJ Support Palliat Care. 2018;8(3):314–24. [DOI] [PubMed] [Google Scholar]
- 55. Ho TH, Barbera L, Saskin R, Lu H, Neville BA, Earle CC. Trends in the aggressiveness of end‐of‐life cancer care in the universal health care system of Ontario. Canada J Clin Oncol. 2011;29(12):1587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Odejide OO, Cronin AM, Condron N, Earle CC, Wolfe J, Abel GA. Timeliness of end‐of‐life discussions for blood cancers: a national survey of hematologic oncologists. JAMA Intern Med. 2016;176(2):263–5. [DOI] [PubMed] [Google Scholar]
- 57. Hui D, Bansal S, Park M, Reddy A, Cortes J, Fossella F, et al. Differences in attitudes and beliefs toward end‐of‐life care between hematologic and solid tumor oncology specialists. Ann Oncol. 2015;26(7):1440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jang RW, Caraiscos VB, Swami N, Banerjee S, Mak E, Kaya E, et al. Simple prognostic model for patients with advanced cancer based on performance status. J Oncol Pract. 2014;10(5):e335–41. [DOI] [PubMed] [Google Scholar]
- 59. Button E, Chan RJ, Chambers S, Butler J, Yates P. A systematic review of prognostic factors at the end of life for people with a hematological malignancy. BMC Cancer. 2017;17(1):213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Button E, Chan R, Chambers S, Butler J, Yates P. Signs, symptoms, and characteristics associated with end of life in people with a hematologic malignancy: a review of the literature. Oncol Nurs Forum. 2016;43(5):E178–87. [DOI] [PubMed] [Google Scholar]
- 61. Odejide OO, Salas Coronado DY, Watts CD, Wright AA, Abel GA. End‐of‐life care for blood cancers: a series of focus groups with hematologic oncologists. J Oncol Pract. 2014;10(6):e396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McGrath P. End‐of‐life care in hematology: update from Australia. J Soc Work End Life Palliat Care. 2013;9(1):96–110. [DOI] [PubMed] [Google Scholar]
- 63. LeBlanc TW. Palliative care and hematologic malignancies: old dog, new tricks? J Oncol Pract. 2014;10(6):e404–7. [DOI] [PubMed] [Google Scholar]
- 64. Zimmermann C. Palliative care for patients with hematological malignancies: time for a new model. Leuk Res. 2016;48:78–9. [DOI] [PubMed] [Google Scholar]
- 65. Odejide OO, Cronin AM, Earle CC, Tulsky JA, Abel GA. Why are patients with blood cancers more likely to die without hospice? Cancer. 2017;123(17):3377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Booker R, Dunn S, Earp MA, Sinnarajah A, Biondo PD, Simon JE. Perspectives of hematology oncology clinicians about integrating palliative care in oncology. Curr Oncol. 2020;27(6):313–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheng BHW, Sham MMK, Chan KY, Li CW, Au HY. Intensive palliative care for patients with hematological cancer dying in hospice: analysis of the level of medical care in the final week of life. Am J Hosp Palliat Care. 2015;32(2):221–5. [DOI] [PubMed] [Google Scholar]
- 68. Odejide OO. A policy prescription for hospice care. JAMA. 2016;315(3):257–8. [DOI] [PubMed] [Google Scholar]
- 69. ASH Statement in Support of Palliative Blood Transfusions in Hospice Setting [Internet]. [cited 2022 Jan 31]. Available from: https://www.hematology.org:443/advocacy/policy‐statements/2019/palliative‐blood‐transfusions‐in‐hospice
- 70. ASH President Applauds Introduction of Legislation for Palliative Blood Transfusions ‐ Hematology.org [Internet]. [cited 2022 Jan 31]. Available from: https://www.hematology.org:443/newsroom/press‐releases/2021/ash‐president‐applauds‐introduction‐of‐legislation‐for‐palliative‐blood‐transfusions
- 71. Zimmermann C, Swami N, Krzyzanowska M, Hannon B, Leighl N, Oza A, et al. Early palliative care for patients with advanced cancer: a cluster‐randomised controlled trial. Lancet. 2014;383(9930):1721–30. [DOI] [PubMed] [Google Scholar]
- 72. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med. 2010;363(8):733–42. [DOI] [PubMed] [Google Scholar]
- 73. Temel JS, Greer JA, El‐Jawahri A, Pirl WF, Park ER, Jackson VA, et al. Effects of Early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol. 2017;35(8):834–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Maltoni M, Scarpi E, Dall'Agata M, Zagonel V, Bertè R, Ferrari D, et al. Systematic versus on‐demand early palliative care: results from a multicentre, randomised clinical trial. Eur J Cancer. 2016;65:61–8. [DOI] [PubMed] [Google Scholar]
- 75. Bakitas M, Lyons KD, Hegel MT, Balan S, Brokaw FC, Seville J, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: the Project ENABLE II randomized controlled trial. JAMA. 2009;302(7):741–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Scarpi E, Dall'Agata M, Zagonel V, Gamucci T, Bertè R, Sansoni E, et al. Systematic vs. on‐demand early palliative care in gastric cancer patients: a randomized clinical trial assessing patient and healthcare service outcomes. Support Care Cancer. 2019;27(7):2425–34. [DOI] [PubMed] [Google Scholar]
- 77. Vanbutsele G, Pardon K, Van Belle S, Surmont V, De Laat M, Colman R, et al. Effect of early and systematic integration of palliative care in patients with advanced cancer: a randomised controlled trial. Lancet Oncol. 2018;19(3):394–404. [DOI] [PubMed] [Google Scholar]
- 78. Franciosi V, Maglietta G, Degli Esposti C, Caruso G, Cavanna L, Bertè R, et al. Early palliative care and quality of life of advanced cancer patients‐a multicenter randomized clinical trial. Ann Palliat Med. 2019;8(4):381–9. [DOI] [PubMed] [Google Scholar]
- 79. Groenvold M, Petersen MA, Damkier A, Neergaard MA, Nielsen JB, Pedersen L, et al. Randomised clinical trial of early specialist palliative care plus standard care versus standard care alone in patients with advanced cancer: the Danish palliative care trial. Palliat Med. 2017;31(9):814–24. [DOI] [PubMed] [Google Scholar]
- 80. El‐Jawahri A, Traeger L, Greer JA, VanDusen H, Fishman SR, LeBlanc TW, et al. Effect of inpatient palliative care during hematopoietic stem‐cell transplant on psychological distress 6 months after transplant: results of a randomized clinical trial. J Clin Oncol. 2017;35(32):3714–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Loggers ET, LeBlanc TW, El‐Jawahri A, Fihn J, Bumpus M, David J, et al. Pretransplantation supportive and palliative care consultation for high‐risk hematopoietic cell transplantation patients. Biol Blood Marrow Transplant. 2016;22(7):1299–305. [DOI] [PubMed] [Google Scholar]
- 82. Grudzen CR, Richardson LD, Johnson PN, Hu M, Wang B, Ortiz JM, et al. Emergency department‐initiated palliative care in advanced cancer: a randomized clinical trial. JAMA Oncol. 2016;2(5):591–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Olszewski A. Removing Transfusion Dependence as a Barrier to Hospice Enrollment [Internet]. clinicaltrials.gov; 2021 Nov [cited 2022 Feb 3]. Report No.: NCT05063591. Available from: https://clinicaltrials.gov/ct2/show/NCT05063591
- 84. Case Comprehensive Cancer Center . A Pilot Study to Determine the Effects of Early Palliative Care Integration on Patients With Newly Diagnosed Multiple Myeloma [Internet]. clinicaltrials.gov; 2021 Jun [cited 2022 Feb 3]. Report No.: NCT04248244. Available from: https://clinicaltrials.gov/ct2/show/NCT04248244
- 85. Ofran Y, Bar‐Sela G, Toledano M, Kushnir I, Moalem B, Gil W, et al. Palliative care service incorporated in a hematology department: a working model fostering changes in clinical practice. Leuk Lymphoma. 2019;60(8):2079–81. [DOI] [PubMed] [Google Scholar]
- 86. Zimmermann C, Ryan S, Hannon B, Saltman A, Rodin G, Mak E, et al. Team‐based outpatient early palliative care: a complex cancer intervention. BMJ Support Palliat Care. 2019;1–10. [DOI] [PubMed] [Google Scholar]
- 87. Devlin B, Agnew A. An evaluation of a domiciliary blood transfusion service for palliative care patients in Northern Ireland. Community Pract. 2008;81(7):32–5. [PubMed] [Google Scholar]
- 88. Benson K. Home is where the heart is: do blood transfusions belong there too? Transfus Med Rev. 2006;20(3):218–29. [DOI] [PubMed] [Google Scholar]
- 89. Worster B, Swartz K. Telemedicine and palliative care: an increasing role in supportive oncology. Curr Oncol Rep. 2017;19(6):37. [DOI] [PubMed] [Google Scholar]
- 90. Calton B, Abedini N, Fratkin M. Telemedicine in the time of coronavirus. J Pain Symptom Manag. 2020;60(1):e12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ritchey KC, Foy A, McArdel E, Gruenewald DA. Reinventing palliative care delivery in the era of COVID‐19: how telemedicine can support end of life care. Am J Hosp Palliat Care. 2020;37(11):992–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wentlandt K, Krzyzanowska MK, Swami N, Rodin GM, Le LW, Zimmermann C. Referral practices of oncologists to specialized palliative care. J Clin Oncol. 2012;30(35):4380–6. [DOI] [PubMed] [Google Scholar]
- 93. Hausner D, Tricou C, Mathews J, Wadhwa D, Pope A, Swami N, et al. Timing of palliative care referral before and after evidence from trials supporting early palliative care. Oncologist. 2021;26(4):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zimmermann C, Pope A, Hannon B, Krzyzanowska MK, Rodin G, Li M, et al. Phase II trial of symptom screening with targeted early palliative care for patients with advanced cancer. J Natl Compr Cancer Netw. 2021;20(4):361–70.e3. [DOI] [PubMed] [Google Scholar]
- 95. McDonald J, Swami N, Hannon B, Lo C, Pope A, Oza A, et al. Impact of early palliative care on caregivers of patients with advanced cancer: cluster randomised trial. Ann Oncol. 2017;28(1):163–8. [DOI] [PubMed] [Google Scholar]
- 96. Maltoni M, Scarpi E, Dall'Agata M, Schiavon S, Biasini C, Codecà C, et al. Systematic versus on‐demand early palliative care: a randomised clinical trial assessing quality of care and treatment aggressiveness near the end of life. Eur J Cancer. 2016;69:110–8. [DOI] [PubMed] [Google Scholar]
- 97. Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Heist RS, et al. Effect of early palliative care on chemotherapy use and end‐of‐life care in patients with metastatic non‐small‐cell lung cancer. J Clin Oncol. 2012;30(4):394–400. [DOI] [PubMed] [Google Scholar]
- 98. Temel JS, Greer JA, Admane S, Gallagher ER, Jackson VA, Lynch TJ, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non‐small‐cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol. 2011;29(17):2319–26. [DOI] [PubMed] [Google Scholar]
- 99. El‐Jawahri A, Greer JA, Pirl WF, Park ER, Jackson VA, Back AL, et al. Effects of early integrated palliative care on caregivers of patients with lung and gastrointestinal cancer: a randomized clinical trial. Oncologist. 2017;22(12):1528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Vanbutsele G, Van Belle S, Surmont V, De Laat M, Colman R, Eecloo K, et al. The effect of early and systematic integration of palliative care in oncolOgy on quality of life and health care use near the end of life: a randomised controlled trial. Eur J Cancer. 2020;124:186–93. [DOI] [PubMed] [Google Scholar]
- 101. Bakitas MA, Tosteson TD, Li Z, Lyons KD, Hull JG, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the enable iii randomized controlled trial. J Clin Oncol. 2015;33(13):1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tanzi S, Luminari S, Cavuto S, Turola E, Ghirotto L, Costantini M. Early palliative care versus standard care in haematologic cancer patients at their last active treatment: study protocol of a feasibility trial. BMC Palliat Care. 2020;19(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Scarfò L, Karamanidou C, Doubek M, Garani‐Papadatos T, Didi J, Pontikoglou C, et al. MyPal ADULT study protocol: a randomised clinical trial of the MyPal ePRO‐based early palliative care system in adult patients with haematological malignancies. BMJ Open. 2021;11(11):e050256. [DOI] [PMC free article] [PubMed] [Google Scholar]


