Abstract
A woman's reproductive history is strongly associated with her risk of ovarian cancer. However, it is unclear how pregnancies of different duration impact a woman's ovarian cancer risk, and therefore, what part of a pregnancy explains the protective effect. Using a cohort of all Danish women followed from 1968 to 2018, with prospectively registered information on reproductive history (eg, gestational duration of pregnancies, tubal ligation and resection and hormonal pharmaceutical use), we investigated the effect of pregnancy duration on ovarian cancer risk. We adjusted for potential confounders, such as age at pregnancy and time since pregnancy, using log‐linear Poisson regression to isolate the effect of pregnancy duration on ovarian cancer risk. Among 2.5 million Danish women with 4.4 million pregnancies, a pregnancy was associated with a reduction of ovarian cancer risk of 21% (95% CI, 14%‐28%), 26% (95% CI, 21%‐31%), 12% (95% CI, 7%‐17%) and 3% (95% CI, −5% to 11%) compared to one less, for the first, second, third and fourth pregnancy, respectively (P < .001 for heterogeneity), with similar effects of induced abortions, spontaneous abortions and childbirths. Sensitivity analysis of age at pregnancy, time since pregnancy and other potential confounders did not change these findings. The reduced ovarian cancer risk associated with pregnancy is primarily driven by the first three pregnancies, with similar effects of induced abortion, spontaneous abortions and childbirth, suggesting that mainly exposure to early pregnancy factors, and not pregnancy duration, protect against ovarian cancer.
Keywords: abortion, childbirth, cohort study, ovarian cancer, pregnancy
What's new?
Full‐term pregnancies are known to be associated with a decreased risk of ovarian cancer. However, it remains unclear how pregnancies of shorter durations influence ovarian cancer risk. In this 50‐year nationwide cohort study of 2.5 million women, the authors found that the reduced ovarian cancer risk associated with pregnancy is primarily driven by the first three pregnancies, with similar effects of induced abortion, spontaneous abortions and childbirth. The results imply that exposure to early pregnancy factors, and not pregnancy duration, is the main protective mechanism against ovarian cancer.
Abbreviations
- CI
confidence interval
- CRS
Danish Civil Registration System
- ICD
International Classification of Diseases
1. INTRODUCTION
Ovarian cancer has the worst prognosis of the common female cancers with limited effects of current screening and treatment modalities. 1 , 2 Yet, it is well‐established that both a woman's life‐time use of oral contraceptives and her number of pregnancies are associated with a strong decreased risk of ovarian cancer. However, while there is firm evidence of a dose‐response relationship between duration of oral contraceptive use and decreased ovarian cancer risk, 3 , 4 , 5 it is not clear how duration of pregnancy influences ovarian cancer risk. 6 A recent pooled analysis of 15 case‐control studies suggests that even incomplete pregnancies lasting <6 months reduce ovarian cancer risk, 7 but it is unknown if the protection associated with pregnancy is a consequence of a minimal duration of each pregnancy or the total duration of all pregnancies.
Using 50 years of nationwide follow‐up for ovarian cancer in a full population cohort of 2.5 million women with prospectively registered and highly detailed information on reproductive factors (eg, gestational duration of pregnancies resulting in childbirth, gestational duration at time of induced abortion, spontaneous abortions, tubal ligations and resections and hormonal pharmaceutical use), we investigated the association between pregnancy duration (by pregnancy type and number of months pregnant) and ovarian cancer risk. The effect of pregnancy duration was analyzed by comparing effects of pregnancies resulting in childbirth and shorter pregnancies (ie, induced and spontaneous abortions), in addition to estimating effects of three additional months of pregnancy, irrespective of pregnancy type. To narrow in on the effect of pregnancy duration on ovarian cancer risk, we also considered other potentially important factors, such as maternal age at pregnancy, time since pregnancy and histological subtype of ovarian cancer.
2. METHODS
2.1. Population cohort
We created a nationwide population cohort of all Danish women, born from 1935 and aged 12 years or more, from 2 April 1968, until 31 December 2018, using the Danish Civil Registration System (CRS) which contains prospectively registered demographic information on all Danish residents. 8 The CRS assigns a unique identification number for residents and allows linkage to family members, including cohabitating children starting from 1968 in addition to linkage to other health and population registries (eg, The National Registry of Induced Abortions, The Medical Birth Registry, The Danish National Patient Registry and socioeconomic registries at Statistics Denmark).
2.2. Study variables
The National Registry of Induced Abortion includes information on procedural date and week of gestation for induced abortions in Denmark, starting from 1973, while the Medical Birth Register includes week of gestation for all childbirths in Denmark starting from 1978, as previously described. 9 The Danish National Patient Registry holds prospectively registered information on spontaneous abortions, endometriosis and other disease categories diagnosed in hospital settings. Information on tubal ligation (including tubectomy and tubal resection), oophorectomy and hysterectomy was registered according to the Danish Classification of Surgical Procedures and Therapies from 1977 to 1995, and from 1996 onwards according to the Nordic Medico‐Statistical Committee Classification of Surgical Procedures. 10 Use of oral contraceptives and hormone replacement therapy is registered in The Danish National Prescription Registry starting in 1995, which contains information on all filled prescriptions. 11 Information on socioeconomic covariates such as educational attainment, urbanicity and marital status was obtained from Statistics Denmark 12 (see Table 1 for categorization). For further information on coding of specific study variables and study timeline see Table S1 and Figure 1.
TABLE 1.
Person‐years and ovarian cancer events according to number of induced abortions, number of childbirths, age at first pregnancy, age at latest pregnancy, duration of latest pregnancy, time since latest pregnancy, attained age, birth cohort, educational attainment, marital status and urbanicity, from 2 April 1968 to 31 December 2018
| Characteristic | Person‐years (in 1000s) (%) | Ovarian cancer events (%) |
|---|---|---|
| Total cohort | 74 885 (100.0%) | 8566 (100.0%) |
| Number of induced abortions | ||
| 0 | 63 555 (84.9%) | 7194 (84.0%) |
| 1 | 8513 (11.4%) | 1035 (12.1%) |
| ≥2 | 2818 (3.8%) | 337 (3.9%) |
| Number of childbirths | ||
| 0 | 30 644 (40.9%) | 1624 (19.0%) |
| 1 | 11 303 (15.1%) | 1608 (18.8%) |
| 2 | 21 827 (29.1%) | 3452 (40.3%) |
| 3 | 8567 (11.4%) | 1417 (16.5%) |
| ≥4 | 2544 (3.4%) | 465 (5.4%) |
| Age at first pregnancy (years) | ||
| <30 | 41 802 (90.1%) | 6367 (89.8%) |
| ≥30 | 4586 (9.9%) | 722 (10.2%) |
| Age at latest pregnancy (years) | ||
| <30 | 27 097 (58.4%) | 3921 (55.3%) |
| ≥30 | 19 292 (41.6%) | 3168 (44.7%) |
| Duration of latest pregnancy (weeks) | ||
| Nulligravid women | 28 497 (38.1%) | 1477 (17.2%) |
| <12 | 7158 (9.6%) | 1010 (11.8%) |
| 12‐21 | 163 (0.2%) | 24 (0.3%) |
| 22‐36 | 1084 (1.4%) | 98 (1.1%) |
| ≥37 | 19 261 (25.7%) | 1588 (18.5%) |
| Missing gestational duration | 18 722 (25.0%) | 4369 (51.0%) |
| Time since latest pregnancy (years) | ||
| <10 | 22 229 (47.9%) | 806 (11.4%) |
| ≥10 | 24 159 (52.1%) | 6283 (88.6%) |
| Attained age (years) | ||
| <50 | 68 294 (91.2%) | 5476 (63.9%) |
| ≥50 | 6591 (8.8%) | 3090 (36.1%) |
| Birth cohort | ||
| 1935‐1939 | 5862 (7.8%) | 2025 (23.6%) |
| 1940‐1944 | 7346 (9.8%) | 1910 (22.3%) |
| 1945‐1949 | 8788 (11.7%) | 1731 (20.2%) |
| 1950‐1959 | 16 330 (21.8%) | 1835 (21.4%) |
| 1960‐ | 36 560 (48.8%) | 1065 (12.4%) |
| Educational attainment a | ||
| Primary schooling | 34 819 (46.5%) | 3520 (41.1%) |
| Short basic education | 25 182 (33.6%) | 3096 (36.1%) |
| Higher education | 14 884 (19.9%) | 1950 (22.8%) |
| Marital status b | ||
| Married | 34 052 (45.5%) | 5359 (62.6%) |
| Divorced | 6030 (8.1%) | 1235 (14.4%) |
| Widowed | 1812 (2.4%) | 700 (8.2%) |
| Unmarried | 32 991 (44.1%) | 1272 (14.9%) |
| Urbanicity c | ||
| Rural | 38 348 (51.2%) | 3857 (46.0%) |
| Urban | 36 537 (48.8%) | 4526 (54.0%) |
Missing educational attainment status (8985 k person‐years and 175 events) was categorized as primary schooling.
No registered marital status was categorized as unmarried.
Information on urbanicity was available from 1 January 1976. In total 7232 k person‐years and 183 events had missing information on urbanicity. Missing information on urbanicity after 1 January 1976, was categorized as urban.
FIGURE 1.
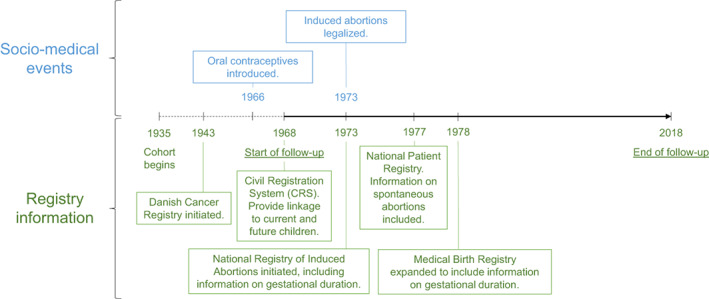
Overview of study timeline [Color figure can be viewed at wileyonlinelibrary.com]
2.3. Outcomes
The Danish Cancer Registry has since 1943 registered incidence of cancer in Denmark and is considered close to complete. 13 Ovarian cancer was defined by the ICD‐7 coding system until 1977 and by ICD‐10 coding system from 1978. Histological subtypes from 1978 were specified by ICD‐O‐3 morphology codes as described previously. 14
2.4. Subjects
The cohort was followed from 2 April 1968, for diagnosis of ovarian cancer, death or emigration until 31 December 2018. Subjects were censored at oophorectomy (both unilateral and bilateral) but also hysterectomy to avoid potential misclassification of hysterectomies that included a concurrent oophorectomy.
2.5. Statistical analyses
We estimated incidence rate ratios (relative risks) of ovarian cancer using log‐linear Poisson regression, adjusting analyses for effects of age, calendar period (in 10‐year intervals) and interaction between calendar period and age, in addition to other potential confounders. First, we classified pregnancy (including both induced abortions and childbirths) as a categorical variable and estimated relative effects of pregnancies using either no pregnancies or the previous pregnancy as reference (as described previously 9 ). To assess differences in effects by pregnancy number, we compared a model with identical effects of each pregnancy to a model with categorical effects by pregnancy number. Second, we estimated effects of pregnancy as categorical variable by pregnancy type, including spontaneous abortions with information from 1 January 1977. To assess differences in effects by pregnancy type, we compared models with same effects of each pregnancy, regardless of type, by each individual pregnancy number and overall, to models with categorical effects of pregnancy number and type. Furthermore, we estimated pregnancy effects by ovarian cancer subtype and compared overall differences between pregnancy types for each subtype.
Additionally, we investigated effect of induced abortions and childbirths by specific gestational duration, and assessed difference in weekly duration of pregnancy. Further, to investigate an effect of time span between pregnancies, we explored differences in ovarian cancer risk by time span between the first and the latest pregnancy, using <5 years between pregnancies as reference, among women with two, three or four pregnancies, respectively. Finally, to explore the extent of the association between pregnancy and ovarian cancer, we estimated the relative risk of ovarian cancer among women with one, two or three pregnancies, compared to nulligravid women, by time since latest pregnancy.
In sensitivity analyses, we investigated general models with continuous effect of pregnancy duration, maternal age at pregnancy and time since pregnancy. In these models we investigated individual effects of pregnancy number, pregnancy timing and ovarian cancer subtype. For induced abortions and childbirths with missing information on gestational duration we used mode imputation to determine pregnancy duration (8 weeks for induced abortions and 40 weeks for childbirths). In further sensitivity analyses, we considered specific effects of number of induced abortions, oral contraceptive use, hormone replacement therapy, hysterectomy, clinical infertility, follow‐up calendar period and socioeconomic factors, by pregnancy number and pregnancy type. Finally, we investigated potential implications of underlying fecundity by analyzing the effect of one additional third pregnancy among women who had 2 years or less between their first and second pregnancy, representing high ability to conceive (ie, high fecundity). All analyses were performed in SAS© version 9.4 using PROC GENMOD.
3. RESULTS
Our main cohort included 2 457 932 women who were followed for 74 885 334 person‐years (in average 30.5 years). The women experienced 4 189 565 pregnancies of which 702 302 were induced abortions and 3 487 263 were childbirths. During the 50‐year follow‐up, 8566 women developed ovarian cancer (see Table 1 for full demographic description of the cohort).
Using nulligravid women as reference, pregnancy was associated with 21% (95% CI, 14%‐28%), 42% (95% CI, 37%‐47%), 49% (95% CI, 44%‐53%) and 51% (95% CI, 45%‐55%) risk reduction for respectively one, two, three and four or more pregnancies (Table 2). Comparing with one pregnancy less, each pregnancy was associated with a risk reduction of 21% (95% CI, 14%‐28%), 26% (95% CI, 21%‐31%), 12% (95% CI, 7%‐17%) and 3% (95% CI, −5%‐11%) for the first, second, third and fourth pregnancy, respectively (P < .001 for heterogeneity). A similar pattern was found when only considering childbirths (see Table S2).
TABLE 2.
Ovarian cancer risk by number of pregnancies, with no pregnancies and previous pregnancy as reference, respectively
| Pregnancy number | Adjusted relative risk (95% CI) with nulligravid as reference a | Adjusted relative risk (95% CI) with previous pregnancy as reference a , b |
|---|---|---|
| Nulligravid | 1 (ref.) | — |
| First | 0.79 (0.72‐0.86) | 0.79 (0.72‐0.86) |
| Second | 0.58 (0.53‐0.63) | 0.74 (0.69‐0.79) |
| Third | 0.51 (0.47‐0.56) | 0.88 (0.83‐0.93) |
| Fourth or more | 0.49 (0.45‐0.55) | 0.97 (0.89‐1.05) |
Adjusted for age, calendar period, tubal ligation/resection, endometriosis, marital status, educational attainment, urbanicity and interaction between calendar period and age, educational attainment, marital status and urbanicity.
P < .001 for heterogeneity.
Investigating the effect of pregnancy type on ovarian cancer risk (Table 3), including information on spontaneous abortions from 1977, we found no differences between the effects of induced abortion, spontaneous abortion and childbirth, for the first (P = .51), second (P = .51), third (P = .17), fourth pregnancy (P = .86) or overall (P = .40). In addition, we investigated effects of pregnancy type on the most common ovarian cancer subtypes and found no overall differences between abortions and childbirths (Table 4). Nevertheless, our subtype specific results indicate that for clear‐cell ovarian cancer a fourth pregnancy might also be protective. Investigating effects of specific gestational duration of induced abortions and childbirths (see Table S3), we found little indication of difference by gestational duration stratified by pregnancy number and type.
TABLE 3.
Ovarian cancer risk by pregnancy number and type, compared to one pregnancy less, including information on spontaneous abortions
| Pregnancy number and type a | Adjusted relative risk (95% CI) by pregnancy type, compared to one pregnancy less b | Test for difference |
|---|---|---|
| First pregnancy | ||
| Induced abortion | 0.85 (0.73‐1.00) | P = .51 |
| Spontaneous abortion | 0.84 (0.69‐1.02) | |
| Childbirth | 0.79 (0.72‐0.86) | |
| Second pregnancy | ||
| Induced abortion | 0.74 (0.64‐0.86) | P = .51 |
| Spontaneous abortion | 0.86 (0.72‐1.02) | |
| Childbirth | 0.74 (0.69‐0.79) | |
| Third pregnancy | ||
| Induced abortion | 0.95 (0.86‐1.04) | P = .17 |
| Spontaneous abortion | 0.88 (0.72‐1.08) | |
| Childbirth | 0.85 (0.80‐0.91) | |
| Fourth pregnancy | ||
| Induced abortion | 1.01 (0.89‐1.14) | P = .86 |
| Spontaneous abortion | 0.91 (0.69‐1.20) | |
| Childbirth | 0.95 (0.86‐1.05) | |
| Test for overall difference c | P = .40 |
Median duration of registered spontaneous abortions was previously estimated to 11 gestational weeks, 30 while median duration of induced abortions and childbirths was eight and 40 gestational weeks, respectively.
Adjusted relative risk compared to one pregnancy less, regardless of pregnancy type. Adjusted for age, calendar period, tubal ligation/resection, endometriosis, marital status, educational attainment, urbanicity and interaction between calendar period and age, educational attainment, marital status and urbanicity. Information on spontaneous abortions was available for 42 years from 1 January 1977 to 31 December 2018, with 240 303 spontaneous abortions registered in this time period.
Test for overall difference between spontaneous abortions, induced abortions and childbirths, based on follow‐up from 1 January 1977 to 31 December 2018. Tests for differences between induced abortions and childbirths based on follow‐up from 2 April 1968, are described in see Table S13.
TABLE 4.
Ovarian cancer risk by pregnancy number and type, compared to one pregnancy less, by subtype of ovarian cancer based on follow‐up from 1 January 1978
| Pregnancy number and type | Clear‐cell | Endometrioid | Mucinous | Serous |
|---|---|---|---|---|
| (Nevents = 471) | (Nevents = 1186) | (Nevents = 1003) | (Nevents = 5178) | |
| Adj. relative risk (95% CI) a | Adj. relative risk (95% CI) a | Adj. relative risk (95% CI) a | Adj. relative risk (95% CI) a | |
| First pregnancy | ||||
| Abortion, any | 0.77 (0.44‐1.32) | 0.71 (0.51‐1.00) | 0.70 (0.49‐1.01) | 1.06 (0.90‐1.24) |
| Childbirth | 0.77 (0.55‐1.08) | 0.63 (0.50‐0.79) | 0.86 (0.67‐1.12) | 0.86 (0.77‐0.98) |
| Second pregnancy | ||||
| Abortion, any | 0.93 (0.57‐1.50) | 0.72 (0.51‐1.02) | 0.89 (0.63‐1.25) | 0.83 (0.71‐0.97) |
| Childbirth | 0.58 (0.44‐0.77) | 0.67 (0.55‐0.81) | 0.75 (0.61‐0.93) | 0.81 (0.74‐0.89) |
| Third pregnancy | ||||
| Abortion, any | 0.93 (0.60‐1.43) | 0.78 (0.58‐1.05) | 0.87 (0.65‐1.17) | 0.98 (0.87‐1.11) |
| Childbirth | 0.73 (0.54‐1.00) | 0.85 (0.69‐1.04) | 0.84 (0.68‐1.03) | 0.82 (0.75‐0.90) |
| Fourth pregnancy | ||||
| Abortion, any | 0.55 (0.29‐1.08) | 0.97 (0.68‐1.38) | 1.10 (0.78‐1.55) | 0.98 (0.85‐1.14) |
| Childbirth | 0.55 (0.32‐0.95) | 0.89 (0.66‐1.21) | 1.05 (0.78‐1.40) | 0.99 (0.87‐1.12) |
| Test for overall difference b | P = .65 | P = .95 | P = .83 | P = .08 |
Adjusted relative risk compared to one pregnancy less, regardless of pregnancy type. Adjusted for age, calendar period, tubal ligation/resection, endometriosis, marital status, educational attainment, urbanicity and interaction between calendar period and age, educational attainment, marital status and urbanicity. Information on ovarian cancer subtypes was available for 41 years from 1 January 1978 to 31 December 2018.
Test for overall difference between any abortion and childbirth. More specifically, the test is comparing the model as presented in Table 4 with an alternative model where there are identical effects of abortion and childbirth within each parity.
To explore potential effects of timing of pregnancies, we investigated differences in ovarian cancer risk by time span between the first pregnancy and the latest pregnancy, among women with two, three or four pregnancies (see Table S4). We found no difference in ovarian cancer risk by increasing time span between the pregnancies among women with two (P trend = .67), three (P trend = .57) or four pregnancies (P trend = .62).
Examining the relative risk of ovarian cancer by time since latest pregnancy, we found the protective effects associated with pregnancy attenuated over time (Figure 2). However, even 40 years or more after their latest pregnancy, women with two or three pregnancies had a reduced relative risk of ovarian cancer of 30% (95% CI, 20%‐39%) and 43% (95% CI, 31%‐53%), respectively, compared to nulligravid women.
FIGURE 2.
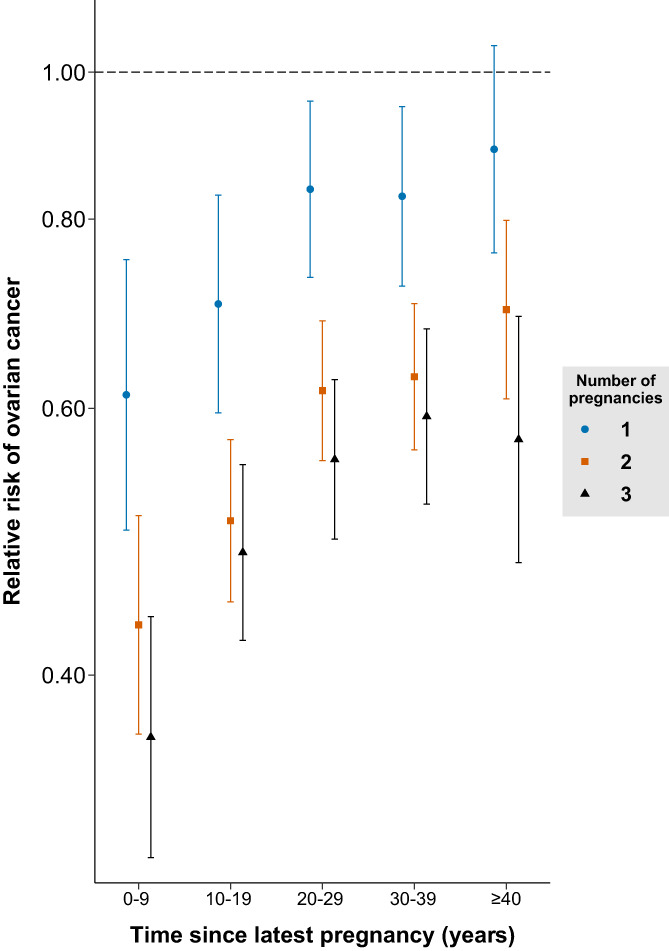
Relative risk of ovarian cancer by time since latest pregnancy compared to nulligravid women, stratified by number of pregnancies. Adjusted for age, calendar period, tubal ligation/resection, endometriosis, marital status, educational attainment, urbanicity and interaction between calendar period and age, educational attainment, marital status and urbanicity [Color figure can be viewed at wileyonlinelibrary.com]
In detailed sensitivity analyses we explored individual and combined effects of continuous gestational duration, maternal age at pregnancy and time since pregnancy (see Tables S5 to S7). This alternative modeling approach allowed us to investigate effects of continuous pregnancy duration on risk of ovarian cancer risk overall and ovarian cancer subtypes. Beyond the effect of becoming pregnant, three additional months of pregnancy were only associated with 5% (95% CI, 3%‐7%) reduced relative risk of ovarian cancer, with no difference by pregnancy number (P = .88), pregnancy type (P = .89) or ovarian cancer subtype (P = .49). For maternal age at pregnancy no substantial effect was found. On the contrary, for time since pregnancy, we found attenuation of pregnancy effects by increased time since pregnancy (−5% [95% CI, −4% to −7%] reduced relative risk per 10 years increased time since pregnancy).
In additional sensitivity analyses, we looked into the effects of number of induced abortions (see Table S8), oral contraceptive use (see Table S9), hormonal replacement therapy (see Table S10), clinical infertility (see Table S11), hysterectomy (see Table S12), follow‐up calendar period (see Table S13) and different socioeconomic factors (see Table S14) and found no substantial confounding effect of any of these factors. Furthermore, supplementary analyses of five or more pregnancies and exclusively age‐adjusted analyses provided no substantially different estimates compared to our main results (Tables S15 to S17). Finally, we found no indication of a fecundity effect, as a pregnancy was associated with reduced ovarian cancer risk even among women with a similar fecundity level (see Table S18).
4. DISCUSSION
In a nationwide cohort study of 2.5 million women with follow‐up for ovarian cancer over 50 years, we found pregnancy to be associated with a marked reduction in ovarian cancer risk. The protective association was, however, primarily driven by the first three pregnancies, with similar effects by different pregnancy types and durations. Our study thereby suggests that predominately factors associated with early pregnancy and not the total duration of pregnancies, reduce a woman's ovarian cancer risk.
Our study is to our knowledge the longest cohort study ever conducted on reproductive factors and ovarian cancer risk. In addition, with a total of 8566 ovarian cancer events, our cohort study has approximately 10 times more events than a similar EPIC cohort study, which included 786 ovarian cancer events in their analysis of induced abortion and ovarian cancer risk. 15 Using prospectively collected nationwide information from the Danish national registries, we had the advantage of a complete population, long‐term follow‐up, no recall bias and little, if any, relevant misclassification. Furthermore, the precise information on timing and gestational duration of individual pregnancies allowed us to consider potential effects related to pregnancy order, pregnancy timing, maternal age at pregnancy and time since pregnancy, alone and in combination.
The cohort was, however, limited to 24 years of follow‐up for oral contraceptive use. Nevertheless, using individual‐level information for all women born in 1978 who had complete follow‐up for oral contraception use from 16 to 40 years of age, we were able to finely estimate cumulative oral contraception use by number of induced abortions and childbirths, and maternal age at pregnancies, and impute the use for all women born from 1950. Given the stable uptake of oral contraceptives among women in Denmark from the 1960s, 16 we have little reason to believe the pattern of oral contraceptive use markedly changed over time, and our analysis substantiate no important confounding effect of oral contraceptive use by pregnancy type on ovarian cancer risk. If anything, a previous study suggests less stringent adherence to oral contraceptives among women with repeated induced abortions, 17 which would bias our results toward an even more similar effect of induced abortions and childbirths, as longer duration of oral contraceptive use is associated with a decreased life‐time risk of ovarian cancer. 3 Support for such a scenario is also indicated by our sensitivity analysis in which the small group of women with two or more induced abortions was excluded and the effect of induced abortion and childbirth was nearly identical. In this analysis, were all estimates for induced abortions pertain to a single exposure to an induced abortion, we found significantly reduced risk of ovarian cancer relating to induced abortion, regardless of whether it occurred as a woman's first, second or third pregnancy. Finally, a recent large pooled case‐control analysis by Lee et al similarly found a strongly reduced ovarian cancer risk associated with exposure to incomplete pregnancies even when adjusting for oral contraceptive use. 7 Nevertheless, our study suggested stronger protective effects of complete than incomplete pregnancies. However, the study did not investigate the role of pregnancy order or specific gestational duration, and there was no differentiation between spontaneous and induced abortions.
We found no meaningful indications of a protective effect on ovarian cancer risk of additional pregnancies after the third pregnancy. However, our cohort had no information on breastfeeding, which has been associated with a reduced risk of ovarian cancer. 18 This aspect of reproductive life could bias our results as women with four or more children might have different breastfeeding patterns compared to women with fewer children. 19 The observed minimal effect of the fourth and additional pregnancies could also be due to the lower duration of oral contraceptive use among women with many pregnancies. Nevertheless, similar minute effects of more than three pregnancies were indicated by Lee et al, who adjusted for oral contraceptive use and breastfeeding. 7 Furthermore, the finding is consistent with a study of grand multiparous women that did not find any additional childbirths beyond the fifth childbirth to be associated with any reduction in ovarian cancer risk. 20 Taken together, these results suggest an important role of pregnancy order, whereby the reduction in ovarian cancer risk is primarily obtained with the first three pregnancies.
Three large observational studies have previously looked into differential effects of childbirths on ovarian cancer histological subtypes. 7 , 21 , 22 All three studies found protective effects of childbirth on the risk of each major subtype of ovarian cancer subtype (ie, serous, mucinous, endometrioid and clear‐cell ovarian cancer), which also was found in our study. Nevertheless, the previous studies found heterogeneity of childbirth effects by ovarian cancer subtype, with results suggesting more protection from endometrioid and clear‐cell ovarian cancer with exposure to childbirth. This tendency was also seen in our analysis of risk of ovarian cancer subtypes, but with regards to the difference between abortions and childbirths, no statistically significant difference for each ovarian cancer subtype was found. Furthermore, in our alternative modeling approach were we investigated effects of continuous pregnancy duration (by three additional months of pregnancy) on ovarian cancer risk, we did not find statistical different effects by ovarian cancer subtype. Thus, even larger studies and meta‐analyses are needed to look into potential differential effects of pregnancy duration on the risk of ovarian cancer subtypes.
The small impact observed of gestational duration is underlined by the relatively strong protective associations with both induced and spontaneous abortions which have median durations of eight and 11 gestational weeks, respectively, compared to the median 40 weeks of pregnancies resulting in childbirth. Furthermore, the discovery of a protective association between short‐term pregnancies and ovarian cancer risk mirrors recent findings on endometrial cancer. 23 , 24 Together, these findings support that short‐term exposure to a pregnancy can result in long‐term reduced risk of malignancy. However, in contrast to endometrial cancer, the protective association with ovarian cancer is primarily driven by the first three pregnancies.
Three main hypotheses have tried to explain the protective role of pregnancy on ovarian cancer risk; “the incessant ovulation hypothesis” stressing the number of ovarian cycles suppressed during each pregnancy, 25 “the gonadotropin hypothesis” stressing suppressed effects of pituitary gonadotropins during pregnancy, 26 and “the cell clearance hypothesis” stressing the removal of accumulated premalignant cells during a woman's latest pregnancy. 27 Our findings go strongly against the incessant ovulation hypothesis and the gonadotropins hypothesis, as we only find minor effect of pregnancy duration (ie, number of suppressed ovarian cycles), either across or within pregnancy type, and as pregnancies beyond the third seem to have little effect. Furthermore, the incessant ovulation hypothesis has fallen out of favor since most ovarian cancers are thought to have tubal origin. 6 The competing theory, the cell clearance hypothesis, is supported by two of our findings. First, we find that the initial pregnancies are associated with the strongest reduction in risk, while later pregnancies have little effect, in line with fewer accumulated premalignant cells at the beginning of each additional pregnancy. Second, we find attenuation of the protective effect of pregnancy by increasing time since pregnancy, congruent with a renewed accumulation of premalignant cells following pregnancy. However, we found no meaningful protective effect of higher maternal age at pregnancy (ie, a longer extent of potential clearance of premalignant cells), when adjusted for time since pregnancy. Likewise, comparing time span between pregnancies among women with an identical number of pregnancies, we found no trend whereby an increasing time span between pregnancies was associated with lower risk of ovarian cancer. Thus, none of the main theories adequately fits the epidemiological findings.
The risk reduction associated with pregnancy could instead be a result of underlying fecundity, whereby a pregnancy serves as a marker for a healthy reproductive system, as some nulliparous women are nulliparous due to reduced fecundity. Nevertheless, we found a protective effect of even spontaneous abortions, which are not attributed to a healthy reproductive system. Furthermore, an additional third pregnancy had a markedly protective effect even among women with a comparable high level of fecundity.
To accommodate these findings we introduce a new hypothesis, termed “the dormant cell hypothesis.” Given the similar effects of induced abortions, spontaneous abortions and childbirths, we propose that primary protective changes occur early in pregnancy. Unlike the clearance hypothesis, we suggest that these changes do not eliminate premalignant cells, but modify the cells of the distal fallopian tube epithelium, where most ovarian cancers are thought to arise, 28 into a dormant, less active, state. The dormant state is subsequently reinforced by additional pregnancies, with saturation following the first two to three pregnancies and little added benefit hereafter. Following a woman's last pregnancy, the dormant state is gradually lost over decades, in accordance with attenuation of risk reduction by increased time since pregnancy, reestablishing potential for malignant transformation of epithelial cells. Similarly, the notion of a dormant state has recently been used to describe ovarian cancer precursor lesions with low degree of proliferation, 29 despite cancerous genetic alterations. The many hormonal changes during pregnancy, both systemically and locally within the reproductive organs, could results in such changes of the epithelium. The specific mechanism behind a lower degree of proliferation in the dormant state is unknown, but epigenetic alteration or other intracellular changes within the fallopian tube epithelium could explain this phenomenon. Our hypothesis is therefore concordant with recent pathological findings on the origin of ovarian cancer, which also suggests that a “dormant” epithelial cell state protects against tumor development. Nevertheless, more research into specific biological mechanisms which can explain our epidemiological findings are needed.
We believe our results add to the understanding of the relationship between pregnancy and ovarian cancer risk and provide evidence for protective effects of early pregnancy factors, unrelated to pregnancy duration. Our findings furthermore indicate that a transient biological factor in early pregnancy reduces ovarian cancer risk.
AUTHOR CONTRIBUTIONS
Anders Husby conceived the study, contributed to the study design, classified register data and performed statistical analysis, interpreted the study results, drafted the article and is the guarantor of the study. Jan Wohlfahrt contributed to the study design, planned statistical analysis, oversaw the conduct of the statistical analysis, interpreted the study results and revised the article. Mads Melbye contributed to the study design, interpreted the study results and revised the article. All authors had access to all of the data and take full responsibility for the integrity of the data, the accuracy of the data analysis and the finished article. The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
Our study was supported by The Danish Cancer Society (grant No R167‐A10791 to AH and MM). The funder had no role in the design of study, conduct of the study or the decision to submit the article for publication. All researchers acted independently from the study sponsor in all aspects of the study.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
As the study was based on de‐identified information form the Danish national registers and as study participants are never contacted, consent from the Danish research bioethics committees are not required. The study's use of register data was covered by the approval from the Danish Data Protection Agency for register based studies conducted by Statens Serum Institut (approval No 2015‐57‐0102).
Supporting information
Appendix S1 Supporting Information.
Husby A, Wohlfahrt J, Melbye M. Pregnancy duration and ovarian cancer risk: A 50‐year nationwide cohort study. Int J Cancer. 2022;151(10):1717‐1725. doi: 10.1002/ijc.34192
Funding information The Danish Cancer Society, Grant/Award Number: R167‐A10791
DATA AVAILABILITY STATEMENT
The data used in the study can be obtained by submitting a research protocol to the Danish Data Protection Agency (Datatilsynet) and, if permission is granted, by applying to the Ministry of Health's Research Service (Forskerservice) and Statistics Denmark (Danmarks Statistik) for access to the data. Further information is available from the corresponding author upon request.
REFERENCES
- 1. Grossman DC, Curry SJ, Owens DK, et al. Screening for ovarian cancer US preventive services task force recommendation statement. JAMA. 2018;319(6):588‐594. doi: 10.1001/jama.2017.21926 [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284‐296. doi: 10.3322/caac.21456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collaborative Group on Epidemiological Studies of Ovarian Cancer . Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. Lancet. 2008;371(9609):303‐314. doi: 10.1016/S0140-6736(08)60167-1 [DOI] [PubMed] [Google Scholar]
- 4. Iversen L, Fielding S, Lidegaard Ø, Mørch LS, Skovlund CW, Hannaford PC. Association between contemporary hormonal contraception and ovarian cancer in women of reproductive age in Denmark: prospective, nationwide cohort study. BMJ. 2018;362:k3609. doi: 10.1136/BMJ.K3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schrijver LH, Antoniou AC, Olsson H, et al. Oral contraceptive use and ovarian cancer risk for BRCA1/2 mutation carriers: an international cohort study. Am J Obstet Gynecol. 2021;22:e1‐e17. doi: 10.1016/j.ajog.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Troisi R, Bjørge T, Gissler M, et al. The role of pregnancy, perinatal factors and hormones in maternal cancer risk: a review of the evidence. J Intern Med. 2018;283(5):430‐445. doi: 10.1111/joim.12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee AW, Rosenzweig S, Wiensch A, et al. Expanding our understanding of ovarian cancer risk: the role of incomplete pregnancies. J Natl Cancer Inst. 2020;7:301‐308. doi: 10.1093/jnci/djaa099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pedersen CB, Gøtzsche H, Møller JO, Mortensen PB. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441‐449. [PubMed] [Google Scholar]
- 9. Husby A, Wohlfahrt J, Øyen N, Melbye M. Pregnancy duration and breast cancer risk. Nat Commun. 2018;9(1):4255. doi: 10.1038/s41467-018-06748-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449‐490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pottegård A, Schmidt SAJ, Wallach‐Kildemoes H, Sørensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2016;25(3):dyw213. doi: 10.1093/ije/dyw213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7_suppl):91‐94. doi: 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- 13. Storm HH, Michelsen EV, Clemmensen IH, Pihl J. The Danish Cancer Registry—history, content, quality and use. Dan Med Bull. 1997;44(5):535‐539. [PubMed] [Google Scholar]
- 14. Gottschau M, Mellemkjaer L, Hannibal CG, Kjaer SK. Ovarian and tubal cancer in Denmark: an update on incidence and survival. Acta Obstet Gynecol Scand. 2016;95(10):1181‐1189. doi: 10.1111/aogs.12948 [DOI] [PubMed] [Google Scholar]
- 15. Braem MGMM, Onland‐Moret NC, Schouten LJ, et al. Multiple miscarriages are associated with the risk of ovarian cancer: results from the European prospective investigation into cancer and nutrition. PLoS One. 2012;7(5):e37141. doi: 10.1371/journal.pone.0037141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lidegaard Ø. Slides. Presentation. http://www.lidegaard.dk/Slides/OCepidem/PP07-11-20en.pdf. Accessed October 23, 2018.
- 17. Heikinheimo O, Gissler M, Suhonen S. Age, parity, history of abortion and contraceptive choices affect the risk of repeat abortion. Contraception. 2008;78(2):149‐154. doi: 10.1016/j.contraception.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 18. Babic A, Sasamoto N, Rosner BA, et al. Association between breastfeeding and ovarian cancer risk. JAMA Oncol. 2020;6(6):e200421. doi: 10.1001/jamaoncol.2020.0421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Der G, Batty GD, Deary IJ. Effect of breast feeding on intelligence in children: prospective study, sibling pairs analysis, and meta‐analysis. BMJ. 2006;333(7575):945. doi: 10.1136/BMJ.38978.699583.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinkula M, Pukkala E, Kyyrönen P, Kauppila A. Incidence of ovarian cancer of grand multiparous women—a population‐based study in Finland. Gynecol Oncol. 2006;103(1):207‐211. doi: 10.1016/j.ygyno.2006.02.025 [DOI] [PubMed] [Google Scholar]
- 21. Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888‐2898. doi: 10.1200/JCO.2016.66.8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gaitskell K, Green J, Pirie K, et al. Histological subtypes of ovarian cancer associated with parity and breastfeeding in the prospective million women study. Int J Cancer. 2018;142(2):281‐289. doi: 10.1002/ijc.31063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jordan SJ, Na R, Weiderpass E, et al. Pregnancy outcomes and risk of endometrial cancer: a pooled analysis of individual participant data in the epidemiology of endometrial cancer consortium. Int J Cancer. 2021;148(9):2068‐2078. doi: 10.1002/ijc.33360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Husby A, Wohlfahrt J, Melbye M. Pregnancy duration and endometrial cancer risk: Nationwide cohort study. BMJ. 2019;366:l4693. doi: 10.1136/bmj.l4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fathalla MF. Incessant ovulation—a factor in ovarian neoplasia? Lancet (London, England). 1971;2(7716):163. [DOI] [PubMed] [Google Scholar]
- 26. Lukanova A, Kaaks R. Endogenous hormones and ovarian cancer: epidemiology and current hypotheses. Cancer Epidemiol Biomarkers Prev. 2005;14(1):98‐107. [PubMed] [Google Scholar]
- 27. Adami H‐O, Lambe M, Persson I, et al. Parity, age at first childbirth, and risk of ovarian cancer. Lancet. 1994;344(8932):1250‐1254. doi: 10.1016/S0140-6736(94)90749-8 [DOI] [PubMed] [Google Scholar]
- 28. Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433‐443. doi: 10.1097/PAS.0b013e3181cf3d79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shih IM, Wang Y, Wang TL. The origin of ovarian cancer species and precancerous landscape. Am J Pathol. 2021;191(1):26‐39. doi: 10.1016/j.ajpath.2020.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lohse SR, Farkas DK, Lohse N, et al. Validation of spontaneous abortion diagnoses in the Danish National Registry of Patients. Clin Epidemiol. 2010;2:247‐250. doi: 10.2147/CLEP.S13815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data used in the study can be obtained by submitting a research protocol to the Danish Data Protection Agency (Datatilsynet) and, if permission is granted, by applying to the Ministry of Health's Research Service (Forskerservice) and Statistics Denmark (Danmarks Statistik) for access to the data. Further information is available from the corresponding author upon request.


