Summary
Exposure to particulate matter (PM), a major component of air pollution, is associated with exacerbation of chronic respiratory disease, and infectious diseases such as community‐acquired pneumonia. Although PM can cause adverse health effects through direct damage to host cells, our previous study showed that PM can also impact bacterial behaviour by promoting in vivo colonization. In this study we describe the genetic mechanisms involved in the bacterial response to exposure to black carbon (BC), a constituent of PM found in most sources of air pollution. We show that Staphylococcus aureus strain USA300 LAC grown in BC prior to inoculation showed increased murine respiratory tract colonization and pulmonary invasion in vivo, as well as adhesion and invasion of human epithelial cells in vitro. Global transcriptional analysis showed that BC has a widespread effect on S. aureus transcriptional responses, altering the regulation of the major virulence gene regulators Sae and Agr and causing increased expression of genes encoding toxins, proteases and immune evasion factors. Together these data describe a previously unrecognized causative mechanism of air pollution‐associated infection, in that exposure to BC can increase bacterial colonization and virulence factor expression by acting directly on the bacterium rather than via the host.
Introduction
Air pollution is the world's largest single global environmental health risk with an estimated 90% of people worldwide breathing polluted air, this pollution is responsible for over 7 million deaths per year (World Health Organization − News Release, 2018). It is the result of natural and anthropogenic activity, with increased urbanization resulting in significant increases in types and concentrations of pollutants (Manisalidis et al., 2020). Particulate matter (PM) is a major component of air pollution, with particles of <2.5 μm causing the most serious adverse health effects due to deposition in the upper respiratory tract and the ability to enter the lower respiratory tract and bloodstream (Cohen et al., 2017; McNeil, 2019).
PM exposure is strongly associated with cancer and cardiovascular diseases, and exacerbation of chronic respiratory disease, such as COPD and asthma (Cohen et al., 2017). There is also an association with infectious disease, with community‐acquired pneumonia rates most affected (Neupane et al., 2010; Qiu et al., 2014), but less well known is the impact on infective endocarditis (Hsieh et al., 2019), infection of cystic fibrosis patients (Psoter et al., 2015; Psoter et al., 2017), otitis media (Park et al., 2018), chronic rhinosinusitis (Schwarzbach et al., 2020) and adverse effects on chronic skin diseases (Dijkhoff et al., 2020). High levels of PM exposure also alter respiratory microbiome diversity (Li et al., 2017; Li et al., 2019; Mariani et al., 2018; Mariani et al., 2020; Rylance et al., 2016; Wang et al., 2019).
In seeking to explain how PM adversely affects chronic and infectious diseases, research has focused on direct damage to host tissue caused by PM exposure, including increased inflammation, and oxidative stress (Lee et al., 2021). It is also known that in infection, PM can potentiate disease by repressing the immune system (Castranova et al., 2001; Liu et al., 2019; Migliaccio et al., 2013; Shears et al., 2020; Yang et al., 2001) and by disruption of epithelial function (Liu et al., 2019; Misiukiewicz‐Stepien & Paplinska‐Goryca, 2021). The possibility that PM may directly affect bacteria had not received attention until recently. In Hussey et al. (2017) we showed that air pollution does have a direct impact on bacterial behaviour.
Direct exposure of Staphylococcus aureus and Streptococcus pneumoniae to black carbon (BC), a by‐product of biomass burning and a major constituent of PM (Bell et al., 2007), results in major changes in bacterial biofilm formation and antibiotic susceptibility (Hussey et al., 2017). Additionally, we found that in mice simultaneously exposed to S. pneumoniae and BC there was increased bacterial dissemination to the lungs (Hussey et al., 2017). The instillation of BC into the mice did not cause detectable tissue damage, indicating that BC acts as a signal that alters bacterial behaviour (Hussey et al., 2017). Subsequent studies also showed that direct bacterial exposure to a variety of PM sources also increased biofilm formation (Woo et al., 2018; Yadav et al., 2020) and increased S. pneumoniae nasopharyngeal colonization and dissemination to the lungs and middle ear of mice (Yadav et al., 2020). None of these studies sought to determine the biological mechanisms involved in the bacterial response to PM exposure, but the BC‐induced changes could be a key contributing factor in how air pollutants cause increased lower respiratory tract infectious disease.
Here we report investigation of not only the impact of BC on nasopharyngeal colonization and invasion by the community‐acquired, methicillin‐resistant S. aureus (CA‐MRSA) strain USA300 LAC but also the genetic mechanisms involved. We show that, relative to S. aureus alone, simultaneous inoculation of BC and S. aureus into the nasopharynx increases S. aureus numbers in the nasopharynx, lungs and blood of mice, and increases S. aureus adhesion to human respiratory epithelial cells. Staphylococcus aureus grown in BC prior to inoculation also showed increased murine respiratory tract colonization and invasion in vivo as well as adhesion and invasion of human epithelial cells in vitro supporting the hypothesis that BC acts as a hitherto unconsidered signal that has a direct effect on S. aureus behaviour. Global transcriptional analysis showed that BC does indeed have a widespread effect on S. aureus transcriptional responses, altering the regulation of the major virulence gene regulators Sae and Agr and causing increased expression of genes for toxins, proteases and immune evasion factors.
Experimental procedures
Bacterial strains and growth conditions
The methicillin‐resistant S. aureus USA300 LAC was used in this study (Kennedy et al., 2008). Transduction with Phage 11 was used to move the bursa aurealis agrB::Tn (strain ΦΕ95) transposon insertion mutation from the Nebraska Transposon Mutant Library (Bae et al., 2008; Fey et al., 2013) and the saeS::Tn917 from strain Newman sae::Tn917 (Goerke et al., 2001) into USA300 LAC. A double mutant strain LAC agr::tet/∆saePQRS was kindly provided by A. Horswill (University of Colorado). Mutant strains were confirmed by PCR using gene‐specific primers (Table S1). Unless otherwise stated, bacteria were grown in Tryptic Soy Broth (TSB; Beckton Dickinson) statically at 37°C in 5% vol./vol. CO2.
Black carbon
BC (Sigma‐Aldrich product number 699632) was dispersed in sterile dH2O at 2–10 mg ml−1. The particle size of the powder was <500 nm and it contained <500 ppm trace metals. BC is an ideal model particulate for this study as BC does not affect bacterial growth (Hussey et al., 2017), unlike purified synthetic nanoparticles such as Carbon Black which are generally toxic to bacteria (Al‐Jumaili et al., 2017).
Murine colonization model
Experiments were carried out in accordance with the UK Home Office Project Licence P7B01C07A. Female 8‐week‐old outbred CD1 mice from Charles River, UK were used. Animals were allowed to acclimatize for 1 week prior to the experiments. Animals were housed in groups of five, maintained on a 12 h dark/light cycle and allowed unrestricted access to food and water. Prior to use, bacteria were grown in TSB in the presence and absence of 100 μg ml−1 BC, to mid‐exponential phase, and stored in aliquots at −80°C. For use, frozen aliquots were thawed, washed and resuspended in PBS. Mice were intranasally infected with 15 μl containing 1 × 107 CFU S. aureus USA300, or 1 × 107 CFU LAC mixed with 105 μg of BC as previously described (Hussey et al., 2017). After infection, the mice showed no signs of disease over the following 7 days. At days 1 and 7 post‐infection the numbers of bacteria in the nasopharynx, lungs and blood of preselected animals were assessed in homogenized tissue by serial dilution and plating (Hussey et al., 2017). Significance was determined using a Kruskal–Wallis test with Dunn's multiple comparison.
RNA extraction
Staphylococcus aureus strains were grown to late‐exponential phase in TSB, with and without 100 μg ml−1 BC. To preserve RNA integrity, cultures were treated with RNAprotect (Qiagen) and cells were pelleted and stored at −80°C as per the manufacturer's instructions. Bacteria were lysed in 200 μl Tris EDTA (TE) buffer containing 100 μg ml−1 lysostaphin and 50 μg ml−1 proteinase K final concentration. 600 μl of Trizol reagent was added and cells were then mechanically disrupted using an MP Biomedicals FastPrep Instrument and Lysing Matrix B tubes (MP Biomedicals). BC particles were removed by centrifugation at 12 000g and RNA extracted from the bacteria in the supernatant using a Direct‐zol RNA Miniprep Plus Kit (Zymogen) following the manufacturer's instructions. Samples were further treated with TURBO DNA‐free (Ambion) to ensure complete removal of DNA, which was confirmed via qPCR. RNA concentrations were determined using a Nanodrop spectrophotometer.
RNAseq
RNA quality and integrity were assessed using a 2100 Bioanalyser and RNA 6000 Nano chip (Agilent), to ensure a minimum RNA integrity value of 8 (Table S4). Samples were depleted for ribosomal RNA and libraries were prepared using ScriptSeq RNA Library Preparation before paired‐end sequencing on an Illumina NextSeq550.
RNAseq data quality was assessed using FastQC (v. 0.11.5). Trimmomatic (v. 0.36) was used to remove adaptor sequences, and the read correction tool SOAPec (v. 2.01) was used to identify and repair errors in the read data. The reads were mapped to the S. aureus USA300 FRP3757 genome (accession no. CP000255) using HISAT2 (v 2.1.0) and the transcriptome was assembled using STRINGTIE (v. 1.3.3b). The R package DESeq2 was used to test differential gene expression between the samples, and gene expression is expressed as the Log2 Fold Change (L2FC) in expression relative to growth without BC. The screening threshold for the results was set at >1 or <−1 L2FC using an adjusted p‐value (pADJ) of 0.001.
To determine whether any functional groups of genes were significantly upregulated or downregulated in response to BC, GO Enrichment Analysis was carried out and the Fisher's exact test with a p‐value ≤0.05 was used to test the enrichment in each category (Ashburner et al., 2000; Gene, 2021; Mi et al., 2019). Additional gene function data, including TIGRFAM functional groups, were extracted for each locus from the AureoWiki database, which provides a pan‐genome approach to functional annotation of genes (Fuchs et al., 2018).
Quantitative reverse transcriptase PCR
Total RNA was converted into cDNA using Superscript IV VILO Master Mix reverse transcriptase (Invitrogen), and 0.5 ng of cDNA was used for each qPCR reaction. qRT‐PCR was done using SYBR Green Master Mix (Applied Biosystems) in a 7300 Fast System (Applied Biosystems) following the manufacturer's instructions. Relative gene expression for each of the sample genes (for primer details see Table S1) was normalized to the expression of the endogenous control genes gyrB and 16S rRNA and expressed relative to the LAC wild‐type strain cultured without BC, using the ∆∆Ct method to calculate RQ (2−∆∆Ct) (Livak & Schmittgen, 2001). Significance was determined by a Kruskal–Wallis test with Dunn's multiple comparison test (*p < 0.05, **p < 0.01).
Alpha‐haemolysin activity assay
To prepare erythrocytes, heparinised rabbit blood was diluted with 20 vol. of PBS and centrifuged at 3000g for 5 min to pellet the cells. Erythrocytes were washed once in 20 vol. PBS and then resuspended in 6 vol. PBS. To prepare the bacteria S. aureus USA300 LAC was grown to late‐exponential phase in TSB, with and without 100 μg ml−1 BC. Cultures were centrifuged at 3000g for 5 min to pellet the cells, and supernatants were filter sterilized using a 0.2 μm filter membrane. Cell pellets were washed twice in an equal volume of PBS. To measure haemolytic activity an equal volume of prepared erythrocytes and either supernatant or cell suspension were mixed and incubated at 37°C for 30 min. Cells were also mixed with 100 μg ml−1 BC suspended in PBS to determine the haemolytic activity of BC alone. Cell suspensions were centrifuged at 120g for 7 min to pellet intact erythrocytes. The absorbance of the suspension supernatant was measured at 450 nm. A PBS control was used to measure spontaneous haemolysis and SDS was used to measure total erythrocyte lysis. Percentage of total haemolytic activity was calculated as follows:
Data are presented as the mean of three independent biological replicates (±SEM) and significance was determined by two‐way ANOVA.
Epithelial cell adhesion, invasion and persistence
For bacterial adhesion and invasion studies, the human Type II‐like bronchial epithelial cell line A549 was used. 24‐well tissue culture plates were seeded with 1 × 105 cells in RPMI with 1% vol./vol. foetal bovine calf serum (FBS) and grown to 70%–100% confluency prior to inoculation with S. aureus. A549 cells were inoculated with 1 × 107 CFU of S. aureus under the following conditions: (i) S. aureus LAC alone, (ii) S. aureus LAC plus 100 μg ml−1 of BC or (iii) S. aureus LAC grown in the presence of 100 μg ml−1 BC. Bacterial cells were washed twice in PBS and diluted to 1 × 107 CFU/50 μl in PBS prior to A549 inoculation. All doses were confirmed by serial dilution and plating. Infected cells were incubated at 37°C in 5% vol./vol. CO2 for 2 h. For adhesion assays, cells were washed in PBS and lysed in 1% vol./vol. Triton‐X‐100 for 10 min. Bacterial CFU was determined by serial dilution and plating. For invasion and persistence assays, 2 h post‐inoculation A549 cells were washed and resuspended in RPMI containing 300 μg ml−1 gentamicin for 2 h (invasion) before washing and lysing the cells as described above, followed by serial dilution and plating to determine CFU (Richards et al., 2015). Data are presented as the mean of at least three independent biological replicates (±SEM) and significance was determined by one‐way ANOVA with Tukey's multiple comparison test.
Cytotoxicity
A549 cytotoxicity was measured by the presence of lactate dehydrogenase (LDH) in the culture medium. LDH release was measured using CyQuant LDH Cytotoxicity Assay Kit (Invitrogen) as per the manufacturer's instructions. LDH activity was assayed in supernatant from uninfected cells (spontaneous damage), cells infected with bacteria alone and with 100 μg ml−1 BC and cells exposed to 100 μg ml−1 BC alone (to determine cell damage from BC specifically). Absorbance was measured at 490 and 680 nm (background) and the background value was subtracted from the 490 nm reading to give LDH activity. % cytotoxicity was calculated as follows:
Data are presented as the mean of three independent biological replicates (±SEM) and significance was determined by one‐way ANOVA with Tukey's multiple comparison test.
Results
Exposure of S. aureus to BC prior to inoculation increases bacterial numbers in the respiratory tract
Previous studies have shown that bacterial dissemination from the nasopharynx to murine or rat lungs is induced when the animals are exposed to different forms of PM before bacterial inoculation or when bacteria and PM are simultaneously inoculated (Hussey et al., 2017; Shears et al., 2020; Yadav et al., 2020; Zhao et al., 2014). However, the direct effect of PM on bacterial behaviour during colonization was not fully established because the presence of significant levels of PM within the host could potentiate colonization by several mechanisms including damaging host tissue, by acting as a vehicle to support bacterial dissemination through the respiratory tract, or by supporting bacterial growth.
Our previous work showed that BC increased colonization and invasion by S. pneumoniae; however, it was not established whether this was due to direct effects on the bacterium and/or to effects on the host. Biologically relevant concentrations of BC are used that are at the lower end of the range of total amounts (96–378 μg day−1) of air pollution PM reported to be inhaled and deposited in the human respiratory tract each day (Chalvatzaki et al., 2018). To establish if BC directly affects the in vivo behaviour of S. aureus, mice were intranasally inoculated with the bacterium grown in the presence of BC but with the BC particles removed by dilution prior to inoculation. Mice were also inoculated with S. aureus LAC alone and LAC simultaneously inoculated with BC.
Staphylococcus aureus pre‐grown in BC prior to inoculation (gBC) significantly increased staphylococci in the nasopharynx (Figure 1A) and lungs (Figure 1B) at day 7 post‐infection period compared to the control without BC (p < 0.05). When S. aureus were co‐inoculated together with BC (LAC + BC) a significant increase in S. aureus in the nasopharynx and lungs by 7 days post‐infection was also observed (Figure 1A and B both p < 0.01 compared to the control). In contrast, only after co‐inoculation of BC and S. aureus were there more staphylococci in the blood compared to the BC control (Figure 1C, p < 0.05). None of the mice had visible signs of disease and all survived throughout the experiment. Together these data indicate that the increase in infection of the nasopharynx and lungs by S. aureus is caused by the BC acting directly on the bacterium rather than via the host, but the presence of BC is important for bacterial invasion to the blood.
Fig. 1.
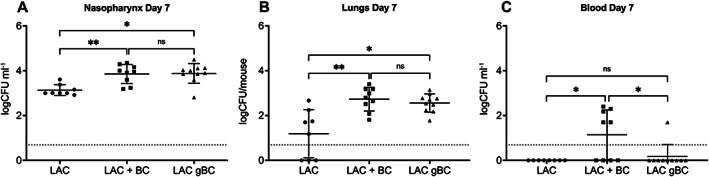
Exposure of S. aureus to BC results in increased respiratory tract colonization in mice. Female CD1 mice were intranasally inoculated with 15 μl containing 107 S. aureus LAC (−BC), 107 LAC with 105 μg BC (+BC) or 107 CFU LAC pre‐grown in the presence of 100 μg ml−1 BC (gBC). After 7 days, bacteria were recovered from the nasopharynx (A), lungs (B) and blood (C) and plated to determine the bacterial CFUs. No mice showed any clinical signs of infection. Data are presented as logCFU ml−1 for nasopharyngeal washes and blood, and logCFU/mouse for lungs. The dotted line marks the limit of detection. Data were analysed using a Kruskal–Wallis test with Dunn's multiple comparison (*p < 0.05, **p < 0.01).
Staphylococcus aureus pre‐grown in BC show increased adhesion and invasion of epithelial cells in vitro
Because S. aureus can invade non‐professional phagocytes thereby avoiding aspects of the immune system (Garzoni & Kelley, 2008), we determined whether BC alters adherence or invasion of S. aureus to human respiratory epithelial cells. The type II‐like bronchial epithelial cells, A549, were exposed to S. aureus LAC grown in the same conditions as the murine colonization.
There was a significant increase in numbers of S. aureus adhering to (Figure 2A, p < 0.0001) and invading (Figure 2B, p < 0.01) A549 cells when grown with BC (gBC) prior to inoculation compared to bacteria grown in the absence of BC (−BC), an observation consistent with a direct effect of BC on the bacteria. There was also increased adhesion of S. aureus to A549 cells when simultaneously inoculated with BC (+BC) (Figure 2A, p < 0.01) but no significant change in invasion. Importantly inoculation with BC alone did not affect A549 cell viability (Figure 2C). Incubation of A549 with S. aureus LAC caused 18% cytotoxicity but there was no significant increase in cytotoxicity on A549 cells during co‐inoculation with BC or when S. aureus were pre‐grown in BC (Figure 2C). Together these data demonstrate that increased S. aureus epithelial adhesion and invasion is caused by direct interaction of BC with the bacteria and does not involve gross changes to the epithelial cells.
Fig. 2.
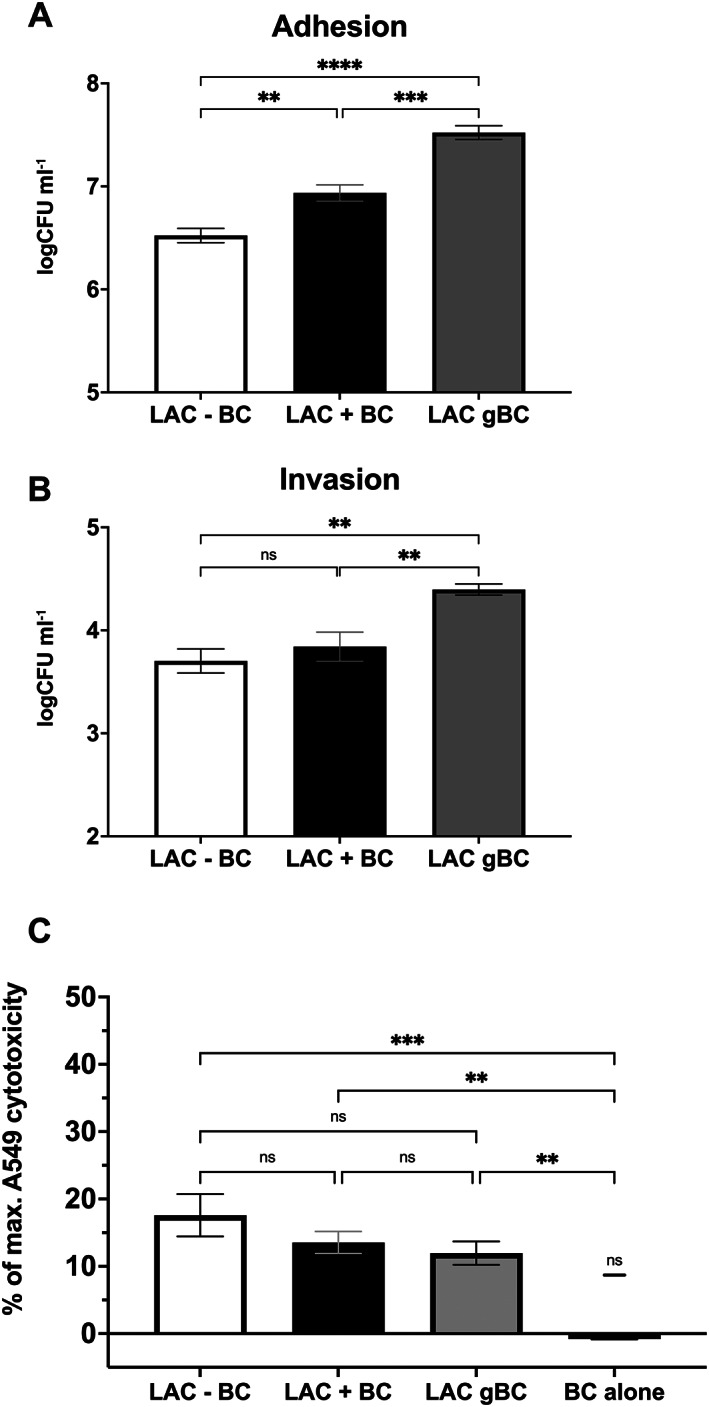
Exposure to BC results in increased adhesion, invasion and persistence within human epithelial cells. Staphylococcus aureus LAC adhesion (A), and invasion (B) of human lung epithelial A549 cells was measured using a gentamicin protection assay. Monolayers of 1 × 105 A549 cells in 24‐well plates were infected at a MOI of 100. Data are presented as logCFU ml−1 and error bars represent 1 SEM of at least five biological repeats. Significance was determined by one‐way ANOVA with Dunnett's multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001). (C) Cytotoxicity was measured through LDH release from A549 cells after 2 h exposure to S. aureus and/or BC. % cytotoxicity is calculated relative to spontaneous cell death (0%) and maximum cell death (100% cell lysis). Significance was determined by one‐way ANOVA with Tukey's multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
BC induces expression of S. aureus genes for toxins and proteases and the SOS response
Having shown that BC alters the phenotype of S. aureus, we used transcriptome sequencing (RNAseq) to investigate the pattern of gene expression induced using the same BC exposure growth conditions as the colonization experiments. RNAseq analysis identified 52 staphylococcal genes that showed a significant increase in expression (Table 1 and Table S2) and 63 genes that showed a significant decrease in expression (Table 2 and Table S3) after exposure to BC.
Table 1.
Genes significantly upregulated at a fold change >2 in response to BC.
| Locus tag (SAUSA300) | Gene name | Product description | Fold change |
|---|---|---|---|
| RS11190 | kdpA | Potassium‐transporting ATPase subunit A | 6.30 |
| RS09620 | splA | Serine protease SplA | 4.88 |
| RS10530 | chp | Chemotaxis‐inhibiting protein CHIPS | 4.75 |
| RS09610 | splC | Serine protease SplC | 4.40 |
| RS09615 | splB | Serine protease SplB | 4.04 |
| RS09595 | splF | Serine protease SplF | 3.99 |
| RS05720 | hla | Alpha‐haemolysin | 3.81 |
| RS09605 | splD | Serine protease SplD | 3.75 |
| RS10525 | scn | Complement inhibitor SCIN‐A | 3.60 |
| RS15090 | – | Phenol‐soluble modulin PSM‐alpha‐3 | 3.53 |
| RS15730 | – | Phenol‐soluble modulin PSM‐alpha‐4 | 3.50 |
| RS05795 | psm_2 | Beta‐class phenol‐soluble modulin | 3.45 |
| RS15735 | – | Phenol‐soluble modulin PSM‐alpha‐2 | 3.44 |
| RS00185 | – | DUF1643 domain‐containing protein | 3.29 |
| RS15740 | – | Phenol‐soluble modulin PSM‐alpha‐1 | 3.07 |
| RS06445 | glpD | Glycerol‐3‐phosphate dehydrogenase/oxidase | 2.96 |
| RS01705 | gehB | YSIRK domain‐containing triacylglycerol lipase Lip2/Geh | 2.90 |
| RS13080 | hlgB | Bi‐component gamma‐haemolysin HlgAB/HlgCB subunit B | 2.87 |
| RS06715 | Hypothetical protein | 2.86 | |
| RS09600 | splE | Serine protease SplE | 2.83 |
| RS06840 | mucB | DNA repair protein MucB | 2.78 |
| RS00515 | plc | Phosphatidylinositol‐specific phospholipase C | 2.68 |
| RS05790 | psm_1 | Beta‐class phenol‐soluble modulin | 2.68 |
| RS02340 | metQ2 | Dipeptide ABC transporter glycylmethionine‐binding lipoprotein | 2.63 |
| RS01835 | fepC | Iron permease FTR1 family protein | 2.61 |
| RS06370 | recA | DNA recombination/repair protein RecA | 2.61 |
| RS04005 | uvrA | Excinuclease ABC subunit UvrA | 2.61 |
| RS04395 | ear | DUF4888 domain‐containing protein | 2.57 |
| RS02335 | metP2 | ABC transporter permease | 2.53 |
| RS14170 | nrdG | Anaerobic ribonucleoside‐triphosphate reductase activating protein | 2.46 |
| RS04985 | comK1 | Competence protein ComK | 2.42 |
| RS13075 | hlgC | Bi‐component gamma‐haemolysin HlgCB subunit C | 2.36 |
| RS10660 | – | HNH endonuclease | 2.34 |
| RS10495 | MAP domain‐containing protein | 2.30 | |
| RS10845 | lukG | Bi‐component leukocidin LukGH subunit G | 2.29 |
| RS01825 | fepA | EfeM/EfeO family lipoprotein | 2.27 |
| RS02330 | metN2 | Methionine ABC transporter ATP‐binding protein | 2.15 |
| RS10505 | hlb‐1 | Sphingomyelin phosphodiesterase | 2.14 |
| RS04000 | uvrB | Excinuclease ABC subunit UvrB | 2.13 |
| RS14555 | – | S‐adenosyl‐l‐methionine hydroxide adenosyltransferase family protein | 2.13 |
| RS06710 | lexA | Transcriptional repressor LexA | 2.11 |
| RS13060 | sbi | Immunoglobulin‐binding protein Sbi | 2.11 |
| RS10420 | – | YolD‐like family protein | 2.10 |
| RS07540 | Panton‐Valentine bi‐component leukocidin subunit F | 2.09 | |
| RS08875 | infC | Translation initiation factor IF‐3 | 2.09 |
| RS04990 | – | IDEAL domain‐containing protein | 2.07 |
| RS03850 | nrdF | Class 1b ribonucleoside‐diphosphate reductase subunit beta | 2.05 |
| RS03840 | nrdI | Class Ib ribonucleoside‐diphosphate reductase assembly flavoprotein NrdI | 2.05 |
| RS11200 | kdpD | Sensor histidine kinase KdpD | 2.04 |
| RS08675 | recJ | Single‐stranded‐DNA‐specific exonuclease RecJ | 2.04 |
| RS06750 | sbcC | SMC family ATPase | 2.04 |
| RS10850 | lukH | Bi‐component leukocidin LukGH subunit H | 2.03 |
Adjusted p‐values for all genes are <0.001.
Table 2.
Genes significantly downregulated at a fold change <−2 in response to BC.
| Locus tag (SAUSA300) | Gene name | Product description | Fold change |
|---|---|---|---|
| RS14135 | betB | Betaine‐aldehyde dehydrogenase | −6.96 |
| RS01470 | – | Hypothetical protein | −4.43 |
| RS14005 | – | TIGR04197 family type VII secretion effector | −3.83 |
| RS09670 | epiA | Gallidermin/nisin family lantibiotic | −3.78 |
| RS05670 | ecb | Complement convertase inhibitor Ecb | −3.75 |
| RS02705 | pdxT | Pyridoxal 5′‐phosphate synthase glutaminase subunit PdxT | −3.66 |
| RS00995 | – | Alpha‐keto acid decarboxylase family protein | −3.59 |
| RS05755 | argF | Ornithine carbamoyltransferase | −3.53 |
| RS11395 | ptpB | Low‐molecular‐weight protein arginine phosphatase | −3.51 |
| RS02700 | pdxS | Pyridoxal 5′‐phosphate synthase lyase subunit PdxS | −3.35 |
| RS05990 | – | Hypothetical protein | −3.34 |
| RS14130 | betA | Oxygen‐dependent choline dehydrogenase | −3.34 |
| RS13660 | ddh | d‐lactate dehydrogenase | −3.15 |
| RS12650 | – | DUF805 domain‐containing protein | −3.15 |
| RS02320 | mccA | Cysteine synthase family protein | −3.14 |
| RS00905 | – | Hypothetical protein | −3.09 |
| RS11900 | – | Aldo/keto reductase | −2.95 |
| RS05495 | – | YlbG family protein | −2.87 |
| RS00665 | – | MFS transporter | −2.81 |
| RS06680 | katA | Catalase | −2.80 |
| RS05760 | arcC1 | Carbamate kinase | −2.78 |
| RS11905 | – | MerR family transcriptional regulator | −2.78 |
| RS05690 | efb | Fibrinogen‐binding protein | −2.76 |
| RS14030 | – | Glyoxalase/bleomycin resistance/extradiol dioxygenase family protein | −2.73 |
| RS02920 | sdrD | MSCRAMM family adhesin SdrD | −2.66 |
| RS01460 | – | ABC transporter permease | −2.66 |
| RS13140 | Type I toxin‐antitoxin system Fst family toxin | −2.62 | |
| RS12640 | – | Sodium ABC transporter permease | −2.61 |
| RS11230 | – | Hypothetical protein | −2.60 |
| RS00560 | – | Oleate hydratase | −2.51 |
| RS04765 | – | DUF2929 domain‐containing protein | −2.50 |
| RS01490 | esxA | WXG100 family type VII secretion effector EsxA | −2.47 |
| RS13655 | frp | NAD(P)H‐dependent oxidoreductase | −2.40 |
| RS07865 | – | DUF1672 domain‐containing protein | −2.40 |
| RS14315 | isaB | Immunodominant staphylococcal antigen IsaB | −2.36 |
| RS14615 | – | Arylamine N‐acetyltransferase | −2.34 |
| RS01485 | – | CHAP domain‐containing protein | −2.31 |
| RS02975 | proP | Proline/betaine transporter | −2.31 |
| RS01230 | – | DUF488 domain‐containing protein | −2.25 |
| RS13045 | gpmA | Phosphoglycerate mutase | −2.24 |
| RS01455 | – | ABC transporter ATP‐binding protein | −2.22 |
| RS04855 | pepF | Oligoendopeptidase F | −2.21 |
| RS02030 | nfrA | NADPH‐dependent oxidoreductase | −2.21 |
| RS08610 | – | LLM class flavin‐dependent oxidoreductase | −2.17 |
| RS00910 | – | DUF4242 domain‐containing protein | −2.16 |
| RS01620 | – | DUF4064 domain‐containing protein | −2.15 |
| RS06940 | – | Oligoendopeptidase F | −2.15 |
| RS00915 | ssuB | ABC transporter ATP‐binding protein | −2.15 |
| RS11450 | – | DUF2529 domain‐containing protein | −2.14 |
| RS02105 | – | SDR family oxidoreductase | −2.14 |
| RS03315 | mntC | Metal ABC transporter substrate‐binding protein | −2.14 |
| RS10360 | nadE | Ammonia‐dependent NAD(+) synthetase | −2.13 |
| RS06665 | – | Hypothetical protein | −2.10 |
| RS06380 | – | Hypothetical protein | −2.09 |
| RS02970 | – | HAD family hydrolase | −2.08 |
| RS05620 | trxA | Thioredoxin | −2.07 |
| RS13480 | Hypothetical protein | −2.06 | |
| RS04995 | lplA1 | Lipoate–protein ligase | −2.05 |
| RS12320 | fdhD | Formate dehydrogenase accessory sulfurtransferase FdhD | −2.04 |
| RS09050 | – | Class I SAM‐dependent methyltransferase | −2.04 |
| RS00990 | – | Isochorismatase family protein | −2.02 |
| RS02100 | – | DUF1304 domain‐containing protein | −2.01 |
| RS01240 | hmp | Nitric oxide dioxygenase | −2.00 |
Adjusted p‐values for all genes are <0.001.
Gene ontology (GO) enrichment analysis was used to determine which GO groupings of biological processes (BP), molecular functions (MF) and cellular components (CC) were statistically over‐represented in the presence of BC compared with the absence of BC. The genes upregulated in response to BC showed significant over‐representation of 19 BP and six MF genes (Figure 3). In the BP category the top four terms (cell killing GO:0001906, cytolysis in other organisms GO:0051715, killing of cells of other organisms GO:0031640 and haemolysis in another organism GO:0044179) are all involved in cell killing. This is mirrored in the MF, in that the most overrepresented term is toxin activity (GO:0090729). Other BPs that show over‐representation include those involved in the response to environmental changes and DNA damage and repair. There was no significant over‐representation of any biological processes or molecular functions in the negatively regulated genes and no cellular components in either growth condition.
Fig. 3.
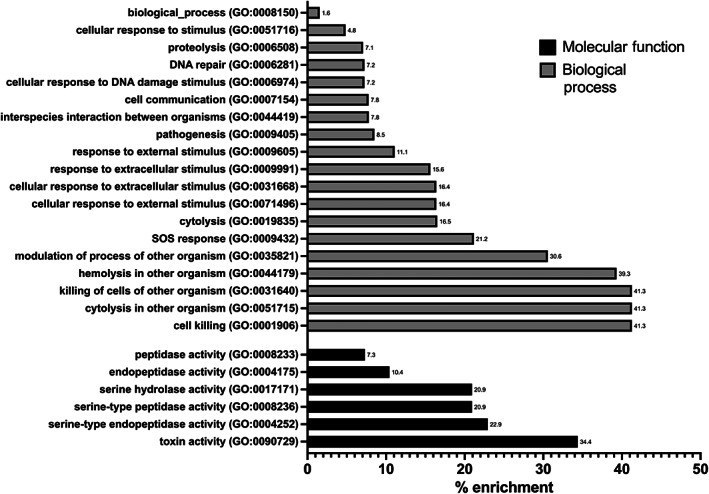
BC exposure results in increased expression of genes involved in toxin production, proteases and DNA replication and repair. Gene ontology (GO) enrichment analysis (A) of 52 genes upregulated in response to BC. The chart shows the over‐representation of each GO term within the dataset as a % enrichment.
The differentially expressed genes were also grouped based on their main function, using the associated TIGRFAM number which automatically groups proteins, based on sequence homology, into functional families and provides most‐likely functions for hypothetical and unannotated genes (Haft et al., 2013; Haft et al., 2018) (Tables S2 and S3 and Figure 4). Of the 52 genes upregulated in response to BC, 16 (30.1%) are involved in toxin production, immune evasion, or pathogenesis, 11 genes (21.1%) are involved in DNA metabolism, replication, recombination and repair, and eight genes (15%) play a role in protein synthesis, degradation and repair (Figure 4A). The remaining genes mainly play roles in cellular processes, cell envelope and signalling, transport and binding, and six genes (11%) are currently undefined. Of the 63 genes downregulated in response to BC, the largest represented groups contain 10 genes (15.9%) involved in central metabolism and eight genes (12.7%) involved in energy metabolism, with 22 undefined genes (35%) (Figure 4B).
Fig. 4.
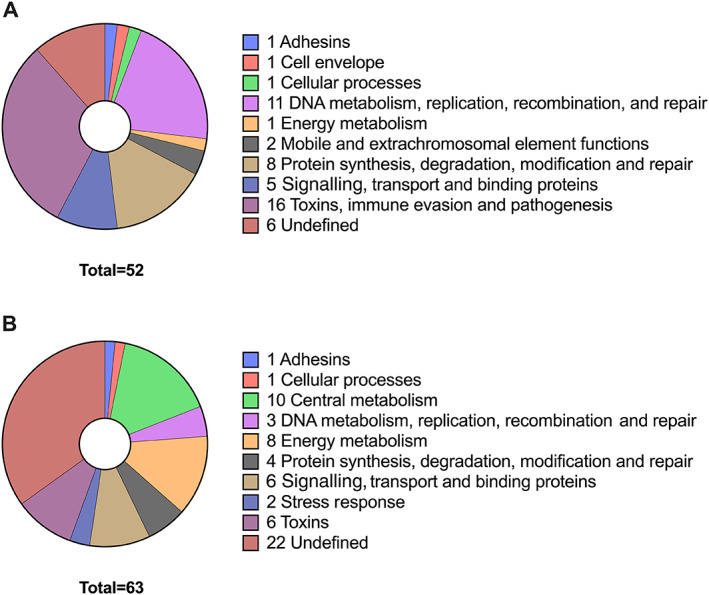
BC exposure results in differential regulation in S. aureus. Pie chart organizing (A) the 52 upregulated genes and (B) the 63 downregulated genes, by their main TIGRFAM function.
GO analysis showed that genes associated with pathogenesis are induced by BC. We observed twofold to sixfold induction of the serine protease genes (splA, B, C, D, E, F), and genes for toxins and immune evasion (hla, hlb‐1, hlgBC, gehB, scn, chp, plc, psm alpha‐1, 2, 3, 4, psm beta‐1, 2, sbi, lukF‐PV, lukG, H). It is notable that all these genes are regulated by either the Agr quorum‐sensing system (Bronesky et al., 2016; Kavanaugh & Horswill, 2016; Le & Otto, 2015) or the SaeRS two‐component regulatory system (Liu et al., 2016; Voyich et al., 2009) or both (Table 1).
Several genes involved in DNA repair show a twofold to 2.6‐fold induction in response to BC exposure. These include the genes for the UvrAB nucleotide excision repair endonuclease, the UmuC error‐prone polymerase V and ribonucleotide reductase, HNH endonuclease, Yol‐D family protein and single‐stranded‐DNA‐specific exonuclease RecJ that form an integral part of the SOS response (Podlesek & Žgur Bertok, 2020).
The other genes induced by BC are for glycerol‐3‐phosphate dehydrogenase/oxidase (glpD), the FepABC haem utilization system (fepAC) (Turlin et al., 2013), the dipeptide methionine transporter (metNPQ2) (Wade et al., 2004) and the potassium transporter and regulator (kdpA, kdpD) (Xue et al., 2011). These genes are regulated by CcpA, the iron repressor protein Fur, the cysteine metabolism regulator CymR and KdpDE two‐component regulator respectively (Fuchs et al., 2018; Nagarajan & Elasri, 2007).
BC represses expression of genes for stress responses and metabolism in S. aureus
GO analysis did not show any significant over‐representation of downregulated genes, unlike the upregulated genes. Several genes that are repressed in BC are typically induced in response to different stresses, including those involved in oxidative stress (katA, trxA), osmotic stress (glycine betaine synthesis betAB, proline/betaine transporter proP), sulfur metabolism (cysM, proP, ssuB) and nitrosative stress (hmp, ldh).
Exposure to BC also results in repression of genes for some adhesins (sdrD, efb) and an immune evasion factor (ecb). Interestingly, although the stress response (betAB, ldh, proP) and metabolic genes (argF, ptpB, pepF, nadE) that are repressed by Agr are also repressed by BC, the majority of the adhesin genes normally repressed by Agr showed no change in expression (e.g. fnbA, fnbB, emp, spa; refs), suggesting that the Agr regulon is only partially affected by BC. It is also noteworthy that BC exposure partially repressed the regulons of other global regulators [e.g. CymR (cysM), SigB (proP), GraRS (ldh, entB) PdxR (pdxS, pdxT), RexAB (frp, ldh), PerR (katA, trxA) [Fuchs et al., 2018; Nagarajan & Elasri, 2007]] showing that BC acts as a signal that induces a newly described pattern of S. aureus global gene expression.
To establish whether BC induces gene expression at lower BC concentrations, qRT‐PCR was used to determine S. aureus gene expression in response to BC at 5, 50 and 100 μg ml−1. The expression of the virulence genes chp, splF, betB, ecb, and epiA all showed a clear concentration‐dependent effect in response to BC (Figure 5) showing that BC can induce S. aureus gene expression at low concentrations.
Fig. 5.
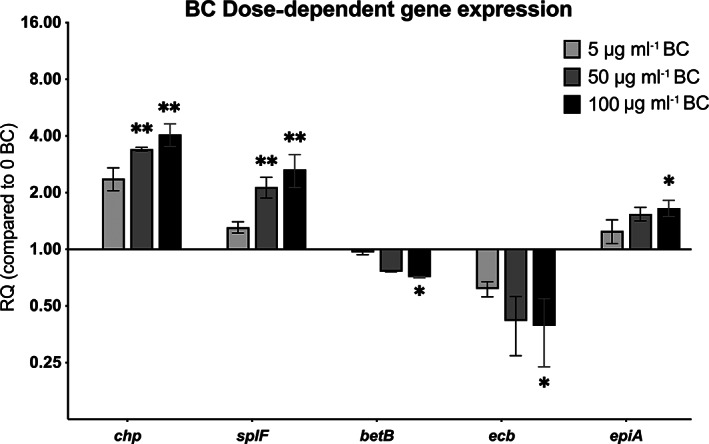
Gene expression changes in response to BC are dose dependent. Relative fold change in S. aureus USA300 gene expression grown in the presence of 5, 50 and 100 μg ml−1 BC. RQ is the fold change in expression relative to –BC. Significance of each concentration compared to 0 BC was determined by Kruskal–Wallis test with Dunn's multiple comparison test (*p < 0.05, **p < 0.01).
BC‐induced transcriptional changes correspond to increased haemolysis
The transcriptional analysis showed that BC alters the expression of only subsets of the Agr and Sae regulons. This conclusion from the RNAseq analysis was tested using qRT‐PCR. Of the Agr and Sae responsive genes investigated, there were significant changes in expression of the Agr (hla; Figure 6A, p < 0.05) and Sae (chp; Figure 6A, p < 0.01) regulated genes for toxin and immune evasion factors, in agreement with the RNAseq data, but none of the other Agr‐ and Sae‐regulated adhesins (clfA, fnbA, fnbB, emp) showed significant change in response to BC (p > 0.05), also in agreement with the RNA seq analysis (Figure 6A). The agrBDCA and sae operons also showed no significant increase in expression in response to BC (data not shown), whereas RNAIII showed a significant twofold increase in expression (Figure 6A, p < 0.05). Furthermore, PerR‐regulated oxidative stress genes (Horsburgh et al., 2001) also showed differential regulation in response to BC, with decreased transcription of katA (Figure 6A, p < 0.05) but no change in that of the sodA gene. All genes tested showed the same response to BC in S. aureus grown to mid‐logarithmic or post‐exponential phase (data not shown). Overall, these data confirm the RNAseq analysis and show that BC induces a hitherto unseen pattern of gene expression.
Fig. 6.
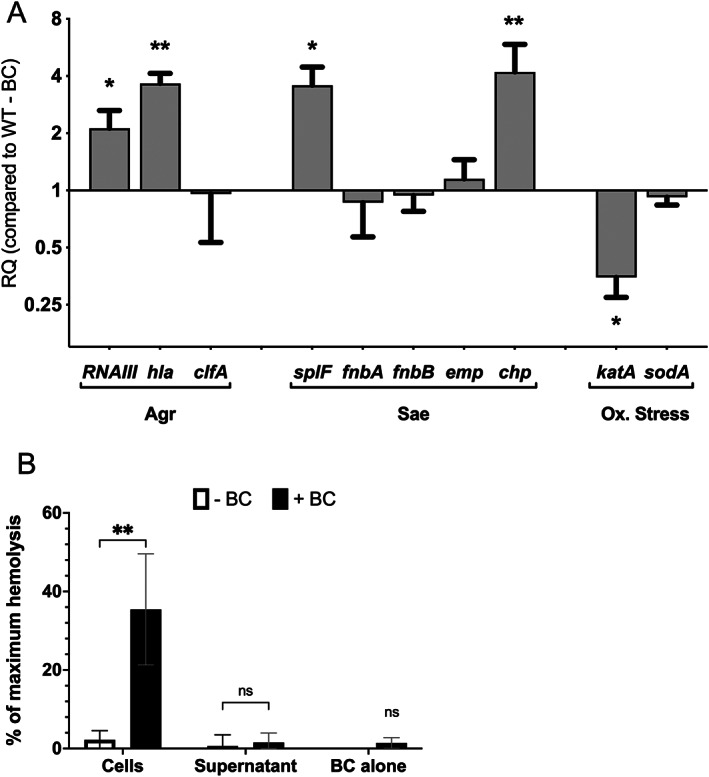
BC induces unexpected patterns of gene expression in key virulence and stress regulons.
A. Relative fold change in S. aureus USA300 gene expression grown in the presence of 100 μg ml−1 BC. Effector genes are grouped based on their primary regulator (Agr, Sae or Oxidative stress). RQ is the fold change in expression relative to –BC. Significance of each concentration compared to 0 BC was determined by Kruskal–Wallis test with Dunn's multiple comparison test (*p < 0.05, **p < 0.01).
B. Haemolysis activity of S. aureus USA300 cells and culture supernatant after growth with and without 100 μg ml−1 BC. Haemolytic activity was measured as haemoglobin released from prepared rabbit erythrocytes cells after 30 min exposure to S. aureus cells or supernatant. The data are presented as % of maximum haemolysis and is calculated relative to spontaneous haemolysis (PBS, 0%) and maximum haemolysis of the cells (SDS, 100%). Significance was determined by two‐way ANOVA (*p < 0.05).
The hla gene, which encodes α‐toxin a pore‐forming haemolysin that binds to the membrane of erythrocytes causing haemolysis (Seilie & Bubeck Wardenburg, 2017), showed increased transcription in response to BC (Figure 6A). To establish that induced transcription results in increased toxin activity, the haemolytic activity of either washed bacterial cells or supernatants of S. aureus LAC grown with and without 100 μg ml−1 BC was tested using a rabbit erythrocyte assay. SDS was used as a control to completely lyse the erythrocytes (100% lysis) and haemolytic activity is presented as a percentage of total haemoglobin released. BC and TSB medium were used as negative controls. Staphylococcus aureus cells grown in TSB with BC showed a significant increase in haemolytic activity compared to TSB without BC (Figure 6B, p < 0.05), whereas BC alone had no effect on haemolysis. Interestingly, we do not see any change in haemolytic activity in the supernatant from the same bacterial cultures (Figure 6B). Thus, BC does indeed cause an increase in toxin activity.
BC‐induced transcriptional changes require functional Agr and Sae regulators
To investigate the role of the Agr and SaeRS regulators in the bacterial response to BC, S. aureus LAC agrB and saeS transposon insertion mutants were constructed as described in the methods. With these S. aureus LAC mutants, the transcription of the Agr‐regulated psmβ, the Sae‐regulated chp and the dual Agr‐ and Sae‐ regulated lukS‐PV and splF genes in response to BC was investigated. The expression of kdpD was also investigated because kdpD is indirectly induced by Agr through repression of the repressor Rot, but it is not regulated by Sae (Xue et al., 2011). In the absence of BC, transcriptional analysis confirmed previous studies of Agr and Sae regulation of these genes (Cheung et al., 2011; Liu et al., 2016). The expression of lukS and splF show twofold decrease in expression in both the agr and sae mutants, the psmβ gene was not expressed in the agr mutant, while chp was not expressed in the sae mutant, and kdpD showed no major change in expression in either the agr or sae mutants (Figure 7).
Fig. 7.
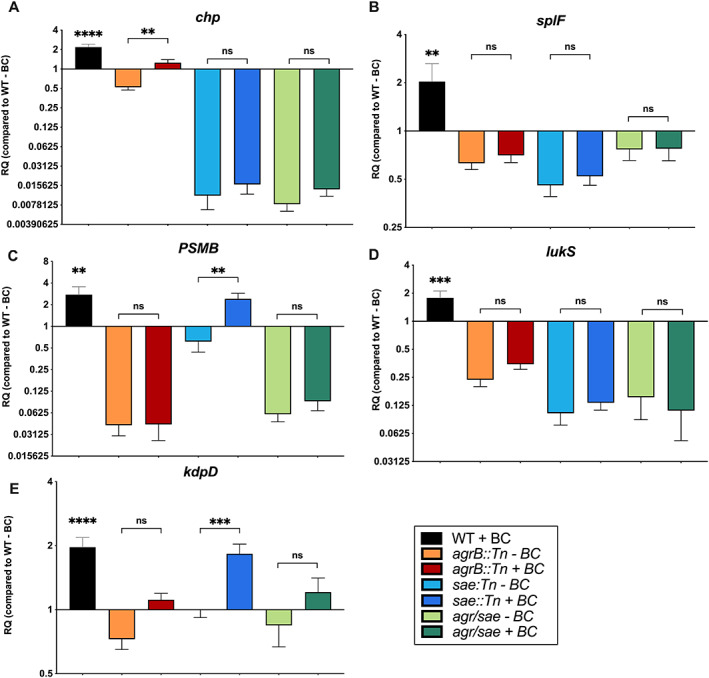
The transcriptional regulators agr and sae are involved in the BC regulation of some but not all BC‐induced genes. Transcriptional response of S. aureus genes in response to BC in USA300 LAC WT, agrB::Tn, sae::Tn and agr::tet/∆saePQRS mutant strains. RQ is the fold change in expression in each strain relative to the WT – BC. Significance was determined by one‐way ANOVA with Tukey's multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Interestingly, in the presence of BC, significant induction of chp gene transcription remained in the agr mutant (Figure 7A, p < 0.01) and psmβ and kdpD were still significantly induced by BC in the sae mutant (Figure 7C, p < 0.01; Figure 7E, p < 0.001). In contrast, there was no significant BC induction of the lukS, psmβ or splF gene expression in the agr mutant or chp, splF and lukS in the sae mutant. The involvement of Agr and Sae in response to BC was verified in an agr/sae double mutant, which showed decreased expression of the Agr/Sae‐regulated genes (Figure 7). It is noteworthy that these data show that either Agr or Sae are required for BC induction of chp, kdpD and psmβ and that the BC response is facilitated by both genes either together or separately.
BC induction of S. aureus epithelial cell invasion is via a sae‐independent mechanism
Our data show that BC increases S. aureus adhesion and invasion to human epithelial cells. The ability of S. aureus to adhere to and invade non‐professional phagocytes has been reported to be dependent on Sae induction of the adhesin genes fnbB, fnbA, eap and atl (Hirschhausen et al., 2010; Liang et al., 2006). To investigate the role of Sae and Agr in the BC‐mediated increase in S. aureus adhesion and invasion, A549 were exposed to wild type S. aureus LAC, and agrB and sae mutants pre‐grown in the presence and absence of BC.
As shown in Figure 8, although BC significantly increases S. aureus LAC adhesion to A549 cells (Figure 8A, p < 0.05), neither the agrB nor sae mutants showed significant changes in adhesion compared to the wild type in the presence or absence of BC (Figure 8A), although the agr mutant shows a small decrease in the response compared to LAC and the sae mutant. In the absence of BC, the agr mutant showed a significant increase in invasion (Figure 8B, p < 0.05), whereas the sae mutant showed a significant decrease in invasion (Figure 8B, p < 0.0001), confirming previous studies of the roles of Sae and Agr in S. aureus invasion of epithelial cells (Liang et al., 2006; Wesson et al., 1998). In the presence of BC, there was no significant change in S. aureus invasion in the agr mutant compared to the wild type (Figure 8B). In contrast, there was a significant increase in BC‐induced S. aureus invasion in the sae mutant (Figure 8B, p < 0.001) demonstrating that BC mediates staphylococci invasion via Sae and Agr‐independent mechanisms.
Fig. 8.
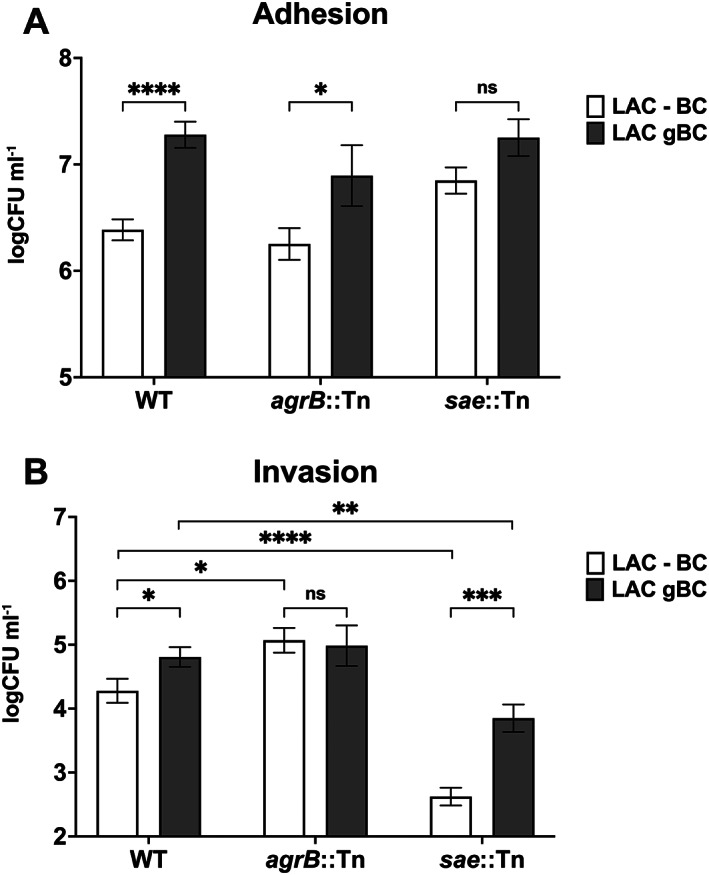
The effect of BC exposure on host cell interaction involves the Sae regulatory system. Staphylococcus aureus LAC adhesion (A) and invasion (B) of human lung epithelial A549 cells by S. aureus LAC WT, agrB::Tn and sae::Tn mutants in response to BC was measured using a gentamycin protection assay. Cells were infected at a MOI of 100 on 1 × 105 A549 monolayers in 24 well plates. Data are presented as logCFU ml−1 of recovered cells, and error bars represent 1 SEM of at least five biological repeats. Significance was determined by two‐way ANOVA with Dunnett's multiple comparison test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Discussion
BC is a major component of PM in air pollution (Bell et al., 2007). Here we show that BC increases S. aureus colonization of the murine respiratory tract and increases the bacterium's adhesion to human respiratory epithelial cells and its invasion of these cells. Our data show that increased colonization is due to the direct impact of BC on the bacteria and occurs in the absence of any detected BC mediated effects on the host. BC has a widespread effect on S. aureus global transcription causing increased expression of genes for toxins, proteases, and immune evasion factors critical for dissemination and colonization. Together these data provide evidence for a new causative mechanism of the detrimental effects of air pollution in that air pollutants directly change bacterial gene expression altering their invasive capacity and their ability to colonize and disseminate within the respiratory tract.
There is growing epidemiological evidence that PM exposure increases the risk of infectious diseases that can be caused or exacerbated by S. aureus; for example, community‐acquired pneumonia, that is increasingly caused by CA‐MRSA (Pivard et al., 2021), infective endocarditis (Hsieh et al., 2019), cystic fibrosis (Psoter et al., 2015; Psoter et al., 2017), chronic rhinosinusitis (Schwarzbach et al., 2020) and chronic skin diseases (Dijkhoff et al., 2020). Exposure to atmospheric PM has great potential to affect the activities of S. aureus because the bacterium persistently or transiently colonizes the anterior nares and the skin (Pivard et al., 2021), where it will be exposed to PM.
In this study we show that simultaneous inoculation of S. aureus and biologically relevant concentrations of BC causes increased infection of murine lungs and increased nasopharyngeal colonization. These new data agree with our previous observations with S. pneumoniae exposed to BC (Hussey et al., 2017) and confirm a wider phenomenon of the impact of BC on bacterial respiratory tract colonization, that also been recently shown with other types of particulate pollutant (Liu et al., 2019; Shears et al., 2020; Woo et al., 2018; Yadav et al., 2020).
It is notable that we showed that pre‐growth of S. aureus in BC prior to infection induces a significant increase in staphylococcal murine respiratory tract colonization. The increased colonization is maintained for at least 7 days without further administration of BC. To our knowledge, this is the first study to pre‐grow the bacteria with PM prior to inoculation. All previous studies have either pre‐exposed the host to PM or inoculated PM and bacteria together (Liu et al., 2019; Shears et al., 2020; Woo et al., 2018; Yadav et al., 2020). These publications hypothesised that increased bacterial colonization was due to PM binding to the bacteria thereby promoting transmission throughout the respiratory tract, or PM providing metabolites to support bacterial growth or PM‐mediated toxicity damaging epithelial integrity (Liu et al., 2019; Shears et al., 2020; Yadav et al., 2020).
In contrast, our data demonstrate a novel explanation of the detrimental effects of air pollution in that BC directly alters bacterial behaviours to increase colonization. Supporting this conclusion, the concentration of BC used in our studies does not have a visible effect on host tissue and does not promote S. aureus growth (Hussey et al., 2017) and murine colonization is promoted even when S. aureus are pre‐grown with BC and BC has not been directly administered to the mice. BC is a particulate compound that can leach compounds or adsorb solutes from the extracellular milieu, potential chemical and particle effects on bacterial behaviour are currently under investigation in our laboratory. Together these data demonstrate that major effects of particulates on the host are not essential for increased bacterial colonization. Similar conclusions come from the work with A549 cells since there was no detectable impact on A549 cell viability and yet S. aureus pre‐grown in BC prior to infection show a significant increase in staphylococcal adhesion and invasion of these cells.
RNAseq analysis confirms that exposure to BC alters a collection of S. aureus genetic responses that have multiple deleterious effects on the host's ability to combat infection and play important roles in colonization and dissemination (Pivard et al., 2021). BC increases the transcription of genes for cytotoxins that lyse immune cells (lukGH, hlgBC, α and β‐psms) (Collins et al., 2020; Tromp & van Strijp, 2020), for factors that inhibit complement (scin, sbi) (Sultan et al., 2018; Pivard et al., 2021) and prevent phagocyte recruitment (chemotaxis‐inhibiting protein chp), and are important for S. aureus survival in human blood and neutrophils (phospholipase C, plc) (White et al., 2014). BC also highly induces expression of the Spl protease genes that play a role in mucin degradation and lung adaptation, with an spl mutant showing decreased lung dissemination a rabbit model of pneumonia (Paharik et al., 2016).
Interestingly, BC also induces the SOS response regulators (lexA, recA) and effectors (uvrAB, umuC, hnh, yolD, recJ and nrdIFG). The SOS response is important for the induced expression of genes important for survival and colonization of the host including DNA repair, virulence and immune evasion (Podlesek & Žgur Bertok, 2020). Typically, the SOS response is induced by RecA sensing impairment of bacterial growth and intracellular DNA damage and then initiating the self‐cleavage of the LexA repressor protein (Podlesek & Žgur Bertok, 2020), but effect on growth does not seem to be the trigger here because BC does not inhibit S. aureus growth and there is no evidence of DNA damage and the transcriptional data do not show other stress responses being activated.
On the contrary, BC represses several genes that are typically induced in response to different stresses, including those involved in oxidative stress (katA, trxA), osmotic stress (glycine betaine synthesis betAB, proline/betaine transporter proP), sulfur metabolism (cysM, proP, ssuB) and nitrosative stress (hmp, ldh) (Fuchs et al., 2018; Nagarajan & Elasri, 2007). It must be noted though that the negatively regulated genes do not show such a strong uniform response as the genes induced by BC with there being no significant over‐representation of downregulated genes from any functional group.
The BC induction of the toxin, protease and immune evasion genes (lukGH, hlgBC, α and β‐psms, hla, scin, sbi, chp) is likely to occur through the activity of the Agr and Sae two‐component regulators (Cheung et al., 2011; Geiger et al., 2008) that typically control the expression of these genes. The S. aureus Agr quorum‐sensing system is important for the switch from a colonizing state to a more aggressive invasive state through induced expression of toxins and the factors required for dissemination (Jenul & Horswill, 2019). Toxin and immune evasion gene expression is also activated by the Sae regulatory system (Geiger et al., 2008).
Both Agr and Sae have cell membrane located sensors, the activity of which can be influenced by a range of different environmental conditions (Geiger et al., 2008; Kavanaugh & Horswill, 2016), although the exact mechanisms involved have not been fully elucidated. It is possible that BC directly interacts with Agr and Sae by either altering environmental signals such as the Agr quorum‐sensing signal concentrations or activating the membrane‐bound sensors to induce gene expression.
The role of Agr and Sae in BC induction of the toxin and immune evasion genes was confirmed by transcriptional analysis of sae and agr mutants that showed that both Sae and Agr are associated with BC induction of gene expression. The pattern of response differs between the tested genes with either the Agr or Sae regulator or both being required for BC‐mediated gene regulation. Importantly, BC appears to induce only parts of the Agr and Sae regulons. For example, the expression of adhesin genes that would typically be repressed by Agr (e.g. spa) (Cheung et al., 2011) and or induced by Sae (e.g. fnbA, fnbB, emp) (Mainiero et al., 2010) were not altered in the RNAseq or the qRT‐PCR analysis. Therefore, the data suggest that exposure of S. aureus to BC prior to or during colonization of the nares would induce a previously unrecognized regulatory response that increases invasive disease, which is distinct from previously described patterns of induction of the Agr and Sae regulons.
BC induction of cytotoxins contrasts with the gene regulatory effects observed with other pollutants, e.g. cigarette smoke extract (CSE). As with BC, CSE increases S. aureus epithelial cell adhesion and invasion (Kulkarni et al., 2012; Lacoma et al., 2019; McEachern et al., 2015) but in contrast to BC, CSE represses Agr resulting in increased adhesins (Kulkarni et al., 2012) and repressed cytotoxin expression (Lacoma et al., 2019).
BC caused a significant increase in haemolysis confirming that BC‐induced hla transcriptional changes correspond to increased α‐toxin activity. Interestingly, haemolysis only increased when red blood cells were treated with whole cells and not with the growth culture supernatant. This is surprising because α‐toxin is typically secreted and would be expected to be found in the culture supernatant (Seilie & Bubeck Wardenburg, 2017). However, our data suggest that although there is an increase in the level of Hla activity in response to BC, the toxin remains associated with the cell surface of the bacteria rather than being released from the cell. Staphylococcus aureus USA300 cell‐associated toxin activity has recently been shown for other toxins with cellular location being dependent on a process that involves the cell membrane lipid, lysyl‐phosphatidylglycerol and lipoteichoic acid (Brignoli et al., 2022; Zheng et al., 2021). This suggests that BC may influence the cell envelope which could have interesting implications for antibiotic activity.
Furthermore, our data suggest that BC induces a novel mechanism for increased invasion of epithelial cells. Typically, S. aureus invasion of epithelial cells involves Sae‐dependent mechanisms involving the fibronectin‐binding proteins, lipases and toxin‐induced changes in the cytoskeleton (Josse et al., 2017). The only surface proteins showing induced expression in response to BC in the RNAseq data that are not induced by Agr or Sae are the EfeM/EfeO family lipoprotein (fepA) and a Map domain protein both of which have no known role in S. aureus invasion. Therefore, the novel mechanism for S. aureus invasion induced by BC requires further investigation.
In conclusion, we have provided substantial evidence supporting the novel contention that a single air pollutant, at concentrations non‐harmful to bacteria or the host, can specifically alter bacterial behaviour and would be expected to have adverse health outcomes. This concept has significant implications for mitigating air pollution toxicity and subsequent adverse health effects, because the currently held hypotheses are restricted to the belief that the toxicity of particle pollutants causes adverse effects by damaging the host directly and that control of pollutant levels need only be limited to concentrations below those that are toxic to humans. This study shows that adverse effects can occur at apparently non‐toxic concentrations of pollutant that can alter bacterial behaviour to potentiate infectious disease.
Supporting information
Table S1. Primers used in this study.
Table S2. Genes significantly upregulated at a L2FC >1 in response to BC, grouped into TIGRFAM functional groups (Main). * entries do not have an official TIGRFAM entry and have been annotated based on literature review of their functions
Table S3. Genes significantly downregulated at a L2FC < ‐1 in response to BC, grouped into TIGRFAM functional groups (Main) * entries do not have an official TIGRFAM entry and have been annotated based on literature review of their functions
Table S4. RNA integrity and concentrations of samples sent for RNAseq.
ACKNOWLEDGEMENTS
USA300 JE2 and JE2 agr::Tn strains were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) Program: USA300 supported under NIAID/NIH Contract No. HHSN272200700055C. We thank Prof. Alexander Horswill, University of Colorado, for generously sending us the USA300 LAC agr::tet/∆saePQRS double mutant. We thank Andrew Briscoe at the Core Research Laboratories, Natural History Museum for the RNAseq library preparation and sequencing. J.P. and S.J.K.H. were supported by a Leverhulme Trust grant (RPG‐2015‐183) awarded to J.A.M., P.W.A., J.M.K., P.S.M.; L.C. was supported by a National Centre for Atmospheric Science Air Pollution Science Training Studentship Programme. L.P. was supported by MRC DTP IMPACT studentship.
REFERENCES
- Al‐Jumaili, A. , Alancherry, S. , Bazaka, K. , and Jacob, M.V. (2017) Review on the antimicrobial properties of carbon nanostructures. Materials 10: 1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner, M. , Ball, C.A. , Blake, J.A. , Botstein, D. , Butler, H. , Cherry, J.M. , et al. (2000) Gene ontology: tool for the unification of biology. Nat Genet 25: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae, T. , Glass, E.M. , Schneewind, O. , and Missiakas, D. (2008) Generating a collection of insertion mutations in the Staphylococcus aureus genome using bursa aurealis . In Microbial gene essentiality: protocols and bioinformatics, Osterman, A.L. , and Gerdes, S.Y. (eds). Totowa: NJ, Humana Press, pp. 103–116. [DOI] [PubMed] [Google Scholar]
- Bell, M.L. , Dominici, F. , Ebisu, K. , Zeger, S.L. , and Samet, J.M. (2007) Spatial and temporal variation in PM2.5 chemical composition in the United States for health effects studies. Environ Health Perspect 115: 989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignoli, T. , Douglas, E. , Duggan, S. , Fagunloye, O.G. , Adhikari, R. , Aman, M.J. , and Massey, R.C. (2022) Wall techoic acids facilitate the release of toxins from the surface of Staphylococcus aureus . bioRxiv 10.1101/2022.01.31.478600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronesky, D. , Wu, Z. , Marzi, S. , Walter, P. , Geissmann, T. , Moreau, K. , et al. (2016) Staphylococcus aureus RNAIII and its regulon link quorum sensing, stress responses, metabolic adaptation, and regulation of virulence gene expression. Annu Rev Microbiol 70: 299–316. [DOI] [PubMed] [Google Scholar]
- Castranova, V. , Ma, J.Y. , Yang, H.M. , Antonini, J.M. , Butterworth, L. , Barger, M.W. , et al. (2001) Effect of exposure to diesel exhaust particles on the susceptibility of the lung to infection. Environ Health Perspect 109: 609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalvatzaki, E. , Chatoutsidou, S. , Mammi‐Galani, E. , Almeida, S. , Gini, M. , Eleftheriadis, K. , et al. (2018) Estimation of the personal deposited dose of particulate matter and particle‐bound metals using data from selected European cities. Atmosphere 9: 248. [Google Scholar]
- Cheung, G.Y.C. , Wang, R. , Khan, B.A. , Sturdevant, D.E. , Otto, M. , and Payne, S.M. (2011) Role of the accessory gene regulator agr in community‐associated methicillin‐resistant Staphylococcus aureus pathogenesis. Infect Immun 79: 1927–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, A.J. , Brauer, M. , Burnett, R. , Anderson, H.R. , Frostad, J. , et al. (2017) Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet (British edition) 389: 1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, M.M. , Behera, R.K. , Pallister, K.B. , Evans, T.J. , Burroughs, O. , Flack, C. , et al. (2020) The accessory gene sae P of the Sae R/S two‐component gene regulatory system impacts Staphylococcus aureus virulence during neutrophil interaction. Front Microbiol 11: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhoff, I.M. , Drasler, B. , Karakocak, B.B. , Petri‐Fink, A. , Valacchi, G. , Eeman, M. , and Rothen‐Rutishauser, B. (2020) Impact of airborne particulate matter on skin: a systematic review from epidemiology to in vitro studies. Part Fibre Toxicol 17: 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey, P.D. , Endres, J.L. , Yajjala, V.K. , Widhelm, T.J. , Boissy, R.J. , Bose, J.L. , and Bayles, K.W. (2013) A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio 4: 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, S. , Mehlan, H. , Bernhardt, J. , Hennig, A. , Michalik, S. , Surmann, K. , et al. (2018) Aureo Wiki ‐ the repository of the Staphylococcus aureus research and annotation community. Int J Med Microbiol 308: 558–568. [DOI] [PubMed] [Google Scholar]
- Garzoni, C. , and Kelley, W.L. (2008) Staphylococcus aureus: new evidence for intracellular persistence. Trends Microbiol 17: 59–65. [DOI] [PubMed] [Google Scholar]
- Geiger, T. , Goerke, C. , Mainiero, M. , Kraus, D. , and Wolz, C. (2008) The virulence regulator Sae of Staphylococcus aureus: promoter activities and response to phagocytosis‐related signals. J Bacteriol 190: 3419–3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene, O.C. (2021) The gene ontology resource: enriching a GOld mine. Nucleic Acids Res 49: D325–D334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerke, C. , Fluckiger, U. , Steinhuber, A. , Zimmerli, W. , and Wolz, C. (2001) Impact of the regulatory loci agr, sar A and sae of Staphylococcus aureus on the induction of α‐toxin during device‐related infection resolved by direct quantitative transcript analysis. Mol Microbiol 40: 1439–1447. [DOI] [PubMed] [Google Scholar]
- Haft, D.H. , DiCuccio, M. , Badretdin, A. , Brover, V. , Chetvernin, V. , O'Neill, K. , et al. (2018) Ref Seq: an update on prokaryotic genome annotation and curation. Nucleic Acids Res 46: D851–D860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haft, D.H. , Selengut, J.D. , Richter, R.A. , Harkins, D. , Basu, M.K. , and Beck, E. (2013) TIGRFAMs and genome properties in 2013. Nucleic Acids Res 41: D387–D395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhausen, N. , Schlesier, T. , Schmidt, M.A. , Götz, F. , Peters, G. , and Heilmann, C. (2010) A novel staphylococcal internalization mechanism involves the major autolysin Atl and heat shock cognate protein Hsc 70 as host cell receptor. Cell Microbiol 12: 1746–1764. [DOI] [PubMed] [Google Scholar]
- Horsburgh, M.J. , Clements, M.O. , Crossley, H. , Ingham, E. , and Foster, S.J. (2001) Per R controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus . Infect Immun 69: 3744–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, F. , Huang, C. , Lin, S. , Sun, J. , Yen, T. , and Chang, C. (2019) Short‐term exposure to particulate matters is associated with septic emboli in infective endocarditis. Medicine 98: e17899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey, S.J.K. , Purves, J. , Allcock, N. , Fernandes, V.E. , Monks, P.S. , Ketley, J.M. , et al. (2017) Air pollution alters Staphylococcus aureus and Streptococcus pneumoniae biofilms, antibiotic tolerance and colonization. Environ Microbiol 15: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenul, C. , and Horswill, A.R. (2019) Regulation of Staphylococcus aureus virulence. Microbiol Spectr 7(2). 10.1128/microbiolspec.gpp3-0031-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse, J. , Laurent, F. , and Diot, A. (2017) Staphylococcal adhesion and host cell invasion: fibronectin‐binding and other mechanisms. Front Microbiol 8: 2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh, J.S. , and Horswill, A.R. (2016) Impact of environmental cues on staphylococcal quorum sensing and biofilm development. J Biol Chem 291: 12556–12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, A.D. , Otto, M. , Braughton, K.R. , Whitney, A.R. , Chen, L. , Mathema, B. , et al. (2008) Epidemic community‐associated methicillin‐resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci U S A 105: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, R. , Antala, S. , Wang, A. , Amaral, F.E. , Rampersaud, R. , LaRussa, S.J. , et al. (2012) Cigarette smoke increases Staphylococcus aureus biofilm formation via oxidative stress. Infect Immun 80: 3804–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoma, A. , Edwards, A.M. , Young, B.C. , Domínguez, J. , Prat, C. , and Laabei, M. (2019) Cigarette smoke exposure redirects Staphylococcus aureus to a virulence profile associated with persistent infection. Sci Rep 9: 10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le, K.Y. , and Otto, M. (2015) Quorum‐sensing regulation in staphylococci – an overview. Front Microbiol 6: 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. , Lee, P. , Choi, S. , An, M. , and Jang, A. (2021) Effects of air pollutants on airway diseases. Int J Environ Res Public Health 18: 9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Yang, J. , Saffari, A. , Jacobs, J. , Baek, K.I. , Hough, G. , et al. (2017) Ambient ultrafine particle ingestion alters gut microbiota in association with increased atherogenic lipid metabolites. Sci Rep 7: 42906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Sun, Y. , An, Y. , Wang, R. , Lin, H. , Liu, M. , et al. (2019) Air pollution during the winter period and respiratory tract microbial imbalance in a healthy young population in Northeastern China. Environ Pollut 246: 972–979. [DOI] [PubMed] [Google Scholar]
- Liang, X. , Yu, C. , Sun, J. , Liu, H. , Landwehr, C. , Holmes, D. , and Ji, Y. (2006) Inactivation of a two‐component signal transduction system, Sae RS, eliminates adherence and attenuates virulence of Staphylococcus aureus . Infect Immun 74: 4655–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Chen, X. , Dou, M. , He, H. , Ju, M. , Ji, S. , et al. (2019) Particulate matter disrupts airway epithelial barrier via oxidative stress to promote Pseudomonas aeruginosa infection. J Thorac Dis 11: 2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Yeo, W. , and Bae, T. (2016) The Sae RS two‐component system of Staphylococcus aureus . Genes 7: 81.27706107 [Google Scholar]
- Livak, K.J. , and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2(−∆∆C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Mainiero, M. , Goerke, C. , Geiger, T. , Gonser, C. , Herbert, S. , & Wolz, C. (2010) Differential target gene activation by the Staphylococcus aureus two‐component system saeRS . J Bacteriol, 192, 613–623. 10.1128/jb.01242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisalidis, I. , Stavropoulou, E. , Stavropoulos, A. , and Bezirtzoglou, E. (2020) Environmental and health impacts of air pollution: a review. Front Public Health 8: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, J. , Favero, C. , Carugno, M. , Pergoli, L. , Ferrari, L. , Bonzini, M. , et al. (2020) Nasal microbiota modifies the effects of particulate air pollution on plasma extracellular vesicles. Int J Environ Res Public Health 17: 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani, J. , Favero, C. , Spinazzè, A. , Cavallo, D.M. , Carugno, M. , Motta, V. , et al. (2018) Short‐term particulate matter exposure influences nasal microbiota in a population of healthy subjects. Environ Res 162: 119–126. [DOI] [PubMed] [Google Scholar]
- McEachern, E.K. , Hwang, J.H. , Sladewski, K.M. , Nicatia, S. , Dewitz, C. , Mathew, D.P. , et al. (2015) Analysis of the effects of cigarette smoke on staphylococcal virulence phenotypes. Infect Immun 83: 2443–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, V.F. (2019) Addressing the global air pollution crisis: chemistry's role. Trends Chem 1: 5–7. [Google Scholar]
- Mi, H. , Muruganujan, A. , Ebert, D. , Huang, X. , and Thomas, P.D. (2019) PANTHER version 14: more genomes, a new PANTHER GO‐slim and improvements in enrichment analysis tools. Nucleic Acids Res 47: D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio, C.T. , Kobos, E. , King, Q.O. , Porter, V. , Jessop, F. , and Ward, T. (2013) Adverse effects of wood smoke PM2.5 exposure on macrophage functions. Inhal Toxicol 25: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiukiewicz‐Stepien, P. , and Paplinska‐Goryca, M. (2021) Biological effect of PM10 on airway epithelium‐focus on obstructive lung diseases. Clin Immunol 227: 108754. [DOI] [PubMed] [Google Scholar]
- Nagarajan, V. , and Elasri, M.O. (2007) SAMMD: Staphylococcus aureus microarray meta‐database. BMC Genomics 8: 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupane, B. , Jerrett, M. , Burnett, R.T. , Marrie, T. , Arain, A. , and Loeb, M. (2010) Long‐term exposure to ambient air pollution and risk of hospitalization with community‐acquired pneumonia in older adults. Am J Respir Crit Care Med 181: 47–53. [DOI] [PubMed] [Google Scholar]
- Paharik, A.E. , Salgado‐Pabon, W. , Meyerholz, D.K. , White, M.J. , Schlievert, P.M. , and Horswill, A.R. (2016) The Spl serine proteases modulate Staphylococcus aureus protein production and virulence in a rabbit model of pneumonia. mSphere 1: e00208‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M. , Han, J. , Jang, M. , Suh, M. , Lee, J.H. , Oh, S.H. , and Park, M.K. (2018) Air pollution influences the incidence of otitis media in children: a national population‐based study. PLoS One 13: e0199296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivard, M. , Moreau, K. , and Vandenesch, F. (2021) Staphylococcus aureus arsenal to conquer the lower respiratory tract. mSphere. 6(3). 10.1128/msphere.00059-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlesek, Z. , and Žgur Bertok, D. (2020) The DNA damage inducible SOS response is a key player in the generation of bacterial persister cells and population wide tolerance. Front Microbiol 11: 1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psoter, K.J. , De Roos, A.J. , Mayer, J.D. , Kaufman, J.D. , Wakefield, J. , and Rosenfeld, M. (2015) Fine particulate matter exposure and initial Pseudomonas aeruginosa acquisition in cystic fibrosis. Ann Am Thorac Soc 12: 385–391. [DOI] [PubMed] [Google Scholar]
- Psoter, K.J. , De Roos, A.J. , Wakefield, J. , Mayer, J.D. , and Rosenfeld, M. (2017) Air pollution exposure is associated with MRSA acquisition in young U.S. children with cystic fibrosis. BMC Pulm Med 17: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, H. , Tian, L.W. , Pun, V.C. , Ho, K. , Wong, T.W. , and Yu, I.T.S. (2014) Coarse particulate matter associated with increased risk of emergency hospital admissions for pneumonia in Hong Kong. Thorax 69: 1027. [DOI] [PubMed] [Google Scholar]
- Richards, R.L. , Haigh, R.D. , Pascoe, B. , Sheppard, S.K. , Price, F. , Jenkins, D. , et al. (2015) Persistent Staphylococcus aureus isolates from two independent cases of bacteremia display increased bacterial fitness and novel immune evasion phenotypes. Infect Immun 83: 3311–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylance, J. , Kankwatira, A. , Nelson, D.E. , Toh, E. , Day, R.B. , Lin, H. , et al. (2016) Household air pollution and the lung microbiome of healthy adults in Malawi: a cross‐sectional study. BMC Microbiol 16: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbach, H.L. , Mady, L.J. , and Lee, S.E. (2020) What is the role of air pollution in chronic rhinosinusitis? Immunol Allergy Clin N Am 40: 215–222. [DOI] [PubMed] [Google Scholar]
- Seilie, E.S. , and Bubeck Wardenburg, J. (2017) Staphylococcus aureus pore‐forming toxins: the interface of pathogen and host complexity. Semin Cell Dev Biol 72: 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears, R.K. , Jacques, L.C. , Naylor, G. , Miyashita, L. , Khandaker, S. , Lebre, F. , et al. (2020) Exposure to diesel exhaust particles increases susceptibility to invasive pneumococcal disease. J Allergy Clin Immunol 145: 1272–1284.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan, A.R. , Swierstra, J.W. , Lemmens‐den Toom, N.A. , Snijders, S.V. , Hansenová Maňásková, S. , Verbon, A. , et al. (2018) Production of Staphylococcal Complement Inhibitor (SCIN) and other immune modulators during the early stages of Staphylococcus aureus biofilm formation in a mammalian cell culture medium. Infect Immun 86. 10.1128/iai.00352-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tromp, A.T. , and van Strijp, J.A.G. (2020) Studying staphylococcal leukocidins: a challenging endeavour. Front Microbiol 11: 611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlin, E. , Debarbouille, M. , Augustyniak, K. , Gilles, A. , and Wandersman, C. (2013) Staphylococcus aureus Fep A and Fep B proteins drive heme iron utilization in Escherichia coli. PLoS One 8: 56529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyich, J. , Vuong, C. , DeWald, M. , Nygaard, T. , Kocianova, S. , Griffith, S. , et al. (2009) The Sae R/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus . J Infect Dis 199: 1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, A.W. , Zhang, R.‐g. , Zhou, M. , Joachimiak, G. , Gornicki, P. , Missiakas, D. , and Joachimiak, A. (2004) The membrane‐associated lipoprotein‐9 Gmp C from Staphylococcus aureus binds the dipeptide Gly Met via side chain interactions. Biochemistry 43: 16193–16202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Cheng, H. , Wang, D. , Zhao, B. , Zhang, J. , Cheng, L. , et al. (2019) Airway microbiome is associated with respiratory functions and responses to ambient particulate matter exposure. Ecotoxicol Environ Saf 167: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson, C.A. , Liou, L.E. , Todd, K.M. , Bohach, G.A. , Trumble, W.R. , and Bayles, K.W. (1998) Staphylococcus aureus Agr and Sar global regulators influence internalization and induction of apoptosis. Infect Immun 66: 5238–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, M.J. , Boyd, J.M. , Horswill, A.R. , and Nauseef, W.M. (2014) Phosphatidylinositol‐specific phospholipase C contributes to survival of Staphylococcus aureus USA300 in human blood and neutrophils. Infect Immun 82: 1559–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, S.H. , Lee, S.M. , Park, K.C. , Park, G.N. , Cho, B. , Kim, I. , et al. (2018) Effects of fine particulate matter on Pseudomonas aeruginosa adhesion and biofilm formation in vitro. Biomed Res Int 2018: 6287932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization − News Release . (2018) 9 out of 10 people worldwide breathe polluted air, but more countries are taking action.
- Xue, T. , You, Y. , Hong, D. , Sun, H. , and Sun, B. (2011) The Staphylococcus aureus Kdp DE two‐component system couples extracellular K+ sensing and Agr Signalling to infection programming. Infect Immun 79: 2154–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav, M.K. , Go, Y.Y. , Jun, I. , Chae, S. , and Song, J. (2020) Urban particles elevated Streptococcus pneumoniae biofilms, colonization of the human middle ear epithelial cells, mouse nasopharynx and transit to the middle ear and lungs. Sci Rep 10: 5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, H.M. , Antonini, J.M. , Barger, M.W. , Butterworth, L. , Roberts, B.R. , Ma, J.K. , et al. (2001) Diesel exhaust particles suppress macrophage function and slow the pulmonary clearance of listeria monocytogenes in rats. Environ Health Perspect 109: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. , Li, W. , Gao, Y. , Li, J. , and Wang, H. (2014) Exposure to particulate matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells. Toxicology (Amsterdam) 325: 180–188. [DOI] [PubMed] [Google Scholar]
- Zheng, X. , Marsman, G. , Lacey, K.A. , Chapman, J.R. , Goosmann, C. , Ueberheide, B.M. , & Torres, V.J. (2021). The cell envelope of Staphylococcus aureus selectively controls the sorting of virulence factors. Nature Commun 12. 10.1038/s41467-021-26517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Primers used in this study.
Table S2. Genes significantly upregulated at a L2FC >1 in response to BC, grouped into TIGRFAM functional groups (Main). * entries do not have an official TIGRFAM entry and have been annotated based on literature review of their functions
Table S3. Genes significantly downregulated at a L2FC < ‐1 in response to BC, grouped into TIGRFAM functional groups (Main) * entries do not have an official TIGRFAM entry and have been annotated based on literature review of their functions
Table S4. RNA integrity and concentrations of samples sent for RNAseq.


