Abstract
RNA aptamers can be genetically encoded in cells to probe and manipulate cellular function. The usefulness of aptamers in mammalian cells is limited by low accumulation and degradation by ribonucleases. Expression of circular RNA aptamers using the Tornado expression system achieves high stability and an abundance of intracellular RNA aptamers. With this method, RNA aptamers with otherwise minimal activity become potent inhibitors. Here, we describe protocols to characterize circular RNA aptamers expressed using Tornado. Included are methods to assess stability, abundance, subcellular localization, and target binding of circular RNA aptamers.
Keywords: circular RNA, expression system, protein binding, RNA aptamers
1. INTRODUCTION
RNA aptamers are a promising class of RNAs that form complex three-dimensional structures that tightly bind a target of molecular or protein of interest. RNA aptamers can bind and interact with a range of targets that includes small molecules [1, 2], metabolites [3, 4], and proteins [5–7].
While similar in function to antibodies, RNA aptamers are easier and less time-consuming to develop. A process called systematic enrichment of ligands by enrichment exponentially [8, 9] (SELEX) is fairly straightforward and does not required specialized reagents or equipment. Aptamer sequences with high binding affinity for proteins can be isolated from starting pools with as many as 1015 different RNA sequences. Much like antibodies, RNA aptamers not only bind to their protein targets, but can also block the protein’s function and inhibit their activity [10–12]. Unlike antibodies, RNA aptamers can be readily genetically encoded which allows them to be expressed in cells and therefore used as novel tool compounds for regulating cellular functions. Additionally, aptamers could potentially be used as an expressed RNA in gene therapy, thus forming the basis of potentially novel therapeutics. Thus, the promise of RNA aptamers is of a genetically encoded biomolecule that can be rapidly developed to inhibit protein targets.
With this in mind, the primary issue with RNA aptamers is instability. RNAs are inherently unstable especially since they are susceptible to decay by exoribonucleases, which degrade RNAs from their 5’ or 3’ ends. To address the instability problem, there has been extensive research into RNA modifications [13–15] that block ribonuclease activity and prevent degradation. This includes phosphate backbone alterations and modifications to the ribose of each nucleotide. One problem with this solution is that SELEX does not tolerate many of these modifications during transcription or reverse transcription. If these modifications are added after SELEX, they can fundamentally change the folding and function of an aptamer, which may require extensive optimization. Additionally, these modifications are not encoded during transcription in cells, and therefore modified aptamers need to be chemically synthesized rather than genetically encoded.
A potential solution is RNA circularization. Circular RNAs are the same as linear RNA but with the 5’ and 3’ ends covalently attached through a phosphodiester bond. The result of lacking 5’ and 3’ ends is a highly stabilized RNA because it is impossible for exoribonucleases to degrade circular RNAs. While linear aptamers have short half-lives no more than a few hours [16], circular aptamers are very long lived [16], sometimes remaining in cells for multiple weeks [17]. Furthermore, circular aptamers are physically constrained, which can improve folding and binding to the target [18]. This is especially true for aptamers wherein their 5’ and 3’ ends form a helix or are in close proximity.
We have recently described a highly efficient approach to genetically encoding circular RNA aptamers. There are various methods to transcribe circular RNAs in cells, most of which hijack either the tRNA [16, 19] or mRNA [20] splicing pathways. None of these methods generate intracellular levels of circular aptamers beyond a few hundred nM [18]. However, the Tornado (Twister-optimized RNA for durable overexpression) expression system uses rapidly cleaving ribozymes to efficiently produce an ideal substrate for an endogenous RNA ligase [18]. Transcription of circular RNA aptamers using Tornado leads to 200 times more of the aptamer than if it were linearly expressed. Depending on the RNA sequence, Tornado-expressed aptamers are concentrated enough (~10 μM) [18] to stoichiometrically bind all of the target protein molecules.
Here, we describe protocols for generating and validating the expression of circular RNA aptamers and for testing their binding activity. These protocols benefit from tagging the RNAs with Broccoli, a fluorogenic RNA that activates the fluorescence of its cognate dye, DFHBI [21]. Alongside the following protocols, a cellular assay that specifically tests target inhibition or aptamer efficacy can be used to fully characterize the behavior of a circular RNA aptamer.
2. MATERIALS
-
Cloning reagents and equipment
pAV parent vectors for circular aptamer and circular Broccoli-aptamer RNAs (Addgene, cat# 124362)
NotI (New England Biolabs, cat# R0138)
SacII (New England Biolabs, cat# R0145)
FastDigest KflI (Thermo Fisher, cat# FD2164)
-
Standard cloning reagents, for example
Taq DNA polymerase (New England Biolabs, cat# E5000)
Deoxynucleotide mix (dNTPs) (Sigma Aldrich, cat# D7295)
Quick Ligase kit (New England Biolabs, cat# M200)
Agarose (Sigma Aldrich, cat# D7295)
-
50x TAE buffer
2 M tris base (Sigma Aldrich, cat# 648310)
1 M glacial acetic acid (Sigma Aldrich, cat# A6283)
0.05 M EDTA, pH 8.0 (Sigma Aldrich, cat# EDS-100G)
PCR thermocycler
agarose gel casting and running apparatus
LB agar (Thermo Fisher, cat# 22700025)
LB broth base (Thermo Fisher, cat# 12780052)
Carbenicillin (Sigma Aldrich, cat# C1389)
37 °C Bacterial incubator
-
Circular RNA aptamer expression
HEK293T cells (ATCC, cat# CRL-3216)
Dulbecco’s Modified Eagle Medium (DMEM) (Thermo Fisher, cat# 11054001)
Penicillin/Streptomycin (Thermo Fisher, cat# 15140122)
Glutamax (Thermo Fisher, cat# 35050061)
Fetal Bovine Serum (FBS) (Thermo Fisher, cat# 26140)
TrypLE (Thermo Fisher, cat# 12563011)
Phosphate Buffered Saline (PBS) (Thermo Fisher, cat# 70011044)
FuGENE HD (Promega, cat# E2311)
-
Trizol LS (Thermo Fisher, cat# 10296010)
Trizol LS contains phenol and chloroform, which present health and safety hazards. Please refer to the Safety Data Sheet for appropriate use.
Isopropanol (Sigma Aldrich, cat# I9516)
Biosafety cabinet
Tissue culture incubator
Actinomycin D (Sigma Aldrich, cat# A9415)
-
Denaturing PAGE gel
Low Range ssRNA ladder (New England Biolabs, cat# N0364S)
Novex TBE-Urea sample buffer (Thermo Fisher, cat# LC6876)
Novex TBE-Urea gels (Thermo Fisher, cat# EC6875)
-
10x TBE
1 M tris base (Sigma Aldrich, cat# 648310)
1 M boric acid (Sigma Aldrich, cat# B6768)
0.02 M EDTA, pH 8.0 (Sigma Aldrich, cat# EDS-100G)
PAGE gel running apparatus
PCR thermocycler or 80 °C heat block
-
Broccoli staining solution
40 mM HEPES-K pH 7.4 (Sigma Aldrich, cat# 54457)
100 mM KCl (Sigma Aldrich, cat# P9541)
1 mM MgCl2 (Sigma Aldrich, cat# M8266)
10 μM DFHBI, DFHBI-1T or DFHBI-BI (Lucerna Technologies cat# 400, 410, or 600)
SYBR Gold Nucleic Acid Gel Stain (Invitrogen cat# S11494)
Fluorescence gel imager
-
Live cell circular RNA detection
Fluorescence microscope with EGFP filter set
FluoroBrite medium (Thermo Fisher, cat# A1896701)
Hoechst stain (bisbenzimide H 33342) (Sigma Aldrich, cat# B2261)
DFHBI or its derivatives, DFHBI-1T or BI (Lucerna Technologies, cat# 400, 410, or 600)
-
Linear and circular RNA preparation
Ampliscribe T7 Transcription Kit (Lucigen, cat#AS3107)
Fluor-coated TLC plate for UV shadowing
Gel Crushing tube (IST Engineering Inc., cat# 3388–100
DEPC-treated nuclease-free water (Sigma Aldrich, cat# 7732–18-5)
-
Extraction buffer
10mM HEPES pH 6.8
300 mM NaCl
1 mM EDTA
Costar Spin-X tube filters (Corning, cat# 8161)
Ethanol (Sigma Aldrich, cat# 1085430250)
GlycoBlue (Thermo Fisher, cat# AM9515)
RtcB Ligase (New England Biolabs, cat# M0458S)
RNA clean and concentrator kit (Zymo Research, cat# R1013)
-
Electrophoretic mobility shift assay
Yeast tRNA (Thermo Fisher, cat# AM7119)
Bovine Serum Albumin (BSA) (Sigma Aldrich, cat# A2153)
RNAse OUT (Thermo Fisher, cat# 10777019)
Purified protein target (Genscript or other)
Novex TBE minigels, 6% (Thermo Fisher, cat# EC6265BOX)
-
6x native PAGE buffer
30% glycerol
0.1% bromophenol blue
-
10x TBE
1 M tris base (Sigma Aldrich, cat# 648310)
1 M boric acid (Sigma Aldrich, cat# B6768)
0.02 M EDTA, pH 8.0 (Sigma Aldrich, cat# EDS-100G)
Hybond N+ membrane (GE Healthcare, cat# RPN203B)
UV cross-linker
Proteinase K (New England Biolabs, cat# P8107S)
Chemiluminescence Nucleic Acid Detection Kit (Thermo Fisher, cat# 89880)
-
5’ biotin-labeled DNA probe for the RNA aptamer sequence (Integrated DNA Technologies)
If the RNA sequences include Broccoli as designed in section 3.2.1, the following sequence is an appropriate probe: 5’-GCCCACACTCTACTCGACA-3’
3. METHODS
3.1. Cloning aptamer into Tornado
Each aptamer and aptamer variant are cloned into a pAV vector at the NotI and SacII site between the 5’ and 3’ ribozymes as previously described [18]. The resulting transcript is driven by a U6 promoter and ends at a U6 terminator. The transcript contains the aptamer flanked by each ribozyme, which autocatalytically cleave prior to circularization by RtcB.
For fluorescently-tagged circular RNA, aptamers can be cloned into a pAV vector encoding F30-Broccoli at the KflI site. This transcript contains F30 [22] (a three-way RNA junction that facilitates folding of the aptamers), Broccoli, and the aptamer of interest (Fig. 1A). The transcript is also driven by the U6 promoter and includes the two flanking ribozymes that lead to circularization. Broccoli-containing constructs are required for imaging aptamers in cells and recommended for experiments in sections 3.2.2, 3.3.1, and 3.3.2.
Figure 1:

Measuring stability and abundance of Broccoli-tagged circular RNA aptamers (A) A drawing is shown of a circular RNA aptamer construct that includes the Broccoli aptamer sequence to aid with RNA detection. The F30 3-way junction improves folding of each independent aptamer. The Tornado stem is the short sequence that remains after circularization. (B) A typical result of a circular RNA stability measurement is shown. In the DFHBI-1T-stained image (right) the circular RNA is abundant and resistant to treatment with actinomycin D. The linear aptamer is barely detected (see contrast-adjusted image of the dotted region) and disappears after actinomycin D treatment. (C) The expression levels of different circular RNA aptamer sequence variants are compared by denaturing PAGE. The signal from each band corresponds to the abundance of the circular RNA in cells (compare with total RNA bands in SYBR Gold image).
3.2. Validating expression of circular RNA aptamers
3.2.1. Transfection, imaging circular aptamer localization in cells
All cell cultures are maintained in a tissue culture incubator at 37 °C with 5% CO2 in growth media (DMEM supplemented with 1x Pen-Strep, 1x Glutamax, and 10% FBS).
Each plasmid is transfected into HEK293T cells using FuGENE HD according to the manufacturer’s protocol. A 12-well plate with 200,000 cells in each well is plated 24 h prior to transfection.
-
The cells are trypsinized 72 h after transfection and can be plated onto imaging dishes to test for aptamer localization. Aptamer localization will only work with constructs that include a fluorogenic RNA tag, such as Broccoli (see section 3.1)
Optional: Imaging circular aptamer localization in cells, as in Figure 2A. Glass bottom dishes should be coated with poly-D-lysine for several hours before washing with PBS, drying, and then plating trypsinized cells. 40,000 cells should be plated per cm2 of the imaging dish surface area in standard growth media. One day later, the growth media should be aspirated and replaced with FluoroBrite media containing 1 μg/mL Hoechst stain and 10 μM DFHBI, DFHBI-1T, or BI and incubated for 30 minutes. Broccoli can be imaged in live cells using a standard EGFP filter set (470 nm excitation and 525 nm emission). If using DHFBI or DFHBI-1T, the fluorescence signal is susceptible to reversible photobleaching, while the BI dye is more resistant to this effect. Remaining cells can be used in section 3.2.2.
Figure 2:
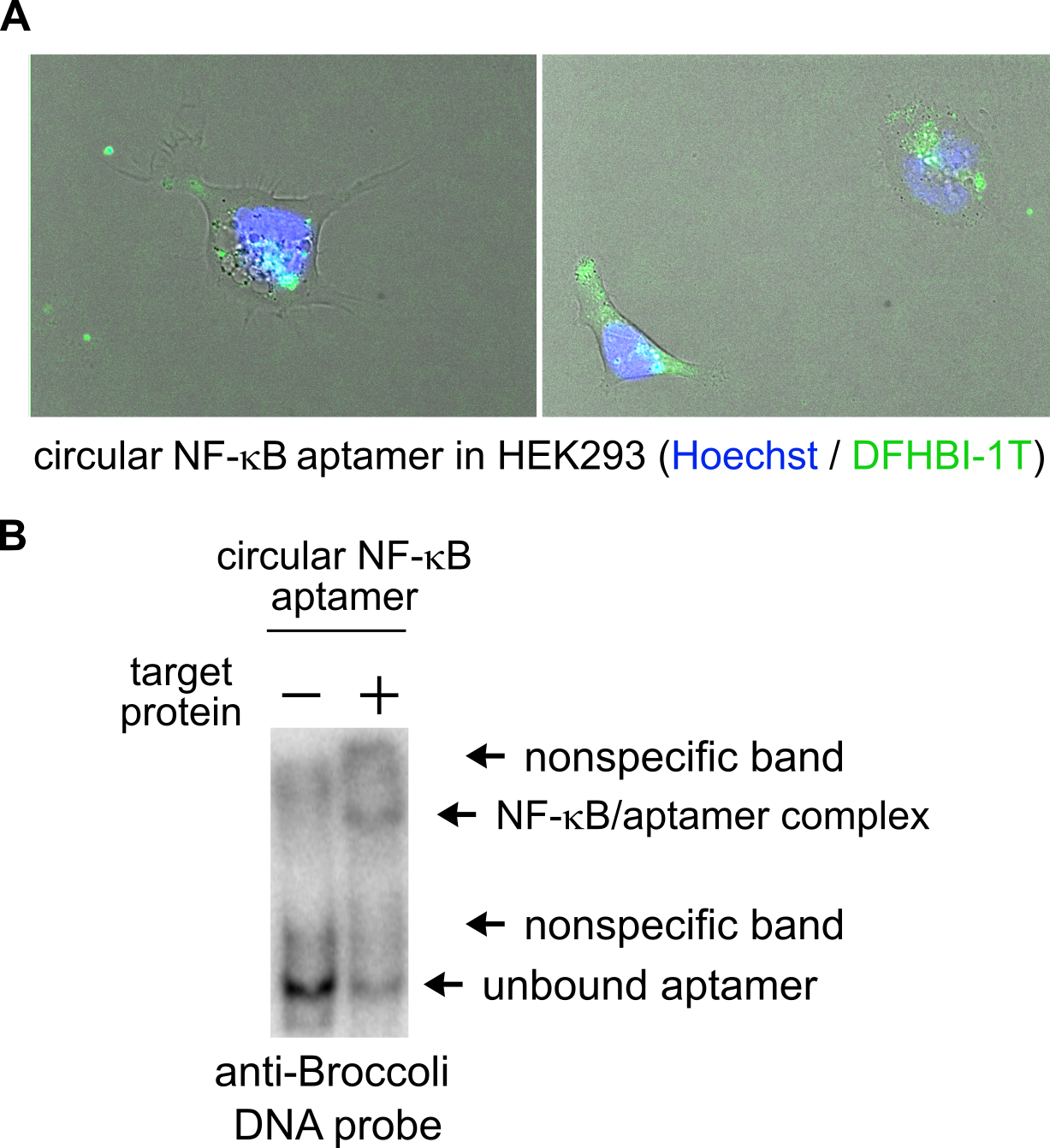
Detection of circular RNA aptamer’s subcellular localization and binding to target (A) Representative images show the subcellular localization of a circular NF-κB RNA aptamer when visualized by fluorescence microscopy. DFHBI-1T labeling (green) reveals localization of the aptamer and Hoechst staining (blue) indicates cell nuclei. (B) The circular NF-κB aptamer binds to its protein target by EMSA. When incubating the circular aptamer with its target, most of the Broccoli-containing RNA is shifted to a higher mobility. Higher mobility corresponds to complex formation and target binding by the aptamer.
3.2.2. Measuring abundance and stability of circular RNA aptamers
To ensure that Broccoli RNAs are circular, actinomycin D is used to test their stability. Actinomycin D blocks RNA transcription leading to loss of unstable RNAs over time, while stable RNAs remain as in Figure 1B.
Plate remaining cells from section 3.2.1 at a 1:4 density into two wells of a 12-well plate for each condition. Early the next day, add μg/mL actinomycin D pre-diluted 1:10 in PBS. Collect total cellular RNA 6 hours later in the next step.
To collect total cellular RNA, process each set of cells using Trizol LS according to the manufacturer’s instructions. The mixture of cells in Trizol LS can be stored for several weeks at −20 °C. (Note: Trizol LS is hazardous. Please refer to the Safety Data Sheet for proper handling and disposal instructions.)
Circular RNA aptamers can be detected by denaturing polyacrylamide gel electrophoresis (PAGE). Total cellular RNA from the previous step is first separated by using in PAGE minigels containing 6–8 M urea. 10% polyacrylamide gels are best suited to separating small RNAs.
Pre-run the PAGE minigels at 300 V for 30 minutes. Meanwhile, prepare 1–5 μg of total cellular RNA for each transfected plasmid with the denaturing PAGE buffer to a total volume of 10–20 μL. Heat each sample along with 500 ng of the ladder to 80 °C for 10 minutes. When the samples have cooled and the minigels are finished pre-runnning, clear the urea from each well of the minigel with a p1000 pipette. Then, load samples and run the minigel at 250 V for 30 minutes.
After the minigel is finished running, carefully transfer it to an inverted pipette tip box or plastic tray. Rinse the gel with deionized water for 5 minutes and repeat 2–3 times with fresh deionized water. Then incubate the gel with 10 mL of the Broccoli staining solution for 30 minutes. After incubation, this solution can be stored at 4 °C and reused at least 3 times. Image the gel using Alexa488 settings or equivalent (470 nm excitation and 530 nm emission). The band intensities in this image (as in Figure 2C) correspond to the relative abundances of Broccoli containing RNAs [22]. Circular RNA aptamers can only be visualized using this method when using constructs that include Broccoli (see section 3.1)
Next, the total cellular RNA bands can be visualized. After washing 3×5 minutes with deionized water, stain the minigel with 15 mL of 1x SYBR Gold for 10 minutes. Then image the minigel using SYBR Gold settings (302 nm excitation and 590 nm emission). Visualized bands correspond to total RNA as in Figure 2C).
3.3. Validate binding of circular RNA aptamer
3.3.1. Prepare circular and linear RNA aptamers
DNA templates for transcription can be derived by PCR of the plasmids generated in section 3.1. Choosing a construct that includes Broccoli can be useful when identifying the correct band in later steps. Use a forward primer that begins with the T7 promoter sequence (5’-gtataatacgactcactatag-3’), followed by a sequence that binds at the beginning of the first ribozyme. A reverse complementary primer that binds to the end of the second ribozyme is the reverse primer. Templates for transcribing the linear RNA must also include the T7 promoter sequence in the forward primer.
With DNA templates prepared, the circular and linear RNA aptamers are generated using the Ampliscribe T7 transcription kit. Prepare scaled up 60 μL reactions according the manufacturer’s protocol.
The transcription reactions should be purified on TBE-Urea minigels. Each reaction will need to be loaded across multiple wells of the gel. Follow the steps 4–7 in section 3.2.2 using the transcription reaction in place of total cellular RNA.
After running and imaging the purification gels for Broccoli and total RNA bands, excise the linear RNA bands and the pre-circularized RNA bands from the gel. Identifying the RNA bands is aided by UV shadowing with the gel on a layer of plastic wrap. The gel mobility rate of the pre-circularized RNA is predicable, as opposed to the mobility of circular RNAs.
Extract the RNA from the gel slices. Fires, transfer gel slices to gel breaker tubes in 1.7 mL microfuge tubes, then centrifuge at 12,000 g for 1 minute. Discard the gel breaker tube and add 300 μL Extraction buffer to the gel pieces. Flash freeze tube on dry ice for 5 minutes, then incubate while shaking at 37 °C for 1 hour. Repeat the flash freeze and incubation steps, then transfer the liquid portion of the gel mixture to a SpinX column using a cut 1 mL tip. Centrifuge at 17,000 g for 1 minute.
Transfer the extracted RNA eluate to a new microfuge tube and ethanol precipitate the RNA. First add 2 μL of GlycoBlue and mix, followed by 2.5 volumes of 4 °C ethanol. Incubate for 5 minutes, then centrifuge at 17,000 g for 30 minutes at 4 °C. You should see a blue pellet. Carefully discard the supernatant, add 1 mL of 70% ethanol and centrifuge 17,000 g for 10 minutes. Remove all of the supernatant without disturbing the pellet and allow to dry for 5 minutes. Resuspend the pellet in 20 μL of nuclease free water.
Next, prepare an RtcB reaction with the pre-circularized RNA. This reaction contains 20 pmoles of RNA and components supplied with this enzyme (2 μL of 10x RtcB ligase buffer, 2 μL of 10mM MnCl2, 2μL of 1 mM GTP, and 1μL of RtcB) to a total volume of 20μL. Incubate at 37 °C for 1 hour.
Lastly, clean up the circular RNA from the RtcB reaction using an RNA clean and concentrator kit according to the manufacturer’s protocol and elute RNA in 10 μL.
3.3.2. Prepare native gel for electrophoretic mobility shift assay (EMSA)
An EMSA is used to assess binding of linear and circular RNA aptamers prepared in section 3.3.1 to purified protein targets. The number of samples will depend on the number of aptamers and different concentrations of protein that will be tested, but be sure to include and sample without the protein target.
Pre-run a native TBE PAGE gel for 30 minutes at 100 V. Meanwhile, prepare each RNA diluted in 5 μL and heat to 80 °C for 5 minutes, then cool. Add components required for the aptamer’s specific binding conditions, but be sure to include 0.25 μL RNAse OUT, 0.25mg/mL BSA, 0.5 μg yeast tRNA, and 5% glycerol to a total volume of 15 μL per reaction. After adding the protein target, incubate at room temperature for 20 minutes and wash the wells of the gel with a syringe or p1000 pipette tip. Then load the gel and run at 100 V for 1 hour or until the bromophenol blue band reaches the bottom of the gel.
Next, transfer the gel to a Hybond N+ membrane in 0.5x TBE overnight night 100 mA at 4 °C. The next day, remove the blot and cross-link for one minute at a distance of about 10 cm. Incubate the blot with 50 μg/mL Proteinase K in 10 mL PBS while shaking for 1.5 hours. Discard liquid and incubate for 15 minutes with PBS while shaking, then repeat with fresh PBS.
Next, follow the steps for the Chemiluminescent Nucleic Acid detection kit to block, probe and expose the blot. Use the 5’ biotin-labeled DNA probe that is reverse complementary to the aptamer sequence. Chemiluminescent bands represent Broccoli-containing RNAs and RNA/protein complexes as in Figure 2B. The RNA-only band will be visible in the lane without the protein target and may also be visible in lanes with the target present. Slow mobility bands should only appear in lanes where the target protein is present representing aptamer/protein complexes. The brightness of these bands can be used to determine the percent of ligand that is bound to the target. By constructing a Scatchard plot, these values along with the known protein concentrations for each condition, can be used to calculate each RNA’s binding affinity.
4. NOTES
Aptamer circularization works best when the 5’ and 3’ ends of the aptamer form a helix. When the aptamer is inserted into the vector for circular RNA expression by this helix, circularization is less likely to affect aptamer folding and function.
Since the circular RNAs are transcribed by polymerase III, it is important to avoid termination sequences specific to this polymerase. The canonical polymerase III termination sequence is poly(T) sequence; however, termination also occurs at degenerate poly(T) as well [23]. When possible, avoid these sequences with mutations that eliminate premature termination and preserve aptamer function.
Circular RNAs run at unpredictable mobilities, even in denaturing PAGE gels. Depending on the percentage of polyacrylamide and the size of the circular RNA aptamer, it may have mobilities higher or lower than predicted based on nucleotide length.
ACKNOWLEDGEMENT
This work was supported by NIH grant R35NS111631 to S.R.J.
S.R.J. is the co-founder of Lucerna Technologies and has equity in this company. Lucerna has licensed technology related to Spinach, Broccoli, and other RNA-fluorophore complexes. S.R.J. and J.L.L. are founders of Chimerna Therapeutics and have equity in this company.
REFERENCES
- 1.Weigand JE, Suess B (2007) Tetracycline aptamer-controlled regulation of pre-mRNA splicing in yeast. Nucleic Acids Res 35:4179–4185. 10.1093/nar/gkm425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paige JS, Wu KY, Jaffrey SR (2011) RNA mimics of green fluorescent protein. Science 333:642–646. 10.1126/science.1207339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenison RD, Gill SC, Pardi A, Polisky B (1994) High-resolution molecular discrimination by RNA. Science (80- ) 263:1425–1429. 10.1126/science.7510417 [DOI] [PubMed] [Google Scholar]
- 4.Sazani PL, Larralde R, Szostak JW (2004) A small aptamer with strong and specific recognition of the triphosphate of ATP. J Am Chem Soc 126:8370–8371. 10.1021/ja049171k [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srisawat C, Engelke DR (2002) RNA affinity tags for purification of RNAs and ribonucleoprotein complexes. Methods 26:156–161. 10.1016/S1046-2023(02)00018-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahsai AW, Wisler JW, Lee J, et al. (2016) Conformationally selective RNA aptamers allosterically modulate the beta2-adrenoceptor. Nat Chem Biol 12:1–11. 10.1038/nchembio.2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alam KK, Chang JL, Lange MJ, et al. (2018) Poly-Target Selection Identifies Broad-Spectrum RNA Aptamers. Mol Ther - Nucleic Acids 13:605–619. 10.1016/j.omtn.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–22. 10.1038/346818a0 [DOI] [PubMed] [Google Scholar]
- 9.Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment:RNA ligands to bacteriophage T4 DNA polymerase. Science (80- ) 249:505–510. 10.1126/science.2200121 [DOI] [PubMed] [Google Scholar]
- 10.Seiwert SD, Nahreini TS, Aigner S, et al. RNA aptamers as pathway-specific MAP kinase inhibitors. 833–843 [DOI] [PubMed]
- 11.Wurster SE, Maher LJ (2008) Selection and characterization of anti-NF-κB p65 RNA aptamers. RNA 14:1037–1047. 10.1261/rna.878908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rentmeister A, Bill A, Wahle T, et al. (2006) RNA aptamers selectively modulate protein recruitment to the cytoplasmic domain of β-secretase BACE1 in vitro. RNA 12:1650–1660. 10.1261/rna.126306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang J, Lee MS, Watowich SJ, Gorenstein DG (2007) Combinatorial selection of a RNA thioaptamer that binds to Venezuelan equine encephalitis virus capsid protein. 581:2497–2502. 10.1016/j.febslet.2007.04.072 [DOI] [PubMed] [Google Scholar]
- 14.Rusconi CP, Scardino E, Layzer J, et al. (2002) RNA aptamers as reversible antagonists of coagulation factor IXa. 419:0–4. 10.1038/nature00947.1. [DOI] [PubMed] [Google Scholar]
- 15.Lin Y, Nieuwlandt D, Magallanez A, et al. (1996) High-affinity and specific recognition of human thyroid stimulating hormone (hTSH) by in vitro-selected 2’-amino-modified RNA. Nucleic Acids Res 24:3407–3414. 10.1093/nar/24.17.3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Z, Filonov GS, Noto JJ, et al. (2015) Metazoan tRNA introns generate stable circular RNAs in vivo. RNA 21:1554–1565. 10.1261/rna.052944.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Memczak S, Jens M, Elefsinioti A, et al. (2013) Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 495:333–8. 10.1038/nature11928 [DOI] [PubMed] [Google Scholar]
- 18.Litke JL, Jaffrey SR (2019) Highly efficient expression of circular RNA aptamers in cells using autocatalytic transcripts. Nat Biotechnol 37:667–675. 10.1038/s41587-019-0090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puttaraju M, Been MD (1992) Group I permuted intron-exon (PIE) sequences self-splice to produce circular exons. Nucleic Acids Res 20:5357–64. 10.1093/nar/20.20.5357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeck WR, Sorrentino JA, Wang K, et al. (2013) Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 19:141–157. 10.1261/rna.035667.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filonov GS, Moon JD, Svensen N, Jaffrey SR (2014) Broccoli: Rapid selection of an RNA mimic of green fluorescent protein by fluorescence-based selection and directed evolution [SUPPLEMENT]. J Am Chem Soc 136:16299–16308. 10.1021/ja508478x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filonov GS, Kam CW, Song W, Jaffrey SR (2015) In-Gel Imaging of RNA Processing Using Broccoli Reveals Optimal Aptamer Expression Strategies. Chem Biol 22:649–660. 10.1016/j.chembiol.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orioli A, Pascali C, Quartararo J, et al. (2011) Widespread occurrence of non-canonical transcription termination by human RNA polymerase III. Nucleic Acids Res 39:5499–5512. 10.1093/nar/gkr074 [DOI] [PMC free article] [PubMed] [Google Scholar]


