Abstract
Reaction of the 6π‐electron aromatic four‐membered heterocycle (IPr)2C2P2 (1) (IPr=1,3‐bis(2,6‐diisopropylphenyl)‐1,3‐dihydro‐2H‐imidazol‐2‐ylidene) with [Fe2CO9] gives the neutral iron tricarbonyl complex [Fe(CO)3‐η3‐{(IPr)2C2P2}] (2). Oxidation with two equivalents of the ferrocenium salt, [Fe(Cp)2](BArF24), affords the dicationic tricarbonyl complex [Fe(CO)3‐η4‐{(IPr)2C2P2}](BArF24)2 (4). The one‐electron oxidation proceeds under concomitant loss of one CO ligand to give the paramagnetic dicarbonyl radical cation complex [Fe(CO)2‐η4‐{(IPr)2C2P2}](BArF24) (5). Reduction of 5 allows the preparation of the neutral dicarbonyl complex [Fe(CO)2‐η4‐{(IPr)2C2P2}] (6). An analysis by various spectroscopic techniques (57Fe Mössbauer, EPR) combined with DFT calculations gives insight into differences of the electronic structure within the members of this unique series of iron carbonyl complexes, which can be either described as electron precise or Wade–Mingos clusters.
Keywords: Cluster Compounds, Density Functional Calculations, Donor–Acceptor Systems, Iron, Phosphorus
Bis‐imidazolium‐diphosphete‐diide, (IPr)2C2P2, acts as an electronically remarkably flexible ligand to iron carbonyl fragments, giving access to complexes of the general formula [Fe(CO) x {(IPr)2C2P2}] n+ (x=2, 3; n=0, +1, +2). Redox events involve either the metal ion or C2P2 ligand, as demonstrated by EPR, Mössbauer and computational analyses and characterize (IPr)2C2P2 as a redox active ligand.

Introduction
The discovery of ferrocene, [Fe(Cp)2]=Fc, [1] revolutionized the understanding of the interaction between a transition metal and a cyclic multi‐hapto‐bound ligand with a conjugated π‐electron system. [2] The bonding in ferrocene can be explained with a molecular orbital correlation diagram, in which symmetry‐adapted occupied and vacant orbitals at the FeII center and the Cp− ligand interact. [3] Alternative descriptions exist, [4] and one of them considers Fc being composed of two nido‐clusters with the Fe center as vertex‐sharing atom. [5] One way to probe the interaction between a metal center and its ligand is to investigate the redox behavior. [6] Mild oxidation agents oxidize Fc to the corresponding mono cation, ferrocenium, Fc+. [12] Ferrocene derivatives, however, can be converted to the dication [13] or monoanion. [14] All redox events mainly involve the iron center supporting a description, in which an Fe n+ (n=1, 2, 3, 4) ion interacts with two Cp− rings. Another way to experimentally probe the electronic effect of a ligand L and the electron density at a metal center, is to investigate complexes, which—apart from L—carry carbonyls as co‐ligands. A high electron density at the metal center is reflected by a low CO stretching frequency due to M→CO back‐bonding. [15] Figure 1 shows selected 18 valence electron (18 VE) configured complexes relevant to this work. The cationic complex [Fe(CO)3(Cp)]+ (I) shows IR vibrational absorption bands at νCO=2120, 2068 cm−1, [7] (Table 1) indicating rather weak d(Fe)→π*CO back‐donation, in accord with the assumption that the iron ion is rather electron poor and has the formal oxidation state +2. Reduction of I by two electrons is accompanied by loss of CO and leads to the formally isoelectronic, highly sensitive anionic complex [Fe(CO)2(Cp)]− (II; 18 VE) (νCO=1880, 1845 cm−1; Table 1), where the CO stretching frequencies have been shifted by more than 200 cm−1 to lower frequencies with respect to I indicating strong d(Fe)→π*CO back‐donation. [8] This is also true for neutral highly reactive and rare iron arene complexes such as [Fe(CO)2(C6Me6)] (III; 18 VE) with νCO=1954, 1892 cm−1 (Table 1). [9] In both complexes, II and III, a formal oxidation state of zero can be assigned to iron. On the contrary, the iron cyclobutadiene tricarbonyl complex [Fe(CO)3(C4H4)] (IV; 18 VE), [10] also known as Pettit′s reagent, shows stretching frequencies at higher wavenumbers νCO=2055, 1985 cm−1 (Table 1) indicating that the—in unbound form anti‐aromatic—C4H4 ring is a rather weak electron donor. Neutral IV decomposes upon oxidation under loss of C4H4 [16] but bulky electron‐donating substituents in the ring, which lower νCO up to 30 cm−1, allow to stabilize the oxidized products. [17]
Figure 1.

Selected iron carbonyl complexes I, [7] II, [8] III, [9] IV, [10] and V. [11] Counter anion for all species from this work: BArF24 −.
Table 1.
Selected analytical data. CO stretching frequencies were measured by ATR‐IR. Values marked with a rhombus (♦) were measured by transmission‐IR in saturated n‐hexane solution. Values marked with an asterisk (*) were measured by transmission‐IR in acetonitrile solution. Values marked with a dot (•) were measured by transmission‐IR in saturated THF solution. For detailed spectra and calculations see the Supporting Information. †: centroid of the coordinated PCP‐plane was constructed. Mössbauer and IR data calculated by DFT are given in italics.
|
Compound |
CO‐stretch freq. exp. [cm−1] |
CO‐stretch freq. calc. [cm−1] |
CIpr−Cring bond length [Å] |
N−C(−N) bond length [Å] |
Fe‐centroid distance [Å] |
57Fe Mössbauer isomer shift [mms−1] |
57Fe Mössbauer quad. splitting [mms−1] |
|---|---|---|---|---|---|---|---|
|
1 |
– |
– |
Ø 1.387(3) [23] |
Ø 1.375(3) [23] |
– |
– |
– |
|
2 |
*1983♦, 1917♦, 1904♦ |
1976, 1922, 1913 |
1.437(5) 1.340(6) |
Ø 1.369(4) Ø 1.403(4) |
1.909(1)† |
0.05 (−0.03) |
1.14 (−0.94) |
|
3 |
2015*, 1948* |
2018, 1969, 1960 |
– |
– |
– |
– (0.18) |
– (1.52) |
|
4 |
2083,* 2035* |
2070, 2028, 2023 |
Ø 1.449(4) |
Ø 1.355(3) |
1.825(1) |
0.11 (0.02) |
1.40 (1.24) |
|
5 |
1966*, 1903* |
1978, 1930 |
Ø 1.432(5) |
Ø 1.355(5) |
1.804(2) |
0.27 (0.20) |
1.06 (0.94) |
|
6 |
1870, 1807* |
1897, 1850 |
Ø 1.409(4) |
Ø 1.364(4) |
1.776(1) |
0.12 (0.05) |
1.44 (−1.49) |
|
I |
2120, 2068 [7] |
2127, 2083 |
– |
– |
1.710(1) [27] |
0.05 [28] (0.07) |
1.88 [28] (1.75) |
|
II |
1881•, 1808•, 1864•, 1770•[8a] |
1875, 1819 |
– |
– |
1.7284(15) [29] |
(0.26) |
(−2.76) |
|
III |
1954, 1892 [9] |
1970, 1920 |
– |
– |
– |
(0.22) |
(−1.89) |
|
IV |
2055, 1985 [10] |
2049, 1991, 1986 |
– |
– |
1.770(1) [30] |
0.02 [31] (0.00) |
1.54 [31] (1.52) |
|
V |
– |
2040, 1992, 1982 |
– |
– |
– |
(0.00) |
(1.14) |
Sandwich complexes with π‐conjugated heterocycles, in which a CR unit has been replaced by a phosphorus center—a research area fueled by the diagonal relationship between carbon and phosphorus [18] —have been widely studied. [19] While iron complexes with η6‐bound six‐membered rings (CR)6−x P x are relatively rare, [20] those with five‐membered η5‐bound phospholide rings (CR)5−x P x are numerous.[ 19 , 21 ]
Often, anodic (positive) shifts of the redox potential were observed [21b] and the overall conclusion is that replacement of a CR unit by phosphorus decreases the σ‐electron donating properties of the heterocycle while the π‐accepting properties increase strongly. [21c] It is assumed that the redox processes take place mainly at the iron center. [19] Iron complexes with π‐conjugated four‐membered rings, such as 1‐, 1,2‐ and 1,3‐diphosphacyclobutadienes or even [P4]2− as ligands, are known [22] but few like the complex [Fe(CO)3‐η4‐(tBu2C2P2)] V, [11] related to Pettit's reagent IV, contain carbonyls as co‐ligands. The calculated νCO=2040, 1992, 1982 cm−1 of V (Table 1) are quite similar to IV; thus, indicating rather weak M→CO back‐donation.
The recently reported (IPr)2C2P2 (1) can be considered as an N‐heterocyclic carbene stabilized cyclic “dicarbondiphosphide” A or, more appropriately, as a bis(imidazolium)‐1,3‐diphosphete‐diide B with a formal 2− charge in the C2P2 ring and positive charges on every IPr (IPr=1,3‐bis(2,6‐diisopropylphenyl)‐1,3‐dihydro‐2H‐imidazol‐2‐ylidene) unit. Both descriptions lead to a central aromatic 6π‐electron system delocalized over the C2P2 ring. Heterocycle 1 is easily oxidized at E°=−0.451 V (vs. Fc/Fc+) to its persistent radical cation [23] and binds to M(CO)3 fragments (M=Cr, Mo) as electron donating ligand. [24]
Here, we report that 1 not only allows to prepare neutral iron dicarbonyl complexes, which—in contrast to arene complexes—are remarkably stable but also show a rich redox chemistry. Specifically, a comparison between [Fe(CO)3‐η4‐{(IPr)2C2P2}]2+ and [Fe(CO)2‐η4‐{(IPr)2C2P2}] will show that subtle differences in the structure may lead to significant differences in the electronic structure of the central FeC2P2 core.
Results and Discussion
Heterocycle 1 reacted with [Fe2CO9] in mesitylene at 130 °C to give [Fe(CO)3{η3‐(IPr)2C2P2}] 2 in two hours (Scheme 1). The product is isolated as dark blue powder (73 % yield), which always contained a second, minor species (≤5 %; [δ(31P{1H})=δ=18.2 ppm (FeP); δ=−98.6 ppm (P), 2 J PP=15 Hz)]) that could not be further characterized (for details see the Supporting Information, Figures S1–S4). The major compound 2 shows one broadened 31P NMR resonance [δ(31P{1H})=79.2 ppm; Δν1/2=85 Hz] indicating hindered rotation of the Fe(CO)3 moiety (for variable temperature NMR spectra and solid‐state NMR spectra see the Supporting Information, Figures S6–S8). A single crystal of 2 could be obtained and allowed to determine the structure by X‐ray diffraction methods (see below). Cyclic voltammetry (CV) of solutions of 2 in THF (containing 0.1 M (nBu4N)PF6 as electrolyte; scan rate: 100 mV s−1) showed two quasi‐reversible redox waves at −0.83 V and −1.18 V vs. Fc/Fc+ (Figure S11). Chemical oxidation of 2 with two equivalents of Fc[BArF24] (BArF24=tetrakis[3,5‐bis(trifluoromethyl)phenyl]borate) in 1,2‐difluorobenzene (DFB) gave the dicationic tricarbonyl complex [Fe(CO)3{η4‐(IPr)2C2P2}](BArF24)2 (4) [δ(31P{1H})=57.1 ppm], which is obtained after recrystallization from 1,2‐DFB/n‐hexane as analytically pure, golden crystals (Scheme 1). From the separation of the two redox waves by ΔΔE=0.35 V a small equilibrium constant of K disp=1.24×10−6 for the disproportionation of the one‐electron oxidation product [Fe(CO)3{η3‐(IPr)2C2P2}]⋅+ (3) into 2 and 4 is calculated suggesting that the radical cation 3 may be observed at least spectroscopically. Indeed, mixing of equimolar amounts of 2 and 4 in a polar solvent (fluorobenzene, 1,2‐DFB, or acetonitrile) resulted in the immediate formation of a new species 3 (Scheme 1), which is NMR‐silent but is characterized by two new absorptions at νCO=2015 and 1948 cm−1 in the IR spectrum. Furthermore, an EPR spectrum was recorded (see below and Figures S16 and S17 in the Supporting Information). Complex 3 is a transient species that loses CO with a half‐life of about τ 1/2≈4 h to form the stable iron dicarbonyl radical cation [Fe(CO)2{η4‐(IPr)2C2P2}]⋅+ (BArF24) (5) (Scheme 1). The CV of a solution of 5 in THF [0.1 M (nBu4N)PF6 as electrolyte; scan rate: 100 mV s−1] shows a quasi‐reversible redox wave at a potential of −1.29 V vs. Fc/Fc+ (for details see the Supporting Information, Figure S26). Reduction of 5 with KC8 in a mixture of n‐hexane/TMEDA (TMEDA=N,N,N′,N′‐tetramethyl‐ethane‐1,2‐diamine) gave the neutral dicarbonyl compound 6 (Scheme 1). [25] Note that 6 could not be obtained by thermal or photochemical removal of CO from the tricarbonyl complex 2 (see Figure S9 in the Supporting Information for TGA‐MS‐IR spectra) in agreement with DFT calculations, which indicate that CO loss from 2 is strongly endergonic (ΔG=18.7 kcal mol−1) while CO dissociation from 3 (ΔG=−1.4 kcal mol−1) is slightly exergonic. The lowering of the CO binding energy by as much as 20.1 kcal mol−1 is a consequence of the reduced d(Fe)→π*CO back‐bonding in the radical cation 3. The 31P{1H} NMR spectra of complexes 4 [δ(31P{1H})=58.3 ppm] and 6 [δ(31P{1H})=48.5 ppm] show a singlet resonance in the range also observed for [Cr(CO)3{η4‐(IPr)2C2P2}] [δ(31P)=64.7 ppm] and [Co(CO)2{η4‐(IPr)2C2P2}]+ [δ(31P)=66.3 ppm], in which the C2P2 ring is symmetrically η4‐coordinated.
Scheme 1.
Possible descriptions of 1 as(A) and (B). Reaction of ligand 1 with [Fe2(CO)9] provides complex 2. Oxidation of 2 with Fc(BArF24) (BArF24=tetrakis[3,5‐bis(trifluoro‐methyl)phenyl]borate) yields FeI complex 3. Radical cation 3 either loses CO leading to the formation of radical cation 5, or can be oxidized a second time by one electron under formation of 4. Reduction of 5 with KC8/TMEDA leads to formation of the neutral dicarbonyl complex 6.
Single crystals for X‐ray diffraction analysis could be obtained for the iron complexes 2, 4, 5, and 6. In 2, ligand 1 coordinates to the Fe(CO)3 fragment in an η3‐fashion via the two phosphorus atoms and one carbon atom of the central C2P2 ring (Figure 2A). The exocyclic C1−C6 bond [1.340(6) Å] is significantly shorter than the C2−C7 bond [1.437(5) Å]; thus, indicating that the IPr unit bound to C2 is best described as an imidazolium group and the coordinated PCP unit serves as 4π‐electron donor comparable to an allyl ligand. This assignment is supported by DFT calculations, which indicate a formal double bond between C1 and C6 and a single bond between C2 and C7 (see the Supporting Information). The C2P2 ring is not planar but adopts a butterfly conformation [fold angle along the P−P axis 25.2(3)°]. In complexes 4 (Figure 2B), 5 (Figure 2C), and 6 (Figure 2D), the central C2P2 ring is η4‐coordinated to the iron centers. The CIpr−Cring bonds between the IPr moieties and the central C2P2 ring are rather long [4: 1.449(4) Å, 5: 1.432(5) Å, 6: 1.409(4) Å] indicating imidazolium character of the IPr units and, in return, electron donation into the C2P2 ring. As expected, the distance between the iron center and the ring centroid (ct), Fe–ct, is shorter in 4 [1.825(1) Å], 5 [1.804(2) Å], and 6 [1.776(1) Å] in comparison to the Cr complex [Cr(CO)3{η4‐(IPr)2C2P2}] (Cr–ct=1.929(1) Å]. The Co–ct bond in [Co(CO)2{η4‐(IPr)2C2P2}]+ (Co–ct=1.806(1) Å] shows a similar length. A shortening of the Fe–ct bond in going from the tricarbonyl complex 4 to the dicarbonyl complex 5 can be ascribed to the loss of a CO ligand (although this effect is certainly counter‐balanced by decreasing the formal charge from +2 to +1). The even shorter Fe–ct bond in neutral 6, however, is astonishing given that 6 was generated from 5 by one‐electron reduction.
Figure 2.
Solid‐state structures of iron complexes 2 (A), 4 (B), 5 (C), and 6 (D). Hydrogen atoms, counter ions and solvent molecules are omitted for clarity. Selected metrical parameters can be found in Table 1. [26]
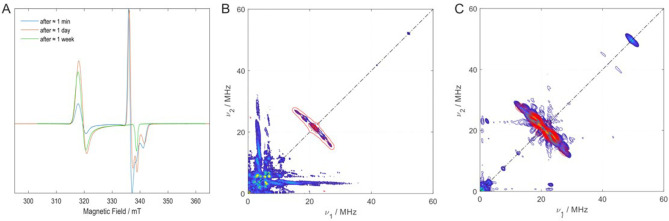
The stretching frequencies, νCO, of the CO groups follow the expected trends (Table 1). In the tricarbonyl complexes 2, 3, and 4, the increasing positive charge correlates with increasing stretching frequencies due to diminished d(Fe)→π*CO orbital back‐bonding. The opposite is observed when the dicarbonyl radical cation 5 (νCO=1970, 1908 cm−1) is reduced to the neutral complex 6 (νCO=1870, 1807 cm−1). Note that—with the exception of dicationic 4—the νCO are observed at significantly lower wavenumbers than in comparable iron tricarbonyl complexes with conjugated cyclic hydrocarbons as ligands, such as Cp− in I or C4H4 in III. Also, [Fe(CO)3(tBu2C2P2)] IV (νCO=2040, 1990 cm−1) has higher νCO stretching frequencies than all complexes with 1 as ligand. The same trend is observed when the dicarbonyl complexes [Fe(CO)2(C6Me6)] II and [Fe(CO)2‐η4‐{(IPr)2C2P2}] 6 are compared. The latter complex shows the lowest stretching frequencies of all complexes reported here; indicating especially strong electron donation of (IPr)2C2P2 1 to the Fe(CO)2 unit. Only the anionic complex [Fe(CO)2(Cp)]− shows similar low stretching frequencies (see above and Table 1). [8] The paramagnetic complexes [Fe(CO)3{η3‐(IPr)2C2P2}]⋅+ (3) and [Fe(CO)2{η4‐(IPr)2C2P2}]⋅+ (5) were further investigated by EPR spectroscopy in frozen fluorobenzene/toluene solution (1 : 1 mixture). For 3, an axial g‐tensor with the principal values g x =g y =2.0166 and gz=1.9858 (giso=2.0062) was obtained (Figure 3A and Figure S17). Complex 5 also shows an axial g‐tensor, however, the g‐values (g x =g y =2.1287, g z =2.0021, giso=2.0865) are larger compared to 3 and giso deviates more strongly from the free‐electron value (g=2.0023) indicating a larger spin‐density on the iron center in 5. The pulse EPR technique HYSCORE, [32] allows to determine 31P hyperfine couplings (HFC's) in the two complexes. Because of the hindered rotation of the Fe(CO)3 or Fe(CO)2 units in 3 and 5, respectively, in frozen solution the complexes become unsymmetric and therefore show two sets of 31P HFCs (Figure 3B, C and Figure S16). The g‐tensors obtained from DFT calculations [3: g11=1.9906, g22=2.0158, g33=2.01890 (giso=2.0084); 5: g11=2.0038, g22=2.0087, g33=2.1045 (giso=2.0653)] are in reasonable agreement with the experimental data and validate the electronic structure models. Specifically, that 3 contains a η3‐bound (IPr)2C2P2 ligand (like in 2) and is a 17 VE complex. The spin‐population in 3 is distributed over the iron center, the non‐coordinating carbon atom of the C2P2 ring and the nitrogen atoms of the respective IPr moiety (Fe 0.63, P −0.02 and −0.03, N 0.1 each, C 0.27). For 5, likewise a 17 VE complex but with a η4‐bound (IPr)2C2P2 ligand, a significantly greater spin‐density is located on Fe [Fe 1.27, P −0.03 each, N <0.01, C in C2P2 <0.01], which is in agreement with the larger experimental giso value. These data suggest that the oxidation from 2 to 3 does not take place solely at the iron center but rather involves the whole complex indicating that (IPr)2C2P2 1 behaves as a redox non‐innocent ligand. In contrast, in the redox pair 5/6, the redox event solely takes place at the iron center and 1 acts as a non‐redox active innocent ligand in this case.
Figure 3.
EPR spectra and 31P hyperfine couplings of 3 and 5. A) Reaction of 3 to 5 as monitored by cw EPR. (ca. 9.5 GHz, 20 K, frozen solution). Blue spectrum: after≈1 min, red spectrum after≈1 day, green spectrum after≈one week. Note that the final spectrum (green) shows a single spectral component and thereby allows for an unambiguous resonance assignment and g‐tensor determination. HYSCORE spectra (ca. 34.4 GHz) of 3 (B) and 5 (C) in blue/yellow obtained at the spectral maximum of the respective component and simulations (red). Both spectra show clear ridges due to 31P couplings centered around the 31P nuclear Zeeman frequency of 20 and 21 MHz in (B) and (C), respectively. The simulated 31P hyperfine coupling (HFC) tensors (red) are (4.4, 3.2, 1.5) MHz, (1.6, 5.5, 3.0) MHz in 3 and (−8.0, 7.4, 1.7) MHz, (−0.5, 5.8, 1.0) MHz in 5. Due to their initial low modulation depth, 31P nuclear frequencies were enhanced by matching pulses in C (see the Supporting Information). The calculated 31P HFC values [3: (−21.8, 12.6, −12.3) MHz, (−11.1, 1.5, 12.0) MHz; 5: (7.7, 16.4, −23.0) MHz, (27.0, 16.0, −20.2) MHz] deviate from the experimental ones but the symmetry of the tensors is reproduced. The two different sets of 31P HFCs result from hindered rotation of the Fe(CO)3 or Fe(CO)2 units in 3 and 5, respectively.
Zero‐field 57Fe Mössbauer spectra were recorded for solid samples of the (IPr)2C2P2 complexes 2, 4, 5, and 6 at 77 K (see the spectrum of 6 in Figure 4 as example) and the calculated 57Fe Mössbauer parameters are in good agreement with the experimental data (Table 1). For comparison, the experimental and/or calculated data for the complexes with cyclic hydrocarbon ligands (I–IV) and [Fe(CO)3(tBu2C2P2)] V were added in Table 1. Despite the significant differences with respect to structure and charge, all complexes—with the exception of 5—have rather similar 57Fe Mössbauer parameters. Unfortunately, the range of isomer shifts, δ, for these complexes lies within the ones typically observed for both Fe0 and FeII low‐spin complexes [33] and suggests similar physical oxidation states (electron densities) at the iron centers. The relatively high local symmetry of most complexes—with the exception of II (local Cs symmetry)—is reflected by a quadrupole splitting ΔE Q<2 mms−1. The isomer shift δ=0.27 mms−1 of the paramagnetic complex [Fe(CO)2{η4‐(IPr)2C2P2}](BArF24) (5), with its 17‐valence electron configuration at the iron center, reflects the deviation from a closed‐shell Fe0 or FeII (S=0) compound to a one‐electron oxidized species with S=1/2.
Figure 4.

Zero‐field 57Fe Mössbauer spectrum of a solid sample of 6 at 77 K. The measured data (circles) were fitted using least‐square fitting of the Lorentzian signals (red line).
A comparison of the electronic structure between the dicationic iron tri(carbonyl) complex [Fe(CO)3{η4‐(IPr)2C2P2}]2+ (4) and the neutral dicarbonyl complex [Fe(CO)2{η4‐(IPr)2C2P2}] (6) is especially intriguing (a detailed bonding analysis of the tricarbonyl complex [Fe(CO)3{η3‐(IPr)2C2P2}] (2), which is related to [Fe(CO)3(η4‐arene)] complexes will be published elsewhere). Both, 4 and 6, have the same valence electron (VE) count of 34 within the central FeC2P2 core under the assumption that each IPr is bound via a dative two‐electron bond to the C2P2 ring. [23] 4: VE=8(Fe)+18(C2P2)+4(2 IPr)+6(3 CO)−2 (positive charge)=34; 6: VE=8(Fe)+18(C2P2)+4(2×IPr)+4 (2 CO)=34. The polyhedral skeletal electron pair theory (PSEP), formulated by Wade and Mingos, [34] predicts that the dicationic tricarbonyl complex 4 can be described as a nido‐cluster, in which 14 e=7 electron pairs of the total of 34 VE's are used for bonding within the FeC2P2 core. On the other hand, the dicarbonyl complex 6 fulfills the EAN rule for electron precise clusters [b= (18 n+8 m)−VE= (18+32)−34)=8; n=number of transition metal centers; m=number of main group element centers; b=number of 2‐center‐2‐electron‐shared bonds within the m+n cluster core]. Consequently, 6 can be viewed as a square pyramidal electron precise FeC2P2 cluster, in which every edge corresponds to an electron‐shared, 2‐center‐2‐electron (2c2e) bond.
In order to verify this assessment, the wave functions of 4, and 6 were analyzed by means of intrinsic bond orbitals (IBO) [35] and their localized‐orbital centroids (LOCs), [36] using the ORCA program package. [37]
The IBO calculations for 4 show three filled non‐bonding 3d orbitals at Fe and three bonding orbitals describing the interaction between the iron center and the η4‐bound C2P2 ring (see Figure 5A). One of these orbitals shows an LOC in between the Fe and C2P2 ring that accounts for a delocalized covalent P−Fe−P interaction; thus, making a clear assignment of an oxidation state to the iron center in 4 difficult. The other two LOCs are closer to the carbon centers and can be interpreted as localized dative Fe←C bonds (for details see the Supporting Information, Figure S29). This is in accord with the description of 4 as five‐vertex nido‐cluster with a square pyramidal structure, where from the n+2=7 skeletal electron pairs three are used for bonding between the Fe center and the C2P2 ring (shown as light‐blue dots) and four between the C and P centers (these LOCs are indicated by green dots). The NICS (nucleus‐independent chemical shift) value at the center of the FeC2P2 pyramid in 4 is −32.9 indicating the presence of spherical aromaticity.
Figure 5.

Localised orbital centroids (LOCs) for substances 4 (A), 6 (B), I (C), II (D), and III (E). For detailed calculation results see the Supporting Information, section 3.2. Note that a truncated model was used for III where the methyl groups were replaced by hydrogen atoms.
The electronic structure of the dicarbonyl complex 6 is different when compared to 4. Three non‐bonding 3d orbitals are located at the iron center and three orbitals accounting for dative ligand‐to‐metal donation, Fe←C2P2, are found with LOCs strongly located towards the C2P2 plane (see Figure 5B). In addition, one LOC lies close to the Fe center and originates from a filled 3d orbital, which is strongly involved in π‐back‐bonding to the LUMO of the C2P2 moiety (see the Supporting Information, section 3.2). As a result, (IPr)2C2P2 (1) does not only serve as a strong 6π‐donor ligand but also as a π‐accepting ligand in 6. These acceptor properties are enabled by the relatively small HOMO–LUMO gap of 2.4 eV in 1. Furthermore, the LUMO of (IPr)2C2P2 (1) exhibits CIpr−Cring bonding character and partial occupation of this orbital by electron back‐donation leads to a shortening of these bonds. This specific back‐bonding interaction is absent in the dication 4, explaining the initially surprising lengthening of the Fe–ct distance in 4 compared to 6 (see above). This different electronic structure of 6 results in a lower spherical aromaticity compared to 4, reflected by a lower NICS value of −20.0 in the center of the C2P2Fe pyramid. We propose therefore to describe the dicarbonyl complex 6 as an electron‐precise “classical” organometallic complex between an Fe0 center to which the (IPr)2C2P2 ring acts prevalently as a π‐donor ligand.
For comparison, we also inspected the iron complexes I (Figure 5C), II (Figure 5D), and III (Figure 5E) carrying either a Cp− ring or benzene as 6π‐aromatic ligand using the same theoretical approach. Clearly, the three LOCs close to the carbon centers characterize these ligands as classical π‐electron donor ligands, [4] while three (I), respectively, four (II, III) LOCs at the Fe center indicate FeII or Fe0 oxidation states in I or II, III.
Finally, the reaction energies ΔE r for the ligand exchange reactions 1 and 2 where calculated in order to evaluate the binding energies of (IPr)2C2P2 using the neutral dicarbonyl complex 6 as specific example:
| (1) |
| (2) |
The interaction between the Fe(CO)2 fragment and the η4‐bound (IPr)2C2P2 ligand in 6 is about 30 kcal mol−1 stronger than the iron η6‐benzene bond in complex III and about as strong as the interaction between the η5‐cyclopentadienide anion and Fe in complex II. These data clearly indicate that (IPr)2C2P2 may bind very tightly to transition metal fragments.
Conclusion
The following conclusions can be drawn from the series of iron carbonyl complexes with (IPr)2C2P2 (1) as ligand: a) The molecule 1 acts as an unusually strong electron donating ligand. This is in contrast to other phosphorus containing heterocycles, which are often referred to as electron accepting ligands. b) As ligand, 1 is electronically remarkably flexible, which relates this compound to redox non‐innocent ligands.[ 6 , 38 ] c) The very similar 57Fe Mössbauer isomer shifts δ indicate that the iron nuclei in 4 and 6 must be located in rather similar electronic environments. Regardless, while neutral [Fe(CO)3{η4‐(IPr)2C2P2}] 6 can be viewed as a classical coordination compound with iron in its oxidation state zero, the dication [Fe(CO)2{η4‐(IPr)3C2P2}]2+ in 4 is better described as square pyramidal nido‐cluster, where the oxidation state at the iron center remains uncertain due to the more covalent character of the bonds within the Fe2C2P2 core. This is reflected in the experimental data: Reduction of 4 involves the whole FeC2P2 core and leads to the expected opening of the cluster structure to give 3, in which the binding mode of 1 is reduced from η4 to η3. d) The use of 1 as ligand opens the possibility to prepare electron‐rich neutral iron complexes with cyclic conjugated 6π‐aromatic ligands that are significantly more stable than related iron arene complexes.
Conflict of interest
The authors declare no conflict of interest.
1.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNF; grant 2‐77199‐18) and the ETH Zürich (grant 0‐20406‐18). PC gratefully acknowledges funding by the Deutsche Forschungsgemeinschaft (DFG, grant 438203135). K.M. thanks the Friedrich‐Alexander‐Universität Erlangen‐Nürnberg (FAU) for generous financial support and H.G. thanks FAU for the participation in the FAU Visiting Professorship programme. We thank Dr. M. Wöhrle for valuable assistance with the X‐ray diffraction measurements and Dr. R. Verel for valuable help with acquisition and interpretation of NMR data. Open Access funding provided by Eidgenössische Technische Hochschule Zürich.
Dedicated to Professor John Nixon
M. T. Scharnhölz, P. Coburger, L. Gravogl, D. Klose, J. J. Gamboa-Carballo, G. Le Corre, J. Bösken, C. Schweinzer, D. Thöny, Z. Li, K. Meyer, H. Grützmacher, Angew. Chem. Int. Ed. 2022, 61, e202205371; Angew. Chem. 2022, 134, e202205371.
Contributor Information
Peter Coburger, Email: pcoburger@inorg.chem.ethz.ch.
Hansjörg Grützmacher, Email: hgruetzmacher@ethz.ch.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.
- 1a. Kealy T. J., Pauson P. L., Nature 1951, 168, 1039–1040; [Google Scholar]
- 1b. Miller S. A., Tebboth J. A., Tremaine J. F., J. Chem. Soc. 1952, 632–635. [Google Scholar]
- 2. Okuda J., Eur. J. Inorg. Chem. 2017, 217–219. [Google Scholar]
- 3. Mayor-López M. J., Weber J., Chem. Phys. Lett. 1997, 281, 226–232. [Google Scholar]
- 4. Rayón V. M., Frenking G., Organometallics 2003, 22, 3304–3308. [Google Scholar]
- 5. Jemmis E. D., Balakrishnarajan M. M., Pancharatna P. D., J. Am. Chem. Soc. 2001, 123, 4313–4323. [DOI] [PubMed] [Google Scholar]
- 6. de Bruin B., Gualco P., Paul N. D., Ligand Design in Metal Chemistry: Reactivity and Catalysis, Wiley, Hoboken, 2016, pp. 176–204. [Google Scholar]
- 7. Busetto L., Angelici R. J., Inorg. Chim. Acta 1968, 2, 386–390. [Google Scholar]
- 8.
- 8a. Clifford A. F., Mukherjee A. K., J. Inorg. Nucl. Chem. 1963, 25, 1065–1066; [Google Scholar]
- 8b. Giuseppetti M. E., Cutler A. R., Organometallics 1987, 6, 970–973; [Google Scholar]
- 8c. Ellis J. E., Flom E. A., J. Organomet. Chem. 1975, 99, 263–268. [Google Scholar]
- 9. Weber S. R., Brintzinger H. H., J. Organomet. Chem. 1977, 127, 45–54. [Google Scholar]
- 10. Emerson G. F., Watts L., Pettit R., J. Am. Chem. Soc. 1965, 87, 131–133. [Google Scholar]
- 11. Binger P., Biedenbach B., Schneider R., Regitz M., Synthesis 1989, 1989, 960–961. [Google Scholar]
- 12. Woodward R. B., Rosenblum M., Whiting M. C., J. Am. Chem. Soc. 1952, 74, 3458–3459. [Google Scholar]
- 13. Malischewski M., Adelhardt M., Sutter J., Meyer K., Seppelt K., Science 2016, 353, 678–682. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Walawalkar M. G., Pandey P., Murugavel R., Angew. Chem. Int. Ed. 2021, 60, 12632–12635; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 12740–12743; [Google Scholar]
- 14b. Goodwin C. A. P., Giansiracusa M. J., Greer S. M., Nicholas H. M., Evans P., Vonci M., Hill S., Chilton N. F., Mills D. P., Nat. Chem. 2021, 13, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.
- 15a. Eschenbroich C., Organometallchemie, 6. ed., Teubner, Leibzig, 2008; [Google Scholar]
- 15b. Eschenbroich C., Organometallics, 3. ed., Wiley-VCH, Weinheim, 2016. [Google Scholar]
- 16.
- 16a. Donaldson W. A., Encyclopedia of Reagents for Organic Synthesis, Wiley, Hoboken, 2001; [Google Scholar]
- 16b. Johnson R. P., The Chemistry of Cyclobutanes, Wiley, Hoboken, 2005, pp. 589–616. [Google Scholar]
- 17. Minegishi S., Komatsu K., Kitagawa T., Organometallics 2011, 30, 1002–1007. [Google Scholar]
- 18. Dillon K. B., Mathey F., Nixon J. F., Phosphorus: The Carbon Copy, Wiley, New York, 1998. [Google Scholar]
- 19. Bartsch R., Datsenko S., Ignatiev N. V., Müller C., Nixon J. F., Pickett C. J., J. Organomet. Chem. 1997, 529, 375–378. [Google Scholar]
- 20.
- 20a. Rezaei Rad B., Chakraborty U., Mühldorf B., Sklorz J. A. W., Bodensteiner M., Müller C., Wolf R., Organometallics 2015, 34, 622–635, and references therein; [Google Scholar]
- 20b. Böhm D., Knoch F., Kummer S., Schmidt U., Zenneck U., Angew. Chem. Int. Ed. Engl. 1995, 34, 198–201; [Google Scholar]; Angew. Chem. 1995, 107, 251–254; [Google Scholar]
- 20c. Eggers K., Heinemann F. W., Hennemann M., Clark T., Binger P., Zenneck U., C. R. Chim. 2010, 13, 1203–1212, and references therein. [Google Scholar]
- 21.
- 21a. Petrov A. V., Zagidullin A. A., Bezkishko I. A., Khrizanforov M. N., Kholin K. V., Gerasimova T. P., Ivshin K. A., Shekurov R. P., Katsyuba S. A., Kataeva O. N., Budnikova Y. H., Miluykov V. A., Dalton Trans. 2020, 49, 17252–17262; [DOI] [PubMed] [Google Scholar]
- 21b. Lemoine P., Gross M., Braunstein P., Mathey F., Deschamps B., Nelson J. H., Organometallics 1984, 3, 1303–1307; [Google Scholar]
- 21c. De Lauzon G., Mathey F., Simalty M., J. Organomet. Chem. 1978, 156, C33-C36. [Google Scholar]
- 22.
- 22a. Wolf R., Ehlers A. W., Khusniyarov M. M., Hartl F., de Bruin B., Long G. J., Grandjean F., Schappacher F. M., Pottgen R., Slootweg J. C., Lutz M., Spek A. L., Lammertsma K., Chem. Eur. J. 2010, 16, 14322–14334; [DOI] [PubMed] [Google Scholar]
- 22b. Lyaskovskyy V., Elders N., Ehlers A. W., Lutz M., Slootweg J. C., Lammertsma K., J. Am. Chem. Soc. 2011, 133, 9704–9707; [DOI] [PubMed] [Google Scholar]
- 22c. Chirila A., Wolf R., Slootweg J. C., Lammertsma K., Coord. Chem. Rev. 2014, 270–271, 57–74; [Google Scholar]
- 22d. Efraty A., Chem. Rev. 1977, 77, 691–744; [Google Scholar]
- 22e. Wang Z. C., Qiao L., Sun Z. M., Scheer M., J. Am. Chem. Soc. 2022, 144, 6698–6702. [DOI] [PubMed] [Google Scholar]
- 23. Li Z., Chen X., Andrada D. M., Frenking G., Benko Z., Li Y., Harmer J. R., Su C. Y., Grützmacher H., Angew. Chem. Int. Ed. 2017, 56, 5744–5749; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 5838–5843. [Google Scholar]
- 24. Li Z., Chen X., Liu L. L., Scharnhölz M. T., Grützmacher H., Angew. Chem. Int. Ed. 2020, 59, 4288–4293; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 4318–4323. [Google Scholar]
- 25.Alternatively, 2 can be oxidized with Fc[PF6] to form 3[PF6] and reduced after loss of a CO ligand. An advantage of this procedure is the lesser solubility of K[PF6], which leads to a cleaner product.
- 26.Deposition Numbers 2089380, 2089381, 2089382 and 2096257 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 27. Gress M. E., Jacobson R. A., Inorg. Chem. 1973, 12, 1746–1749. [Google Scholar]
- 28. Long G. J., Alway D. G., Barnett K. W., Inorg. Chem. 1978, 17, 486–489. [Google Scholar]
- 29. Hey-Hawkins E., von Schnering H. G., Z. Naturforsch. B 1991, 46, 621–624. [Google Scholar]
- 30. Harvey P. D., Schaefer W. P., Gray H. B., Gilson D. F. R., Butler I. S., Inorg. Chem. 1988, 27, 57–59. [Google Scholar]
- 31. Herber R. H., King R. B., Ackermann M. N., J. Am. Chem. Soc. 1974, 96, 5437–5441. [Google Scholar]
- 32.
- 32a. Jeschke G., Rakhmatullin R., Schweiger A., J. Magn. Reson. 1998, 131, 261–271; [DOI] [PubMed] [Google Scholar]
- 32b. Höfer P., Grupp A., Nebenführ H., Mehring M., Chem. Phys. Lett. 1986, 132, 279–282. [Google Scholar]
- 33.
- 33a. Gütlich P., Bill E., Trautwein A. X., Mössbauer Spectroscopy and Transition Metal Chemistry, Springer, Heidelberg, 2011; [Google Scholar]
- 33b. Collins R. L., Pettit R., J. Am. Chem. Soc. 1963, 85, 2332–2333. [Google Scholar]
- 34.
- 34a. Wade K., J. Chem. Soc. D 1971, 792–793; [Google Scholar]
- 34b. Mingos D. M. P., Acc. Chem. Res. 1984, 17, 311–319; [Google Scholar]
- 34c. Rudolph R. W., Acc. Chem. Res. 1976, 9, 446–452. [Google Scholar]
- 35. Knizia G., J. Chem. Theory Comput. 2013, 9, 4834–4843. [DOI] [PubMed] [Google Scholar]
- 36. Gimferrer G. C.-V. M., Salvador P., Molecules 2020, 25, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neese F. W. F., Becker U., Riplinger C., J. Chem. Phys. 2020, 152, 224108. [DOI] [PubMed] [Google Scholar]
- 38. Fritz M., Schneider S. in The Periodic Table II: Catalytic, Materials, Biological and Medical Applications (Ed.: Mingos D. M. P.), Springer International Publishing, Cham, 2019, pp. 1–36. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.