Abstract
Aim
Spouse bereavement is one of life's greatest stresses and has been suggested to trigger or accelerate cognitive decline and dementia. However, little information is available about the potential brain pathologies underlying the association between spouse bereavement and cognitive decline. We aimed to investigate that lifetime spouse bereavement is associated with in vivo human brain pathologies underlying cognitive decline.
Methods
A total of 319 ever‐married older adults between the ages of 61 and 90 years underwent comprehensive clinical assessments and multimodal brain imaging including [11C] Pittsburgh compound B‐positron emission tomography (PET), AV‐1451 PET, [18F] fluorodeoxyglucose‐PET, and magnetic resonance imaging. Participants were classified as experiencing no spouse bereavement or spouse bereavement, and comparisons using propensity score matching (59 cases and 59 controls) were performed.
Results
Spouse bereavement was significantly associated with higher cerebral white matter hyperintensity (WMH) volume compared with no spouse bereavement. Interaction and subsequent subgroup analyses showed that spouse bereavement was significantly associated with higher WMH in the older (>75 years) subgroup and among those with no‐ or low‐skill occupations. In addition, spouse bereavement at 60 years or older affects WMH volume compared with no spouse bereavement, whereas spouse bereavement at younger than 60 years did not. No group differences were observed in other brain pathologies between spouse bereavement categories.
Conclusions
The findings suggest that the spouse bereavement may contribute to dementia or cognitive decline by increasing cerebrovascular injury, particularly in older individuals and those with no‐ or low‐skill occupations.
Keywords: neurodegeneration, spouse bereavement, white matter hyperintensities
Spouse bereavement is a major life event and is regarded as one of life's greatest stresses. 1 , 2 Extreme stress from spouse bereavement has been repeatedly suggested to trigger or accelerate cognitive decline and dementia. 3 , 4 , 5 , 6 , 7 Previous cross‐sectional studies reported that bereaved older individuals performed worse on tests of memory, attention, and executive function when compared with nonbereaved individuals. 3 , 4 One cohort study demonstrated significantly greater cognitive decline among individuals with a history of spouse bereavement. 5 A meta‐analysis of 15 studies also showed that those who had experienced spouse bereavement had a 20% greater risk of developing dementia during 3 to 15 years of follow‐up. 7
Nevertheless, little information is available on the neuropathological changes underlying the association between the experience of spouse bereavement and cognitive decline. Some studies have suggested that cardiovascular disease or events are a main biological adverse response to spouse bereavement. 8 , 9 Thus, spouse bereavement may be associated with other forms of vascular injury including cerebrovascular disease.
Therefore, we first aimed to test the hypothesis that spouse bereavement is associated with cerebrovascular injury in nondemented older adults. Cerebral white matter hyperintensities (WMH) on magnetic resonance imaging (MRI) were used as a measure of cerebrovascular injury. 10 , 11 We additionally explored the relationship of spouse bereavement with in vivo Alzheimer disease (AD) pathologies including cerebral beta‐amyloid protein (Aβ) deposition, tau deposition, and AD‐signature neurodegeneration because some preclinical studies using AD transgenic mouse models also showed that stress elevates Aβ 12 , 13 , 14 or tau pathologies, 15 , 16 , 17 and a recent study reported that being widowed was associated with accelerated Aβ‐related cognitive decline. 6 Furthermore, the relationship of spouse bereavement with whole brain and hippocampal volume was explored considering numerous previous reports on association between chronic stress and brain atrophy. 18 , 19 , 20
Methods
Participants
The present study was performed as part of the KBASE (Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer's Disease), an ongoing prospective cohort study. 21 KBASE aimed to search for new AD biomarkers and investigate how multifaceted lifetime experiences and bodily changes contribute to the brain changes related to AD. As of November 2016, a total of 319 ever‐married older adults between the ages of 61 and 90 years were initially enrolled in the study. All participants were not demented, i.e. were cognitively normal (CN) or exhibited mild cognitive impairment (MCI). Participants were recruited through four recruitment sites around Seoul, South Korea. Potentially eligible individuals who participated in a dementia screening program at two public centers for dementia prevention and management or visited memory clinics at two university hospitals (i.e. Seoul National University Hospital [SNUH] and Seoul National University‐Seoul Metropolitan Government [SNU‐SMG] Boramae Medical Center) around Seoul, South Korea, were informed about study participation and those who volunteered were invited for an assessment of eligibility. In addition, volunteers from the community were recruited through advertisements through an online homepage, posters, and brochures provided at main recruitment sites and word of mouth (recommended by other participants, family members, friends, or acquaintances). The CN group consisted of participants with a Clinical Dementia Rating (CDR) 22 score of 0 and no diagnosis of MCI or dementia. All individuals with MCI met the current consensus criteria for amnestic MCI and had a CDR score of 0.5. The current consensus criteria for amnestic MCI are as follows: (1) memory complaints confirmed by an informant; (2) objective memory impairments; (3) preserved global cognitive function; (4) independence in functional activities; and (5) no dementia. With regard to criterion 2, the age‐, education‐, and sex‐adjusted z scores for at least one of four episodic memory tests were <−1.0. The four memory tests were the Word List Memory, Word List Recall, Word List Recognition, and Constructional Recall tests, which are included in the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD‐K) neuropsychological battery. 23 The exclusion criteria were as follows: (1) presence of a major psychiatric illness; (2) significant neurological or medical conditions that could affect mental function; (3) contraindications for MRI; (4) illiteracy; (5) the presence of significant visual/hearing difficulties and/or severe communication or behavioral problems that would make clinical examinations or brain scans difficult; and (6) taking an investigational drug. The presence of any item included in the exclusion criteria was determined by research clinicians referring to the results of laboratory examinations and MRI, as well as the clinical data collected by trained nurses during systematic interviews of participants and their reliable informants during the screening period. More detailed information on the recruitment of the KBASE cohort is presented in a previous report from the research group. 21
Since age and sex, which are likely to have prominent confounding effects on the relationship of spouse bereavement with brain pathologies, differed substantially between groups with and without lifetime experience of spouse bereavement (Table 1), we used propensity score–matching methods 24 to generate more balanced groups having similar age and sex characteristics. Propensity scores are conditional probabilities of belonging to a particular group, given a set of observed background characteristics (i.e. age and sex in our propensity score–matching model). Finally, 59 individuals with and 59 without lifetime experience of spouse bereavement were included, as shown in Table 1.
Table 1.
Participant characteristics with and without spouse bereavement †
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| No spouse bereavement | Spouse bereavement | P‐value | No spouse bereavement | Spouse bereavement | P‐value | |
| No. | 260 | 59 | 59 | 59 | ||
| Age (years) | 72.32 (6.21) | 76.05 (5.76) | <0.001 ‡ | 75.58 (5.21) | 76.05 (5.76) | 0.640 ‡ |
| Age at spouse bereavement, No. (%) | <0.001 § | <0.001 § | ||||
| <60 years | 0 (0.00) | 29 (49.15) | 0 (0.00) | 29 (49.15) | ||
| ≥60 years | 0 (0.00) | 30 (50.85) | 0 (0.00) | 30 (50.85) | ||
| Women, No. (%) | 126 (48.46) | 47 (79.66) | 0.001 § | 48 (81.36) | 47 (79.66) | 0.816 § |
| Education (years) | 0.001 § | 0.108 § | ||||
| 0–6 | 59 (22.69) | 25 (42.37) | 18 (30.51) | 25 (42.37) | ||
| 7–12 | 101 (38.85) | 26 (44.07) | 24 (40.68) | 26 (44.07) | ||
| 13+ | 100 (38.46) | 8 (13.56) | 17 (28.81) | 8 (13.56) | ||
| APOE4 positivity, No. (%) | 58 (22.31) | 16 (27.59) | 0.390 § | 16 (27.12) | 16 (27.59) | 0.955 § |
| Clinical diagnosis, CN, No. (%) | 169 (65.00) | 38 (64.41) | 0.931 § | 32 (54.24) | 38 (64.41) | 0.261 § |
| Other bereavement, No. (%) | ||||||
| Close family members | 244 (93.85) | 55 (93.22) | 0.772 ¶ | 54 (91.53) | 55 (93.22) | 0.717 ¶ |
| Close friends | 115 (44.23) | 23 (38.98) | 0.463 § | 20 (33.90) | 23 (38.98) | 0.566 § |
| Divorce or separation, No. (%) | 13 (5.00) | 3 (5.08) | 1.000 ¶ | 2 (3.39) | 3 (5.08) | 1.000 ¶ |
| Remarriage, No. (%) | 1 (0.38) | 2 (3.39) | 0.089 ¶ | 0 (0.00) | 2 (3.39) | 0.496 ¶ |
| MOS‐SSS overall score | 71.54 (16.32) | 71.76 (16.08) | 0.924 ‡ | 71.76 (16.08) | ||
| Physical activity score, No. (%) | 0.944 § | 0.737 § | ||||
| High | 77 (36.32) | 14 (34.15) | 19 (42.22) | 14 (34.15) | ||
| Medium | 68 (32.08) | 13 (31.71) | 13 (28.89) | 13 (31.71) | ||
| Low | 67 (31.60) | 14 (34.15) | 13 (28.89) | 14 (34.15) | ||
| GDS score | 6.62 (6.22) | 7.58 (6.17) | 0.286 ‡ | 6.46 (5.47) | 7.58 (6.17) | 0.300 ‡ |
| BMI | 24.50 (3.02) | 24.37 (2.93) | 0.766 ‡ | 24.74 (2.80) | 24.37 (2.93) | 0.489 ‡ |
| Smoking status, No. (%) | 0.013 § | 1.000 § | ||||
| Never | 171 (66.02) | 50 (84.75) | 51 (86.44) | 50 (84.75) | ||
| Former | 76 (29.34) | 9 (15.25) | 8 (13.56) | 9 (15.25) | ||
| Smoker | 12 (4.63) | 0 (0.00) | 0 (0.00) | 0 (0.00) | ||
| Alcohol drinking status, No. (%) | 0.004 § | 1.000 § | ||||
| Never | 137 (52.90) | 45 (76.27) | 45 (76.27) | 45 (76.27) | ||
| Former | 40 (15.44) | 3 (5.08) | 4 (6.78) | 3 (5.08) | ||
| Drinker | 82 (31.66) | 11 (18.64) | 10 (16.95) | 11 (18.64) | ||
| Occupational complexity, No. (%) | 0.044 § | 0.116 § | ||||
| None | 47 (18.15) | 14 (23.73) | 19 (32.20) | 14 (23.73) | ||
| Skill level 1 | 16 (6.18) | 6 (10.17) | 4 (6.78) | 6 (10.17) | ||
| Skill level 2 | 76 (29.34) | 23 (38.98) | 15 (25.42) | 23 (38.98) | ||
| Skill level 3 | 33 (12.74) | 8 (13.56) | 4 (6.78) | 8 (13.56) | ||
| Skill level 4 | 87 (33.59) | 8 (13.56) | 17 (28.81) | 8 (13.56) | ||
| Annual income, No. (%) | 0.100 § | 0.732 § | ||||
| <MCL | 24 (9.23) | 5 (8.47) | 5 (8.47) | 5 (8.47) | ||
| ≥MCL, <2 MCL | 115 (44.23) | 35 (59.32) | 31 (52.54) | 35 (59.32) | ||
| ≥2 MCL | 121 (46.54) | 19 (32.20) | 23 (38.98) | 19 (32.20) | ||
| Vascular risk | ||||||
| Hypertension, No. (%) | 128 (49.23) | 41 (69.49) | 0.005 § | 34 (57.63) | 41 (69.49) | 0.181 § |
| Diabetes, No. (%) | 49 (18.85) | 12 (20.34) | 0.792 § | 8 (13.56) | 12 (20.34) | 0.326 § |
| Coronary artery disease, No. (%) | 16 (6.15) | 3 (5.08) | 1.000 ¶ | 5 (8.47) | 3 (5.08) | 0.717 ¶ |
| Hyperlipidemia, No. (%) | 90 (34.62) | 29 (49.15) | 0.040 ¶ | 26 (44.07) | 29 (49.15) | 0.580 ¶ |
| Transient ischemic attack, No. (%) | 3 (1.15) | 0 (0.00) | 1.000 ¶ | 1 (1.69) | 0 (0.00) | 1.000 ¶ |
| Stroke, No. (%) | 0 (0.00) | 0 (0.00) | NA | 0 (0.00) | 0 (0.00) | NA |
| Vascular risk score | 1.10 (1.00) | 1.44 (0.90) | 0.017 ‡ | 1.25 (0.99) | 1.44 (0.90) | 0.286 ‡ |
| Undernutrition | 46 (17.69) | 11 (18.64) | 0.819 § | 14 (23.73) | 11 (18.64) | 0.530 § |
| WMH volume, cm3 | 12.52 (10.74) | 15.72 (13.03) | 0.048 ‡ | 11.10 (7.67) | 15.72 (13.03) | 0.021 ‡ |
| Cerebral Aβ deposition | ||||||
| Aβ retention, SUVR | 1.32 (0.37) | 1.31 (0.32) | 0.791 ‡ | 1.37 (0.37) | 1.31 (0.32) | 0.348 ‡ |
| Cerebral tau deposition | ||||||
| AV‐1451, SUVR (n = 86) | 1.59 (0.69) (n = 71) | 1.42 (0.29) (n = 15) | 0.352 ‡ | 1.62 (0.92) (n = 13) | 1.42 (0.29) (n = 15) | 0.420 ‡ |
| AD‐neurodegeneration | ||||||
| AD‐CM, SUVR | 1.39 (0.13) | 1.38 (0.13) | 0.530 ‡ | 1.39 (0.15) | 1.38 (0.13) | 0.585 ‡ |
| AD‐CT (mm) | 2.79 (0.22) | 2.73 (0.21) | 0.056 ‡ | 2.75 (0.21) | 2.73 (0.21) | 0.491 ‡ |
| HVa, cm3 | −1.21 (1.06) | −1.34 (1.09) | 0.398 ‡ | −1.22 (1.07) | −1.34 (1.09) | 0.558 ‡ |
| WBV, cm3 | 0.73 (0.34) | 0.74 (0.31) | 0.047 ‡ | 0.73 (0.03) | 0.74 (0.31) | 0.580 ‡ |
| CERAD‐NP test | ||||||
| VF | 14.52 (4.52) | 12.78 (4.43) ‡ | 0.008 | 13.56 (4.54) | 12.78 (4.43) | 0.347 ‡ |
| BNT | 11.43 (2.39) | 10.12 (2.59) ‡ | <0.001 | 11.17 (2.33) | 10.12 (2.59) | 0.022 ‡ |
| CP | 9.73 (1.44) | 9.54 (1.61) ‡ | 0.385 | 9.61 (1.46) | 9.54 (1.61) | 0.811 ‡ |
| WLM | 15.77 (6.41) | 14.000 (7.49) ‡ | 0.066 | 15.56 (6.26) | 14.00 (7.49) | 0.223 ‡ |
| WLR | 5.05 (2.33) | 4.73 (2.45) ‡ | 0.353 | 4.81 (2.49) | 4.73 (2.45) | 0.852 ‡ |
| WLRc | 7.98 (2.34) | 8.19 (2.23) ‡ | 0.532 | 7.63 (0.271) | 8.19 (2.23) | 0.223 ‡ |
| CR | 5.76 (3.56) | 4.32 (3.16) ‡ | 0.005 | 4.80 (3.54) | 4.32 (3.16) | 0.444 ‡ |
| MMSE | 25.35 (3.30) | 24.66 (3.55) ‡ | 0.154 ‡ | 24.71 (3.66) | 24.66 (3.55) | 0.939 ‡ |
| TS | 70.13 (16.52) | 63.68 (17.65) ‡ | 0.008 | 67.03 (16.68) | 63.68 (17.65) | 0.291 ‡ |
Unless otherwise indicated, data are expressed as mean (standard deviation).
By t test.
By chi‐square test.
By Fisher exact test.
Aβ, beta‐amyloid; AD, Alzheimer disease; AD‐CM, Alzheimer disease signature cerebral glucose metabolism; AD‐CT, Alzheimer disease signature cortical thickness; APOE4, apolipoprotein ε4; BMI, body mass index; BNT, Boston naming test; CERAD‐NP, consortium to establish a registry for Alzheimer disease neuropsychological battery; CN, cognitively normal; CP, construction praxis; CR, constructional recall; GDS, Geriatric Depression Scale; HVa, adjusted hippocampal volume; MCL, minimum cost of living; MMSE, Mini‐Mental State Examination; MOS‐SSS, Medical Outcomes Study‐Social Support Survey; SUVR, standardized uptake value ratio; TS, total score of the CERAD‐NP; VF, verbal fluency; WBV, whole brain volume; WLM, word list memory; WLR, word list recall; WLRc, word list recognition; WMH, white matter hyperintensity.
The study protocol was approved by the institutional review boards of SNUH (C‐1401‐027‐547) and SNU‐SMG Boramae Medical Center (26–2015‐60), Seoul, South Korea, and was performed in accordance with the recommendations of the current version of the Declaration of Helsinki. All patients gave written informed consent.
Clinical and neuropsychological assessments
Trained board‐certified psychiatrists administered standardized clinical assessments to all participants based on the KBASE clinical assessment protocol, which incorporated the CERAD‐K clinical assessment. 21 A clinical neuropsychologist or trained psychometrist also administered a comprehensive neuropsychological assessment battery to the participants, following a standardized protocol incorporating the CERAD‐K neuropsychological battery.
A CERAD total score (TS) was generated by summing the scores of six tests in the CERAD neuropsychological battery including the Verbal Fluency, modified Boston Naming Test, Word List Memory, Constructional Praxis, Word List Recall, and Word List Recognition. 25 CERAD‐TS was selected as a measure of global cognitive function.
Assessment of spouse bereavement and related conditions
Information (yes/no) on lifetime experience of spouse bereavement was obtained from all participants through systematic interviews with the participants and their reliable informants by trained nurses. If the answer was yes, the age of the bereavement experience was also documented. To analyze the effects of the age of the bereavement experience, we divided those with spouse bereavement into subgroups, i.e. spouse bereavement at <60 years versus spouse bereavement at ≥60 years. Information (yes/no) on a death of a close family member and a close friend, divorce, separation, and remarriage was also obtained.
Assessment of potential confounders or modulators
The association between spouse bereavement and brain pathologies may be influenced or modulated by various other conditions. Therefore, we systematically evaluated all participants for potential confounders or modulators, such as undernutrition, depression, social support, annual income, occupational complexity, vascular risk, body mass index (BMI), alcohol intake, smoking, physical activity, and apolipoprotein E (APOE) genotyping. The detailed procedures for assessment of these potential confounders or modulators are described in Method S1.
Measurement of WMH
All participants underwent MRI scans with fluid‐attenuated inversion recovery using a 3.0T Biograph mMR (PET‐MR) scanner (SiemensUSA) according to the manufacturer's guidelines. We followed the validated automatic procedure reported previously. 26 Briefly, the procedure consisted of 11 steps, i.e. spatial coregistration of T1 and FLAIR images, fusion of T1 and FLAIR images, segmentation of T1, attainment of transformation parameters, deformation and obtainment of the white matter mask, obtainment of FLAIR within the white matter mask, intensity normalization of the masked FLAIR, nomination of candidate WMH with a designated threshold, creation of a junction map, and elimination of the junction. The current processing procedure had two modifications compared with the original study: (i) an optimal threshold of 70 was applied, as it was more suitable for our data than the threshold of 65 used in the original study; and, (ii) given that individuals with acute cerebral infarcts were not enrolled in our sample, we did not use diffusion‐weighted imaging in the current automated procedure. Using the final WMH candidate image, the WMH volume was extracted in the native space in each patient. More specifically, the lobar regions of interest (ROIs) template was adapted from a previously published minimal deformation template. 27 The acquired transformation parameter for each subject from the automated procedure was applied to the template to transform the lobar ROIs template into native space to be used for extracting WMH volumes in each lobe.
Measurement of cerebral Aβ deposition
All participants underwent simultaneous three‐dimensional (3D) [11C] Pittsburgh compound B (PiB)‐positron emission tomography (PET) and a 3D T1‐weighted MRI scan using the abovementioned 3.0T PET‐MR scanner (Siemens). The details of the PiB‐PET imaging acquisition and preprocessing were previously described. 28 An automatic anatomical labeling algorithm and a region‐combining method 29 were applied to determine ROIs to characterize the PiB retention levels in the frontal, lateral parietal, posterior cingulate–precuneus, and lateral temporal regions. The standardized uptake value ratio (SUVR) for each ROI was calculated by dividing the mean value for all voxels within each ROI by the mean cerebellar uptake value in the same image. A global cortical ROI consisting of the four ROIs was also defined and a global Aβ retention value was generated by dividing the mean value for all voxels of the global cortical ROI by the mean cerebellar uptake value in the same image. 29 , 30
Measurement of cerebral tau deposition
A subset of patients underwent [18F] AV‐1451 PET scans using a Biograph TruePoint 40 PET/CT scanner (Siemens), in accordance with the manufacturer's guidelines. While all of the other neuroimaging scans were performed during the baseline visit, AV‐1451 PET imaging was performed at an average of 2.5 years after the baseline visit. The details of AV‐1451 PET imaging acquisition and preprocessing were previously described. 28 To estimate cerebral tau deposition, we quantified the AV‐1541 SUVR of an a priori ROI of “AD‐signature regions” of tau accumulation, which was composed of a size‐weighted average of partial volume‐corrected uptake in entorhinal, amygdala, parahippocampal, fusiform, inferior temporal, and middle temporal ROIs in accordance with the method used in a previous report. 31 The AV‐1451 SUVR of the abovementioned ROI was used as an outcome variable for cerebral tau deposition.
Measurement of AD‐signature neurodegeneration and brain volume
All participants underwent [18F] fluorodeoxyglucose (FDG)‐PET imaging using the abovementioned PET‐MRI machine. The details of the FDG‐PET image acquisition and preprocessing were previously described. 28 AD‐signature FDG ROIs, such as the angular gyri, posterior cingulate cortex, and inferior temporal gyri, which are sensitive to changes associated with AD, 32 were determined. AD‐signature cerebral glucose metabolism (AD‐CM) was defined as the voxel‐weighted mean SUVR extracted from the AD‐signature FDG ROIs. The details of MRI acquisition and preprocessing were previously described. 28 AD‐signature cortical thickness (AD‐CT) was defined as the mean cortical thickness values obtained from AD‐signature regions, including the entorhinal, inferior temporal, middle temporal, and fusiform gyrus, as previously described. 32 An adjusted hippocampal volume (HVa) was calculated as the unstandardized residual from the linear regression of total hippocampal volume versus the total intracranial volume of the reference group (the young CN group of the study cohort). 33 HVa indicates the volume deviated from the expected total hippocampal volume according to the ICV in young CN individuals. For the whole brain volume (WBV), we chose to use the “ratio of brain segmentation volume to estimated ICV” variable output from FreeSurfer recon‐all segmentation with manual correction.
Statistical analysis
To test the hypothetical associations between spouse bereavement and WMH, multiple linear regression analysis with the spouse bereavement group as the independent variable and WMH volume as a dependent variable was performed. In the analysis, WMH volume was used after natural log‐transformation to achieve normal distributions. Three models were tested for stepwise control of the potential confounders other than age and sex that could affect the relationships between spouse bereavement and the biomarkers. The first model (model I) did not include any covariate. The second model (model II) included clinical diagnosis (CN versus MCI), vascular risk score (VRS), BMI, APOE4, and undernutrition. The third model (model III) included the covariates in the second model plus education, Geriatric Depression Scale (GDS) score, Medical Outcomes Study‐Social Support Survey (MOS‐SSS) score, annual income, occupational complexity, alcohol intake status, and smoking status, which have been considered possible confounders in previous studies. 1 , 6 , 8 , 34 , 35 To explore the effects of age of the bereavement experience, the same analyses were separately performed for the two relevant subgroups (i.e. spouse bereavement at <60 years versus spouse bereavement at ≥60 years). In all analyses, no spouse bereavement was used as a reference. As sensitivity analyses, the same analyses were also performed for: (i) participants without death of a close friend, (ii) those with neither divorce nor marital separation, and (iii) those without remarriage. Additional exploratory analyses were performed for the neuroimaging biomarkers showing significant associations with spouse bereavement in the above analyses as follows. To investigate the modulating effects of age (younger [≤75 years] versus older [>75 years]), sex, APOE4 positivity, clinical diagnosis, education, GDS score, MOS‐SSS score, annual income, occupational complexity, VRS, BMI, physical activity, alcohol intake, and smoking on the association between spouse bereavement and the neuroimaging biomarker(s), the same regression analyses were repeated including a two‐way interaction term between spouse bereavement and each of the factors mentioned above as an additional independent variable. We additionally investigated the association between spouse bereavement and cognitive performance using a multiple linear regression model with spouse bereavement as an independent variable, cognitive test as a dependent variable, and the potential covariates. Then, the same regression model was analyzed again while controlling for WMH volume as additional covariates in order to examine whether the relationship between spouse bereavement and cognitive impairment was mediated by WMH. For the purpose of exploration, we additionally analyzed the association between spouse bereavement and other neuroimaging biomarkers (Aβ and tau retention, AD‐CM, AD‐CT, HVa, or WBV) using the same multiple regression models used for WMH. Global Aβ retention was used after natural log‐transformation to achieve normal distributions. Statistical analyses were performed using SPSS Statistics version 27 (IBM).
Results
Participant characteristics
Participants' demographic and clinical characteristics are presented in Table 1. Before the propensity score matching, 260 of the 319 participants were categorized as the no spouse bereavement group and 59 as the spouse bereavement group. After the matching, 59 of 118 participants were categorized as the no spouse bereavement group and 59 as the spouse bereavement group (29 with spouse bereavement at <60 years and 30 with spouse bereavement at ≥60 years).
Association of spouse bereavement with WMH
The spouse bereavement group showed greater WMH volume compared with the no spouse bereavement group independent of the covariates (Table 2 and Fig. 1a). Furthermore, individuals with spouse bereavement at ≥60 years had greater WMH volume than those without spouse bereavement, whereas those with spouse bereavement at <60 years did not (Table 3 and Fig. 1b). The same analyses including only participants with no death of a close friend showed similar results in terms of the association between spouse bereavement and WMH volume (Table S1). The results were also similar after excluding those with divorce or marital separation (Table S2) and those who had remarried (Table S3).
Table 2.
Results of multiple linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume (N = 118)
| No spouse bereavement | Spouse bereavement | ||
|---|---|---|---|
| n = 59 | |||
| n = 59 | |||
| B (95% CI) | P‐value | ||
| WMH volume, cm3 | |||
| Model I † | Reference | 0.074 (0.006–0.142) | 0.034 |
| Model II‡ | Reference | 0.082(0.012–0.152) | 0.021 |
| Model III § | Reference | 0.090 (0.016–0.164) | 0.018 |
Unadjusted.
Adjusted for clinical diagnosis, vascular risk score (VRS), body mass index (BMI), apolipoprotein ε4 (APOE4), and undernutrition.
Adjusted for clinical diagnosis, VRS, BMI, APOE4, undernutrition, education, occupational complex, annual income, Geriatric Depression Scale (GDS) score, Medical Outcomes Study‐Social Support Survey (MOS‐SSS) score, alcohol intake status, and smoking status.
CI; confidence interval; WMH, white matter hyperintensity.
Fig. 1.
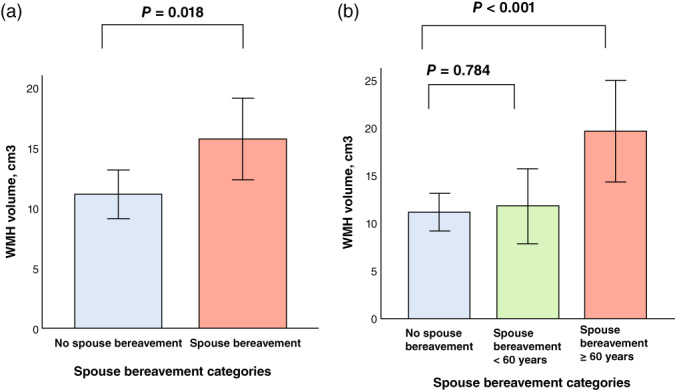
Bar plots of the associations of spouse bereavement categories. (a) No spouse bereavement vs spouse bereavement, and (b) no spouse bereavement vs spouse bereavement aged <60 years vs spouse bereavement at ≥60 years with white matter hyperintensity (WMH) volume in participants. Multiple linear regression analyses were performed after adjusting for all potential covariates.Values are presented as the mean of WMH volume, and error bars represent standard errors.
Table 3.
Results of multiple linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume (N = 118)
| No spouse bereavement | Spouse bereavement at <60 years | Spouse bereavement at ≥60 years | |||
|---|---|---|---|---|---|
| n = 59 | |||||
| n = 30 | |||||
| n = 29 | |||||
| B (95% CI) | P‐value | B (95% CI) | P‐value | ||
| WMH volume, cm3 | |||||
| Model I † | Reference | 0.006 (−0.075 to 0.087) | 0.880 | 0.141 (0.061 to 0.222) | 0.001 |
| Model II ‡ | Reference | 0.011 (−0.070 to 0.093) | 0.787 | 0.157 (0.074 to 0.240) | <0.001 |
| Model III § | Reference | 0.012 (−0.076 to 0.101) | 0.784 | 0.160 (0.075 to 0.246) | <0.001 |
Unadjusted.
Adjusted for clinical diagnosis, vascular risk score (VRS), body mass index (BMI), apolipoprotein ε4 (APOE4), and undernutrition.
Adjusted for clinical diagnosis, VRS, BMI, APOE4, undernutrition, education, occupational complex, annual income, Geriatric Depression Scale (GDS) score, Medical Outcomes Study‐Social Support Survey (MOS‐SSS) score, alcohol intake status, and smoking status.
BMI, body mass index; CI, confidence interval; WMH, white matter hyperintensity.
Influence of potential modulators on the association between spouse bereavement and WMH
The interactions of spouse bereavement with age and occupational complexity were significant, indicating that age and occupational complexity independently modulated the association between spouse bereavement and WMH volume (Table S4). Further subgroup analyses showed that spouse bereavement was significantly associated with higher WMH in the older (>75 years) subgroup but not in the younger (≤75 years) one, and in the no‐ or low‐skill occupational subgroup but not in the high‐skill group (Table S5; Fig. 2a,b). The interactions of spouse bereavement with sex, APOE4 positivity, clinical diagnosis, education, GDS score, MOS‐SSS score, annual income, VRS, BMI, physical activity, alcohol intake, and smoking were not significant (Table S4).
Fig. 2.
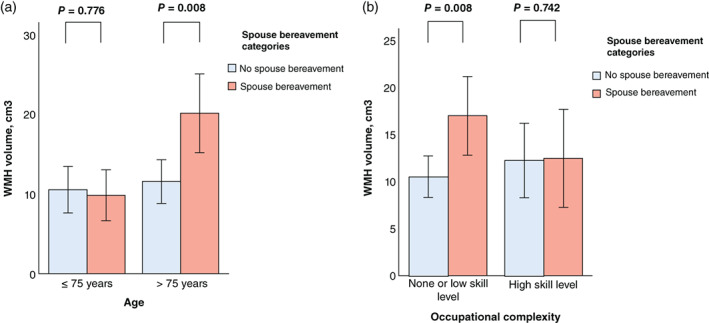
Bar plots of the associations of spouse bereavement categories with white matter hyperintensity (WMH) volume according to (a) age and (b) occupational complexity. Multiple linear regression analyses were performed after adjusting for all potential covariates. Values are presented as the mean of WMH volume, and error bars represent standard errors. [Correction added on Aug 01, 2022, after first online publication: “<75 years” has been amended to “>75 years”.]
Association between spouse bereavement, WMH, and cognitive performance
The spouse bereavement group was associated with lower cognitive performance as assessed by CERAD‐TS compared with no spouse bereavement (Table S6). In addition, WMH volume was inversely associated with CERAD‐TS (Table S7). When WMH volume was controlled as an additional covariate, the relationship between spouse bereavement and CERAD‐TS was not significant any more (Table S6).
Association of spouse bereavement with other neuroimaging biomarkers
Independent of the models, no differences were observed in Aβ and tau deposition, AD‐CM, AD‐CT, HVa, and WBV between the group with and that without spouse bereavement (Tables 4 and 5).
Table 4.
Results of multiple linear regression analyses for assessing the relationships between stratified spouse bereavement and Aβ, AV‐1451, AD‐CM, AD‐CT, HVa, or WBV (N = 118)
| No spouse bereavement | Spouse bereavement | ||
|---|---|---|---|
| n = 59 | |||
| n = 59 | |||
| B (95% CI) | P‐value | ||
| Aβ retention, SUVR | |||
| Model I † | Reference | −0.049 (−0.130 to 0.032) | 0.231 |
| Model II ‡ | Reference | −0.030 (−0.094 to 0.035) | 0.366 |
| Model III § | Reference | −0.025 (−0.092 to 0.041) | 0.456 |
| AV‐1451, SUVR | |||
| Model I † | Reference | −0.204 (−0.717 to 0.308) | 0.420 |
| Model II ‡ | Reference | −0.181 (−0.720 to 0.359) | 0.493 |
| Model III § | Reference | 0.053 (−0.563 to 0.669) | 0.856 |
| AD‐CM, SUVR | |||
| Model I † | Reference | −0.011 (−0.064 to 0.041) | 0.664 |
| Model II ‡ | Reference | −0.021 (−0.070 to 0.029) | 0.411 |
| Model III § | Reference | −0.024 (−0.076 to 0.028) | 0.359 |
| AD‐CT, mm | |||
| Model I † | Reference | −0.026 (−0.105 to 0.053) | 0.518 |
| Model II ‡ | Reference | −0.044 (−0.117 to 0.029) | 0.233 |
| Model III § | Reference | −0.052 (−0.125 to 0.021) | 0.061 |
| HVa, cm3 | |||
| Model I † | Reference | −0.114 (−0.519 to 0.290) | 0.577 |
| Model II ‡ | Reference | −0.213 (−0.565 to 0.139) | 0.232 |
| Model III § | Reference | −0.284 (−0.634 to 0.067) | 0.111 |
| WBV | |||
| Model I † | Reference | 0.002 (−0.010 to 0.015) | 0.707 |
| Model II ‡ | Reference | 0.001 (−0.011 to 0.013) | 0.881 |
| Model III § | Reference | −0.002 (−0.014 to 0.010) | 0.775 |
Unadjusted.
Adjusted for clinical diagnosis, vascular risk score (VRS), body mass index (BMI), apolipoprotein ε4 (APOE4), and undernutrition.
Adjusted for clinical diagnosis, VRS, BMI, APOE4, undernutrition, education, occupational complex, annual income, Geriatric Depression Scale (GDS) score, Medical Outcomes Study‐Social Support Survey (MOS‐SSS) score, alcohol intake status, and smoking status.
Aβ, beta‐amyloid; AD‐CM, Alzheimer disease signature cerebral glucose metabolism; AD‐CT, Alzheimer disease signature cortical thickness; CI; confidence interval; HVa, adjusted hippocampal volume; SUVR, standardized uptake value ratio; WBV, whole brain volume.
Table 5.
Results of multiple linear regression analyses for assessing the relationships between stratified spouse bereavement and Aβ, AV‐1451, AD‐CM, AD‐CT, Hva, or WBV (N = 118)
| No spouse bereavement | Spouse bereavement at <60 years | Spouse bereavement at ≥60 years | |||
|---|---|---|---|---|---|
| n = 59 | |||||
| n = 29 | n = 30 | ||||
| B (95% CI) | P‐value | B (95% CI) | P‐value | ||
| Aβ retention, SUVR | |||||
| Model I † | Reference | −0.088 (−0.186 to 0.010) | 0.079 | −0.010 (−0.108 to 0.088) | 0.840 |
| Model II ‡ | Reference | −0.072 (−0.149 to 0.005) | 0.068 | 0.015 (−0.064 to 0.093) | 0.708 |
| Model III § | Reference | −0.074 (−0.156 to 0.007) | 0.074 | 0.020 (−0.059 to 0.099) | 0.624 |
| AV‐1451, SUVR | |||||
| Model I† | Reference | −0.265 (−0.992 to 0.461) | 0.459 | −0.174 (−0.754 to 0.407) | 0.544 |
| Model II ‡ | Reference | −0.431 (−1.214 to 0.353) | 0.265 | −0.069 (−0.668 to 0.529) | 0.811 |
| Model III § | Reference | −0.234 (−1.166 to 0.699) | 0.597 | 0.156 (−0.516 to 0.828) | 0.624 |
| AD‐CM, SUVR | |||||
| Model I † | Reference | −0.014 (−0.078 to 0.050) | 0.671 | −0.009 (−0.073 to 0.055) | 0.779 |
| Model II ‡ | Reference | −0.019 (−0.079 to 0.041) | 0.539 | −0.023 (−0.084 to 0.039) | 0.467 |
| Model III § | Reference | −0.021 (−0.085 to 0.044) | 0.530 | −0.027 (−0.090 to 0.035) | 0.390 |
| AD‐CT, mm | |||||
| Model I † | Reference | 0.006 (−0.092 to 0.105) | 0.899 | −0.056 (−0.152 to 0.040) | 0.251 |
| Model II ‡ | Reference | −0.008 (−0.098 to 0.081) | 0.856 | −0.080 (−0.168 to 0.009) | 0.079 |
| Model III § | Reference | −0.022 (−0.113 to 0.070) | 0.639 | −0.077 (−0.164 to 0.009) | 0.080 |
| HVa, mm3 | |||||
| Model I † | Reference | 0.146 (−0.396 to 0.689) | 0.594 | −0.237 (−0.773 to 0.299) | 0.382 |
| Model II‡ | Reference | −0.009 (−0.488 to 0.469) | 0.969 | −0.406 (−0.903 to 0.092) | 0.109 |
| Model III § | Reference | −0.066 (−0.554 to 0.422) | 0.789 | −0.483 (−0.971 to 0.006) | 0.053 |
| WBV | |||||
| Model I † | Reference | 0.001 (−0.014 to 0.017) | 0.850 | 0.003 (−0.012 to 0.018) | 0.678 |
| Model II ‡ | Reference | 0.001 (−0.015 to 0.016) | 0.928 | 0.001 (−0.014 to 0.016) | 0.879 |
| Model III § | Reference | −0.004 (−0.09 to 0.012) | 0.652 | <0.001 (−0.015 to 0.014) | 0.971 |
Unadjusted.
Adjusted for clinical diagnosis, vascular risk score (VRS), body mass index (BMI), apolipoprotein ε4 (APOE4), and undernutrition.
Adjusted for clinical diagnosis, VRS, BMI, APOE4, undernutrition, education, occupational complex, annual income, Geriatric Depression Scale (GDS) score, Medical Outcomes Study‐Social Support Survey (MOS‐SSS) score, alcohol intake status, and smoking status.
Aβ, beta‐amyloid; AD‐CM, Alzheimer disease signature cerebral glucose metabolism; AD‐CT, Alzheimer disease signature cortical thickness; CI, confidence interval; HVa, adjusted hippocampal volume; SUVR, standardized uptake value ratio; WBV, whole brain volume.
Discussion
The present study shows that lifetime experience of spouse bereavement was associated with increased WMH volume, but not with AD neuroimaging markers, in nondemented older adults. The association of spouse bereavement with WMH observed in the present study may be explained by the following potential mechanisms. First, the loss of a spouse is considered one of the most stressful life events. 1 , 2 , 36 The experience of spouse bereavement can cause a severe acute or chronic stress reaction and emotional sequalae such as depression, which may subsequently contribute to cerebrovascular changes, resulting in increased WMH. Evidence indicates that stress and depression elicit multifaceted dysfunction in the cerebral microcirculation, which plays a critical role in brain health and the pathogenesis of stress‐related cerebrovascular events. 37 , 38 Second, a widowed state after spouse bereavement may lead to lower socioeconomic status and poor health care utilization. 39 Both may result in poor management of vascular risks, which could contribute to cerebrovascular changes and subsequent increased WMH. However, given the association between spouse bereavement and WMH was observed even after controlling the annual income, the degree of social support, nutritional status, and VRS, the possibility appears not so high.
Unlike the association with WMH, additional exploratory analyses showed that the experience of spouse bereavement was not associated with any other neuroimaging biomarkers including AD‐related ones, indicating that it may not directly affect AD‐specific brain changes in older adults. One cohort study reported that widowed adults with higher baseline cortical Aβ levels exhibited steeper cognitive decline. 6 However, similar to our finding, the authors of that study reported no difference in brain Aβ between the groups with and without spouse bereavement. As they suggested, spouse bereavement may modulate AD pathology‐related cognitive decline by affecting the brain or cognitive reserves, but not by affecting AD pathologies themselves. Although WBV was not related to spouse bereavement, white matter degeneration as indicated by WMH volume can impair the cognitive reserves. 40 , 41 Therefore, together with our finding for WMH, the decreased reserves associated with white matter degeneration may synergistically aggravate cognitive function in individuals with spouse bereavement when AD pathologies are present in the brain.
The individuals who had experienced spouse bereavement at ≥60 years had greater WMH than those without spouse bereavement, whereas those who had experienced it <60 years of age did not. This finding implies that the age‐related vulnerability of the brain to stress or cerebrovascular changes at the time of bereavement is more important than the time elapsed since bereavement or the chronicity of influence. Similarly, current age also moderated the relationship between bereavement and WMH volume; spouse bereavement was significantly associated with higher WMH volume in the older (>75 year) subgroup but not in the younger (≤75 years) subgroup. This finding additionally indicates that vulnerability to bereavement‐related white matter injury depends not only on the age of bereavement but also on current age.
In addition to age, lifetime occupation also moderated the relationship between bereavement and WMH volume, with spouse bereavement significantly associated with higher WMH only in individuals with no‐ or low‐ skill occupations and not in those with higher skill occupations. Given that the moderation effect of occupational complexity was significant even after annual income and social support were controlled, the association is apparently not simply attributable to economic difficulty or poorer social support resulting from spouse loss. Furthermore, because the National Health Insurance system in Korea covers nearly all people in the country, the lack of access to adequate health care caused by the loss of a spouse with better health insurance 42 may not clearly explain the moderation effect of occupational level. The brains of those with no‐ or low‐skill occupations may be more vulnerable to stress or cerebrovascular disease because of lower brain reserves. 43
We additionally observed a significant relationship between spouse bereavement and poorer cognitive performance, replicating previous reports. 3 , 4 When WMH volume was adjusted as an additional covariate, the relationship between spouse bereavement and cognitive impairment was not significant any more, further supporting the possibility that spouse bereavement may contribute to the development of dementia or cognitive decline via cerebrovascular injury in older adults.
Strengths and Limitations
The present study had some strengths. First, to our knowledge, this is the first study to elucidate the association of spouse bereavement with brain pathologies in living human. Second, the study included a relatively large number of participants who were well characterized through comprehensive clinical assessments including systematic interviews for detailed history about spouse bereavement, the death of close family members and close friends, divorce, separation, and remarriage, in addition to multimodal brain imaging to assess in vivo AD pathologies and WMH. Third, we used propensity score–matching methods to create more balanced groups of similar age and sex and to minimize the potential confounding effect of age and sex on the relationship of spouse bereavement with brain pathologies. Additionally, various other potential confounders were systematically controlled in the statistical models to clarify the association between spouse bereavement and brain pathologies as clearly as possible. The findings from the present study were not changed even after controlling for all potential confounders and were confirmed by sensitivity analyses conducted after excluding participants with bereavement of close friends, those reporting divorce or marital separation, and those who had remarried.
Nevertheless, the present study had several limitations that should be considered. First, as this was a cross‐sectional study, we could not confirm a causal relationship between spouse bereavement and brain WMH. Further long‐term follow‐up studies are required to clarify the causal relationships. Second, we did not consider the duration or severity of bereavement reaction and other family members who lived with the patients, although those factors may have an impact on the relationship between spouse bereavement and brain change. Third, information about bereavement and related topics was obtained through clinical interviews, raising some concern about recall bias, especially for participants with MCI. However, although individuals with MCI have some problem with recent memory, their remote memory tends to be well preserved. 44 Therefore, it is not likely that individuals with MCI reported a history of spouse bereavement less accurately, as such history mainly depends on remote memory rather than recent memory. Furthermore, even when we controlled for clinical diagnosis (CN versus MCI) as an additional covariate (Tables 2, 3, 4, 5; Tables S1–S7), the results were still similar. Additionally, we interviewed reliable informants as well as the participants. Third, the present study excluded participants with a history of stroke or severe vascular lesions including infarcts and hemorrhages on brain MRI. Therefore, we could not assess the effect of spouse bereavement on individuals with severe cerebrovascular disease. Further studies are required to clarify these effects in those with high cerebrovascular burdens. Finally, tau PET was applied at an average of 2.5 years from the baseline visit, whereas other neuroimaging scans were performed at baseline. This temporal gap may have influenced the association between spouse bereavement and tau. When we controlled for the temporal gap as an additional covariate, however, the results did not change. In addition, only a subset of participants (13 without spouse bereavement versus 15 with spouse bereavement after propensity score matching) underwent tau PET. This relatively reduced sample size for tau PET may have decreased the statistical power and contributed to the null result for the relationship between spouse bereavement and tau deposition.
In conclusion, the findings from the present study suggest that the experience of spouse bereavement may contribute to dementia or cognitive decline by increasing cerebrovascular injury rather than by aggravating AD pathologies, particularly in older individuals and those with no‐ or low‐skill occupations. More attention should be paid to spouse bereavement–related brain health problems.
Disclosure statement
The authors declare that they have no competing interests.
Author contributions
J.W.K. and D.Y.L. conceived and designed the study. J.W.K., M.S.B., D.Y., J.H.L., M.J.K., G.J., J.Y.L., K.M.K., C.H.S., Y.S.L., Y.K.K., and D.Y.L. were involved in acquisition, or analysis and interpretation of the data and helped to draft the manuscript. J.W.K., M.S.B., D.Y., J.H.L., and D.Y.L. were major contributors in writing the manuscript and critically revising the manuscript for intellectual content. D.Y.L, served as principal investigator and supervised the study. All authors read and approved the final manuscript.
Supporting information
Appendix S1. Supporting Information
Method S1. Assessment of potential confounders or modulators.
Table S1. Results of multiple logistic and linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume in participants without death of close friend(s) (n = 75).
Table S2. Results of multiple logistic and linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume in participants with neither divorce nor separation (n = 113).
Table S3. Results of multiple logistic and linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume in participants without remarriage (n = 116).
Table S4. Results of multiple linear regression analyses including the interaction term between spouse bereavement and age (or sex or APOE4 positivity or clinical diagnosis or VRS or BMI or undernutrition or education or GDS score or occupational complexity or annual income or physical activity or MOS‐SSS score or alcohol intake or smoking) status predicting WMH volume (N = 118).
Table S5. Results of the multiple linear regression analysis for assessing the relationships between spouse bereavement and WMH volume according to participants' age and occupational complexity (N = 118).
Table S6. Results of the multiple linear regression analyses for assessing the relationships between stratified spouse bereavement and cognitive performance (N = 118).
Table S7. Results of multiple linear regression analyses for assessing the relationships between WMH volume and cognitive performance (N = 118).
Acknowledgments
This study was supported by a grant from the Ministry of Science, ICT, and Future Planning, Republic of Korea (grant number NRF‐2014M3C7A1046042), grants of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers HI18C0630 and HI19C0149), and a grant from the National Institute of Aging (U01AG072177). The funding source had no role in the study design, data collection, data analysis, data interpretation, writing of the manuscript, or decision to submit it for publication. We thank all of the members of the KBASE Research Group for their contribution. Members of the KBASE Research Group are listed elsewhere (http://kbase.kr). We sincerely thank the patients for their participation in this study. The precursor of [18F] AV‐1451 was provided by AVID Radiopharmaceuticals.
APPENDIX A.
A.1. Authors
| Name | Location | Role | Contribution |
|---|---|---|---|
| Jee Wook Kim, MD, PhD | Hallym University Dongtan Sacred Heart Hospital, Hwaseong, Republic of Korea | First author | Study concept and design; analysis, and interpretation of data; and drafting and critically revising the manuscript for intellectual content |
| Min Soo Byun, MD, PhD | Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Jun Ho Lee, MD | National Center for Mental Health, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Dahyun Yi, PhD | Medical Research Center Seoul National University, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Min Jung Kim, MD | Eulji University Nowon Eulji Medical Center, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Gijung Jung, RN, PhD | Seoul National University Hospital, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Jun‐Young Lee, MD, PhD | SMG‐SNU Boramae Medical Center, Seoul National University College of Medicine, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Yun‐Sang Lee, PhD | Seoul National University College of Medicine, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Yu Kyeong Kim, MD, PhD | SMG‐SNU Boramae Medical Center, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Koung Mi Kang, MD | Seoul National University Hospital, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Chul‐Ho Sohn, MD, PhD | Seoul National University Hospital, Seoul, Republic of Korea | Author | Acquisition, analysis, and interpretation of data; and critically revising the manuscript for intellectual content |
| Dong Young Lee, MD, PhD | Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea | Corresponding author | Study concept and design; acquisition, analysis, and interpretation of data; and drafting and critically revising the manuscript for intellectual content |
A.2. Coinvestigators
Coinvestigators are listed in elsewhere (http://kbase.kr).
| Name | Location | Role | Contribution |
|---|---|---|---|
| Dong Young Lee, MD, PhD | Seoul National University College of Medicine | Principal investigator | Designed and conceptualized the cohort study, led and supervised the cohort study, coordinated communication among study cores and study sites, acquired funding |
| Min Soo Byun, MD, PhD | Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Republic of Korea | Core PI Clinical & Executive | Supervised and coordinated the Clinical and Executive core of the cohort study |
| Dahyun Yi, PhD | Medical Research Center Seoul National University | Core PI Neuropsychology | Supervised and coordinated the Neuropsychological Core of the cohort study |
| Yu Kyeong Kim, MD, PhD | SMG‐SNU Boramae Medical Center | Core PI PET | Supervised and coordinated the PET Core of the cohort study |
| Chul‐Ho Sohn, MD, PhD | Seoul National University College of Medicine | Core PI MRI | Supervised and coordinated the MRI Core of the study |
| Inhee Mook‐Jung, PhD | Seoul National University College of Medicine | Core PI Biomarker | Supervised and coordinated the Biomarker Core of the study |
| Murim Choi, PhD | Seoul National University | Core PI Genetics | Supervised and coordinated the Genetic Core of the study |
| Yu Jin Lee, MD, PhD | Seoul National University College of Medicine | Core PI Sleep | Supervised and coordinated the Sleep Core of the study |
| Seokyung Hahn, PhD | Seoul National University College of Medicine | Core PI Biostatistics | Supervised and coordinated the Biostatistics Core of the study |
| Hyun Jung Kim, MD | Changsan Convalescent Hospital | Coinvestigator | Performed clinical assessment of participants and quality control of the clinical data |
| Mun Young Chang, MD | Chung‐Ang University College of Medicine | Coinvestigator | Coordinated an add‐on study of the main cohort study |
| Seung Hoon Lee, MD | Daerim St. Mary's Hospital | Coinvestigator | Performed clinical assessment of participants and quality control of the clinical data |
| Na Young Han, MD | Dongrae Medical Center | Coinvestigator | Performed clinical assessment of participants and quality control of the clinical data |
| Jisoo Pae, MD, PhD | Genome & Company | Coinvestigator | Coordinated an add‐on study of the main cohort study |
| Hansoo Park, MD, PhD | Genome & Company | Coinvestigator | Coordinated an add‐on study of the main cohort study |
| Jee Wook Kim, MD, PhD | Hallym University Dongtan Sacred Heart Hospital | Coinvestigator | Coordinated a study site and performed participants recruitment and quality control of the clinical data |
| Young Min Choe, MD | Hallym University Dongtan Sacred Heart Hospital | Coinvestigator | Performed recruitment and clinical assessment of participants and monitoring of the clinical data |
| Jong‐Min Lee, PhD | Hanyang University | Coinvestigator | Coordinated an add‐on study of the main cohort study |
| Dong Woo Lee, MD, PhD | Inje University Snaggye Paik Hospital | Coinvestigator | Coordinated a study site and recruited participants of the cohort study |
| Bo Kyung Sohn, MD | Inje University Snaggye Paik Hospital | Coinvestigator | Coordinated a study site and recruited participants of the cohort study, performed clinical data analysis |
| Seok Woo Moon, MD, PhD | Konkuk University Chungju Hospital | Coinvestigator | Coordinated a study site and performed clinical data analysis |
| Seung‐Ho Ryu, MD, PhD | Konkuk University Medical Center | Coinvestigator | Coordinated a study site and recruited participants |
| Man Ho Choi, PhD | Korea Institute of Science and Technology | Coinvestigator | Supervised and coordinated the MRI Core of the study |
| Hyewon Baek, MD | Kyunggi Provincial Hospital for the Elderly | Coinvestigator | Performed clinical assessment of participants and quality control of the clinical data |
| Yoon‐Keun Kim, MD, PhD | MD Healthcare Inc. | Coinvestigator | Coordinated an add‐on study of the main cohort study |
| Kang Ko, MD | National Center for Mental Health | Coinvestigator | Performed clinical assessment of participants and quality control of the clinical data |
| Jong‐Won Kim, MD, PhD | Samsung Medical Center | Coinvestigator | Supervised and performed genetic analysis |
| Shin Gyeom Kim, MD, PhD | Soonchunhyang University Hospital Bucheon | Coinvestigator | Coordinated a study site and performed clinical data analysis |
| Sun‐Ho Han, PhD | Seoul National University | Coinvestigator | Coordinated blood sample repository, performed blood‐biomarker‐related analysis |
| Joo‐Youn Cho, PhD | Seoul National University | Coinvestigator | Coordinated and performed blood‐biomarker‐related analysis |
| Jae Sung Lee, PhD | Seoul National University | Coinvestigator | Coordinated and performed PET image data‐related analysis |
| Yun‐Sang Lee, PhD | Seoul National University | Coinvestigator | Coordinated the acquisition of the PET data and related logistics |
| Jong Inn Woo, MD, PhD | Seoul National University | Coinvestigator | Supervised and advised the cohort study |
| Sang Eun Kim, MD, PhD | Seoul National University Bundang Hospital | Coinvestigator | Coordinated the production of PET radiotracer |
| Byung Chul Lee, PhD | Seoul National University Bundang Hospital | Coinvestigator | Coordinated the production of PET radiotracer |
| Gi Jeong Cheon, MD, PhD | Seoul National University Hospital | Coinvestigator | Coordinated the acquisition of the PET data |
| Koung Mi Kang, MD | Seoul National University Hospital | Coinvestigator | Participated in the acquisition and clinical interpretation of the MRI/MRA data |
| Jee‐Eun Park, MD, PhD | Seoul National University Hospital | Co‐investigator | Performed clinical and sleep‐related data analysis |
| Hyeong Gon Yu, MD, PhD | Seoul National University Hospital | Coinvestigator | Coordinated an add‐on study of the main cohort study |
| Jun‐Young Lee, MD, PhD | SMG‐SNU Boramae Medical Center | Coinvestigator | Coordinated a study site and performed participants recruitment |
| Hyo Jung Choi, MD | Coinvestigator | Performed clinical assessment of participants and quality control of the clinical data | |
| Kwangsoo Kim, Ph.D | Seoul National University Hospital | Coinvestigator | Supervised and performed biostatistics data analysis |
| Jun Ho Lee, MD | Seoul National University Hospital | Coinvestigator | Coordinated participant recruitment and follow‐up, performed clinical assessment of participants, quality control of the clinical data analysis |
| Sung Wook Park, MD, PhD | Seoul National University Hospital | Research fellow | Performed an add‐on study and data analysis |
| So Yeon Jeon, MD | Seoul National University Hospital | Research fellow | Coordinated participant recruitment and follow‐up, performed clinical assessment of participants, quality control of the clinical data |
| Woo Jin Kim, MD, PhD | Seoul National University Hospital | Research fellow | Performed clinical assessment of participants and quality control of the clinical data |
| Hak Young Kim | Seoul National University Hospital | Psychologist | Performed neuropsychological assessment of participants, quality control. and preprocessing of the data |
| Haejung Joung | Seoul National University Hospital | Psychologist | Performed neuropsychological assessment of participants. Quality control and preprocessing of the data |
| Younghwa Lee | Seoul National University Hospital |
Psychologist |
Performed neuropsychological assessment of participants, quality control, and preprocessing of the data |
| Donghwi Hwang | Seoul National University | Image analyst | Performed PET data analysis |
| Seung Kwan Kang | Seoul National University | Image analyst | Performed PET data analysis |
| Seong A Shin | Seoul National University | Image analyst | Performed PET data pre‐processing |
| Jeong Yeon Hwang, MD | Seoul National University | Data analyst | Performed sleep‐related data analysis |
| Jong‐Chan Park | Seoul National University | Data analyst | Performed blood‐biomarker related analysis |
| Jong‐Ho Park | Samsung Medical Center | Genetic data analyst | Performed genetic data analysis |
| Jieun Seo | Seoul National University | Genetic data analyst | Performed genetic data analysis |
| Gi Jung Jung | Seoul National University Hospital | Research coordinator | Coordinated participants recruitment, follow‐up and assessment among sites, performed clinical assessment of participants and data monitoring |
| Min Jeong Kim | Seoul National University Hospital | Research coordinator | Coordinated participants recruitment, performed clinical assessment of participants |
| Han Na Lee | Seoul National University Hospital | Research coordinator | Coordinated participants recruitment, follow‐up and assessment among sites, performed clinical assessment of participants and data monitoring |
| Yun Jung Hwang | Seoul National University Hospital | Researcher | Performed the clinical data analysis |
| Joon Hyung Jung, MD | Seoul National University Hospital | Researcher | Performed the clinical data analysis |
| Kiyoung Sung, MD | Seoul National University Hospital | Researcher | Performed the clinical data analysis |
| Eun Hye Kim | Seoul National University | Research assistant | Coordinated and performed the collection and preprocessing of blood samples |
| Han Byul Choi | National Research Center for Dementia | Administrative staff | Coordinated participant recruitment and provided administrative support |
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/El42lo.
Membership of the KBASE Research Group are listed in the Appendix.
Data Availability Statement
The data of the current study are not freely accessible because the institutional review board of Seoul National University Hospital prohibits public data‐sharing for privacy reasons. However, the data may be available from the independent data‐sharing committee of the KBASE Research Group on reasonable request, after approval by the institutional review board. Requests for data access can be submitted to the administrative coordinator of the KBASE group by e‐mail (kbasecohort@gmail.com); the coordinator is independent of the authors.
References
- 1. Buckley T, Sunari D, Marshall A, Bartrop R, McKinley S, Tofler G. Physiological correlates of bereavement and the impact of bereavement interventions. Dialogues Clin. Neurosci. 2012; 14: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah SM, Carey IM, Harris T, Dewilde S, Victor CR, Cook DG. The effect of unexpected bereavement on mortality in older couples. Am. J. Public Health 2013; 103: 1140–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosnick CB, Small BJ, Burton AM. The effect of spousal bereavement on cognitive functioning in a sample of older adults. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2010; 17: 257–269. [DOI] [PubMed] [Google Scholar]
- 4. Ward L, Mathias JL, Hitchings SE. Relationships between bereavement and cognitive functioning in older adults. Gerontology 2007; 53: 362–372. [DOI] [PubMed] [Google Scholar]
- 5. Shin SH, Kim G, Park S. Widowhood status as a risk factor for cognitive decline among older adults. Am. J. Geriatric Psychiatry 2018; 26: 778–787. [DOI] [PubMed] [Google Scholar]
- 6. Biddle KD, Jacobs HIL, d'Oleire Uquillas F et al. Associations of widowhood and beta‐amyloid with cognitive decline in cognitively unimpaired older adults. JAMA Netw. Open 2020; 3: e200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sommerlad A, Ruegger J, Singh‐Manoux A, Lewis G, Livingston G. Marriage and risk of dementia: Systematic review and meta‐analysis of observational studies. J. Neurol. Neurosurg. Psychiatry 2018; 89: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parkes CM, Benjamin B, Fitzgerald RG. Broken heart: A statistical study of increased mortality among widowers. Br. Med. J. 1969; 1: 740–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carey IM, Shah SM, DeWilde S, Harris T, Victor CR, Cook DG. Increased risk of acute cardiovascular events after partner bereavement: A matched cohort study. JAMA Intern. Med. 2014; 174: 598–605. [DOI] [PubMed] [Google Scholar]
- 10. Williamson W, Lewandowski AJ, Forkert ND et al. Association of Cardiovascular Risk Factors with MRI indices of cerebrovascular structure and function and white matter Hyperintensities in young adults. JAMA 2018; 320: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: Systematic review and meta‐analysis. BMJ 2010; 341: c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience 2004; 127: 601–609. [DOI] [PubMed] [Google Scholar]
- 13. Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid‐beta via corticotropin‐releasing factor and neuronal activity. Proc. Natl. Acad. Sci. U. S. A. 2007; 104: 10673–10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devi L, Alldred MJ, Ginsberg SD, Ohno M. Sex‐ and brain region‐specific acceleration of beta‐amyloidogenesis following behavioral stress in a mouse model of Alzheimer's disease. Mol. Brain 2010; 3: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sotiropoulos I, Sousa N. Tau as the converging protein between chronic stress and Alzheimer's disease synaptic pathology. Neurodegener Dis 2016; 16: 22–25. [DOI] [PubMed] [Google Scholar]
- 16. Joshi YB, Chu J, Pratico D. Stress hormone leads to memory deficits and altered tau phosphorylation in a model of Alzheimer's disease. J. Alzheimer's Dis.: JAD 2012; 31: 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carroll JC, Iba M, Bangasser DA et al. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin‐releasing factor receptor‐dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci. 2011; 31: 14436–14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lambert HK, McLaughlin KA. Impaired hippocampus‐dependent associative learning as a mechanism underlying PTSD: A meta‐analysis. Neurosci. Biobehav. Rev. 2019; 107: 729–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stein‐Behrens B, Mattson MP, Chang I, Yeh M, Sapolsky R. Stress exacerbates neuron loss and cytoskeletal pathology in the hippocampus. J. Neurosci. 1994; 14: 5373–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saavedra Perez HC, Ikram MA, Direk N et al. Cognition, structural brain changes and complicated grief. A population‐based study. Psychol. Med. 2015; 45: 1389–1399. [DOI] [PubMed] [Google Scholar]
- 21. Byun MS, Yi D, Lee JH et al. Korean brain aging study for the early diagnosis and prediction of Alzheimer's disease: Methodology and baseline sample characteristics. Psychiatry Investig. 2017; 14: 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology 1993; 43: 2412–2414. [DOI] [PubMed] [Google Scholar]
- 23. Lee DY, Lee KU, Lee JH et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J. Int. Neuropsychol. Soc.: JINS 2004; 10: 72–81. [DOI] [PubMed] [Google Scholar]
- 24. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav. Res. 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seo EH, Lee DY, Lee JH et al. Total scores of the CERAD neuropsychological assessment battery: Validation for mild cognitive impairment and dementia patients with diverse etiologies. Am. J. Geriatric Psychiatry 2010; 18: 801–809. [DOI] [PubMed] [Google Scholar]
- 26. Tsai JZ, Peng SJ, Chen YW et al. Automated segmentation and quantification of white matter hyperintensities in acute ischemic stroke patients with cerebral infarction. PloS One 2014; 9: e104011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kochunov P, Lancaster JL, Thompson P et al. Regional spatial normalization: Toward an optimal target. J. Comput. Assist. Tomogr. 2001; 25: 805–816. [DOI] [PubMed] [Google Scholar]
- 28. Park JC, Han SH, Yi D et al. Plasma tau/amyloid‐beta1‐42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer's disease. Brain: J. Neurol. 2019; 142: 771–786. [DOI] [PubMed] [Google Scholar]
- 29. Reiman EM, Chen K, Liu X et al. Fibrillar amyloid‐beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2009; 106: 6820–6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choe YM, Sohn BK, Choi HJ et al. Association of homocysteine with hippocampal volume independent of cerebral amyloid and vascular burden. Neurobiol. Aging 2014; 35: 1519–1525. [DOI] [PubMed] [Google Scholar]
- 31. Jack CR Jr, Wiste HJ, Weigand SD et al. Defining imaging biomarker cut points for brain aging and Alzheimer's disease. Alzheimers Dement. 2017; 13: 205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jack CR Jr, Wiste HJ, Weigand SD et al. Age‐specific population frequencies of cerebral beta‐amyloidosis and neurodegeneration among people with normal cognitive function aged 50‐89 years: A cross‐sectional study. Lancet Neurol. 2014; 13: 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JH, Byun MS, Yi D et al. Sex‐specific association of sex hormones and gonadotropins, with brain amyloid and hippocampal neurodegeneration. Neurobiol. Aging 2017; 58: 34–40. [DOI] [PubMed] [Google Scholar]
- 34. Ennis J, Majid U. "death from a broken heart": A systematic review of the relationship between spousal bereavement and physical and physiological health outcomes. Death Stud. 2019; 45: 538–551. [DOI] [PubMed] [Google Scholar]
- 35. Forbes HJ, Wong AYS, Morton C et al. Partner bereavement and detection of dementia: A UK‐based cohort study using routine health data. J. Alzheimer's Dis.: JAD 2019; 72: 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmes TH, Rahe RH. The social readjustment rating scale. J. Psychosom. Res. 1967; 11: 213–218. [DOI] [PubMed] [Google Scholar]
- 37. Burrage E, Marshall KL, Santanam N, Chantler PD. Cerebrovascular dysfunction with stress and depression. Brain Circul. 2018; 4: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brooks S, Branyan KW, DeVallance E et al. Psychological stress‐induced cerebrovascular dysfunction: The role of metabolic syndrome and exercise. Exp. Physiol. 2018; 103: 761–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jin L, Chrisatakis NA. Investigating the mechanism of marital mortality reduction: The transition to widowhood and quality of health care. Demography 2009; 46: 605–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brickman AM, Siedlecki KL, Muraskin J et al. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiol. Aging 2011; 32: 1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alber J, Alladi S, Bae HJ et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimers Dement (N Y) 2019; 5: 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang J, Birkenmaier J, Kim Y. Job loss and unmet health care needs in the economic recession: Different associations by family income. Am. J. Public Health 2014; 104: e178–e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smart EL, Gow AJ, Deary IJ. Occupational complexity and lifetime cognitive abilities. Neurology 2014; 83: 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Leyhe T, Muller S, Milian M, Eschweiler GW, Saur R. Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer's disease. Neuropsychologia 2009; 47: 2464–2469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Method S1. Assessment of potential confounders or modulators.
Table S1. Results of multiple logistic and linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume in participants without death of close friend(s) (n = 75).
Table S2. Results of multiple logistic and linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume in participants with neither divorce nor separation (n = 113).
Table S3. Results of multiple logistic and linear regression analyses for assessing the relationships between stratified spouse bereavement and WMH volume in participants without remarriage (n = 116).
Table S4. Results of multiple linear regression analyses including the interaction term between spouse bereavement and age (or sex or APOE4 positivity or clinical diagnosis or VRS or BMI or undernutrition or education or GDS score or occupational complexity or annual income or physical activity or MOS‐SSS score or alcohol intake or smoking) status predicting WMH volume (N = 118).
Table S5. Results of the multiple linear regression analysis for assessing the relationships between spouse bereavement and WMH volume according to participants' age and occupational complexity (N = 118).
Table S6. Results of the multiple linear regression analyses for assessing the relationships between stratified spouse bereavement and cognitive performance (N = 118).
Table S7. Results of multiple linear regression analyses for assessing the relationships between WMH volume and cognitive performance (N = 118).
Data Availability Statement
The data of the current study are not freely accessible because the institutional review board of Seoul National University Hospital prohibits public data‐sharing for privacy reasons. However, the data may be available from the independent data‐sharing committee of the KBASE Research Group on reasonable request, after approval by the institutional review board. Requests for data access can be submitted to the administrative coordinator of the KBASE group by e‐mail (kbasecohort@gmail.com); the coordinator is independent of the authors.


