Abstract
Introduction
The development of inhibitors with factor VIII (FVIII) replacement therapy is one of the most common and challenging complications of haemophilia A (HA) treatment, jeopardising treatment efficacy and predisposing patients to high risks of morbidity and mortality. The management of patients with inhibitors is particularly challenging in countries where resources are limited.
Aim
To provide a comprehensive summary of the management of HA with inhibitors while focusing on differences in practice between Western and non‐Western countries and how resource scarcity can impact HA management, leading to suboptimal outcomes in patients with inhibitors.
Methods
Summary of key evidence and regional expert opinion.
Results
We address, particularly, the diagnosis of and testing for inhibitors, as well as the epidemiology of inhibitors, including incidence, prevalence and disease burden. Secondly, we provide an overview of the current treatment landscape in HA with inhibitors regarding the eradication of inhibitors with immune tolerance induction and the treatment and prevention of bleeding with bypassing agents, non‐factor replacement agents and other experimental therapies. This is complemented with insights from the authors around the applicability of, and challenges associated with, such therapies in their settings of practice.
Conclusions
We conclude by proposing some key steps towards bridging the gaps in the management of HA with inhibitors in resource‐limited countries, including: (1) the collection of quality data that can inform healthcare reforms and policies; (2) improving disease knowledge among healthcare practitioners and patients with the aim of standardising disease management across centres and (3) working towards promoting equal access to HA care and therapies for everyone.
Keywords: bypassing agents, factor replacement therapy, haemophilia A, immune tolerance induction, inhibitors, non‐factor replacement agents
1. INTRODUCTION
Replacement therapy with factor VIII (FVIII) concentrates is the standard of care for treating acute bleeding episodes and preventing long‐term bleeding in haemophilia A (HA). 1 , 2 , 3 The development of neutralising antibodies or inhibitors following treatment with FVIII replacement therapy is one of the most common and challenging complications of HA treatment, 4 compromising the treatment's haemostatic efficacy and predisposing patients to high risks of morbidity and mortality. 5
Despite the availability of management standards for HA and the recent development of multiple innovative therapeutic approaches that can prevent or treat bleeding and reduce disease burden in patients, such as extended half‐life products, non‐factor replacement products and gene therapy, treatment of patients with inhibitors remains particularly challenging in some countries owing to resource constraints at both diagnostic and therapeutic levels, rendering international guidelines for care unfeasible and hardly achievable. In addition, there are limited data on experience with management options in HA patients with inhibitors that can guide the development of local recommendations in such resource‐limited settings.
With this background, the aim of this review is to provide an overview of the burden and management of HA with inhibitors while summarising published data from Western and non‐Western countries – particularly those where authors have long‐standing experience (Italy, UK, Turkey, India and Egypt) – to allow comparisons between standards of care (Table 1). The review will also feature insights from the authors’ own experience and approach to management in the real‐life setting to uncover challenges and opportunities in different regions and, finally, delineate areas of improvement in HA management to achieve enhanced care in patients with inhibitors.
TABLE 1.
Summary of differences in standards of care and inhibitor management between Western and non‐Western countries as reported by the authors
| UK | Italy | Turkey | India | Egypt | |
|---|---|---|---|---|---|
| Inhibitor diagnosis and testing |
|
|
|
|
|
| Morbidity/mortality associated with inhibitors |
|
|
|
|
|
| ITI adoption |
|
|
|
||
| BPA use |
|
|
|
|
|
| Use of non‐factor replacement agents |
|
|
|
|
|
aPCC, activated prothrombin complex concentrate; BPAs, bypassing agents; ED, exposure day; FVIII, factor VIII; ITI, immune tolerance induction; PUP, previously untreated patient; QoL, quality of life; rFVIIa; recombinant factor VIIa; WFH, World Federation of Hemophilia.
2. WHERE DO WE STAND?
2.1. Diagnosis and monitoring of inhibitors
Screening and testing for inhibitor development is an essential aspect of any comprehensive haemophilia programme and allows timely and adequate medical treatment and management of inhibitors; nevertheless, many centres around the world, particularly in countries with limited resources, do not have inhibitor testing resources or expertise, 6 or testing is too expensive to be performed regularly.
In our experience, patients in the UK are regularly evaluated, up to four times a year, as part of a comprehensive programme to monitor bleeding history, joint health, dental health and HIV status, among others, which consequently allows routine screening and timely detection of inhibitors. Likewise, in Italy, previously untreated patients (PUPs) are evaluated every five exposure days (EDs) up to 50 EDs, as recommended by the International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Committee (SSC). 7 Nevertheless, more physicians are initiating replacement therapy very early nowadays, which is resulting in fewer bleeding episodes and wider intervals between episodes; this warrants further evaluation and standardisation of the inhibitor development concept and, particularly, inhibitor testing. In our experience in Egypt, routine inhibitor screening is performed biannually following the World Federation of Hemophilia (WFH) recommendations. 6 In Turkey, the national inhibitor screening programme was launched 15 years ago, and educational workshops were set up for regional laboratory technicians. Nowadays, all centres have expert laboratory technicians, and inhibitor screening and testing is performed routinely in most of them every five EDs up to 50 EDs. Nevertheless, as in India, to avoid high testing costs, some centres provide cumulative screening tests by incubated mixing before performing the Nijmegen‐modified Bethesda assay (NBA) tests, which are only conducted for confirmation of the mixing studies. In India, inhibitor testing is performed opportunistically, only in patients who do not respond to treatment. Mass inhibitor testing is sometimes carried out with support from industry or in community‐based camps and provides only a snapshot of inhibitor status in the country. In addition, city‐to‐city variations and inequities in resource allocation exist across India; therefore, very few laboratories have the capacity or even the expertise to perform inhibitor testing. Furthermore, the majority of these laboratories lack centralisation, which makes reliable and standardised testing and reading even more challenging.
2.2. Incidence and prevalence of inhibitor development
Given that one‐third of inhibitors can be transient or successfully eliminated with appropriate treatment, the most accurate epidemiological measure of inhibitor development usually comes from prospective studies, including newly diagnosed PUPs, who are tested regularly for the presence of inhibitors. Globally, such studies have reported a cumulative incidence of inhibitor development in severe HA ranging widely between 2% and 52%, with most commonly reported figures falling between 20% and 40%. 5 , 8 , 9 , 10 , 11
Data from non‐Western countries are mainly based on cross‐sectional or retrospective studies. One recent study from Egypt reported a 22% prevalence of FVIII inhibitors in patients with severe HA, 12 while another reported a prevalence of 12% in moderate to severe disease. 13 In turkey, a large recent study, including 657 patients with severe HA, found 15.8% to be positive for inhibitors. 14 Other reports also indicated an overall inhibitor prevalence of around 14% and a prevalence of 16.7% in patients with severe disease. 15 , 16 Based on our clinical observation, inhibitor incidence in severe HA ranges between 15% and 20%, with almost 10% of PUPs developing inhibitors with plasma‐derived FVIIIs and 20%–25% with recombinants factors. Findings from the Survey of Inhibitors in Plasma‐Product Exposed Toddlers (SIPPET) trial, demonstrating that rFVIII products were associated with a higher risk of inhibitor development than plasma‐derived products in PUPs with HA, 11 together with those from the national Turkish registry, have led to updating of the Turkish haemophilia guidelines 17 to recommend the use of plasma‐derived products instead of recombinant ones. Nowadays, most Turkish paediatric haematologists are moving to recombinant FVIII products after the completion of 50 EDs with plasma products, which has significantly decreased the likelihood of inhibitor development. Previously, patients were developing inhibitors every year, but no new inhibitor cases have been reported for 4 years now. This was demonstrated in a preliminary analysis from the PUP‐SWITCH study, which has not reported inhibitor development to date in any of the first 39 patients who received a median of 111 EDs of rFVIII following a switch from plasma‐derived products after a median of 60 EDs. 18
In India, figures were similar or slightly lower, with one study conducted on a large series of patients with bleeding disorders reporting a prevalence of inhibitors of 6.1% from a total of 1285 HA patients. 19 Another study conducted through community‐based camps in haemophilia treatment centres in various parts of the country found that inhibitors were present in 19.5% of 447 evaluated patients, with high‐titre inhibitors detected in 35.6% of them. 20 Also, a recent study in 2019 recorded a prevalence of 26.2% and 7.40% for severe and moderate disease, respectively. 21
Such rates may be considered relatively low compared with commonly reported figures from Western countries. Several factors could explain this observation. Firstly, the study designs are different: prevalence studies can only capture the number of patients with inhibitors at one point in time, leaving out patients who develop inhibitors at a later point in their lives, those who develop transient inhibitors and those who had them successfully eliminated, thereby underestimating the actual numbers. One would also question the accuracy and robustness of inhibitor data from such countries, given that testing resources and expertise are scarce. Secondly, sample sizes were variable across the studies and were sometimes very small, which could also have affected the representativeness of these figures. Moreover, as inhibitors develop in patients who are treated with replacement therapy, small numbers could also reflect undertreatment in these countries/regions in particular. This seems to be at least the situation in India, where, based on our experience, patients receive delayed and only infrequent episodic replacement therapy secondary to delays in seeking treatment during bleeding episodes, which rarely results in peak treatment moments and thus subsequently leads to inhibitor development, especially in younger patients. Finally, geographical or ethnic differences in risk factors for inhibitor development could also be responsible for observed variations in epidemiology, although such differences were not detected across the 14 countries in the SIPPET trial. 11
3. WHY ARE WE CONCERNED?
Inhibitor development in HA has been associated with significant morbidity, including deterioration in quality of life (QoL), as well as with inflated healthcare costs. 22 Haemophilic arthropathy is a main clinical presentation in severe haemophilia as a consequence of recurrent haemarthrosis, 23 and its burden is greater in patients with inhibitors than in those without. A multicentre, case–control study across five European countries (France, Germany, Italy, Spain and UK) evaluated orthopaedic complications and QoL in patients with severe HA with and without inhibitors. 24 The study showed a higher level of arthropathy as assessed by Gilbert and Pettersson scores in patients with inhibitors compared with those without. From patient interviews, significantly more patients with inhibitors suffered joint pain and reduced mobility, with a greater need for wheelchairs and walking aids. Hospitalisation due to orthopaedic or musculoskeletal bleeding was also significantly more common in patients with inhibitors. Patients with inhibitors reported having significantly greater difficulties with mobility, daily activities, and pain/discomfort and had more absence from work or school because of orthopaedic complications than those without. 24 In another retrospective study involving haemophilia centres in Italy, inhibitor status was a strong independent predictor of intracranial haemorrhage (2.5‐fold increased risk). 25 In fact, based on observations from our practice in Italy, patients mainly suffered from poor QoL, which usually translated into high absenteeism from work or school; nevertheless, the availability of extended half‐life products and non‐replacement therapies during the past couple years has remarkably helped improve the QoL of patients who can now lead a close to normal life.
Examining data from non‐Western countries, a study conducted at a tertiary‐care teaching hospital in western India showed that patients with HA and inhibitors experienced several bleeding complications, including haemarthroses, haematomas, haematuria, neurological complications, mucous membrane haemorrhage, and dental and surgical bleeding. These happened at numerically higher rates in patients with inhibitors than in those without. 21 Although there is a scarcity of outcomes data in patients with inhibitors in India, we recognise from our experience that mortality due to cerebral bleeds is anecdotally elevated in this cohort. The prevalence of joint damage and pseudo‐tumours is also high in these patients because of untreated bleeds. Likewise, in Turkey, patients develop arthropathies and have poorer QoL than patients without inhibitors. In Egypt, patients experience severe complications, which similarly, are usually less common in those without inhibitors. The Epidemiological Study on Haemophilia Care and Orthopaedic Status in Developing Countries evaluated haemophilia‐related orthopaedic disease burden in 282 males with severe haemophilia from Algeria, India, Morocco, Oman and South Africa. The study included patients with and without inhibitors, treated on demand for bleeding. 26 Interestingly, the study showed that outcomes were similar and equally suboptimal in patients both with and without inhibitors, including in Hemophilia Joint Health score, Pettersson score and mean annualised bleeding rate (25.8 in the total population). Moreover, QoL scores were similar between the two groups. Based on these results, which contrasted with findings from Western countries, indicating that the absence of inhibitors is related to improved outcomes, the authors concluded that both patient groups (with and without inhibitors) were receiving suboptimal care in these resource‐limited countries. The finding was also confirmed by the very low rates of prior prophylactic therapy (11% of adults and 4% of paediatric patients). This indicates that treatment optimisation may be an overall unmet need rather than restricted to patients with inhibitors.
Data on mortality have been conflicting, with some studies reporting an increased risk of death among patients with inhibitors compared with those without inhibitors, 27 , 28 , 29 and others not showing any difference between the two groups. 30 , 31 It is difficult to comment on these inconsistencies, especially when no temporal trend that could reflect advances in treatment modalities could be observed. Nevertheless, one explanation could be the adoption of different definitions and classifications of inhibitor status in the various studies. In addition, the results might also be reflective of the quality of care that these patients are receiving in the corresponding countries. In Turkey, mortality in patients with inhibitors is low also because of prophylaxis treatment in this group.
There is consistent evidence that the cost of treating haemophilia with inhibitors significantly exceeds that of treating haemophilia without inhibitors. 26 , 32 , 33 , 34 In resource‐limited countries, where data are generally limited, this is further complicated by the limited availability and access to haemostatic treatment and haemophilia care in general, including orthopaedic care, leading to yet poorer outcomes in this group of patients, hence the importance of continuous research into inhibitor development to better understand the status of haemophilia care in these countries, with the aim of improving patients’ outcomes.
4. WHAT ARE OUR TREATMENT OPTIONS?
There are two aspects to the management of patients with inhibitors: the treatment and prevention of bleeding episodes, which requires the adoption of alternative haemostatic agents that can overcome the effect of the inhibitors, and the eradication of inhibitor production through the induction of immune tolerance.
4.1. Immune tolerance induction
Immune tolerance induction (ITI) is the standard of care for the eradication of inhibitors and is considered the most effective approach to managing the majority of patients with severe HA who develop inhibitors. 35 , 36 , 37 , 38 , 39 Successful eradication of inhibitors has been reported in 50–80% of patients in ITI observational studies. 40 , 41 , 42 , 43 , 44 , 45 ITI therapy generally consists of regular and long‐term administration of FVIII concentrate, often at high doses, to downregulate the anti‐FVIII antibody response, resulting in desensitisation and immune tolerance. Proposed mechanisms of tolerance induction include T‐cell exhaustion through overstimulation and, eventually, T‐cell anergy, inhibition of FVIII‐specific memory B‐cell differentiation and formation of anti‐idiotypic antibodies. 46
The best candidates for ITI therapy are children with recently diagnosed inhibitors. Adults with persistent inhibitors may also be suitable candidates for ITI therapy, depending on clinical profile and bleeding phenotype. 47 ITI therapy is usually not considered for patients with low‐titre and low‐responding antibodies or those with transient inhibitors, who can usually continue with replacement therapy for the prevention and treatment of bleeds, but it is recommended as soon as possible in those with inhibitor titres between 5 and 10 Bethesda units (BU). 48 For patients with inhibitor titres of more than 10 BU, it was previously recommended that ITI therapy be delayed until the titres decrease to less than 10 BU; nevertheless, some evidence suggests starting ITI immediately to improve the success rate in these patients. 35 , 49 After successful ITI, patients are able to resume factor replacement therapies for prophylaxis and acute bleeding. Several regimens for ITI have been described, with different dosing schedules and combined therapies 50 , 51 , 52 , 53 , 54 , 55 , 56 ; the selection of an ITI regimen is usually considered on a case‐by‐case basis. 57
The International ITI study is the only randomised controlled trial to date to evaluate the efficacy of ITI in patients with severe HA and good risk features (historical inhibitor titres <200 BU and immediate pre‐ITI titres <10 BU), comparing a low‐dose daily FVIII regimen of 50 IU/kg three times weekly with a high‐dose regimen of 200 IU/kg. 55 The study demonstrated an almost 70% overall success rate in achieving tolerance, with no statistically significant difference between the two arms. The time to negative inhibitor titre and normal FVIII recovery was shorter in the high‐dose group. 55 A multivariate analysis showed that the peak inhibitor titre on ITI was, alone, a significant predictor of ITI outcome, while peak titre pre‐ITI and peak titre on ITI predicted time taken to achieve tolerance. 55 Some FVIII genotypes were also found to be associated with increased likelihood of ITI success, while a long (>5‐year) interval between inhibitor diagnosis and ITI initiation, as well as interruption of ITI therapy, were predictors of ITI failure. 58 The North American Immune Tolerance Registry provides outcome data on ITI in patients with severe HA and poor risk features, reporting that 40% of patients with a pre‐ITI titre of 10 BU or higher achieved successful tolerance, compared with 83% of those with pre‐ITI titres lower than 10 BU. 41 Several groups and organisations have proposed recommendations for the implementation of ITI in patients with HA. 37 , 38 In Europe, access and use of ITI has generally increased in the last decade, although this is more notable in the western versus central parts of the continent. 59 Nevertheless, Europe is currently witnessing a paradigm shift with the recent introduction of alternative non‐factor replacement therapies, whereby both physicians and patients are opting for less complex and demanding prophylactic therapies with improved regimens, thus avoiding the use of ITI. Although the introduction of these therapies, particularly in PUPs, is facilitating initial treatment in children, several questions remain to be answered. Specifically, there is debate over whether patients who previously had their inhibitors eradicated and were switched to non‐replacement therapy should still receive factor replacement therapy at certain intervals in order to avoid a second occurrence of inhibitors. No clear recommendations exist on how to treat these patients and how to address an inhibitor relapse in case it occurs.
Few studies have also reported experience with ITI in non‐Western countries. A study conducted in Turkey surveyed eight paediatric haematology centres about the success of six low‐dose ITI plasma‐derived FVIII regimens in paediatric HA patients with high‐titre inhibitors. The survey results showed that 21 patients were treated with low‐dose ITI between 1997 and 2006, of whom only five achieved complete immune tolerance within a median time of 6 months, indicating a low ITI success rate of 23.3%. Seven patients achieved partial tolerance, whereas the low‐dose ITI treatment failed in the remainder despite decreased inhibitor levels. 54 On the other hand, a recent case report from Turkey described the success of low‐dose ITI (50 IU/kg, 3 days a week) in neutralising inhibitors in a patient with severe HA who had been on on‐demand therapy for the first 8 years of his life and in whom secondary prophylaxis was initiated after haemophilic arthropathy. 60 Based on these observations and the lack of reimbursement schemes for these regimens, only low‐dose ITI with plasma‐derived FVIII (50 IU/kg thrice weekly for 6 months, and a decreasing dose for another 6 months) is being adopted in Turkey, and sometimes no ITI at all, while high dose ITI is only available through clinical trials. In reference to our experience, one of the patients who developed high‐responding inhibitors to rFVIII received ITI therapy with plasma‐derived FVIII for 6 months (50 IU/kg thrice weekly), but inhibitors did not disappear until 12 months after the end of ITI, possibly as a result of secondary prophylaxis with bypassing agents (BPAs). The successful use of low‐dose ITI in a small single‐centre patient cohort has also been reported in India. 61 This was mainly achieved through close collaboration with European centres of excellence. Experience suggested some important considerations for effective planning and initiation of ITI, including: (1) calculating upfront the quantity of clotting factor concentrates required and ensuring continuous supply to avoid any interruptions and subsequent failure of therapy; (2) securing some BPAs for management of breakthrough bleeds and (3) counselling the patient's family and/or caregiver about the benefits and risks of ITI. 61 However, no large‐scale real‐world data on ITI experience from India are available, but it is known that ITI is not done adequately in that country as it requires financial resources, constant availability of factor and patient commitment, all of which are lacking.
4.2. Bypassing agents
For the treatment of bleeding episodes in haemophilia with inhibitors, BPAs provide haemostasis through pathways that avoid the need for FVIII to generate thrombin. The two currently available BPAs are activated prothrombin complex concentrate (aPCC) and recombinant factor VIIa (rFVIIa).
aPCC is a plasma‐derived BPA comprising prothrombin complex factors, prothrombin, FVII, FIX and FX, largely in their inactivated form, with the exception of FVII, which is available in greater amounts in its activated form. 62 The bypassing haemostatic effect of aPCC is achieved through the provision of prothrombin and FXa, which can induce immediate thrombin generation and clot formation. 63 The recommended dose for treating bleeding episodes is 50–100 IU/kg every 6–12 hours and should not exceed 200 IU/kg to minimise the risk of thrombotic complications. 63 In more than 50% of patients on regular aPCC treatment, inhibitor titres gradually decrease with exposure. Up to 30% may show a variable anamnestic increase of inhibitor levels caused by the presence in the product of small amounts of FVIII; however, the anamnestic response is not associated with a reduction in clinical efficacy. 64
rFVIIa is a single‐chain glycoprotein produced in genetically modified baby hamster kidney cells. rFVIIa is able to directly activate FX and increase thrombin generation on the surface of activated platelets in the absence of FVIII or FIX. 65 , 66 Given its relatively short half‐life, rFVIIa has been initially approved at a dose of 90 μg/kg every 2–3 hours until haemostasis is achieved. A single bolus of 270 μg/kg has also been used in clinical practice to reduce the frequency of infusions and allow longer intervals between them. This dose has been proven to be comparable to three 90 μg/kg doses administered every 3 hours and is safe, with a low risk of thrombosis. 67 , 68
In a controlled head‐to‐head comparison of aPCC and rFVIIa, the resolution of bleeding events occurred equally in the two groups at a rate of 70%–80%. 69 Patients might experience an improved response to one BPA compared with the other over time, 70 and in 10%–20% of the bleeds not effectively managed with one BPA, sequential regimens of both aPCC and rFVIIa may be necessary to control the bleeds. 71 However, particular attention should be given to elderly patients with comorbidities.
Although the clinical benefit associated with the use of aPCC and rFVIIa is not as evident as with FVIII prophylaxis in patients without inhibitors, these have proven efficacy in prophylactic regimens in patients with inhibitors before starting ITI, during ITI and after failure of ITI. 4 Two randomised clinical trials have demonstrated a 60%–72% reduction in bleeding episodes with prophylactic treatment versus on‐demand treatment with aPCC, 72 , 73 while only one has evaluated the efficacy of prophylactic versus on‐demand treatment with rFVIIa, reporting up to 60% reduction in bleeding episodes from the pre‐prophylactic period with the prophylactic regimen. 74
Surgery is a high‐risk procedure for patients with inhibitors. For decades, BPAs have been the mainstay treatment to ensure haemostasis in surgical interventions, and several studies have proven their efficacy and safety in preventing bleeding pre‐ and post‐operatively, 75 , 76 , 77 , 78 , 79 , 80 , 81 although in some patients, haemostasis might not be reached with rFVIIa and aPCC alone. The WFH recommends that patients with HA and high‐responding inhibitors undergoing surgery or an invasive procedure be given BPA therapy (rFVIIa and aPCC interchangeably) and be closely monitored for thrombosis. 6
The above‐reported data from Turkey highlights the longstanding successful experience with BPAs for the management of bleeding peri‐ and post‐operatively, which has allowed the performance of elective and urgent orthopaedic surgery for the past 20 years. It was apparent that surgical procedures for patients with inhibitors must be done only in haemophilia expert centres. A retrospective multicentre registry including 17 Turkish centres between 2000 and 2007 reported the clinical experience with aPCC in the treatment of acute bleeding and surgical haemostasis in patients with HA after its introduction in Turkey in 2000. The study reported a haemostatic efficacy rate of 86% with aPCC in surgical procedures, with no occurrences of thromboembolic complications or other adverse events. 81 Another study reported the 8‐year experience of a single centre with aPCC and/or rFVIIa in treating 30 patients with severe HA with high‐responding inhibitors who required surgery. Results showed that minor and major surgeries were successfully and safely performed with no thromboembolic or major complications. 80 Likewise, in our experience in Egypt, patients receive either aPCC or rFVIIa as pre‐ and post‐surgical interventions based on the WFH recommendations. 6 It is not the case in India, where BPAs remain expensive and are not widely available, so physicians try to delay elective surgery as much as possible to avoid their use; real‐world data on experience with these agents in emergency settings are scarce.
Limitations associated with the use of BPAs mainly include the possible risk of thrombotic events, 82 , 83 their short half‐life in view of their elevated costs, especially when used in the prophylaxis setting, 84 as well as the lack of an easily available laboratory test for monitoring their efficacy, which makes patient outcomes unpredictable. 4 These limitations, together with the emergence of novel non‐factor‐based prophylactic agents, are shifting the preventive treatment of inhibitor‐related bleeds towards the use of newer agents with improved mode and frequency of administration.
BPAs are accessible in most countries in Europe, but reporting of use has been generally inconsistent, 59 while their availability is limited in less resourceful countries. When treating bleeding episodes in India, some cost‐effective alternatives are used, such as platelet transfusions along with BPAs to increase thrombin generation, although the risk of complications when combining therapies remains high. 61 One study from Turkey 85 reported the successful use of a modified treatment protocol with rFVIIa and aPCC to treat bleeding in five patients with inhibitors. The proposed modified protocol aimed to mitigate the drugs’ costs while preventing any further bleeding risk or complications in the patients. It begins with rFVIIa over the first 3 days, followed by aPCC from the fourth until the seventh or 14th day, when less frequent administration over a longer period is sufficient to stabilise the patient; for this purpose, aPCC is a cheaper option than rFVIIa. Based on our experience in Turkey, prophylactic aPCC regimens are effective in decreasing the incidence of bleeds; nowadays, around two‐third of patients with high‐responding inhibitors are on secondary prophylaxis with aPCC while a small proportion are on rFVIIa because of the need of preapproval from the ministry of health. In addition, BPAs have been used successfully in elective and emergency surgical interventions. The government has been reimbursing both types of BPAs for the past 20 years, with some limitations.
4.3. Non‐factor replacement agents
Non‐factor replacement agents were developed to overcome the challenges experienced with replacement therapy mainly relating to the intravenous route of administration and potential inhibitor development. New non‐replacement agents do not contain FVIII, hence they do not trigger any immune reaction against these proteins, and they cannot be neutralised by pre‐existing inhibitory anti‐FVIII antibodies. 86 Their key features encompass their subcutaneous administration, their similar effectiveness in patients regardless of inhibitor status, their longer duration of action and, consequently, the extended steady‐state haemostatic effect that they can achieve with fewer injections and a low drug volume. 87 , 88 , 89 , 90
Emicizumab is the first non‐factor replacement therapy approved for the prophylactic treatment of patients with HA with and without inhibitors. It is a humanised bispecific monoclonal antibody that mimics the function of activated FVIII. 91 Data on the efficacy of emicizumab are derived from an extensive clinical programme of Phase III trials (HAVEN 1, HAVEN 2 and HAVEN 4) in patients with HA with and without inhibitors, 87 , 92 , 93 which reported significant reductions in annualised bleeding rates and other bleeding endpoints in paediatric and adult patients when treated with emicizumab prophylaxis after switching from on‐demand or prophylactic BPA treatment. Recent real‐world studies have reported similar efficacy outcomes with emicizumab and a tolerable safety profile. 94 , 95 , 96 , 97 Emicizumab was also shown to improve health‐related QoL in patients with inhibitors. 92 , 97
To date, emicizumab is the most extensively researched non‐factor replacement drug; less data are available for the other drugs that are still under investigation, including fitusiran, 88 a small interfering ribonucleic acid that inhibits the synthesis of antithrombin, and monoclonal antibodies that target tissue factor pathway inhibitor, 89 , 90 the primary regulator of the tissue factor–FVIIa complex.
The concept of these therapies is that the extent of haemostatic correction they provide with a prophylaxis regimen is almost similar to that of patients with mild haemophilia 88 , 98 , 99 ; therefore, breakthrough bleeding can still occur, and the conferred protection might not be adequate for some surgical interventions. Consequently, in such events, additional haemostatic agents such as factor concentrates or BPA might be needed. 86 Moreover, the occasional need for additional procoagulants in the case of breakthrough bleeding or surgery has been linked to an increased thrombotic risk. 86 , 87 Following this observation, some risk‐mitigation plans were brought forward to reduce the risk of thrombotic complications when using emicizumab prophylaxis in combination with other procoagulants, 86 including warnings against the concomitant use of aPCC. 100 Finally, unlike for replacement therapy, no routine, standardised laboratory test exists for the monitoring of non‐replacement drugs, and long‐term clinical data are limited, although most recent data report good efficacy and safety results internationally. 94 , 95 , 96 , 97
Following the favourable outcomes achieved in emicizumab's clinical programme and in the real world, most of the European patients with inhibitors have now been switched to emicizumab. In contrast, the experience in Turkey with emicizumab is limited to eight paediatric patients who were enrolled in HAVEN 292 for 3 years. The Ministry of Health approved emicizumab at the end of 2019; however, it is still not reimbursed and thus not widely accessible. In Egypt, physicians have been using it for 14 months, with very good results so far. In India, emicizumab is available through patient access programs only, making it affordable to patients, especially that it is given at adjusted reduced doses, and it is unlikely to be licensed in the near future given its high cost, especially with the anticipated shift in focus of the government amidst the COVID‐19 pandemic.
4.4. Other therapies
New and enhanced variants of BPAs aimed at providing high bleeding protection with improved treatment regimens are now under investigation. These include marzeptacog alfa, an rFVIIa variant with enhanced procoagulant activity and a longer duration of action compared with wild‐type rFVIIa, 101 and SerpinPC, a bioengineered serine protease inhibitor that specifically inhibits activated protein C. 102
Gene therapy is another promising approach to treating and potentially curing HA, and initial trials have proven sufficient safety and efficacy to expand experimental testing in larger Phase III trials. 103 , 104 , 105 , 106 Most recently, valoctocogene roxaparvovec, an adeno‐associated virus type 5 (AAV5) gene therapy, has been under investigation in an ongoing Phase I/II trial, which has shown sustained therapeutic benefit for up to 5 years following treatment. 107 Nevertheless, some challenges and safety concerns have arisen with gene therapy, which might limit wide application in the future. 86 Moreover, patients with inhibitors have commonly been excluded from gene therapy trials. Although promising, these therapies are still under investigation and are not yet adopted in the management of HA.
5. FINAL THOUGHTS
Over the past few decades, haemophilia treatment has witnessed great advances that have remarkably improved outcomes in patients. While we have been successful at overcoming some of the existing challenges associated with inhibitor management, some still linger, and others are surfacing with the shift towards enhanced therapeutic options and are acting as constraints to the delivery of ideal care (Table 1, Figure 1). This is most burdensome in non‐Western countries, where the availability of resources plays a major role in shaping the quality of care that patients receive.
FIGURE 1.
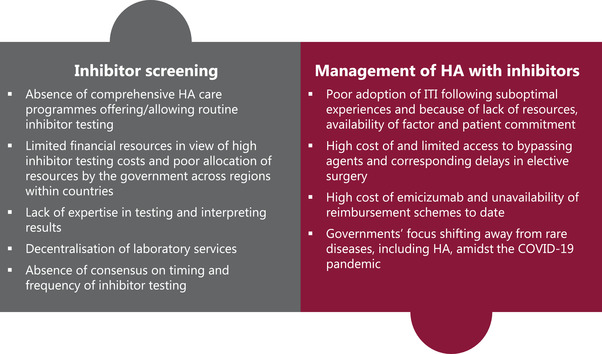
Key challenges in the screening and management of haemophilia A with inhibitors in non‐Western countries. HA, haemophilia A; ITI, immune tolerance induction
One of the major gaps in these countries is the scarcity of good‐quality data on HA management. Such data can help identify potential and existing challenges in the diagnosis and management of inhibitor cases in association with morbidity and mortality data and can inform healthcare reforms and policies. National registries and centre‐level research can provide valuable insights into actual clinical practice and the associated challenges and gaps. Areas of interest are: (1) the adoption of the WFH recommendations in HA management; (2) acute bleeding management, including patients’ perspectives on management approaches, protection of patients with a prophylaxis scheme and the impact on QoL, with a focus on morbidity parameters; (3) ITI therapy, including healthcare practitioners and patients’ acceptance and adoption patterns to understand drivers, challenges and success rates and (4) surgical management of HA with inhibitors, including current protocols and local experiences. On the other hand, the lack of data records compromises patients’ eligibility for clinical trial enrolment, in the settings where clinical trials are the only option for patients access to therapy.
Poor disease knowledge among both healthcare professionals and patients is another major constraint contributing to poor outcomes in HA with inhibitors. Patients and their caregivers should be educated on the seriousness of the disease and on how to recognise bleeds and promptly seek medical care to avoid bleeding complications and long‐term morbidity, as well as to increase their confidence in caring for themselves or their patients. Educational programmes targeted at healthcare professionals actively engaged in delivering care to HA patients should be set up to improve disease awareness to specifically recognise red flags that prompt immediate medical care and to work on standardising disease management across centres. This is also achieved through the transfer of knowledge and expertise from successful practices to settings where basic optimal care is still lagging.
Finally, access to therapy is one of the most challenging obstacles that resource‐limited countries are faced with. Factor replacement therapies, BPAs and non‐factor‐based therapies are costly treatments, and access to these is limited by government reimbursement capacity and plans. Routine inhibitor testing and screening is also expensive and not equally available to everyone, with regional disparities within resource‐limited countries. Pragmatic planning at government, health authority and payer levels is essential to close access gaps within countries and ensure equitable government resource allocation based on needs. This is achieved by support and engagement from expert medical bodies and non‐governmental organisations, as well as education on the severity and burden of disease and inhibitors specifically, to ensure that policies are driven by scientific evidence (Figure 2).
FIGURE 2.

Future directions towards achieving improved care in haemophilia A with inhibitors in non‐Western countries. HA, haemophilia A; ITI, immune tolerance induction; WFH, World Federation of Hemophilia
CONFLICTS OF INTEREST
Flora Peyvandi has received speaker fees for participating in educational symposia and advisory boards for Biomarin, Roche, Sanofi and Sobi. Kaan Kavakli has received consultant fees for participating in advisory boards for Takeda, Novo Nordisk and Roche. Savita Rangarajan has received consultant fees for advisory roles for Reliance Life Sciences, Pfizer, Sanofi and Sigilon, speaker fees for participating in speakers’ bureau for Shire, Takeda and Pfizer, and other fees for travel, accommodation and expenses from Reliance Life Sciences, Shire and Takeda. Amal El‐Beshlawy has no conflicts of interest to disclose.
6.
ACKNOWLEDGEMENTS
All authors have contributed equally to this manuscript. The authors thank Sarah Keyrouz of AMICULUM for medical editorial assistance. Financial support for medical editorial assistance was provided by Takeda Pharmaceuticals FZCO.
Open access funding provided by BIBLIOSAN.
[Correction added on 25 November 2022, after first online publication: BIBLIOSAN funding statement has been added.]
Peyvandi F, Kavakli K, El‐Beshlawy A, Rangarajan S. Management of haemophilia A with inhibitors: A regional cross‐talk. Haemophilia. 2022;28:950–961. 10.1111/hae.14638
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- 1. Escuriola‐Ettingshausen C, Auerswald G, Königs C, et al. Optimizing the management of patients with haemophilia A and inhibitors in the era of emicizumab: recommendations from a German expert panel. Haemophilia. 2021;27:e305‐e313. [DOI] [PubMed] [Google Scholar]
- 2. Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. Lancet. 2016;388:187‐197. [DOI] [PubMed] [Google Scholar]
- 3. Berntorp E, Shapiro AD. Modern haemophilia care. Lancet. 2012;379:1447‐1456. [DOI] [PubMed] [Google Scholar]
- 4. Linari S, Castaman G. Concomitant use of RFVIIA and emicizumab in people with hemophilia A with inhibitors: current perspectives and emerging clinical evidence. Ther Clin Risk Manag. 2020;16:461‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franchini M, Tagliaferri A, Mengoli C, Cruciani M. Cumulative inhibitor incidence in previously untreated patients with severe hemophilia A treated with plasma‐derived versus recombinant factor VIII concentrates: a critical systematic review. Crit Rev Oncol Hematol. 2012;81:82‐93. [DOI] [PubMed] [Google Scholar]
- 6. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1‐158. [DOI] [PubMed] [Google Scholar]
- 7. White GCI, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia: recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the international society on thrombosis and haemostasis. Thromb Haemost. 2001;85:560. [PubMed] [Google Scholar]
- 8. Volkers P, Hanschmann KM, Calvez T, et al. Recombinant factor VIII products and inhibitor development in previously untreated patients with severe haemophilia A: combined analysis of three studies. Haemophilia. 2019;25:398‐407. [DOI] [PubMed] [Google Scholar]
- 9. Gouw SC, van der Bom JG, Ljung R, et al. Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 2013;368:231‐239. [DOI] [PubMed] [Google Scholar]
- 10. Calvez T, Chambost H, Claeyssens‐Donade S, et al. Recombinant factor VIII products and inhibitor development in previously untreated boys with severe hemophilia A. Blood. 2014;124:3398‐3408. [DOI] [PubMed] [Google Scholar]
- 11. Peyvandi F, Mannucci PM, Garagiola I, et al. A randomized trial of factor VIII and neutralizing antibodies in hemophilia A. N Engl J Med. 2016;374:2054‐2064. [DOI] [PubMed] [Google Scholar]
- 12. Sherief LM, Gaber OA, Youssef HM, et al. Factor VIII inhibitor development in Egyptian hemophilia patients: does intron 22 inversion mutation play a role? Ital J Pediatr. 2020;46:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sadek H, Youssry I, Ibrahim NSE, Abou‐Elalla AA, Atef G, Mousa SM. The development of FVIII inhibitors in relation to IL10 gene polymorphism in hemophilia A Egyptian pediatric patients. Fetal Pediatr Pathol. 2017;36:184‐189. [DOI] [PubMed] [Google Scholar]
- 14. Kavakli K, Aktuǧlu G, Kemahli S, et al. Inhibitor screening for patients with hemophilia in Turkey. Turk J Hematol. 2006;23:25‐32. [PubMed] [Google Scholar]
- 15. Atik T, Işık E, Onay H, et al. Factor 8 gene mutation spectrum of 270 patients with hemophilia A: identification of 36 novel mutations. Turk J Hematol. 2020;37:145‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Öner N, Gürsel T, Kaya Z, et al. Inherited coagulation disorders in Turkish children: a retrospective, single‐center cohort study. Transfus Apher Sci. 2020;59:102728. [DOI] [PubMed] [Google Scholar]
- 17. Türk Hematoloji Derneği . Ulusal Hemofili Tanı ve Tedavi Kılavuzu Askıda. Türk Hematoloji Derneği; 2021. Accessed January 2, 2022. https://www.thd.org.tr/1/haberler/1105/ulusal‐hemofili‐tani‐ve‐tedavi‐kilavuzu‐askida [Google Scholar]
- 18. Miri S, Kaan Kavakli R, Halimeh S, et al. Inhibitor development upon switch from plasma‐derived to recombinant FVIII in PUPs with severe haemophilia A: preliminary analysis of the PUPSWITCH study. Haematologica. 2021;106(suppl 1):1‐35.33386710 [Google Scholar]
- 19. Pinto P, Shelar T, Nawadkar V, et al. The epidemiology of FVIII inhibitors in Indian haemophilia A patients. Indian J Hematol Blood Transfus. 2014;30:356‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. David S, Nair SC, Singh GS, et al. Prevalence of FVIII inhibitors in severe haemophilia A patients: effect of treatment and genetic factors in an Indian population. Haemophilia. 2019;25:67‐74. [DOI] [PubMed] [Google Scholar]
- 21. Shah S, Patel T, Bhatnagar N, Gajjar M, Shah M, Tripathi S. “Prevalence of inhibitors in hemophilia patients and its clinical implications”: a study of 276 patients in western India. Glob J Transfus Med. 2019;4:168‐174. [Google Scholar]
- 22. Walsh CE, Jiménez‐Yuste V, Auerswald G, Grancha S. The burden of inhibitors in haemophilia patients. Thromb Haemost. 2016;116(suppl 1):S10‐S17. [DOI] [PubMed] [Google Scholar]
- 23. Dobón M, Lucía JF, Aguilar C, et al. Value of magnetic resonance imaging for the diagnosis and follow‐up of haemophilic arthropathy. Haemophilia. 2003;9:76‐85. [DOI] [PubMed] [Google Scholar]
- 24. Morfini M, Haya S, Tagariello G, et al. European study on orthopaedic status of haemophilia patients with inhibitors. Haemophilia. 2007;13:606‐612. [DOI] [PubMed] [Google Scholar]
- 25. Zanon E, Iorio A, Rocino A, et al. Intracranial haemorrhage in the Italian population of haemophilia patients with and without inhibitors. Haemophilia. 2012;18:39‐45. [DOI] [PubMed] [Google Scholar]
- 26. Gupta N, Benbouzid A, Belhani M, et al. HAEMOcare: the first international epidemiological study measuring burden of hemophilia in developing countries. TH Open. 2019;3:e190‐e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walsh CE, Soucie JM, Miller CH. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90:400‐405. [DOI] [PubMed] [Google Scholar]
- 28. Darby SC, Keeling DM, Spooner RJD, et al. The incidence of factor VIII and factor IX inhibitors in the hemophilia population of the UK and their effect on subsequent mortality, 1977–99. J Thromb Haemost. 2004;2:1047‐1054. [DOI] [PubMed] [Google Scholar]
- 29. Diamondstone LS, Aledort LM, Goedert JJ. Factors predictive of death among HIV‐uninfected persons with haemophilia and other congenital coagulation disorders. Haemophilia. 2002;8:660‐667. [DOI] [PubMed] [Google Scholar]
- 30. Soucie JM, Nuss R, Evatt B, et al. Mortality among males with hemophilia: relations with source of medical care. Blood. 2000;96:437‐442. [PubMed] [Google Scholar]
- 31. Rasi V, Ikkala E. Haemophiliacs with factor VIII inhibitors in Finland: prevalence, incidence and outcome. Br J Haematol. 1990;76:369‐371. [DOI] [PubMed] [Google Scholar]
- 32. Guh S, Grosse SD, Mcalister S, Kessler CM, Soucie JM, Disabilities D. Health care expenditures for Medicaid‐covered males with haemophilia in the United States, 2008. Haemophilia. 2012;18:276‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thorat T, Neumann PJ, Chambers JD. Hemophilia burden of disease: a systematic review of the cost‐utility literature for hemophilia. J Manag Care Spec Pharm. 2018;24:632‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rocha P, Carvalho M, Lopes M, Araújo F. Costs and utilization of treatment in patients with hemophilia. BMC Health Serv Res. 2015;15:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DiMichele DM, Hoots WK, Pipe SW, Rivard GE, Santagostino E. International workshop on immune tolerance induction: consensus recommendations. Haemophilia. 2007;13(suppl 1):1‐22. [DOI] [PubMed] [Google Scholar]
- 36. Brackmann HH, Lenk H, Scharrer I, Auerswald G, Kreuz W. German recommendations for immune tolerance therapy in type A haemophiliacs with antibodies. Haemophilia. 1999;5:203‐206. [DOI] [PubMed] [Google Scholar]
- 37. Collins PW, Chalmers E, Hart DP, et al. Diagnosis and treatment of factor VIII and IX inhibitors in congenital haemophilia: (4th edition) UK Haemophilia Centre Doctors Organization. Br J Haematol. 2013;160:153‐170. [DOI] [PubMed] [Google Scholar]
- 38. Valentino LA, Kempton CL, Kruse‐Jarres R, Mathew P, Meeks SL, Reiss UM. US guidelines for immune tolerance induction in patients with haemophilia A and inhibitors. Haemophilia. 2015;21:559‐567. [DOI] [PubMed] [Google Scholar]
- 39. di Michele DM. Immune tolerance induction in haemophilia: evidence and the way forward. J Thromb Haemost. 2011;9(suppl 1):216‐225. [DOI] [PubMed] [Google Scholar]
- 40. Coppola A, Margaglione M, Santagostino E, et al. Factor VIII gene (F8) mutations as predictors of outcome in immune tolerance induction of hemophilia A patients with high‐responding inhibitors. J Thromb Haemost. 2009;7:1809‐1815. [DOI] [PubMed] [Google Scholar]
- 41. DiMichele DM, Kroner BL, Adair S, et al. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87:52‐57. [PubMed] [Google Scholar]
- 42. DiMichele D. The North American Immune Tolerance Registry: contributions to the thirty‐year experience with immune tolerance therapy. Haemophilia. 2009;15:320‐328. [DOI] [PubMed] [Google Scholar]
- 43. Haya S, López MF, Aznar JA, Batlle J. Immune tolerance treatment in haemophilia patients with inhibitors: the Spanish registry. Haemophilia. 2001;7:154‐159. [DOI] [PubMed] [Google Scholar]
- 44. Lenk H. The German registry of immune tolerance treatment in hemophilia – 1999 update. Haematologica. 2000;85:45‐47. [PubMed] [Google Scholar]
- 45. Mariani G, Scheibel E, Nogao T, et al. Immunetolerance as treatment of alloantibodies to factor VIII in hemophilia. The International Registry of Immunetolerance Protocols. Semin Hematol. 1994;31(suppl 4):62‐64. [PubMed] [Google Scholar]
- 46. Waters B, Lillicrap D. The molecular mechanisms of immunomodulation and tolerance induction to factor VIII. J Thromb Haemost. 2009;7:1446‐1456. [DOI] [PubMed] [Google Scholar]
- 47. Coppola A, di Minno MND, Santagostino E. Optimizing management of immune tolerance induction in patients with severe haemophilia A and inhibitors: towards evidence‐based approaches. Br J Haematol. 2010;150:515‐528. [DOI] [PubMed] [Google Scholar]
- 48. Kempton CL, Meeks SL. Toward optimal therapy for inhibitors in hemophilia. Hematology Am Soc Hematol Educ Program. 2014;2014:364‐371. [DOI] [PubMed] [Google Scholar]
- 49. Nakar C, Manco‐Johnson MJ, Lail A, et al. Prompt immune tolerance induction at inhibitor diagnosis regardless of titre may increase overall success in haemophilia A complicated by inhibitors: experience of two US centres. Haemophilia. 2015;21:365‐373. [DOI] [PubMed] [Google Scholar]
- 50. Mauz‐Korholz C, Korholz D, Gobel U. Rapid elimination of a high‐titered F VIII inhibitor by high dose recombinant F VIII combined with high dose immunoglobulin infusion. Thromb Haemost. 1997;78:959. [PubMed] [Google Scholar]
- 51. Mingot‐Castellano ME, Álvarez‐Román MT, López‐Fernández MF, et al. Spanish consensus guidelines on prophylaxis with bypassing agents for surgery in patients with haemophilia and inhibitors. Eur J Haematol. 2016;96:461‐474. [DOI] [PubMed] [Google Scholar]
- 52. Mauser‐Bunschoten EP, Nieuwenhuis HK, Roosendaal G, van den Berg HM. Low‐dose immune tolerance induction in hemophilia A patients with inhibitors. Blood. 1995;86:983‐988. [PubMed] [Google Scholar]
- 53. Berntorp E, Astermark J, Carlborg E. Immune tolerance induction and the treatment of hemophilia. Malmö protocol update. Haematologica. 2000;85(suppl 10):48‐51. [PubMed] [Google Scholar]
- 54. Unuvar A, Kavakli K, Baytan B, et al. Low‐dose immune tolerance induction for paediatric haemophilia patients with factor VIII inhibitors. Haemophilia. 2008;14:315‐322. [DOI] [PubMed] [Google Scholar]
- 55. Hay CRM, DiMichele DM. The principal results of the International Immune Tolerance Study: a randomized dose comparison. Blood. 2012;119:1335‐1344. [DOI] [PubMed] [Google Scholar]
- 56. Malec LM, Journeycake J, Ragni MV. Extended half‐life factor VIII for immune tolerance induction in haemophilia. Haemophilia. 2016;22:e552‐e554. [DOI] [PubMed] [Google Scholar]
- 57. Athale AH, Marcucci M, Iorio A. Immune tolerance induction for treating inhibitors in people with congenital haemophilia A or B. Cochrane Database Syst Rev. 2014:CD010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Witmer C, Young G. Factor VIII inhibitors in hemophilia A: rationale and latest evidence. Ther Adv Hematol. 2013;4:59‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Noone D, O'Mahony B, Peyvandi F, Makris M, Bok A. Evolution of haemophilia care in Europe: 10 years of the principles of care. Orphanet J Rare Dis. 2020;15:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ay Y, Ersin T, Yesim O, et al. Successful immune tolerance induction with low‐dose coagulation factor VIII in a patient with hemophilia A from a developing country. Blood Coagul Fibrinolysis. 2016;27:729‐731. [DOI] [PubMed] [Google Scholar]
- 61. Seth T. Experience of immune tolerance induction therapy for hemophilia A patients with inhibitors from a single center in India. Indian J Hematol Blood Transfus. 2020;36:458‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Turecek PL, Váradi K, Gritsch H, Schwarz HP. FEIBA: mode of action. Haemophilia. 2004;10(suppl 2):3‐9. [DOI] [PubMed] [Google Scholar]
- 63. Négrier C, Gomperts ED, Oldenburg J. The history of FEIBA: a lifetime of success in the treatment of haemophilia complicated by an inhibitor. Haemophilia. 2006;12(suppl 5):4‐13. [Google Scholar]
- 64. Negrier C, Goudemand J, Sultan Y, Bertrand M, Rothschild C, Lauroua P. Multicenter retrospective study on the utilization of FEIBA in France in patients with factor VIII and factor IX inhibitors. Thromb Haemost. 1997;77:1113‐1119. [PubMed] [Google Scholar]
- 65. Monroe DM, Hoffman M, Oliver JA, Roberts HR. Platelet activity of high‐dose factor VIIa is independent of tissue factor. Br J Haematol. 1997;99:542‐547. [DOI] [PubMed] [Google Scholar]
- 66. Hoffman M, Monroe DM, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high‐dose activated factor VII. Blood Coagul Fibrinolysis. 1998;9(suppl 1):S61‐S65. [PubMed] [Google Scholar]
- 67. Kavakli K, Makris M, Zulfikar B, et al. Home treatment of haemarthroses using a single dose regimen of recombinant activated factor VII in patients with haemophilia and inhibitors. A multi‐centre, randomized, double‐blind, cross‐over trial. Thromb Haemost. 2006;95:600‐605. [PubMed] [Google Scholar]
- 68. Santagostino E, Mancuso ME, Rocino A, Mancuso G, Scaraggi F, Mannucci PM. A prospective randomized trial of high and standard dosages of recombinant factor VIIa for treatment of hemarthroses in hemophiliacs with inhibitors. J Thromb Haemost. 2006;4:367‐371. [DOI] [PubMed] [Google Scholar]
- 69. Astermark SJ, Donfield SM, DiMichele DM, et al. A randomized comparison of bypassing agents in hemophilia complicated by an inhibitor: the FEIBA NovoSeven Comparative (FENOC) Study. Blood. 2007;109:546‐551. [DOI] [PubMed] [Google Scholar]
- 70. Hayashi T, Tanaka I, Shima M, et al. Unresponsiveness to factor VIII inhibitor bypassing agents during haemostatic treatment for life‐threatening massive bleeding in a patient with haemophilia A and a high responding inhibitor. Haemophilia. 2004;10:397‐400. [DOI] [PubMed] [Google Scholar]
- 71. Gringeri A, Fischer K, Karafoulidou A, Klamroth R, López‐Fernández MF, Mancuso E. Sequential combined bypassing therapy is safe and effective in the treatment of unresponsive bleeding in adults and children with haemophilia and inhibitors. Haemophilia. 2011;17:630‐635. [DOI] [PubMed] [Google Scholar]
- 72. Leissinger C, Gringeri A, Antmen B, et al. Anti‐inhibitor coagulant complex prophylaxis in hemophilia with inhibitors. N Engl J Med. 2011;365:1684‐1692. [DOI] [PubMed] [Google Scholar]
- 73. Antunes SV, Tangada S, Stasyshyn O, et al. Randomized comparison of prophylaxis and on‐demand regimens with FEIBA NF in the treatment of haemophilia A and B with inhibitors. Haemophilia. 2014;20:65‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Konkle BA, Ebbesen LS, Erhardtsen E, et al. Randomized, prospective clinical trial of recombinant factor VIIa for secondary prophylaxis in hemophilia patients with inhibitors. J Thromb Haemost. 2007;5:1904‐1913. [DOI] [PubMed] [Google Scholar]
- 75. Ju HY, Jang HL, Park YS. The efficacy of bypassing agents in surgery of hemophilia patients with inhibitors. Blood Res. 2015;50:173‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Valentino LA, Cooper DL, Goldstein B. Surgical experience with rFVIIa (NovoSeven) in congenital haemophilia A and B patients with inhibitors to factors VIII or IX. Haemophilia. 2011;17:579‐589. [DOI] [PubMed] [Google Scholar]
- 77. Polyanskaya T, Zorenko V, Karpov E, Sampiev M, Mishin G, Vasiliev D. Experience of recombinant activated factor VII usage during surgery in patients with haemophilia with inhibitors. Haemophilia. 2012;18:997‐1002. [DOI] [PubMed] [Google Scholar]
- 78. Négrier C, Lienhart A, Numerof R, et al. SURgical interventions with FEIBA (SURF): international registry of surgery in haemophilia patients with inhibitory antibodies. Haemophilia. 2013;19:e143‐e150. [DOI] [PubMed] [Google Scholar]
- 79. Ingerslev J. Efficacy and safety of recombinant factor VIIa in the prophylaxis of bleeding in various surgical procedures in hemophilic patients with factor VIII and factor IX inhibitors. Semin Thromb Hemost. 2000;26:425‐432. [DOI] [PubMed] [Google Scholar]
- 80. Balkan C, Karapinar D, Aydogdu S, et al. Surgery in patients with haemophilia and high responding inhibitors: izmir experience. Haemophilia. 2010;16:902‐909. [DOI] [PubMed] [Google Scholar]
- 81. Zülfikar B, Aydogan G, Salcioglu Z, et al. Efficacy of FEIBA for acute bleeding and surgical haemostasis in haemophilia A patients with inhibitors: a multicentre registry in Turkey. Haemophilia. 2012;18:383‐391. [DOI] [PubMed] [Google Scholar]
- 82. Roberts HR. Clinical experience with activated factor VII: focus on safety aspects. Blood Coagul Fibrinol. 1998;9(suppl 1):9819041. [PubMed] [Google Scholar]
- 83. Ehrlich HJ, Henzl MJ, Gomperts ED. Safety of factor VIII inhibitor bypass activity (FEIBA®): 10‐year compilation of thrombotic adverse events. Haemophilia. 2002;8:83‐90. [DOI] [PubMed] [Google Scholar]
- 84. Ljung R. Aspects of prophylactic treatment of hemophilia. Thromb J. 2016;14(suppl 1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Malkan UY, Aksu S. Combination of NovoSeven and FEIBA in hemophiliac patients with inhibitors. Open Med. 2018;13:618‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mancuso ME, Mahlangu JN, Pipe SW. The changing treatment landscape in haemophilia: from standard half‐life clotting factor concentrates to gene editing. Lancet. 2021;397:630‐640. [DOI] [PubMed] [Google Scholar]
- 87. Oldenburg J, Mahlangu JN, Kim B, et al. Emicizumab prophylaxis in hemophilia A with inhibitors. N Engl J Med. 2017;377:809‐818. [DOI] [PubMed] [Google Scholar]
- 88. Pasi KJ, Rangarajan S, Georgiev P, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med. 2017;377:819‐828. [DOI] [PubMed] [Google Scholar]
- 89. Shapiro AD, Angchaisuksiri P, Astermark J, et al. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood. 2019;134:1973‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cardinal M, Kantaridis C, Zhu T, et al. A first‐in‐human study of the safety, tolerability, pharmacokinetics and pharmacodynamics of PF‐06741086, an anti‐tissue factor pathway inhibitor mAb, in healthy volunteers. J Thromb Haemost. 2018;16:1722‐1731. [DOI] [PubMed] [Google Scholar]
- 91. Kitazawa T, Igawa T, Sampei Z, et al. A bispecific antibody to factors IXa and X restores factor VIII hemostatic activity in a hemophilia A model. Nat Med. 2012;18:1570‐1574. [DOI] [PubMed] [Google Scholar]
- 92. Young G, Liesner R, Chang T, et al. A multicenter, open‐label phase 3 study of emicizumab prophylaxis in children with hemophilia A with inhibitors. Blood. 2019;134:2127‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pipe SW, Shima M, Lehle M, et al. Efficacy, safety, and pharmacokinetics of emicizumab prophylaxis given every 4 weeks in people with haemophilia A (HAVEN 4): a multicentre, open‐label, non‐randomised Phase 3 study. Lancet Haematol. 2019;6:e295‐e305. [DOI] [PubMed] [Google Scholar]
- 94. Shang A, Selak Bienz N, Gadiraju R, Chang T, Kuebler P. Real‐world safety of emicizumab: the first interim analysis of the European Haemophilia Safety Surveillance (EUHASS) database. Blood. 2020;136(suppl 1):29‐30. [Google Scholar]
- 95. McCary I, Guelcher C, Kuhn J, et al. Real‐world use of emicizumab in patients with haemophilia A: bleeding outcomes and surgical procedures. Haemophilia. 2020;26:631‐636. [DOI] [PubMed] [Google Scholar]
- 96. Barg AA, Avishai E, Budnik I, et al. Emicizumab prophylaxis among infants and toddlers with severe hemophilia A and inhibitors – a single‐center cohort. Pediatr Blood Cancer. 2019;66:e27886. [DOI] [PubMed] [Google Scholar]
- 97. Ebbert PT, Xavier F, Seaman CD, Ragni MV. Emicizumab prophylaxis in patients with haemophilia A with and without inhibitors. Haemophilia. 2020;26:41‐46. [DOI] [PubMed] [Google Scholar]
- 98. Shima M, Hanabusa H, Taki M, et al. Factor VIII – mimetic function of humanized bispecific antibody in hemophilia A. N Engl J Med. 2016;374:2044‐2053. [DOI] [PubMed] [Google Scholar]
- 99. Eichler H, Angchaisuksiri P, Kavakli K, et al. Concizumab restores thrombin generation potential in patients with haemophilia: pharmacokinetic/pharmacodynamic modelling results of concizumab Phase 1/1b data. Haemophilia. 2019;25:60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Genentech Inc . Highlights of prescribing information. Hemlibra. 2017. Accessed October 24, 2021. https://www.gene.com/download/pdf/hemlibra_prescribing.pdf
- 101. Gruppo RA, Malan D, Kapocsi J, et al. Phase 1, single‐dose escalating study of marzeptacog alfa (activated), a recombinant factor VIIa variant, in patients with severe hemophilia. J Thromb Haemost. 2018;16:1984‐1993. [DOI] [PubMed] [Google Scholar]
- 102. Polderdijk SGI, Baglin TP, Huntington JA. Targeting activated protein C to treat hemophilia. Curr Opin Hematol. 2017;24:446‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rangarajan S, Walsh L, Lester W, et al. AAV5–factor VIII gene transfer in severe hemophilia A. N Engl J Med. 2017;377:2519‐2530. [DOI] [PubMed] [Google Scholar]
- 104. High KA, George LA, Eyster ME, et al. A Phase 1/2 trial of investigational Spk‐8011 in hemophilia A demonstrates durable expression and prevention of bleeds. Blood. 2018;132(suppl 1):487. [Google Scholar]
- 105. Pasi KJ, Rangarajan S, Mitchell N, et al. Multiyear follow‐up of AAV5‐hFVIII‐SQ gene therapy for hemophilia A. N Engl J Med. 2020;382:29‐40. [DOI] [PubMed] [Google Scholar]
- 106. Nathwani AC, Tuddenham E, Chowdary P, et al. Preliminary results of a Phase I/II dose escalation trial of gene therapy for haemophilia A using a novel human factor VIII variant. Blood. 2018;132(suppl 1):489. [Google Scholar]
- 107. Pasi KJ, Rangarajan S, Robinson TM, et al. Hemostatic response is maintained for up to 5 years following treatment with valoctocogene roxaparvovec, an AAV5‐hFVIII‐SQ gene therapy for severe hemophilia A [abstract]. Res Pract Thromb Haemost. 2021;5. Accessed January 19, 2022. https://abstracts.isth.org/abstract/hemostatic‐response‐is‐maintained‐for‐up‐to‐5‐years‐following‐treatment‐with‐valoctocogene‐roxaparvovec‐an‐aav5‐hfviii‐sq‐gene‐therapy‐for‐severe‐hemophilia‐a/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


