Abstract
Stachybotrys chartarum is a toxigenic fungus that has been associated with human health concerns, including pulmonary hemorrhage and hemosiderosis. This fungus produces a hemolysin, stachylysin, which in its apparent monomeric form has a molecular mass of 11,920 Da as determined by matrix-assisted laser desorption ionization–time of flight mass spectrometry. However, it appears to form polydispersed aggregates, which confounds understanding of the actual hemolytically active form. Exhaustive dialysis or heat treatment at 60°C for 30 min inactivated stachylysin. Stachylysin is composed of about 40% nonpolar amino acids and contains two cysteine residues. Purified stachylysin required more than 6 h to begin lysing sheep erythrocytes, but by 48 h, lysis was complete. Stachylysin also formed pores in sheep erythrocyte membranes.
Hemolysins are molecules that are designated as such because they have the ability to lyse erythrocytes (RBCs). It is now recognized that the biological significance of these toxins goes beyond their lysis of RBCs to their more general ability to form pores in many cells (4). Today, the consensus is that the majority of relevant bacterial pathogens produce pore-forming proteins (4). Hemolysins have been isolated and purified from many bacterial pathogens, and they are generally important virulence factors (3–45, 13, 17, 18, 22, 25).
It is often thought that bacterial cytolysins act primarily by killing host cells, but nonlethal reactions in other cells, including endothelial and immune cells, occur as a result of exposure to these toxins (4, 6). Many bacterial hemolysins create pores not only in RBCs, but also in the membranes of nucleated cells (e.g., neutrophils, monocytes, and endothelial cells) (2, 10, 20), and can affect the aggregation of platelets (12). These hemolysin-mediated responses affect the pathophysiology of the host.
A number of fungal pathogens produce hemolysins (11, 19, 26). Recently, production of a hemolysin by the toxigenic fungus Stachybotrys chartarum was described (23). S. chartarum is a toxigenic fungus that has been associated with human health concerns, including pulmonary hemorrhage and hemosiderosis (PH) in infants in Cleveland, Ohio (7). In this paper, we describe the isolation, purification, and characterization of a pore-forming hemolysin, stachylysin, produced by S. chartarum.
MATERIALS AND METHODS
Purification of hemolysin.
S. chartarum conidia of strain 58-06 were produced after 5 weeks of growth on sterile wall board, as previously described (23). Approximately 105 conidia in a 100-μl suspension were used to inoculate 500 ml of tryptic soy broth (TSB) (Becton Dickinson, Sparks, Md.) in a 1-liter flask placed on an incubator shaker (LabLine, Inc. Melrose Park, Ill.) set at 36 ± 1°C and mixed at 200 rpm/min. After 7 days of incubation, the cells and debris were removed from the culture by centrifugation for 15 min at 5,000 × g in an RC5 centrifuge (Dupont Instruments, Newton, Conn.), and the supernatant was recovered. The supernatant was centrifuged in a Millipore Centricon plus 80 filter apparatus with a molecular mass cutoff of 50 kDa (Millipore, Bedford, Mass.) at 4,000 × g for 15 min in an RC5 centrifuge. The concentrate was recovered according to the manufacturer's instructions. The concentrate was then subjected to gel filtration with Sephadex G 100-50 (Sigma, St. Louis, Mo.) hydrated in 0.2M sodium azide for 5 days and giving a final bed of 0.5 by 14 cm. The concentrate was added to the top of the gel filtration column with 0.2 M sodium azide as solvent. Fractions (0.25 ml) were collected at 1.5 ml per h with a fraction collector (ISCO, Lincoln, Nebr.). Then 10 μl of each fraction was plated on sheep's blood agar (SBA) (Becton Dickinson) and incubated at 37°C; hemolysis was noted at 48 h. This gel filtration process was repeated two more times with the five most hemolytically active fractions.
After the final gel filtration, the five most hemolytically active fractions were combined. In some cases, these fractions were desalted twice or three times by using the D-Salt polyacrylamide 6000 desalting column (Pierce, Rockford, Ill.). The final desalted solution was frozen at −80°C and lyophilized with a Spin Vac (Savant Instruments, Farmingdale, N.Y.). The lyophilized pellet was resuspended in sterile water for further analysis or to use in other experiments. In other cases, the five most hemolytically active fractions were placed in dialysis tubing (Spectrum Laboratories, Inc., Laguna Hills, Calif.) with a molecular mass cutoff of 3,000 Da and dialyzed over 48 h against eight 1,000-ml changes of sterile, deionized water. In some cases, this preparation was lyophilized, 10 μl of TSB was added to the pellet, and the solution was tested for hemolytic activity.
Electrophoresis.
Gel electrophoresis was performed with Metaphor Agarose (BMA, Rockland, Maine) according to the manufacturer's instructions. A 4% resolving gel containing 500 mM Tris base, 160 nM boric acid, and 1 M urea was run in a Tris-boric acid buffer (pH 8.5) with 0.1% sodium dodecyl sulfate (SDS). The gels were run at 50 V for 6 h and then stained with Coomassie G-250. The molecular mass standards used were Promega (Madison, Wis.) high and low-range protein molecular mass markers.
MALDI-TOF MS analysis.
A KOMPACT SEQ (Kratos Analytical, Ramsey, N.J.) matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometer was used for MALDI-TOF mass spectrometry (MS). Mass spectra were acquired in the positive linear high-power mode, and pulsed extraction was used to improve resolution. Calibration was done with apomyoglobin and angiotensin II (Sigma). The MALDI target was spotted with 0.25 μl of the sample solution. Before the sample dried, 0.25 μl of matrix solution was applied on top of the sample. The resultant spot was allowed to air dry. The matrix solution was made up of 10 mg of 3,5-dimethoxy-4-hydroxy-cinnamic acid per ml in 50% acetonitrile in water containing 0.05% trifluoroacetic acid.
Characterization of the hemolytic activity.
The concentration of stachylysin in solution was determined by using the DC protein assay (Bio-Rad, Hercules, Calif.). To determine the rate of hemolysis, 20.0 μg of purified stachylysin per ml in water was spotted onto an SBA plate. The plate was incubated at 37°C and photographed at intervals during the incubation. The temperature of inactivation was determined by incubating solutions of 20.0 μg of purified stachylysin per ml in a water bath at 40, 50, and 60°C for 30 min and then plating on SBA and determining the presence or absence of hemolysis.
TEM observations of stachylysin-treated RBCs.
Approximately 0.2 μg of purified stachylysin was added to a 50-μl suspension of sheep RBCs (Becton Dickinson) and incubated statically at 37°C. After 48 h, the stachylysin-treated cells, or untreated cells which were handled identically to the treated cells, were placed on carbon-coated grids, stained with 1% phosphotungstic acid, and examined by transmission electron microscopy (TEM) (JEOL 1200EX).
Amino acid analysis.
Amino acid analysis was performed by the W. M. Keck Facility at Yale University by using a Beckman 7300 amino acid analyzer (Beckman Coulter, Inc., Fullerton, Calif.).
RESULTS
Stachylysin description.
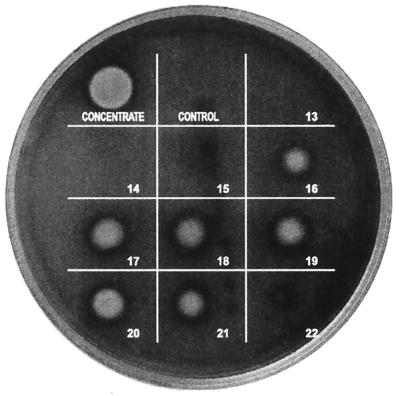
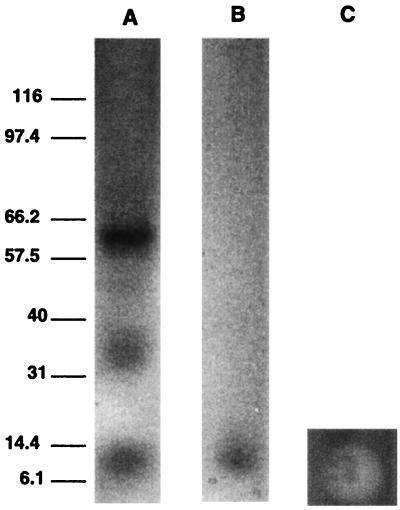
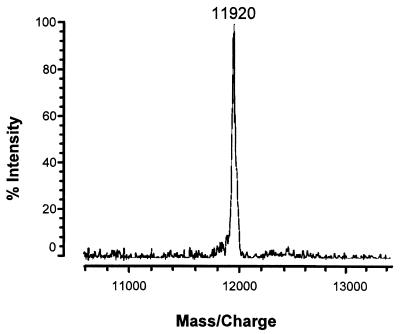
Figure 1 shows the hemolytic activity on SBA of 10-μl spots of gel filtration fractions 13 to 22 from the gel filtration of the hemolysin from S. chartarum isolate 58-06. The active fractions are 16 through 21 (Fig. 1). Gel electrophoresis (after lyophilization) of the combined active fractions, while still in 0.2 M sodium azide, showed a major band at about 60 kDa, two lighter bands at approximately 36 and 12 kDa (Fig. 2A), and a very light band at approximately 48 kDa (not obvious in the photograph). In some preparations, even higher-molecular-mass bands were observed. If the fractions were double desalted only, an approximately 12-kDa band was observed (Fig. 2B), and this was hemolytically active (Fig. 2C). This double-desalted fraction was examined by MALDI-TOF MS and showed a single protein band at 11,920 ± 10 Da (Fig. 3). The error (±10 Da) is the peak width at half the peak height. In this preparation, there were a large sodium peak and a small potassium peak. When this fraction was desalted a third time, the protein ran in the gel (with SDS) with an apparent molecular mass of 6 kDa (not shown).
FIG. 1.
Test for hemolytic activity in gel filtration fractions. Strain 58-06 of S. chartarum was grown in TSB. The proteins greater than 50- kDa were concentrated as described in Materials and Methods and then subjected to gel filtration in 0.2 M sodium azide. Fractions (0.25 ml) were collected, and 10 μl of each fraction was plated on SBA (Becton Dickinson) and incubated at 37°C for 48 h. The spot labeled as “concentrate” was applied before gel filtration. Ten microliters of the 0.2 M sodium azide solution was used as the control.
FIG. 2.
Gel electrophoresis (after lyophilization) of combined hemolytically active fractions in 0.2 M sodium azide (A). Gel electrophoresis (with SDS) after double desalting and lyophilization (B). Molecular mass standards in kilodaltons are shown on the left. (C) Lysis response to 10 μl of doubly-desalted stachylysin solution applied to SBA and then incubated at 37°C for 48 h.
FIG. 3.
MALDI-TOF mass spectrum of the doubly-desalted stachylysin fractions. Mass spectra were acquired in positive linear high-power mode, and pulsed extraction was used to improve resolution. Calibration was done with apomyoglobin and angiotensin II. The MALDI target was spotted with 0.25 μl of the sample solution. Before the sample dried, 0.25 μl of matrix solution was applied on top of the sample. The resultant spot was allowed to air dry. The matrix solution was 10 mg of 3,5-dimethoxy-4-hydroxy-cinnamic acid per ml in 50% acetonitrile in water containing 0.05% trifluoroacetic acid.
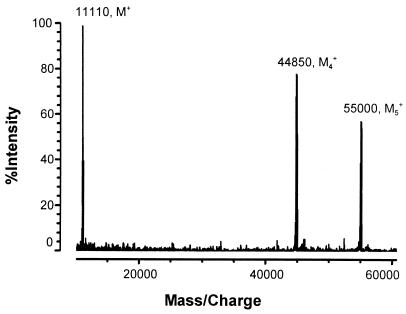
When the fractions were exhaustively dialyzed against sterile deionized water, the protein lost its hemolytic activity. The MALDI-TOF MS analysis of the dialyzed preparation indicated three peaks, designated M1 at 11,140 Da, M4 at 44,890 Da, and M5 at 55,060 Da (Fig. 4). There was no evidence in the MALDI-TOF MS analysis of the dialyzed fraction of sodium or potassium. Addition of TSB back to the inactivated hemolytic preparation did not restore hemolytic activity.
FIG. 4.
MALDI-TOF mass spectrum of the exhaustively dialyzed stachylysin fractions. Mass spectra were acquired in the positive linear high-power mode, and pulsed extraction was used to improve resolution. Calibration was done with apomyoglobin and angiotensin II. The MALDI target was spotted with 0.25 μl of the sample solution. Before the sample dried, 0.25 μl of matrix solution was applied on top of the sample. The resultant spot was allowed to air dry. The matrix solution was 10 mg of 3,5-dimethoxy-4-hydroxy-cinnamic acid per ml in 50% acetonitrile in water containing 0.05% trifluoroacetic acid.
Characterization of the hemolytic activity.
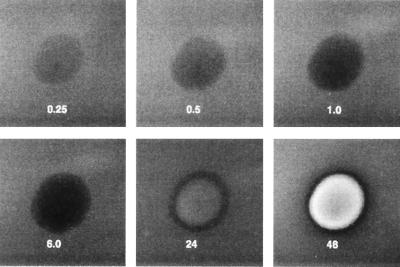
When 0.2 μg of purified stachylysin in 10 μl of sterile deionized water was placed on SBA, the treated area began to turn dark in about 15 min (Fig. 5), and by 6 h, the spot had become black. After 24 h of incubation at 37°C, lysis had begun, and by 48 h, it was complete. Stachylysin was inactivated by incubation at 60°C for 30 min. Similar treatments at 40 or 50°C had no effect on the hemolytic activity of stachylysin.
FIG. 5.
Rate of stachylysin hemolysis of sheep RBCs. Approximately 0.2 μg of purified stachylysin in 10 μl of sterile, deioinzed water was spotted onto an SBA plate. The plate was incubated at 37°C and photographed at intervals of 0.25, 0.5, 1.0, 6.0, 24, and 48 h during the incubation.
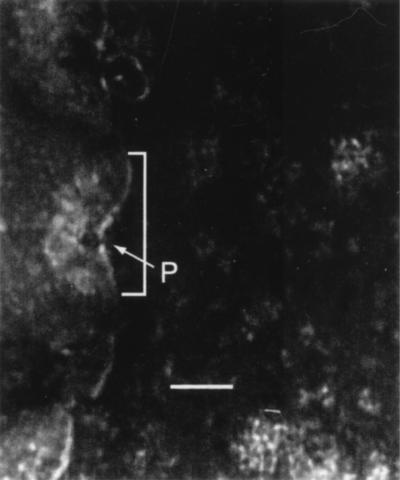
The edge of a stachylysin-treated sheep RBC (37°C for 48 h) is shown in Fig. 6. Stachylysin caused pores to form in the RBC membrane, which were about 5 nm in diameter (P in Fig. 6). Each pore was at the center of a ring-like depression in the membrane about 200 to 300 nm across (shown by a bracket in Fig. 6). Untreated sheep RBCs maintained a smooth, uniform cell membrane integrity.
FIG. 6.
TEM observations of stachylysin-treated RBCs. Approximately 0.2 μg of purified stachylysin was added to a 50-μl suspension of sheep RBCs and incubated statically at 37°C. After 48 h, the stachylysin-treated cells were placed on carbon-coated grids, stained with 1% phosphotungstic acid, and then examined by TEM (JEOL 1200EX). The bracketed area shows the membrane depression resulting from the stachylysin pore-forming process, and P is the actual pore. The scale bar is 100 nm.
Amino acid analysis.
Table 1 shows the amino acid analysis of stachylysin. The sum of the masses of the amino acids in the peptide gave a calculated molecular mass of about 11,904 Da. About 40% of the amino acids are hydrophobic. There are two cysteine residues in each monomer.
TABLE 1.
Amino acid analysis of stachylysin, as determined by the Yale University W. M. Keck Facility
| Amino acid | No. of residues |
|---|---|
| Hydrophobic | |
| Alanine | 9 |
| Isoleucine | 4 |
| Leucine | 5 |
| Methionine | 1 |
| Phenylalanine | 3 |
| Proline | 12 |
| Tryptophan | 0 |
| Tyrosine | 3 |
| Valine | 8 |
| Hydrophilic | |
| Arginine | 2 |
| Asparagine/aspartic acid | 9 |
| Glutamine/glutamic acid | 12 |
| Histidine | 3 |
| Lysine | 3 |
| Serine | 13 |
| Threonine | 17 |
| Other | |
| Cysteine | 2 |
| Glycine | 8 |
| Total | 114 |
DISCUSSION
In the parlance of bacterial nomenclature, stachylysin would be called a β-hemolysin, since it completely lyses the RBCs, leaving a cleared ring around the colony (23). The Candida albicans hemolysin and Asp-hemolysin from Aspergillus fumigatus would apparently also be called β-hemolysins (14, 26). These hemolysins have been associated with fungal virulence (14, 26).
To preliminarily investigate the solution state form of stachylysin, agarose gel electrophoresis was performed with the active hemolysin. Judging from the intensity of the bands, the dominant form is approximately 60 kDa (Fig. 2A). Lighter bands are also observed in Fig. 2A, at about 12, 36, and 48 kDa. By deduction, the 60-, 48-, 36-, and 12-kDa bands were assigned to the pentamer, tetramer, trimer, and monomer, respectively. When the fractions were partially desalted and run in an SDS gel, only the monomer was detected (Fig. 2B). This monomer has a mass of approximately 11,920 Da, based on the MALDI-TOF MS analysis (Fig. 3). A large sodium peak and a smaller potassium peak (not shown) were detected by MALDI-TOF MS. The mass determined by MALDI-TOF MS compares well to the amino acid analysis, which gave a calculated molecular mass of about 11,904 Da, as well as to the approximate 12-kDa assignment from agarose gel electrophoresis. This 12-kDa assignment was confounded when more of the salt was removed by a third desalting, resulting in a protein with an apparent molecular mass of 6 kDa in an SDS gel.
Extensive dialysis caused a significant change in mass observations based on MALDI-TOF MS (Fig. 4), sodium and potassium peaks disappeared, and the hemolysin was inactivated. The mass of the monomer peak was decreased by about 820 U, possibly indicating a chemical change in the monomer. Two peaks in Fig. 4 were tentatively assigned as the irreversibly aggregated tetramer and pentamer. Irreversible aggregation has been implicated in the inactivation of the E. coli α-hemolysin (21). The culture medium, TSB, contains 0.085 M sodium chloride and 0.014 M dipotassium phosphate. It appears that sodium and/or potassium is critical to the stability and integrity of stachylysin and may even affect the way the protein runs in a nonreducing SDS electrophoresis gel. Once the hemolytic activity was lost by removing the salt, after extensive dialysis, returning it to TSB did not restore activity.
When S. chartarum was grown in TSB, it seems likely that the dominant form of the protein was the pentamer or larger complex, because the protein was retained by the 50-kDa cutoff filter used in the purification process. However, the physical form of the stachylysin hemolysin in solution is not known (i.e., whether it exists as a monomer, a covalently bound polymer, or a noncovalent aggregate). However, it is instructive to consider that the E. coli α-hemolysin, which has been extensively studied (21), is reported to exist in solution as noncovalent polydispersed aggregates.
Stachylysin is a surprisingly slow-acting hemolytic agent (Fig. 5). Most bacterial hemolysins act in a matter of minutes to produce lysis (8), but stachylysin takes hours to days. However, the rapid darkening of the SBA in 15 min suggests that some change resulting in the reduction of hemoglobin has occurred. The highly polar nature of the protein suggests an ability to integrate into the cell membrane, causing the change in the cell membrane seen around the pore (Fig. 6).
It is worth pointing out that the relationship between the aggregated form of the protein in solution and the active form of the protein on the RBC membrane is not known. In practice, the exact form of the protein in solution may not be as important as its form once it binds to the RBC membrane during pore formation. If other pore-forming hemolysins are considered, it is reported that for alpha-toxin (4), the hemolysin exists in monomeric form in solution and then forms aggregates on the cell membrane, leading to pore formation, as demonstrated through molecular engineering of mutant hemolysins. For E. coli α-hemolysin, it is reported (21) that in solution, the α-hemolysin exits as polydispersed aggregates, but the active form is the monomer. The actual demonstration of the solution state of stachylysin is beyond the scope of this paper, but it is conceivable that, like the α-hemolysin, the stachylysin exists as aggregates, predominantly trimers and pentamers. Like both α-hemolysin and alpha-toxin, stachylysin may be in the monomeric form during the active process of pore formation.
In many ways, stachylysin seems to be similar to some bacterial hemolysins. Like the hemolysin produced by the group B streptococci (GBS), it is a β-hemolysin (16) and it is a pore-forming toxin (15). However, the number of cysteine residues is high compared to typical bacterial hemolysins, which usually contain no or at most one cysteine (4). Hemolysins have been shown to have great significance in the virulence of many bacterial pathogens. The GBS β-hemolysin damages microvascular endothelial cells and causes alveolar edema and hemorrhage in an infant's lungs (1, 9). Recently, it was demonstrated that isolates of S. chartarum from houses in Cleveland, Ohio, where children developed PH and an isolate from the lung of a PH victim in Texas produced significantly more stachylysin than strains isolated from control houses in Cleveland (24). The role and significance of stachylysin in PH are now under investigation.
ACKNOWLEDGMENTS
We thank Armah de la Cruz for advice and use of equipment. We also thank Melvin Sparks for technical assistance. The TEM work of Pat Clark is gratefully noted here.
This work was supported by funding from the U.S. EPA's National Center for Environmental Assessment's “Children at Risk Program,” which is gratefully acknowledged.
REFERENCES
- 1.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: W. B. Saunders; 1995. pp. 980–1054. [Google Scholar]
- 2.Bhakdi S, Greulich S, Muhly M, Korom S, Schmidt G. Effects of E. coli hemolysin on human monocytes: cytocidal action and stimulation of the interleukin-1 release. J Clin Investig. 1990;85:1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Weller U, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 5.Cavalieri S J, Bohach G A, Snyder I S. Escherichia coli α-hemolysin characteristics and probable role in pathogenicity. Microbiol Rev. 1984;48:326–343. doi: 10.1128/mr.48.4.326-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ermert L, Rousseau S, Schutte H, Birkenmeyer R G, Grimminger F, Bhakdi S, Duncker H R, Seeger W. Induction of severe vascular leakage by low doses of Escherichia coli hemolysin in perfused rabbit lungs. Lab Investig. 1992;66:362–369. [PubMed] [Google Scholar]
- 7.Etzel R A, Montana E, Sorenson W G, Kullman G J, Allan T M, Dearborn D G. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch Pediatr Adolesc Med. 1998;152:757–762. doi: 10.1001/archpedi.152.8.757. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson M R, Xu X-J, Houston C W, Peterson J W, Coppenhaver D H, Popov V L, Chopra A K. Hyperproduction, purification, and mechanism of action of the cytotoxic enterotoxin produced by Aeromonas hydrophila. Infect Immun. 1997;65:4299–4308. doi: 10.1128/iai.65.10.4299-4308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson R L, Nizet V, Rubens C E. Group B streptococcal β-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res. 1999;45:626–634. doi: 10.1203/00006450-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Grimminger F, Sibelius U, Bhakdi S, Suttorp N, Seeger W. Escherichia coli hemolysin is a potent inductor of phosphoinositide hydrolysis and related metabolic responses in human neutrophils. J Clin Investig. 1991;88:1531–1539. doi: 10.1172/JCI115463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henrici A T. An endotoxin from Aspergillus fumigatus. J Immunol. 1939;36:319–338. [Google Scholar]
- 12.Iwaki M, Kamachi K, Heveker N, Konda T. Suppression of platelet aggregation by Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1999;67:2763–2768. doi: 10.1128/iai.67.6.2763-2768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson P, Lindberg M, Haraldsson I, Waldström T. Virulence of Staphylococcus aureus in a mouse mastitis model: studies of alpha hemolysin, coagulase, and protein A as possible virulence determinants with protoplast fusion and gene cloning. Infect Immun. 1985;49:765–769. doi: 10.1128/iai.49.3.765-769.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manns J M, Mosser D M, Buckley H R. Production of a hemolytic factor by Candida albicans. Infect Immun. 1994;62:5154–5156. doi: 10.1128/iai.62.11.5154-5156.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nizet V, Gibson R L, Chi E Y, Framson P E, Hulse M, Rubens C E. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Immun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nizet V, Gibson R L, Rubens C E. The role of group B streptococci β-hemolysin expression in newborn lung injury. In: Horaud T, et al., editors. Streptococci and the host. New York, N.Y: Plenum Press; 1997. pp. 627–630. [DOI] [PubMed] [Google Scholar]
- 17.O'Reilly M, Azavedo J C S, Kennedy S, Foster J T. Inactivation of the alpha hemolysin gene of Staphylococcus aureus 8325-4 by site directed mutagenesis and studies on the expression of its haemolysin. Microb Pathog. 1986;1:125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 18.Ou Said A M, Contrepois M G, Vartanian M D, Girardeau J. Virulence factors and markers in Escherichia coli from calves with bacteremia. Am J Vet Res. 1988;49:1657–1660. [PubMed] [Google Scholar]
- 19.Salvin S B. Hemolysin from the yeast-like phases of some pathogenic fungi. Proc Soc Exp Biol Med. 1951;76:52–54. doi: 10.3181/00379727-76-18653. [DOI] [PubMed] [Google Scholar]
- 20.Seeger W, Obernitz R, Thomas M, Walmrath D, Suttorn N, Holland I B, Grimminger F, Eberspacher B, Hugo F, Bhakdi S. Lung vascular injury after administration of viable hemolysin-forming Escherichia coli in isolated rabbit lungs. Am Rev Respir Dis. 1991;143:797–785. doi: 10.1164/ajrccm/143.4_Pt_1.797. [DOI] [PubMed] [Google Scholar]
- 21.Soloaga A, Ramirez J M, Goni F M. Reversible denaturation, self assembly, and membrane activity of Escherichia coli α-hemolysin, a protein stable in 6 M urea. Biochemistry. 1998;37:6387–6393. doi: 10.1021/bi9730994. [DOI] [PubMed] [Google Scholar]
- 22.Van Der Vijer J C M, Van Es-Boom M M, Michel M F. A study of the virulence factors with induced mutants of Staphylococcal aureus. J Med Microbiol. 1975;8:279–287. doi: 10.1099/00222615-8-2-279. [DOI] [PubMed] [Google Scholar]
- 23.Vesper S J, Dearborn D G, Yike I, Sorenson W G, Haugland R A. Hemolysis, toxicity, and randomly amplified polymorphic DNA analysis of Stachybotrys chartarum strains. Appl. Envir on. Microbiol. 1999;65:3175–3181. doi: 10.1128/aem.65.7.3175-3181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vesper S J, Dearborn D G, Elidemir O, Haugland R A. Quantification of siderophore and hemolysin from Stachybotrys chartarum strains, including a strain isolated from the lung of a child with pulmonary hemorrhage and hemosiderosis. Appl Environ Microbiol. 2000;66:2678–2681. doi: 10.1128/aem.66.6.2678-2681.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welch R A, Dellinger E P, Minshew B, Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature (London) 1981;294:665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- 26.Yokota K, Shimada H, Kamaguchi A, Sakaguchi O. Studies on the toxin of Aspergillus fumigatus VII. Purification and some properties of hemolytic toxin (Asp-hemolysin) from culture filtrates and mycelia. Microbiol Immunol. 1977;21:11–22. doi: 10.1111/j.1348-0421.1977.tb02803.x. [DOI] [PubMed] [Google Scholar]