Abstract
Arctic terrestrial herbivores influence tundra carbon and nutrient dynamics through their consumption of resources, waste production, and habitat‐modifying behaviors. The strength of these effects is likely to change spatially and temporally as climate change drives shifts in herbivore abundance, distribution, and activity timing. Here, we review how herbivores influence tundra carbon and nutrient dynamics through their consumptive and nonconsumptive effects. We also present evidence for herbivore responses to climate change and discuss how these responses may alter the spatial and temporal distribution of herbivore impacts. Several current knowledge gaps limit our understanding of the changing functional roles of herbivores; these include limited characterization of the spatial and temporal variability in herbivore impacts and of how herbivore activities influence the cycling of elements beyond carbon. We conclude by highlighting approaches that will promote better understanding of herbivore effects on tundra ecosystems, including their integration into existing biogeochemical models, new applications of remote sensing techniques, and the continued use of distributed experiments.
Keywords: consumptive effects, element cycling, nonconsumptive effects, species interactions, tundra
Here we review how herbivores influence tundra carbon and nutrient dynamics through their consumptive and non‐consumptive effects. We also present evidence for herbivore responses to climate change and discuss how these responses may alter the spatial and temporal distribution of herbivore impacts. We conclude by highlighting several approaches that will promote better understanding of herbivore effects on tundra ecosystems, including their integration into existing biogeochemical models, new applications of remote sensing techniques, and the continued use of distributed experiments.
![]()
INTRODUCTION
Terrestrial food webs were recognized as having a central role in carbon and nutrient cycling in arctic ecosystems nearly a century ago. In their seminal paper, Summerhayes and Elton 1 conceptualized the nitrogen (N) cycle for the Bear Island High‐Arctic food web, including trophic transfer of N between aboveground‐belowground habitats and across terrestrial‐aquatic systems. Yet, over the following decades, the functional roles of animals––defined as their impacts on carbon (C) and nutrient dynamics––were largely missing from theoretical and empirical ecological work, because arctic food webs gained a reputation for being “simple” after a version of the Summerhayes and Elton 1 figure was reproduced in Elton's 1927 2 text on Animal Ecology. A perception of terrestrial arctic food webs as being largely inconsequential for C and nutrient cycling (hereafter referred to as “element cycling”) was furthered by a historical focus by arctic ecosystem ecologists on abiotic constraints and resource availability (i.e., bottom‐up regulation) 3 rather than on effects of consumers (i.e., top‐down regulation) despite acknowledgment that both simultaneously affect tundra ecosystems. Thus, with a few notable exceptions (e.g., plant consumption by vertebrate herbivores), there have been limited integrative efforts among organismal and ecosystem ecologists to characterize the broader functional roles played by arctic terrestrial animals (Figure 1).
FIGURE 1.
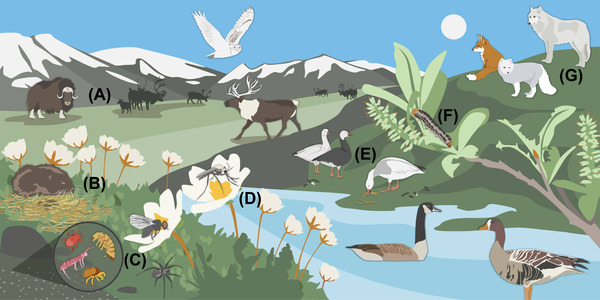
Representative functional groups of animals from the arctic terrestrial food web, including (A) large mammal herbivores, (B) small mammal herbivores, (C) invertebrate detritivores and predators, (D) biting insects, (E) avian herbivores, (F) invertebrate herbivores, and (G) vertebrate predators.
Recently, the structure and function of arctic communities are being revisited. For example, there is growing recognition that tundra food webs contain more complexity than formerly realized due to some of the largest invertebrate diversity pools having been masked by unresolved nodes in previously drawn webs. 4 , 5 This has led to new work aimed at better characterizing the species interactions within these communities. 4 , 6 Increasing appreciation that terrestrial organisms can have wide‐ranging impacts on C and nutrient cycling 7 , 8 , 9 is also motivating new studies on these processes with vertebrates and invertebrates across the Arctic. 10 , 11 , 12 , 13
At the same time, arctic ecosystems and the organisms within them are responding to rapid and dramatic climate change. Although temperatures are increasing globally, the Arctic is warming notably faster than many other areas. 14 Some effects of this rapid warming include changing precipitation regimes, increased greenhouse gas fluxes, reductions in sea ice extent, and advancing plant phenology. 15 , 16 In addition, the Arctic is experiencing an increased frequency of major disturbances, such as wildfire and winter rain‐on‐snow events. 17 With all of these varied ecological disturbances, the fate of large stores of C contained in permafrost soils is of particular concern. 18 As microbial activity increases under warmer conditions, this C could be released to the atmosphere as carbon dioxide (CO2) and methane (CH4). Satellite observations also indicate that photosynthetic biomass is increasing in the Arctic 19 from greater plant growth and higher abundance of deciduous shrubs 20 , 21 as a consequence of numerous factors, including increased nutrient availability. 22 Although vegetation changes could offset some of the increased soil C emissions, herbivore activity is likely to moderate these processes (e.g., Ref. 23).
Responses by arctic animals to warming and other types of environmental change include changes in abundance and diversity, distribution, and timing and intensity of activity (Figure 2; see Ref. 16). Such responses are likely to alter the functional roles of arctic organisms, 24 , 25 , 26 changing the strength and timing of their localized impacts on elemental cycling of C, N, and P and ultimately affecting land–atmosphere feedbacks (Figure 2). A major challenge in quantifying these effects is that both animal responses to climate change and their ecosystem impacts are often context‐dependent and spatially heterogeneous. Achieving a better understanding of potential feedbacks among climate change, animal responses, and element cycling is particularly important in the Arctic, because even small changes could have meaningful impacts on global biogeochemical cycles when scaled across the entire region. 18
FIGURE 2.
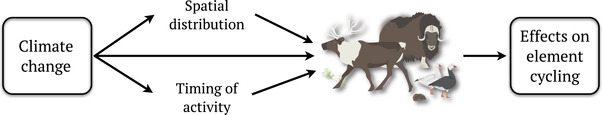
Climate change is modifying the spatial and temporal distribution of herbivore impacts on element cycling in terrestrial tundra ecosystems. Herbivore responses to climate change reflect changes in both abiotic and biotic conditions and include shifts in their distribution, interannual population cycles, and the seasonal timing and local intensity of activity. These changes subsequently alter species‐specific consumptive and nonconsumptive impacts on localized element dynamics.
In this paper, we aim to advance our understanding of the changing functional roles of arctic terrestrial animals and identify priority areas for future research. We focus on herbivores because their ecosystem impacts and climate change responses are the best documented of any animal group in the Arctic, but we also consider how changing interactions among herbivores and other consumer groups may alter their impacts. We review and synthesize recent evidence of how (1) herbivores contribute to element cycling, (2) climate change drives spatial and temporal changes in herbivore activity, and (3) these changes might alter herbivore impacts on element cycling in the future. Using geese as a case study, we highlight how these various factors may interact to affect tundra ecosystem functioning. We also discuss (4) how herbivores mediate existing plant and soil responses to climate change, which can result in further feedbacks to climate change. Finally, we present several remaining gaps in our knowledge on these topics and provide suggestions as to how they might be addressed to stimulate further research on the consequences of animal responses to environmental change in rapidly changing arctic ecosystems.
EFFECTS OF HERBIVORES ON TERRESTRIAL ELEMENT CYCLING
The Arctic is a low‐productivity system where bottom‐up, nutrient‐driven processes are typically thought to be more important than top‐down effects exerted by animals. However, research findings over recent decades show that tundra element cycling is influenced by top‐down consumptive and nonconsumptive effects of tundra herbivores (Figure 3). Here, we consider herbivore consumptive effects to be the direct and indirect effects associated with feeding on live plant biomass (i.e., herbivory). Waste production, which provides nutrient inputs to soils, is a direct consequence of consumption and thus also considered a consumptive effect. Although nonconsumptive effects are often not explicitly included in food webs, 27 behaviors that cause physical disturbance to soil or affect the breakdown of litter can also impact element cycling. Some of these behaviors include trampling, digging, and creating tunnels and hay piles. Lastly, herbivores influence element cycling by inducing changes in plant quality and composition through both the direct and indirect effects of their consumptive and nonconsumptive activities.
FIGURE 3.
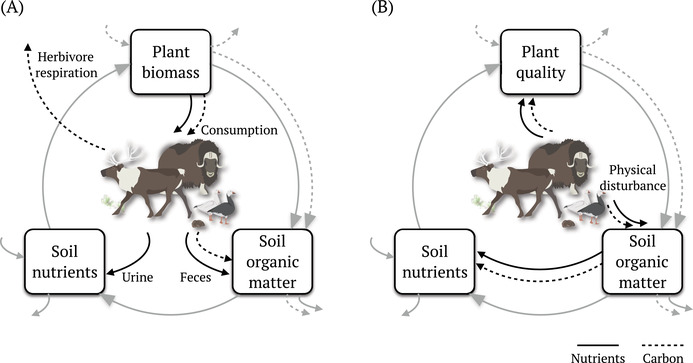
Herbivore effects on element cycling in arctic tundra ecosystems. Gray lines show nutrient flow between plants, soil organic matter, and soil nutrients in the absence of herbivores, and black lines show how herbivores affect nutrient pools in each of these compartments. Solid and dashed lines are indicative of nutrient (N, P) and carbon flow, respectively. Consumptive effects in (A) are due to direct consumption of plant matter. Nonconsumptive effects in (B) include physical disturbance that modifies soil structure through trampling (Rangifer, muskox, and geese), building hay piles (voles), or grubbing (geese). Changes in plant quality (shown in panel B) due to direct and indirect effects of herbivore activity include changes in plant functional traits, plant nutrient content or stoichiometry, and plant community composition (see text for detail).
Arctic terrestrial systems are characterized by only a few species or functional groups with measurable ecosystem impacts (Figure 1). Key groups of mammalian herbivores include two species of large mammal (caribou or reindeer [hereafter Rangifer] and muskox) and a few species of small mammals (primarily voles and lemmings). Although both mammal groups are active year‐round, large mammal effects are more dispersed, because Rangifer and some muskox herds are migratory and may not follow the same migration routes every year. Small mammal home ranges are much smaller, so their ecosystem impacts tend to be localized. Impacts of some small mammal species also vary widely between years due to regular fluctuations in population densities across the Arctic and Subarctic regions (e.g., see Refs. 28 and 29). Overall arctic avian diversity is low; however, diversity of some groups, such as geese and shorebirds, is highest at arctic latitudes. 30 Most of these species are migratory and present only in the summer when densities can become quite high. Research connecting the avian community to arctic element cycling has largely been with geese on the coastal plain; for this reason, we focus on geese as well. Lastly, invertebrates have the highest local abundances and diversity of any herbivore group in the Arctic but are active only in summertime.
We review the evidence for consumptive and nonconsumptive effects of these herbivore groups in tundra ecosystems and consider how herbivore‐induced shifts in plant community composition and/or quality may affect element cycling (Figure 3). Given that effects are caused by herbivore activity, we assume localized impacts on element cycling scale with density (Figure 2). We also briefly consider how consumers from other trophic levels have the potential to modify herbivore impacts on element dynamics.
Consumptive effects
Biomass consumption
Herbivore impacts on tundra plant biomass, nutrient dynamics, and ecosystem C exchange vary depending on the local composition of the herbivore community, herbivore activity levels, and the timing or consistency of their consumptive effects. Overall, the collective community of mammalian herbivores tends to reduce primary production. Experimental mammal exclusions have repeatedly shown that plant biomass is higher in the absence of herbivores in numerous arctic and sub‐arctic ecosystems, including heathlands, 28 , 31 coastal meadows, 32 and mountain snowbeds. 33 However, these effects can vary seasonally due to differential foraging and human management patterns 34 between summer and winter for some herbivores, such as Rangifer. Herbivore effects also vary spatially. For example, results from a recent meta‐analysis indicated that Rangifer consistently have negative effects on lichen but effects on other plant growth forms differ by study location. 35 , 36 With the exception of their calving grounds, 37 grazing impacts of Rangifer are also variable in any given location between years due to variation in annual migration routes, with impacts being lower in low‐density years. 36 Muskox herbivory also causes reductions in plant biomass in wetland communities, but the magnitude of this effect can depend on whether the population moves between winter and summer ranges. 38
In contrast, small mammal impacts are extremely localized but occur throughout the year, because most species are present and active year‐round. In the case of wet tundra, long‐term experimental exclusion of small mammals caused the area to transform from being a C sink into a C source because the lack of herbivory led to litter buildup, which decreased the net ecosystem exchange of C. 39 The annual magnitude of small mammal effects on plant biomass and C‐uptake varies with their population cycles and is likely ecosystem specific. 40 However, following peak rodent years, herbivory effects can be large enough to detect via satellite recorded normalized difference vegetation index. 28
Geese also have large effects on vegetation structure and ecosystem function, particularly in the arctic coastal plain. 41 Direct consumption of plant material is one of the main effects of geese activity on tundra C dynamics. Geese can consume 80% of aboveground net primary production (NPP) in grazed marshes, 42 and these high rates of plant consumption have been shown to result in decreased net ecosystem C uptake. 11 Due to their intense impacts, recent increases in goose population sizes are associated with widespread degradation of arctic wetlands. In a study of nine sites, goose activity caused a large expanse (35,000 ha) of intertidal saltmarsh to transition to an alternative stable state of unvegetated sediment. 43
Background levels of invertebrate herbivory in the tundra are very low and thus, invertebrate herbivores have limited direct effects on C cycling through C consumption. 10 , 44 , 45 However, recent evidence from the Subarctic indicates that background insect herbivory can still significantly weaken CO2 uptake by plants, thus reducing the potential for northern ecosystems to be C sinks under warmer conditions. 46 In addition, occasional larval moth outbreaks in West Greenland have had severe negative consequences for ecosystem productivity. 47 , 48 Outbreaks in subarctic and boreal forest, which are more frequent, also have large impacts on food webs and element cycling, 49 , 50 and can potentially drive ecosystem transitions. 51 Some evidence suggests that these particular types of outbreaks may spread to the tundra in the future. 52
Waste production
Animal waste production converts nutrients from plant material or animal prey into forms that are more available for plant and microbial uptake (i.e., inorganic and simple organic forms found in urine and feces 53 ). Thus, herbivore waste impacts soil chemistry 54 and increases nutrient availability for tundra plants. For example, urine‐derived nutrients are readily absorbed by cryptogams, vascular plants, and the soil microbial community 55 and can alter their stoichiometry. 56 Soil nutrient availability tends to be higher in areas with animal waste, as shown through fecal addition experiments from geese 57 and Rangifer. 58 Increased soil nutrient availability associated with Rangifer feces can have localized impacts on plant productivity, plant composition, microbial biomass, and microbial methane production. 58 , 59 Soil nutrients are elevated near lemming and ground squirrel burrows 60 compared to nearby, undisturbed areas as well.
Herbivore movement between areas of resource consumption and waste production also transfers nutrients between locations. For example, Rangifer were found to modify nutrient distribution across the landscape through their migration and daily movements. 61 Muskox transfer significant amounts of nitrogen between community types by consuming vegetation in wet fen areas but preferentially defecating in drier snow beds. 62 The spatial extent and distribution of these impacts depend on animal size, behavior, and movement distance. For instance, while feeding impacts of voles and lemmings can be spatially diffuse, many species defecate in latrines 63 that concentrate waste and nutrients in small areas. Carcasses also create nutrient hotspots, although the nutrients are not as immediately available as those from other waste products. 60 In extreme cases, aquatic subsidies of invertebrate carcasses can even fertilize tundra soils and stimulate plant growth and litter decomposition. 64 , 65
Nonconsumptive effects
Physical disturbances by herbivores, such as trampling, alter element cycling through effects on soil structure, plant cover, and plant composition (reviewed in Ref. 12). Studies on the effects of trampling in tundra ecosystems have largely focused on large mammals, showing that large mammal activity reduces the abundance of trampling‐sensitive dwarf shrubs, 66 , 67 bryophytes, 68 and lichens. 69 Trampling also compacts soil, 70 which reduces abundances of soil biota. 70 , 71 Although less well studied, geese trampling has some similar effects to that of large mammals. 72 Small mammals have localized trampling effects through their repeated use of trails (i.e., runways), 63 which reduces vegetation cover and compacts soils.
Trampling effects on vegetation cover and composition can have cascading consequences for ecosystem properties. Reduced trampling after the extinction of large herbivores may have been a driving force behind the large‐scale vegetation transition of the Pleistocene Steppe to a low productivity tundra. 12 Recent work from muskox exclosure experiments in wet tundra showed trampling significantly reduced moss cover, raised the water table, and decreased soil temperature. 68 There was also greater CO2 uptake, lower CH4 emissions, 73 and lower plant C and N pools 68 in trampled areas compared to those inside herbivore exclosures. In some cases, trampling effects can even increase resource availability for the soil microbial community. By trampling litter into coastal arctic soils, geese activity was shown to increase N‐mineralization. 72
Herbivores also influence element cycling through burrowing, tunneling, and by constructing haypiles or other types of winter nests. By creating burrows, small mammals, such as lemmings 63 and arctic ground squirrels, 60 move soil between horizons. This activity increases nutrient availability locally and helps to make previously inaccessible materials available to microbes and plants. 74 Soil biota activity also modifies soil structure, which improves the circulation of nutrients, gases, and water. 75 , 76 In contrast, disruptions to the soil organic layer caused by goose foraging for rhizomes early in the growing season causes soil erosion and loss of organic C and N. 41
Effects on plant community composition and traits
Herbivore‐driven changes in plant community composition are caused by a combination of direct and indirect consumptive and nonconsumptive effects. A direct effect of consumption on plant species composition may include selective foraging that reduces the abundance of a particular plant species, while an indirect effect of consumption would be a change in competitive outcomes within the plant community. Evidence from the Arctic generally supports ecological theory predicting that as palatable plant species are removed by herbivores and become less competitive, the composition of the plant community shifts toward less palatable species. 77 This process has been recognized numerous times through reductions in graminoid and forb biomass caused by various herbivores, including Rangifer, 78 geese, 79 and lemmings. 32 Herbivory outcomes can be idiosyncratic, though, and in some cases, plant communities shift toward more palatable species. For example, vole presence was associated with increased graminoid abundance in multiple tundra types. 33 , 80 Long‐term, intensive Rangifer activity was also shown to cause a shift from heath to grass‐dominated vegetation. 34 Changes in plant composition can also arise from nonconsumptive activities by herbivores. For example, haypiles created from graminoid tissue reduce living graminoid abundance (direct effect), while changes in ecosystem properties from trampling could affect plant competitive outcomes (indirect effect).
Lower shrub abundances are a well‐documented example of a plant compositional shift caused by herbivores in tundra ecosystems. Tall deciduous shrubs have expanded in numerous areas in response to reduced Rangifer abundances. 78 , 81 , 82 Similarly, high densities of domesticated Rangifer can reverse such shrub expansion. 83 Exclusion of small mammals also increased deciduous and evergreen shrub abundance in tundra 31 , 80 and the forest‐tundra ecotone. 84 Herbivore‐induced reductions in shrub abundance are broadly relevant to arctic element dynamics, because they could counteract some effects of climate‐induced shrub expansion. 21 However, in some cases, interactions among shrubs and vertebrate herbivores are weak 85 , 86 or dependent on ecosystem type. 80 Moth outbreaks in Greenland also dramatically reduce the biomass and aboveground production of deciduous shrubs, 47 , 48 but it remains unexplored whether low levels of background invertebrate herbivory 10 , 44 affect community composition more generally.
Due to differences in N‐content between tundra plant species and growth forms, herbivore‐driven changes in plant composition can alter community‐level N content. 87 , 88 In some cases, indirect effects of herbivory can have larger impacts on overall N‐content of aboveground plant biomass than direct effects of consumption. 34 Nutrient contents of individual plant species are influenced by herbivory as well; this has been documented through increased graminoid N content caused by geese grazing 42 and decreased N content in shrub leaves infested with gall mites. 89 Changes in plant nutrient concentrations at the species or community level also have cascading effects on processes, such as decomposition. Increased N‐content of plant tissue and consequently, of litter, is one of the primary mechanisms behind observed increases in litter decomposition and nutrient cycling in grazed areas. 82 , 88 Likewise, by promoting dominance of certain plant types, such as evergreen dwarf shrubs, herbivores can induce shifts in soil chemistry and microbial community composition that promote soil C sequestration. 90
If herbivore‐induced changes to the plant community result in lower biomass or photosynthetic capacity, C dynamics may be affected as well. For example, CO2 uptake of an arctic wet meadow was reduced due to barnacle geese activities that decreased graminoid abundance but sustained moss cover. 79 Exclusion of Rangifer increased the biomass of the deciduous shrub Betula nana, resulting in increased community‐level photosynthesis. 91 Reduced herbivory associated with the exclusion of small mammals similarly caused higher plant biomass and led to increased litter accumulation and slower decomposition. 32 , 33 Recent work also showed that the structure and function of the methanotroph community within peat soils depended on previous exposure to goose grazing, which could have implications for C‐cycling. 92 In addition, invertebrate herbivores may have an underappreciated role in influencing C dynamics at the individual plant level. Moth outbreaks in Greenland were associated with lower production and reduced structural support of the common shrub Salix glauca due to changes in the lignin and carbohydrate contents of the plant fibers. 93 Invertebrate herbivory was also documented as driving increased plant emissions of volatile organic compounds (VOCs). 94
Variation in herbivore effects caused by higher trophic levels
Beyond activities such as waste production or denning that directly affect tundra nutrients, 95 arctic predators and parasites indirectly influence element cycling through interactions with herbivores. Specifically, by altering herbivore density, activity, movement, or traits, organisms in higher trophic levels can alter the strength, timing, and location of herbivore impacts (Figures 2 and 3). The extent to which these interactions matter for element cycling at the regional scale remains unclear. However, predator activity has been associated with increased plant biomass in areas with particularly strong predator–prey interactions. 96 , 97 Tundra productivity has also been shown to be higher on islands with vertebrate predators, 98 suggesting the occurrence of trophic cascades in those areas. Among invertebrates, experimental increases in predatory spiders did not affect localized herbivore‐induced plant damage in Greenland 99 but were found to lead to higher soil N‐availability in Alaska. 25 In other cases, although nutrient cycling has not been explicitly addressed, it seems plausible that predators and parasites could have cascading ecosystem impacts. For example, fox activity was associated with landscape distribution of snow geese, 100 a species with extensive impacts on element cycling. Many small mammal population cycles are likely driven in part by top‐down effects of predators as well. 101 For instance, increases in arctic fox populations from greater reliance on marine resources during low lemming years could delay the recovery of lemming populations 102 and their associated impacts. Even the risk of predation reduced snowshoe hare survival in boreal ecosystems, 103 as well as the survival 104 and breeding 105 of ungulates in alpine tundra. Likewise, parasitic infections 106 and harassment by biting flies 107 reduce Rangifer herbivory. Biting insects may also alter spatial and temporal effects of Rangifer by influencing their movement and migration timing. 108 , 109 While predator and parasite effects on ecosystems are generally understudied in tundra ecosystems, their interactions with herbivores have potential to cause changes in local biogeochemistry.
HERBIVORE RESPONSES TO CLIMATE CHANGE
By altering when and where animals are physically present and active on the landscape, climate change is modifying the strength and timing of their impacts on arctic ecosystems (Figure 2). Herbivore responses to climate change include shifts in annual and interannual population densities, seasonal timing of activity, distribution, and key functional traits (Table 1). Temperature changes may even drive shifts in herbivore community structure, as recently shown for vertebrate herbivore groups within the boreal forest and arctic tundra. 110 While some herbivore responses result directly from climate change and may be density‐independent (e.g., warmer temperatures affecting animal physiology or plant resources; effects of greater frequency of wildfire), others are density‐dependent (e.g., increased exposure to novel pathogens). Regardless of the mechanism, changes in individual and population‐level traits can alter the consumptive and nonconsumptive effects of herbivores with subsequent consequences for local element dynamics (Figure 2).
TABLE 1.
Representative examples of how major groups of terrestrial arctic organisms are predicted to or are already responding to climate change in ways that could affect element cycling (see main text for additional discussion)
| Organism | Abundance | Distribution | Phenology |
|---|---|---|---|
| Large mammal herbivores | |||
| Small mammal herbivores |
|
||
| Geese |
|
||
| Invertebrates |
|
|
|
| Vertebrate predators |
|
In this section, we review general knowledge of climate change responses for several key groups of herbivores (large and small mammalian herbivores, geese, and invertebrates). In each case, we consider the recent evidence for how climate change is affecting their population densities, spatial distribution, and timing of activity. Then, in the following section, we provide examples of how these climate change responses could alter herbivore consumptive and nonconsumptive effects on element cycling.
Large mammals
Changes in climate, land‐use, and herding management have had synergistic effects on Rangifer and muskox, 111 but generalizations across the Arctic are challenging due to variation in responses by individual populations. These two species may also respond to different climate change cues, so insights gained from one likely do not apply to the other. 112 For example, recent studies indicate Rangifer herds have been declining globally, 113 , 114 suggesting reductions in the strength of their consumptive and nonconsumptive ecosystem impacts. However, Rangifer utilize a wide range of habitats (both within and among years) and their abundances fluctuate across space and time, suggesting their impacts also fluctuate widely. 35 , 36 , 37 Evidence also indicates some Rangifer ranges are shifting or may shift in the near future to account for changing resource availability (reviewed in Ref. 114). A recent study of the Porcupine Caribou Herd in North America suggests as spring phenology advances, the herd will shift its geographic range to access necessary resources during and after calving. 115 In addition, many Rangifer populations use ice‐covered landscapes to facilitate long distance movements in winter. 114 As ice becomes less common with warming, migratory routes might become extended to avoid open water. This would impose energetic costs on those populations and in turn, cause stronger effects on tundra areas that were historically less impacted.
For Rangifer and muskoxen, changing seasonality and weather patterns may force behavioral changes to ensure adequate food availability. In general, less is known about muskox phenology responses than for Rangifer, but earlier plant green‐up associated with warmer temperatures has been correlated with greater muskox abundance and higher rates of plant consumption. 16 Long‐term data from Greenland showed the local muskox population experienced substantial fluctuations in number and population structure over a recent 18‐year period driven by spring snow patterns; 116 however, not all muskox populations display similar fluctuations (reviewed in Ref. 38). Some evidence suggests that reproductive timing of Rangifer may be flexible, which could partially buffer them from some elements of climate change. In a review of potential mismatches between the timing of Rangifer calving and plant green‐up, the authors found that earlier spring onset could benefit Rangifer by providing higher quality forage during the calving season. 114 Results from the Arctic Animal Movement Archive 117 revealed the timing of Rangifer calving in some populations shifted earlier in warmer years, suggesting they might maintain reproductive success if they can adapt to warmer summers (Table 1; see Ref. 118). If these shifts align with greater food availability, calf survival may increase as well. However, decreases in winter food abundance may counteract some of those effects. Predicting overall annual responses to climate change by large mammals remains challenging due to this combination of shifting phenologies by both the herbivores and their resources.
Extreme events associated with climate change have impacted some large mammal populations. Rangifer are negatively affected by the increasing frequency of tundra fires, because lichens, their primary winter food source, are very slow to recover from wildfire. 119 Increasing rain on snow events 120 also negatively affects grazing herbivores and reduces their ecosystem impacts by limiting access to vegetation during winter and early spring. 16 , 114 , 121
Large mammal responses to climate change are particularly pronounced in the southern areas of their distributions. 122 Direct and indirect human influences in these areas may have compounding effects on Rangifer and muskox as climate change continues. For example, concentrated human activities in the southern Arctic may have detrimental effects on Rangifer populations that will drive them and their associated impacts northward. 123 Though most muskox populations do not migrate, there is evidence that some may be migrating southward in Canada, which would also shift the distribution of their ecosystem impacts (Table 1).
Small mammal herbivores
Multiyear studies suggest that climate change may alter the natural population cycles of some lemmings and voles (i.e., microtine rodents; Table 1). In particular, changing winter weather patterns have been associated with reductions in population peaks and population sizes. 124 , 125 Near Utqiaġvik, Alaska, lemming populations historically peaked every 3–5 years, 29 but there have been no large population peaks in the last decade. 126 However, understanding changes in small mammal population cycles continues to be a challenge because climate change responses are highly context dependent. Temporal patterns in population cycles vary between regions with different climatic conditions, with those in some areas more strongly linked to growing season than winter 127 and little evidence of change in others. 128
Climate change is also driving range expansions among some small mammal species. Subsequent shifts in local community composition among these groups could have consequences for element cycling. As the Arctic warms, some research suggests that currently dominant species might be replaced by locally subdominant or novel species as their distributions expand. 129 Small mammal species inhabiting northern coastlines are already experiencing range contractions and changes in species interactions as southerly species move northward. 130 Models predict that species with more northerly distributions will be those most negatively affected by climate change; some of these northern small mammal species may lose over 25% of habitat space as they shift their ranges further northward (Table 1 and Ref. 130). Subsequent changes in community composition will result in overall changes to small mammal impacts on elemental cycling, because the various species of small mammals have different diets, 131 periodicity in their population cycles, 132 and peak population sizes (e.g., brown vs. collared lemmings 133 ).
Changing seasonality is likely to affect small mammals differently than some of the other groups reviewed here because they remain locally present and active year‐round in the tundra. An exception is arctic ground squirrels, a group that hibernates during winter but has been shown to end hibernation and breed earlier in areas with early snowmelt. 134 However, there is still a general lack of data about arctic small mammal phenology, because key life history events (e.g., breeding and reproduction) for many species occur under snow during wintertime. 135 The extent to which changing seasonality might affect the phenology of these species remains an open area of research.
Avian herbivores: Geese
Geese are one of the most well‐studied groups of birds in the Arctic due to their large numbers and ecological impact. Population of several goose species have increased over the past few decades in North America, Greenland, and Svalbard and are expected to continue increasing (Table 1; see case study below). Geese ranges and the types of habitats they utilize are also shifting with climate change and habitat degradation. Specifically, habitat degradation caused by high geese densities in breeding grounds has expanded occupancy into less degraded areas over recent decades, indirectly fueling continued population growth. 136 As the minimum frost‐free period increases on Svalbard, pink‐footed geese are also predicted to expand their breeding range to the north and east. 137
Similar to other migratory groups, climate change has the potential to disrupt the timing of major life history events and species interactions for geese and other tundra‐breeding birds. Despite their growing numbers, warmer temperatures and changes in plant phenology may ultimately have some negative effects on geese populations in the future. Earlier geese arrival to their arctic breeding grounds does not always match the more advanced dates of plant green‐up (Table 1 and Refs. 138, 139, 140, 141). Key snow goose phenology events (e.g., nesting and fledging timing) have also not advanced fast enough to track changes in vegetation phenology, such as the timing of high N content in plants. 136 , 138 , 141 For multiple species, increasing mismatches between peak gosling hatch and peak forage quality 138 , 141 has led to lower gosling survival. 136
Invertebrate herbivores
Temperature, snowmelt, soil moisture, and plant composition are among the most important drivers of invertebrate composition in the Arctic (e.g., Refs. 142 and 143). As these conditions change, invertebrates are responding in kind. Long‐term data from Greenland indicate that overall invertebrate community composition is changing, 6 , 144 , 145 particularly in arid habitats. 144 , 145 Warming is largely expected to benefit herbivores due to increased plant biomass and alleviation of harsh abiotic conditions (Table 1). However, general assessments of invertebrate responses across the Arctic remain limited by a lack of long‐term data from most regions and because abiotic conditions are rarely measured in microhabitats of relevance to the organisms themselves. 142 , 143 Characterizing the effects of climate change is also a challenge because invertebrate responses in diversity and abundance display high degrees of variation. 144 For example, while abundances of some pollinator species are declining, 146 others exhibit high spatial and interannual variability with no clear population trends (e.g., Lepidoptera in Iceland and Greenland 143 ). Differences in responses among groups are likely due to shifting species interactions and complicated life histories for many species, including the use of multiple habitats. Recent efforts aimed at teasing apart the relative impacts of abiotic versus biotic drivers are a promising way forward to better characterize direct and indirect effects of climate change within this group. 6
Despite projections of northward range expansion by more southerly species, there are only a few examples of invertebrate range shifts or new species introductions in the Arctic (Table 1). A notable example is the winter moth (Operophtera brumata), a species prone to outbreaks that has expanded its distribution northward into the low‐arctic zone in Fennoscandia in response to warmer temperatures. 52 , 147 Range expansions by other invertebrate pests, parasites, and disease vectors 148 (Table 1) may also impose novel stressors on wildlife with indirect effects on tundra nutrients. However, knowledge of species boundaries, distributions, and even basic ecology remains limited for most arctic invertebrates. 143 , 149 Without updated distribution maps and the establishment of more invertebrate monitoring programs, detection of northward range expansions and new introductions will remain a challenge. 143
Most research on changes in arctic invertebrate phenology has been in the context of potential disruptions to food resources for birds and pollination. Many species of arctic invertebrates experience long periods of winter dormancy, with emergence timing and seasonal activity patterns largely driven by snowmelt and air temperature. 150 As snowmelt occurs earlier and temperatures rise, activity windows for some groups are shifting earlier and becoming extended (Table 1). For example, evidence suggests the biomass and activity of herbivores and other canopy‐dwelling invertebrates 151 track those of their host plant resources. On the other hand, lower pollinator abundances 146 and shorter flowering seasons 152 have shrunk pollination time windows, 153 which could have long‐term impacts on plant community composition.
EFFECTS OF CLIMATE CHANGE ON HERBIVORE IMPACTS
As global change alters the spatial and temporal distribution of herbivore activity (Table 1), effects of herbivores on element cycling will also change (Figure 2). In some cases, effects may be relatively straightforward, such as increases or decreases in abundance that cause stronger or weaker species‐specific consumptive and nonconsumptive impacts on plant and soil nutrients. For example, declines in small mammals 124 or Rangifer 114 would reduce their localized impacts on element cycling. However, the consequences of many climate change responses will not be so straightforward. Just as climate change responses vary spatially and temporally, so too will herbivore impacts. For example, shifts in migration routes or species distributions will change the strength of herbivore activity in any given location. Locations of some migratory species’ summer breeding and foraging grounds are also changing, as are distributions for some resident herbivore species (Table 1). Increased energetic costs associated with extended migration routes (e.g., Rangifer) 114 may have other cascading effects on plants via changes in feeding intensity and forage selection as well.
Climate change is also influencing the timing of herbivore impacts within years and the strength of population‐level impacts between years. Phenological shifts by some groups, such as geese, include changes in migration timing and earlier arrival to breeding grounds (Table 1 and Figure 4). Such shifts in herbivore activity timing have the potential to disrupt the highly seasonal progression of tundra element availability. 154 Changes in interannual abundances also contribute to the temporal variability in herbivore impacts on ecosystem processes. This may be particularly relevant for herbivorous rodents, which undergo regular population cycles every 3–5 years, and can have 250% higher density in peak years compared to low years. 29 Recent evidence suggests that population cycles are being suppressed due to climate change (Table 1), which could reduce the overall impacts on landscape nutrient dynamics in the long‐term. In contrast, more frequent insect herbivore outbreaks 52 , 147 could drive higher interannual variability in standing plant biomass, with consequences for primary productivity and nutrient cycling.
FIGURE 4.
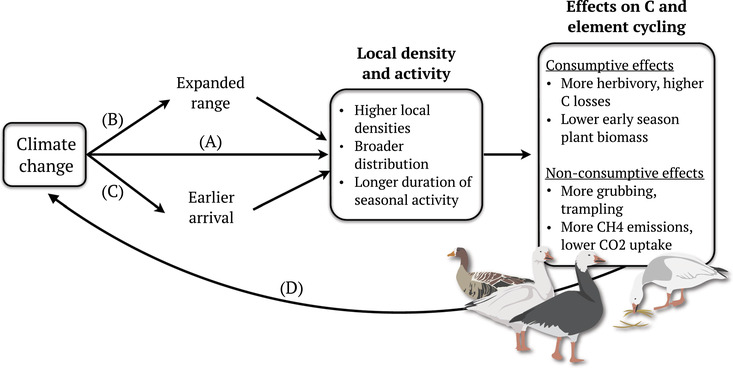
Geese are key arctic herbivores whose densities and activity are changing due to rapid climate change in the Arctic. Changes in the local densities of geese (A), their spatial distribution (B), and timing of activity (C) reflect responses to changing conditions. These responses, in turn, alter the consumptive and nonconsumptive effects of geese on element cycling and can result in feedbacks (D) to climate change.
Changes in species interactions due to climate change may also have unexpected consequences for element dynamics. Differential responses to climate change could decouple interactions among consumers and higher trophic levels, cause trophic mismatches, and alter existing trophic cascades. For example, recent experimental work showed that warming can reverse the indirect effects of spiders on decomposition due to changes in invertebrate predator–prey dynamics. 25 Although similar examples for herbivores are limited, substantial indirect evidence suggests that changes in species interactions will modify the strength of herbivore impacts on nutrient cycling. For instance, temperature increases have been associated with higher predation intensity across the Arctic, suggesting that climatic warming may cause a switch from bottom‐up to top‐down regulation of herbivore populations in some areas. 155 Greater predation pressure associated with warming could thus reduce overall herbivore activity and impacts on element cycling. The density, activity, and impacts of some large mammals may also be reduced as the prevalence of various parasites, diseases, and insect harassment increases in a warming Arctic. 38 , 114 , 148 , 156
In other cases, changes in species interactions could weaken existing trophic cascades or change them altogether. Due to a combination of changing precipitation regimes 157 and increased black fly attacks, 158 some avian predators have experienced reduced nesting success. Weakened predation pressure by these groups could lead to higher densities of small mammal prey, thereby strengthening local herbivore consumptive and nonconsumptive effects. As nonarctic large mammals, such as moose and white‐tailed deer, expand northward, exposure to novel pathogens 114 could negatively impact arctic herbivore populations and reduce their overall ecosystem impacts. Finally, species‐specific responses to climate change could alter competitive interactions that result in changes to element cycling. For example, climate‐driven reductions in lemming populations indirectly reduce geese effects on local element dynamics, because when lemming abundances are low, geese experience higher predation pressure and reduced breeding success 159 (Figure 4).
Geese case study
In the following section, we use geese to provide examples of how climate‐driven changes in spatial and temporal variation in herbivore activity could have cascading effects on element dynamics. Geese make an excellent case study group, because climate‐driven changes in their densities (Figure 4A), distribution (Figure 4B), and activity timing (Figure 4C) are already having measurable impacts on tundra ecosystems.
Populations of several geese species are increasing due to multiple interacting drivers in their wintering grounds and summer breeding areas (Table 1). For example, hunting bans and agricultural change have created more favorable conditions in their wintering grounds. 160 , 161 Progressively earlier spring onset and earlier goose arrival to arctic breeding grounds 138 , 141 have also contributed to population increases. In particular, the combination of advanced goose phenology and higher food availability during the early spring nesting period has led to earlier hatching dates, 162 higher egg production, and reports of improved hatchling success. 163 , 164 These responses have resulted in increased geese densities and more intense early season activity (Figure 4). Geese are also progressively impacting larger areas of tundra; changing climatic conditions are facilitating range expansion 137 and geese are expanding into new areas as habitats become degraded due to high population densities. 136
More geese translates to higher consumption of plant material 42 and increased intensity of nonconsumptive activities, such as grubbing 41 and trampling. 72 The dramatic increases in geese populations have caused proportional increases in geese impacts on soil C and N stocks. 43 Notably, earlier grazing by geese on their breeding grounds can have even larger effects on ecosystem C‐fluxes 11 , 26 and soil N 165 than the earlier start to the growing season. For example, higher consumption of plants early in the season reduces overall plant biomass and decreases overall seasonal CO2 uptake by the plant community. 11 , 166 Early season feeding by geese can also reduce vegetation height and cause higher soil respiration. 26 Effects of geese activity have even been found to amplify warming‐associated increases in methane emissions from coastal wetlands, 11 which could potentially cause feedbacks to climate change (Figure 4D).
HERBIVORE‐DRIVEN CHANGES IN ECOSYSTEM RESPONSES TO CLIMATE CHANGE
Climate change has different effects on tundra biogeochemistry depending on whether herbivores are present or not. Specifically, mounting evidence suggests that herbivore activity can dampen or exacerbate existing plant and soil responses to climate change (e.g., Ref. 167) and even have unexpected feedbacks to climate change. For example, by reducing shrub growth, 23 , 83 herbivores ranging from small to large mammals have been found to counteract the effects of climate‐induced shrub expansion in the Arctic. 20 , 168 , 169 Herbivores may also contribute to the resilience of shrub‐dominated systems to warming; in one case, after long‐term, experimental warming treatments were discontinued, warming‐induced shrub growth was reduced in the presence of herbivores but continued in areas where they were absent. 170 Extreme outbreaks of insect herbivores also dramatically reduce standing plant biomass and have lasting impacts on nutrient cycling. 47 , 52 Experimental work suggests that tundra ecosystems may have reduced resilience to such outbreaks under future climate change. 171 Plant community responses to climate‐associated increased nutrient availability 31 may also be amplified by small mammal activity. For example, herbivory was found to reverse the impacts of warming on plant diversity and to modify the combined effects of fertilization and warming. 172
In addition to influencing plant community responses, herbivores have been shown to mediate plant responses to climate change at the individual level. Plants exposed to both long‐term warming and mimicked moth herbivory emitted far higher levels of VOCs than those exposed to either warming or herbivory in isolation, 94 suggesting that invertebrate herbivory will exacerbate plant VOC emissions as the Arctic warms.
Direct effects of climate change on soil C and nutrients are also mediated by herbivore activity. Depending on whether areas have been grazed or not and the associated changes in soil microclimate, increasing nutrient availability had differential impacts on soil microbial cycling of C and N. 173 Grazing geese were found to exacerbate warming‐associated increases in methane emissions in coastal wetlands as well. 26 Reindeer grazing history can also alter the composition of soil microbial communities 90 , 174 and influence microbial responses to global change drivers, such as warming and nutrient availability. 175 Although evidence is limited, these examples suggest that changes in localized herbivore impacts could modify direct effects of climate change on element cycling.
FUTURE RESEARCH PRIORITIES AND SUGGESTED APPROACHES
Significant gaps remain in our understanding of how climate change is affecting herbivore impacts on element dynamics in the Arctic. Research over the past several decades has primarily focused on consumptive impacts of herbivores, while impacts of nonconsumptive effects have been less studied. Existing evidence is also biased toward vertebrates and particular geographic areas and thus does not represent the full range of ecological contexts or climatic changes experienced by herbivores across the Arctic. 176 In addition, much of our knowledge of consumer impacts on element cycling in the tundra remains limited to C. Multiple arctic ecosystems are suggested to be N‐limited or co‐limited by N and P, while others are more P‐limited. 177 , 178 , 179 Long‐term Rangifer grazing has been found to shift whole plant communities from being N‐limited to P‐limited. 180 Work from the boreal forest also suggests that even in unproductive ecosystems, invertebrate herbivore‐mediated N and P fluxes to the soil can be comparable to those from plant litterfall 181 (but see Ref. 182). Better knowledge of how herbivores contribute to the dynamics of these understudied nutrients will be increasingly important under changing climate scenarios.
As described earlier, climate change is affecting herbivore activity—and, therefore, impacts—both spatially and temporally. Herbivore responses to climate change reflect combinations of reactions to changing environmental conditions and interactions with other species, which can result in indirect effects of climate that are often challenging to quantify. 6 , 110 , 144 , 183 Further complicating matters is that both responses to climate change and subsequent effects on nutrient cycling are likely to be context‐dependent and vary with environmental conditions across the heterogeneous arctic landscape. For example, while warming is expected to lead to higher rates of invertebrate herbivory due to higher abundances (Table 1), predicted increases in precipitation may reduce these impacts in some areas. 184 In many cases, there is not even consistency in climate change responses among closely related species at the same location, 144 which limits our ability to generalize across groups. In addition, while functional diversity of arctic animals varies regionally, 185 most of our knowledge about species responses to climate change comes from just a few places that are not representative of the full suite of environmental conditions across the Arctic. 186 , 187 Impacts of large herbivores, such as Rangifer and some predators (e.g., wolves and red foxes), may also vary depending on management regimes. As effects of climate change continue to manifest differently in different parts of the Arctic, predicting species—and food web—effects on element dynamics will depend on our ability to characterize these context dependencies.
A key component of this is improving our understanding of the temporal drivers of herbivore abundance and activity. Data from outside the summer season are especially lacking given the expectation that changing fall, winter, and spring conditions 188 will have widespread effects on population dynamics of both year‐round 124 and summer‐active animals. 144 , 145 As snowmelt occurs earlier and growing seasons become longer, the duration of activity time for summer‐active animals will increase. These changes will continue to modify seasonal food web composition 189 and subsequent ecosystem impacts. 11 , 165 Some groups, such as voles and muskox, feed year‐round, but their winter activities are understudied. Repeated field sampling across multiple years in more locations is necessary to better understand long‐term trends and the subsequent ecosystem consequences. This is especially important in efforts to differentiate indirect versus direct effects of climate change and to capture the overall temporal trends that can be inconsistent due to the many interacting factors driving consumer populations in the Arctic (e.g., Refs. 144 and 190).
In addition, intra‐ and interannual patterns in herbivore activity are disrupted by the increasing frequency of acute disturbances in the Arctic. These disturbances are not well understood or easily predicted, but they are likely to cause short‐ and long‐term changes to species interactions and localized animal impacts. 191 For example, the frequency and intensity of tundra fires are increasing 192 and may have significant effects on short‐ or even long‐term herbivore densities. 193 More frequent rain on snow events may also reduce herbivore impacts on element cycling by limiting grazer access to vegetation and dampening small mammal population peaks. 124 , 125 In all cases, there is potential for mismatches in the timing of species interactions, the result of which could have cascading effects on element cycling.
Lastly, while vertebrate herbivore impacts are fairly well characterized, those of other trophic levels remain largely unexplored. Estimating the overall food web‐driven effects on ecosystem‐level processes will require the collection of standardized biomass estimates across functional and trophic groups, which to our knowledge has not been conducted for all vertebrate and invertebrate components of the arctic food web in any location. Although the impacts of their activities may not be as immediately apparent as those of herbivores, predators can change the heterogeneity of element distribution across the landscape even when abundances are low. 95 , 194 For example, denning activities and waste production by red foxes near the Arctic treeline caused an increase in localized soil N, soil P, and plant richness. 195 Brown bear predation on salmon in SW Alaska also has transient effects on soil nutrients through the transfer of marine‐derived nutrients to riparian habitats. 196 Detritivores are important drivers of decomposition and nutrient cycling 9 , 197 that could indirectly regulate soil biogeochemistry via changes in microbial activity 65 , 76 , 198 as well. It is unclear whether direct and indirect effects of organisms within other trophic levels are detectable at the regional scale. However, this is an area that warrants further investigation, particularly as these groups continue to respond in variable ways to climate change (Table 1).
Many of the knowledge gaps discussed above can be approached using distributed experiments, models, remote sensing, meta‐analysis, and traditional ecological knowledge. In almost all cases, interdisciplinary teams of scientists and arctic residents are needed to incorporate data from multiple scales, disciplines, and techniques. Given that field research in the Arctic is expensive and logistically difficult, these approaches can help guide targeted field campaigns to maximize the use of existing resources, data, and infrastructure. In addition, broader use and dissemination of data could engage more scientists, students, and the general public in arctic research.
Some of the issues associated with scaling from local to regional scales are being addressed using distributed experiments and meta‐analysis techniques. Collaborative, coordinated networks of field scientists can enable researchers to overcome some logistical challenges and extrapolate results to more locations. Such an approach has proven particularly successful in the Arctic for groups such as the International Tundra Experiment, ShrubHub, the Herbivory Network, and the Network for Arthropods of the Tundra. The Herbivory Network recently published standardized protocols for characterizing herbivore activity at three different spatial scales in the tundra, 13 as well as an evaluation of where herbivore effects on plants have been measured. 176 These efforts will hopefully spur additional measurements that can be compared across spatial scales within and among study areas to better characterize herbivore impacts. Organizations like INTERACT also foster these collaborations. However, even with the growth of these collaborative approaches, establishing and securing additional adaptive long‐term monitoring programs (e.g., COAT in Norway) and funding continues to be extremely important in the Arctic. Ensuring that field data from published studies and long‐term research sites (e.g., NEON and Long‐Term Ecological Research Network sites in the United States or BioBasis Programme locations in Greenland) are continually made publicly available is also an important means to foster meta‐analytic approaches and provide opportunities for scientists unable to physically travel to the Arctic to contribute to this work.
Pronounced heterogeneity in animal and plant responses to climate change has also demonstrated the need to incorporate measurements at multiple spatial scales. 199 Increased use of integrated satellite and plot‐level remote sensing data is a promising approach to help quantify and better understand this heterogeneity. For example, technological advances in remote sensing and unmanned aerial vehicles are increasingly being used in the Arctic to track animal movements to gain a better understanding of migration patterns, habitat use, and impacts. 28 , 40 , 48 , 117 , 200 New approaches are also being used to quantify insect abundances, interactions, and ecosystem impacts on finer spatial and temporal scales. 201 , 202 Further incorporation of the presence and movement of consumers across trophic levels with plant and soil measures made at various scales could elucidate some of the outstanding questions related to spatial and temporal variation in their impacts.
Another critical approach to better understand the role of consumers in the Arctic is to incorporate animal effects into existing biogeochemical models. There are multiple nutrient cycling models calibrated for different types of tundra (e.g., TEM, 203 GEM, 204 and MEL 205 ) at different scales. By incorporating consumptive and nonconsumptive interactions (Figure 3) into these models, they can test hypotheses about the various roles of animals in arctic ecosystems. A recent example of this illustrated how a biogeochemical model can underestimate tundra ecosystem responses to increased temperature and CO2 if grazing effects by small mammals are not considered explicitly. 206 Model development and calibration phases can then be used to prioritize targeted field campaigns to gather additional data to improve model fit and understand how model predictions differ across tundra types and locations.
Finally, incorporation of multiple knowledge types, including local, traditional, and scientific knowledge (e.g., Ref. 207), would contribute to building a richer understanding of the historical role of herbivores and other animals within arctic ecosystems and how their impacts are changing over time. Meaningful engagement of Indigenous Peoples and the codevelopment of new arctic research has been the focus of several recent workshops in the United States and elsewhere as the scientific community increasingly recognizes the value of traditional knowledge.
CONCLUSION
Herbivores are not typically considered in models of element cycling for the Arctic, but the findings from the work reviewed here suggest this should be revisited. Through their consumptive and nonconsumptive effects, herbivores influence local C stocks and nutrient availability and can even move elements across large geographic areas. The extent to which herbivore impacts are important at larger spatial scales or how they may influence regional element cycling is unknown but an important area of ongoing research. As the Arctic continues to change, understanding the shifting roles of terrestrial consumers in ecosystem functioning will become increasingly critical. Given that the Arctic plays a disproportionate role in global biogeochemistry, these changes could have meaningful ecological consequences. We suggest that (1) additional long‐term monitoring of animal populations, including during fall, winter, and spring; (2) incorporating herbivores and other consumers into ecosystem models; (3) using existing research infrastructure and methods in new collaborative ways; and (4) incorporating traditional ecological knowledge and local expertise will help address some of these critical knowledge gaps.
AUTHOR CONTRIBUTIONS
All authors contributed to the writing and revising of the manuscript and approved the submitted version.
COMPETING INTERESTS
The authors have no competing interests to disclose.
ACKNOWLEDGMENTS
We thank Austin Roy and Nicole Williamson for assistance with this manuscript and two anonymous reviewers who provided helpful feedback on previous versions. Figure 1 and the herbivore illustrations in Figures 2, 3, 4 were created by Elise Imbeau. Figure 3 was modified from a figure by Ed Rastetter. This work was supported by funding to L.G. (DEB 1637459 and OPP 1603760) and J.R.M. (OPP 1603677) from the National Science Foundation.
Koltz, A. M. , Gough, L. , & McLaren, J. R. (2022). Herbivores in Arctic ecosystems: Effects of climate change and implications for carbon and nutrient cycling. Ann NY Acad Sci., 1516, 28–47. 10.1111/nyas.14863
REFERENCES
- 1. Summerhayes, V. S. , & Elton, C. S. (1923). Contributions to the ecology of Spitsbergen and Bear Island. Journal of Ecology, 11, 214–286. [Google Scholar]
- 2. Elton, C. (1927). Animal ecology. Sidgwick & Jackson. [Google Scholar]
- 3. Shaver, G. R. , Billings, W. D. , Stuart Chapin, F. , Giblin, A. E. , Nadelhoffer, K. J. , Oechel, W. C. , Rastetter, E. B. , & Chapin, F. S. (1992). Global change and the carbon balance of arctic ecosystems. Bioscience, 42, 433–441. [Google Scholar]
- 4. Wirta, H. K. , Vesterinen, E. J. , Hambäck, P. A. , Weingartner, E. , Rasmussen, C. , Reneerkens, J. , Schmidt, N. M. , Gilg, O. , & Roslin, T. (2015). Exposing the structure of an Arctic food web. Ecology and Evolution, 5, 3842–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hodkinson, I. D. , & Coulson, S. J. (2004). Are high Arctic terrestrial food chains really that simple?–The Bear Island food web revisited. Oikos, 106, 427–431. [Google Scholar]
- 6. Abrego, N. , Roslin, T. , Huotari, T. , Ji, Y. , Schmidt, N. M. , Wang, J. , Yu, D. W. , & Ovaskainen, O. (2021). Accounting for species interactions is necessary for predicting how arctic arthropod communities respond to climate change. Ecography, 44, 885–896. [Google Scholar]
- 7. Hodkinson, I. D. , Webb, N. R. , & Coulson, S. J. (2002). Primary community assembly on land–The missing stages: Why are the heterotrophic organisms always there first? Journal of Ecology, 90, 569–577. [Google Scholar]
- 8. Schmitz, O. J. , & Leroux, S. J. (2020). Food webs and ecosystems: Linking species interactions to the carbon cycle. Annual Review of Ecology, Evolution, and Systematics, 51, 271–295. [Google Scholar]
- 9. Mccary, M. A. , & Schmitz, O. J. (2021). Invertebrate functional traits and terrestrial nutrient cycling: Insights from a global meta‐analysis. Journal of Animal Ecology, 90, 1714–1726. [DOI] [PubMed] [Google Scholar]
- 10. Rheubottom, S. I. , Barrio, I. C. , Kozlov, M. V. , Alatalo, J. M. , Andersson, T. , Asmus, A. L. , Baubin, C. , Brearley, F. Q. , Egelkraut, D. D. , Ehrich, D. , Gauthier, G. , Svala Jónsdóttir, I. , Konieczka, S. , Lévesque, E. , Olofsson, J. , Prevéy, J. S. , Slevan‐Tremblay, G. , Sokolov, A. , Sokolova, N. , … Hik, D. S. (2019). Hiding in the background: Community‐level patterns in invertebrate herbivory across the tundra biome. Polar Biology, 42, 1881–1897. [Google Scholar]
- 11. Leffler, A. J. , Beard, K. H. , Kelsey, K. C. , Choi, R. T. , Schmutz, J. A. , & Welker, J. M. (2019). Delayed herbivory by migratory geese increases summer‐long CO2 uptake in coastal western Alaska. Global Change Biology, 25, 277–289. [DOI] [PubMed] [Google Scholar]
- 12. Tuomi, M. , Väisänen, M. , Ylänne, H. , Brearley, F. Q. , Barrio, I. C. , Bråthen, K. A. , Eischeid, I. , Forbes, B. C. , Jónsdóttir, I. S. , Kolstad, A. L. , Macek, P. , Bon, M. P. , Speed, J. D. M. , Stark, S. , Svavarsdóttir, K. , Thórsson, J. , & Bueno, C. G. (2021). Stomping in silence: Conceptualizing trampling effects on soils in polar tundra. Functional Ecology, 35, 306–317. [Google Scholar]
- 13. Barrio, I. C. , Ehrich, D. , Soininen, E. M. , Ravolainen, V. T. , Bueno, C. G. , Gilg, O. , Koltz, A. M. , Speed, J. D. M. , Hik, D. S. , Mörsdorf, M. , Alatalo, J. M. , Angerbjörn, A. , Bêty, J. , Bollache, L. , Boulanger‐Lapointe, N. , Brown, G. S. , Eischeid, I. , Giroux, M. A. , Hájek, T. , … Jónsdóttir, I. S. (2021). Developing common protocols to measure tundra herbivory across spatial scales. Arctic Science, 10.1139/as-2020-0020 [DOI] [Google Scholar]
- 14. IPCC . (2013). Climate change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. In Stocker, T. F. , Qin, D. , Plattner, G.‐K. , Tignor, M. , Allen, S. K. , Boschung, J. , Nauels, A. , Xia, Y. , Bex, V. , & Midgley, P. M. (Eds.) Cambridge, UK and New York: Cambridge University Press, 1535 pp. [Google Scholar]
- 15. Prevéy, J. , Vellend, M. , Rüger, N. , Hollister, R. D. , Bjorkman, A. D. , Myers‐Smith, I. H. , Elmendorf, S. C. , Clark, K. , Cooper, E. J. , Elberling, B. O. , Fosaa, A. M. , Henry, G. H. R. , Høye, T. T. , Jónsdóttir, I. S. , Klanderud, K. , Lévesque, E. , Mauritz, M. , Molau, U. , Natali, S. M. , … Rixen, C. (2017). Greater temperature sensitivity of plant phenology at colder sites: Implications for convergence across northern latitudes. Global Change Biology, 23, 2660–2671. [DOI] [PubMed] [Google Scholar]
- 16. Post, E. , Alley, R. B. , Christensen, T. R. , Macias‐Fauria, M. , Forbes, B. C. , Gooseff, M. N. , Iler, A. , Kerby, J. T. , Laidre, K. L. , Mann, M. E. , Olofsson, J. , Stroeve, J. C. , Ulmer, F. , Virginia, R. A. , & Wang, M. (2019). The polar regions in a 2 C warmer world. Science Advances, 5, eaaw9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Box, J. E. , Colgan, W. T. , Christensen, T. R. , Schmidt, N. M. , Lund, M. , Parmentier, F.‐J. W. , Brown, R. , Bhatt, U. S. , Euskirchen, E. S. , Romanovsky, V. E. , Walsh, J. E. , Overland, J. E. , Wang, M. , Corell, R. W. , Meier, W. N. , Wouters, B. , Mernild, S. , Mård, J. , Pawlak, J. , & Olsen, M. S. (2019). Key indicators of Arctic climate change: 1971–2017. Environmental Research Letters, 14, 045010. [Google Scholar]
- 18. Schuur, E. A. G. , Mcguire, A. D. , Schädel, C. , Grosse, G. , Harden, J. W. , Hayes, D. J. , Hugelius, G. , Koven, C. D. , Kuhry, P. , Lawrence, D. M. , Natali, S. M. , Olefeldt, D. , Romanovsky, V. E. , Schaefer, K. , Turetsky, M. R. , Treat, C. C. , & Vonk, J. E. (2015). Climate change and the permafrost carbon feedback. Nature, 520, 171–179. [DOI] [PubMed] [Google Scholar]
- 19. Myers‐Smith, I. H. , Kerby, J. T. , Phoenix, G. K. , Bjerke, J. W. , Epstein, H. E. , Assmann, J. J. , John, C. , Andreu‐Hayles, L. , Angers‐Blondin, S. , Beck, P. S. A. , Berner, L. T. , Bhatt, U. S. , Bjorkman, A. D. , Blok, D. , Bryn, A. , Christiansen, C. T. , Cornelissen, J. H. C. , Cunliffe, A. M. , Elmendorf, S. C. , … & Wipf, S. (2020). Complexity revealed in the greening of the Arctic. Nature Climate Change, 10, 106–117. [Google Scholar]
- 20. Myers‐Smith, I. H. , Forbes, B. C. , Wilmking, M. , Hallinger, M. , Lantz, T. , Blok, D. , Tape, K. D. , Macias‐Fauria, M. , Sass‐Klaassen, U. , Lévesque, E. , Boudreau, S. , Ropars, P. , Hermanutz, L. , Trant, A. , Collier, L. S. , Weijers, S. , Rozema, J. , Rayback, S. A. , Schmidt, N. M. , … Hik, D. S. (2011). Shrub expansion in tundra ecosystems: Dynamics, impacts and research priorities. Environmental Research Letters, 6, 045509. [Google Scholar]
- 21. Elmendorf, S. C. , Henry, G. H. R. , Hollister, R. D. , Björk, R. G. , Boulanger‐Lapointe, N. , Cooper, E. J. , Cornelissen, J. H. C. , Day, T. A. , Dorrepaal, E. , Elumeeva, T. G. , Gill, M. , Gould, W. A. , Harte, J. , Hik, D. S. , Hofgaard, A. , Johnson, D. R. , Johnstone, J. F. , Jónsdóttir, I. S. , Jorgenson, J. C. , … & Wipf, S. (2012). Plot‐scale evidence of tundra vegetation change and links to recent summer warming. Nature Climate Change, 2, 453–457. [Google Scholar]
- 22. Syndonia Bret‐Harte, M. , Shaver, G. R. , & Stuart Chapin, F. (2002). Primary and secondary stem growth in arctic shrubs: Implications for community response to environmental change. Journal of Ecology, 90, 251–267. [Google Scholar]
- 23. Christie, K. S. , Bryant, J. P. , Gough, L. , Ravolainen, V. T. , Ruess, R. W. , & Tape, K. D. (2015). The role of vertebrate herbivores in regulating shrub expansion in the arctic: A synthesis. Bioscience, 65, 1123–1133. [Google Scholar]
- 24. Cirtwill, A. R. , Roslin, T. , Rasmussen, C. , Olesen, J. M. , & Stouffer, D. B. (2018). Between‐year changes in community composition shape species’ roles in an Arctic plant–pollinator network. Oikos, 127, 1163–1176. [Google Scholar]
- 25. Koltz, A. M. , Classen, A. T. , & Wright, J. P. (2018). Warming reverses top‐down effects of predators on belowground ecosystem function in Arctic tundra. Proceedings of the National Academy of Sciences, 115, E7541–E7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelsey, K. C. , Leffler, A. J. , Beard, K. H. , Choi, R. T. , Schmutz, J. A. , & Welker, J. M. (2018). Phenological mismatch in coastal western Alaska may increase summer season greenhouse gas uptake. Environmental Research Letters, 13, 044032. [Google Scholar]
- 27. Kéfi, S. , Berlow, E. L. , Wieters, E. A. , Navarrete, S. A. , Petchey, O. L. , Wood, S. A. , Boit, A. , Joppa, L. N. , Lafferty, K. D. , Williams, R. J. , Martinez, N. D. , Menge, B. A. , Blanchette, C. A. , Iles, A. C. , & Brose, U. (2012). More than a meal… integrating non‐feeding interactions into food webs. Ecology Letters, 15, 291–300. [DOI] [PubMed] [Google Scholar]
- 28. Olofsson, J. , Tømmervik, H. , & Callaghan, T. V. (2012). Vole and lemming activity observed from space. Nature Climate Change, 2, 880–883. [Google Scholar]
- 29. Batzli, G. O. , White, R. G. , MacLean, Jr. S. F. , Pitelka, F. A. , & Collier, B. D. (1980). The herbivore‐based trophic system. In Brown, P. C. M. J. , Tieszen, L. L. , & Bunnell, F. L. (Eds.) An arctic ecosystem: The coastal tundra at Barrow, Alaska (pp. 335–410). Stroudsburg, PA: Dowden, Hutchinson & Ross, Inc. [Google Scholar]
- 30. Smith, P. A. , Mckinnon, L. , Meltofte, H. , Lanctot, R. B. , Fox, A. D. , Leafloor, J. O. , Soloviev, M. , Franke, A. , Falk, K. , Golovatin, M. , Sokolov, V. , Sokolov, A. , & Smith, A. C. (2020). Status and trends of tundra birds across the circumpolar Arctic. Ambio, 49, 732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gough, L. , Moore, J. C. , Shaver, G. R. , Simpson, R. T. , & Johnson, D. R. (2012). Above‐ and belowground responses of arctic tundra ecosystems to altered soil nutrients and mammalian herbivory. Ecology, 93, 1683–1694. [DOI] [PubMed] [Google Scholar]
- 32. Johnson, D. R. , Lara, M. J. , Shaver, G. R. , Batzli, G. O. , Shaw, J. D. , & Tweedie, C. E. (2011). Exclusion of brown lemmings reduces vascular plant cover and biomass in Arctic coastal tundra: Resampling of a 50+ year herbivore exclosure experiment near Barrow, Alaska. Environmental Research Letters, 6, 045507. [Google Scholar]
- 33. Virtanen, R. (2000). Effects of grazing on above‐ground biomass on a mountain snowbed, NW Finland. Oikos, 90, 295–300. [Google Scholar]
- 34. Olofsson, J. , Stark, S. , & Oksanen, L. (2004). Reindeer influence on ecosystem processes in the tundra. Oikos, 105, 386–396. [Google Scholar]
- 35. Bernes, C. , Bråthen, K. A. , Forbes, B. C. , Speed, J. D. M. , & Moen, J. (2015). What are the impacts of reindeer/caribou (Rangifer tarandus L.) on arctic and alpine vegetation? A systematic review. Environmental Evidence, 4, 4. [Google Scholar]
- 36. Campeau, A. B. , Rickbeil, G. J. M. , Coops, N. C. , & Côté, S. D. (2019). Long‐term changes in the primary productivity of migratory caribou (Rangifer tarandus) calving grounds and summer pasture on the Quebec‐Labrador Peninsula (Northeastern Canada): The mixed influences of climate change and caribou herbivory. Polar Biology, 42, 1005–1023. [Google Scholar]
- 37. Nicholson, K. L. , Arthur, S. M. , Horne, J. S. , Garton, E. O. , & Del Vecchio, P. A. (2016). Modeling caribou movements: Seasonal ranges and migration routes of the central Arctic herd. PLoS One, 11, e0150333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cuyler, C. , Rowell, J. , Adamczewski, J. , Anderson, M. , Blake, J. , Bretten, T. , Brodeur, V. , Campbell, M. , Checkley, S. L. , Cluff, H. D. , Côté, S. D. , Davison, T. , Dumond, M. , Ford, B. , Gruzdev, A. , Gunn, A. , Jones, P. , Kutz, S. , Leclerc, L.‐M. , … Ytrehus, B. (2020). Muskox status, recent variation, and uncertain future. Ambio, 49, 805–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lara, M. J. , Johnson, D. R. , Andresen, C. , Hollister, R. D. , & Tweedie, C. E. (2017). Peak season carbon exchange shifts from a sink to a source following 50+ years of herbivore exclusion in an Arctic tundra ecosystem. Journal of Ecology, 105, 122–131. [Google Scholar]
- 40. Siewert, M. B. , & Olofsson, J. (2021). UAV reveals substantial but heterogeneous effects of herbivores on Arctic vegetation. Scientific Reports, 11, 19468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mclaren, J. R. , & Jefferies, R. L. (2004). Initiation and maintenance of vegetation mosaics in an Arctic salt marsh. Journal of Ecology, 92, 648–660. [Google Scholar]
- 42. Cargill, S. M. , & Jefferies, R. L. (1984). The effects of grazing by lesser snow geese on the vegetation of a sub‐arctic salt marsh. Journal of Applied Ecology, 21, 669–686. [Google Scholar]
- 43. Jefferies, R. L. , Jano, A. P. , & Abraham, K. F. (2006). A biotic agent promotes large‐scale catastrophic change in the coastal marshes of Hudson Bay. Journal of Ecology, 94, 234–242. [Google Scholar]
- 44. Barrio, I. C. , Lindén, E. , Beest, M. T. e. , Olofsson, J. , Rocha, A. , Soininen, E. M. , Alatalo, J. M. , Andersson, T. , Asmus, A. , Boike, J. , Bråthen, K. A. , Bryant, J. P. , Buchwal, A. , Guillermo Bueno, C. , Christie, K. S. , Denisova, Y. V. , Egelkraut, D. , Ehrich, D. , Fishback, L. A. , … Kozlov, M. V. (2017). Background invertebrate herbivory on dwarf birch (Betula glandulosa‐nana complex) increases with temperature and precipitation across the tundra biome. Polar Biology, 40, 2265–2278. [Google Scholar]
- 45. Koltz, A. M. , Asmus, A. , Gough, L. , Pressler, Y. , & Moore, J. C. (2018). The detritus‐based microbial‐invertebrate food web contributes disproportionately to carbon and nitrogen cycling in the Arctic. Polar Biology, 41, 1531–1545. [Google Scholar]
- 46. Silfver, T. , Heiskanen, L. , Aurela, M. , Myller, K. , Karhu, K. , Meyer, N. , Tuovinen, J.‐P. , Oksanen, E. , Rousi, M. , & Mikola, J. (2020). Insect herbivory dampens Subarctic birch forest C sink response to warming. Nature Communications, 11, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lund, M. , Raundrup, K. , Westergaard‐Nielsen, A. , López‐Blanco, E. , Nymand, J. , & Aastrup, P. (2017). Larval outbreaks in West Greenland: Instant and subsequent effects on tundra ecosystem productivity and CO2 exchange. Ambio, 46, 26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Prendin, A. L. , Carrer, M. , Karami, M. , Hollesen, J. , Pedersen, N. B. , Pividori, M. , Treier, U. A. , Westergaard‐Nielsen, A. , Elberling, B. O. , & Normand, S. (2020). Immediate and carry‐over effects of insect outbreaks on vegetation growth in West Greenland assessed from cells to satellite. Journal of Biogeography, 47, 87–100. [Google Scholar]
- 49. Sandén, H. , Mayer, M. , Stark, S. , Sandén, T. , Nilsson, L. O. , Jepsen, J. U. , Wäli, P. R. , & Rewald, B. (2020). Moth outbreaks reduce decomposition in subarctic forest soils. Ecosystems, 23, 151–163. [Google Scholar]
- 50. Metcalfe, D. B. , Cherif, M. , Jepsen, J. U. , Vindstad, O. P. L. , Kristensen, J. Å. , & Belsing, U. (2019). Ecological stoichiometry and nutrient partitioning in two insect herbivores responsible for large‐scale forest disturbance in the Fennoscandian subarctic. Ecological Entomology, 44, 118–128. [Google Scholar]
- 51. Vindstad, O. P. L. , Jepsen, J. U. , Ek, M. , Pepi, A. , & Ims, R. A. (2019). Can novel pest outbreaks drive ecosystem transitions in northern‐boreal birch forest? Journal of Ecology, 107, 1141–1153. [Google Scholar]
- 52. Jepsen, J. U. , Hagen, S. B. , Ims, R. A. , & Yoccoz, N. G. (2008). Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: Evidence of a recent outbreak range expansion. Journal of Animal Ecology, 77, 257–264. [DOI] [PubMed] [Google Scholar]
- 53. Bardgett, R. D. , & Wardle, D. A. (2003). Herbivore‐mediated linkages between aboveground and belowground communities. Ecology, 84, 2258–2268. [Google Scholar]
- 54. Sitters, J. , Bakker, E. S. , Veldhuis, M. P. , Veen, G. F. , Venterink, H. O. , & Vanni, M. J. (2017). The stoichiometry of nutrient release by terrestrial herbivores and its ecosystem consequences. Frontiers in Earth Science, 5, 32. [Google Scholar]
- 55. Barthelemy, H. , Stark, S. , Michelsen, A. , & Olofsson, J. (2018). Urine is an important nitrogen source for plants irrespective of vegetation composition in an Arctic tundra: Insights from a 15N‐enriched urea tracer experiment. Journal of Ecology, 106, 367–378. [Google Scholar]
- 56. Li, L. , Zhang, J. , He, X. Z. , & Hou, F. (2021). Different effects of sheep excrement type and supply level on plant and soil C: N: P stoichiometry in a typical steppe on the loess plateau. Plant and Soil, 462, 45–58. [Google Scholar]
- 57. Bazely, D. R. , & Jefferies, R. L. (1985). Goose faeces: A source of nitrogen for plant growth in a grazed salt marsh. Journal of Applied Ecology, 22, 693–703. [Google Scholar]
- 58. Barthelemy, H. , Stark, S. , & Olofsson, J. (2015). Strong responses of subarctic plant communities to long‐term reindeer feces manipulation. Ecosystems, 18, 740–751. [Google Scholar]
- 59. Laiho, R. , Penttilä, T. , & Fritze, H. (2017). Reindeer droppings may increase methane production potential in subarctic wetlands. Soil Biology and Biochemistry, 113, 260–262. [Google Scholar]
- 60. Mckendrick, J. D. , Batzli, G. O. , Everett, K. R. , & Swanson, J. C. (1980). Some effects of mammalian herbivores and fertilization on tundra soils and vegetation. Arctic and Alpine Research, 12, 565–578. [Google Scholar]
- 61. Olofsson, J. (2009). Effects of simulated reindeer grazing, trampling, and waste products on nitrogen mineralization and primary production. Arctic, Antarctic, and Alpine Research, 41, 330–338. [Google Scholar]
- 62. Mosbacher, J. B. , Kristensen, D. K. , Michelsen, A. , Stelvig, M. , & Schmidt, N. M. (2016). Quantifying muskox plant biomass removal and spatial relocation of nitrogen in a high arctic tundra ecosystem. Arctic, Antarctic, and Alpine Research, 48, 229–240. [Google Scholar]
- 63. Batzli, G. O. , Pitelka, F. A. , & Cameron, G. N. (1983). Habitat use by lemmings near Barrow, Alaska. Ecography, 6, 255–262. [Google Scholar]
- 64. Gratton, C. , Hoekman, D. , Dreyer, J. , & Jackson, R. D. (2017). Increased duration of aquatic resource pulse alters community and ecosystem responses in a subarctic plant community. Ecology, 98, 2860–2872. [DOI] [PubMed] [Google Scholar]
- 65. Mccary, M. A. , Kasprzak, M. D. , Botsch, J. C. , Hoekman, D. , Jackson, R. D. , & Gratton, C. (2021). Aquatic insect subsidies influence microbial composition and processing of detritus in near‐shore subarctic heathland. Oikos, 130, 1523–1534. [Google Scholar]
- 66. Egelkraut, D. , Barthelemy, H. , & Olofsson, J. (2020). Reindeer trampling promotes vegetation changes in tundra heathlands: Results from a simulation experiment. Journal of Vegetation Science, 31, 476–486. [Google Scholar]
- 67. Zimov, S. A. , Chuprynin, V. I. , Oreshko, A. P. , Chapin, F. S. , Reynolds, J. F. , & Chapin, M. C. (1995). Steppe‐tundra transition: A herbivore‐driven biome shift at the end of the Pleistocene. American Naturalist, 146, 765–794. [Google Scholar]
- 68. Mosbacher, J. B. , Michelsen, A. , Stelvig, M. , Hjermstad‐Sollerud, H. , & Schmidt, N. M. (2019). Muskoxen modify plant abundance, phenology, and nitrogen dynamics in a high Arctic fen. Ecosystems, 22, 1095–1107. [Google Scholar]
- 69. Heggenes, J. , Odland, A. , Chevalier, T. , Ahlberg, J. , Berg, A. , Larsson, H. , & Bjerketvedt, D. K. (2017). Herbivore grazing—or trampling? Trampling effects by a large ungulate in cold high‐latitude ecosystems. Ecology and Evolution, 7, 6423–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Andriuzzi, W. S. , & Wall, D. H. (2017). Responses of belowground communities to large aboveground herbivores: Meta‐analysis reveals biome‐dependent patterns and critical research gaps. Global Change Biology, 23, 3857–3868. [DOI] [PubMed] [Google Scholar]
- 71. Ilum Sørensen, L. , Mikola, J. , Kytöviita, M.‐M. , & Olofsson, J. (2009). Trampling and spatial heterogeneity explain decomposer abundances in a sub‐arctic grassland subjected to simulated reindeer grazing. Ecosystems, 12, 830–842. [Google Scholar]
- 72. Zacheis, A. , Ruess, R. W. , & Hupp, J. W. (2002). Nitrogen dynamics in an Alaskan salt marsh following spring use by geese. Oecologia, 130, 600–608. [DOI] [PubMed] [Google Scholar]
- 73. Falk, J. M. , Schmidt, N. M. , Christensen, T. R. , & Ström, L. (2015). Large herbivore grazing affects the vegetation structure and greenhouse gas balance in a high arctic mire. Environmental Research Letters, 10, 045001. [Google Scholar]
- 74. Ballová, Z. , Pekárik, L. , Píš, V. , & Šibík, J. (2019). How much do ecosystem engineers contribute to landscape evolution? A case study on Tatra marmots. Catena, 182, 104121. [Google Scholar]
- 75. Coleman, D. C. , & Wall, D. H. (2015). Soil fauna: Occurrence, biodiversity, and roles in ecosystem function. Soil Microbiology, Ecology and Biochemistry, 4, 111–149. [Google Scholar]
- 76. FAO, ITPS, GSBI . State of knowledge of soil biodiversity — Status, challenges and potentialities. Rome: FAO. [Google Scholar]
- 77. Díaz, S. , Lavorel, S. , Mcintyre, S. , Falczuk, V. , Casanoves, F. , Milchunas, D. G. , Skarpe, C. , Rusch, G. , Sternberg, M. , Noy‐Meir, I. , Landsberg, J. , Zhang, W. , Clark, H. , & Campbell, B. D. (2007). Plant trait responses to grazing–A global synthesis. Global Change Biology, 13, 313–341. [Google Scholar]
- 78. Cahoon, S. M. P. , Sullivan, P. F. , Post, E. , & Welker, J. M. (2012). Large herbivores limit CO2 uptake and suppress carbon cycle responses to warming in West Greenland. Global Change Biology, 18, 469–479. [Google Scholar]
- 79. Sjögersten, S. , Wal, R. , Loonen, M. J. J. E. , & Woodin, S. J. (2011). Recovery of ecosystem carbon fluxes and storage from herbivory. Biogeochemistry, 106, 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Roy, A. , Suchocki, M. , Gough, L. , & Mclaren, J. R. (2020). Above‐ and belowground responses to long‐term herbivore exclusion. Arctic, Antarctic, and Alpine Research, 52, 109–119. [Google Scholar]
- 81. Andruko, R. , Danby, R. , & Grogan, P. (2020). Recent growth and expansion of birch shrubs across a low Arctic landscape in continental Canada: Are these responses more a consequence of the severely declining caribou herd than of climate warming? Ecosystems, 23(7), 1362–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Väisänen, M. , Ylänne, H. , Kaarlejärvi, E. , Sjögersten, S. , Olofsson, J. , Crout, N. , & Stark, S. (2014). Consequences of warming on tundra carbon balance determined by reindeer grazing history. Nature Climate Change, 4, 384–388. [Google Scholar]
- 83. Verma, M. , Bühne, H. S. , Lopes, M. , Ehrich, D. , Sokovnina, S. , Hofhuis, S. P. , & Pettorelli, N. (2020). Can reindeer husbandry management slow down the shrubification of the Arctic? Journal of Environmental Management, 267, 110636. [DOI] [PubMed] [Google Scholar]
- 84. Olofsson, J. , Hulme, P. E. , Oksanen, L. , & Suominen, O. (2004). Importance of large and small mammalian herbivores for the plant community structure in the forest tundra ecotone. Oikos, 106, 324–334. [Google Scholar]
- 85. Bråthen, K. A. , Gonzalez, V. T. , & Yoccoz, N. G. (2018). Gatekeepers to the effects of climate warming? Niche construction restricts plant community changes along a temperature gradient. Perspectives in Plant Ecology, Evolution and Systematics, 30, 71–81. [Google Scholar]
- 86. Min, E. , Wilcots, M. E. , Naeem, S. , Gough, L. , Mclaren, J. R. , Rowe, R. J. , Rastetter, E. B. , Boelman, N. T. , & Griffin, K. L. (2021). Herbivore absence can shift dry heath tundra from carbon source to sink during peak growing season. Environmental Research Letters, 16, 024027. [Google Scholar]
- 87. Jessen, M.‐T. , Kaarlejärvi, E. , Olofsson, J. , & Eskelinen, A. (2020). Mammalian herbivory shapes intraspecific trait responses to warmer climate and nutrient enrichment. Global Change Biology, 26, 6742–6752. [DOI] [PubMed] [Google Scholar]
- 88. Tuomi, M. , Stark, S. , Hoset, K. S. , Väisänen, M. , Oksanen, L. , Murguzur, F. J. A. , Tuomisto, H. , Dahlgren, J. , & Bråthen, K. A. (2019). Herbivore effects on ecosystem process rates in a low‐productive system. Ecosystems, 22, 827–843. [Google Scholar]
- 89. Mosbacher, J. B. , Schmidt, N. M. , & Michelsen, A. (2013). Impacts of eriophyoid gall mites on arctic willow in a rapidly changing Arctic. Polar Biology, 36, 1735–1748. [Google Scholar]
- 90. Ylänne, H. , Madsen, R. L. , Castaño, C. , Metcalfe, D. B. , & Clemmensen, K. E. (2021). Reindeer control over subarctic treeline alters soil fungal communities with potential consequences for soil carbon storage. Global Change Biology, 27, 4254–4268. [DOI] [PubMed] [Google Scholar]
- 91. Metcalfe, D. B. , & Olofsson, J. (2015). Distinct impacts of different mammalian herbivore assemblages on arctic tundra CO2 exchange during the peak of the growing season. Oikos, 124, 1632–1638. [Google Scholar]
- 92. Rainer, E. M. , Seppey, C. V. W. , Hammer, C. , Svenning, M. M. , & Tveit, A. T. (2021). The influence of above‐ground herbivory on the response of arctic soil methanotrophs to increasing CH4 concentrations and temperatures. Microorganisms, 9, 2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prendin, A. L. , Carrer, M. , Pedersen, N. B. , Normand, S. , Hollesen, J. , Treier, U. A. , Pividori, M. , & Thygesen, L. G. (2021). Chemical signature of Eurois occulta L. outbreaks in the xylem cell wall of Salix glauca L. in Greenland. Science of the Total Environment, 764, 144607. [DOI] [PubMed] [Google Scholar]
- 94. Li, T. , Holst, T. , Michelsen, A. , & Rinnan, R. (2019). Amplification of plant volatile defence against insect herbivory in a warming Arctic tundra. Nature Plants, 5, 568–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gharajehdaghipour, T. , Roth, J. D. , Fafard, P. M. , & Markham, J. H. (2016). Arctic foxes as ecosystem engineers: Increased soil nutrients lead to increased plant productivity on fox dens. Scientific Reports, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Krebs, C. J. , Danell, K. , Angerbjörn, A. , Agrell, J. , Berteaux, D. , Bråthen, K. A. , Danell, Ö. , Erlinge, S. , Fedorov, V. , Fredga, K. , Hjältén, J. , Högstedt, G. , Jónsdóttir, I. S. , Kenney, A. J. , Kjellén, N. , Nordin, T. , Roininen, H. , Svensson, M. , Tannerfeldt, M. , & Wiklund, C. (2003). Terrestrial trophic dynamics in the Canadian Arctic. Canadian Journal of Zoology, 81, 827–843. [Google Scholar]
- 97. Legagneux, P. , Gauthier, G. , Berteaux, D. , Bêty, J. , Cadieux, M.‐C. , Bilodeau, F. , Bolduc, E. , McKinnon, L. , Tarroux, A. , Therrien, J.‐F. , Morissette, L. , & Krebs, C. J. (2012). Disentangling trophic relationships in a high Arctic tundra ecosystem through food web modeling. Ecology, 93, 1707–1716. [DOI] [PubMed] [Google Scholar]
- 98. Aunapuu, M. , Dahlgren, J. , Oksanen, T. , Grellmann, D. , Oksanen, L. , Olofsson, J. , Rammul, Ü. , Schneider, M. , Johansen, B. , & Hygen, H. O. (2008). Spatial patterns and dynamic responses of arctic food webs corroborate the exploitation ecosystems hypothesis (EEH). American Naturalist, 171, 249–262. [DOI] [PubMed] [Google Scholar]
- 99. Visakorpi, K. , Wirta, H. K. , Ek, M. , Schmidt, N. M. , & Roslin, T. (2015). No detectable trophic cascade in a high‐Arctic arthropod food web. Basic and Applied Ecology, 16, 652–660. [Google Scholar]
- 100. Clermont, J. , Grenier‐Potvin, A. , Duchesne, É. , Couchoux, C. , Dulude‐de Broin, F. , Beardsell, A. , Bêty, J. , & Berteaux, D. (2020). The predator activity landscape predicts the anti‐predator behavior and distribution of prey in a tundra community. Ecosphere, 12(12), e03858. [Google Scholar]
- 101. Fauteux, D. , Stien, A. , Yoccoz, N. G. , Fuglei, E. , & Ims, R. A. (2021). Climate variability and density‐dependent population dynamics: Lessons from a simple high Arctic ecosystem. Proceedings of the National Academy of Sciences, 118, e2106635118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roth, J. D. (2003). Variability in marine resources affects arctic fox population dynamics. Journal of Animal Ecology, 72, 668–676. [DOI] [PubMed] [Google Scholar]
- 103. Macleod, K. J. , Krebs, C. J. , Boonstra, R. , & Sheriff, M. J. (2018). Fear and lethality in snowshoe hares: The deadly effects of non‐consumptive predation risk. Oikos, 127, 375–380. [Google Scholar]
- 104. Gehr, B. , Hofer, E. J. , Ryser, A. , Vimercati, E. , Vogt, K. , & Keller, L. F. (2018). Evidence for nonconsumptive effects from a large predator in an ungulate prey? Behavioral Ecology, 29, 724–735. [Google Scholar]
- 105. Broin, F. D. , Hamel, S. , Mastromonaco, G. F. , & Côté, S. D. (2020). Predation risk and mountain goat reproduction: Evidence for stress‐induced breeding suppression in a wild ungulate. Functional Ecology, 34, 1003–1014. [Google Scholar]
- 106. Koltz, A. M. , Civitello, D. J. , Becker, D. J. , Deem, S. L. , Classen, A. T. , Barton, B. , Brenn‐White, M. , Johnson, Z. E. , Kutz, S. , Malishev, M. , Preston, D. L. , Vannatta, J. T. , Penczykowski, R. M. , & Ezenwa, V. O. (2022). Sublethal effects of parasitism on ruminants can have cascading consequences for ecosystems. Proceedings of the National Academy of Sciences, 119, e2117381119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Witter, L. A. , Johnson, C. J. , Croft, B. , Gunn, A. , & Gillingham, M. P. (2012). Behavioural trade‐offs in response to external stimuli: Time allocation of an Arctic ungulate during varying intensities of harassment by parasitic flies. Journal of Animal Ecology, 81, 284–295. [DOI] [PubMed] [Google Scholar]
- 108. Gurarie, E. , Hebblewhite, M. , Joly, K. , Kelly, A. P. , Adamczewski, J. , Davidson, S. C. , Davison, T. , Gunn, A. , Suitor, M. J. , Fagan, W. F. , & Boelman, N. (2019). Tactical departures and strategic arrivals: Divergent effects of climate and weather on caribou spring migrations. Ecosphere, 10, e02971. [Google Scholar]
- 109. Joly, K. , Couriot, O. , Cameron, M. D. , & Gurarie, E. (2020). Behavioral, physiological, demographic and ecological impacts of hematophagous and endoparasitic insects on an arctic ungulate. Toxins, 12, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Speed, J. D. M. , Chimal‐Ballesteros, J. A. , Martin, M. D. , Barrio, I. C. , Vuorinen, K. E. M. , & Soininen, E. M. (2021). Will borealization of Arctic tundra herbivore communities be driven by climate warming or vegetation change? Global Change Biology, 27, 6568–6577. [DOI] [PubMed] [Google Scholar]
- 111. Barrio, I. C. , Bueno, C. G. , Gartzia, M. , Soininen, E. M. , Christie, K. S. , Speed, J. D. M. , Ravolainen, V. T. , Forbes, B. C. , Gauthier, G. , Horstkotte, T. , Hoset, K. S. , Høye, T. T. , Jónsdóttir, I. S. , Lévesque, E. , Mörsdorf, M. A. , Olofsson, J. , Wookey, P. A. , & Hik, D. S. (2016). Biotic interactions mediate patterns of herbivore diversity in the Arctic. Global Ecology and Biogeography, 25, 1108–1118. [Google Scholar]
- 112. Eikelenboom, M. , Higgins, R. C. , John, C. , Kerby, J. , Forchhammer, M. C. , & Post, E. (2021). Contrasting dynamical responses of sympatric caribou and muskoxen to winter weather and earlier spring green‐up in the Arctic. Food Webs, 27, e00196. [Google Scholar]
- 113. Fauchald, P. , Park, T. , Tømmervik, H. , Myneni, R. , & Hausner, V. H. (2017). Arctic greening from warming promotes declines in caribou populations. Science Advances, 3, e1601365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mallory, C. D. , & Boyce, M. S. (2018). Observed and predicted effects of climate change on Arctic caribou and reindeer. Environmental Reviews, 26, 13–25. [Google Scholar]
- 115. Severson, J. P. , Johnson, H. E. , Arthur, S. M. , Leacock, W. B. , & Suitor, M. J. (2021). Spring phenology drives range shifts in a migratory Arctic ungulate with key implications for the future. Global Change Biology, 27, 4546–4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schmidt, N. M. , Pedersen, S. H. , Mosbacher, J. B. , & Hansen, L. H. (2015). Long‐term patterns of muskox (Ovibos moschatus) demographics in high Arctic Greenland. Polar Biology, 38, 1667–1675. [Google Scholar]
- 117. Davidson, S. C. , Bohrer, G. , Gurarie, E. , LaPoint, S. , Mahoney, P. J. , Boelman, N. T. , Eitel, J. U. H. , Prugh, L. R. , Vierling, L. A. , Grier, J. J. E. , Couriot, O. , Kelly, A. P. , Meddens, A. J. H. , Oliver, R. Y. , Kays, R. , Wikelski, M. , Aarvak, T. , Ackerman, J. T. , Alves, J. A. , … Hebblewhite, M. (2020). Ecological insights from three decades of animal movement tracking across a changing Arctic. Science, 370, 712–715. [DOI] [PubMed] [Google Scholar]
- 118. Mallory, C. D. , Williamson, S. N. , Campbell, M. W. , & Boyce, M. S. (2020). Response of barren‐ground caribou to advancing spring phenology. Oecologia, 192, 837–852. [DOI] [PubMed] [Google Scholar]
- 119. Joly, K. , Dale, B. W. , Collins, W. B. , & Adams, L. G. (2003). Winter habitat use by female caribou in relation to wildland fires in interior Alaska. Canadian Journal of Zoology, 81, 1192–1201. [Google Scholar]
- 120. Bintanja, R. , & Andry, O. (2017). Towards a rain‐dominated Arctic. Nature Climate Change, 7, 263–267. [Google Scholar]
- 121. Putkonen, J. , Grenfell, T. C. , Rennert, K. , Bitz, C. , Jacobson, P. , & Russell, D. (2009). Rain on snow: Little understood killer in the north. EOS, Transactions American Geophysical Union, 90, 221–222. [Google Scholar]
- 122. Elmhagen, B. , Kindberg, J. , Hellström, P. , & Angerbjörn, A. (2015). A boreal invasion in response to climate change? Range shifts and community effects in the borderland between forest and tundra. Ambio, 44, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Vistnes, I. , & Nellemann, C. (2008). The matter of spatial and temporal scales: A review of reindeer and caribou response to human activity. Polar Biology, 31, 399–407. [Google Scholar]
- 124. Kausrud, K. L. , Mysterud, A. , Steen, H. , Vik, J. O. , Østbye, E. , Cazelles, B. , Framstad, E. , Eikeset, A. M. , Mysterud, I. , Solhøy, T. , & Stenseth, N. C. (2008). Linking climate change to lemming cycles. Nature, 456, 93–97. [DOI] [PubMed] [Google Scholar]
- 125. Domine, F. , Gauthier, G. , Vionnet, V. , Fauteux, D. , Dumont, M. , & Barrere, M. (2018). Snow physical properties may be a significant determinant of lemming population dynamics in the high Arctic. Arctic Science, 4, 813–826. [Google Scholar]
- 126. Ott, K. E. , Currier, K. D. , & Branch, E. S. (2012). Monitoring lemming abundance and distribution near Barrow, Alaska. Fairbanks, AK: U.S. Fish & Wildlife Service, Fairbanks Fish and Wildlife Field Office. [Google Scholar]
- 127. Korpela, K. , Delgado, M. , Henttonen, H. , Korpimäki, E. , Koskela, E. , Ovaskainen, O. , Pietiäinen, H. , Sundell, J. , Yoccoz, N. G. , & Huitu, O. (2013). Nonlinear effects of climate on boreal rodent dynamics: Mild winters do not negate high‐amplitude cycles. Global Change Biology, 19, 697–710. [DOI] [PubMed] [Google Scholar]
- 128. Ehrich, D. , Schmidt, N. M. , Gauthier, G. , Alisauskas, R. , Angerbjörn, A. , Clark, K. , Ecke, F. , Eide, N. E. , Framstad, E. , Frandsen, J. , Franke, A. , Gilg, O. , Giroux, M.‐A. , Henttonen, H. , Hörnfeldt, B. , Ims, R. A. , Kataev, G. D. , Kharitonov, S. P. , Killengreen, S. T. , … Solovyeva, D. V. (2020). Documenting lemming population change in the Arctic: Can we detect trends? Ambio, 49, 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Krebs, C. J. , Boonstra, R. , Gilbert, B. S. , Kenney, A. J. , & Boutin, S. (2019). Impact of climate change on the small mammal community of the Yukon boreal forest. Integrative Zoology, 14, 528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Baltensperger, A. P. , & Huettmann, F. (2015). Predicted shifts in small mammal distributions and biodiversity in the altered future environment of Alaska: An open access data and machine learning perspective. PLoS One, 10, e0132054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Batzli, G. O. , & Pitelka, F. A. (1983). Nutritional ecology of microtine rodents: Food habits of lemmings near Barrow, Alaska. Journal of Mammalogy, 64, 648–655. [Google Scholar]
- 132. Predavec, M. , Krebs, C. J. , Danell, K. , & Hyndman, R. (2001). Cycles and synchrony in the collared lemming (Dicrostonyx groenlandicus) in Arctic North America. Oecologia, 126, 216–224. [DOI] [PubMed] [Google Scholar]
- 133. Pitelka, F. A. , & Batzli, G. O. (2007). Population cycles of lemmings near Barrow, Alaska: A historical review. Acta Theriologica, 52, 323–336. [Google Scholar]
- 134. Sheriff, M. J. , Kenagy, G. J. , Richter, M. , Lee, T. , Tøien, Ø. , Kohl, F. , Buck, C. L. , & Barnes, B. M. (2011). Phenological variation in annual timing of hibernation and breeding in nearby populations of Arctic ground squirrels. Proceedings of the Royal Society B: Biological Sciences, 278, 2369–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Krebs, C. J. , Boonstra, R. , & Kenney, A. J. (1995). Population dynamics of the collared lemming and the tundra vole at Pearce Point, Northwest Territories, Canada. Oecologia, 103, 481–489. [DOI] [PubMed] [Google Scholar]
- 136. Aubry, L. M. , Rockwell, R. F. , Cooch, E. G. , Brook, R. W. , Mulder, C. P. H. , & Koons, D. N. (2013). Climate change, phenology, and habitat degradation: Drivers of gosling body condition and juvenile survival in lesser snow geese. Global Change Biology, 19, 149–160. [DOI] [PubMed] [Google Scholar]
- 137. Jensen, R. A. , Madsen, J. , O'connell, M. , Wisz, M. S. , Tømmervik, H. , & Mehlum, F. (2008). Prediction of the distribution of Arctic‐nesting pink‐footed geese under a warmer climate scenario. Global Change Biology, 14, 1–10. [Google Scholar]
- 138. Ross, M. V. , Alisauskas, R. T. , Douglas, D. C. , & Kellett, D. K. (2017). Decadal declines in avian herbivore reproduction: Density‐dependent nutrition and phenological mismatch in the Arctic. Ecology, 98, 1869–1883. [DOI] [PubMed] [Google Scholar]
- 139. Lameris, T. K. , Scholten, I. , Bauer, S. , Cobben, M. M. P. , Ens, B. J. , & Nolet, B. A. (2017). Potential for an Arctic‐breeding migratory bird to adjust spring migration phenology to Arctic amplification. Global Change Biology, 23, 4058–4067. [DOI] [PubMed] [Google Scholar]
- 140. Gauthier, G. , Bêty, J. , Cadieux, M.‐C. , Legagneux, P. , Doiron, M. , Chevallier, C. , Lai, S. , Tarroux, A. , & Berteaux, D. (2013). Long‐term monitoring at multiple trophic levels suggests heterogeneity in responses to climate change in the Canadian Arctic tundra. Philosophical Transactions of the Royal Society B: Biological Sciences, 368, 20120482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Doiron, M. , Gauthier, G. , & Lévesque, E. (2015). Trophic mismatch and its effects on the growth of young in an Arctic herbivore. Global Change Biology, 21, 4364–4376. [DOI] [PubMed] [Google Scholar]
- 142. Høye, T. T. (2020). Arthropods and climate change–Arctic challenges and opportunities. Current Opinion in Insect Science, 41, 40–45. [DOI] [PubMed] [Google Scholar]
- 143. Gillespie, M. A. K. , Alfredsson, M. , Barrio, I. C. , Bowden, J. J. , Convey, P. , Culler, L. E. , Coulson, S. J. , Krogh, P. H. , Koltz, A. M. , Koponen, S. , Loboda, S. , Marusik, Y. , Sandström, J. P. , Sikes, D. S. , & Høye, T. T. (2020). Status and trends of terrestrial arthropod abundance and diversity in the North Atlantic region of the Arctic. Ambio, 49, 718–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Høye, T. T. , Loboda, S. , Koltz, A. M. , Gillespie, M. A. K. , Bowden, J. J. , & Schmidt, N. M. (2021). Nonlinear trends in abundance and diversity and complex responses to climate change in Arctic arthropods. Proceedings of the National Academy of Sciences, 118, e2002557117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Koltz, A. M. , Schmidt, N. M. , & Høye, T. T. (2018). Differential arthropod responses to warming are altering the structure of Arctic communities. Royal Society Open Science, 5, 171503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Loboda, S. , Savage, J. , Buddle, C. M. , Schmidt, N. M. , & Høye, T. T. (2018). Declining diversity and abundance of High Arctic fly assemblages over two decades of rapid climate warming. Ecography, 41, 265–277. [Google Scholar]
- 147. Jepsen, J. U. , Kapari, L. , Hagen, S. B. , Schott, T. , Vindstad, O. P. L. , Nilssen, A. C. , & Ims, R. A. (2011). Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub‐Arctic birch. Global Change Biology, 17, 2071–2083. [Google Scholar]
- 148. Koltz, A. M. , & Culler, L. E. (2021). Biting insects in a rapidly changing Arctic. Current Opinion in Insect Science, 47, 75–81. [DOI] [PubMed] [Google Scholar]
- 149. Hodkinson, I. , Babenko, A. , Behan‐Pelletier, V. , Böcher, J. , Boxshall, G. , Brodo, F. , Coulson, S. J. , De Smet, W. , Dózsa‐Farkas, K. , Elias, S. , Fjellberg, A. , Fochetti, R. , Foottit, R. , Hessen, D. , Hobaek, A. , Holmstrup, M. , Koponen, S. , Liston, A. , Makarova, O. , … Zinovjev, A. (2013). Terrestrial and freshwater invertebrates. In Meltofte, H. , Huntington, H. P. , & Barry, T. (eds.) Arctic biodiversity assessment. Status and trends in Arctic biodiversity (pp. 194–223). [Google Scholar]
- 150. Høye, T. T. , & Forchhammer, M. C. (2008). Phenology of high‐Arctic arthropods: Effects of climate on spatial, seasonal, and inter‐annual variation. Advances in Ecological Research, 40, 299–324. [Google Scholar]
- 151. Sweet, S. K. , Asmus, A. , Rich, M. E. , Wingfield, J. , Gough, L. , & Boelman, N. T. (2015). NDVI as a predictor of canopy arthropod biomass in the Alaskan arctic tundra. Ecological Applications, 25, 779–790. [DOI] [PubMed] [Google Scholar]
- 152. Høye, T. T. , Post, E. , Schmidt, N. M. , Trøjelsgaard, K. , & Forchhammer, M. C. (2013). Shorter flowering seasons and declining abundance of flower visitors in a warmer Arctic. Nature Climate Change, 3, 759–763. [Google Scholar]
- 153. Schmidt, N. M. , Mosbacher, J. B. , Nielsen, P. S. , Rasmussen, C. , Høye, T. T. , & Roslin, T. (2016). An ecological function in crisis? The temporal overlap between plant flowering and pollinator function shrinks as the Arctic warms. Ecography, 39, 1250–1252. [Google Scholar]
- 154. McLaren, J. R. , Darrouzet‐Nardi, A. , Weintraub, M. N. , & Gough, L. (2017). Seasonal patterns of soil nitrogen availability in moist acidic tundra. Arctic Science, 4, 98–109. [Google Scholar]
- 155. Legagneux, P. , Gauthier, G. , Lecomte, N. , Schmidt, N. M. , Reid, D. , Cadieux, M.‐C. , Berteaux, D. , Bêty, J. , Krebs, C. J. , Ims, R. A. , Yoccoz, N. G. , Morrison, R. I. G. , Leroux, S. J. , Loreau, M. , & Gravel, D. (2014). Arctic ecosystem structure and functioning shaped by climate and herbivore body size. Nature Climate Change, 4, 379–383. [Google Scholar]
- 156. Kutz, S. J. , Hoberg, E. P. , Molnár, P. K. , Dobson, A. , & Verocai, G. G. (2014). A walk on the tundra: Host–parasite interactions in an extreme environment. International Journal for Parasitology: Parasites and Wildlife, 3, 198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Robinson, B. G. , Franke, A. , & Derocher, A. E. (2017). Weather‐mediated decline in prey delivery rates causes food‐limitation in a top avian predator. Journal of Avian Biology, 48, 748–758. [Google Scholar]
- 158. Lamarre, V. , Legagneux, P. , Franke, A. , Casajus, N. , Currie, D. C. , Berteaux, D. , & Bêty, J. (2018). Precipitation and ectoparasitism reduce reproductive success in an arctic‐nesting top‐predator. Scientific Reports, 8, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nolet, B. A. , Bauer, S. , Feige, N. , Kokorev, Y. I. , Popov, I. Y. U. , & Ebbinge, B. S. (2013). Faltering lemming cycles reduce productivity and population size of a migratory Arctic goose species. Journal of Animal Ecology, 82, 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Abraham, K. , & Jefferies, R. (1997). High goose populations: Causes, impacts and implications. Arctic ecosystems in peril: Report of the Arctic Goose Habitat Working Group. Arctic Goose Joint Venture Special Publication (pp. 7–72). Washington, DC: US Fish and Wildlife Service. [Google Scholar]
- 161. Fox, A. D. , & Madsen, J. (2017). Threatened species to super‐abundance: The unexpected international implications of successful goose conservation. Ambio, 46, 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Fischer, J. B. , Williams, A. R. , & Stehn, R. A. Nest population size and potential production of geese and spectacled eiders on the Yukon‐Kuskokwim Delta, Alaska, 1985–2016. Unpublished Report. Anchorage, AK: U.S. Fish and Wildlife Service. [Google Scholar]
- 163. Madsen, J. , Jaspers, C. , Tamstorf, M. , Mortensen, C. E. , & Rigét, F. (2011). Long‐term effects of grazing and global warming on the composition and carrying capacity of graminoid marshes for moulting geese in East Greenland. Ambio, 40, 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Layton‐Matthews, K. , Hansen, B. B. , Grøtan, V. , Fuglei, E. , & Loonen, M. J. J. E. (2020). Contrasting consequences of climate change for migratory geese: Predation, density dependence and carryover effects offset benefits of high‐arctic warming. Global Change Biology, 26, 642–657. [DOI] [PubMed] [Google Scholar]
- 165. Choi, R. T. , Beard, K. H. , Kelsey, K. C. , Leffler, A. J. , Schmutz, J. A. , & Welker, J. M. (2020). Early goose arrival increases soil nitrogen availability more than an advancing spring in Coastal Western Alaska. Ecosystems, 23, 1309–1324. [Google Scholar]
- 166. Choi, R. T. , Beard, K. H. , Leffler, A. J. , Kelsey, K. C. , Schmutz, J. A. , & Welker, J. M. (2019). Phenological mismatch between season advancement and migration timing alters Arctic plant traits. Journal of Ecology, 107, 2503–2518. [Google Scholar]
- 167. Olofsson, J. , & Post, E. (2018). Effects of large herbivores on tundra vegetation in a changing climate, and implications for rewilding. Philosophical Transactions of the Royal Society B: Biological Sciences, 373, 20170437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Olofsson, J. , Oksanen, L. , Callaghan, T. , Hulme, P. E. , Oksanen, T. , & Suominen, O. (2009). Herbivores inhibit climate‐driven shrub expansion on the tundra. Global Change Biology, 15, 2681–2693. [Google Scholar]
- 169. Post, E. , & Pedersen, C. (2008). Opposing plant community responses to warming with and without herbivores. Proceedings of the National Academy of Sciences, 105, 12353–12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Kaarlejärvi, E. , Hoset, K. S. , & Olofsson, J. (2015). Mammalian herbivores confer resilience of Arctic shrub‐dominated ecosystems to changing climate. Global Change Biology, 21, 3379–3388. [DOI] [PubMed] [Google Scholar]
- 171. Borg Dahl, M. , Priemé, A. , Brejnrod, A. , Brusvang, P. , Lund, M. , Nymand, J. , Kramshøj, M. , Ro‐Poulsen, H. , & Haugwitz, M. S. (2017). Warming, shading and a moth outbreak reduce tundra carbon sink strength dramatically by changing plant cover and soil microbial activity. Scientific Reports, 7, 16035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kaarlejärvi, E. , Eskelinen, A. , & Olofsson, J. (2013). Herbivory prevents positive responses of lowland plants to warmer and more fertile conditions at high altitudes. Functional Ecology, 27, 1244–1253. [Google Scholar]
- 173. Stark, S. , Männistö, M. K. , Ganzert, L. , Tiirola, M. , & Häggblom, M. M. (2015). Grazing intensity in subarctic tundra affects the temperature adaptation of soil microbial communities. Soil Biology and Biochemistry, 84, 147–157. [Google Scholar]
- 174. Santalahti, M. , Sun, H. , Sietiö, O.‐M. , Köster, K. , Berninger, F. , Laurila, T. , Pumpanen, J. , & Heinonsalo, J. (2018). Reindeer grazing alter soil fungal community structure and litter decomposition related enzyme activities in boreal coniferous forests in Finnish Lapland. Applied Soil Ecology, 132, 74–82. [Google Scholar]
- 175. Ahonen, S. H. K. , Ylänne, H. , Väisänen, M. , Ruotsalainen, A. L. , Männistö, M. K. , Markkola, A. , & Stark, S. (2021). Reindeer grazing history determines the responses of subarctic soil fungal communities to warming and fertilization. New Phytologist, 232, 788–801. [DOI] [PubMed] [Google Scholar]
- 176. Soininen, E. M. , Barrio, I. C. , Bjørkås, R. , Björnsdóttir, K. , Ehrich, D. , Hopping, K. , Kaarlejärvi, E. , Kolstad, A. L. , Abdulmanova, S. , Björk, R. G. , Bueno, C. G. , Eischeid, I. , Higgens, R. F. , Forbey, J. S. , Gignac, C. , Gilg, O. , Herder, M. d. , Holm, H. S. , Hwang, B. C. , … Speed, J. D. M. (2021). Location of studies and evidence of effects of herbivory on Arctic vegetation: A systematic map. Environmental Evidence, 10, 25. [Google Scholar]
- 177. Shaver, G. R. , Laundre, J. A. , Bret‐Harte, M. S. , Chapin, F. S. , Mercado‐Díaz, J. A. , Giblin, A. E. , Gough, L. , Gould, W. A. , Hobbie, S. E. , Kling, G. W. , Mack, M. C. , Moore, J. C. , Nadelhoffer, K. J. , Rastetter, E. B. , & Schimel, J. P. (2014). Terrestrial ecosystems at Toolik Lake, Alaska. In John, E. H. , & George, W. K. (Eds.) Alaska's changing Arctic: Ecological consequences for tundra, streams and lakes (pp. 90–142). New York: Oxford University Press. [Google Scholar]
- 178. Mclaren, J. R. , & Buckeridge, K. M. (2019). Decoupled above‐ and belowground responses to multi‐decadal nitrogen and phosphorus amendments in two tundra ecosystems. Ecosphere, 10, e02735. [Google Scholar]
- 179. Zamin, T. J. , & Grogan, P. (2012). Birch shrub growth in the low Arctic: The relative importance of experimental warming, enhanced nutrient availability, snow depth and caribou exclusion. Environmental Research Letters, 7, 034027. [Google Scholar]
- 180. Sitters, J. , Cherif, M. , Egelkraut, D. , Giesler, R. , & Olofsson, J. (2019). Long‐term heavy reindeer grazing promotes plant phosphorus limitation in arctic tundra. Functional Ecology, 33, 1233–1242. [Google Scholar]
- 181. Metcalfe, D. B. , Crutsinger, G. M. , Kumordzi, B. B. , & Wardle, D. A. (2016). Nutrient fluxes from insect herbivory increase during ecosystem retrogression in boreal forest. Ecology, 97, 124–132. [DOI] [PubMed] [Google Scholar]
- 182. Kristensen, J. A. , Michelsen, A. , & Metcalfe, D. B. (2020). Background insect herbivory increases with local elevation but makes minor contribution to element cycling along natural gradients in the Subarctic. Ecology and Evolution, 10, 11684–11698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Juhasz, C.‐C. , Shipley, B. , Gauthier, G. , Berteaux, D. , & Lecomte, N. (2020). Direct and indirect effects of regional and local climatic factors on trophic interactions in the Arctic tundra. Journal of Animal Ecology, 89, 704–715. [DOI] [PubMed] [Google Scholar]
- 184. Virtanen, R. , Clark, A. T. , Herder, M. , & Roininen, H. (2021). Dynamic effects of insect herbivory and climate on tundra shrub growth: Roles of browsing and ramet age. Journal of Ecology, 109, 1250–1262. [Google Scholar]
- 185. Speed, J. D. M. , Skjelbred, I. Å. , Barrio, I. C. , Martin, M. D. , Berteaux, D. , Bueno, C. G. , Christie, K. S. , Forbes, B. C. , Forbey, J. , Fortin, D. , Grytnes, J.‐A. , Hoset, K. S. , Lecomte, N. , Marteinsdóttir, B. , Mosbacher, J. B. , Pedersen, Å. Ø. , Ravolainen, V. , Rees, E. C. , Skarin, A. , … Soininen, E. M. (2019). Trophic interactions and abiotic factors drive functional and phylogenetic structure of vertebrate herbivore communities across the Arctic tundra biome. Ecography, 42, 1152–1163. [Google Scholar]
- 186. Cameron, E. K. , Sundqvist, M. K. , Keith, S. A. , CaraDonna, P. J. , Mousing, E. A. , Nilsson, K. A. , Metcalfe, D. B. , & Classen, A. T. (2019). Uneven global distribution of food web studies under climate change. Ecosphere, 10, e02645. [Google Scholar]
- 187. Metcalfe, D. B. , Hermans, T. D. , Ahlstrand, J. , Becker, M. , Berggren, M. , Björk, R. G. , Björkman, M. P. , Blok, D. , Chaudhary, N. , Chisholm, C. , Classen, A. T. , Hasselquist, N. J. , Jonsson, M. , Kristensen, J. A. , Kumordzi, B. B. , Lee, H. , Mayor, J. R. , Prevéy, J. , Pantazatou, K. , … Abdi, A. M. (2018). Patchy field sampling biases understanding of climate change impacts across the Arctic. Nature Ecology & Evolution, 2, 1443–1448. [DOI] [PubMed] [Google Scholar]
- 188. Vikhamar‐Schuler, D. , Isaksen, K. , Haugen, J. E. , Tømmervik, H. , Luks, B. , Schuler, T. V. , & Bjerke, J. W. (2016). Changes in winter warming events in the Nordic Arctic Region. Journal of Climate, 29, 6223–6244. [Google Scholar]
- 189. Hutchison, C. , Guichard, F. , Legagneux, P. , Gauthier, G. , Bêty, J. , Berteaux, D. , Fauteux, D. , & Gravel, D. (2020). Seasonal food webs with migrations: Multi‐season models reveal indirect species interactions in the Canadian Arctic tundra. Philosophical Transactions of the Royal Society A, 378, 20190354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Soininen, E. M. , Henden, J.‐A. , Ravolainen, V. T. , Yoccoz, N. G. , Bråthen, K. A. , Killengreen, S. T. , & Ims, R. A. (2018). Transferability of biotic interactions: Temporal consistency of arctic plant–rodent relationships is poor. Ecology and Evolution, 8, 9697–9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Spiller, D. A. , Schoener, T. W. , & Piovia‐Scott, J. (2018). Recovery of food webs following natural physical disturbances. Annals of the New York Academy of Sciences, 1429, 100–117. [DOI] [PubMed] [Google Scholar]
- 192. Hu, F. S. , Higuera, P. E. , Duffy, P. , Chipman, M. L. , Rocha, A. V. , Young, A. M. , Kelly, R. , & Dietze, M. C. (2015). Arctic tundra fires: Natural variability and responses to climate change. Frontiers in Ecology and the Environment, 13, 369–377. [Google Scholar]
- 193. Steketee, J. , Rocha, A. , Gough, L. , Griffin, K. , Klupar, I. , An, R. , Williamson, N. , & Rowe, R. In press. Small herbivores with big impacts: Tundra voles (Microtus oeconomus) alter post‐fire ecosystem dynamics. Ecology, 103(7), e3689. [DOI] [PubMed] [Google Scholar]
- 194. Schmitz, O. J. , Hawlena, D. , & Trussell, G. C. (2010). Predator control of ecosystem nutrient dynamics. Ecology Letters, 13, 1199–1209. [DOI] [PubMed] [Google Scholar]
- 195. Lang, J. A. , Roth, J. D. , & Markham, J. H. (2021). Foxes fertilize the subarctic forest and modify vegetation through denning. Scientific Reports, 11, 3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Holtgrieve, G. W. , Schindler, D. E. , & Jewett, P. K. (2009). Large predators and biogeochemical hotspots: Brown bear (Ursus arctos) predation on salmon alters nitrogen cycling in riparian soils. Ecological Research, 24, 1125–1135. [Google Scholar]
- 197. Wall, D. H. , Bradford, M. A. , John, M. G. S. T. , Trofymow, J. A. , Behan‐Pelletier, V. , Bignell, D. E. , Mark Dangerfield, J. , Parton, W. J. , Rusek, J. , Voigt, W. , Wolters, V. , Gardel, H. Z. , Ayuke, F. O. , Bashford, R. , Beljakova, O. I. , Bohlen, P. J. , Brauman, A. , Flemming, S. , Henschel, J. R. , … Zou, X. (2008). Global decomposition experiment shows soil animal impacts on decomposition are climate‐dependent. Global Change Biology, 14, 2661–2677. [Google Scholar]
- 198. Kristensen, J. A. , Metcalfe, D. B. , & Rousk, J. (2018). The biogeochemical consequences of litter transformation by insect herbivory in the Subarctic: A microcosm simulation experiment. Biogeochemistry, 138, 323–336. [Google Scholar]
- 199. Myers‐Smith, I. H. , Kerby, J. T. , & Phoenix, G. K. (2020). Complexity revealed in the greening of the Arctic. Nature Climate Change, 10, 106–117. [Google Scholar]
- 200. Neumann, W. , Martinuzzi, S. , Estes, A. B. , Pidgeon, A. M. , Dettki, H. , Ericsson, G. , & Radeloff, V. C. (2015). Opportunities for the application of advanced remotely‐sensed data in ecological studies of terrestrial animal movement. Movement Ecology, 3, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Rhodes, M. W. , Bennie, J. J. , Spalding, A. , f french‐Constant, R. H. , & Maclean, I. M. D. Recent advances in the remote sensing of insects. Biological Reviews, 97(1), 343–360. [DOI] [PubMed] [Google Scholar]
- 202. Høye, T. T. , Ärje, J. , Bjerge, K. , Hansen, O. L. P. , Iosifidis, A. , Leese, F. , Mann, H. M. R. , Meissner, K. , Melvad, C. , & Raitoharju, J. (2021). Deep learning and computer vision will transform entomology. Proceedings of the National Academy of Sciences, 118, e2002545117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Mcguire, A. D. , Clein, J. S. , Melillo, J. M. , Kicklighter, D. W. , Meier, R. A. , Vorosmarty, C. J. , & Serreze, M. C. (2000). Modelling carbon responses of tundra ecosystems to historical and projected climate: Sensitivity of pan‐Arctic carbon storage to temporal and spatial variation in climate. Global Change Biology, 6, 141–159. [DOI] [PubMed] [Google Scholar]
- 204. Mckane, R. B. , Rastetter, E. B. , Shaver, G. R. , Nadelhoffer, K. J. , Giblin, A. E. , Laundre, J. A. , & Chapin, F. S. (1997). Climatic effects on tundra carbon storage inferred from experimental data and a model. Ecology, 78, 1170–1187. [Google Scholar]
- 205. Jiang, Y. , Rastetter, E. B. , Rocha, A. V. , Pearce, A. R. , Kwiatkowski, B. L. , & Shaver, G. R. (2015). Modeling carbon–nutrient interactions during the early recovery of tundra after fire. Ecological Applications, 25, 1640–1652. [DOI] [PubMed] [Google Scholar]
- 206. Rastetter, E. , Griffin, K. , Rowe, R. , Gough, L. , McLaren, J. R. , & Boelman, N. T. In press. Model responses to CO2 and warming are underestimated without explicit representation of arctic small‐mammal grazing. Ecological Applications, 32(1), e02478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Peacock, S. J. , Mavrot, F. , Tomaselli, M. , Hanke, A. , Fenton, H. , Nathoo, R. , Aleuy, O. A. , Di Francesco, J. , Aguilar, X. F. , Jutha, N. , Kafle, P. , Mosbacher, J. , Goose, A. , Kutz, S. J. , & Ekaluktutiak Hunters and Trappers Organization, Kugluktuk Angoniatit Association, Olokhaktomiut Hunters and Trappers Committee, & Kutz, S . (2020). Linking co‐monitoring to co‐management: Bringing together local, traditional, and scientific knowledge in a wildlife status assessment framework. Arctic Science, 6, 247–266. [Google Scholar]
- 208. Tape, K. D. , Gustine, D. D. , Ruess, R. W. , Adams, L. G. , & Clark, J. A. (2016). Range expansion of moose in Arctic Alaska linked to warming and increased shrub habitat. PLoS One, 11, e0152636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ims, R. A. , Henden, J.‐A. , & Killengreen, S. T. (2008). Collapsing population cycles. Trends in Ecology & Evolution, 23, 79–86. [DOI] [PubMed] [Google Scholar]
- 210. Cornulier, T. , Yoccoz, N. G. , Bretagnolle, V. , Brommer, J. E. , Butet, A. , Ecke, F. , Elston, D. A. , Framstad, E. , Henttonen, H. , Hörnfeldt, B. , Huitu, O. , Imholt, C. , Ims, R. A. , Jacob, J. , Jędrzejewska, B. , Millon, A. , Petty, S. J. , Pietiäinen, H. , Tkadlec, E. , … Lambin, X. (2013). Europe‐wide dampening of population cycles in keystone herbivores. Science, 340, 63–66. [DOI] [PubMed] [Google Scholar]
- 211. Tape, K. D. , Jones, B. M. , Arp, C. D. , Nitze, I. , & Grosse, G. (2018). Tundra be dammed: Beaver colonization of the Arctic. Global Change Biology, 24, 4478–4488. [DOI] [PubMed] [Google Scholar]
- 212. Tape, K. D. , Flint, P. L. , Meixell, B. W. , & Gaglioti, B. V. (2013). Inundation, sedimentation, and subsidence creates goose habitat along the Arctic coast of Alaska. Environmental Research Letters, 8, 045031. [Google Scholar]
- 213. Strathdee, A. T. , Bale, J. S. , Block, W. C. , Coulson, S. J. , Hodkinson, I. D. , & Webb, N. R. (1993). Effects of temperature elevation on a field population of Acyrthosiphon svalbardicum (Hemiptera: Aphididae) on Spitsbergen. Oecologia, 96, 457–465. [DOI] [PubMed] [Google Scholar]
- 214. Alatalo, J. M. , Jägerbrand, A. K. , Juhanson, J. , Michelsen, A. , & Ľuptáčik, P. (2017). Impacts of twenty years of experimental warming on soil carbon, nitrogen, moisture and soil mites across alpine/subarctic tundra communities. Scientific Reports, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Robinson, S. I. , McLaughlin, Ó. B. , Marteinsdóttir, B. , & O'Gorman, E. J. (2018). Soil temperature effects on the structure and diversity of plant and invertebrate communities in a natural warming experiment. Journal of Animal Ecology, 87, 634–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Coulson, S. J. , Hodkinson, I. D. , Wooley, C. , Webb, N. R. , Block, W. , Worland, M. R. , Bale, J. S. , & Strathdee, A. T. (1996). Effects of experimental temperature elevation on high‐Arctic soil microarthropod populations. Polar Biology, 16, 147–153. [Google Scholar]
- 217. Hodkinson, I. D. , Webb, N. R. , Bale, J. S. , Block, W. , Coulson, S. J. , & Strathdee, A. T. (1998). Global change and Arctic ecosystems: Conclusions and predictions from experiments with terrestrial invertebrates on Spitsbergen. Arctic and Alpine Research, 30, 306–313. [Google Scholar]
- 218. Webb, N. R. , Coulson, S. J. , Hodkinson, I. D. , Block, W. , Bale, J. S. , & Strathdee, A. T. (1998). The effects of experimental temperature elevation on populations of cryptostigmatic mites in high Arctic soils. Pedobiologia, 42, 298–308. [Google Scholar]
- 219. Gillespie, M. A. K. , Alfredsson, M. , Barrio, I. C. , Bowden, J. , Convey, P. , Coulson, S. J. , Culler, L. E. , Dahl, M. T. , Daly, K. M. , Koponen, S. , Loboda, S. , Marusik, Y. , Sandström, J. P. , Sikes, D. S. , Slowik, J. , & Høye, T. T. (2020). Circumpolar terrestrial arthropod monitoring: A review of ongoing activities, opportunities and challenges, with a focus on spiders. Ambio, 49, 704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Bowden, J. J. , Hansen, O. L. P. , Olsen, K. , Schmidt, N. M. , & Høye, T. T. (2018). Drivers of inter‐annual variation and long‐term change in high‐Arctic spider species abundances. Polar Biology, 41, 1635–1649. [Google Scholar]
- 221. Wieczorek, K. , & Chłond, D. (2019). The first detection of the alien species: Green‐peach aphid Myzus (Nectarosiphon) persicae (Insecta, Hemiptera, Aphididae) in the Svalbard archipelago. Polar Biology, 42, 1947–1951. [Google Scholar]
- 222. Wieczorek, K. , & Chłond, D. (2020). Hop‐on, hop‐off: The first record of the alien species crescent‐marked lily aphid (Neomyzus circumflexus) (Insecta, Hemiptera, Aphididae) in Greenland.
- 223. Larsson, C. , Hvidsten, D. , Stuen, S. , Henningsson, A. J. , & Wilhelmsson, P. (2018). Candidatus Neoehrlichia mikurensis in Ixodes ricinus ticks collected near the Arctic Circle in Norway. Parasites & Vectors, 11, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Jenkins, A. , Raasok, C. , Pedersen, B. N. , Jensen, K. , Andreassen, Å. , Soleng, A. , Edgar, K. S. , Lindstedt, H. H. , Kjelland, V. , Stuen, S. , Hvidsten, D. , & Kristiansen, B.‐E. (2019). Detection of Candidatus Neoehrlichia mikurensis in Norway up to the northern limit of Ixodes ricinus distribution using a novel real time PCR test targeting the groEL gene. BMC Microbiology, 19, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Blomgren, E. , Hesson, J. C. , Schäfer, M. L. , & Lundström, J. O. (2018). Pest occurrence of Aedes rossicus close to the Arctic Circle in northern Sweden. Journal of Vector Ecology, 43, 36–43. [DOI] [PubMed] [Google Scholar]
- 226. Kafle, P. , Peller, P. , Massolo, A. , Hoberg, E. , Leclerc, L.‐M. , Tomaselli, M. , & Kutz, S. (2020). Range expansion of muskox lungworms track rapid arctic warming: Implications for geographic colonization under climate forcing. Scientific Reports, 10, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kutz, S. J. , Checkley, S. , Verocai, G. G. , Dumond, M. , Hoberg, E. P. , Peacock, R. , Wu, J. P. , Orsel, K. , Seegers, K. , Warren, A. L. , & Abrams, A. (2013). Invasion, establishment, and range expansion of two parasitic nematodes in the Canadian Arctic. Global Change Biology, 19, 3254–3262. [DOI] [PubMed] [Google Scholar]
- 228. Schaefer, P. (2014). Diversity and ecological structure of northern biting flies.
- 229. Wackett, A. A. , Yoo, K. , & Olofsson, J. (2018). Human‐mediated introduction of geoengineering earthworms in the Fennoscandian arctic. Biological Invasions, 20, 1377–1386. [Google Scholar]
- 230. Blume‐Werry, G. , Krab, E. J. , Olofsson, J. , Sundqvist, M. K. , Väisänen, M. , & Klaminder, J. (2020). Invasive earthworms unlock arctic plant nitrogen limitation. Nature Communications, 11, 1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Reneerkens, J. , Schmidt, N. M. , Gilg, O. , Hansen, J. , Hansen, L. H. , Moreau, J. , & Piersma, T. (2016). Effects of food abundance and early clutch predation on reproductive timing in a high Arctic shorebird exposed to advancements in arthropod abundance. Ecology and Evolution, 6, 7375–7386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Høye, T. T. , Post, E. , Meltofte, H. , Schmidt, N. M. , & Forchhammer, M. C. (2007). Rapid advancement of spring in the high Arctic. Current Biology, 17, R449–R451. [DOI] [PubMed] [Google Scholar]
- 233. Culler, L. E. , Ayres, M. P. , & Virginia, R. A. (2015). In a warmer Arctic, mosquitoes avoid increased mortality from predators by growing faster. Proceedings of the Royal Society B: Biological Sciences, 282, 20151549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Gillespie, M. , Hodkinson, I. D. , Cooper, E. J. , Bird, J. M. , & Jónsdóttir, I. S. (2007). Life history and host–plant relationships of the rare endemic Arctic aphid Acyrthosiphon calvulus in a changing environment. Entomologia Experimentalis Et Applicata, 123, 229–237. [Google Scholar]
- 235. Gillespie, M. A. K. , Jónsdóttir, I. S. , Hodkinson, I. D. , & Cooper, E. J. (2013). Aphid–willow interactions in a high Arctic ecosystem: Responses to raised temperature and goose disturbance. Global Change Biology, 19, 3698–3708. [DOI] [PubMed] [Google Scholar]
- 236. Ávila‐Jiménez, M. L. , & Coulson, S. J. (2011). Can snow depth be used to predict the distribution of the high Arctic aphid Acyrthosiphon svalbardicum (Hemiptera: Aphididae) on Spitsbergen? BMC Ecology, 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Høye, T. T. , Kresse, J.‐C. , Koltz, A. M. , & Bowden, J. J. (2020). Earlier springs enable high‐Arctic wolf spiders to produce a second clutch. Proceedings of the Royal Society B, 287, 20200982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Schmidt, N. M. , Ims, R. A. , Høye, T. T. , Gilg, O. , Hansen, L. H. , Hansen, J. , Lund, M. , Fuglei, E. , Forchhammer, M. C. , & Sittler, B. (2012). Response of an arctic predator guild to collapsing lemming cycles. Proceedings of the Royal Society B: Biological Sciences, 279, 4417–4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Gallant, D. , Lecomte, N. , & Berteaux, D. (2020). Disentangling the relative influences of global drivers of change in biodiversity: A study of the twentieth‐century red fox expansion into the Canadian Arctic. Journal of Animal Ecology, 89, 565–576. [DOI] [PubMed] [Google Scholar]
- 240. Elmhagen, B. , & Rushton, S. P. (2007). Trophic control of mesopredators in terrestrial ecosystems: Top‐down or bottom‐up? Ecology Letters, 10, 197–206. [DOI] [PubMed] [Google Scholar]
- 241. Elmhagen, B. , Berteaux, D. , Burgess, R. M. , Ehrich, D. , Gallant, D. , Henttonen, H. , Ims, R. A. , Killengreen, S. T. , Niemimaa, J. , Norén, K. , Ollila, T. , Rodnikova, A. , Sokolov, A. A. , Sokolova, N. A. , Stickney, A. A. , & Angerbjörn, A. (2017). Homage to Hersteinsson and Macdonald: Climate warming and resource subsidies cause red fox range expansion and Arctic fox decline. Polar Research, 36, 3. [Google Scholar]
- 242. Henden, J.‐A. , Ehrich, D. , Soininen, E. M. , & Ims, R. A. (2021). Accounting for food web dynamics when assessing the impact of mesopredator control on declining prey populations. Journal of Applied Ecology, 58, 104–113. [Google Scholar]
- 243. Rockwell, R. F. , & Gormezano, L. J. (2009). The early bear gets the goose: Climate change, polar bears and lesser snow geese in western Hudson Bay. Polar Biology, 32, 539–547. [Google Scholar]
- 244. Mahoney, P. J. , Joly, K. , Borg, B. L. , Sorum, M. S. , Rinaldi, T. A. , Saalfeld, D. , Golden, H. , David M Latham, A. , Kelly, A. P. , Mangipane, B. , Koizumi, C. L. , Neufeld, L. , Hebblewhite, M. , Boelman, N. T. , & Prugh, L. R. (2020). Denning phenology and reproductive success of wolves in response to climate signals. Environmental Research Letters, 15, 125001. [Google Scholar]


