Abstract
To determine the role of endogenous migration-inhibitory factor (MIF) in the development of protective immunity against cutaneous leishmaniasis, we analyzed the course of cutaneous Leishmania major infection in MIF gene-deficient mice (MIF−/−) and wild-type (MIF+/+) mice. Following cutaneous L. major infection, MIF−/− mice were susceptible to disease and developed significantly larger lesions and greater parasite burdens than MIF+/+ mice. Interestingly, antigen-stimulated lymph node cells from MIF−/− mice produced more interleukin-4 (IL-4) and gamma interferon (IFN-γ) than those from MIF+/+ mice, although the differences were statistically not significant. IFN-γ-activated resting peritoneal macrophages from MIF−/− mice showed impaired macrophage leishmanicidal activity and produced significantly lower levels of nitric oxide and superoxide in vitro. The macrophages from MIF−/− mice, however, produced much more IL-6 than macrophages from wild-type mice. These findings demonstrate that endogenous MIF plays an important role in the development of protective immunity against L. major in vivo. Furthermore, they indicate that the susceptibility of MIF−/− mice to L. major infection is due to impaired macrophage leishmanicidal activity rather than dysregulation of Th1 and Th2 responses.
Most inbred mice are resistant to cutaneous Leishmania major infection and develop small self-resolving lesions (1, 16). It is widely accepted that the ability of genetically resistant mice to resolve cutaneous L. major infection is associated with the development of interleukin-12 (IL-12)-induced Th1 response and gamma interferon (IFN-γ) production. The protective role of IFN-γ has been attributed to its ability to induce the Th1 response, inhibit Th2 differentiation, and enhance macrophage leishmanicidal activity (16).
Migration-inhibitory factor (MIF) is a pleiotropic cytokine that is produced by many cells, including macrophages, T cells, and the pituitary gland, during inflammatory responses. MIF inhibits anti-inflammatory effects of corticosteroids and plays a critical role in pathogenesis of sepsis (3, 4). Additionally, MIF also acts as an enzyme and catalyzes the tautomerization of several substrates (21). Experimental studies using MIF-neutralizing antibodies indicate that this cytokine is involved in pathogenesis of autoimmune diseases such as collagen type II-induced arthritis and immunologically induced kidney disease (11, 14). Recent studies show that MIF counteracts the antitumor activity of p53 and appears to link chronic inflammation and tumor formation (9).
Studies from our laboratory and others indicate that MIF may play a critical role in regulation of host immunity or susceptibility to pathogens. For example, we found that MIF−/− mice cleared gram-negative Pseudomonas aeruginosa bacteria more efficiently than MIF+/+ mice, indicating that MIF is involved in pathogenesis of pulmonary P. aeruginosa infection (4). In contrast, oral administration of MIF alone or together with tumor necrosis factor alpha (TNF-α) via transfected attenuated Salmonella enterica serovar Typhimurium enhanced resistance of BALB/c mice to L. major (23). Furthermore, others demonstrated that MIF with endogenous TNF-α enhances macrophage NO production and induces in vitro killing of L. major by macrophages (10). Interestingly, the same study found that both susceptible BALB/c and resistant C57BL/6 mice express high levels of MIF mRNA in their draining lymph nodes following L. major infection (10). Therefore, we examined the cutaneous growth of L. major infection in MIF gene-deficient C57BL/6 × 129/Sv (MIF−/−) mice and compared it with growth in similarly infected age and sex-matched wild-type (MIF+/+) mice. In addition, we analyzed the leishmanicidal activity of resident macrophages and measured cytokine production by the draining lymph nodes from L. major-infected MIF+/+ and MIF−/− mice. Our results indicate that MIF plays a critical role in the development of protective immunity and control of cutaneous L. major infection.
MATERIALS AND METHODS
Mice.
MIF gene-deficient C57BL/6 × 129/Sv mice were generated by gene targeting as described previously (4). MIF−/− mice were maintained by mating between homozygous mice on the C57BL/6 × 129Sv background. The wild-type littermates were used to generate wild-type MIF+/+ mice of the same age and sex that were used as controls in all experiments. Mice were maintained in the specific-pathogen-free facility at the Harvard School of Public Health according to the guidelines for animal research.
Parasites.
L. major LV39 was maintained by serial passage of parasites in BALB/c mice. Amastigotes isolated from lesions of infected BALB/c mice were grown to stationary phase as described previously (18).
Infection protocol and quantitation of parasite burdens.
Mice 8 to 12 weeks old were injected in the hind footpad with 2 × 106 stationary-phase promastigotes enumerated using a Nuebauer hemacytometer. Lesion growth was monitored by measuring the increase in thickness of the infected footpad using a dial-gauge micrometer (18) at weekly intervals up to 12 weeks after infection and comparing this to the thickness of the contralateral uninfected footpad.
Parasite burdens in the infected footpads were determined by limiting-dilution analysis, as described previously (18).
IL-12 treatment.
Recombinant murine IL-12 (R & D Systems Inc.) was used to treat MIF−/− mice in doses described previously (22). Briefly, 1 μg of IL-12 in 100 μl was administered intraperitoneally to each MIF−/− mouse 1 day prior to L. major infection, followed by a daily dose of 1 μg/per mouse for 5 days thereafter. Control MIF−/− mice received injections of 100 μl of sterile phosphate-buffered saline (PBS).
T-cell proliferation and cytokine assays.
T-cell proliferation was determined as previously described (20). MIF+/+ and MIF−/− mice were sacrificed on weeks 2, 6, and 12 following L. major infection. The inguinal lymph nodes were removed and teased gently to prepare a single-cell suspension. Briefly, 3 × 105 cells were added in triplicate to the wells of sterile 96-well flat-bottomed tissue culture plates (Costar, Cambridge, Mass.) and stimulated with Leishmania antigen (LmAg; 20 μg/ml). Supernatants were collected after 72 h of incubation and analyzed for the production of IL-4 (reagent purchased from Endogen, Cambridge, Mass.; detection limit, 10 pg/ml) and IFN-γ (both reagents purchased from PharMingen, San Diego, Calif.; detection limit, 20 pg/ml) by capture enzyme-linked immunosorbent assay (ELISA) (20). Similarly, supernatants from macrophage cultures were also analyzed for IL-6, IL-10, IL-12, and TNF-α production by ELISA.
Assessment of macrophage leishmanicidal activity.
Resting peritoneal macrophages were added to the wells of 24-well flat-bottomed plates containing 12-mm-diameter glass coverslips. Cells were incubated at 37°C for 4 h to allow macrophages to adhere to the coverslips. Nonadherent cells were removed by gentle washing, and adherent macrophages were infected with L. major promastigotes (parasite/macrophage ratio, 5:1) for 4 h, washed, and cultured in the presence of 200 U of IFN-γ (Genzyme, Cambridge, Mass.) per ml for 72 h. At this time, macrophages were stained by Giemsa stain, and coverslips were mounted upside down on a glass slide; intracellular amastigotes were counted by microscopy.
Assessment of NO2− and O2− production.
The supernatants from the above assays were analyzed for nitrite concentration using Griess reagent as described previously (6). For determining levels of O2−, IFN-γ-treated macrophages were stimulated with phorbol myristate acetate, and superoxide dismutase-inhibitable reduction of Fe3+ cytochrome c to the ferrous form (Fe2+) was measured. The amount of O2− released was calculated as described previously (15).
Statistical significance.
Student's unpaired t test was used to determine the statistical significance of values.
RESULTS AND DISCUSSION
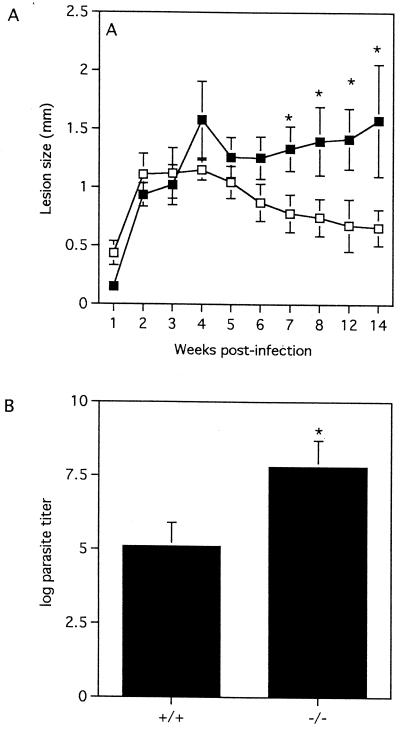
We have previously demonstrated that oral administration of MIF alone or together with IFN-γ and TNF-α via transfected attenuated Salmonella cells conferred significant protection against L. major infection in susceptible BALB/c mice (23). Moreover, others found that MIF enhanced in vitro macrophage leishmanicidal activity (10). In the present study, following infection with 2 × 106 L. major stationary-phase promastigotes, MIF+/+ mice developed lesions that resolved spontaneously by week 12 postinfection (Fig. 1A). In contrast, similarly infected MIF−/− mice displayed smaller lesions than MIF+/+ mice in the early phase of infection but developed large lesions which failed to resolve as infection progressed (Fig. 1A). Concomitantly infected BALB/c mice developed large, rapidly progressive ulcerating lesions by week 6 postinfection (data not shown). At week 12 postinfection, infected footpads from MIF−/− mice also contained significantly more parasites (>100-fold) than those from MIF+/+ mice (Fig. 1B). Taken together, these findings indicate that endogenous MIF plays an important role in the development of protective immunity against L. major in resistant C57BL/6 × 129Sv/Ev mice.
FIG. 1.
MIF−/− mice are relatively more susceptible to cutaneous L. major infection than MIF+/+ mice and develop large lesions. (A) Course of cutaneous L. major infection in MIF−/− (solid squares) and MIF+/+ (open squares) mice following infection with 2 × 106 stationary-phase promastigotes. Disease progression was monitored by measuring the increase in thickness of the infected footpad and comparing this to the thickness of the contralateral uninfected footpad. (B) Footpad parasite burdens in MIF−/− and MIF+/+ mice were determined at week 12 postinfection by limiting-dilution analysis. Data are expressed as the mean titer ± the standard error (SE). Similar results were observed in three and two independent experiments (A and B, respectively).
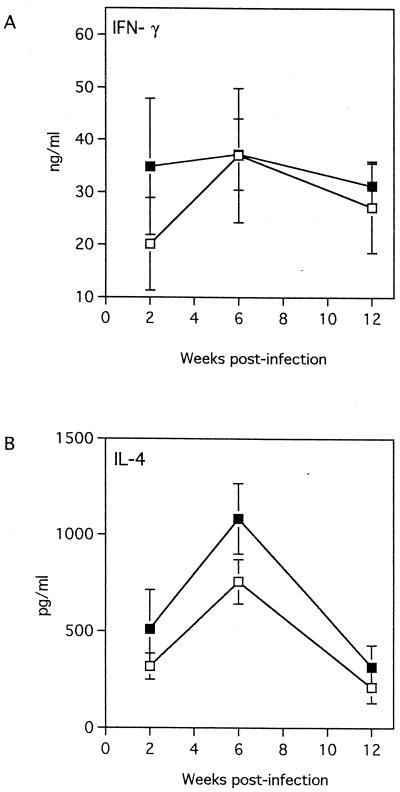
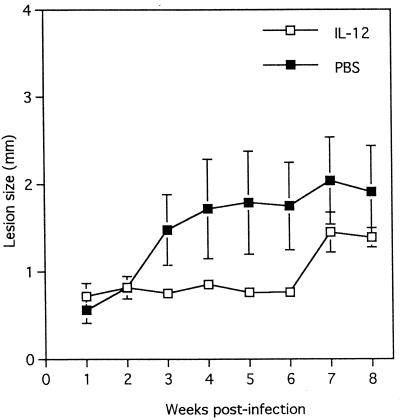
Several studies have demonstrated that control of L. major infection in resistant mice is associated with the development of the IL-12-mediated Th1-like response and the production of IFN-γ, whereas susceptibility to L. major infection is associated with development of a Th2-like response and the production of IL-4 (8, 13, 19). A previous study had demonstrated that MIF plays a critical regulatory role in the activation of T cells (2). Furthermore, MIF was produced by the Leishmania-specific Th2 clone (D10G4.1, L1/1), but not the Th1 (LNC2, B10/B1) clone following in vitro stimulation with mitogen (2). Therefore, we compared IL-4 and IFN-γ production by LmAg-stimulated lymph node cells from L. major-infected MIF−/− and MIF+/+ mice at weeks 2, 6, and 12 postinfection to determine whether susceptibility of MIF−/− mice to L. major is due to an enhanced Th2-like response and/or an impaired Th1-like response. We also measured serum levels of LmAg-specific Th1-associated immunoglobulin G2a (IgG2a) and Th2-associated IgG1 antibodies. Following in vitro stimulation with LmAg, lymph node cells from both L. major-infected MIF−/− and MIF+/+ mice displayed significant but similar levels of proliferative responses (data not shown) and produced significant amounts of IFN-γ and IL-4. Levels of IFN-γ and IL-4 in lymph node cell culture supernatants from MIF−/− mice were either higher or similar to those from MIF+/+ mice, although the differences were not statistically significant (Fig. 2A and B). At week 12 postinfection, MIF+/+ and MIF−/− mice displayed comparable titers of IgG1 (34,400 ± 23,200 and 46,050 ± 14,927 in MIF+/+ and MIF−/− mice, respectively; P < 0.375) and IgG2a (19,200 ± 12,255 and 43,328 ± 17,952 in MIF+/+ and MIF−/− mice, respectively; P < 0.1). Moreover, in one experiment, although administration of recombinant murine IL-12 to MIF−/− mice significantly inhibited lesion growth in the early course of L. major infection between 3 and 6 weeks, these mice eventually developed large lesions comparable to those of control MIF−/− mice treated with PBS (Fig. 3). Although lesion sizes in IL-12-treated MIF−/− mice were smaller than in PBS-treated MIF−/− mice at weeks 7 and 8 postinfection these differences were statistically not significant. As previous studies have clearly demonstrated that IL-12 induces production of IFN-γ from NK cells and T cells, it is most likely that enhanced resistance of IL-12-treated MIF−/− mice against L. major in the early course of infection is due to an increase in IFN-γ production. Nevertheless, taken together, these observations suggest that endogenous MIF may not be involved in the regulation of T-cell activation and production of cytokines IL-4 and IFN-γ following L. major infection.
FIG. 2.
Kinetics of in vitro cytokine production by LmAg-stimulated lymph node cells from MIF−/− (solid squares) and MIF+/+ (open squares) mice. (A) IFN-γ and (B) IL-4 production by draining lymph node cells following in vitro stimulation with LmAg (20 μg/ml) was measured at weeks 2, 6, and 12 postinfection by ELISA. The graph shows the mean (n = 6 to 9 animals) of two separate experiment. Data are expressed as the mean ± SE.
FIG. 3.
Effect of recombinant IL-12 treatment on course of L. major infection in MIF−/− mice. Disease progression was monitored by measuring the increase in the thickness of infected footpad and comparing this to the thickness of the contralateral uninfected footpad. Data are expressed as mean increase in footpad thickness ± SE. Four mice were used in each group.
Macrophages activated by the cytokines IFN-γ and TNF-α are major effector cells involved in killing of Leishmania (12). The leishmanicidal activity of cytokine-activated macrophages has been attributed to their ability to produce microbicidal effector molecules such as nitric oxide and superoxide (12). MIF has been shown to induce TNF-α and NO production in human monocytes (5) and activate murine macrophages to kill Leishmania major in vitro (10). Furthermore, we previously demonstrated that susceptible BALB/c mice that were orally administered MIF together with IFN-γ and TNF-α via transfected attenuated Salmonella express markedly higher levels of nitric oxide synthase 2 in their lymph nodes and developed significantly smaller lesions than control animals (23). Together, these findings indicate that the protective role of MIF in murine leishmaniasis can be attributed to its ability to induce macrophage leishmanicidal activity by increasing NO production.
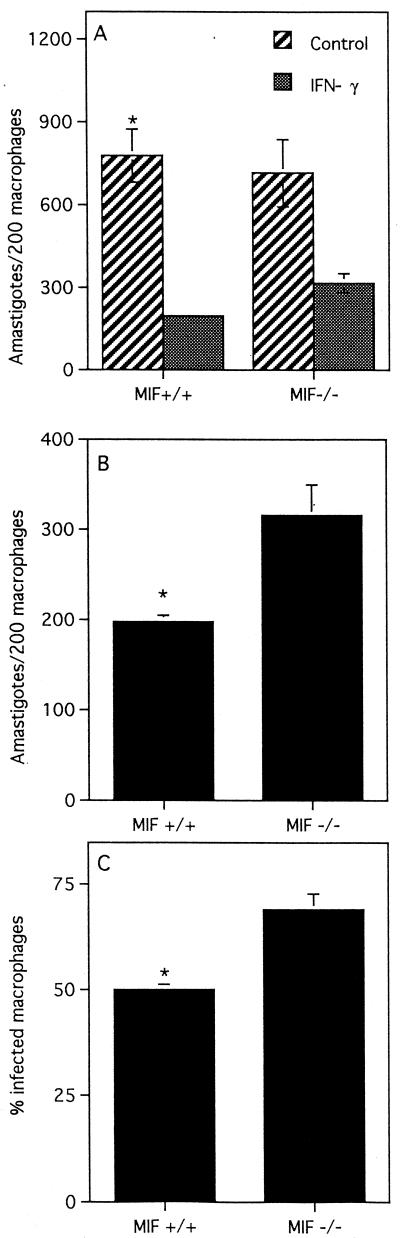
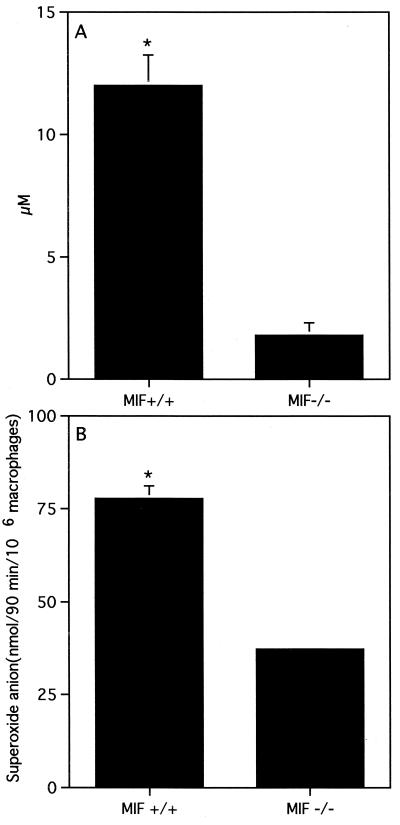
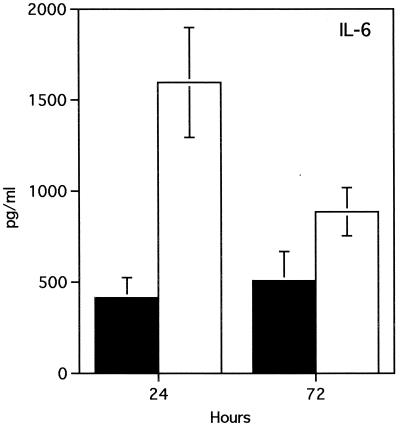
To determine whether the susceptibility of MIF−/− mice to L. major can be attributed to macrophage dysfunction, we compared the ability of resting peritoneal macrophages from MIF−/− and MIF+/+ mice to produce proinflammatory cytokines and kill L. major promastigotes following in vitro stimulation with IFN-γ. Four hours after infection with L. major promastigotes, macrophages from MIF−/− and MIF+/+ mice displayed similar parasite loads (116 ± 13 and 110 ± 18 parasites/200 macrophages in MIF+/+ and MIF−/− macrophages, respectively; P < 0.4), indicating that initial uptake of parasites is not increased in MIF−/− macrophages. After 72 h, IFN-γ induced significant parasite killing in macrophages from both MIF−/− and MIF+/+ mice compared to control macrophages that were not stimulated with IFN-γ (Fig. 4A). At this time point, however, IFN-γ-stimulated macrophages from MIF−/− mice displayed significant impairment of leishmanicidal activity compared to MIF+/+ mice (Fig. 4B and C). Furthermore, impairment of the in vitro leishmanicidal activity of MIF−/− macrophages was associated with significantly lower production of superoxide (two- to threefold) and nitric oxide (fivefold) than MIF+/+ macrophages (Fig. 5A and B). There were no significant differences in the levels of IL-10, transforming growth factor beta (TGF-β), and IL-12 in culture supernatants from MIF+/+ and MIF−/− mice (data not shown). Interestingly, macrophages from MIF−/− mice produced significantly more IL-6 than those from MIF+/+ mice (Fig. 6). In contrast, culture supernatants from LmAg-stimulated lymph node cells from MIF+/+ and MIF−/− mice contained only basal levels of IL-6, suggesting that MIF−/− T cells do not overproduce IL-6. Several studies indicate that IL-6 is a proinflammatory cytokine; others, however, have reported that it can also exhibit immunosuppressive activity and induce differentiation of IL-4-producing CD4+ T cells (17). In fact, IL-6 has been shown to suppress superoxide production in human monocytes and to inhibit their leishmanicidal activity in vitro (7).
FIG. 4.
Resting peritoneal macrophages from MIF−/− mice display impairment of IFN-γ-induced leishmanicidal activity. Macrophages were infected with L. major stationary-phase promastigotes as described in Materials and Methods and stimulated with 200 U of IFN-γ per ml for 72 h. The number of amastigotes per 200 infected macrophages (A and B) and the percentage of infected macrophages (C) were determined microscopically by Giemsa staining. This experiment is representative of two performed. Four to five mice were used in each group, and cells were plated in triplicate.
FIG. 5.
Production of nitric oxide (NO) and superoxide (O2−) is impaired in macrophages from MIF−/− mice. (A) Nitric oxide production and (B) superoxide (O2−) production were measured as described in Materials and Methods. Similar results were observed in two independent experiments. Data are expressed as mean ± SE.
FIG. 6.
Macrophages from MIF−/− mice (open squares) produce significantly more IL-6 than those from MIF+/+ mice (squares). Resting peritoneal macrophages were infected with L. major promastigotes in vitro and stimulated with IFN-γ (200 U/ml). IL-6 production was measured at 12, 24, and 48 h postinfection by ELISA. Data are expressed as the mean of triplicates ± SE. Similar results were observed in three independent experiments.
In our previous paper (4), we reported that thioglycolate-induced macrophages from MIF−/− mice produced the same amount of IL-6 as and a little more NO than wild-type mice. These differences from the present findings are probably due to the much greater stimulus of macrophages used in the earlier study; macrophages induced by thioglycolate may be more activated than resident macrophages. These cells were further stimulated with both lipopolysaccharide (LPS) and IFN-γ rather than with IFN-γ alone, as in the present studies. Therefore, it is perhaps not surprising that thioglycolate-elicited and IFN-γ/LPS-activated peritoneal macrophages from MIF−/− mice killed Leishmania as efficiently as MIF+/+ macrophages (26% ± 10% and 33% ± 11% infected macrophages in MIF+/+ and MIF−/− mice, respectively; P < 0.4). The present protocol using resident macrophages and stimulation with IFN-γ alone is probably more closely related to the physiological reality.
There may be several explanations why IFN-γ-activated MIF−/− macrophage have impaired leishmanicidal activity. First, increased IL-6 production may contribute at least partly to increased susceptibility of MIF−/− mice to L. major by inhibiting macrophage superoxide production, as reported previously (7). Hence, we are currently generating IL-6/MIF double-knockout mice to investigate this further. Second, as MIF has been shown to induce TNF-α, impaired leishmanicidal activity of resident peritoneal macrophages from MIF−/− mice may be due to reduced TNF-α production. This, however, is unlikely in the present study, as TNF-α was either absent or detectable only at basal levels in culture supernatants from both groups (data not shown). Lastly, it is possible that lack of MIF suppresses expression of IFN-γ and/or TNF receptors on macrophages that are essential for mediating the biological functions of these cytokines. We are presently investigating this hypothesis in studies in our laboratory. Nevertheless, together these results indicate that the protective role of endogenous MIF against L. major in resistant mice is mediated by its ability to regulate superoxide and NO production and induce macrophage leishmanicidal activity.
In conclusion, our findings in the present study demonstrate that MIF gene-deficient C57BL/6 × 129Sv/Ev mice are susceptible to cutaneous L. major infection and develop larger lesions and greater parasite burdens than their wild-type counterparts. The susceptibility of MIF−/− mice to L. major is due to impaired IFN-γ-induced macrophage leishmanicidal activity rather than to the lack of Th1 development or enhanced Th2 development. These observations indicate that endogenous MIF is required for optimal activation of macrophages and control of L. major infection in resistant mice. Additionally, the data demonstrate that MIF is not required for activation of T cells and production of Th1-associated IFN-γ and Th2-associated IL-4.
ACKNOWLEDGMENT
This work was funded in part by National Institutes of Health grant AI22532-13.
REFERENCES
- 1.Alexander J, Satoskar A R, Russell D G. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 2.Bacher M, Metz C, Calandra T, Mayer K, Chesney J, Lhoff M, Gemsa D, Donnelly T, Bucala R. An essential role for MIF in T cell activation. Proc Natl Acad Sci USA. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernhagen J, Calandra T, Mitchell R A, Martin S B, Tracey K J, Voelter W, Manouge K R, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 4.Bozza M, Satoskar A R, Lin G, Lu B, Humbles A A, Gerard C, David J R. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. 1999;189:341–346. doi: 10.1084/jem.189.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calandra T, Bernhagen J, Mitchell R A, Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994;179:1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 7.Hatzigeorgiou D E, He S, Sobel J, Grabstein K H, Hafner A, Ho J L. IL-6 down-modulates the cytokine-enhanced antileishmanial activity in human macrophages. J Immunol. 1993;151:3682–3692. [PubMed] [Google Scholar]
- 8.Heinzel F P, Sadick M D, Mutha S S, Locksley R M. Production of interferon-γ, interleukin2, interleukin 4, and interleukin 10 by CD4+ T lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hudson J D, Shoaibi M A, Maestro R, Carnero A, Hannon G J, Beach D H. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J Exp Med. 1999;190:1375–1382. doi: 10.1084/jem.190.10.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juttner S, Bernhagen J, Metz C N, Rollinghoff M, Bucala R, Gessner A. Migration inhibitory factor induces killing of Leishmania major by macrophages: dependent on reactive nitrogen intermediates and endogenous TNF-α. J Immunol. 1998;161:2383–2390. [PubMed] [Google Scholar]
- 11.Lan H, Bacher M, Yang N, Mu W, Nikolic-Paterson D, Metz C, Meinhardt A, Bucala R, Atkins R. The pathogenic role of macrophage migration inhibitory factor in immunologically induced kidney disease in the rat. J Exp Med. 1997;185:1455–1465. doi: 10.1084/jem.185.8.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew F Y, O'Donnell C A. Immunology of leishmaniasis. Adv Parasitol. 1993;32:161–259. doi: 10.1016/s0065-308x(08)60208-0. [DOI] [PubMed] [Google Scholar]
- 13.Locksley R M, Heinzel F P, Holaday B J, Mutha S S, Reiner S L, Sadick M D. Induction of Th1 and Th2 CD4+ subsets during murine Leishmania major infection. Res Immunol. 1991;142:28–38. doi: 10.1016/0923-2494(91)90007-6. [DOI] [PubMed] [Google Scholar]
- 14.Mikulowska A, Metz C, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type-II-induced arthritis in mice. J Immunol. 1997;158:5514–5517. [PubMed] [Google Scholar]
- 15.Pick E, Mizel D. Rapid microassays for the measurement of superoxide and hydrogen peroxide production by macrophages in culture using an automatic enzyme imunoassay reader. J Immunol Methods. 1981;46:211–226. doi: 10.1016/0022-1759(81)90138-1. [DOI] [PubMed] [Google Scholar]
- 16.Reed S G, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin Immunol. 1993;5:524–531. doi: 10.1016/0952-7915(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 17.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell R A. IL-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185:461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoskar A R, Stamm L M, Zhang X, Satoskar A A, Okano M, Terhorst C, David J R, Wang B. Mice lacking NK cells develop an efficient Th1 response and control cutaneous Leishmania major infection. J Immunol. 1999;162:6747–6754. [PubMed] [Google Scholar]
- 19.Scott P, Natovitz P, Coffman R L, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis: T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamm L M, Raisenen-Sokolowski A, Okano M, Russell M E, David J R, Satoskar A R. Mice with STAT6-targeted gene disruption develop a Th1 response and control cutaneous leishmaniasis. J Immunol. 1998;161:6180–6188. [PubMed] [Google Scholar]
- 21.Swope M D, Lolis E. Macrophage migration inhibitory factor: cytokine, hormone, or enzyme? Rev Physiol Biochem Pharmacol. 1999;139:1–32. doi: 10.1007/BFb0033647. [DOI] [PubMed] [Google Scholar]
- 22.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D, McSorley S, Tetley L, Chatfield S, Dougan G, Chan W, Satoskar A, David J R, Liew F Y. Protective effect on Leishmania major infection of migration inhibitory factor, TNF-alpha, and IFN-gamma administered orally via attenuated Salmonella typhimurium. J Immunol. 1998;160:1285–1289. [PubMed] [Google Scholar]