Abstract
Background
Peptidoglycan recognition protein 1 (PGLYRP1) is an antimicrobial and proinflammatory innate immunity protein activated during infections. We aimed to investigate whether PGYLRP1 and associated molecules of the immune response in saliva is a cumulative outcome result of both MI and periodontal inflammation.
Methods and Results
Two hundred patients with MI and another 200 matched non‐MI controls were included. A full‐mouthexamination was conducted to assess periodontal inflammation and collection of stimulated saliva was performed 6 to 10 weeks after the first MI. PGLYRP1, triggering receptor expressed on myeloid cells 1 (TREM‐1), interleukin‐1 beta (IL‐1β) were analyzed by ELISA. Matrix metalloproteinase (MMP)‐8 levels were determined by IFMA. Compared to controls, MI patients showed higher salivary PGLYRP1, but not TREM‐1 levels. The difference in PGLYRP1 levels remained after adjustment for covariates. In MI patients, the PGLYRP1 levels positively correlated with BOP and PPD 4 to 5 mm. Among non‐MI subjects, the levels of PGLYRP1 correlated positively and significantly with BOP and total PPD. Salivary PGLYRP1 concentrations also showed strong positive correlations with levels of TREM‐1, IL‐1β and MMP‐8. In multivariate linear regression analysis, in MI patients, BOP and former smokingstatus displayed an association with salivary PGLYRP1 concentration.
Conclusion
MI patients showed higher salivary PGLYRP1 levels than healthy controls, also after adjusting for smoking, sex, age and periodontal health status. Salivary levels of PGLYRP1 may reflect the overall inflammatory burden to chronic bacterial exposure, possibly underpinning the observed associations between periodontitis and exposure with MI.
Keywords: IL‐1β, MMP‐8, myocardial infarction, periodontitis, PGLYRP1, saliva, TREM‐1
1. INTRODUCTION
Peptidoglycan recognition protein 1 (PGLYRP1) is a secreted antimicrobial and proinflammatory protein, abundant in polymorphonuclear leukocyte granules and releasedon degranulation to counteract microbial infections. 1 High levels of circulating PGLYRP1 have been associated with subclinical atherosclerotic lesions and acute atherosclerotic disease. 2 , 3 , 4 Moreover, global gene expression profiling from patients with acute coronary diseases suggest that PGLYRP1 classifies among highly specific and sensitive diagnostic biomarkers for acute myocardial infarction (MI). 5
MI, a major cause of mortality, may not be fully captured by traditional risk factors and likely to be multifactorial. 6 , 7 , 8 , 9 Periodontitis, a polymicrobial chronic local inflammatory disease, has been associated with a higher risk for MI. 6 , 7 , 8 A number of possible mechanisms for a causative relation between periodontitis and CVD have been discussed. 10 , 11 Atherosclerosis, the primary underlying disease process in MI, is driven by lipid accumulation in the arterial wall and inflammation. 9 Chronic exposure to periodontal bacteria or to their leaked products may exacerbate the inflammatory response within the arterial walls. 12 There could also be proinflammatory mediators, leaking from the periodontal lesion, which have a proatherosclerotic effect, either directly on the vessel wall or indirectly through an increase of the systemic inflammation. 13 , 14
PGLYRP1 specifically functions as a bacterial peptidoglycan (PGN) scavenger, thus provides bactericidal activity. Several oral bacteriaare known to possess of PGNs detected in human atheromatous plaques. 15 , 16 Oral bacterial PGNsmay in return promote arterial inflammation by forming PGLYRP1/PGN complexes. Mechanistically, as a putative ligand of the triggering receptor expressed on myeloid cells 1 (TREM‐1), PGLYRP1 can also activate the TREM‐1 signaling pathway resulting in both local and systemic proinflammatory immune response. 17 , 18 TREM‐1 and PGLYRP1 are upstream regulators of this signaling cascade that amplifies proinflammatory cytokine production, including interleukin‐1 beta (IL‐1β), in response to oral bacterial exposure. 19 , 20 , 21 The cell‐bound TREM‐1 can also be shed into body fluids as soluble TREM‐1 through matrix metalloproteinase (MMP)‐mediated proteolytic cleavage. 20 , 22 , 23 Higher levels of MMP‐8 have been found in in human artheroma 24 (52). According to previous studies salivary MMP‐8 levels are associated with periodontitis. 25
PGLYRP1, TREM‐1, and MMPs can be found in increased amounts in saliva, gingival crevicular fluid (GCF), gingival tissue, and serum of patients with periodontitis when compared to periodontally healthy individuals, and it correlates positively with the levels of putative periodontal pathogens present in subgingival plaque. 26 , 27 , 28 Interestingly, higher levels of circulating TREM‐1 are associated with higher risk of death following MI, 29 independent of associated risk factors. 30 More recent evidence suggested that TREM‐1 may have a role in sepsis driven myocardial dysfunction by enhancing proatherogenic cytokine production. 31 , 32 , 33 Although the role of PGLYRP1/TREM‐1 pathway has been acknowledged in MI, none of these studies included assessment of oral inflammation burden as a potential risk factor. In this aspect, we have previously demonstrated that higher salivary and circulating PGLYRP1/TREM‐1 level was associated with poor periodontal health and higher systemic inflammatory burden among patients with rheumatoid arthritis or higher risk of major cardiovascular events in patients with chronic kidney disease. 21 , 34 , 35 , 36 Thus, salivary levels of PGLYRP1 may reflect the overall inflammatory burden to chronic bacterial exposure, possibly underpinning the observed associations between periodontitis and exposure with MI. We hypothesize that the concentration of PGYLRP1 and associated molecules in saliva is a cumulative outcome of both MI and periodontal inflammation and are reflective of overall inflammatory burden in the mouth. Thus, in the present study, we investigated whether PGYLRP1 and associated molecules of the immune response in saliva are associated with MI.
2. MATERIALS AND METHODS
2.1. Study population, design, and saliva collection
The study protocols were approved by the regional ethical review board (Dnr. 2008/152‐31/2) Karolinska Institutet, Stockholm, Sweden and in line with the STROBE guidelines for observational studies. 37 Patients with a first MI (n = 200) according to the established criteria, 38 below 75 years old were recruited to this study who were admitted to a coronary care unit between May 2010 and December 2011 at 11 hospitals in Sweden. Two‐hundred controls, free from previous MI or heart valve replacement, matched for age, sex, and postal code were recruited from Swedish national population registry. Study participants were excluded if they had undergone cardiac valvular surgery or had language barriers preventing them to complete study procedures. A detailed description of the study procedures has been published earlier. 1 In brief, comprehensive procedural information was presented to the participants and a written informed consent was obtained from all individuals before the inclusion in the study. The study was conducted in accordance with the principles of the Declaration of Helsinki and good clinical practice. The medical history included recoding the diagnoses of hypertension, diabetes mellitus, and stroke. Medications at the time of hospital admission and follow‐up visit were also recorded. Smoking status was obtained and classified as never, former (quit >1 month), current. The presence of a family history of CVD (close relative with CVD below the age of 60 years), other systemic diseases including peripheral artery, rheumatic, pulmonary, and kidney disease, and cancer and depression were based on self‐reported questionnaires.
At first, stimulated saliva samples were collected, followed by a standardized clinical examination protocol including assessment of periodontal health status by probing pocket depth (PPD), bleeding on probing (BOP), dichotomous plaque at four sites (buccal‐proximal‐lingual) per tooth. The sum of all pockets ≥4 mm was also calculated and registered as “total PPD.” As detailed earlier, dental radiographs were taken from all subjects and alveolar bone levels at the mesial‐distal sites were measured from the marginal alveolar bone crest to the tooth apex (total bone height) and from the cemento‐enamel junction to the tooth apex (total root length). 39 , 40 The mean values calculated from the total root length and the total bone height were reported as an indicator of the proportion of existing alveolar bone height. These dental x‐rays were graded by three dentists, and the agreement correlation values were >0.90 with an agreement of 96% of measurements. Based on these dental x‐ray values, the participants were classified into the following groups: healthy (>80% remaining bone); mild to moderate periodontitis (79–66%); and severe periodontitis (<66%). Details regarding the study population and design and saliva collection are detailed in earlier studies. 39 , 40 Briefly, the study participants were asked to abstain eating and dental hygiene practices one hour prior to their dental examination. Stimulated saliva was obtained by chewing paraffin for 10 minutes. Then, the samples were immediately frozen down (−20°C) and later, before the analysis, thawed on ice, vortexed briefly and centrifuged (500 ×g, 5°C) for 5 minutes to obtain the cell‐free saliva supernatants. 40
2.2. Assessment of sTREM‐1, PYGLRP1, and IL‐1β levels in saliva by ELISA
Salivary sTREM‐1,* PGLYRP1† and IL‐1β‡ were measured by target specific enzyme linked immunosorbent assays (ELISAs) as described earlier. 21 , 27 In brief, the ELISA plates were coated with the working concentrations of the capture antibodies (goat antihuman TREM‐1, goat antihuman PGRP‐S, mouse antihuman IL‐1β, respectively) overnight at room temperature (RT). After removal of unbound antibodies by washing three times, the respective standards and the cell‐free saliva supernatants (prediluted in reagent diluent in 1:2 for TREM‐1, 1:60 for PGLYRP1, in 1:12 for IL‐1β) were added to the microplates and incubated at RT for 2 hours. After washing with a washing buffer consisting of 0.05% Tween 20 in sterile phosphate‐buffered saline (PBS, pH 7) three times, biotin‐conjugated antibodies (biotinylated goat antihuman TREM‐1, biotinylated goat antihuman PGRP‐S, biotinylated goat antihuman IL‐1β) were added to each well and incubated for 2 hours at RT, and the unbound antibodies were removed by washing three times. Then, tetramethylbenzidine was added as a substrate solution to each well and incubated for 20 minutes at RT, after which the reaction was stopped by the addition of 2 N H2SO4. The absorbance was measured at 450 nm using a microplate reader with a wavelength correction set at 570 nm to subtract background. The concentrations of TREM1, PGLYRP1, and IL‐1β in each sample were calculated based on a four‐parametric logistic standard curve for each analyte. The standard curve assay range for each ELISA assay was TREM‐1: 93.8 to 6000 pg/mL, PGLYRP1: 15.6 to 1000 pg/mL, and IL‐1β: 3.9 to 250 pg/mL. The detection limit of each assay is 23.1 pg/mL, 12.5 pg/ mL, 1.8 pg/mL for TREM‐1, PGLYRP1, and IL‐1β, respectively. All saliva samples were blinded to the laboratory team and tested in random order. To validate the reliability of the immunoassay results and monitor plate to plate variation, we tested interassay coefficient of variation (CV) by repeatedly running three saliva samples on multiple assay plates. The CVs were <15% (TREM‐1: 14%, PGYLRP‐1: 11%, IL‐1β: 8%).
2.3. Assessment of salivary MMP‐8 levels by IFMA
The MMP‐8 concentrations were determined by an immunofluorescence assay (IFMA) as described earlier. 39 , 40 Themonoclonal MMP‐8 specific antibodies 8708 and 8706§ were used as a catching antibody and a tracer antibody, respectively. The tracer antibodywas labeled using europium‐chelate. The assay buffer contained 20 mM Tris‐HCl, pH 7.5, 0.5 M NaCl, 5 mM CaCl2, 50 μM ZnCl2, 0.5% BSA, 0.05% sodium azide and 20 mg/L diethylenetriaminepentaacetic acid (DTPA). Samples were diluted in assay buffer and incubated for one hour, followed by incubation for one hour with tracer antibody. Enhancement solution was added and after 5 minutes fluorescence was measured using a 1234 Delfia Research Fluorometer.** The mean CV was 7.3%. The detection limit for the assay is 0.08 ng/mL. 39 , 40
2.4. Statistical analysis
All calculations were done in SPSS (Statistical Package for Social Sciences) version 25.†† Differences were considered significant at a probability level of P < 0.05. To compare the ratio of categorical variables, the Chi‐squared test was used. Age, the periodontal clinical parameters, and systemic conditions are expressed as median and interquartile range (IQR). Salivary biomarker levels were log‐transformed before group comparisons or correlations to achieve approximately normal distribution. The group comparisons of the biomarkers were performed using independent‐samples t‐test. Pearson correlations were computed both in the study of relations between the studied markers and clinical and radiographic variables of periodontal status. Multiple linear regressions were used with markers as dependent variables (log‐transformed: IL‐1β, TREM‐1, and PGLYRP1) and as independent variables (clinical, demographic variables, systemic conditions). The independent variables were entered in four different blocks.
3. RESULTS
3.1. Demographic and clinical data of the study population
The demographics of MI patients and non‐MI subjects are already published in our previous studies. 13 , 34 The detailed clinical characteristics are presented in Supporting Information Table S1 (see Table S1 in online Journal of Periodontology). In brief, the mean age of MI patients and non‐MI subjects was 61±8; P > 0.05), and 84% (n = 168; P > 0.05) in both groups were male. Smoking history and the presence of the other systemic diseases including hypertension, diabetes mellitus, kidney disease, respiratory disease, cancer, and rheumatic disease did not differ between the study groups. In contrast, the use of medications including aspirin, angiotensin‐converting enzyme inhibitors (ACEI), beta (β)‐blockers, and statins was significantly higher in the patients at the time for saliva collection and dental assessment compared with the controls (P < 0.05). Among the dental parameters, only the total pathogenic periodontal pocket depth significantly differed between the groups with median 48 mm in the MI group compared with 38 mm in the non‐MI group (P < 0.01). Notably, the patients with MI had higher but non‐significant plaque scores, BOP, and number of sites with PPD 4 to 5 mm than the controls (see Table S1 in online Journal of Periodontology).
3.2. Salivary levels of TREM‐1, PGYLRP‐1, and IL‐1β in MI patients compared with non‐MI subjects
Salivary TREM‐1, PGLYRP1, and IL‐1β were analyzed from a total of 400 subjects (200 MI patients versus 200 non‐MI subjects) using the obtained saliva supernatants. TREM‐1 (ng/mL: min 11.7; max 2579.8), PGLYRP1 (ng/mL: min 254.5; max 30,7763.1), and IL‐1β (ng/mL: min 5.8; max 886.6) were detected in all samples except for three samples of IL‐1β that were under the detection limit. The mean PGLYRP1 levels (Figure 1A) in saliva showed a significant difference (P < 0.001) between MI patients and non‐MI subjects (mean ± SD pg/mL: 26,321 ± 27,237 versus 18,967 ± 27,955) whereas the difference in mean concentration of TREM‐1 (mean ± SD pg/mL: 464.5 ± 456.3 versus 417.8 ± 357.5) and IL‐1β (mean ± SD pg/mL: 112.9 ± 127.0 versus 120.2 ± 132.4) did not reach significance (Figure 1B,C).
FIGURE 1.
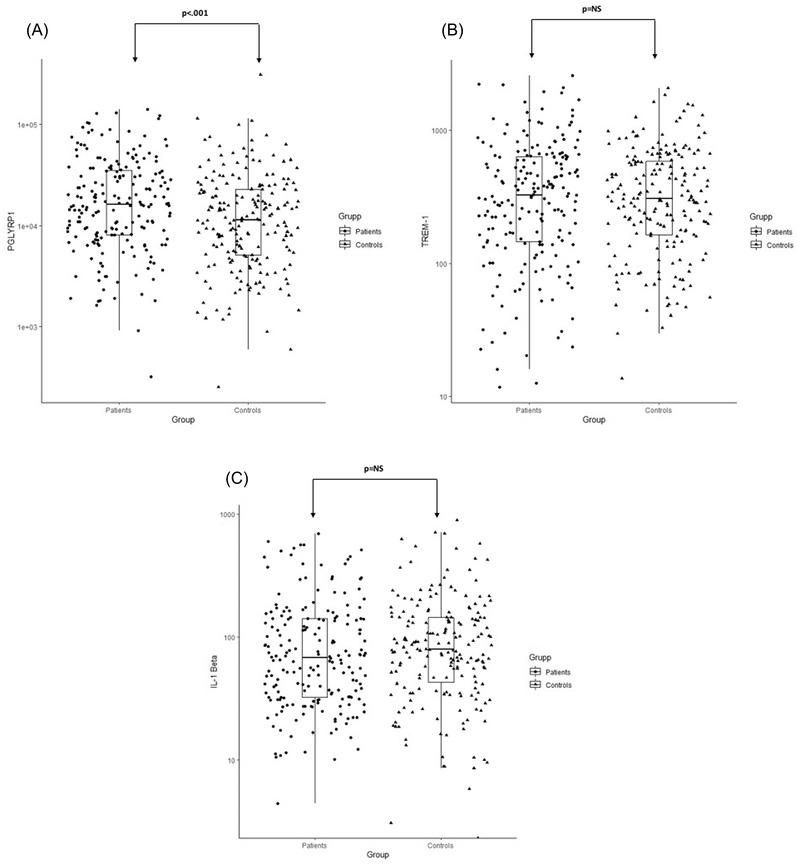
Salivary PGYLRP1 (A), TREM‐1 (B) and IL‐1β (C) concentrations in patients with postmyocardial infarction (MI) and non‐MI subjects. An independent sample t‐test was used for statistical analysis; data reported as log10 converted values. *P < 0.05 was considered as significant
Figure 2A to 2C shows the correlation between salivary levels of PGLYRP1/TREM‐1, IL‐1β/TREM‐1,and IL‐1β/ PGLYRP1 in MI patients and non‐MI subjects. A significant positive correlation was observed in both groups and between analyzed biomarkers: PGLYRP1/TREM‐1 (MI patients: R = 0.73; P < 0.001 and non‐MI subjects: R = 0.65; P < 0.001), IL‐1β/TREM‐1(MI patients: R = 0.63; P < 0.001 and non‐MI subjects: R = 0.62; P < 0.001) and IL‐1β/PGLYRP1 (MI patients: R = 0.60; P < 0.001 and non‐MI subjects: R = 0.54; P < 0.001) in saliva. We further investigated whether there were any correlations between the PGLYRP1 and TREM‐1 concentrations detected in the current study and the previously reported MMP‐8 concentrations in the same saliva samples 34 (Figure 2D,E). There was a strong correlation between PGLYRP1 and MMP‐8 (MI patients: R = 0.60; P < 0.001 and non‐MI subjects: R = 0.5; P < 0.001) and between TREM‐1 and MMP‐8 (MI patients: R = 0.75; P < 0.001 and non‐MI subjects: R = 0.73; P < 0.001).
FIGURE 2.
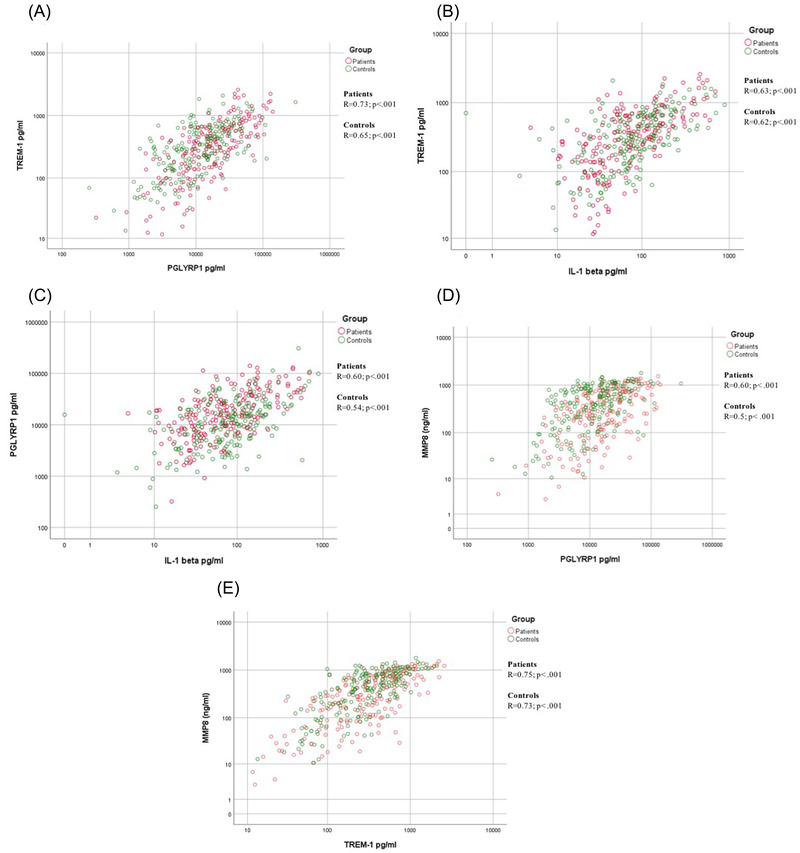
Correlation between salivary levels of PGYLRP1/TREM‐1 (A), IL‐1β/TREM‐1 (B), IL‐1β/PGLYRP1 (C), MMP‐8/ PGYLRP1 (D), MMP‐8/TREM‐1 (E) in patients with postmyocardial infarction (MI) and non‐MI subjects. Pearson correlations were used to investigate the relations between the studied biomarkers. *P < 0.05 was considered as significant
3.3. Salivary sTREM‐1, PGLYRP1, IL‐1β, and periodontal health parameters
The Pearson correlations between log‐transformed salivary levels of IL‐1β, PGLYRP1, TREM‐1 and clinical and radiographic variables of periodontal status in MI patients and non‐MI subjects are outlined in Table 1. In MI patients, there was a significant positive correlation between IL‐1β and BOP (R = 0.24; P < 0.001), PPD 4 to 5 mm (R = 0.18; P < 0.01), and PPD ≥6 mm (R = 0.22; P < 0.01); PGLYRP1 and BOP (R = 0.36; P < 0.001) and PPD 4 to 5 mm (R = 0.22; P < 0.001); and TREM‐1 and BOP (R = 0.35; P < 0.001). Among non‐MI subjects, the levels of IL‐1β showed a slight positive correlation and significant with BOP (R = 0.18; P < 0.01). The salivary PGLYRP1 correlated positively and significantly with BOP (R = 0.23; P < 0.001) and total PPD (R = 0.18; P < 0.01). The TREM‐1 levels of saliva in non‐MI subjects significantly correlated with plaque (R = 0.27; P < 0.0001), BOP (R = 0.36; P < 0.0001), and PPD ≥6 mm (R = 0.18; P < 0.01). However, the radiographic periodontal status did not correlate with the analyzed salivary biomarkers both in MI patients (IL‐1β [R = 0.06; P = 0.37], PGLYRP1 [R = ‐0.02; P = 0.83], TREM‐1 [R = 0.03; P = 0.72]) and non‐MI subjects (IL‐1β [R = 0.04; P = 0.62], PGLYRP1 [R = 0.07; P = 0.29], TREM‐1 [R = 0.01; P = 0.99]).
TABLE 1.
Correlation between salivary levels of log transformed PGLYRP1, TREM‐1 and IL‐1β and clinical variables of periodontitis as well as radiographic periodontal status
| PGLYRP1 | TREM‐1 | IL‐1β | ||||
|---|---|---|---|---|---|---|
| Biomarkers/periodontal parameters | MI patients (n = 200) | non‐MI (n = 200) | MI patients (n = 200) | non‐MI (n = 200) | MI patients (n = 200) | non‐MI (n = 200) |
| Plaque (n sites) | 0.09/NS | 0.16/NS | 0.20/NS | 0.27/< .001 | 0.07/NS | 0.15/NS |
| BOP (n sites) | 0.36/< .001 | 0.23/< .001 | 0.35/< .001 | 0.36/< .001 | 0.24/< .001 | 0.18/< .01 |
| PPD 4–5 mm | 0.22/< .001 | 0.13/NS | 0.16/NS | 0.12/NS | 0.18/< .01 | −0.02/NS |
| PPD ≥6 mm | 0.21/NS | 0.16/NS | 0.16/NS | 0.18/< .01 | 0.22/< .01 | 0.06/NS |
| Total PPD (mm) | 0.11/NS | 0.18/< .01 | 0.02/NS | 0.11/NS | 0.08/NS | −0.05/NS |
| Radiographic periodontal status | −0.02/NS | 0.07/NS | 0.03/NS | 0.01/NS | 0.06/NS | 0.04/NS |
Data shown as R/P‐value, visible plaque, number of sites with bleeding on probing (BOP), probing pocket depths (PPD 4 to 5 mm and ≥6 mm).
The sum of all pockets ≥4 mm was also calculated and registered as “total PPD.” Radiographic periodontal status = the measured alveolar bone loss.
*P < 0.05 was considered as significant.
3.4. Linear regression models
Multiple linear regression analysis evaluated the studied biomarker concentration differences between MI and non‐MI groups based on the independent predictor variables. Salivary PGLYRP1 levels showed significant association with BOP and former smoking status across the different models with R2 of 0.17 (Table 2). BOP and former smoker status were the variables that explained differences in TREM‐1levels across the models and reached a significant level; the maximum R2 was 0.18 (see Table S2 in online Journal of Periodontology). In the fourth multivariate model (R2: 0.10), the salivary IL‐1β showed significant association with BOP and sex (see Table S3 in online Journal of Periodontology).
TABLE 2.
Multiple linear regression analysis evaluating PGLYRP1‐concentration differences (dependent variable) between MI patients and non‐MI groups, based on independent predictor variables (n = 324)
| Unstandardized coefficients | Standardized coefficients | 95% Confidence interval for B | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | B | Std. error | Beta | t | Significance | Lower bound | Upper bound | |
| 1 | (Constant) | 4.018 | .052 | 77.469 | .000 | 3.916 | 4.120 | |
| Group | −.156 | .050 | −.164 | −3.145 | .002 | −.253 | −.058 | |
| PD Mild/moderate | .023 | .057 | .021 | .395 | .693 | −.090 | .136 | |
| PD Severe | −.151 | .097 | −.082 | −1.552 | .122 | −.342 | .040 | |
| BoP | .006 | .001 | .330 | 6.325 | .000 | .004 | .008 | |
| 2 | (Constant) | 4.072 | .223 | 18.295 | .000 | 3.634 | 4.510 | |
| Group | −.138 | .050 | −.145 | −2.740 | .006 | −.237 | −.039 | |
| PD Mild/moderate | .024 | .059 | .022 | .405 | .685 | −.092 | .140 | |
| PD Severe | −.159 | .099 | −.086 | −1.606 | .109 | −.354 | .036 | |
| BoP | .006 | .001 | .333 | 6.279 | .000 | .004 | .008 | |
| Age | −.001 | .003 | −.022 | −.413 | .680 | −.008 | .005 | |
| Sex | −.040 | .070 | −.031 | −.573 | .567 | −.178 | .097 | |
| Present smoker | .017 | .073 | .014 | .229 | .819 | −.127 | .160 | |
| Former smoker | .123 | .056 | .130 | 2.180 | .030 | .012 | .234 | |
| 3 | (Constant) | 4.002 | .231 | 17.352 | .000 | 3.548 | 4.456 | |
| Group | −.109 | .058 | −.114 | −1.886 | .060 | −.222 | .005 | |
| PD Mild/moderate | .029 | .060 | .027 | .494 | .622 | −.088 | .147 | |
| PD Severe | −.158 | .100 | −.086 | −1.583 | .114 | −.354 | .038 | |
| BoP | .006 | .001 | .342 | 6.324 | .000 | .004 | .008 | |
| Age | −.001 | .003 | −.018 | −.340 | .734 | −.008 | .005 | |
| Sex | −.036 | .070 | −.027 | −.511 | .610 | −.175 | .103 | |
| Present smoker | .025 | .074 | .021 | .338 | .736 | −.121 | .172 | |
| Former smoker | .117 | .057 | .123 | 2.052 | .041 | .005 | .229 | |
| Diabetes | −.042 | .103 | −.022 | −.411 | .682 | −.244 | .160 | |
| Hypertension | .026 | .053 | .027 | .503 | .615 | −.077 | .130 | |
| Cholesterol | −.001 | .008 | −.004 | −.079 | .937 | −.017 | .016 | |
| Acetylsalicylic Acid latest 14 days | .074 | .058 | .075 | 1.281 | .201 | −.040 | .188 | |
| 4 | (Constant) | 3.989 | .234 | 17.082 | .000 | 3.529 | 4.448 | |
| Group | −.105 | .058 | −.111 | −1.807 | .072 | −.220 | .009 | |
| PD Mild/moderate | .024 | .060 | .022 | .399 | .690 | −.095 | .143 | |
| PD Severe | −.159 | .100 | −.086 | −1.588 | .113 | −.357 | .038 | |
| BoP | .006 | .001 | .343 | 6.306 | .000 | .004 | .009 | |
| Age | −.001 | .003 | −.014 | −.268 | .789 | −.007 | .006 | |
| Sex | −.035 | .074 | −.027 | −.470 | .639 | −.181 | .111 | |
| Present smoker | .025 | .075 | .021 | .340 | .734 | −.122 | .173 | |
| Former smoker | .120 | .057 | .127 | 2.099 | .037 | .008 | .233 | |
| Diabetes | −.051 | .104 | −.026 | −.489 | .625 | −.256 | .154 | |
| Hypertension | .026 | .053 | .027 | .496 | .620 | −.078 | .130 | |
| Cholesterol | −.001 | .009 | −.006 | −.116 | .908 | −.018 | .016 | |
| Acetylsalicylic Acid latest 14 days | .074 | .058 | .074 | 1.262 | .208 | −.041 | .188 | |
| Kidney disease | −.079 | .104 | −.040 | −.760 | .448 | −.284 | .126 | |
| Lung disease | .002 | .075 | .001 | .022 | .982 | −.146 | .149 | |
| Rheumatic disease | .021 | .073 | .016 | .285 | .776 | −.124 | .166 | |
Values are coefficient 95% confidence intervals. Unit of concentrations of the studied markers were logarithmically transformed.
*P < 0.05 was considered as significant.
4. DISCUSSION
In this study, we demonstrated higher salivary PGLYRP1 levels in post‐MI patients compared to non‐MI individuals, following adjustment according to their periodontal status. Although we did not directly measure the systemic levels of this marker in serum, but rather indirectly measured the total salivary levels in the oral cavity, our findings are in line with observations that PGLYRP1 is a suitable atherosclerosis related biomarker, identified in acute coronary syndrome patients. 29 In addition, systemic PGLYRP1 levels have been independently associated with measures of atherosclerosis in both coronary and peripheral vascular beds and with different stages of development from early increases in wall thickness to protruding plaque to late calcification. 30 The association with subclinical and acute atherosclerosis suggests a potential role for PGLYRP1 as an atherosclerosis related biomarker, possibly suited for diagnostic screening for undetected cardiovascular disease. Potential correlations between serum and salivary levels of PGLYRP1 in MI patients should be further investigated.
Although the effect of MI on the salivary level of PGYLRP1 is noteworthy, one may expect that existing periodontal inflammation and MI are independently associated with an increase in the concentration of PGYLRP1 in saliva. Indeed, elevated salivary PGLYRP1 levels were associated with increased BOP and severity of periodontal pocket measures in both groups. This is in line with earlier observations that local gingival inflammationinfluences the protein concentration, thus demonstrating the importance to consider periodontal status when analyzing biomarkers in saliva. 41 Considering that the salivary inflammatory mediators are mainly derived from the periodontium via influx of gingival crevicular fluid, it is not surprising that the influence of periodontal inflammation on the concentration of PGYLRP1 is more pronounced than the effect of MI. 42 This association is supported by the recent findings that an increase in BOP scores in response to oral biofilm accumulation mirrored PGLYRP1 release into saliva and interventions to remove biofilms resulted in a decrease in its levels. 21
Moreover, the further analysis showed that salivary PGLYRP1 correlated with TREM‐1 levels as well as with salivary levels of MMP‐8 and IL‐1β. Our finding of a positive correlation between these molecules indicates that the number and activation of neutrophils by oral bacteria in saliva has a strong influence on the concentration in saliva. It is noteworthy that salivary TREM‐1 levels and IL‐1β levels were not able to distinguish between MI patients and systemically healthy controls despite their levels might closely reflect the presence of gingival inflammation. 26 , 27 , 43 , 44 , 45 Although PGLYRP1 has the ability to activate TREM‐1 and IL‐1β in the periodontium, 19 , 46 , 47 multiple pathways exist that might influence their expression and stimulation. 48 The recent findings are indicating that TREM‐1 upregulates in plasma of acute MI patients and participates to the deleterious amplification of the inflammatory process that leads to cardiac remodeling and dysfunction. 5 , 49 Another study conducted by Zysset and co‐authors showed that TREM‐1 promotes cardiovascular disease by exacerbating atherosclerosis via enhanced lipid accumulation. 32 One possible explanation for this discrepancy is that the concentrations of salivary TREM‐1 and IL‐1β are inhibited by anti‐inflammatory medications. 20 Their levels might be downregulated in MI at the time of saliva collection due to more frequent use of cardiovascular medications (aspirin, β‐blockade, ACEI, and statins). The intake of these drugs may affect their overall inflammatory burden in saliva, there upon mask the magnitude the observed association. 50 Another potential explanation that saliva samples from the patients in current study were not collected at the admission for MI but at 6 to 10 weeks after the MI. Therefore, additional studies are needed to investigate the screening potential of PGLYRP1 and associated molecules in saliva collected at the time of MI.
5. CONCLUSION
Overall, MI patients showed higher salivary PGLYRP1 levels than non‐MI controls, also after adjusting for smoking, sex, age and periodontal status, but the degree of its specificity remains to be confirmed.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest. However, Timo Sorsa is inventor of US Patent 2017/0023671A1 and the Japan Patent 2016‐554676.
AUTHOR CONTRIBUTION
NR, AG, TS, AN, and NB contributed to the study design, data acquisition, data analysis and interpretation and manuscript writing. All authors gave final approval and agreed to be accountable for all aspects of this work.
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The study was academic initiated. It was financially supported by the Swedish Research Council, Stockholm, the Academy of Finland, Helsinki, the Apollonia Foundation for Dental Research and the Helsinki University Central Hospital Research Foundation, Helsinki and the authors' institutional funds, Stockholm and Helsinki.
Rathnayake N, Gustafsson A, Sorsa T, Norhammar A, Bostanci N; PAROKRANK Steering Committee. Association of peptidoglycan recognition protein 1 to post‐myocardial infarction and periodontal inflammation: A subgroup report from the PAROKRANK (Periodontal Disease and the Relation to Myocardial Infarction) study. J Periodontol. 2022;93:1325–1335. 10.1002/JPER.21-0595
Footnotes
Human TREM‐1 DuoSet ELISA, R&D Systems, Oxon, Great Britain.
Human PGYLRP1 DuoSet ELISA,R&D Systems, Oxon, Great Britain.
Human IL‐1β DuoSet ELISA, R&D Systems, Oxon, Great Britain.
Medix Biochemica, Kauniainen, Finland.
Wallac, Turku, Finland.
SPSS Inc., Chicago, IL.
DATA AVAILABILITY STATEMENT
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
- 1. Dziarski R, Platt KA, Gelius E, Steiner H, Gupta D. Defect in neutrophil killing and increased susceptibility to infection with nonpathogenic gram‐positive bacteria in peptidoglycan recognition protein‐S (PGRP‐S)‐deficient mice. Blood. 2003;102:689‐697. [DOI] [PubMed] [Google Scholar]
- 2. Brownell NK, Khera A, de Lemos JA, Ayers CR, Rohatgi A. Association between peptidogly can recognition protein‐1 and incident atherosclerotic cardiovascular disease events: the Dallas Heart Study. J Am Coll Cardiol. 2016;67:2310‐2312. [DOI] [PubMed] [Google Scholar]
- 3. Rohatgi A, Ayers CR, Khera A, et al. The association between peptidoglycan recognition protein‐1 and coronary and peripheral atherosclerosis: observations from the Dallas Heart Study. Atherosclerosis. 2009;203:569‐575. [DOI] [PubMed] [Google Scholar]
- 4. Gada E, Owens AW, Gore MO, et al. Discordant effects of rosiglitazone on novel inflammatory biomarkers. Am Heart J. 2013;165:609‐614. [DOI] [PubMed] [Google Scholar]
- 5. Park HJ, Noh JH, Eun JW, et al. Assessment and diagnostic relevance of novel serum biomarkers for early decision of ST‐elevation myocardial infarction. Oncotarget. 2015;6:12970‐12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ryden L, Buhlin K, Ekstrand E, et al. Periodontitis increases the risk of a first myocardial infarction: a report from the PAROKRANK Study. Circulation. 2016;133:576‐583. [DOI] [PubMed] [Google Scholar]
- 7. Jonsson D, Orho‐Melander M, Demmer RT, et al. Periodontal disease is associated with carotid plaque area: the Malmo Offspring Dental Study (MODS). J Intern Med. 2020;287:301‐309. [DOI] [PubMed] [Google Scholar]
- 8. Sanz M, Marco Del Castillo A, Jepsen S, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47:268‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawler PR, Bhatt DL, Godoy LC, et al. Targeting cardiovascular inflammation: next steps in clinical translation. Eur Heart J. 2021;42:113‐131. [DOI] [PubMed] [Google Scholar]
- 10. Lockhart PB, Bolger AF, Papapanou PN, et al. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520‐2544. [DOI] [PubMed] [Google Scholar]
- 11. Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol 2000. 2020;83:90‐106. [DOI] [PubMed] [Google Scholar]
- 12. Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554‐1560. [DOI] [PubMed] [Google Scholar]
- 13. D'Aiuto F, Parkar M, Nibali L, Suvan J, Lessem J, Tonetti MS. Periodontal infections cause changes in traditional and novel cardiovascular risk factors: results from a randomized controlled clinical trial. Am Heart J. 2006;151:977‐984. [DOI] [PubMed] [Google Scholar]
- 14. Roth GA, Moser B, Roth‐Walter F, et al. Infection with a periodontal pathogen increases mononuclear cell adhesion to human aortic endothelial cells. Atherosclerosis. 2007;190:271‐281. [DOI] [PubMed] [Google Scholar]
- 15. Pinon‐Esteban P, Nunez L, Moure R, et al. Presence of bacterial DNA in thrombotic material of patients with myocardial infarction. Sci Rep. 2020;10:16299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laman JD, Schoneveld AH, Moll FL, van Meurs M, Pasterkamp G. Significance of peptidoglycan, a proinflammatory bacterial antigen in atherosclerotic arteries and its association with vulnerable plaques. Am J Cardiol. 2002;90:119‐123. [DOI] [PubMed] [Google Scholar]
- 17. Tammaro A, Derive M, Gibot S, Leemans JC, Florquin S, Dessing MC. TREM‐1 and its potential ligands in non‐infectious diseases: from biology to clinical perspectives. Pharmacol Ther. 2017;177:81‐95. [DOI] [PubMed] [Google Scholar]
- 18. Read CB, Kuijper JL, Hjorth SA, et al. Cutting edge: identification of neutrophil PGLYRP1 as a ligand for TREM‐1. J Immunol. 2015;194:1417‐1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bostanci N, Abe T, Belibasakis GN, Hajishengallis G. TREM‐1 isupregulated in experimental periodontitis, and its blockade inhibits IL‐17A and RANKL expression and suppresses bone loss. J Clin Med. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bostanci N, Belibasakis GN. Doxycycline inhibits TREM‐1 induction by Porphyromonas gingivalis . FEMS Immunol Med Microbiol. 2012;66:37‐44. [DOI] [PubMed] [Google Scholar]
- 21. Silbereisen A, Hallak AK, Nascimento GG, et al. Regulation of PGLYRP1 and TREM‐1 during progression and resolution of gingival inflammation. JDR Clin Trans Res. 2019;4:352‐359. [DOI] [PubMed] [Google Scholar]
- 22. Gibot S, Cravoisy A. Soluble form of the triggering receptor expressed on myeloid cells‐1 as a marker of microbial infection. Clin Med Res. 2004;2:181‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gomez‐Pina V, Soares‐Schanoski A, Rodriguez‐Rojas A, et al. Metalloproteinases shed TREM‐1 ectodomain from lipopolysaccharide‐stimulated human monocytes. J Immunol. 2007;179:4065‐4073. [DOI] [PubMed] [Google Scholar]
- 24. Herman MP, Sukhova GK, Libby P, et al. Expression of neutrophil collagenase (matrix metalloproteinase‐8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899‐1904. [DOI] [PubMed] [Google Scholar]
- 25. Sorsa T, Tjaderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med. 2006;38:306‐321. [DOI] [PubMed] [Google Scholar]
- 26. Bisson C, Massin F, Lefevre PA, Thilly N, Miller N, Gibot S. Increased gingival crevicular fluid levels of soluble triggering receptor expressed on myeloid cells (sTREM)‐1 in severe periodontitis. J Clin Periodontol. 2012;39:1141‐1148. [DOI] [PubMed] [Google Scholar]
- 27. Bostanci N, Ozturk VO, Emingil G, Belibasakis GN. Elevated oral and systemic levels of soluble triggering receptor expressed on myeloid cells‐1 (sTREM‐1) in periodontitis. J Dent Res. 2013;92:161‐165. [DOI] [PubMed] [Google Scholar]
- 28. Willi M, Belibasakis GN, Bostanci N. Expression and regulation of triggering receptor expressed on myeloid cells 1 in periodontal diseases. Clin Exp Immunol. 2014;178:190‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishii K, Hamamoto H, Imamura K, et al. Porphyromonas gingivalis peptidoglycans induce excessive activation of the innate immune system in silkworm larvae. J Biol Chem. 2010;285:33338‐33347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang YK, Tang JN, Shen YL, et al. Prognostic utility of soluble TREM‐1 in predictingmortality and cardiovascular events in patients with acute myocardial infarction. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boufenzer A, Lemarie J, Simon T, et al. TREM‐1 mediates inflammatory injury and cardiac remodeling following myocardial infarction. Circ Res. 2015;116:1772‐1782. [DOI] [PubMed] [Google Scholar]
- 32. Zysset D, Weber B, Rihs S, et al. TREM‐1 links dyslipidemia to inflammation and lipid deposition in atherosclerosis. Nat Commun. 2016;7:13151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu Q, Johnson EM, Lam RK, et al. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nat Immunol. 2019;20:1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arenius I, Ruokonen H, Ortiz F, et al. The relationship between oral diseases and infectious complications in patients under dialysis. Oral Dis. 2020;26:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 35. Nylund KM, Ruokonen H, Sorsa T, et al. Association of the salivary triggering receptor expressed on myeloid cells/its ligand peptidoglycan recognition protein 1 axis with oral inflammation in kidney disease. J Periodontol. 2018;89:117‐129. [DOI] [PubMed] [Google Scholar]
- 36. Ortiz F, Nylund KM, Ruokonen H, et al. Salivary biomarkers of oral inflammation are associated with cardiovascular events and death among kidney transplant patients. Transplant Proc. 2020;52:3231‐3235. [DOI] [PubMed] [Google Scholar]
- 37. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453‐1457. [DOI] [PubMed] [Google Scholar]
- 38. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551‐2567. [DOI] [PubMed] [Google Scholar]
- 39. Rathnayake N, Buhlin K, Kjellstrom B, et al. Saliva and plasma levels of cardiac‐related biomarkers in post‐myocardial infarction patients. J Clin Periodontol. 2017;44:692‐699. [DOI] [PubMed] [Google Scholar]
- 40. Rathnayake N, Gustafsson A, Norhammar A, et al. Salivary matrix metalloproteinase‐8 and ‐9 and myeloperoxidase in relation to coronary heart and periodontal diseases: a Subgroup Report from the PAROKRANK Study (Periodontitis and Its Relation to Coronary Artery Disease). PLoS One. 2015;10:e0126370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bostanci N, Selevsek N, Wolski W, et al. Targeted proteomics guided by label‐free quantitative proteome analysis in saliva reveal transition signatures from health to periodontal disease. Mol Cell Proteomics. 2018;17:1392‐1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis–a review. J Clin Periodontol. 2000;27:453‐465. [DOI] [PubMed] [Google Scholar]
- 43. Ortiz F, Nylund KM, Ruokonen H, et al. Salivary biomarkers of oral inflammation are associated with cardiovascular events and death among kidney transplant patients. Transplant Proc. 2020;52(10):3231‐3235. [DOI] [PubMed] [Google Scholar]
- 44. Yucel ZPK, Silbereisen A, Emingil G, et al. Salivary biomarkers in the context of gingival inflammation in children with cystic fibrosis. J Periodontol. 2020. [DOI] [PubMed] [Google Scholar]
- 45. Raivisto T, Heikkinen AM, Silbereisen A, et al. Regulation of salivary peptidoglycan recognition protein1 in adolescents. JDR Clin Trans Res. 2020:5(4):332‐341. [DOI] [PubMed] [Google Scholar]
- 46. Dziarski R, Gupta D. Review: mammalian peptidoglycan recognition proteins (PGRPs) in innate immunity. Innate Immun. 2010;16:168‐174. [DOI] [PubMed] [Google Scholar]
- 47. Guan R, Mariuzza RA. Peptidoglycan recognition proteins of the innate immune system. Trends Microbiol. 2007;15:127‐134. [DOI] [PubMed] [Google Scholar]
- 48. Bostanci N, Thurnheer T, Belibasakis GN. Involvement of the TREM‐1/DAP12 pathway in the innate immune responses to Porphyromonas gingivalis . Mol Immunol. 2011;49:387‐394. [DOI] [PubMed] [Google Scholar]
- 49. Jeremie L, Amir B, Marc D, Sebastien G. The Triggering Receptor Expressed on Myeloid cells‐1: a new player during acute myocardial infarction. Pharmacol Res. 2015;100:261‐265. [DOI] [PubMed] [Google Scholar]
- 50. Donos N, Calciolari E, Brusselaers N, Goldoni M, Bostanci N, Belibasakis GN. The adjunctive use of host modulators in non‐surgical periodontal therapy. A systematic review of randomized, placebo‐controlled clinical studies. J Clin Periodontol. 2020;47(Suppl 22):199‐238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.


