Abstract
Psoriasis poses a substantial economic burden by reducing the work productivity of affected patients. We aimed to evaluate the negative impact of plaque psoriasis on work productivity and effectiveness of brodalumab in improving work productivity impairment in real‐life employed patients. This analysis was conducted in employed patients from ProLOGUE, an open‐label, multicenter, prospective cohort study (Japan Registry of Clinical Trials identifier: jRCTs031180037). Outcomes included association of Work Productivity and Activity Impairment‐Psoriasis (WPAI‐PSO) domain scores with scores from various patient‐reported outcome measures or Psoriasis Area and Severity Index (PASI) scores at baseline. Change from baseline in WPAI‐PSO domain scores following brodalumab treatment was also evaluated. Of the 73 patients enrolled, 51, 48, and 40 patients were considered employed at baseline, Week 12, and Week 48 of brodalumab treatment, respectively. In the model adjusted by age and sex, the work productivity loss score correlated with the Dermatology Life Quality Index (DLQI), itch Numeric Rating Scale (NRS), Patient Health Questionnaire‐8 (PHQ‐8), and skin pain NRS scores (partial Spearman correlation coefficient [ρ] = 0.608, 0.510, 0.461, and 0.424, respectively); presenteeism score correlated with the DLQI, itch NRS, and skin pain NRS scores (ρ = 0.568, 0.500, and 0.403, respectively); and activity impairment score correlated with the DLQI and PHQ‐8 scores (ρ = 0.530 and 0.414, respectively). None of the WPAI‐PSO domain scores correlated with the PASI score. All WPAI‐PSO domain scores (except absenteeism) significantly reduced from baseline to Weeks 12 (p < 0.0001) and 48 (p < 0.001) with brodalumab treatment. In conclusion, work productivity impairment in psoriasis was associated with various subjective symptoms that can be captured using patient‐reported outcome measures. Brodalumab treatment improved work productivity in real‐life employed patients with plaque psoriasis.
Keywords: brodalumab, employment, patient‐reported outcome measures, psoriasis, work performance
1. INTRODUCTION
Psoriasis is a chronic, immune‐mediated inflammatory skin disease affecting approximately 0.84% of the world's population (64.6 million individuals as of 2017) 1 and 0.34% of the Japanese population, with 60.0% of the patients being men. 2 Psoriasis not only has a profound negative impact on the physical, social, and psychological well‐being of patients 3 but also poses a substantial economic burden on healthcare systems, patients, and their families. 4 , 5 A case–control study in Japan showed that most patients with psoriasis, especially men, present for their first clinical visit between 30 and 69 years of age, 6 which are considered the most economically productive years of life. The costs of treating psoriasis with topical or systemic medication alone or biologics 7 , 8 and indirect costs associated with unemployment and reduced working ability add to the financial burden. 5
Previous randomized controlled trials (RCTs) have demonstrated that biologic therapies are associated with significant improvement in work productivity/reduced work impairment and a reduction in associated costs in patients with moderate‐to‐severe psoriasis. 9 , 10 , 11 However, there are limited reports on the effect of brodalumab, a human anti‐interleukin‐17 receptor monoclonal antibody approved for the treatment of plaque psoriasis, 12 , 13 , 14 on work productivity.
Patient‐reported outcomes (PROs) are established tools used by physicians and patients for shared decision‐making on treatment preferences, optimizing treatment strategies and outcomes, and assessing patient satisfaction toward a treatment strategy. 15 , 16 , 17 Work productivity outcomes are commonly assessed using the Work Productivity and Activity Impairment (WPAI) questionnaire—a PRO‐based quantitative measure of health‐related loss in work productivity. 18 , 19 It measures absenteeism (work time missed), presenteeism (impairment at work/reduced on‐the‐job effectiveness), work productivity loss (WPL; overall work impairment/absenteeism plus presenteeism), and activity impairment (AI; impairment in regular daily activities other than work) 7 days prior to questionnaire completion. WPAI has been adapted to specific diseases/health problems; WPAI‐Psoriasis (WPAI‐PSO) is a validated version of the questionnaire used to collect patient responses on the impact of psoriasis on work‐related and regular activities. 19 , 20
Therefore, using PROs to assess work productivity and effectiveness of biologic treatment in improving work productivity can provide further insights into the impact of psoriasis on real‐life patients. Using various PROs, the single‐arm, interventional, open‐label, multicenter, prospective ProLOGUE study 21 , 22 (Japan Registry of Clinical Trials identifier: jRCTs031180037) assessed the effectiveness of brodalumab in real‐life Japanese patients with plaque psoriasis. In this analysis of the ProLOGUE study, we assessed the associations between PROs and work productivity to evaluate the negative impact of plaque psoriasis on work productivity and the effectiveness of brodalumab in improving work productivity impairment in real‐life employed patients.
2. METHODS
2.1. Study design, patients, and treatment
The design and key eligibility criteria of the ProLOGUE study have been reported previously. 21 , 22 The ProLOGUE study was conducted at 15 facilities across Japan from October 2017 to March 2020 and included patients (aged ≥18 years) with plaque psoriasis who had no peripheral arthritis symptoms, could self‐administer brodalumab, and had not appropriately responded to existing systemic treatments. 21 Patients received brodalumab 210 mg subcutaneously on Day 1 and at Weeks 1 and 2, followed by once every 2 weeks. Patients were treated in daily clinical practice per the Japanese drug package insert, and no criteria were set for concomitant or prohibited therapies. 22
This study was first reviewed and approved by the research ethics committee of each participating facility. Following enforcement of the Japanese act for the conduct of clinical research funded by pharmaceutical companies in April 2018, the study was reviewed and approved by the Certified Review Board of Nippon Medical School Foundation. All patients provided written informed consent for participation in the study.
2.2. Outcomes
The outcomes assessed were correlations between WPAI‐PSO domain scores and scores from other PRO measures or the Psoriasis Area and Severity Index (PASI) scores at baseline, change from baseline in WPAI‐PSO domain scores following brodalumab treatment, and income opportunity loss because of WPL in employed patients.
2.3. Assessments
Patients' employment status and work‐associated problems due to psoriasis during the preceding 7 days were assessed at baseline and at Weeks 12 and 48 of brodalumab treatment using the WPAI‐PSO questionnaire 19 with the help of an electronic PRO system. The WPAI‐PSO questionnaire comprised six questions. Patients who responded “yes” to the WPAI‐PSO question 1 “Are you currently employed (working for pay)?” were deemed employed. Patients answering “yes” to question 1 at baseline; at baseline and Week 12; and at baseline, Week 12, and Week 48 were considered employed at baseline, Week 12, and Week 48, respectively. The domain scores for absenteeism, presenteeism, WPL, and AI were calculated on a scale of 0.0%–100.0% each, with higher scores indicating more impairment or lower productivity. 19 Patients reporting a score of greater than 0.0% in any domain were considered as having impairment in that WPAI‐PSO domain.
Other scores that were captured using an electronic PRO system before baseline medical examination were current dermatology‐specific health‐related quality of life (HRQoL), measured using the Dermatology Life Quality Index (DLQI); 23 itching and skin pain levels during the preceding 24 h, assessed using the itch Numeric Rating Scale (NRS) 24 , 25 and skin pain NRS 26 scores, respectively (range: 0 [no itch/pain] to 10 [worst imaginable itch/pain]); patients' satisfaction with their medication over the preceding 2–3 weeks or since the most recent use, assessed using the Treatment Satisfaction Questionnaire for Medication‐9 (TSQM‐9) domain scores (effectiveness, convenience, and global satisfaction; 0.0%–100.0% each); 27 anxiety and depressive symptoms during the preceding 2 weeks, assessed using the Generalized Anxiety Disorder‐7 (GAD‐7) score (range, 0–21) 28 and the Patient Health Questionnaire‐8 (PHQ‐8) score (range, 0–24), 29 respectively (higher scores indicating more severe symptoms); sleep problems during the preceding 4 weeks, assessed using the Sleep Problems Index‐II (SPI‐II) score (range, 0–100; higher scores indicating fewer sleep‐related problems); 30 and general health status, assessed on the day of the survey using the European Quality of Life 5‐Dimensions 5‐Levels Utility Index (EQ‐5D‐5L UI) score (range, −0.025 to 1.000; higher scores indicating higher health utility), calculated using the Japanese tariff. 31 The PASI score was assessed by the attending physician.
2.4. Statistical analyses
All patients except those who did not receive brodalumab were included in the full analysis set. This analysis was conducted in those patients in the full analysis set who were employed according to question 1 of the WPAI‐PSO questionnaire. Correlations between each WPAI‐PSO domain score and the PASI score as well as scores from other PRO measures were assessed using the partial Spearman correlation coefficient ([ρ]; absolute value ≥0.70, strongly correlated; ≥0.40 to <0.70, correlated; ≥0.20 to <0.40, weakly correlated; <0.20, not correlated), 32 with a significance level of p < 0.05 (two‐tailed). The covariates used for adjustment were age and sex. The WPAI‐PSO domain scores and PASI and DLQI scores of employed patients at baseline were compared with those at Weeks 12 and 48 of brodalumab treatment using the Wilcoxon signed‐rank test.
Based on the 2017 national wage data in Japan published by the National Tax Agency, Commissioner's Secretariat, Planning Division (stratified by age category and sex), 33 the income opportunity loss (loss of annual income per person owing to WPL) at baseline and Week 48 for each employed patient was calculated using the reference value that matched the age category (20–24, 25–29, 30–34, 35–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, and ≥70 years) or sex.
Analyses were performed using last observation carried forward, where discontinuations (patients who withdrew from the study) up to Week 12 were recorded as Week 12 data and those between Weeks 12 and 48 were recorded as Week 48 data. No other imputation for missing values was performed. In addition, all analyses were exploratory in nature; hence, the analysis did not account for multiple comparisons. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
3. RESULTS
3.1. Patient disposition and baseline characteristics
Of the 73 patients originally enrolled in this study, 21 51 (69.9%), 48 (65.8%), and 40 (54.8%) were deemed employed at baseline, Week 12, and Week 48, respectively (Figure 1). Among the employed patients at baseline, 44 (86.3%) were men. The median (quartile [Q]1–Q3) age was 54.0 (42.0–62.0) years; body weight, 70.0 (63.0–80.2) kg; and body mass index, 24.5 (22.9–28.0) kg/m2 (Table 1). Median (Q1–Q3) PASI, DLQI, and WPAI‐PSO AI scores at baseline were 12.6 (9.2–15.3), 7.0 (3.0–10.0), and 10.0% (0.0%–50.0%), respectively.
FIGURE 1.
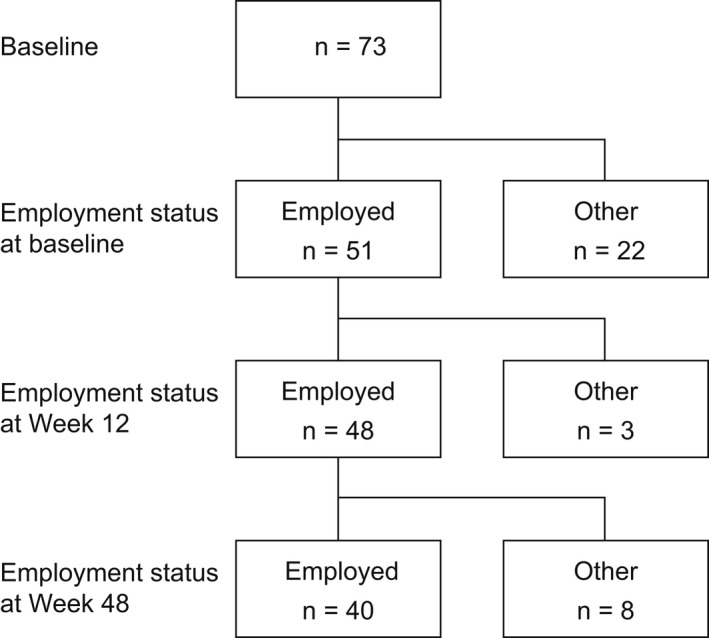
Patient disposition. “Employed” refers to patients who responded “yes” to question 1 of the WPAI‐PSO questionnaire. “Other” refers to patients who responded “no” to question 1 of the WPAI‐PSO questionnaire. 19 WPAI‐PSO, Work Productivity and Activity Impairment‐Psoriasis.
TABLE 1.
Baseline demographics and characteristics of employed patients
| Category | All patients (n = 51) |
|---|---|
| Male | 44 (86.3) |
| BMI a , ≥25 kg/m2 | 22 (45.8) |
| Smoking | 37 (72.5) |
| Drinking | 46 (90.2) |
| Prior biologics use | 21 (41.2) |
| Age, years | 54.0 (42.0–62.0) |
| Disease duration, years | 16.0 (8.0–24.0) |
| Age of onset, years | 33.0 (25.0–48.0) |
| Body weight a , kg | 70.0 (63.0–80.2) |
| BMI a , kg/m2 | 24.5 (22.9–28.0) |
| PASI score | 12.6 (9.2–15.3) |
| DLQI score | 7.0 (3.0–10.0) |
| WPAI‐PSO AI, % | 10.0 (0.0–50.0) |
Note: Data are presented as n (%) or median (Q1–Q3).
Abbreviations: BMI, body mass index; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; Q, quartile; WPAI‐PSO AI, activity impairment domain of the Work Productivity and Activity Impairment‐Psoriasis questionnaire.
n = 48 as body weight and BMI data were missing for three patients.
3.2. Correlation between WPAI‐PSO domain scores and PRO and PASI scores at baseline
In the adjusted model, the WPL score correlated with the DLQI, itch NRS, PHQ‐8, and skin pain NRS scores (ρ = 0.608, 0.510, 0.461, and 0.424, respectively) and weakly correlated with the GAD‐7, SPI‐II, EQ‐5D‐5L UI, TSQM‐9 effectiveness, and TSQM‐9 convenience scores (ρ = 0.329, −0.372, −0.330, −0.309, and − 0.283, respectively; Table 2). No correlation was found between the WPL and TSQM‐9 global satisfaction scores (ρ = −0.189). The presenteeism score correlated with the DLQI, itch NRS, and skin pain NRS scores (ρ = 0.568, 0.500, and 0.403, respectively) and weakly correlated with the PHQ‐8, GAD‐7, TSQM‐9 convenience, EQ‐5D‐5L UI, and SPI‐II scores (ρ = 0.358, 0.309, −0.286, −0.285, and − 0.283, respectively). In contrast, the absenteeism score weakly correlated with only the PHQ‐8 and SPI‐II scores (ρ = 0.367 and − 0.390, respectively). The AI score correlated with the DLQI and PHQ‐8 scores (ρ = 0.530 and 0.414, respectively) and weakly correlated with the GAD‐7 and TSQM‐9 convenience scores (ρ = 0.337 and − 0.298, respectively).
TABLE 2.
Correlation (ρ) between WPAI‐PSO domain scores and symptom‐related, HRQoL‐related, and satisfaction‐related PRO and PASI scores at baseline
| Scores | Unadjusted model | Adjusted model | ||||||
|---|---|---|---|---|---|---|---|---|
| WPL | Presenteeism | Absenteeism | AI | WPL | Presenteeism | Absenteeism | AI | |
| PASI | 0.335 (0.0161) | 0.36 (0.0094) | 0.242 (0.0872) | 0.363 (0.0088) | 0.228 (0.1155) | 0.253 (0.0789) | 0.244 (0.0905) | 0.251 (0.0814) |
| Itch NRS | 0.527 (<0.0001) | 0.516 (0.0001) | 0.133 (0.3516) | 0.329 (0.0184) | 0.51 (0.0002) | 0.5 (0.0003) | 0.127 (0.3831) | 0.277 (0.0536) |
| Skin pain NRS | 0.443 (0.0011) | 0.423 (0.002) | 0.153 (0.2833) | 0.263 (0.0626) | 0.424 (0.0024) | 0.403 (0.0041) | 0.145 (0.3208) | 0.209 (0.149) |
| GAD‐7 | 0.429 (0.0017) | 0.415 (0.0025) | 0.137 (0.3394) | 0.445 (0.0011) | 0.329 (0.0209) | 0.309 (0.0307) | 0.128 (0.3809) | 0.337 (0.0178) |
| PHQ‐8 | 0.523 (<0.0001) | 0.441 (0.0012) | 0.352 (0.0114) | 0.481 (0.0004) | 0.461 (0.0009) | 0.358 (0.0115) | 0.367 (0.0095) | 0.414 (0.0031) |
| SPI‐II | −0.333 (0.017) | −0.253 (0.0736) | −0.388 (0.0049) | −0.153 (0.2847) | −0.372 (0.0084) | −0.283 (0.0485) | −0.39 (0.0055) | −0.174 (0.2311) |
| DLQI | 0.692 (<0.0001) | 0.667 (<0.0001) | 0.203 (0.1534) | 0.641 (<0.0001) | 0.608 (<0.0001) | 0.568 (<0.0001) | 0.228 (0.1143) | 0.53 (<0.0001) |
| EQ‐5D‐5L UI | −0.364 (0.0087) | −0.325 (0.02) | −0.146 (0.305) | −0.287 (0.0409) | −0.33 (0.0204) | −0.285 (0.0474) | −0.147 (0.3149) | −0.244 (0.0913) |
| TSQM‐9: effectiveness | −0.267 (0.0585) | −0.236 (0.0961) | −0.176 (0.218) | −0.162 (0.2553) | −0.309 (0.0308) | −0.275 (0.0557) | −0.189 (0.1943) | −0.206 (0.1552) |
| TSQM‐9: convenience | −0.349 (0.012) | −0.354 (0.0108) | −0.067 (0.6391) | −0.357 (0.0102) | −0.283 (0.0487) | −0.286 (0.0463) | −0.074 (0.6133) | −0.298 (0.0373) |
| TSQM‐9: global satisfaction | −0.108 (0.4514) | −0.077 (0.5914) | −0.078 (0.5852) | 0.037 (0.7949) | −0.189 (0.1934) | −0.157 (0.2803) | −0.09 (0.5395) | −0.036 (0.8046) |
Note: Data are presented as ρ (p value).
Abbreviations: AI, activity impairment; DLQI, Dermatology Life Quality Index; EQ‐5D‐5L UI, European Quality of Life 5‐Dimensions 5‐Levels Utility Index; GAD‐7, Generalized Anxiety Disorder‐7; HRQoL, health‐related quality of life; NRS, Numeric Rating Scale; PASI, Psoriasis Area and Severity Index; PHQ‐8, Patient Health Questionnaire‐8; PRO, patient‐reported outcome; SPI‐II, Sleep Problems Index‐II; TSQM‐9, Treatment Satisfaction Questionnaire for Medication‐9; WPAI‐PSO, Work Productivity and Activity Impairment‐Psoriasis; WPL, work productivity loss; ρ, partial Spearman correlation coefficient.
None of the WPAI‐PSO domain scores showed correlation with the PASI score in the adjusted analysis, although a weak correlation was observed between the PASI and WPL, presenteeism, and AI domain scores in the unadjusted analysis.
3.3. Work productivity impairment
Of the 51 employed patients at baseline, 31 (60.8%) reported WPL (score >0.0% either in the WPAI‐PSO absenteeism and/or presenteeism domains), with 21 (41.2%), 2 (3.9%), and 8 (15.7%) patients reporting impairment because of presenteeism alone, absenteeism alone, and both absenteeism and presenteeism, respectively (Figure 2). Impairment in the WPAI‐PSO AI domain (score >0.0%) was reported by 32 (62.7%) patients (data not shown).
FIGURE 2.
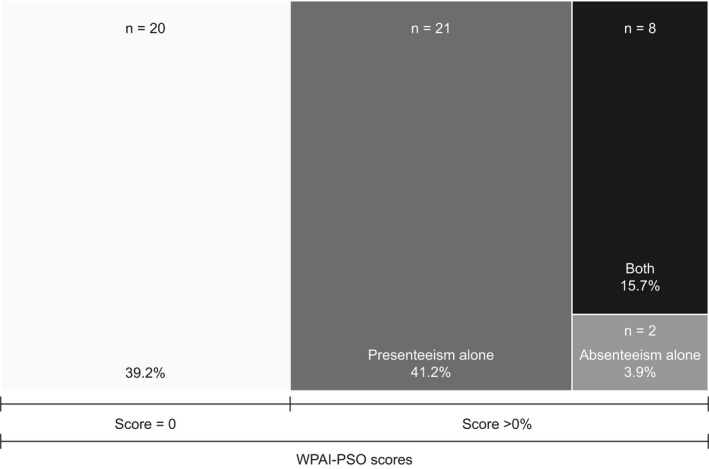
Work productivity impairment in employed patients at baseline. Data represent patients with work productivity impairment (WPAI‐PSO score >0.0%) in the absenteeism or presenteeism domains. WPAI‐PSO, Work Productivity and Activity Impairment‐Psoriasis.
3.4. Change from baseline in WPAI‐PSO domain scores following brodalumab treatment
A statistically significant reduction from baseline in all WPAI‐PSO domain scores, except absenteeism (p = 0.4868), was observed at Week 12 (p < 0.0001 for WPL, presenteeism, and AI, each; Figure 3a) and Week 48 (p = 0.0002, 0.0005, and <0.0001 for WPL, presenteeism, and AI, respectively; Figure 3b) of brodalumab treatment. In employed patients with impaired WPAI‐PSO domain scores (>0.0%) at baseline, WPL, presenteeism, and AI scores significantly decreased from baseline to Week 12 (p < 0.0001 for all; Figure 3c) and Week 48 (WPL, p < 0.0001; presenteeism, p = 0.0002; AI, p < 0.0001; Figure 3d) of brodalumab treatment. The absenteeism score did not show any significant reduction in employed patients, irrespective of their WPAI‐PSO scores at baseline. In employed patients with no impairment in the WPAI‐PSO domain scores (0.0%) at baseline, no significant difference from baseline was observed following brodalumab treatment (data not shown). The PASI and DLQI scores of employed patients significantly decreased from baseline to Weeks 12 and 48 (p < 0.0001 for each) of brodalumab treatment (Table S1 in Appendix S1).
FIGURE 3.
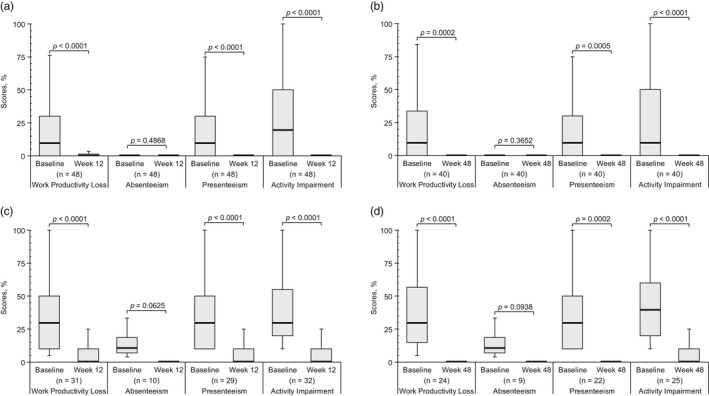
Change from baseline in the WPAI‐PSO domain scores: (a) baseline vs Week 12 in all employed patients, (b) baseline vs Week 48 in all employed patients, (c) baseline vs Week 12 in employed patients with impaired WPAI‐PSO domain score (>0.0%) at baseline, and (d) baseline vs Week 48 in employed patients with impaired WPAI‐PSO domain score (>0.0%) at baseline. Top whisker represents Q3 + (1.5 × IQR) or the maximum, whichever is lower; bottom whisker represents Q1 − (1.5 × IQR) or the minimum, whichever is higher; top border of the box represents Q3; bottom border of the box represents Q1; middle (bolded) line of the box represents the median. IQR, interquartile range; Q, quartile; WPAI‐PSO, Work Productivity and Activity Impairment‐Psoriasis.
3.5. Income opportunity loss
Income opportunity loss owing to WPL in employed patients significantly decreased from baseline to Week 48 (p = 0.0002) of brodalumab treatment (Figure 4). Although income opportunity loss was observed to numerically decrease at Week 48 of brodalumab treatment on stratifying patients by age (25–44 and 45–64 years) and sex (Figure S1), statistical analyses to confirm these findings were not performed. Furthermore, no income opportunity loss owing to WPL at both baseline and Week 48 was observed in patients aged ≥65 years (Figure S1) because the WPL domain score was 0.0% in this age group (data not shown).
FIGURE 4.
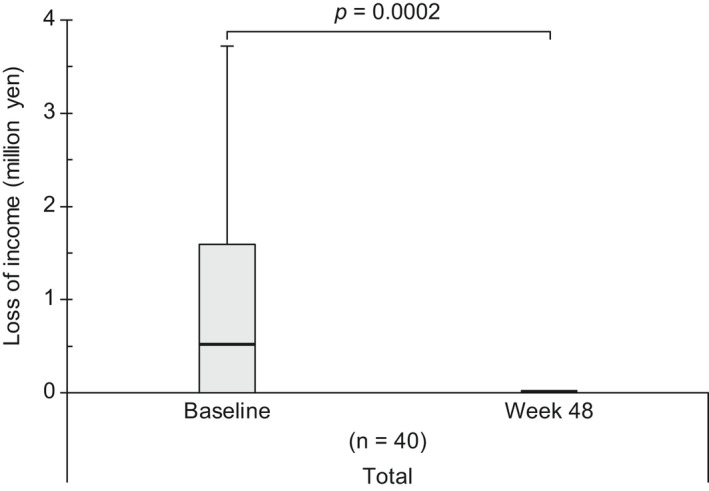
Change in income opportunity loss (loss of annual income per person) because of WPL from baseline to Week 48 in all employed patients. Top whisker represents Q3 + (1.5 × IQR) or the maximum, whichever is lower; bottom whisker represents Q1 – (1.5 × IQR) or the minimum, whichever is higher; top border of the box represents Q3; bottom border of the box represents Q1; middle (bolded) line of the box represents the median. IQR, interquartile range; Q, quartile; WPL, work productivity loss.
4. DISCUSSION
Work productivity is a significant challenge for employed patients with psoriasis. 34 , 35 , 36 Treatment with biologics has shown efficacy in improving work productivity in patients with moderate‐to‐severe psoriasis in several RCTs. 10 , 37 , 38 Results of the current analysis of the ProLOGUE study confirmed improved work productivity in Japanese employed patients with psoriasis who were treated with brodalumab in daily clinical practice, as illustrated by reductions in the WPL, presenteeism, and AI domain scores from baseline to Weeks 12 and 48 of brodalumab treatment.
In the current analysis, approximately 40% of the employed patients at baseline did not show WPL. The median baseline WPL score was 10.0% for all employed patients and 30.0% for employed patients with impaired WPAI‐PSO domain score at baseline, which is lower than the mean WPL score at baseline (40.2%) reported in a post‐marketing surveillance of a biologic agent in Japanese patients with psoriatic arthritis. 39 The difference in the baseline WPL score between the studies can be attributed to the exclusion of patients with peripheral arthritis from the present study because previous research has suggested an association between psoriatic arthritis and reduced work productivity. 40 , 41 In contrast to previous research findings derived from patients with psoriasis treated with biologics, 41 , 42 no association was observed between improvement in the PASI score and reduction in WPL in the current analysis, which could be supported by results of other real‐life studies. 39 , 43 The lack of association between these two factors observed in some of the real‐life studies, including ours, can be attributed to lower baseline PASI scores in real‐life settings (mean, 9.0–9.5; 39 , 43 median, 12.1 21 ) compared with those recorded in the RCT (mean, 21.5–21.7) 44 or the use of models adjusted by age and sex in our analysis and a previous real‐life study. 43 Indeed, in the unadjusted model of the current analysis, the PASI score and WPL domain score showed a weak correlation.
We observed a decrease in income opportunity loss due to WPL in employed patients with psoriasis following brodalumab treatment, which remained consistent in patients aged <65 years. As psoriasis is more common in patients aged 30–69 years, 6 an aggressive therapy including treatment with biologics may be needed for patients in their prime working years to improve their clinical condition and minimize indirect costs resulting from WPL.
Our study has some limitations. First, the number of patients, especially women, with an impaired absenteeism domain score at baseline was limited. Second, we did not collect information on patients' working status (part‐ or full‐time) or type of occupation, making it difficult to evaluate the impact of these factors on the current results. Lastly, our results are indicative of only a partial cost to patients with psoriasis because they only show the inferred opportunity loss by the patients themselves (the expected impact on their income), not accounting for other expenses such as indirect (caregivers and transportation) and direct (medical fees) costs.
In summary, the impairment in work productivity in real‐life employed patients with psoriasis was associated with various subjective symptoms that can be captured using PROs. Therefore, WPAI‐PSO can be an important source of information for shared decision‐making between patients with psoriasis, most of whom are in their prime working years, and physicians for choosing and optimizing treatment options. Treatment with brodalumab resulted in improved work productivity and minimized annual income loss due to WPL in employed patients with psoriasis.
CONFLICT OF INTEREST
H. Saeki reports grants from Kyowa Kirin during the study period; grants, personal fees, and nonfinancial support from Kyowa Kirin, Mitsubishi Tanabe Pharma, Taiho Pharmaceutical, Maruho, TOKIWA Pharmaceutical, Torii Pharmaceutical, and Eisai outside the submitted work; and personal fees and nonfinancial support from Sanofi, Celgene, and KYORIN Pharmaceutical outside the submitted work. Y. Kanai is an employee of Kyowa Kirin. K. Murotani reports grants from Kyowa Kirin during the study period. K. Ito reports grants from Kyowa Kirin during the study period and personal fees from Kyowa Kirin, Mitsubishi Tanabe Pharma, Sato Pharmaceutical, Ushio, Amgen, Janssen Pharmaceutical, AbbVie, Eisai, Sanofi, Eli Lilly Japan, Maruho, Nippon Kayaku, Taiho Pharmaceutical, and Novartis Pharma outside the submitted work. T. Miyagi reports grants from Kyowa Kirin during the study period; grants, personal fees, and nonfinancial support from AbbVie, Kaken Pharmaceutical, Maruho, Boehringer Ingelheim, Eisai, Celgene, Eli Lilly Japan, Novartis Pharma, and Taiho Pharmaceutical outside the submitted work; grants and personal fees from Daiichi Sankyo, Sanofi, Ono Pharmaceutical, Mitsubishi Tanabe Pharma, and Otsuka Pharmaceutical outside the submitted work; personal fees and nonfinancial support from Janssen Pharmaceutical; grants from Actelion, Earth Corporation, Teijin Pharma, LEO Pharma, and Sato Pharmaceutical outside the submitted work; and personal fees from CSL Behring outside the submitted work. H. Takahashi reports grants from Kyowa Kirin during the study period. Y. Tada reports grants from Kyowa Kirin during the study period; grants, personal fees, and nonfinancial support from Kyowa Kirin, Eli Lilly Japan, AbbVie, Maruho, Celgene, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma, Novartis Pharma, Sanofi, UCB Japan, Torii Pharmaceutical, LEO Pharma, Eisai, Kaken Pharmaceutical, Pfizer, Ushio, Meiji Seika Pharma, Nippon Boehringer Ingelheim, JIMRO, Bristol Myers Squibb, and TOKIWA Pharmaceutical outside the submitted work; grants and nonfinancial support from Kanebo Cosmetics, MSD, Ono Pharmaceutical, Pola Pharma, Nihon Pharmaceutical, Smith & Nephew, and Sato Pharmaceutical outside the submitted work; personal fees and nonfinancial support from Janssen Pharmaceutical outside the submitted work; grants from Japan Blood Products Organization, Mochida Healthcare, Oshimatsubaki, and Shionogi outside the submitted work; and personal fees from Chugai Pharmaceutical outside the submitted work. M. Higashiyama reports grants from Kyowa Kirin during the study period; personal fees and nonfinancial support from LEO Pharma outside the submitted work; and personal fees from Kyowa Kirin, AbbVie, Celgene, Taiho Pharmaceutical, Torii Pharmaceutical, Mitsubishi Tanabe Pharma, Eli Lilly Japan, Novartis Pharma, Maruho, and Janssen Pharmaceutical outside the submitted work. Y. Hashimoto reports grants from Kyowa Kirin during the study period and personal fees from Kyowa Kirin, Eisai, AbbVie, Eli Lilly Japan, Nippon Kayaku, Janssen Pharmaceutical, Taiho Pharmaceutical, Torii Pharmaceutical, LEO Pharma, Maruho, UCB Japan, Novartis Pharma, and Celgene outside the submitted work. H. Kitabayashi is an employee of Kyowa Kirin and owns stock in the company. S. Imafuku reports grants from Kyowa Kirin during the study period; grants and personal fees from AbbVie, Eisai, Kaken Pharmaceutical, Kyowa Kirin, Sato Pharmaceutical, Sanofi, Taiho Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Tsumura, Torii Pharmaceutical, Nippon Zoki Pharmaceutical, Novartis Pharma, Maruho, and LEO Pharma outside the submitted work; grants from Pola Pharma outside the submitted work; and personal fees from Astellas, Eli Lilly Japan, MSD, Otsuka Pharmaceutical, Ono Pharmaceutical, Sun Pharma, GSK, JIMRO, Celgene, Daiichi Sankyo, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Japan Blood Products Organization, Pfizer, Bristol Myers Squibb, Meiji Seika Pharma, Janssen Pharmaceutical, and UCB Japan outside the submitted work.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This study was funded by Kyowa Kirin Co., Ltd. Employees from Kyowa Kirin Co., Ltd., were involved in the design and conduct of the study, management and interpretation of the data, and review of the manuscript. Medical writing support was provided by Mami Hirano, MS, and Archana Patkar, PhD, of Cactus Life Sciences (part of Cactus Communications) and funded by Kyowa Kirin Co., Ltd. Statistical analysis support, in the form of statistical analysis plan preparation, analysis program creation, and analysis data quality control, was provided by Masashi Suzuki of I'cros Co., Ltd., and funded by Kyowa Kirin Co., Ltd.
Saeki H, Kanai Y, Murotani K, Ito K, Miyagi T, Takahashi H, et al. Work productivity in real‐life employed patients with plaque psoriasis: Results from the ProLOGUE study. J Dermatol. 2022;49:970–978. 10.1111/1346-8138.16517
DATA AVAILABILITY STATEMENT
We are unable to share the data at this time because the study began enrolling patients before January 1, 2019, and informed consent does not specify data sharing, although it does mention secondary use.
REFERENCES
- 1. AlQassimi S, AlBrashdi S, Galadari H, Hashim MJ. Global burden of psoriasis ‐ comparison of regional and global epidemiology, 1990 to 2017. Int J Dermatol. 2020;59:566–71. [DOI] [PubMed] [Google Scholar]
- 2. Kubota K, Kamijima Y, Sato T, Ooba N, Koide D, Iizuka H, et al. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ. 2015;5:e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999;41:401–7. [DOI] [PubMed] [Google Scholar]
- 4. Chen KC, Hung ST, Yang CW, Tsai TF, Tang CH. The economic burden of psoriatic diseases in Taiwan. J Dermatol Sci. 2014;75:183–9. [DOI] [PubMed] [Google Scholar]
- 5. Villacorta R, Teeple A, Lee S, Fakharzadeh S, Lucas J, McElligott S. A multinational assessment of work‐related productivity loss and indirect costs from a survey of patients with psoriasis. Br J Dermatol. 2020;183:548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naito R, Imafuku S. Distinguishing features of body mass index and psoriasis in men and women in Japan: a hospital‐based case‐control study. J Dermatol. 2016;43:1406–11. [DOI] [PubMed] [Google Scholar]
- 7. Takahashi H, Satoh K, Takagi A, Iizuka H. Economic burden of psoriatic patients in Japan: analysis from a single outpatient clinic. J Dermatol. 2017;44:1024–6. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi H, Satoh K, Takagi A, Iizuka H. Cost‐efficacy and pharmacoeconomics of psoriatic patients in Japan: analysis from a single outpatient clinic. J Dermatol. 2019;46:478–81. [DOI] [PubMed] [Google Scholar]
- 9. Kimball AB, Yu AP, Signorovitch J, Xie J, Tsaneva M, Gupta SR, et al. The effects of adalimumab treatment and psoriasis severity on self‐reported work productivity and activity impairment for patients with moderate to severe psoriasis. J Am Acad Dermatol. 2012;66:e67–76. [DOI] [PubMed] [Google Scholar]
- 10. Armstrong AW, Lynde CW, McBride SR, Ståhle M, Edson‐Heredia E, Zhu B, et al. Effect of ixekizumab treatment on work productivity for patients with moderate‐to‐severe plaque psoriasis: analysis of results from 3 randomized phase 3 clinical trials. JAMA Dermatol. 2016;152:661–9. [DOI] [PubMed] [Google Scholar]
- 11. Warren RB, Halliday A, Graham CN, Gilloteau I, Miles L, McBride D. Secukinumab significantly reduces psoriasis‐related work impairment and indirect costs compared with ustekinumab and etanercept in the United Kingdom. J Eur Acad Dermatol Venereol. 2018;32:2178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti‐interleukin‐17‐receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–9. [DOI] [PubMed] [Google Scholar]
- 13. Nakagawa H, Niiro H, Ootaki K. Japanese brodalumab study group. Brodalumab, a human anti‐interleukin‐17‐receptor antibody in the treatment of Japanese patients with moderate‐to‐severe plaque psoriasis: efficacy and safety results from a phase II randomized controlled study. J Dermatol Sci. 2016;81:44–52. [DOI] [PubMed] [Google Scholar]
- 14. Greig SL. Brodalumab: first global approval. Drugs. 2016;76:1403–12. [DOI] [PubMed] [Google Scholar]
- 15. Gorelick J, Shrom D, Sikand K, Renda L, Burge R, Dworkin C, et al. Understanding treatment preferences in patients with moderate to severe plaque psoriasis in the USA: results from a cross‐sectional patient survey. Dermatol Ther. 2019;9:785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strohal R, Prinz JC, Girolomoni G, Nast A. A patient‐centred approach to biological treatment decision making for psoriasis: an expert consensus. J Eur Acad Dermatol Venereol. 2015;29:2390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tada Y, Ishii K, Kimura J, Hanada K, Kawaguchi I. Patient preference for biologic treatments of psoriasis in Japan. J Dermatol. 2019;46:466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–65. [DOI] [PubMed] [Google Scholar]
- 19. Margaret Reilly Associates, Inc . Work productivity and activity impairment questionnaire: Psoriasis (WPAI:PSO) [Internet]. [cited 2022 March 9]. http://www.reillyassociates.net/WPAI‐PSORIASIS‐English‐US_.doc
- 20. Wu JJ, Lin C, Sun L, Goldblum O, Zbrozek A, Burge R, et al. Minimal clinically important difference (MCID) for work productivity and activity impairment (WPAI) questionnaire in psoriasis patients. J Eur Acad Dermatol Venereol. 2019;33:318–24. [DOI] [PubMed] [Google Scholar]
- 21. Imafuku S, Kanai Y, Murotani K, Nomura T, Ito K, Ohata C, et al. Utility of the dermatology life quality index at initiation or switching of biologics in real‐life Japanese patients with plaque psoriasis: results from the ProLOGUE study. J Dermatol Sci. 2021;101:185–93. [DOI] [PubMed] [Google Scholar]
- 22. Imafuku S, Ohata C, Okubo Y, Tobita R, Saeki H, Mabuchi T, et al. Effectiveness of brodalumab in achieving treatment satisfaction for patients with plaque psoriasis: the ProLOGUE study. J Dermatol Sci. 2022;105:176–84. [DOI] [PubMed] [Google Scholar]
- 23. Finlay AY, Khan GK. Dermatology life quality index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. [DOI] [PubMed] [Google Scholar]
- 24. Naegeli AN, Flood E, Tucker J, Devlen J, Edson‐Heredia E. The worst itch numeric rating scale for patients with moderate to severe plaque psoriasis or psoriatic arthritis. Int J Dermatol. 2015;54:715–22. [DOI] [PubMed] [Google Scholar]
- 25. Kimball AB, Luger T, Gottlieb A, Puig L, Kaufmann R, Nikaï E, et al. Impact of ixekizumab on psoriasis itch severity and other psoriasis symptoms: results from 3 phase III psoriasis clinical trials. J Am Acad Dermatol. 2016;75:1156–61. [DOI] [PubMed] [Google Scholar]
- 26. Ljosaa TM, Stubhaug A, Mork C, Moum T, Wahl AK. Improvement in psoriasis area and severity index score predicts improvement in skin pain over time in patients with psoriasis. Acta Derm Venereol. 2013;93:330–4. [DOI] [PubMed] [Google Scholar]
- 27. Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated treatment satisfaction questionnaire for medication (TSQM‐9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med. 2006;166:1092–7. [DOI] [PubMed] [Google Scholar]
- 29. Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ‐8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–73. [DOI] [PubMed] [Google Scholar]
- 30. Allen RP, Kosinski M, Hill‐Zabala CE, Calloway MO. Psychometric evaluation and tests of validity of the medical outcomes study 12‐item sleep scale (MOS sleep). Sleep Med. 2009;10:531–9. [DOI] [PubMed] [Google Scholar]
- 31. Ikeda S, Shiroiwa T, Igarashi A, Noto S, Fukuda T, Saito S, et al. Developing a Japanese version of the EQ‐5D‐5L value set. J Natl Inst Public Health. 2015;64:47–55. [Google Scholar]
- 32. Germanotta M, Gower V, Papadopoulou D, Cruciani A, Pecchioli C, Mosca R, et al. Reliability, validity and discriminant ability of a robotic device for finger training in patients with subacute stroke. J Neuroeng Rehabil. 2020;17:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. National Tax Agency, Commissioner's Secretariat, Planning Division . 2017 statistics on average wage in private companies (in Japanese) [internet]. [cited 2022 March 9]. https://www.nta.go.jp/publication/statistics/kokuzeicho/minkan2017/pdf/000.pdf
- 34. Chan B, Hales B, Shear N, Ho V, Lynde C, Poulin Y, et al. Work‐related lost productivity and its economic impact on Canadian patients with moderate to severe psoriasis. J Cutan Med Surg. 2009;13:192–7. [DOI] [PubMed] [Google Scholar]
- 35. Pearce DJ, Singh S, Balkrishnan R, Kulkarni A, Fleischer AB, Feldman SR. The negative impact of psoriasis on the workplace. J Dermatolog Treat. 2006;17:24–8. [DOI] [PubMed] [Google Scholar]
- 36. Fowler JF, Duh MS, Rovba L, Buteau S, Pinheiro L, Lobo F, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772–80. [DOI] [PubMed] [Google Scholar]
- 37. Reich K, Foley P, Han C, McElligott S, Muser E, Li N, et al. Guselkumab improves work productivity in patients with moderate‐to‐severe psoriasis with or without depression and anxiety: results from the VOYAGE 2 comparator study versus adalimumab. J Dermatolog Treat. 2020;31:617–23. [DOI] [PubMed] [Google Scholar]
- 38. Li N, Teeple A, Muser E, You Y, Song M, Armstrong AW. Work/study productivity gain and associated indirect cost savings with guselkumab compared with adalimumab in moderate‐to‐severe psoriasis: results from the VOYAGE 1 study. J Dermatolog Treat. 2022;33:278–83. [DOI] [PubMed] [Google Scholar]
- 39. Nakagawa H, Tanaka Y, Sano S, Kameda H, Taniguchi A, Kashiwagi T, et al. Real‐world postmarketing study of the impact of adalimumab treatment on work productivity and activity impairment in patients with psoriatic arthritis. Adv Ther. 2019;36:691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimball AB, Bensimon AG, Guerin A, Yu AP, Wu EQ, Okun MM, et al. Efficacy and safety of adalimumab among patients with moderate to severe psoriasis with co‐morbidities: subanalysis of results from a randomized, double‐blind, placebo‐controlled, phase III trial. Am J Clin Dermatol. 2011;12:51–62. [DOI] [PubMed] [Google Scholar]
- 41. Hayashi M, Saeki H, Ito T, Fukuchi O, Umezawa Y, Katayama H, et al. Impact of disease severity on work productivity and activity impairment in Japanese patients with psoriasis. J Dermatol Sci. 2013;72:188–91. [DOI] [PubMed] [Google Scholar]
- 42. Feldman SR, Zhao Y, Gilloteau I, Graham CN, Miles L, McBride D, et al. Higher psoriasis skin clearance is associated with lower annual indirect costs in the United States: a post hoc analysis from the CLEAR study. J Manag Care Spec Pharm. 2018;24:617–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmitt JM, Ford DE. Work limitations and productivity loss are associated with health‐related quality of life but not with clinical severity in patients with psoriasis. Dermatology. 2006;213:102–10. [DOI] [PubMed] [Google Scholar]
- 44. Blauvelt A, Reich K, Tsai TF, Tyring S, Vanaclocha F, Kingo K, et al. Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate‐to‐severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol. 2017;76:60–9.e9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
We are unable to share the data at this time because the study began enrolling patients before January 1, 2019, and informed consent does not specify data sharing, although it does mention secondary use.


