Abstract
Background
Interventions for head/neck cancer (HNC) survivors may not address their cancer‐related and general health needs.
Methods
Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guided this systematic review of studies from 2000 to 2021 of interventions targeting cancer survivors treated with curative‐intent, using MEDLINE, Embase, Emcare, and PsycINFO. Interventions were categorized into domains of the Quality of Cancer Survivorship Care Framework to characterize the scope and quality of interventions.
Results
We identified 28 studies for inclusion: 13 randomized and 15 non‐randomized. Most targeted surveillance/management of physical effects (n = 24) including 13 that also targeted psychosocial effects. Four studies addressed prevention/surveillance for recurrence/new cancers, one addressed health promotion/disease prevention, and one addressed chronic medical conditions. Most studies (n = 27) had medium‐high risk of bias.
Conclusions
There are few high‐quality studies addressing HNC survivorship. Future rigorously designed studies should address broader areas of care, including chronic disease management and health promotion/disease prevention.
Keywords: cancer treatment effects, head and neck cancer, oropharynx cancer, radiation therapy, survivorship
1. INTRODUCTION
The population of head and neck cancer (HNC) survivors is growing, due to both improvements in treatment and the changing epidemiology of the disease. Human papillomavirus (HPV)‐associated HNC, which is rising in incidence, has a better prognosis than non‐HPV related HNC. 1 With improvements in patient survival, there is a growing population of HNC survivors that have cancer‐related effects that extend years beyond treatment. 2 Survivors of HNC have unique needs compared to survivors of other cancers. The aerodigestive anatomic location of the tumor influences eating, breathing, speaking, and appearance. Long‐term effects of HNC treatment are wide‐ranging and often serious, encompassing numerous physical conditions that are critical to daily functioning. Psychosocial effects are also significant, with HNC survivors experiencing high rates of depression and suicide, 3 fear of cancer recurrence, 3 and financial toxicity. 4 , 5 , 6 , 7 Both recurrence and subsequent malignancies are common, especially among HNC survivors with heavy alcohol and tobacco use. 8 Furthermore, HNC survivors may have pre‐existing comorbidities that require ongoing medical management and health promotion to reduce risk. With such complex ongoing health issues, HNC survivors require coordinated care beyond treatment completion.
The recently developed Quality of Cancer Survivorship Care Framework describes five domains of cancer survivorship care, all of which are relevant to HNC survivors. 9 The domains include: (1) surveillance and management of physical effects; (2) surveillance and management of psychosocial effects; (3) prevention and surveillance for recurrences and new cancers; (4) chronic disease management; (5) health promotion and disease prevention. The framework also includes contextual domains of the health care delivery system that influence cancer survivorship care quality including clinical structure, communication and decision making, care coordination, and patient/caregiver experience. The effect of survivorship care across these domains can be ascertained by health outcomes, which include function/health‐related quality of life, emergency/hospitalization, costs, and mortality. Even though HNC survivors represent a complex population that require high‐quality survivorship care across all domains, it is unclear how to address these needs, particularly in long‐term follow‐up after treatment and acute recovery. We performed a systematic review of the literature to identify, characterize, and assess the evidence, and identify gaps for interventions.
2. METHODS
The protocol for this review was registered on PROSPERO (registration ID: CRD42021269566), and the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) guidelines were followed. 10 Electronic searches were conducted across four databases (MEDLINE, Embase, Emcare, and PsycINFO) for primary studies published in English between January 1, 2000 and November 12, 2021. The search strategy (Supplementary Data S1) included key words and MeSH terms related to head and neck neoplasms, survivorship, symptom management, and survivorship needs captured in the quality framework (Supplementary Data S1).
2.1. Study selection
The patient population included adults (≥18 years) without active disease who completed curative‐intent treatment for HNC. Tumors could be of any histology from the following cancer sites: larynx, hypopharynx, oropharynx, oral cavity, nasopharynx, nasal cavity, salivary glands, and paranasal sinuses. Eligible studies included randomized and non‐randomized primary studies of interventions that began after completion of treatment with a study endpoint assessed at least 12 months following completion of therapy or cancer diagnosis (when date of treatment completion was not available). Studies were included if some patients had <12‐month follow‐up since cancer treatment, if details were given on the proportion of patients with at least 12 months follow‐up. Studies could have a control group, comparison with standard of care or with another intervention, no comparator/control group, or pre‐intervention/historical controls. We excluded editorials, reviews, meta‐analyses, opinion pieces, case reports, study protocols, conference abstracts and retrospective reviews of interventions or practices.
Covidence systematic review software 11 was used to facilitate article screening, study selection and data extraction. Two reviewers (any two of PD, KM, MM, LN, TS, DM, RV, SC, or JW) screened titles and abstracts. Full‐text articles were also independently evaluated for inclusion by two reviewers (any two of the aforementioned), and disagreements were resolved by consensus. When more than one paper was published from a single trial, the endpoints were reviewed, critically appraised, and the data combined, such that each trial is listed only once in Table 1.
TABLE 1.
Characteristics of included trials (n = 28)
| Study | Country | Study design | Number of participants | Intervention type | Outcome | Disease site | Setting | Risk of bias |
|---|---|---|---|---|---|---|---|---|
| Randomized trials (n = 13) | ||||||||
| Alamoudi 2018 12 | Canada | Randomized controlled trial | 20 | Submental liposuction | Lymphedema | Oropharynx, oral cavity, larynx, neck, nasal cavity, | Hospital | High |
| Bhatia 2017 13 | United States | Randomized controlled trial | 176 | 13 Cis‐retinoic acid | Prevention of second primary cancer | Oropharynx, oral cavity, larynx, hypopharynx | Hospital | Medium |
| Cramer 2021 14 | United States | Randomized controlled trial, post hoc analysis | 171 | Lung cancer screening | Incidence of second primary lung cancer | Oropharynx, oral cavity, larynx, nasal cavity, sinus | Hospital | Medium |
| Guglielmo 2020 15 | Italy | Randomized controlled trial | 32 | Ginseng | Fatigue | Oral cavity, oropharynx, larynx, hypopharynx, nasopharynx, paranasal sinus, salivary, unknown primary | Hospital | High |
| Jansen 2020 16 | Netherlands | Randomized controlled trial | 92 | Guided self‐help program | Swallow/communication | HNC NOS | Hospital | Medium |
| Kaae 2020 17 | Denmark | Randomized controlled trial | 91 | Chewing gum | Dry mouth | Oropharynx, oral cavity | Hospital | High |
| McNeely 2015 18 | Canada | Randomized controlled trial | 52 | Resistance exercise | Shoulder dysfunction | Oropharynx, oral, larynx, hypopharynx, thyroid, other. | Hospital | Medium |
| Millgard 2020 19 | Sweden | Randomized controlled trial | 74 | Voice rehabilitation | Voice quality | Larynx | Hospital | High |
| Pereira 2020 20 | Brazil | Randomized controlled trial | 40 | Pilocarpine spray | Dry mouth | HNC NOS | Hospital | Medium |
| Schutte 2021 21 | Netherlands | Randomized controlled trial | 134 | Stepped care program | Sexual interest/enjoyment | Oropharynx, oral cavity, larynx, hypopharynx, other | Hospital | High |
| Tang 2011 22 | China | Randomized controlled trial | 43 | Rehab therapy | Trismus and dysphagia | Nasopharynx | Hospital and home | High |
| Vadcharavivad 2013 23 | Thailand | Randomized controlled trial | 50 | Saliva substitute | Dry mouth | HNC NOS | Hospital | High |
| Wu 2019 24 | Australia | Randomized controlled trial | 41 | Endoscopic dilation | Dysphagia | HNC NOS | Hospital | Low |
| Non‐randomized prospective studies (N = 15) | ||||||||
| Al‐Bazie 2016 25 | Saudi Arabia | Single arm prospective study | 89 | Perioperative antibiotics and antibacterial mouthwash | Prevention of osteoradionecrosis after dental extractions | Nasopharynx, oral cavity, maxilla | Hospital | High |
| Chan 2004 26 | China | Non‐randomized experimental study | 29 | Alpha‐tocopherol | Cognitive function for temporal lobe necrosis | Nasopharynx | Hospital | Medium |
| Chen 2020 27 | Taiwan | Single‐arm prospective study | 175 | Endoscopic surveillance | Metachronous esophageal squamous cell carcinoma | Oropharynx, oral cavity, larynx, hypopharynx | Hospital | High |
| DeLeeuw 2013 28 | Netherlands | Non‐randomized experimental study | 160 | Nurse‐led additional follow‐up consults | Psychosocial adjustment and HRQOL | Oropharynx, oral cavity, larynx, hypopharynx, other | Hospital | Medium |
| Dholam 2011 29 | India | Single arm prospective study | 12 | Implant‐retained dental prosthesis into reconstructed maxillae and mandibles | Quality of life questionnaires and speech assessment software | HNC NOS | Hospital | High |
| Fong 2014 30 | Hong Kong | Non‐randomized experimental study | 52 | Qigong training | HRQOL, physical | Nasopharynx | Community and home‐based | Medium |
| Fong 2014 31 | ||||||||
| Kraaijenga 2017 32 | Netherlands | Single‐arm prospective study | 18 | Swallowing exercise program | Dysphagia | Oropharynx, oral cavity, hypopharynx, larynx, neck, parotid | Hospital | High |
| Liu 2021 33 | Taiwan | Parallel arm prospective study | 217 | Carotid duplex ultrasound | Carotid artery stenosis progression | Nasopharynx, HNC NOS | Hospital | High |
| Manne 2020 34 | United States | Single‐arm prospective study | 66 | Web‐based tool | Feasibility, preliminary impact on health/QOL outcomes | Oropharynx, oral cavity | Hospital, community | High |
| Martin‐Harris 2015 35 | United States | Single‐arm prospective study | 30 | Respiratory‐swallow training | Dysphagia related QOL, spirometry | Oropharynx, oral cavity, nasopharynx, larynx/hypopharynx | Hospital | High |
| Montalvo 2020 36 | Sweden | Single‐arm prospective study | 15 | Therabite | Trismus | HNC NOS | Hospital | High |
| Mozzati 2014 37 | Italy | Non‐randomized experimental study | 20 | Plasma rich growth factors | Healing post‐extraction | Oropharynx, oral cavity, larynx, ‘bone’ | Hospital | High |
| Nativ‐Zelter 2021 38 | United States | Single‐arm prospective study | 10 | Autologous muscle‐derived cell therapy | Safety, dysphagia (secondary) | Oropharynx | Hospital | High |
| Pauli 2016 39 | Sweden | Cohort study | 100 | Therabite® | Trismus | Oropharynx, oral cavity, nasopharynx, HNC NOS | Hospital and community (control) | High |
| Sterba 2019 40 | United States | Single arm prospective study | 52 | SNAP (Survivorship Needs Assessment Planning Tool) | Feasibility and short‐term change in psychosocial outcomes | Oropharynx, oral cavity, larynx, other | Hospital | High |
Abbreviations: HNC, head and neck cancer; HRQOL, health‐related quality of life; NOS, not otherwise specified.
2.2. Data extraction
The Quality of Cancer Survivorship Care Framework 9 was used to inform the development of the data extraction fields. Information on the following was extracted: study characteristics (country, year, study aim, study design, methods), study population (tumor site, number of participants, treatment modality), intervention information (aim, targeted symptom or concern, survivorship framework domain and health care outcome measures, type of intervention, components, timing and duration) and outcome (outcomes measured, timing of outcome measurement, effect of intervention). Data extraction was pilot tested by all authors to ensure consistency. Thereafter, data were extracted independently, and then collated and checked for consistency and inaccuracies.
2.3. Data synthesis and critical appraisal
Due to the anticipated heterogeneity of the included studies, narrative synthesis was used to summarize the data. Studies were critically appraised by two reviewers to assess for bias using the Joanna Briggs Institute (JBI) critical appraisal tools corresponding to each study design. 41 Each of these tools evaluates elements of study design and reporting of findings that may reflect the quality and rigor of the original research.
3. RESULTS
3.1. Study selection
A flow diagram of study identification is provided in Figure 1. The search identified 7395 studies. After removal of duplicates and screening of titles and abstracts and subsequent full text review, 28 studies were included for critical appraisal and are shown in Table 1. These include 13 randomized trials (including one post hoc analysis 14 ) and 15 non‐randomized studies.
FIGURE 1.
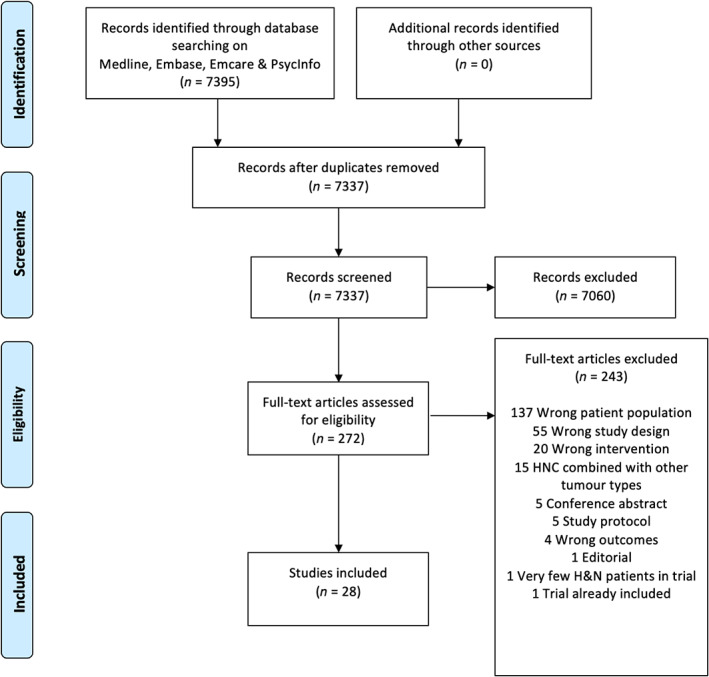
PRISMA flowchart [Color figure can be viewed at wileyonlinelibrary.com]
3.2. Study population
Most studies included patients with heterogeneous cancer types or did not specify the HNC subsites: six studies were limited to the specific sites of the nasopharynx, 22 , 26 , 30 , 31 larynx 19 and oropharynx. 38 Receipt of cancer treatment, including radiation therapy (RT), surgery, or chemotherapy, was reported for most studies. Among the 28 studies, 17 included patients treated with radiation therapy with or without surgery/chemotherapy, 12 , 15 , 17 , 19 , 20 , 22 , 23 , 24 , 25 , 26 , 30 , 31 , 33 , 36 , 37 , 38 , 39 and 3 included patients treated with surgery with combinations of RT/chemotherapy 16 , 18 , 29 ; other studies included a combination of treatment modalities 28 , 35 , 40 or did not specify. 27 Hospital/academic setting was the site of patient recruitment and intervention training for all studies except for two that had a community‐based component of the intervention. 30 , 31 , 34 Eligible patients were generally identified from records at head and neck oncology clinics. The studies were most commonly from North America, Europe, and Asia, mainly the United States (n = 6), Netherlands (n = 5), Sweden (n = 3), Canada (n = 2), China (n = 2), and Italy (n = 2).
3.3. Quality of the evidence
Studies were appraised for risk of bias as shown in Table 1 and Supplementary Tables 1–3. Most had a medium to high risk of bias. Among the 13 randomized studies, there were 12 with a medium 13 , 14 , 16 , 18 , 20 to high 12 , 15 , 17 , 19 , 21 , 22 , 23 risk of bias, and only one study with a low 24 risk of bias. The most common sources of bias were lack of concealment of allocation, heterogeneity of baseline participant characteristics, or unclear/lack of blinding of the participants, assessors, or those delivering the study intervention. Additional reasons for introduction of bias included incomplete information on follow‐up of participants, 15 limited information on power calculations, 14 , 21 , 22 and lack of target accrual 13 or patient attrition. 19
The 15 non‐randomized studies included 12 with a high risk of bias 25 , 27 , 29 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 and three with a medium risk of bias. 26 , 28 , 30 , 31 Common reasons for introducing bias included lack of planned sample size/power calculations or pre‐specified endpoints. Follow‐up was frequently incomplete due to low participation in the intervention or loss to follow‐up with lack of adequate description or analysis to account for loss to follow‐up. 28 , 30 , 31 , 32 , 34 , 35 , 36
3.4. Survivorship domains
Interventions were grouped into the domains as specified by the Quality of Cancer Survivorship Care Framework 9 (Table 2) and described below.
TABLE 2.
Quality of cancer survivorship care framework domains
| Study | Surveillance and management of physical effects | Surveillance and management of psychosocial effects | Prevention and surveillance for recurrence and new cancers | Surveillance and management of chronic medical conditions | Health promotion and disease prevention |
|---|---|---|---|---|---|
| Alamoudi 2018 12 | ✓ | ✓ | |||
| Al‐Bazie 2016 25 | ✓ | ||||
| Bhatia 2017 13 | ✓ | ||||
| Chan 2004 26 | ✓ | ✓ | |||
| Chen 2020 27 | ✓ | ||||
| Cramer 2021 14 | ✓ | ||||
| DeLeeuw 2013 28 | ✓ | ✓ | |||
| Dholam 2011 29 | ✓ | ✓ | |||
| Fong 2014, 31 Fong 2014 30 | ✓ | ✓ | ✓ | ✓ | |
| Guglielmo 2020 15 | ✓ | ✓ | |||
| Jansen 2020 16 | ✓ | ✓ | |||
| Kaae 2020 17 | ✓ | ||||
| Kraaijenga 2017 32 | ✓ | ✓ | |||
| Liu 2021 33 | ✓ | ||||
| Manne 2020 34 | ✓ | ✓ | ✓ | ||
| Martin‐Harris 2015 35 | ✓ | ||||
| McNeely 2015 18 | ✓ | ✓ | |||
| Millgard 2020 19 | ✓ | ||||
| Montalvo 2020 36 | ✓ | ||||
| Mozzati 2014 37 | ✓ | ||||
| Nativ‐Zeltzer 2021 38 | ✓ | ||||
| Pauli 2016 39 | ✓ | ✓ | |||
| Pereira 2020 20 | ✓ | ✓ | |||
| Schutte 2021 21 | ✓ | ✓ | |||
| Sterba 2019 40 | ✓ | ||||
| Tang 2011 22 | ✓ | ||||
| Vadcharavivad 2013 23 | ✓ | ||||
| Wu 2019 24 | ✓ |
3.4.1. Surveillance and management of physical effects
Most interventions (n = 24) focused on surveillance and management of physical effects, with 13 of those studies also addressing surveillance and management of psychosocial effects (described below). The physical domains targeted by the 11 randomized studies included: speech and swallow function and trismus, 16 , 19 , 22 , 24 dry mouth, 17 , 20 , 23 fatigue, 15 shoulder dysfunction, 18 sexual function 21 and lymphedema. 12 Of these, seven randomized controlled trials (RCTs) reported statistically significant results, including one trial with a low‐risk of bias showing an improvement in dysphagia after endoscopic dilatation for patients treated with RT with or without total laryngectomy. 24 Two studies had a medium risk of bias, and showed improvements in shoulder pain and function with a progressive resistance exercise training program, 18 and swallowing‐related QOL measures after a guided self‐help exercise program. 16 Four additional studies had a high risk of bias 12 , 17 , 22 , 23 focusing on appearance after submental liposuction, 12 dry mouth after chewing gum intervention, 17 trismus and dysphagia after speech and swallow rehabilitation exercise therapy, 22 and dry mouth with use of a hospital prepared saliva substitute. 23
The 13 non‐randomized studies targeting physical effects of cancer therapy focused on improving trismus and dysphagia, 32 , 35 , 36 , 38 , 39 carotid stenosis surveillance, 33 prevention of dental complications and osteoradionecrosis, 25 , 37 cognitive function, 26 health‐related quality of life after implant‐retained dental prostheses into reconstructed mandibles, 29 and patient‐reported physical symptoms and role functioning. 28 , 30 , 31 , 34 All non‐randomized studies had a medium to high risk of bias. Included non‐randomized studies examined the effect of an oral opening device on trismus, 36 , 39 antibiotic use around teeth extraction after RT, 25 healing in post‐extraction sockets treated with plasma‐rich growth factors, 37 dysphagia following autologous muscle derived stem cell therapy, 38 and swallowing following respiratory‐swallow training. 35 Additional non‐randomized studies reported the use of alpha‐tocopherol use on neurocognitive function, 26 and carotid ultrasound in predicting progressive carotid artery stenosis. 33
3.4.2. Surveillance and management of psychosocial effects
Thirteen studies targeted surveillance and management of psychosocial effects (Table 2). Four studies focused on psychosocial outcomes of cancer treatment as the primary study outcome, including one RCT with a high risk of bias 21 and three non‐randomized studies with a medium 28 to high risk of bias. 34 , 40 The RCT studied sexual interest after a stepped care program intervention targeting psychological distress; this trial did not show a statistically significant effect. 21 The non‐randomized studies looked at the effect of a nurse‐led intervention on psychosocial adjustment and health‐related quality of life (HRQOL) showing no statistically significant difference between groups, 28 the effect on self‐efficacy with a web‐based tool showing an improvement with descriptive statistics but no tests of significance, 34 and a statistically significant improvement in depression, unmet needs, and survivorship knowledge in both survivors and care‐givers. 40 Of note, this was the only study identified by this systematic review that targeted an intervention to the patient‐caregiver dyad rather than the survivor alone. Most of the 13 studies assessed psychosocial effects as secondary outcomes using surveys such as the EORTC‐QLQ‐H&N35 to ascertain the multi‐dimensional effect of an intervention targeting physical effects of cancer treatment (see Table 3 for measures of outcome).
TABLE 3.
Detailed study outcomes of randomized and non‐randomized studies (n = 28)
| Study | Interval from treatment to intervention c | Comparison | Intervention type | Outcomes | Measures | Results | Conclusions |
|---|---|---|---|---|---|---|---|
| Randomized trials (n = 13) | |||||||
| Alamoudi 2018 12 | 30 ± 12 months | Intervention versus observation | Submental liposuction | Appearance/Lymphedema |
MBOE a DAS‐59 (Derriford Appearance Scale) |
SS improvement in both scales | Submental liposuction vs. no intervention associated with improvement in patient‐reported appearance |
| Bhatia 2017 13 | 1‐61 months | Intervention versus placebo | 13 Cis‐retinoic acid | Prevention of second primary cancer |
Number of secondary primary tumors (SPT) & time to diagnosis of SPT a OS |
N‐SS difference in SPT or time to SPT | 13‐CRA did not reduce SPT in underpowered trial |
| Cramer 2021 14 |
Intervention group: median 9 years (IQR 6–13 years) CXR: median 10 years (IQR 6–17 years) |
Low‐dose CT (LDCT) versus chest‐x‐ray (CXR) | Lung cancer screening | Incidence of second primary lung cancer |
Incidence of second primary lung cancer (SPLC) a Incidence of a second primary HNC, combined SPHNC or SPLC, OS, incidence of abnormal imaging findings |
N‐SS difference in SPLC identified on LDCT compared to CXR SS‐higher incidence of SPLC in HNC survivors compared to other |
Post hoc analysis of a RCT did not show SS difference in SPLC in LDCT in HNC subgroup; SS higher SPLC in HNC survivors |
| Guglielmo 2020 15 | ≥12 months | Intervention versus placebo | Ginseng | Fatigue | BFI a | No SS difference in BFI from baseline to post‐intervention | Ginseng did not reduce patient‐reported fatigue |
| Jansen 2020 16 |
78%: 6 months–5 years 22%: <6 months |
Intervention versus self‐care education program alone | Guided self‐help exercise program and self‐care education program | Swallow/communication |
SWAL‐QOL a SHI (speech handicap index) Shoulder problems (SDQ) PAM EORTC QLQ‐C30 EORTC QLQ‐H&N35 |
SS improvement in SWAL‐QOL in intervention group N‐SS improvement in other domains Time since cancer treatment moderated effectiveness of intervention on speech problems |
Guided self‐help exercise program improvement patient‐reported swallowing function |
| Kaae 2020 17 |
75%: 6–24 months 25%: 36–60 months |
Intervention versus CAU | Chewing gum | Dry mouth |
EORTC QLQ‐H&N35 “dry mouth” question a GRIX UWS and SWS sialometry |
SS reduction improvement in primary endpoint N‐SS difference in other measures |
Chewing gum associated with improvement with dry mouth question on EORTC‐QLQ‐HN35 |
| McNeely 2015 18 |
44%: ≥18 months 42%: <9 months 15%: 9–17 months |
Intervention versus CAU, option to crossover | Progressive resistance exercise training | Shoulder dysfunction |
SPADI a Upper extremity strength Shoulder ROM FACT‐An NDII |
SS improvement in all measures | Progressive resistance exercise training reduced patient‐reported shoulder pain and disability and improved muscle strength/endurance |
| Millgard 2020 19 | Follow‐up extended to 2 years | Intervention versus CAU | Voice rehabilitation | Voice quality |
CPPS a GRBAS sale |
N‐SS differences in measures | Voice rehab may have positive effects but N‐SS correlation found between CPPS and perceptual parameters of GRBAS |
| Pereira 2020 20 | 2–6 years | Intervention versus placebo | Pilocarpine spray | Dry mouth |
SWSF a XI OHIP‐14 |
N‐SS difference in measures | Topical pilocarpine spray did no lead to SS difference in measures of xerostomia |
| Schutte 2021 21 |
46%: >12 months 37%: >7 months 18%: 7–12 months |
Intervention versus CAU | Stepped care program targeting psychological distress | Sexual interest/enjoyment | Sexuality symptom subscale of EORTC QLQ‐H&N35 a | N‐SS improvement | SC targeting psychological distress did not reduce problems with sexuality. Interventions specifically targeting sexuality are recommended |
| Tang 2011 22 | Mean 4.6 years for intervention versus 4.8 years for control | Intervention versus CAU | Rehabilitation exercise therapy | Trismus and dysphagia |
Water swallow test b LENT/SOMA IID |
SS‐improvement in all measures | Swallow and trismus therapy improved swallow function and reduced severity of trismus |
| Vadcharavivad 2013 23 | ≥1 year | Intervention versus commercially available saliva substitute | In‐hospital prepared saliva substitute | Dry mouth | XeQoLS a | SS inferior score in intervention group | Commercially available saliva substitute was better than the hospital‐prepared formulation |
| Wu 2019 24 | ≥1 year | Intervention versus sham | Endoscopic dilation | Dysphagia |
SSQ score + satisfactory global assessment by swallow therapist a SAE Dysphagia relapse |
SS improvement in all measures, no SAEs | Dilation improves swallowing function |
| Non‐randomized prospective studies (N = 15) | |||||||
| Al‐Bazie 2016 25 | 12–33 months | None | Perioperative antibiotics (oral amoxicillin) and antibacterial mouthwash | Prevention of osteoradionecrosis after dental extractions |
No. extracted teeth b Osteoradionecrosis (no further definition) |
232 extractions (average 2.6 teeth/patient) and no ORN | No patients using the antibiotic protocol had ORN after extractions |
| Chan 2004 26 |
Intervention: mean 15.47 years (SD 5.3 years) Control: 13.80 years (7.45) |
Matched control group | Alpha‐tocopherol | Cognitive function for temporal lobe necrosis |
Cantonese MMSE b Category Fluency Test Hong Kong List Learning Test (HKLLT) Visual Reproduction subtest of the Wechsler Memory Scale‐III (WMS‐III VR) Cognitive Flexibility Test Self‐evaluation questionnaire |
SS improvement in MSSE, and verbal and visual memory, and executive function N‐SS difference between groups in attention, language, or self‐reported improvement |
Alpha‐tocopherol may improve cognitive function |
| Chen 2020 27 | Mean 33 months | None | Endoscopic surveillance | Metachronous esophageal squamous cell carcinoma | Biospy‐proven dysplasia or squamous cell carcinoma | Metachronous esophageal squamous cell neoplasms ESCN) developed in 11.4% patients (17 low‐grade dysplasia, 3 squamous cell carcinoma. Median time to ESCN was 33 ± 22.9 months | Endoscopic surveillance can detect ESCN |
| DeLeeuw 2013 28 | Intervention extended to 12 months post‐treatment | CAU group recruited in preceding year | Nurse‐led additional follow‐up consults | Psychosocial adjustment and HRQOL |
PAIS‐SR b EORTC QLQ‐C30 and QLQ‐H&N35 |
N‐SS difference between groups | Nurse‐led consultations had a positive but not SS effect on HRQOL |
| Dholam 2011 29 | ≥1 year | No | Implant‐retained dental prosthesis into reconstructed maxillae and mandibles | HRQOL, and speech |
EORTC QLQ‐H&N 35 and EORTC QLQ‐C30 b Dr. Speech Software |
N‐SS improvement in pre‐intervention versus post‐intervention assessment, even if numerically improved | QOL parameters did not markedly change after implant retained prosthesis reconstruction even if individual parameters numerically improved |
| Fong 2014 31 | Mean 12.5 years in intervention group versus 8.4 years in control group | Self‐selected volunteers who did CAU | Qigong training | HRQOL, physical |
EORTC QLQ‐H&N, QLQ‐C30 b Blood flow velocity Arterial resistance by Doppler ultrasound Functional aerobic capacity measured by walking distance and self‐report of fatigue Palmar skin temperature measurement |
NS‐SS difference between intervention and control group for EORTC QLQ measures SS higher diastolic blood flow, lower arterial blood flow resistance, and higher palmar skin temperature, and functional aerobic capacity |
Tai Chi Qigong program may improve arterial hemodynamics and functional aerobic capacity |
| Fong 2014 30 | |||||||
| Kraaijenga 2017 32 | ≥88%: ≥2 years | None | Swallowing exercise program | Dysphagia |
Feasibility and compliance a SWAL‐QOL EQ‐5D Interincisal opening FOIS VFS parameters PAS IOPI Dynamometer for jaw muscle strength |
High compliance (97%) and completion rate (88%) SS‐not reported, but descriptive statistics for numeric improvements in strength in various muscles |
Feasibility and compliance for a swallowing exercise program can be high with some objective and subjective effects of muscle strength and swallow function despite most being at least 2 years post‐treatment |
| Liu 2021 33 | Mean 8.81 years (SD 4.66) in high plaque (HP) group and 9.56 years (SD 3.67) in low plaque (LP) group | At enrolment, 2 groups created: high‐plaque group versus low‐plaque group | Carotid duplex ultrasound (CDU) | Carotid artery stenosis (CAS) progression | >50% stenosis on B‐mode CDU with compatible hemodynamic pattern in any ICA or CCA on a follow‐up CDU study b | HP group had a SS higher frequency of CAS progression and N‐SS increased future ischemic stroke | Patients with total plaque sore of ≥7 on CDU are susceptible to CAS progression and should have close monitoring |
| Manne 2020 34 | 1–3 years | None | Web‐based tool: “Empowered Survivor” | Feasibility, preliminary impact on health/QOL outcomes |
22‐item scale composed for the study to represent confidence in managing different aspects of self‐care a 10‐item scale used previously by study group for assessing preparedness for oral and oropharyngeal survivorship EORTC QLQ‐HN35 Study‐specific measure for performance and thoroughness of oral self‐exam, maintenance of exercise, and action/coping planning, activation, and information needs Supportive Care Needs Survey |
82% pts viewed intervention Descriptive statistics showed increased self‐efficacy, preparedness for survivorship, HRQOL, rates of oral self‐exam, and other secondary endpoints |
The web‐based survivorship empowerment tool showed a beneficial impact on multiple domains |
| Martin‐Harris 2015 35 | >1 year | None | Respiratory‐swallow training | Dysphagia related QOL, spirometry |
Respiratory‐swallow phase pattern b MBSImP PAS MDADI |
SS improvement in optimal phase swallowing patterning, and component scores of MBSimP including laryngeal vestibular closure, tongue base retraction, and pharyngeal residue SS improvement in PAS and MDADI |
Improvements in respiratory‐swallowing coordination can be trained in patients with chronic dysphagia with favorable effects on airway protection and bolus clearance |
| Montalvo 2020 36 | Mean 6.2 years (range 0.7–14.8) | None | Therabite | Trismus |
MIO b Gothenburg Trismus Questionnaire (GTQ) EORTC QLQ C30 and EORTC QLQ‐H&N35 |
SS improvement in MIO and individual domains in the other questionnaires | Structured exercise with the jaw‐mobilizing device was beneficial for patients with trismus |
| Mozzati 2014 37 | Mean 4.1 ± 2.5 years | Same patient, contralateral extraction sockets with CAU | Plasma rich growth factors | Healing post‐extraction | Healing index (HI), residual socket volume (RSV), postoperative complications b | Intervention showed SS‐better RSV and HI and no postoperative complications (bone exposure) | Plasma rich in growth factors accelerated mucosal healing and avoided post‐extraction bone exposure |
| Nativ‐Zelter 2021 38 | Mean 11.5 years, (SD 7.6) | No | Autologous muscle‐derived cell therapy | Safety (phase I trial with efficacy measurements), dysphagia |
IOPI a PAS Pharyngeal constriction ratio Pharyngo‐esophageal segment (PES) opening Pharyngeal transit time Pharyngeal peak pressure EAT‐10 VHI‐10 |
No SAEs SS increase in tongue pressure. N‐SS change in other metrics |
Injection with autologous muscle‐derived cell therapy was feasible and safe and was accompanied by increase in tongue strength |
| Pauli 2016 39 |
Includes 2‐year f/u The 10‐week Intervention was 3–6 months post‐treatment |
Control group receiving CAU (no structured trismus‐focused program | Therabite® | Trismus |
MIO a Gothenburg Trismus Questionnaire (GTQ) EORTC QLQ C30 and EORTC QLQ‐H&N35 |
SS higher MIO and GTQ at 2‐year follow‐up in intervention group. Individual domains in other questionnaires had SS differences | There is a positive persistent effect of jaw opening exercises on trismus and patient reported outcomes |
| Sterba 2019 40 |
9 patients: >12 months 6 patients: 6–12 months 11 patients: 0–6 months |
No | SNAP (Survivorship Needs Assessment Planning Tool) | Feasibility and short‐term change in psychosocial outcomes |
PROMIS (depression) a Cancer Survivors/Partners Unmet Needs instruments PLANS Dyadic coping inventory Zarit Burden Inventory FOCUS—2 single items Other study‐specific surveys |
SS improvement in scores for depression, unmet needs, and survivorship knowledge in survivors and caregivers NS‐SS change in symptom distress and management |
The SNAP tool is feasible and able to address dyads' needs; the tool merits further testing in a clinical trial |
Abbreviations: BFI, brief fatigue inventory; CAU, care as usual; CPPS, smoothed cepstral peak prominence; EAT‐10, Eating Assessment Tool; EORTC‐QLQ, European Organization for Research and Treatment of Cancer generic and HNC‐specific health‐related quality of life measures; EQ‐5D, European Quality of Life 5 Dimensional Questionnaire; FACT‐An scale, Functional Assessment of Cancer Therapy‐Anemia scale; FOCUS, National Cancer Institute Follow‐up Care Use and Health Outcomes of Cancer Survivors; FOIS, functional oral intake scale; GRBAS, Grade, Roughness, Breathiness, Asthenia and Strain scale; GRIX, Groningen Radiation‐Induced Xerostomia questionnaire; HNC, head and neck cancer; HRQOL, health‐related quality of life; IID, interincisal distance; IOPI, Iowa Oral Performance Instrument; LENT/SOMA, Late Effects Normal Tissue/Subjective, Objective, Management, Analytic scales; MBOE, Modified blepharoplasty Outcomes Evaluation; MBSImP, Modified Barium Swallow Impairment Profile; MDADI, MD Anderson Dysphagia Inventory; MIO, maximal interincisal opening; MMSE, Mini‐Mental Status Examination; NDII, neck dissection impairment index; No., number; NOS, not otherwise specified; N‐SS, non‐statistically significant; OHIP‐14, Oral Health Impact Profile; PAIS‐SR, Psycho‐social Adjustment to Illness Scale‐Self Report; PAM, patient activation measure; PAS, penetration aspiration scale; PLANS, Preparing for Life As a New Survivor; PROMIS, Patient‐Reported Outcomes Measure Information System; ROM, range of motion; SAE, serious adverse event; SDQ, shoulder disability questionnaire; SHI, speech handicap index; SPADI, shoulder pain and disability index; SS, statistically significant; SSQ, Sydney Swallow Questionnaire; SWAL‐QOL, swallowing quality of life questionnaire; SWSF, stimulated whole saliva flow; UWS, unstimulated whole saliva; VFS, video fluoroscopy; VHI, Voice Handicap Index; XeQoLS, Xerostomia Quality of Life Scale; XI, Xerostomia Inventory.
Primary endpoint.
Primary endpoint not specifically stated in methods.
Time from treatment to intervention is given, time from diagnosis is given if specific time from treatment not given.
3.4.3. Prevention and surveillance for recurrence and new cancers
Four interventional studies, including two RCTs with a medium risk of bias, 13 , 14 and two non‐randomized experimental studies with a high risk of bias 27 , 34 reported on prevention and surveillance for recurrence and new cancers. The two RCTs were both underpowered and did not show a statistically significant benefit of the intervention. One of these was the ECOG‐ACRIN chemoprevention trial that closed early due to slow accrual and did not show a benefit of a synthetic vitamin A derivative for prevention of second primary cancers in HNC survivors. 13 The other was a post hoc analysis of the National Lung Screening Trial, which demonstrated the high incidence of second primary lung cancer among HNC survivors. 14 In this study, there was a non‐statistically significant increase in detection of lung cancer and survival with low‐dose CT compared to chest x‐ray surveillance.
The two non‐randomized trials with a high risk of bias included a single‐arm study designed to assess detection of metachronous esophageal squamous cell neoplasms in HNC survivors using endoscopic surveillance. 27 The other was an eHealth intervention to teach patients to self‐screen for recurrent or second primary oral or skin lesions, showing increased engagement in oral self‐exams to screen for recurrence or second primary tumors. 34
3.4.4. Chronic medical conditions/health promotion and disease prevention
We found only one study that touched on the general health‐related domains. This study, with a high risk of bias, examined the effect of Tai Chi Qigong on improving measures of arterial hemodynamics and functional aerobic capacity. Tai Chi had a statistically significant benefit for physical measures, 30 but no significant benefit on quality‐of‐life measures (using the EORTC QLQ‐C30 and QLQ‐H&N35 instruments). 31
3.5. Health care outcomes
Study outcome measures were categorized according to four previously described outcome measures identified in the Quality of Cancer Survivorship Care Framework including health‐related quality of life/function, emergency services/hospitalizations, costs, and mortality. 9 All studies assessed the HRQOL/function outcomes (Table 3). Only two studies assessed mortality outcomes as secondary endpoints. 13 , 14 No studies assessed outcomes of emergency services/hospitalizations and costs.
4. DISCUSSION
This systematic review identified 13 randomized trials and 15 non‐randomized prospective studies, mostly with medium to high risk of bias, focusing on interventions for HNC survivors at least 1 year after curative‐intent treatment. These survivorship interventions were characterized into the five quality domains of the Quality of Cancer Survivorship Care Framework demonstrating an emphasis on surveillance and management of physical and psychosocial effects of cancer treatment, with particular focus on management rather than surveillance. Few studies evaluated interventions addressing surveillance and management of chronic medical conditions and health promotion and disease prevention. Outcomes almost exclusively addressed HRQOL/function rather than costs, financial toxicity, health care utilization, or mortality. We identified numerous gaps in HNC survivorship research including under‐represented domains of survivorship care, and methodologic gaps in study design, conduct, and analysis that introduce risk of bias.
Our findings emphasize a lack of prospective data with low risk of bias regarding interventions for HNC survivors that span beyond the acute phase of treatment. Our identification of so few high quality interventions highlights the lack of evidence in the current guidelines for HNC survivorship care, 42 , 43 in which most of the supporting evidence is based on level three data (case control or prospective cohort studies) or expert opinion. 42 However, we did identify a few studies with low to medium risk of bias that have clinical implications and may be considered for incorporation into survivorship guidelines. Specifically, endoscopic dilation can lead to improvement in dysphagia in select patients at risk of pharyngo‐esophageal junction stricture. 24 Tailored rehabilitation exercises targeting shoulder dysfunction can improve function and HRQOL, 18 which aligns with a recent systematic review identifying the beneficial effects of physical rehabilitation in cancer survivorship. 44 And a self‐help exercises program suggested that dysphagia‐related QOL may improve modestly, even among long‐term survivors. 16 Even a few studies with a high risk of bias may be considered as routine components of survivorship care, due to the relatively low risk of harm. These include oral opening exercises for trismus and specific swallowing exercise programs. Unfortunately, variations between studies in dysphagia‐targeted interventions limit generalizability of interventions. Integration of movement‐based programs such as Tai Chi in a survivorship program may also have beneficial effects on general health maintenance and chronic disease prevention through reduction in measures of hypertension and improved aerobic capacity. 30
We identified very few studies targeting common HNC psychosocial symptoms and conditions, specifically fatigue, neurocognitive function, depression, sexual health, and coping. Only two small studies of Internet‐based tools specifically targeted depression and unmet survivorship needs, both showing favorable effects, but requiring more definitive clinical trials with longer follow‐up to demonstrate benefit. 34 , 40 Additionally, we did not identify interventions addressing hearing loss 45 and renal dysfunction associated with cisplatin‐induced kidney injury, 46 which are both important side effects of treatment with chemotherapy that impact long‐term physical health and function. Additionally, despite the prevalence of sleep‐related breathing disorders in patients with HNC after treatment, 47 , 48 we did not find studies targeting obstructive sleep apnea or other causes of sleep complaints.
We found health outcomes to address function and quality of life, rather than costs, health care utilization and mortality. Studies are needed that investigate and intervene on cost and financial toxicity, a recognized concern for HNC patients that are particularly vulnerable given the high rate of workforce exit 4 , 5 and gaps in dental coverage. 49 Due to the high prevalence of chronic medical conditions, subsequent cancers, smoking and other symptoms specific to HNC survivors, hospitalization and emergency‐department utilization, and mortality are needed.
In addition to characterizing the limited high‐quality clinical evidence for the existing HNC survivorship literature, we uncovered a number of methodological gaps, including study design (e.g., integrity of randomization and concealment, lack of blinding of participants and/or outcome assessors), study populations (e.g., small sample sizes, patient heterogeneity), intervention (e.g., limited in scope, hospital based rather than community‐based), and outcome measures (e.g., lack of pre‐specified clinically meaningful endpoints, and loss to follow‐up without characterization or analysis of impact). These methodological gaps are described below with recommendations for future study design.
First, most of the identified studies enrolled survivors in a hospital‐based or academic setting, with few focused on patients in their home/community. As such, the findings may not be generalizable to the population of HNC survivors in a rural or community‐based setting. Recruitment and study conduct may have the highest yield of eligible patients in the clinic setting. However, as time from treatment completion increases, some patients may be lost to follow‐up for various reasons including discharge, travel time or distance to clinic, and competing health or social circumstances. This may limit participation of follow‐up in trials that study endpoints that may occur years after treatment.
Second, study retention and attrition are major limitations to many of the studies we identified. Attrition among HNC survivors and caregivers was characterized in a recent study that identified the most common causes as mortality, logistical, physical, and psychological‐related reasons. 50 As patients become less mobile or have more comorbidities, there is a lower likelihood of travel to the hospital setting or participation in multi‐timepoint surveys or interventions. Future studies may address these gaps of follow‐up by engaging survivors in the community using web‐based recruitment and interventions. 51 Another proposed solution to loss‐to‐follow‐up is to oversample specific subgroups such as those with higher comorbidity or higher risk of mortality. 50
Third, most of the studies we reviewed were relatively small, ranging from 10 to 217 (median 52) participants. This is of particular importance due to the heterogeneity of HNC survivors that receive a range of treatments with physical, psychological, socioeconomic, and other late effects that differ substantially based on patient‐factors, cancer‐extent and treatments. For example, patients that received laryngectomy may face more difficulty with communication and social isolation than patients treated for early‐stage tonsil cancer who are expected to have good swallowing and speech outcomes when treated appropriately. 52 A patient treated with radiation for early glottic larynx cancer would be expected to have limited dental complications from treatment which is focused just on the larynx, compared to a patient treated with surgery and radiation to the mandible for an oral cavity cancer. Sample size and heterogeneity present challenges that limit study power. Including patients with multiple tumor sites, stages, and treatments into the same study may bias the study, most often toward the null, depending on the outcome and study design. Use of large‐scale clinical research networks such as PCORnet®, a US‐based infrastructure bridging multiple health care systems, may enhance the ability to conduct patient‐centered research in the “real‐world” setting and may facilitate enrollment of larger patient cohorts. Further, collaborative groups and consortiums may improve the ability to conduct large well‐powered studies. Unfortunately, we found that even the largest published randomized control trial in our review, the ECOG chemoprevention trial, was underpowered due to slow‐accrual. 13
As mentioned earlier, a major challenge to studying HNC survivorship is the long latency between the treatment and some targeted health outcomes, including stroke, critical carotid stenosis, hypertension, pituitary endocrinopathy, and other potential late effects. This requires very long follow‐up, and it is difficult to design a feasible interventional trial with an outcome that may take more than a decade to manifest. Therefore, trials are needed with intermediary endpoints, such as optimization of cardiac risk factors, specifically targeting chronic disease management, including diabetes, dyslipidemia, and hypertension as well as health promotion and disease prevention, which could include interventions targeting reduction in tobacco, alcohol, weight management, and age‐appropriate cancer screening.
Limitations to our study should be acknowledged. It is possible that our pre‐specified study inclusion criteria may have excluded informative interventions. For example, studies that intervened on multiple cancer survivor populations were excluded if there were no results shown specifically for HNC survivors. For interventions to reduce distress, increase smoking cessation activities, or target other behavioral outcomes, we may have excluded interventions that are equally relevant to and beneficial for HNC survivors. However, without demonstrating effects in HNC survivors, the relevance to this population is still untested and should be demonstrated in future research. In addition, the purpose of the study was to focus on interventions of HNC survivors without active cancer and beyond the acute toxicity phase of therapy. Therefore, we excluded studies that either did not specify the time from treatment to the study intervention, or that did not include a study time point at least 12 months after HNC treatment. One excluded study that both included too broad of a population over too wide a time window since treatment was a recent trial looking at eHealth self‐management application termed “Oncokompas” that evaluated the impact of a computer‐based intervention on 625 cancer survivors, including 185 HNC survivors. 53 Because the time from diagnosis or treatment to intervention was not specified for the HNC survivors, we could not ascertain the relevance of this intervention to our population of interest. To inform the care of long‐term HNC survivors, a focus on the post‐treatment stage of survivorship is critical and should be included in eligibility and stratification criteria for future trials on survivorship interventions. Our English language restriction may have resulted in under‐representation of some studies in our review, especially given high rates of oral cancers in South Central and East Asia. 53 Most studies were from the United States, Canada, Europe, China, and India. Global survivorship care for HNC is clearly a topic that needs more representation in the research domain.
Lastly, our systematic review focused on interventions directed at HNC survivors and not health care providers. For example, an excluded paper showed that thyroid function testing to detect hypothyroidism within a year after radiation completion could be increased through clinician education and maintenance of an institutional database. 54 However, in reviewing the literature, we did not find much attention to such interventions in HNC survivorship.
5. CONCLUSION
Most studies identified by this systematic review focused on surveillance and management of physical and psychosocial effects of HNC treatment, though we found significant gaps in addressing common symptoms and conditions within these domains. Surveillance and management of chronic medical conditions as well as health promotion and disease prevention were not addressed. Health care outcomes mainly addressed function and quality of life, rather than mortality, costs, and health care utilization. Studies were medium to high risk of bias and limited by lack of blinding, sample size/power calculations, heterogeneity of patients, and loss to follow‐up. While there are unique challenges to HNC survivorship research related to heterogeneity of cancer types and treatment, comorbidity, and long latency from treatment to health care outcomes, future rigorously designed studies should address broader areas of care, including chronic disease management and health promotion/disease prevention.
AUTHOR CONTRIBUTIONS
All work was performed by the authors only.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
This systematic review adheres to the guidelines provided by the PRISMA report.
Supporting information
Data S1 Supporting information
Margalit DN, Salz T, Venchiarutti R, et al. Interventions for head and neck cancer survivors: Systematic review. Head & Neck. 2022;44(11):2579‐2599. doi: 10.1002/hed.27142
This study was accepted for presentation at ASCO 2022.
REFERENCES
- 1. Mahal BA, Catalano PJ, Haddad RI, et al. Incidence and demographic burden of HPV‐associated oropharyngeal head and neck cancers in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(10):1660‐1667. [DOI] [PubMed] [Google Scholar]
- 2. Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15(9):994‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Osazuwa‐Peters N, Simpson MC, Zhao L, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124(20):4072‐4079. [DOI] [PubMed] [Google Scholar]
- 4. Baddour K, Fadel M, Zhao M, et al. The cost of cure: examining objective and subjective financial toxicity in head and neck cancer survivors. Head Neck. 2021;43(10):3062‐3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mott NM, Mierzwa ML, Casper KA, et al. Financial hardship in patients with head and neck cancer. JCO Oncol Pract. 2022;18:e925‐e937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casswell G, Gough K, Drosdowsky A, et al. Fear of cancer recurrence in survivors of human papillomavirus‐associated oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2021;111(4):890‐899. [DOI] [PubMed] [Google Scholar]
- 7. Kar A, Asheem MR, Bhaumik U, Rao VUS. Psychological issues in head and neck cancer survivors: need for addressal in rehabilitation. Oral Oncol. 2020;110:104859. [DOI] [PubMed] [Google Scholar]
- 8. Fullerton ZH, Butler SS, Mahal BA, et al. Short‐term mortality risks among patients with oropharynx cancer by human papillomavirus status. Cancer. 2020;126(7):1424‐1433. [DOI] [PubMed] [Google Scholar]
- 9. Nekhlyudov L, Mollica MA, Jacobsen PB, Mayer DK, Shulman LN, Geiger AM. Developing a quality of cancer survivorship care framework: implications for clinical care, research, and policy. J Natl Cancer Inst. 2019;111(11):1120‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Covidence Systematic Review Software, by Veritas Health Innovation, Melbourne, Australia [computer program]. https://www.covidence.org. Accessed January 05, 2022.
- 12. Alamoudi U, Taylor B, MacKay C, et al. Submental liposuction for the management of lymphedema following head and neck cancer treatment: a randomized controlled trial. J Otolaryngol Head Neck Surg. 2018;47(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhatia AK, Lee JW, Pinto HA, et al. Double‐blind, randomized phase 3 trial of low‐dose 13‐cis retinoic acid in the prevention of second primaries in head and neck cancer: long‐term follow‐up of a trial of the Eastern Cooperative Oncology Group‐ACRIN Cancer Research Group (C0590). Cancer. 2017;123(23):4653‐4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cramer JD, Grauer J, Sukari A, Nagasaka M. Incidence of second primary lung cancer after low‐dose computed tomography vs chest radiography screening in survivors of head and neck cancer: a secondary analysis of a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2021;28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guglielmo M, Di Pede P, Alfieri S, et al. A randomized, double‐blind, placebo controlled, phase II study to evaluate the efficacy of ginseng in reducing fatigue in patients treated for head and neck cancer. J Cancer Res Clin Oncol. 2020;146(10):2479‐2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jansen F, Eerenstein SEJ, Cnossen IC, et al. Effectiveness of a guided self‐help exercise program tailored to patients treated with total laryngectomy: results of a multi‐center randomized controlled trial. Oral Oncol. 2020;103:104586. [DOI] [PubMed] [Google Scholar]
- 17. Kaae JK, Stenfeldt L, Hyrup B, Brink C, Eriksen JG. A randomized phase III trial for alleviating radiation‐induced xerostomia with chewing gum. Radiother Oncol. 2020;142:72‐78. [DOI] [PubMed] [Google Scholar]
- 18. McNeely ML, Parliament MB, Seikaly H, et al. Sustainability of outcomes after a randomized crossover trial of resistance exercise for shoulder dysfunction in survivors of head and neck cancer. Physiother Can. 2015;67(1):85‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Millgard M, Tuomi L. Voice quality in laryngeal cancer patients: a randomized controlled study of the effect of voice rehabilitation. J Voice. 2020;34(3):486.e413‐486.e422. [DOI] [PubMed] [Google Scholar]
- 20. Pereira RMS, Bastos MDR, Ferreira MP, et al. Topical pilocarpine for xerostomia in patients with head and neck cancer treated with radiotherapy. Oral Dis. 2020;26:1209‐1218. [DOI] [PubMed] [Google Scholar]
- 21. Schutte LER, Melissant HC, Jansen F, et al. Effect of stepped care on sexual interest and enjoyment in distressed patients with head and neck cancer: a randomized controlled trial. Sex Med. 2021;9(1):100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tang Y, Shen Q, Wang Y, Lu K, Wang Y, Peng Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation‐induced dysphagia and trismus. Strahlenther Onkol. 2011;187(1):39‐44. [DOI] [PubMed] [Google Scholar]
- 23. Vadcharavivad S, Boonroung T. Effects of two carboxymethylcellulose‐containing saliva substitutes on post‐radiation xerostomia in head and neck cancer patients related to quality of life. Asian Biomed. 2013;7(2):193‐202. [Google Scholar]
- 24. Wu PI, Szczesniak MM, Maclean J, et al. Endoscopic dilatation improves long‐term dysphagia following head and neck cancer therapies: a randomized control trial. Dis Esophagus. 2019;32(6):1. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Bazie SA, Bahatheq M, Al‐Ghazi M, Al‐Rajhi N, Ramalingam S. Antibiotic protocol for the prevention of osteoradionecrosis following dental extractions in irradiated head and neck cancer patients: a 10 years prospective study. J Cancer Res Ther. 2016;12(2):565‐570. [DOI] [PubMed] [Google Scholar]
- 26. Chan AS, Cheung MC, Law SC, Chan JH. Phase II study of alpha‐tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer. 2004;100(2):398‐404. [DOI] [PubMed] [Google Scholar]
- 27. Chen YH, Wang YK, Chuang YS, et al. Endoscopic surveillance for metachronous esophageal squamous cell neoplasms among head and neck cancer patients. Cancer. 2020;12(12):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Leeuw J, Prins JB, Teerenstra S, Merkx MAW, Marres HAM, Van Achterberg T. Nurse‐led follow‐up care for head and neck cancer patients: a quasi‐experimental prospective trial. Support Care Cancer. 2013;21(2):537‐547. [DOI] [PubMed] [Google Scholar]
- 29. Dholam KP, Bachher GK, Yadav PS, Quazi GA, Pusalkar HA. Assessment of quality of life after implant‐retained prosthetically reconstructed maxillae and mandibles postcancer treatments. Implant Dent. 2011;20(1):85‐94. [DOI] [PubMed] [Google Scholar]
- 30. Fong SSM, Ng SSM, Luk WS, Chung JWY, Leung JCY, Masters RSW. Effects of a 6‐month Tai Chi Qigong program on arterial hemodynamics and functional aerobic capacity in survivors of nasopharyngeal cancer. J Cancer Surviv. 2014;8(4):618‐626. [DOI] [PubMed] [Google Scholar]
- 31. Fong SS, Ng SS, Luk WS, Chung LM, Wong JY, Chung JW. Effects of qigong training on health‐related quality of life, functioning, and cancer‐related symptoms in survivors of nasopharyngeal cancer: a pilot study. eCAM. 2014;2014:495274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kraaijenga SAC, Molen LV, Stuiver MM, et al. Efficacy of a novel swallowing exercise program for chronic dysphagia in long‐term head and neck cancer survivors. Head Neck. 2017;39(10):1943‐1961. [DOI] [PubMed] [Google Scholar]
- 33. Liu CH, Chang JT, Lee TH, et al. Total plaque score helps to determine follow‐up strategy for carotid artery stenosis progression in head and neck cancer patients after radiation therapy. PLoS One. 2021;16(2):e0246684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manne S, Hudson S, Frederick S, et al. e‐Health self‐management intervention for oral and oropharyngeal cancer survivors: design and single‐arm pilot study of empowered survivor. Head Neck. 2020;23:23‐3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martin‐Harris B, McFarland D, Hill EG, et al. Respiratory‐swallow training in patients with head and neck cancer. Arch Phys Med Rehab. 2015;96(5):885‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Montalvo C, Finizia C, Pauli N, Fagerberg‐Mohlin B, Andrell P. Impact of exercise with TheraBite device on trismus and health‐related quality of life: a prospective study. Ear Nose Throat J. 2020;145561320961727:014556132096172. [DOI] [PubMed] [Google Scholar]
- 37. Mozzati M, Gallesio G, Gassino G, Palomba A, Bergamasco L. Can plasma rich in growth factors improve healing in patients who underwent radiotherapy for head and neck cancer? A split‐mouth study. J Craniofac Surg. 2014;25(3):938‐943. [DOI] [PubMed] [Google Scholar]
- 38. Nativ‐Zeltzer N, Kuhn MA, Evangelista L, et al. Autologous muscle‐derived cell therapy for swallowing impairment in patients following treatment for head and neck cancer. Laryngoscope. 2021;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pauli N, Svensson U, Karlsson T, Finizia C. Exercise intervention for the treatment of trismus in head and neck cancer ‐ a prospective two‐year follow‐up study. Acta Oncol. 2016;55(6):686‐692. [DOI] [PubMed] [Google Scholar]
- 40. Sterba KR, Armeson K, Zapka J, et al. Evaluation of a survivorship needs assessment planning tool for head and neck cancer survivor–caregiver dyads. J Cancer Surviv. 2019;13(1):117‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Institute JB . Checklist for Systematic Reviews and Research Syntheses ; 2017. Accessed April 26, 2022. https://jbi.global/critical-appraisal-tools
- 42. Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66(3):203‐239. [DOI] [PubMed] [Google Scholar]
- 43. Nekhlyudov L, Lacchetti C, Siu LL. Head and neck cancer survivorship care guideline: American Society of Clinical Oncology clinical practice guideline endorsement summary. J Oncol Pract. 2018;14(3):167‐171. [DOI] [PubMed] [Google Scholar]
- 44. Sleight AG, Gerber LH, Marshall TF, et al. A systematic review of functional outcomes in cancer rehabilitation research. Arch Phys Med Rehabil. 2022. doi: 10.1016/j.apmr.2022.01.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Theunissen EA, Zuur CL, Bosma SC, et al. Long‐term hearing loss after chemoradiation in patients with head and neck cancer. Laryngoscope. 2014;124(12):2720‐2725. [DOI] [PubMed] [Google Scholar]
- 46. Bhat ZY, Cadnapaphornchai P, Ginsburg K, et al. Understanding the risk factors and long‐term consequences of cisplatin‐associated acute kidney injury: an observational cohort study. PLoS One. 2015;10(11):e0142225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Faiz SA, Balachandran D, Hessel AC, et al. Sleep‐related breathing disorders in patients with tumors in the head and neck region. Oncologist. 2014;19(11):1200‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saesen K, van der Veen J, Buyse B, Nuyts S. Obstructive sleep apnea in head and neck cancer survivors. Support Care Cancer. 2021;29(1):279‐287. [DOI] [PubMed] [Google Scholar]
- 49. D'Souza RN, Collins FS, Murthy VH. Oral health for all ‐ realizing the promise of science. N Engl J Med. 2022;386(9):809‐811. [DOI] [PubMed] [Google Scholar]
- 50. Jansen F, Brakenhoff RH, Baatenburg de Jong RJ, et al. Study retention and attrition in a longitudinal cohort study including patient‐reported outcomes, fieldwork and biobank samples: results of the Netherlands quality of life and biomedical cohort study (NET‐QUBIC) among 739 head and neck cancer patients and 262 informal caregivers. BMC Med Res Methodol. 2022;22(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kelly R, Gordon P, Thompson R, Semple C. Availability and use of web‐based interventions for patients with head and neck cancer: a scoping review. J Cancer Surviv. 2022;1‐18. doi: 10.1007/s11764-022-01168-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nichols AC, Theurer J, Prisman E, et al. Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open‐label, phase 2, randomised trial. Lancet Oncol. 2019;20(10):1349‐1359. [DOI] [PubMed] [Google Scholar]
- 53. van der Hout A, van Uden‐Kraan CF, Holtmaat K, et al. Role of eHealth application Oncokompas in supporting self‐management of symptoms and health‐related quality of life in cancer survivors: a randomised, controlled trial. Lancet Oncol. 2020;21(1):80‐94. [DOI] [PubMed] [Google Scholar]
- 54. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209‐249. [DOI] [PubMed] [Google Scholar]
- 55. Bhatt N, Taufique Z, Kamen E, et al. Improving thyroid function monitoring in head and neck cancer patients: a quality improvement study. Laryngoscope. 2019;28:28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information


