Abstract
The pharmacokinetics of lithium, the gold standard for the treatment of bipolar disorder, are well described in nonpregnant patients. Because lithium is commonly prescribed to women of childbearing age, more data are essential to characterize lithium pharmacokinetics during the perinatal period. Lithium is primarily eliminated by the kidney. As a result, shifts in lithium elimination clearance parallel pregnancy‐related changes in glomerular filtration rate. Lithium's narrow therapeutic window increases the risk for therapeutic failure and toxicity when lithium elimination clearance is altered. To characterize the pharmacokinetics of lithium in pregnancy and postpartum, 3 women treated with lithium for bipolar disorder completed serial blood sampling protocols during each trimester of pregnancy and at least once postpartum. The trajectory of lithium elimination clearance, creatinine clearance, and serum lithium concentrations were determined. Manic, depressive, and anxiety symptoms were also assessed at each study visit. Compared to the nonpregnant state, lithium elimination clearance increased an average of 63.5% by the third trimester. Lithium elimination clearance was inversely related to changes in serum lithium concentration. Mood symptoms worsened with declines in serum lithium concentration. Lithium elimination clearance returned to baseline at 4 to 9 weeks postpartum. To maintain lithium effectiveness during pregnancy and prevent toxicity postpartum, lithium therapeutic drug monitoring and dose adjustments are warranted.
Keywords: bipolar disorder, lithium, pregnancy, pharmacokinetics, pharmacology, postpartum, psychiatric disorders, symptom characterization, women's mental health
Lithium, the gold standard for the treatment of bipolar disorder, has a narrow therapeutic range of 0.4 to 1.0 mEq/L. Concentrations <0.4 mEq/L are not likely to result in a therapeutic response; conversely, concentrations >1.0 mEq/L increase the risk of toxicity. 1 The serum lithium concentration must be individualized to achieve maximum symptom reduction and tolerable side effects. 2 , 3 Serum lithium concentrations fluctuate due to changes in hydration, renal function, diet, concomitant drug interactions, and inconsistent adherence. Although breakthrough episodes of depression or mania are possible despite optimal treatment and consistent adherence, 4 the risk of a recurrence increases when concentrations are subtherapeutic for prolonged periods of time. Consistent with the established guidelines for the treatment of men and nonpregnant women with bipolar disorder, 5 maintenance of effective concentrations reduces the risk of therapeutic failure and toxicity in perinatal women.
During pregnancy, lithium clearance increases in response to profound physiological changes and increased renal excretion. The glomerular filtration rate (GFR) is elevated during pregnancy due to increased cardiac output. 6 Lithium elimination clearance parallels changes in GFR, which increases 50% by gestation week 14. 7 Creatinine clearance (CLCr), a measurable surrogate marker of GFR, is a predictor of lithium elimination clearance. In the only prospective study of perinatal lithium pharmacokinetics, Schou et al 8 reported that the lithium elimination clearance doubled in the final trimester in 3 of 4 healthy pregnant women without mental illness who took a 600‐mg dose of lithium. The lithium elimination clearance was not determined in these women during the first 2 trimesters.
Increases in lithium clearance during pregnancy result in reduced serum lithium concentrations. Data from retrospective observational studies show that serum lithium concentrations decline across pregnancy. 9 , 10 In 1 study, investigators found that serum lithium concentrations increase slightly in the third trimester. 9 Characterization of the trajectory of lithium elimination clearance across pregnancy and after birth is necessary to inform monitoring and dose adjustments. The timing and magnitude of dose increases required to prevent bipolar disorder symptom recurrence while minimizing adverse effects across the perinatal period has not been determined.
We conducted a prospective longitudinal study of lithium pharmacokinetics during pregnancy and postpartum in 3 women with bipolar disorder. We determined changes in lithium elimination clearance and CLCr across pregnancy compared to a nonpregnant baseline and explored the relationship between decreasing serum lithium concentrations and the course of bipolar disorder symptoms during pregnancy.
Methods
Participants
All research activities were approved by the Northwestern University Institutional Review Board in Chicago, Illinois. Participants provided written informed consent before completing research procedures. The study was registered in ClinicalTrials.gov (NCT02490241) and conducted at the Northwestern University Clinical Research Unit and the Asher Center for the Study and Treatment of Depressive Disorders. Eligible participants were pregnant or actively attempting conception, English speaking, and prescribed lithium for the treatment of bipolar disorder (any subtype). The diagnosis was confirmed with a clinician‐administered Mini–International Neuropsychiatric Interview. 11 All participants chose to continue lithium during pregnancy and postpartum with the guidance of their prescriber, who was not involved in the study. Participants continued treatment with a nonstudy psychiatrist and obstetrician‐gynecologist throughout the study. Dose changes were made by the prescriber and based on interpretation of the patient's lithium concentration and symptom presentation.
Women were excluded for substance use within 6 months of enrollment, comorbid medical conditions that might affect serum lithium concentration (eg, renal impairment), iron deficiency anemia (hematocrit <28%), and use of medications known to interact with lithium (eg, diuretics). Stable, treated hypothyroidism was not an exclusion.
Study Procedures
We investigated the steady‐state pharmacokinetics of lithium. Serial blood sampling protocols and urine collection were completed over 24‐ or 12‐hour visits for participants who took their dose once or twice daily, respectively. The protocol was completed during pregnancy trimesters 1, 2, and 3, and once or twice within the first 3 months postpartum and, when possible, preconception.
At each protocol visit, thirteen 3‐mL serial blood samples were drawn from an indwelling peripheral intravenous catheter into a tube that contained no anticoagulant or other additive before dosing and 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 hours after dosing for participants dosed twice daily, and before dosing and 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 hours after dosing for participants dosed once daily. Blood samples were immediately processed, and serum was frozen in a –80°C freezer for 10 to 12 days before analysis. Blood was also obtained for serum creatinine concentration measurement at each visit. Participants were asked to empty their bladder before dosing, and urine was collected throughout the duration of the serial blood sampling protocol to estimate CLCr. Creatine clearance was calculated from the plasma creatinine concentration (Pcr), the urine creatinine concentration (Ucr), the urine volume (Uvol), and the time over which that volume was collected in minutes (Tmin) by the following equation:
A maternal serum and umbilical cord sample was obtained at delivery when possible.
The Inventory of Depressive Symptomatology Scale Self‐Report 12 and the Generalized Anxiety Disorder 7 13 were completed during each study visit. In addition, a clinical mood assessment including a mental status exam and the Young Mania Rating Scale 14 was administered by a clinician. Body mass index and vital signs were obtained at each visit.
Participants provided consent to obtain medical records from their psychiatrist as well as their obstetrician. At each visit, their medication list was updated. After each visit, prescribers were provided with the scores from the Generalized Anxiety Disorder 7, Inventory of Depressive Symptomatology Scale Self‐Report, and Young Mania Rating Scale.
Serum Lithium Ion Concentration Measurement
The lithium ion concentrations of serum samples were measured by NMS Labs (Horsham, Pennsylvania) using a validated inductively coupled plasma optical emission spectroscopy (iCAP 6500 Duo Simultaneous ICP‐OES [inductively coupled plasma atomic emission spectroscopy] instrument; Thermo Scientific, Waltham, Massachusetts) method. A multipoint calibration curve was prepared, and the sample concentration was calculated on the basis of a linear regression curve fit to the signal measured from the standards. A fresh serum calibration curve was prepared daily. Each batch of serum samples analyzed included at least 0.072 and 1.73 mEq/L quality control samples, along with blank matrix and blank reagent controls, to determine the acceptability of each run. For the analyses, 0.5‐mL aliquots of each serum sample were brought to full volume with deionized water and internal standard/diluent. The serum lithium assay was linear from 0.0144 to 2.88 mEq/L with both intra‐assay and interassay coefficients of variation of <10% throughout the linear range. The lower limit of lithium quantification was 0.0144 mEq/L for serum.
Pharmacokinetic Modeling
The serum lithium ion concentration vs time relationships were modeled using the SAAM II software system (SAAM Institute, Seattle, Washington). Individual serum concentration histories resulting from a single oral dose were obtained by a standard pharmacokinetic curve peel and modeled using a 1‐compartment pharmacokinetic model. The SAAM II objective function used was the extended least‐squares maximum likelihood function using data weighted with the inverse of the model‐based variance of the data at the observation times. 15 Model misspecification was sought by visual inspection of the measured and predicted marker concentrations vs time relationships. 15
Results
Four participants enrolled in the study. Three women completed the investigation (1 Black, 1 White, 1 Hispanic) and 1 withdrew. Data from participants who completed the study are detailed here. Participants’ ages ranged from 22 to 39 years, and they all had a confirmed diagnosis of bipolar 1 disorder. One participant completed a visit between 10 and 15 weeks’ gestation. All 3 participants completed visits between 18 and 26 weeks’ gestation and between 31 and 33 weeks’ gestation. One participant completed a preconception visit. Following delivery, all participants were evaluated at 4 to 9 weeks, and 2 also completed an evaluation at 12 weeks or later. A total of 182 samples were collected for serum lithium concentration analysis. Women in the study took lithium twice daily (n = 1) or once daily (n = 2) during pregnancy and postpartum. One participant receiving once daily lithium dosing before pregnancy completed a preconception visit. After switching to twice‐daily dosing in the first trimester of pregnancy, she continued twice‐daily dosing through her postpartum visit. Body mass index ranged from 23.3 to 34.5 kg/m2, with an increase of 0.83 to 3.73 kg/m2 by the end of pregnancy.
The mean (±standard deviation [SD]) baseline (determined before pregnancy or at the second postpartum visit) lithium elimination clearance in these patients was 21.5 ± 3.0 mL/min (Table 1), which is similar to the mean (±SD) lithium elimination clearance in the 8 (6 women and 3 men) patients with normal body habitus in the study of Reiss et al 16 (23.0 ± 6.2 mL/min). Compared to baseline, lithium elimination clearance was increased in all pregnancy trimesters (Table 1). On average, lithium elimination clearance increased 63.5% by the third trimester compared to baseline. Elimination clearance returned to baseline by the time of the first postdelivery visit, which ranged from 4 to 9 weeks postpartum among the participants. The fit of the pharmacokinetic model to the single dose data for patient 1, who received a single daily dose of 1200 mg of lithium in the second and third trimesters of pregnancy and in her 2 postpartum visits, is illustrated in Figure 1. In addition, the pharmacokinetic models of patients 2 and 3, who had dose adjustments or started lithium in pregnancy, respectively, are shown in Figure 1. As expected, decreases in serum lithium concentrations paralleled increases in serum lithium clearance. Lithium elimination clearance paralleled CLCr (Figure 2). Changes in serum lithium concentrations correlated with changes in serum creatinine concentrations (Table 2).
Table 1.
Lithium Pharmacokinetics and Creatinine Clearance
| Patient 1 | Patient 2 | Patient 3 | Mean (SD) | ||
|---|---|---|---|---|---|
| Volume of distribution at steady state, L | Trimester 1 | … | 24.3 | … | … |
| Trimester 2 | 29.1 | 28.1 | 21.7 | 26.3 (4.0) | |
| Trimester 3 | 30.7 | 22.8 | 25.7 | 26.4 (4.0) | |
| Postpartum 1 (4‐9 weeks) | 28.4 | 24.4 | 27.0 | 26.6 (2.0) | |
| Postpartum 2 (>12 weeks) | 29.6 | 23.0 a | 27.9 | 26.8 (3.4) | |
| Lithium elimination clearance, mL/min | Trimester 1 | … | 53.4 | … | … |
| Trimester 2 | 35.3 | 39.2 | 23.5 | 32.7 (8.2) | |
| Trimester 3 | 36.4 | 41.1 | 28.4 | 35.3 (6.4) | |
| Postpartum 1 (4‐9 weeks) | 26.2 | 25.0 | 18.7 | 23.3 (4.0) | |
| Postpartum 2 (>12 weeks) | 21.3 | 24.6 a | 18.6 | 21.5 (3.0) | |
| Lithium elimination half‐life, h | Trimester 1 | … | 5.3 | … | … |
| Trimester 2 | 9.5 | 8.3 | 10.6 | 9.5 (1.2) | |
| Trimester 3 | 9.7 | 6.4 | 10.5 | 8.9 (2.2) | |
| Postpartum 1 (4‐9 weeks) | 12.5 | 11.3 | 16.7 | 13.5 (2.8) | |
| Postpartum 2 (>12 weeks) | 16.0 | 10.8 a | 17.3 | 14.7 (3.4) | |
| Creatinine clearance, mL/min | Trimester 1 | … | 183.8 | … | … |
| Trimester 2 | 191.3 | 147.9 | 179.6 | 172.9 (22.5) | |
| Trimester 3 | 206.3 | 143.1 | 163.6 | 171.0 (32.2) | |
| Postpartum 1 (4‐9 weeks) | 108.5 | 151.8 | 135.9 | 132.1 (22) | |
| Postpartum 2 (>12 weeks) | 133.2 | 133.8 a | 101.3 | 122.8 (18.6) |
SD, standard deviation.
Trimester 1 = 0‐12 weeks; trimester 2 = 13‐26 weeks; and trimester 3 = 27‐40 weeks.
Postpartum 2 data are generally considered to be baseline data. Preconception baseline rather than postpartum baseline pharmacokinetics were determined for this subject.
Figure 1.
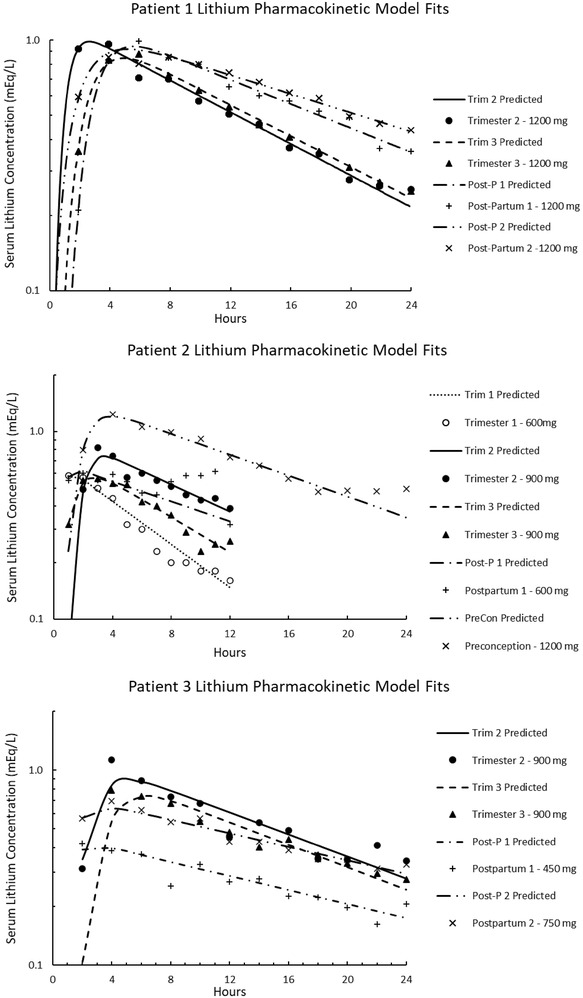
Serum lithium concentration versus time relationship following administration of a 1200‐mg dose of lithium to patient 1 in the second trimester of pregnancy (TRIM 2), the third trimester of pregnancy (TRIM 3), 4‐9 weeks postpartum (Post‐P 1), and at least 12 weeks postpartum (Post‐P 2) and the fit of the 1 compartment model to the data. Patient 2, serum lithium concentration versus time relationship following administration of 1200 mg dose preconception, a 600 mg dose in TRIM 1 and Post‐P 1, and a 900 mg dose in TRIM 2 and TRIM 3 and the fit of the 1 compartment model. Patient 3, serum lithium concentration versus time graph following administration of a 900 mg dose in TRIM 2 and TRIM 3, a 450 mg dose Post‐P 1, and a 750 mg dose Post‐P 2 and the fit to 1 compartment model.
Figure 2.

Lithium clearance vs creatinine clearance.
Table 2.
Lithium and Creatinine Concentrations and Symptom Measurement
| Patient 1 Preconception Dose = 1200 mg Once Daily | Patient 2 Preconception Dose = 1200 mg Once Daily | Patient 3 Preconception Dose = 0 mg daily | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TRI 1 | TRI 2 | TRI 3 | PP 1 | PP 2 | Pre‐conc | TRI 1 | TRI 2 | TRI 3 | PP 1 | TRI 1 | TRI 2 | TRI 3 | PP 1 | PP 2 | |
| Dose, mg | … | 1200 | 1200 | 1200 | 1200 | 1200 | 600/600 | 600/900 c | 600/900 c | 600/600 | … | 900 | 900 | 450 | 750 |
| a Lithium, mEq/L | … | 0.35 | 0.34 | 0.60 | 0.71 | 0.65 | 0.34 | 0.67 | 0.58 | 0.66 | … | 0.56 | 0.47 | 0.4 | 0.52 |
| b C/D ratio | … | 0.03 | 0.03 | 0.05 | 0.06 | 0.06 | 0.03 | 0.05 | 0.04 | 0.06 | … | 0.06 | 0.05 | 0.09 | 0.07 |
| Serum creatinine, mg/dL | … | 0.51 | 0.5 | 0.61 | 0.66 | 0.79 | 0.52 | 0.62 | 0.65 | 0.76 | … | 0.62 | 0.65 | 0.77 | 0.9 |
| IDS‐SR | … | 70 | 74 | 67 | 61 | 5 | 13 | 10 | 11 | 3 | … | 8 | 11 | 14 | 13 |
| GAD‐7 | … | 21 | 21 | 21 | 21 | 3 | 0 | 3 | 1 | 0 | … | 0 | 0 | 0 | 1 |
| YMRS | … | 8 | 8 | 6 | 6 | 0 | 0 | 0 | 0 | 0 | … | 0 | 0 | 0 | 4 |
GAD‐7, Generalized Anxiety Disorder 7; IDS‐SR, Inventory of Depressive Symptomatology Scale Self‐Report; PP, postpartum; TRI, trimester; YMRS, Young Mania Rating Scale.
All dose changes reported were based on prescriber monitoring.
Lithium serum concentrations were obtained at trough (within 10 min of the next scheduled dose); trough is reported as 24‐h concentrations for lithium dosed once daily and at 12 h for lithium dosed twice daily.
C/D ratio calculation: concentration/dose × 100.
Trough serum concentration was obtained before this dose.
Lithium was held at the onset of labor for all 3 participants. Umbilical cord blood samples were collected for the 1 participant who delivered at Northwestern. Lithium serum concentrations obtained from the mother and the umbilical cord were not detectable (lower limit of quantification 0.0144 mEq/L).
Concentration and Dose Changes
Participants took a total daily lithium dose of 900 mg (n = 1) or 1200 mg (n = 2) at the time of their first study visit as prescribed by their psychiatrist. Baseline serum lithium concentrations ranged from 0.52 to 0.71 mEq/L. Patients 1 and 3, who took lithium once a day, had 12‐hour serum lithium concentrations that were 40% to 55% higher than her 24‐hour trough serum lithium concentrations. Serum lithium concentrations were 50% lower at 15 and 26 weeks compared to postpartum baseline concentrations for patient 1. Two participants had dose increases of 20% to 25%.
Mood Symptoms During Pregnancy and Postpartum
Depressive, manic, and anxiety symptoms were related to decreased serum lithium concentrations during pregnancy (Table 2). Patients 1 and 2 reported mixed depressive, manic, and anxiety symptoms including sleep disturbance, irritability, diurnal mood variation, and restlessness. These symptoms continued throughout pregnancy in patient 1, whose psychiatrist did not change her dose. She had persistent symptoms worsening after her 24‐hour serum lithium concentration decreased from a baseline of 0.71 to 0.35 mEq/L (Table 2). Increasing the lithium dose by 300 mg improved symptoms in patient 2. Her symptoms worsened during the first trimester when her 12‐hour lithium concentration declined to 0.34 mEq/L from a baseline 12‐hour concentration of 0.65 mEq/L (Table 2). After the dose increase, she remained asymptomatic throughout the remainder of pregnancy. Patient 3's lithium dose was initiated in pregnancy and titrated to a therapeutic concentration during the first trimester. Shortly after birth, she required a 50% dose decrease to relieve nausea, which was attributed to lithium side effects. By postpartum visit 2, her dose was increased to achieve a more therapeutic effect and remained 20% lower than her dose in pregnancy. The nausea was consistent with adverse effects related to the decrease in lithium elimination clearance and resultant increase in postpartum serum lithium concentration.
Discussion
Our prospective longitudinal investigation extends information on the trajectory of changes in lithium elimination clearance, serum concentrations, and symptom course in pregnant and postpartum women with bipolar disorder. Lithium elimination clearance increased by >50% in all participants by the third trimester of pregnancy and returned to near baseline by 4 to 9 weeks postpartum. Decreases in steady‐state serum lithium concentrations were inversely associated with increases in elimination clearance. The volume of distribution of lithium remained relatively stable throughout pregnancy, which suggests that decreases in lithium concentration were most influenced by the impact of physiological changes on lithium elimination clearance. The changes in CLCr paralleled lithium elimination clearance and were inversely associated with serum lithium concentrations and creatinine concentrations. Symptoms worsened in patients with lithium concentrations that decreased >0.2 mEq/L below their personal baseline concentration and/or below the therapeutic concentration of 0.4 mEq/L.
The changes in lithium elimination clearance and serum lithium concentration we observed during pregnancy are comparable to those reported by others. Consistent with Schou's finding that lithium elimination clearance was higher in the third trimester compared to the nonpregnant baseline, we observed that the mean lithium elimination clearance increased by >50% by the third trimester of pregnancy compared to the nonpregnant baseline. 8 Similar to our finding that serum lithium concentrations decreased as much as 50% during pregnancy, Westin et al 10 reported that concentration‐to‐dose ratios were significantly below baseline (–34%; 95%CI, –44% to –23%) in the third trimester.
The increase in elimination clearance was sustained throughout pregnancy and was stable through the second and third trimesters in all participants. This finding and that of Shou differ from the finding of Wesseloo et al, 9 who determined that the mean serum lithium concentration decreased from preconception through second trimester and increased in the late third trimester. Data from each of our participants show that the trajectory of lithium elimination clearance and corresponding lithium concentration is consistent within patients but varies between patients. Changes in lithium clearance affect serum concentrations, which must serve as the basis of individual dose adjustments, along with consideration of the timing of sample collection and pregnancy‐related physiological changes.
Many women with bipolar disorder experience symptom recurrence during pregnancy despite continuation of medication. 17 The lack of dose adjustment to sustain an effective concentration of mood stabilizers in the face of increased elimination clearance is one reason for symptom worsening and episode recurrence. Consistent with Westin et al., 10 we found that decreases in lithium concentration affected the course of bipolar symptoms.
Standard clinical practice recommends therapeutic drug monitoring of lithium serum concentrations 12 hours after dosing. Blood samples should be collected for serum lithium concentration measurements after the absorption phase (≈3 hours after dosing) and as close as possible to 12 hours after dosing for comparison to standard dosing concentrations. Consistent with the consensus by the International Society of Bipolar Disorders Task Force for nonpregnant patients, we recommend measuring steady‐state (achieved after lithium is taken consistently for a minimum of 5 days) serum concentrations at 12 hours (±1 hour) after dosing for twice‐daily dosing before conception as well as during pregnancy and postpartum for the most accurate determination of serum lithium concentration. 2 Despite the increasingly common practice of dosing lithium once daily instead of twice daily or 3 times a day, the timing of blood sample collection for therapeutic drug monitoring is rarely adjusted. However, for the pregnant patient, we agree with experts who recommend monitoring 24‐hour trough serum concentrations for once‐daily dosing. 18 Nonpregnant patients prescribed lithium once daily have 12‐hour concentrations that are 10% to 15% higher than the trough concentration. 19 We found that the lithium concentration for our patients who took once‐daily doses were grossly overestimated during pregnancy at 12 hours compared to the 24‐hour serum concentration due to the higher elimination clearance during pregnancy (Figure 1). To prevent overestimation of the lithium serum concentration and underdosing, 24‐hour monitoring is most accurate for once‐daily dosing. If the patient has difficulty providing a 12‐hour or 24‐hour blood sample, obtaining serum concentrations at the same time after dosing and as close to 12 or 24 hours after dosing depending on dosing schedule, is effective for serum concentration monitoring during pregnancy and postpartum.
The increased risk of therapeutic failure compels lithium dose adjustments when serum lithium concentrations decrease below the woman's most effective serum lithium concentration established before pregnancy. Our finding that increased lithium elimination clearance is sustained in the second and third trimesters suggests that higher lithium doses can be maintained throughout pregnancy without the need to reduce the lithium dose in the third trimester, if the woman is without pregnancy complications that decrease renal function (ie, preeclampsia, acute renal insufficiency). To maintain stable concentration during pregnancy, serum lithium concentrations should be checked once each trimester.
Although the empirical method has not been validated in pregnancy, it has been used for conservative serum lithium concentration prediction for dose adjustments and may be used to adjust dose when serum lithium concentrations fall below the prepregnancy baseline. According to the empirical method, for every 300‐mg increase in dose, the serum lithium concentration increases by 0.3 ± 0.1 mEq/L. 19 Due to interindividual variability, once the dose is adjusted, serum lithium concentration measurement is repeated in 1 week (or before the next trimester visit) to determine if the patient has achieved the desired concentration. During pregnancy, dose adjustments must account for the increased elimination clearance. Each dose adjustment should be followed by a repeated serum lithium concentration measurement at steady state to ensure that the targeted concentration has been achieved. Because the volume of distribution during pregnancy did not change significantly among our participants, increased once‐daily doses may push peak concentrations into the toxic range and result in adverse side effects such as nausea and excessive sedation. For this reason, twice‐daily dosing is recommended, with dose increases to avoid peak concentration side effects. Use of lithium sustained‐release formulations are an alternative to the immediate‐release formulation but may also require twice daily dosing to minimize peak effects.
After delivery, the lithium elimination clearance quickly decreased and returned to baseline as early as 1 month postpartum and possibly sooner. It has been recommended to obtain serum lithium concentrations after delivery and twice weekly for the first 2 weeks postpartum, 8 which may be appropriate if lithium was initiated during pregnancy. However, if lithium was taken before pregnancy, we found that adjustment of the dose to baseline postpartum did not require more than weekly monitoring or cause adverse effects. Further characterization of the trajectory of lithium elimination clearance throughout pregnancy and postpartum through a prospective approach is an important next step to establishing universal monitoring and dose adjustment guidelines for lithium during the perinatal period.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
Supported by the National Institute of Child Health and Human Development (K23HD087529; Dr Clark).
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author [CTC] at crystal.clark@northwestern.edu, upon reasonable request. The data are not publicly available due to the data containing information that could compromise research participant privacy/consent.
References
- 1. Severus WE, Kleindienst N, Seemuller F, Frangou S, Moller HJ, Greil W. What is the optimal serum lithium level in the long‐term treatment of bipolar disorder–a review? Bipolar Disord. 2008;10(2):231‐237. [DOI] [PubMed] [Google Scholar]
- 2. Nolen WA, Licht RW, Young AH, et al. What is the optimal serum level for lithium in the maintenance treatment of bipolar disorder? A systematic review and recommendations from the ISBD/IGSLI Task Force on treatment with lithium. Bipolar Disord. 2019;21(5):394‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nemeroff CB, Evans DL, Gyulai L, et al. Double‐blind, placebo‐controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry. 2001;158(6):906‐912. [DOI] [PubMed] [Google Scholar]
- 4. Coryell W, Endicott J, Maser JD, Mueller T, Lavori P, Keller M. The likelihood of recurrence in bipolar affective disorder: the importance of episode recency. J Affect Disord. 1995;33(3):201‐206. [DOI] [PubMed] [Google Scholar]
- 5. Tondo L, Alda M, Bauer M, et al. Clinical use of lithium salts: guide for users and prescribers. Int J Bipolar Disord. 2019;7(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Elsevier Saunders; 2006;xxxv:1116. [Google Scholar]
- 7. Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis. 2013;20(3):209‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schou M, Amdisen A, Steenstrup OR. Lithium and pregnancy. II. Hazards to women given lithium during pregnancy and delivery. Br Med J. 1973;2(5859):137‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wesseloo R, Wierdsma AI, van Kamp IL, et al. Lithium dosing strategies during pregnancy and the postpartum period. Br J Psychiatry. 2017;211(1):31‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westin AA, Brekke M, Molden E, Skogvoll E, Aadal M, Spigset O. Changes in drug disposition of lithium during pregnancy: a retrospective observational study of patient data from two routine therapeutic drug monitoring services in Norway. BMJ Open. 2017;7(3):e015738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59(20):22‐33;quiz 34–57. [PubMed] [Google Scholar]
- 12. Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477‐486. [DOI] [PubMed] [Google Scholar]
- 13. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD‐7. Arch Intern Med. 2006;166(10):1092‐1097. [DOI] [PubMed] [Google Scholar]
- 14. Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429‐435. [DOI] [PubMed] [Google Scholar]
- 15. Barrett PH, Bell BM, Cobelli C, et al. SAAM II: simulation, analysis, and modeling software for tracer and pharmacokinetic studies. Metabolism. 1998;47(4):484‐492. [DOI] [PubMed] [Google Scholar]
- 16. Reiss RA, Haas CE, Karki SD, Gumbiner B, Welle SL, Carson SW. Lithium pharmacokinetics in the obese. Clin Pharmacol Ther. 1994;55(4):392‐398. [DOI] [PubMed] [Google Scholar]
- 17. Viguera AC, Whitfield T, Baldessarini RJ, et al. Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry. 2007;164(12):1817‐1824; quiz 1923. 10.1176/appi.ajp.2007.06101639 [DOI] [PubMed] [Google Scholar]
- 18. Reddy DS, Reddy MS. Serum lithium levels: ideal time for sample collection! Are we doing it right? Indian J Psychol Med. 2014;36(3):346‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amdisen A. Serum level monitoring and clinical pharmacokinetics of lithium. Clin Pharmacokinet. 1977;2(2):73‐92. [DOI] [PubMed] [Google Scholar]


