Summary
Outcomes remain poor for patients with relapsed/refractory B‐cell non‐Hodgkin lymphoma (R/R B‐NHL). While chimeric antigen receptor (CAR) T‐cell therapy has revolutionised treatment, a significant proportion of patients relapse or fail to respond. Odronextamab is a CD20 × CD3 bispecific antibody that has demonstrated durable responses and a manageable safety profile in patients with R/R B‐NHL in a first‐in‐human trial (NCT02290951). Here, we document two patients with diffuse large B‐cell lymphoma refractory to CART‐cell therapy. Both achieved complete responses that remain ongoing for ≥2 years following odronextamab. Neither patient experienced Grade ≥3 cytokine release syndrome or Grade ≥3 neurological adverse events during treatment.
Keywords: bispecific antibodies, cellular therapies, clinical trials, non‐Hodgkin lymphoma, tumour immunotherapy
INTRODUCTION
Outcomes are poor for patients with relapsed/refractory diffuse large B‐cell lymphoma (DLBCL), with a median overall survival (OS) of 6.3 months. 1 CD19‐specific chimeric antigen receptor (CAR) T cells provide these patients with significantly improved responses and survival. 2 , 3 , 4 , 5 Outcomes for patients who fail CD19 CART‐cell therapy are poor, (median OS 5.3 months), 6 with no standard of care for subsequent treatment. 7
By targeting a different antigen, CD20 × CD3 bispecific antibodies provide an attractive option following CD19‐directed CART‐cell therapy failure. Odronextamab is a CD20 × CD3 bispecific immunoglobulin G4 antibody, modified to reduce Fc receptor binding. By bridging CD20‐ and CD3‐expressing cells, odronextamab elicits CD20‐specific local T‐cell activation and cytotoxicity. In a first‐in‐human study of odronextamab for non‐Hodgkin lymphoma (N = 145; DLBCL, n = 82) no dose‐limiting toxicities were noted. 8 In patients with DLCBL receiving odronextamab ≥80 mg, the complete response (CR) rate was 53% in the CART‐cell therapy‐naïve group and 27% in post CART‐cell therapy progressors, with CRs durable beyond 20 months.
We report two cases of durable CR to odronextamab in patients with DLBCL refractory to prior CART‐cell therapy. Both patients were enrolled into a phase I dose‐escalation trial (NCT02290951) in which odronextamab was administered intravenously to a goal dose of 80 mg, initially as an escalating weekly split infusion (weeks 1–3), followed by full dose weekly infusion (weeks 4–12); then every 2 weeks until week 36 (24 total doses). Patients were assessed by computed tomography (CT) scan and positron emission tomography (PET)‐CT using the Lugano classification. 9 Regeneron designed the research protocol, which was approved by relevant institutional review boards/ethics committees. The study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki for Studies Involving Human Subjects. All participants provided written informed consent.
The first patient was a 71‐year‐old woman who presented with non‐germinal centre DLBCL with diffuse lymphadenopathy. The patient received rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in combination with lenalidomide after cycle two. 10 End‐of‐treatment PET‐CT demonstrated a CR. The patient relapsed 3 months later, and was refractory to second‐line rituximab, gemcitabine, and oxaliplatin. She was salvaged with third‐line lisocabtagene maraleucel (CD19‐directed CART‐cells), which she tolerated well with no cytokine release syndrome (CRS) or neurotoxicity. She achieved CR at 1 month, followed by relapse 3 months later. Post‐CART‐cell therapy biopsy demonstrated CD19+ DLBCL. She relapsed again following a second CART‐cell infusion.
The patient was subsequently treated with odronextamab per protocol with no major toxicities. Week 5 CT imaging demonstrated a CR and week 9 PET‐CT confirmed complete metabolic response (Figure 1A). The patient received all treatment doses and had an ongoing CR at 42 months of follow‐up (Figure 1B,C).
FIGURE 1.
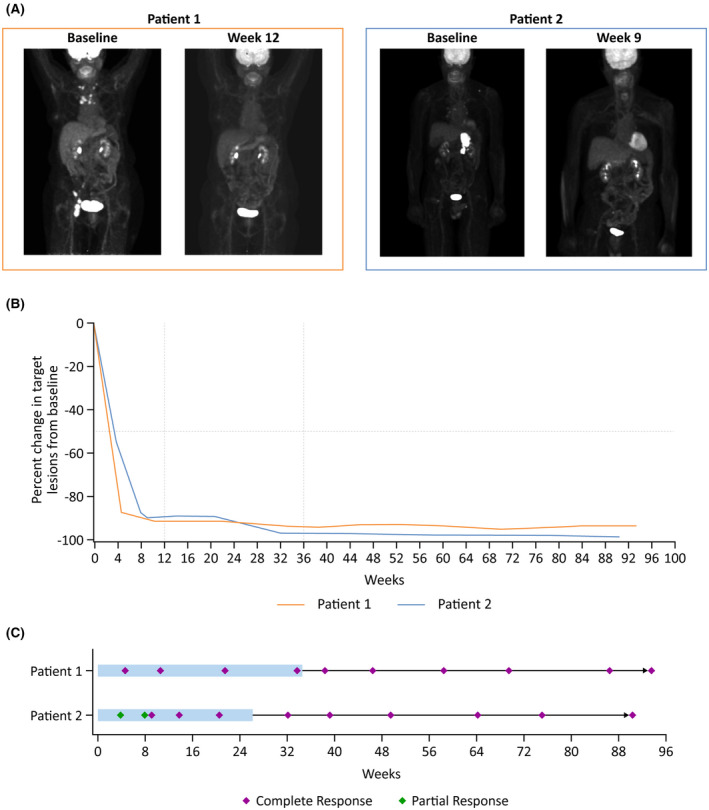
Assessment of response to odronextamab. PET scans (A), change from baseline in tumour size (SPD) (B) and swimmer plot (C). (A) Patient 1 had a complete response at the week 12 assessment by PET (Deauville 1). Patient 2 had a complete response at the week 9 response assessment by PET (Deauville 3). (B) Patient 1 had a complete response by CT measurement at first response assessment. Patient 2 had an initial partial response by CT measurement, but rapid transition to complete response. (C) Both patients had sustained complete responses during and following treatment completion. Shaded bars represent the on‐treatment period. CT, computed tomography; PET, positron emission tomography; SPD, sum of products of diameters.
The second patient was a 69‐year‐old man who initially presented with high‐grade, non‐germinal centre DLBCL. The patient received six cycles of dose‐adjusted etoposide, doxorubicin, cyclophosphamide, vincristine, and prednisone, plus rituximab (da‐EPOCH‐R), resulting in a CR. The patient relapsed 9 months later with abdominal lymphadenopathy. The patient's disease was subsequently refractory to salvage rituximab, ifosfamide, carboplatin, and etoposide. The patient then received lisocabtagene maraleucel, which was complicated by Grade 1 CRS with hypotension. PET imaging 2 months post‐CART‐cell infusion showed persistent disease, and a left adrenal biopsy demonstrated persistent DLBCL. The tumour continued to express CD19 and was free of significant T‐cell infiltration.
The patient was salvaged with odronextamab complicated by fevers following treatment in the first 3 weeks. The week 4 dose was interrupted due to hypotension, managed with intravenous dexamethasone and fluids. Treatment was further interrupted for a pseudomonal infection, and he ultimately received 12 doses of odronextamab in total, which was further complicated by cytopenias and cytomegalovirus infection of the eye resulting in cessation of therapy. The toxicities were likely worsened by recent prior therapy. CT imaging after 4 weeks of odronextamab treatment demonstrated a significant response. A biopsy showed no involvement of the prior lymphoma, and most infiltrating immune cells were CD3+ T cells. A complete metabolic response was achieved by week 9 (Figure 1A). The patient had an ongoing CR at 42 months follow‐up (Figure 1B,C).
T cells transduced with lisocabtagene maraleucel express epidermal growth factor receptor (EGFR) on their surface to allow monitoring with flow cytometry and in situ hybridisation. 11 Prior to odronextamab treatment, CART cells were detected in both the tumour biopsy and peripheral blood of Patient 2 but were not detectable in tumour tissue after odronextamab treatment (Figure 2A). Endogenous and CART‐cell populations were tracked in the peripheral blood at weekly intervals during odronextamab treatment. Endogenous CD3+ T cells, but not CART cells, proliferated and upregulated granzyme B in response to odronextamab. However, no expansion of the CART‐cell population was observed (Figure 2B,C).
FIGURE 2.
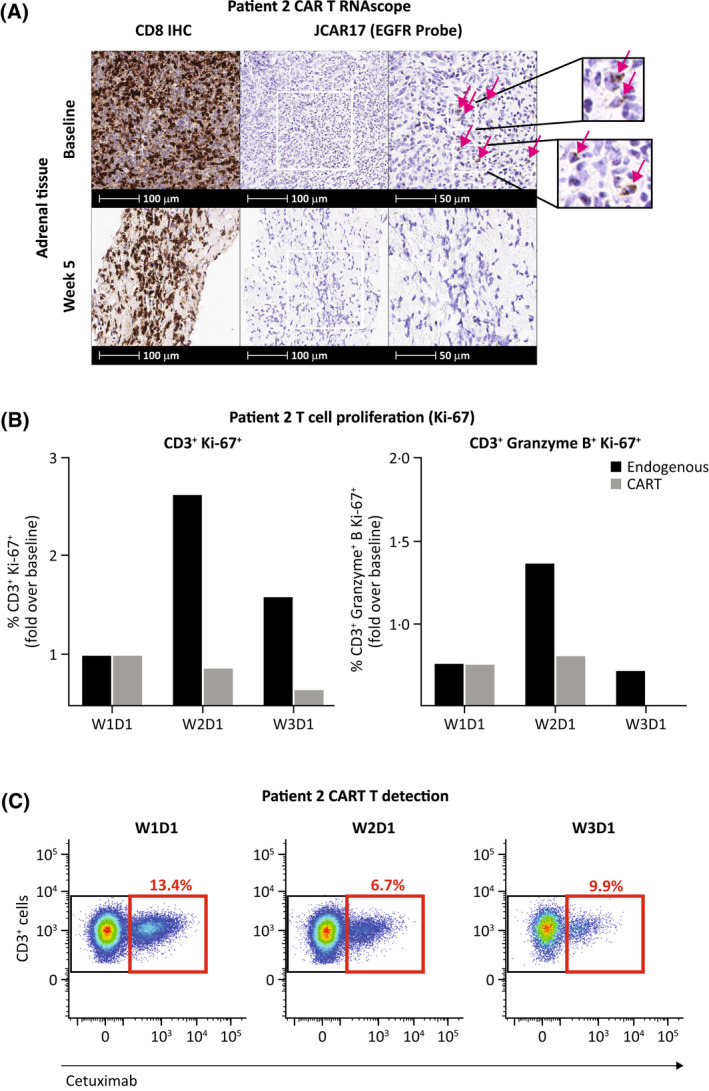
T cell response in Patient 2. T cell populations in adrenal tissue (A) and peripheral blood (B, C). (A) A population of infiltrating T cells was detected in the pre‐treatment, baseline tumour biopsy using the RNAscope method. 14 A subset of these T cells stained positive for the EGFR tag, indicating the presence of residual CART cells. At week 5 of treatment with odronextamab, no CART cells were detected in the tumour. (B) One week after initiating odronextamab therapy, a rapid, initial expansion of granzyme B+, Ki‐67+ CD3+ and proliferating Ki‐67+ CD3+ endogenous T cells, but not cetuximab+ CART cells, was observed in the circulating blood by flow cytometry. (C) No significant expansion of the residual cetuximab+ CART cell population (cetuximab is specific to EGFR) was observed in the circulation across early treatment timepoints. CAR, chimeric antigen receptor; D, day; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry; W, week.
In both patients, transient increases in serum cytokines interferon gamma (IFN‐γ) and interleukin 6 (IL‐6) occurred following the first split infusions of odronextamab with levels returning to baseline prior to day 2 dosing (Figure S1). Transient, low‐level IFN‐γ and IL‐6 elevations were subsequently observed in weeks 3–5, while changes in level were barely detectable after week 6. Serum C‐reactive protein (CRP) levels transiently increased following the split odronextamab infusions in weeks 1 and 2 (Figure S1).
Fixed duration odronextamab was well tolerated and induced durable, CRs in two patients with DLBCL refractory to CART‐cell therapy. In both cases the tumour continued to express CD19, suggesting clearance or exhaustion of the prior CART‐cell therapy. In the second patient there was no clear expansion of CART cells or CART‐cell infiltration into the tumour, following odronextamab therapy. The above observations suggest that odronextamab functioned independently of CART cells in these two patients.
Given the lack of effective treatment options following CART‐cell therapy, the above findings suggest odronextamab could be an effective salvage option. While there are many potential mechanisms of CART‐cell therapy failure, including: poor CART‐cell expansion; CART‐cell exhaustion; poor tumour infiltration; limited cytotoxicity; tumour CD19 down‐regulation; patient immunological response against the CAR; and tumour growth outpacing CART‐cell function, 12 , 13 the independent function of bispecific antibodies has the potential to salvage patients, regardless of the mechanism of failure.
AUTHOR CONTRIBUTIONS
Srikanth R. Ambati and Aafia Chaudhry conceptualised the study. Jon Arnason recruited patients and collected the data. Srikanth R. Ambati, Jurriaan Brouwer‐Visser, Nathalie Fiaschi, Vladimir Jankovic, Gavin Thurston, Raquel P. Deering, Aafia Chaudhry and Stephane Pourpe contributed to data curation and data analysis. Jon Arnason drafted the manuscript. All authors provided critical review, revision, and approval of the manuscript, and the decision to submit for publication.
CONFLICT OF INTEREST
Srikanth R. Ambati, Jurriaan Brouwer‐Visser, Nathalie Fiaschi, Vladimir Jankovic, Gavin Thurston, Raquel P. Deering and Aafia Chaudhry hold stock or stock options for and are employees of Regeneron Pharmaceuticals, Inc. Jon Arnason reports participation on a data safety monitoring board or advisory board for Bristol Myers Squibb, Juno, and Regeneron Pharmaceuticals, Inc., outside the submitted work.
Supporting information
Figure S1
ACKNOWLEDGEMENTS
This study was supported by Regeneron Pharmaceuticals. Editorial support was provided by Lewis Cawkwell of Arc, a division of Spirit Medical Communications Group Limited, and funded by Regeneron Pharmaceuticals.
Weinstock M, Elavalakanar P, Bright S, Ambati SR, Brouwer‐Visser J, Pourpe S, et al. Complete responses to odronextamab in two patients with diffuse large B‐cell lymphoma refractory to chimeric antigen receptor T‐cell therapy. Br J Haematol. 2022;199(3):366–370. 10.1111/bjh.18383
REFERENCES
- 1. Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B‐cell lymphoma: results from the international SCHOLAR‐1 study. Blood. 2017;130(16):1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CART‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med. 2017;377(26):2531–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380(1):45–56. [DOI] [PubMed] [Google Scholar]
- 5. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B‐cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–52. [DOI] [PubMed] [Google Scholar]
- 6. Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, et al. Outcomes of patients with large B‐cell lymphomas and progressive disease following CD19‐specific CART‐cell therapy. Am J Hematol. 2019;94(8):E209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jalbert JJ, Arnason JE, Ge W, Chen C‐I, Ambati S, Wu N, et al. Real‐world treatment patterns among patients with diffuse large B‐cell lymphoma (DLBCL) treated with CD19‐directed chimeric antigen receptor T‐cell therapy (CART). HemaSphere. 2020;4(S1):595 (Abstract EP1269). [Google Scholar]
- 8. Bannerji R, Arnason JE, Advani RH, Brown JR, Allan JN, Ansell SM, et al. Odronextamab, a human CD20×CD3 bispecific antibody in patients with CD20‐positive B‐cell malignancies (ELM‐1): results from the relapsed or refractory non‐Hodgkin lymphoma cohort in a single‐arm, multicentre, phase 1 trial. Lancet Haematol. 2022;9(5):e327–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD, et al. Lenalidomide combined with R‐CHOP overcomes negative prognostic impact of non‐germinal center B‐cell phenotype in newly diagnosed diffuse large B‐cell lymphoma: a phase II study. J Clin Oncol. 2015;33(3):251–7. [DOI] [PubMed] [Google Scholar]
- 11. Reiss DJ, Do T, Kuo D, Gray VE, Olson NE, Lee C‐W, et al. Multiplexed immunofluorescence (IF) analysis and gene expression profiling of biopsies from patients with relapsed/refractory (R/R) diffuse large B cell lymphoma (DLBCL) treated with lisocabtagene maraleucel (liso‐cel) in TRANSCEND NHL 001 reveal patterns of immune infiltration associated with durable response. Blood. 2019;134(Suppl 1):202. [Google Scholar]
- 12. Swanson C, Do T, Merrigan S, Lonning S, Merriam K, Prentiss J, et al. Predicting clinical response and safety of JCAR017 in B‐NHL patients: potential importance of tumor microenvironment biomarkers and CART‐cell tumor infiltration. Blood. 2017;130(Suppl 1):194. [Google Scholar]
- 13. Shah NN, Fry TJ. Mechanisms of resistance to CART cell therapy. Nat Rev Clin Oncol. 2019;16(6):372–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson CM, Zhang B, Miller M, Butko E, Wu X, Laver T, et al. Fully automated RNAscope in situ hybridization assays for formalin‐fixed paraffin‐embedded cells and tissues. J Cell Biochem. 2016;117(10):2201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


