Abstract
Background
Research on hyperhidrosis comorbidities has documented the co‐occurrence of diseases but has not provided information about temporal disease associations.
Objective
To investigate the temporal disease trajectories of individuals with hospital‐diagnosed hyperhidrosis.
Methods
This is a hospital‐based nationwide cohort study including all patients with a hospital contact in Denmark between 1994 and 2018. International Classification of Diseases version‐10 diagnoses assigned to inpatients, outpatients and emergency department patients were collected from the Danish National Patient Register. The main outcome was the temporal disease associations occurring in individuals with hyperhidrosis, which was assessed by identifying morbidities significantly associated with hyperhidrosis and then examining whether there was a significant order of these diagnoses using binomial tests.
Results
Overall, 7 191 519 patients were included. Of these, 8758 (0.12%) patients had localized hyperhidrosis (5674 female sex [64.8%]; median age at first diagnosis 26.9 [interquartile range 21.3–36.1]) and 1102 (0.015%) generalized hyperhidrosis (606 female sex [59.9%]; median age at first diagnosis 40.9 [interquartile range 26.4–60.7]). The disease trajectories comprised pain complaints, stress, epilepsy, respiratory and psychiatric diseases. The most diagnosed morbidities for localized hyperhidrosis were abdominal pain (relative risk [RR] = 121.75; 95% Confidence Interval [CI] 121.14–122.35; P < 0.001), soft tissue disorders (RR = 151.19; 95% CI 149.58–152.80; P < 0.001) and dorsalgia (RR = 160.15; 95% CI 158.92–161.38; P < 0.001). The most diagnosed morbidities for generalized hyperhidrosis were dorsalgia (RR = 306.59; 95% CI 302.17–311.02; P < 0.001), angina pectoris (RR = 411.69; 95% CI 402.23–421.16; P < 0.001) and depression (RR = 207.92; 95% CI 202.21–213.62; P < 0.001). All these morbidities were diagnosed before hyperhidrosis.
Conclusions
This paper ascertains which hospital‐diagnosed morbidities precede hospital‐diagnosed hyperhidrosis. As hyperhidrosis mainly is treated in the primary health care sector, the trajectories suggests that these morbidities may lead to a worse disease course of hyperhidrosis that necessitates treatment in hospitals. Treating these morbidities may improve the disease course of hyperhidrosis.
Introduction
Hyperhidrosis (HH), i.e. pathologically increased sweating, is diagnosed based on patient‐reported symptoms. 1 Etiological classifications divide HH into a primary idiopathic form and a secondary form caused by underlying diseases, medication‐use or substance misuse. 1 Primary HH manifests mainly with localized excessive sweating without an apparent cause, while secondary HH more often manifests with generalized sweating. 2 A particularly challenging aspect of studying the comorbidities of HH is that different studies have used different diagnostics, of which many have not been validated. 3 Nevertheless, research on comorbidities of primary HH has hitherto mostly identified skin infections and psychiatric diseases, 4 , 5 , 6 e.g. depression, anxiety, stress and quality of life impairments. 4 , 5 , 6 , 7 , 8 , 9 However, these findings are based on observational evidence, which is owing to the study designs, limited to reporting on associations between co‐occurring diseases, omitting any temporal aspects. Hence, it remains unknown whether HH develops before or after these associated comorbidities. Yet, such information is crucial for the management of any disease. One way to research the order in which diseases are diagnosed is to determine the temporal disease associations, which requires that two diseases are significantly associated and that one disease, in general, is diagnosed before the other. 10 , 11 , 12 Such an approach may identify unrecognized comorbidities and improve the general understanding of the evolution of HH and its comorbidities. Additional implications include the detection of disease risk factors and severe and possibly preventable outcomes, which can improve patient care. Therefore, the objective of this study is to investigate the disease trajectories of individuals with hospital‐diagnosed hyperhidrosis identified through hospital records between 1994 and 2018 in Denmark.
Materials and methods
Study design
This is a hospital‐based cohort study using full population data from the Danish National Patient Register (DNPR).
The Danish National Patient Register and ICD codes
Individuals who are born in or immigrate to Denmark are assigned a unique Civil Personal Register (CPR) number, which enables the traceability of individuals across Danish registers. 13 , 14 The DNPR has since 1977 collected complete data from all public hospital inpatient records and from private hospital records since 2003. 13 , 14 Data on public hospital outpatient records and emergency departments have been compiled in the DNPR since 1 January 1994, which coincided with the introduction of the International Classification of Diseases (ICD) version‐10 system in hospitals in Denmark. 13 , 14 The ICD‐10 is a system to code and report diseases in hospitals in Denmark. The ICD‐10 diagnoses are assigned to patients once a hospital physician has diagnosed a disease. In this study, we utilized inpatient, outpatient and emergency department data starting on 1 January 1994, and we had access to hospital records until 10 April 2018. The ICD‐8 system preceded the ICD‐10 system in Denmark between 1971 and 1993, in which HH only was described using the code 78819 for HH, without further specification.
Study population
Individuals registered in the DNPR between 1994 and 2018 with the ICD‐10 codes R610 for localized HH, R611 for generalized HH or R619 for unspecified HH were included as HH cases. Comparators were individuals in the DNPR without an ICD‐10 code for HH, with matching age and sex, who had been diagnosed with an ICD‐10 code within the same week as the case individual had been diagnosed with HH.
Variables
Variables were ICD‐10 diagnoses, sex, age at first diagnosis, age at end of follow‐up, and family with HH collected from the DNPR. Sex was coded as female and male sex. Age at first diagnosis was defined as age at receiving the HH diagnosis for the first time and it was coded as a continuous variable. Age at end of follow‐up was defined as age on 10 April 2018, and it was coded as a continuous variable. ICD‐10 codes comprised A, B and G diagnoses, which describe the disease that is responsible for the inpatient, outpatient or emergency hospital contact, other diseases relevant for the hospital contact, and all underlying diseases the patient have, respectively. The reason for choosing these types of diagnoses is to identify all the diseases occurring in the included individuals. The ICD‐10 diagnoses R610, R611 and R619 were used as three separate binary variables indicating having or not having the ICD‐10 codes. Additionally, individuals diagnosed with R610 and R611 were excluded from R619 as the latter was the phenotypically undetermined unspecified HH. Family with HH indicated having or not having first‐degree relatives with the aforementioned ICD‐10 codes for HH and it was constructed based on kinship data from CPR data.
Temporal disease associations and trajectories
The aim was to determine the disease trajectories of individuals with different types of hospital‐diagnosed hyperhidrosis. The method is described extensively in the previous literature. 10 , 11 Firstly, individuals in the DNPR with the ICD‐10 codes R610, R611 or R619 were identified and analysed separately. For each of the study populations with the three separate ICD codes for HH, we analysed the co‐occurrence of ICD‐10 codes with a higher prevalence in the study population compared to the matched comparator population. The strength of the associations of co‐occurring diseases was reported using relative risk (RR), which was calculated using:
In which is the number of individuals with HH who had disease 1 (D1) who then developed disease 2 (D2), which also is known as a pair (D1 → D2). For each pair of diseases in individuals with HH, a comparator group of 10 000 individuals matching on D1, sex, birth decade, type of hospital contact and discharge week were identified. The occurrence of D2 in the comparator group was determined. Only co‐occurring morbidities with a RR > 1.0 were included and 95% CI of the RR was computed through bootstrapping of 20 iterations with 80% of the population. One‐sided binominal tests determined if disease A was diagnosed before disease B or B before A. Statistically significant morbidity pairs with a direction were combined into longer trajectories of three diseases and networks. The alpha level was set to <0.05. To correct for multiple testing, we used a Bonferroni corrected threshold of P‐value <1.21 × 10−9 for finding significant disease associations and a corrected Bonferroni P‐value <1.21 × 10−8 to discover the directionality of those associations. The ICD‐10 codes are defined by the first three characters of the disease code, but it includes all subdivisions of the ICD‐10 code. A list of the ICD‐10 codes with included subdivisions is presented in Table S1.
Descriptive statistics
The distributions of the variables age at first diagnosis and age at end of follow‐up were determined by histograms and depending on normality, presented as mean with standard deviation or median with interquartile range (IQR). The variables sex and family with HH were presented as frequency distributions with percentages. Statistical analysis was conducted using R, version 3.4.0 for Windows (R Project for Statistical Computing).
Results
For results on demographics, see Table 1. Of the 7 191 519 individuals registered in the DNPR between 1 January 1994, and 10 April 2018, 8758 (0.12%) had localized HH; 1102 (0.015%) had generalized HH and 3380 (0.047%) had unspecified HH. Of these, 5 571 264 individuals were alive by the end of follow‐up, of whom 12 322 had a HH diagnosis, which corresponds to an overall prevalence of 0.22% (95% CI 0.22–0.23). Of these 12 322 individuals, 8450 had localized HH, 908 had generalized HH and 3082 had unspecified HH, which equal prevalence of 0.15% (95% CI 0.15–0.15), 0.02% (95% CI 0.02–0.02) and 0.06% (95% CI 0.05–0.06), respectively. There were no missing observations for the variables sex, age at first diagnosis, age at end of follow‐up or family with HH. Overall, 76 patients were diagnosed with HH only in the ICD‐8 system.
Table 1.
Demographics of the study participants with hyperhidrosis
| Localized HH, R610, n = 8758 | Generalized HH, R611, n = 1012 | Unspecified HH, R619, n = 3380 | |
|---|---|---|---|
| Female sex, n (%) | 5673 (64.8) | 606 (59.9) | 2058 (60.9) |
| Male sex, n (%) | 3085 (35.2) | 406 (40.1) | 1322 (39.1) |
| Age at first diagnosis, median (IQR) | 26.9 (21.3–36.1) | 40.9 (26.4–60.7) | 33.2 (23.6–52.6) |
| Age at end of follow‐up, median (IQR) | 36.6 (28.4–46.1) | 47.6 (34.4–67.1) | 43.2 (31.3–61.1) |
| Family with HH, n (%) | 269 (3.1) | 15 (1.5) | 42 (1.2) |
HH, Hyperhidrosis; IQR, interquartile range; n, number.
For presentations of disease trajectory networks of localized, generalized and unspecified HH separately, see Figs. 1, 2, 3. In localized HH, the most diagnosed morbidities were pain in the abdomen and back, soft tissue disorders and reactions to stress. Then, in descending order were chronic diseases of the tonsils, asthma, depression, personality disorders, synovitis or tenosynovitis, non‐suppurative otitis media and epilepsy. For generalized HH, the pattern was similar with pain in the back and angina pectoris being the most diagnosed morbidities, followed by depression and personality disorders.
Figure 1.
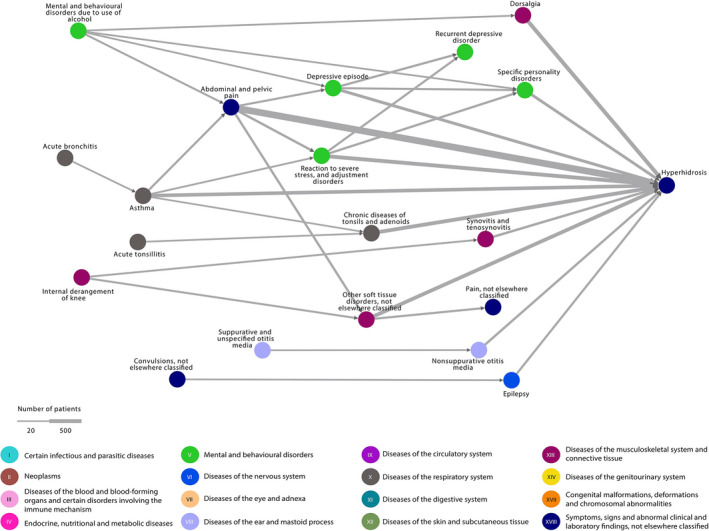
Localized hyperhidrosis disease trajectory network. The nodes represent the diseases and the arrows connecting them, the temporal associations. The colours of the nodes indicate ICD‐10 chapters. The width of the arrow indicates the number of patients following the trajectory. All trajectories include at least 20 patients with localized hyperhidrosis. ICD‐10, International Classification of Diseases version‐10.
Figure 2.
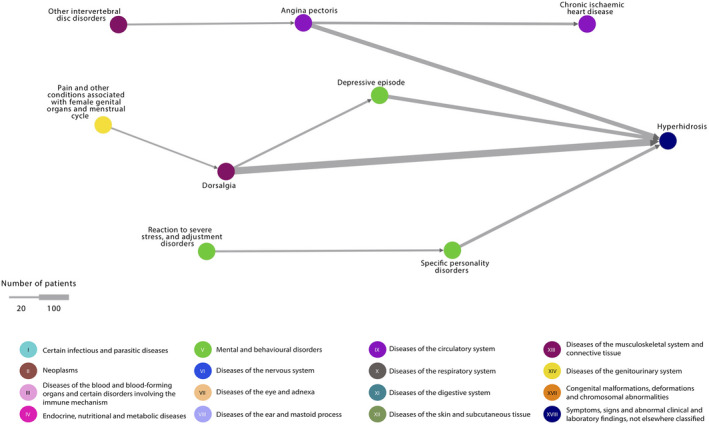
Generalized hyperhidrosis disease trajectory network. The nodes represent the diseases and the arrows connecting them, the temporal associations. The colours of the nodes indicate ICD‐10 chapters. The width of the arrow indicates the number of patients following the trajectory. All trajectories include at least 10 patients with generalized hyperhidrosis. ICD‐10, International Classification of Diseases version‐10.
Figure 3.
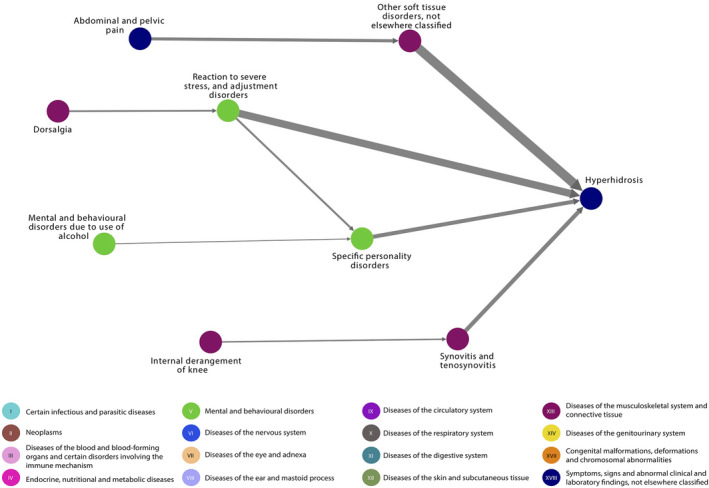
Unspecified hyperhidrosis disease trajectory network. The nodes represent the diseases and the arrows connecting them, the temporal associations. The colours of the nodes indicate ICD‐10 chapters. The width of the arrow indicates the number of patients following the trajectory. All trajectories include at least 20 patients with unspecified hyperhidrosis. ICD‐10, International Classification of Diseases version‐10.
All morbidities were diagnosed before HH. See Table 2 for RR with 95% CI and mean time between disease pairs.
Table 2.
Relative risk and mean time between diagnosing hyperhidrosis and the co‐occurring morbidities
| D1 of pairs (ICD‐10 code) | D2 of pairs (ICD‐10 code) | Individuals in disease pairs, n (%) | Relative risk (95% CI) of temporal associations | P‐value of directionality | Mean time in months between D1 and D2 (95% CI) |
|---|---|---|---|---|---|
| Depressive episode (F32) | Localized hyperhidrosis (R610) | 349 (4.13) | 132.89 (131.60–134.17) | <0.001 | 63.05 (57.78–68.32) |
| Generalized hyperhidrosis (R611) | 69 (0.82) | 207.92 (202.21–213.62) | <0.001 | 84.30 (67.68–100.92) | |
| Reaction to severe stress and adjustment disorders (F43) | Localized hyperhidrosis (R610) | 507 (6.00) | 123.57 (122.63–124.51) | <0.001 | 76.19 (71.37–81.01) |
| Unspecified hyperhidrosis (R619) | 322 (9.53) | 150 (148.94–151.93) | <0.001 | 85.30 (78.30–92.20) | |
| Specific personality disorders (F60) | Localized hyperhidrosis (R610) | 274 (3.24) | 111.35 (109.98–112.72) | <0.001 | 73.47 (66.37–80.56) |
| Generalized hyperhidrosis (R611) | 42 (0.50) | 133.72 (129.89–137.55) | <0.001 | 126.74 (104.07–149.41) | |
| Unspecified hyperhidrosis (R619) | 170 (5.03) | 136.45 (133.99–138.90) | <0.001 | 90.11 (80.03–100.19) | |
| Epilepsy (G40) | Localized hyperhidrosis (R610) | 153 (1.81) | 147.13 (143.52–150.74) | <0.001 | 100.72 (90.20–111.24) |
| Non‐suppurative otitis media (H65) | Localized hyperhidrosis (R610) | 169 (2.00) | 149.86 (147.40–152.32) | <0.001 | 146.90 (136.07–157.73) |
| Angina pectoris (I20) | Generalized hyperhidrosis (R611) | 72 (0.85) | 411.69 (402.23–421.16) | <0.001 | 97.16 (83.97–110.35) |
| Chronic diseases of tonsils and adenoids (J35) | Localized hyperhidrosis (R610) | 493 (5.83) | 118.33 (117.62–119.03) | <0.001 | 108.06 (102.21–113.90) |
| Asthma (J45) | Localized hyperhidrosis (R610) | 455 (5.38) | 146.99 (145.25–148.72) | <0.001 | 115.56 (108.90–122.22) |
| Dorsalgia (M54) | Localized hyperhidrosis (R610) | 553 (6.54) | 160.15 (158.92–161.38) | <0.001 | 73.80 (68.70–78.89) |
| Generalized hyperhidrosis (R611) | 137 (1.62) | 306.59 (302.17–311.02) | <0.001 | 95.15 (83.51–106.79) | |
| Synovitis and tenosynovitis (M65) | Localized hyperhidrosis (R610) | 200 (2.37) | 155.02 (153.05–156.99) | <0.001 | 83.48 (74.94–92.02) |
| Unspecified hyperhidrosis (R619) | 173 (5.12) | 195.58 (192.10–199.05) | <0.001 | 87.98 (78.49–97.48) | |
| Other soft tissue disorders, not elsewhere classified (M79) | Localized hyperhidrosis (R610) | 559 (6.62) | 151.19 (149.58–152.80) | <0.001 | 78.10 (72.97–83.23) |
| Unspecified hyperhidrosis (R619) | 449 (13.28) | 200.32 (198.06–202.58) | <0.001 | 83.55 (77.65–89.45) | |
| Abdominal and pelvic pain (R10) | Localized hyperhidrosis (R610) | 1183 (14.00) | 121.75 (121.14–122.35) | <0.001 | 83.13 (79.69–86.58) |
CI, Confidence interval; D1, Disease 1 in disease a pair, D2, Disease 2 in a disease pair; ICD‐10, 10th revision of the International Classification of Diseases and Related Health Problems; n, Number; RR, Relative Risk.
For disease pairs corrected for age at diagnosis, see Figure S1, which visualizes that individuals with HH are diagnosed earlier with other morbidities than individuals without HH who are diagnosed with the same morbidities.
Discussion
In this hospital‐based nationwide cohort study, we have determined the disease trajectories of hospital‐diagnosed HH. Overall, the results are coherent with previously reported comorbidities, which supports the validity of the method. 4 , 6 , 15 , 16 , 17 Similar to other studies, we have identified an association between HH and depression. 4 , 16 Tentative explanations include the mental burden of primary HH and the social isolation of HH coping mechanisms leading to psychiatric diseases. 18 Also, there may be an indirect effect as one of the adverse effects of antidepressants can be secondary HH. 2 , 19 In addition to depression, this study identifies new potential psychiatric comorbidities of HH including adjustment reactions and personality disorders.
Epilepsy preceded localized HH. In a previous publication, sleep HH has been reported in about 6% of children with epilepsy. 20 Speculatively, this association with epilepsy suggests an underlying central nervous system pathomechanism. The temporality of the association may be because epilepsy is referred to hospitals upon onset while HH is subject to a diagnostic delay and is often treated in the primary health care sector before it is treated in hospitals. 21 , 22 Another explanation of the observed association is that the use of antiepileptic drugs can lead to secondary HH19. Only further studies can verify if there is a link between HH and epilepsy.
Generalized HH was associated with both angina pectoris and chronic ischemic heart disease, although the temporal component with HH was lacking for the latter. This is consistent with a previous study that found an association between HH and an elevated risk of ischemic heart diseases and stroke. 17 It may be speculated that this observed association is caused by sympathetic nervous system overactivity in individuals with HH, cardiac diseases, medications or shared risk factors for cardiac diseases and HH, such as being overweight. 22 , 23 , 24 , 25 Specific studies are required to determine if individuals with HH are at risk of heart diseases.
A previous study found that 347 of 387 (96.6%) patients with primary HH had localized sweating and 13 of 387 (3.4%) had generalized sweating. Therefore, the disease trajectories of localized HH may mostly reflect those of primary HH2. Likewise, the same study found that 17 of 28 (61.0%) patients with secondary HH had localized sweating while 11 of 28 (39%) had generalized sweating. This suggests that generalized HH comprise primary and secondary HH, which is caused by concurrent comorbidities, medication‐use and substance abuse.
The reason for the high RR is that the occurrence of HH in the DNPR is between 0.015% and 0.12%. When calculating the RR of the trajectories, the denominator becomes correspondingly low, which leads to the very high RR we report.
Limitations
The disease trajectories require both a statistically significant association between the diseases and a temporal association. Thus, morbidities that lack the temporal component in their association with HH do not appear in the disease trajectories. Additionally, a proportion of the HH cases had the phenotypically undetermined unspecified HH, which likely is either localized or generalized HH. A more precise classification of these cases is unlikely to change the results, as the morbidities reported for unspecified HH also occurred in the trajectories of localized or generalized HH. Nevertheless, only future hospital contacts can further characterize what kind of HH these individuals have. Also, data from the 76 individuals with HH registered in the DNPR before the implementation of the ICD‐10 system in 1994 were not included. In the ICD‐8, HH was not specified as being localized, generalized or unspecified as in the ICD‐10; therefore, these individuals could not have changed the results on localized or generalized HH. In addition, as these 76 individuals are a small fraction compared to the study population, they were unlikely to change the results significantly. Also, although the ICD codes for HH are assigned to patients by hospital physicians, there is a need for validating their diagnostic properties. Furthermore, prescription‐data could not be linked to the participants, which prevented determining whether drug‐use could explain the trajectories. Finally, as the median ages at end of follow‐up were 37, 43 and 48 years for the different groups of patients with HH, many individuals will likely develop additional comorbidities after the end of the follow‐up.
Generalizability
Individuals with HH and other morbidities only treated in the primary healthcare sector are not represented in the disease trajectories. Therefore, the study results may not be generalizable to individuals with HH only treated outside of hospitals.
Interpretation
This paper ascertains which hospital‐diagnosed morbidities precede hospital‐diagnosed HH. In Denmark, HH is primarily treated in the primary health care sector. This study, however, suggests that individuals diagnosed with these morbidities are subsequently diagnosed with HH in hospitals. This may be because these morbidities lead to a worse disease course of HH and, therefore, are treated in hospitals. Treating these morbidities in individuals with HH may improve the course of HH.
In conclusion, this study reports that pain complaints, stress, epilepsy, respiratory and psychiatric diseases are diagnosed in individuals who later are diagnosed with HH in hospitals. The temporality of the trajectories suggests that these morbidities may lead to a disease course of HH that necessitates hospital treatments. Treating these morbidities in individuals with HH may improve the disease course, ideally obviating the need for hospital treatments. Additional research that seeks to reproduce and explain these findings is warranted.
Ethical approvals
This study has been registered on the UCPH record for The Danish Data Protection Agency (KU 514‐0255‐18‐3000) and approved by The Danish Health Data Authority (FSEID 00003092 and FSEID 0004491). The data of the DNPR are protected by the Danish Act on Processing of Personal Data and can be accessed after application to the Health Data Authority. Consent to participate was not obtained as the register data of the current study was completely anonymized. The current study was performed in accordance with the ethical standards as laid down in the 1975 Declaration of Helsinki, as revised in 1983.
Conflicts of Interest
Mattias AS Henning and Rune Kjærsgaard Andersen report grants from Leo Foundation (grant number LF‐18002). Roc Reguant and Isabella Friis Jørgensen report grants from Novo Nordisk Foundation (the Core grant NNF14CC0001 and the Challenge grant NFF17OC0027594). Gregor B Jemec reports grants and personal fees from Abbvie, personal fees from Coloplast, personal fees from Chemocentryx, personal fees from LEO pharma, grants from LEO Foundation, grants from Afyx, personal fees from Incyte, grants and personal fees from InflaRx, grants from Janssen‐Cilag, grants and personal fees from Novartis, grants and personal fees from UCB, grants from CSL Behring, grants from Regeneron, grants from Sanofi, personal fees from Kymera, personal fees from VielaBio, outside the submitted work. Søren Brunak has ownerships in Intomics A/S, Hoba Therapeutics Aps, Novo Nordisk A/S, Lundbeck A/S and managing board membership in Intomics A/S. Kristina S Ibler reports personal fees from Leo Pharma, Sanofi Genzymes, Astra Zeneca and Eli Lilly. Ole B Pedersen has no conflicts of interest.
Supporting information
Table S1. Subdivisions of the ICD‐10 diagnosis.
Figure S1. Localized hyperhidrosis, generalized hyperhidrosis and unspecified hyperhidrosis disease pairs corrected for age.
Acknowledgements
The generous support of the Leo Foundation Denmark is gratefully acknowledged. Likewise, the Novo Nordisk Foundation is gratefully acknowledged.
Funding sources
Mattias Henning and Rune Kjærsgaard Andersen were provided research grants from Leo Foundation Denmark (grant number LF‐18002). Søren Brunak, Isabella Jørgensen and Roc Reguant were provided grants from the Novo Nordisk Foundation (the Core grant NNF14CC0001 and the Challenge grant NFF17OC0027594). No study sponsor or funder has taken part in the study design, data collection, data analysis or manuscript preparation.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available due to that they are protected by the Danish Act on Processing of Personal Data and can be accessed after application to the Health Data Authority.
References
- 1. Hornberger J, Grimes K, Naumann M et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. J Am Acad Dermatol 2004; 51: 274–286. [DOI] [PubMed] [Google Scholar]
- 2. Walling HW. Clinical differentiation of primary from secondary hyperhidrosis. J Am Acad Dermatol 2011; 64: 690–695. [DOI] [PubMed] [Google Scholar]
- 3. Henning MAS, Thorlacius L, Ibler KS, Jemec GBE. How to diagnose and measure primary hyperhidrosis: a systematic review of the literature. Clin Auton Res 2021; 31: 511–528. [DOI] [PubMed] [Google Scholar]
- 4. Bahar R, Zhou P, Liu Y et al. The prevalence of anxiety and depression in patients with or without hyperhidrosis (HH). J Am Acad Dermatol 2016; 75: 1126–1133. [DOI] [PubMed] [Google Scholar]
- 5. Gross KM, Schote AB, Schneider KK, Schulz A, Meyer J. Elevated social stress levels and depressive symptoms in primary hyperhidrosis. PLoS One 2014; 9: e92412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shayesteh A, Janlert U, Brulin C, Boman J, Nylander E. Prevalence and characteristics of hyperhidrosis in Sweden: a cross‐sectional study in the general population. Dermatology 2016; 232: 586–591. [DOI] [PubMed] [Google Scholar]
- 7. Walling HW. Primary hyperhidrosis increases the risk of cutaneous infection: a case‐control study of 387 patients. J Am Acad Dermatol 2009; 61: 242–246. [DOI] [PubMed] [Google Scholar]
- 8. Henning MAS, Ibler KS, Ostrowski SR et al. Hyperhidrosis and the risk of being treated for skin infections. J Dermatol Treat 2022; 33: 2263–2263. [DOI] [PubMed] [Google Scholar]
- 9. Henning MAS, Ibler KS, Loft I et al. The health‐related quality of life in hyperhidrosis and co‐morbidities. Qual Life Res 2022; 31: 2331–2340. [DOI] [PubMed] [Google Scholar]
- 10. Jensen AB, Moseley PL, Oprea TI et al. Temporal disease trajectories condensed from population‐wide registry data covering 6.2 million patients. Nat Commun 2014; 5: 4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siggaard T, Reguant R, Jørgensen IF et al. Disease trajectory browser for exploring temporal, population‐wide disease progression patterns in 7.2 million Danish patients. Nat Commun 2020; 11: 4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kjærsgaard Andersen R, Jørgensen IF, Reguant R, Jemec GBE, Brunak S. Disease trajectories for hidradenitis suppurativa in the Danish population. JAMA Dermatol 2020; 156: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schmidt M, Schmidt SAJ, Adelborg K et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019; 11: 563–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shayesteh A, Boman J, Janlert U, Brulin C, Nylander E. Primary hyperhidrosis: implications on symptoms, daily life, health and alcohol consumption when treated with botulinum toxin. J Dermatol 2016; 43: 928–933. [DOI] [PubMed] [Google Scholar]
- 16. Kristensen JK, Vestergaard DG, Swartling C, Bygum A. Association of Primary Hyperhidrosis with depression and anxiety: a systematic review. Acta Derm Venereol 2019; 100: adv00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park J‐M, Moon DH, Lee HS, Park J‐Y, Lee J‐W, Lee S. Hyperhidrosis, endoscopic thoracic Sympathectomy, and cardiovascular outcomes: a cohort study based on the Korean Health Insurance Review and Assessment Service database. Int J Environ Res Public Health 2019; 16: 3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Solish N, Wang R, Murray CA. Evaluating the patient presenting with hyperhidrosis. Thorac Surg Clin 2008; 18: 133–140. [DOI] [PubMed] [Google Scholar]
- 19. Cheshire WP, Fealey RD. Drug‐induced hyperhidrosis and Hypohidrosis. Drug Saf 2008; 31: 109–126. [DOI] [PubMed] [Google Scholar]
- 20. Zambrelli E, Turner K, Vignoli A et al. Sleep disturbances in Italian children and adolescents with epilepsy: a questionnaire study. Epilepsy Behav 2020; 106: 107014. [DOI] [PubMed] [Google Scholar]
- 21. Shayesteh A, Gerdsdorff F, Persson M, Brulin C, Nylander E. Navigating in the fog. Facing delays, rejection and ignorance when seeking help for primary hyperhidrosis. Int J Qual Stud Health Well Being 2021; 16: 1930642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henning MAS, Ibler KS, Loft I et al. Epidemiology of hyperhidrosis in Danish blood donors. Acta Derm Venereol 2021; 101: adv00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cercato C, Fonseca FA. Cardiovascular risk and obesity. Diabetol Metab Syndr 2019; 11: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Astman N, Friedberg I, Wikstrom J et al. The association between obesity and hyperhidrosis: a Nationwide, cross sectional study of 2.77 million Israeli adolescents. J Am Acad Dermatol 2019; 81: 624–627. [DOI] [PubMed] [Google Scholar]
- 25. Lee Y‐C, You YK, Lee JH et al. Comparison of EQ‐5D‐3L and metabolic components between patients with hyperhidrosis and the general population: a propensity score matching analysis. Qual Life Res 2021; 30: 2591–2599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Subdivisions of the ICD‐10 diagnosis.
Figure S1. Localized hyperhidrosis, generalized hyperhidrosis and unspecified hyperhidrosis disease pairs corrected for age.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to that they are protected by the Danish Act on Processing of Personal Data and can be accessed after application to the Health Data Authority.


