Abstract
Flippases transport lipids across the membrane bilayer to generate and maintain asymmetry. The human fungal pathogen Candida albicans has 5 flippases, including Drs2, which is critical for filamentous growth and phosphatidylserine (PS) distribution. Furthermore, a drs2 deletion mutant is hypersensitive to the antifungal drug fluconazole and copper ions. We show here that such a flippase mutant also has an altered distribution of phosphatidylinositol 4-phosphate [PI(4)P] and ergosterol. Analyses of additional lipid transporters, i.e. the flippases Dnf1-3, and all the oxysterol binding protein (Osh) family lipid transfer proteins, i.e. Osh2-4 and Osh7, indicate that they are not critical for filamentous growth. However, deletion of Osh4 alone, which exchanges PI(4)P for sterol, in a drs2 mutant can bypass the requirement for this flippase in invasive filamentous growth. In addition, deletion of the lipid phosphatase Sac1, which dephosphorylates PI(4)P, in a drs2 mutant results in a synthetic growth defect, suggesting that Drs2 and Sac1 function in parallel pathways. Together, our results indicate that a balance between the activities of two putative lipid transporters regulates invasive filamentous growth, via PI(4)P. In contrast, deletion of OSH4 in drs2 does not restore growth on fluconazole, nor on papuamide A, a toxin that binds PS in the outer leaflet of the plasma membrane, suggesting that Drs2 has additional role(s) in plasma membrane organization, independent of Osh4. As we show that C. albicans Drs2 localizes to different structures, including the Spitzenkörper, we investigated if a specific localization of Drs2 is critical for different functions, using a synthetic physical interaction approach to restrict/stabilize Drs2 at the Spitzenkörper. Our results suggest that the localization of Drs2 at the plasma membrane is critical for C. albicans growth on fluconazole and papuamide A, but not for invasive filamentous growth.
Author summary
Candida albicans is a human opportunistic fungal pathogen that diverged from Saccharomyces cerevisiae ~250 million years ago, in which a switch from budding growth to filamentous hyphal growth is associated with virulence. We sought here to understand how membrane expansion in these hyperpolarized filamentous cells is regulated by lipid transport mechanisms, and in particular flippases that transport phospholipids across lipid bilayers, thus generating membrane lipid asymmetry. Deletion of one of these flippases results in cells defective for hyphal invasive growth, cell wall integrity and virulence, as well as increased sensitivity to the most commonly used antifungal drug fluconazole. We show that this flippase localizes to different cellular compartments and our results suggest that the plasma membrane localization is critical for growth on fluconazole, but not for invasive filamentous growth and cell wall integrity. Strikingly, our data reveal that a genetic interaction between homologs of two different lipid transporters, previously identified in S. cerevisiae budding growth, regulates C. albicans hyphal growth. An attractive possibility that merits investigation is whether this is a conserved feature of the fungal kingdom.
Introduction
Polarized growth is an essential process that is regulated, in particular, by cooperative interactions between key establishment proteins and specific lipids at the plasma membrane (PM). For instance in the baker’s yeast Saccharomyces cerevisiae, dynamic nanoclusters of the Rho-GTPase Cdc42 are regulated by multivalent interactions between its sole activator Cdc24, the scaffold protein Bem1 and anionic lipids, including PS [1–3]. Similarly, in the fission yeast Schizosaccharomyces pombe, the localization and function of the two essential Rho-GTPases, Cdc42 and Rho1, depend on polarized PS distribution [4]. The importance of anionic lipids, and PS in particular, for cellular function and signaling encompasses kingdoms from fungi, to mammals [5] to plants [6]. In addition, PS has been reported to be critical for virulence in a broad spectrum of microbial pathogens (reviewed in [7,8]). In particular, in the human fungal pathogen Candida albicans, the PS synthase Cho1, which is conserved in fungi but does not have an ortholog in Humans, is required for virulence in a murine candidiasis model [9]. A cho1 deletion mutant also exhibits increased exposure of β(1,3)-glucan via up-regulation of cell wall MAPK cascades, facilitating its detection by innate immune cells [10]. PS is synthesized in the endoplasmic reticulum (ER) and its cellular distribution is regulated both by lipid transfer proteins (LTPs), which function at contact sites between the ER and the target cellular compartments, and lipid transporters, such as flippases that establish PS asymmetry between membrane leaflets (reviewed in [11]).
Lipid flippases are P4-ATPases, only found in eukaryotes, where they are similar in domain structures from fungi to Humans (reviewed in [12]). These proteins actively transport phospholipids from the external/luminal to cytoplasmic membrane leaflets and play an important role in polarized growth (reviewed in [13]). There are 14 P4-ATPases in Humans and only 5 in the yeast S. cerevisiae (Dnf1-3, Drs2, Neo1), with most of them regulated by non-catalytic subunits from the Cdc50/Lem3 family. For example, in S. cerevisiae, Cdc50 forms a functional complex with Drs2 [14,15] and very recently, the cryo-electron microscopy structure of different conformations of this complex was resolved at 2.8 to 3.7 Å [16]. This complex, which is well characterized, both in vitro and in vivo, primarily transports PS [17,18]. The role of lipid flippases on membrane curvature and in trafficking has been extensively studied in S. cerevisiae, as well as in mammals (reviewed in [19]). In S. cerevisiae, Neo1, which is the sole essential flippase [20], localizes to the Golgi and endomembranes, Drs2 and Dnf3 to the trans-Golgi network (TGN) and Dnf1-2 to the PM [17,21,22]. A recent study indicates that Dnf3 can also be found at the PM in a cell-cyle dependent fashion, where it regulates, together with Dnf1-2, S. cerevisiae pseudohyphal growth [23]. Furthermore, in response to pheromone, it was shown that Dnf1, Dnf2 and Dnf3 localize to the schmoo tip, while Drs2 remains at the Golgi [24]. Interestingly, in the filamentous fungi Aspergillus nidulans and Fusarium graminearum, it was shown that the Drs2 homolog, DnfB, localizes primarily to a cluster of vesicles at the hyphal apex, called the Spitzenkörper (SPK), which is characteristic of fungi that grow in a filamentous form [25,26]. Together, these data indicate that flippases can localize to different compartments in specific conditions of polarized growth.
LTPs and more specifically oxysterol-binding protein (OSBP)-related proteins (ORPs), are also important for membrane lipid composition, via non-vesicular traffic (reviewed in [27]), including for PS distribution (reviewed in [11]). LTPs can bind specific ligands such as PI(4)P, PS and sterol. For example, in S. cerevisiae, there are 7 oxysterol-binding homology (Osh) proteins, among which Osh6/Osh7 transports PS from cortical ER (cER) and PM [28], in counter-exchange with PI(4)P [29]. On the other hand, another Osh protein, Kes1, which is homolog to Osh4 and transports sterol in counter-exchange with PI(4)P [30], was proposed to act antagonistically with Drs2 to regulate the sterol distribution between PM and internal membranes in S. cerevisiae [31].
Filamentous fungi are highly polarized organisms, in which the role of flippases has been investigated. In A. nidulans, the homologs of Dnf1 and Drs2, i.e. DnfA and DnfB, regulate growth and PS asymmetry [25], while in Magnaporthe grisea, MgAPT2, one of the 4 aminophospholipid translocase (APT) encoding genes related to Drs2, is required for plant infection but not filamentous growth [32]. In F. graminearum, flippases play redundant as well as distinct roles in vegetative growth, where FgDnfA is critical, stress response, reproduction and virulence [26,33,34]. In the human fungal pathogen C. albicans, deletion of either Dnf1 or Dnf2 results in moderate increase sensitivity to copper [35]. The drs2 deletion mutant is hypersensitive to copper [35], as well as to the antifungal drug fluconazole [36]. Deletion of Drs2 also results in altered PS distribution and impaired filamentous growth [36], and that of its Cdc50 subunit in altered filamentous growth and reduced virulence in a murine candidiasis model [37]. Here, we sought to determine more specifically the role of Drs2 in morphogenesis and whether PS asymmetry is critical for this process.
Our results show that in response to serum, of the four flippases Dnf1-3 and Drs2, only Drs2 is critical for filamentous growth, with Dnf2 having a minor role in this process. Filamentous growth was largely restored in the drs2 mutant upon deletion of the LTP Osh4, but not by that of other Osh proteins (Osh2, Osh3 and Osh7). Furthermore, our results demonstrate that the distribution of PS and of the phosphatidylinositol phosphate PI(4)P, which are both altered in the drs2 mutant, is restored upon deletion of OSH4. These data indicate that the requirement for flippase activity across the lipid bilayer during filamentous growth can be specifically bypassed by lipid exchange between membrane compartments.
Results
Drs2 has a unique role in C. albicans
In A. nidulans, the Drs2 homolog DnfB, is not critical for hyphal growth [25]. In contrast, in C. albicans, Drs2 is required for filamentous growth, whereas budding growth is largely unaffected (mean doubling time of 90 min, compared to 80 min in the wild-type strain) [36]. We investigated the importance of other flippases, i.e. Dnf1, Dnf2 and Dnf3, using loss of function mutants that we generated (S1A Fig). As illustrated in Fig 1A, a drs2 deletion mutant was not invasive on serum-containing media. In contrast, deletion of DNF1 or DNF3 did not alter serum-induced invasive growth, while deletion of DNF2 resulted in reduced invasive growth, which was further reduced in a double dnf1dnf2 deletion mutant. Over-expression of DNF2 restored invasive growth in the dnf2 and dnf1 dnf2 mutants, but not in the drs2 mutant, suggesting that Dnf2 and Drs2 do not functionally overlap in this invasive growth process. Upon serum-induced hyphal growth in liquid media (Fig 1B–1D), the dnf1, dnf2 and dnf3 deletion mutants produced hyphae similar to the wild-type cells, with hyphal formation in the dnf1 dnf2 cells somewhat reduced. The average hyphal filament length at 90 min was, nonetheless, slightly reduced in dnf2 cells as well as in dnf1 dnf2 (14 ± 3 μm and 13 ± 3 μm, respectively) compared to the WT, dnf1 and dnf3 cells (20 ± 4 μm, 18 ± 4 μm and 20 ± 4 μm, respectively). These results are in agreement with very recent data, which also show that both a dnf2 and a dnf1 dnf2 mutant exhibit reduced invasive filamentous growth in response to the nutrient poor spider media, another inducer of filamentous growth [38]. These data indicate that, in contrast to the drs2 deletion mutant, dnf1, dnf2 and dnf3 deletion mutants undergo filamentous growth similar to the wild-type cells, albeit with a reduced efficiency for dnf2. Furthermore, Fig 2A and 2B shows that the dnf1-3 mutants also grew similar to the wild-type cells in the presence of the cell wall perturbants calcofluor white (CFW) and congo red (CR), the antifungal drugs, caspofungin (Caspo) and fluconazole (FCZ), and the detergent sodium dodecyl sulfate (SDS), with only the dnf2 mutant growth somewhat reduced on 0.05% SDS. In contrast, the drs2 mutant was hypersensitive to SDS, in addition to CFW and FCZ [36]. The growth defect of drs2 cells on FCZ could result from a mislocalization of multi-drug transporters, such as Cdr1 [39]. Fig 2C shows that, although a Cdr1-GFP fusion protein was detected at the PM in drs2 cells, 79 ± 8% of the cells had internal Cdr1 signal, in contrast to wild-type and complemented cells, suggesting that PM targeting of MDR is partially impaired in drs2 cells. Together, these data indicate that Drs2 has a unique role in C. albicans.
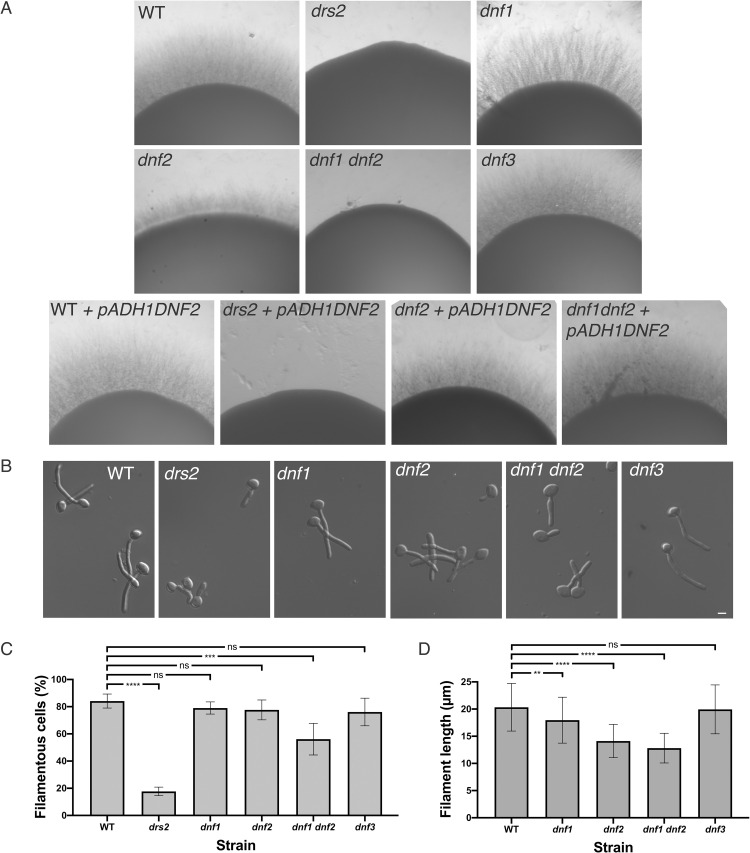
Fig 1. Drs2 is critical for hyphal invasive growth.
A) Invasive filamentous growth cannot be restored by overexpression of DNF2 in the drs2/drs2 mutant. The indicated strains, WT (PY4861), drs2/drs2 (drs2, PY3375), dnf1/dnf1 (dnf1, PY6235), dnf2/dnf2 (dnf2, PY5814), dnf3/dnf3 (dnf3, PY5801), dnf1/dnf1 dnf2/dnf2 (dnf1 dnf2, PY6400), WT + pADH1DNF2 (PY5005), drs2/drs2 + pADH1DNF2 (drs2 + pADH1DNF2, PY5003), dnf2/dnf2 + pADH1DNF2 (dnf2 + pADH1DNF2, PY5919), and dnf1/dnf1 dnf2/dnf2 + pADH1DNF2 (dnf1 dnf2 + pADH1DNF2, PY5922), were grown on agar-containing YEPD with serum and images were taken after 6 days. Similar results were observed in 2 independent experiments. B) DRS2 is specifically required for hyphal growth in response to serum. Cells from the indicated strains were incubated with serum for 90 min at 37°C. Bars are 5 μm. C) and D). Graphs represent the percentage of hyphae (C) and the filament length (D) in the indicated strains grown as in 1B. The percentage of hyphae was 84 ± 5%, 79 ± 4%, 78 ± 7%, 76 ± 10%, 56 ± 12% and 18 ± 3% for wild-type and the dnf1, dnf2, dnf3, dnf1 dnf2 and drs2 deletion mutants, respectively (average of 3 experiments with n ~ 150 cells each). The filament length was measured from the junction between cell body and filament (error bars indicate the mean +/- the SD of 3 experiments, n ~ 50 cells each). **, P < 0.01; ***, P < 0.0005; **** P < 0.0001; ns, not significant.
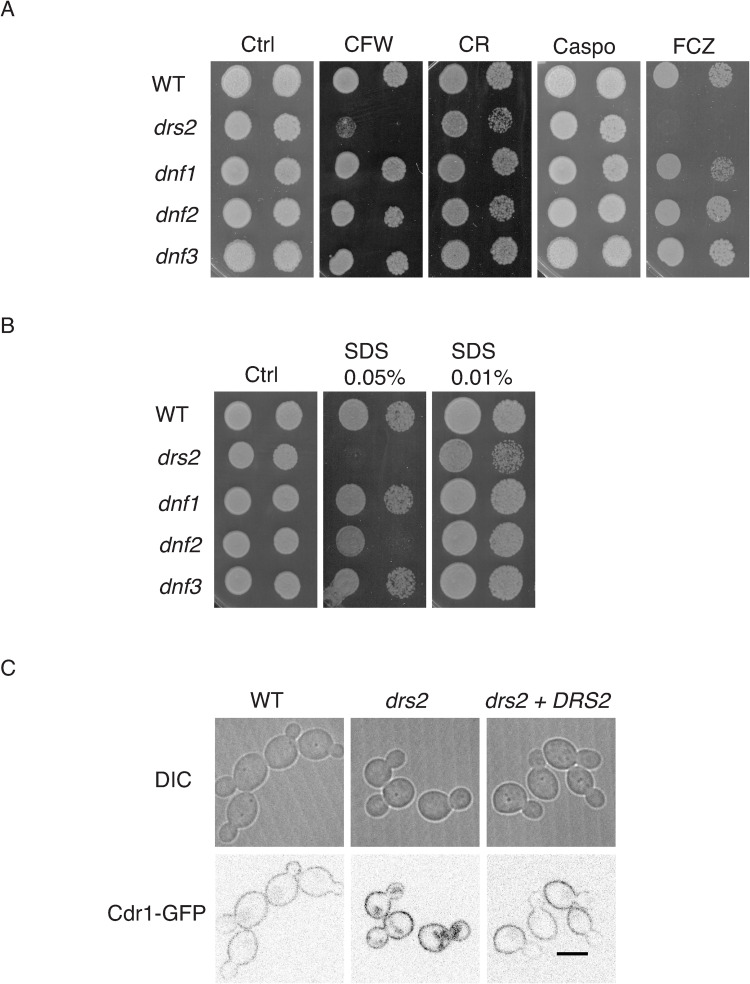
Fig 2. Drs2 has a unique role in C. albicans.
(A). The drs2 mutant has specific increased susceptibility to fluconazole and calcofluor white. Serial dilutions of indicated strains, as in Fig 1, were spotted on YEPD media (Ctrl) containing 25 μg/ml calcofluor white (CFW), 400 μg/ml Congo red (CR), 125 ng/ml caspofungin (Caspo) or 5 μg/ml fluconazole (FCZ). Images were taken after 2 days at 30°C. Similar results were observed in 2 independent experiments. (B). The drs2 mutant has increased susceptibility to SDS. Serial dilutions of indicated strains, grown as in 2A, were spotted on YEPD media (Ctrl) containing 0.01% or 0.05% SDS. (C). PM targeting of the multidrug ABC transporter Cdr1 is altered in drs2 cells. Central z-sections of representative wild-type (WT, PY6393), drs2/drs2 (drs2, PY6395) and drs2/drs2 + pDRS2DRS2 (drs2 + DRS2, PY6646) cells expressing Cdr1-GFP are shown.
Drs2 is critical for hyphal extension after septin ring formation
In response to serum, drs2 cells appear to initiate filamentous growth, although they were unable to form hyphae [36] and invasive colonies (Fig 1A), and reintroduction of a copy of DRS2 in this mutant restored invasive growth (Fig 3A). We examined this mutant in a murine model for systemic candidiasis and Fig 3B shows that the drs2 mutant causes no lethality, compared to the wild-type and complemented strains, at 16 days post injection. At the transcriptional level, hyphal growth is controlled by the hyphal specific cyclin HGC1, which is further regulated by the transcription factor UME6 [40]. Both hgc1 and ume6 deletion mutants are defective in hyphal extension and attenuated for virulence in a mouse infection model [41,42]. Quantitative RT-PCR analyses in Fig 3C show that, upon serum exposure, HGC1 and UME6 were up-regulated in the drs2 mutant compared to budding cells (>100-fold for HGC1 and 40-fold for UME6), with a level of induction of HGC1 slightly reduced compared to that of the wild-type cells. Similarly, ECE1, which encodes the peptide toxin candidalysin and whose expression correlates with cell elongation [43], HWP1, which encodes a hyphal cell wall protein associated with hyphal development [44] and ALS3, which encodes an agglutinin-like (Als) adhesin [45], were all up-regulated in the drs2 mutant upon serum exposure, with levels slightly reduced (3- to 5-fold) compared to the control cells. Only the induction of SAP6, which encodes a secreted acid protease [46], was substantially reduced (~ 10-fold) in drs2. Together, these data indicate that the drs2 mutant hyphal growth defect is unlikely due to the modest reduction of HGC1 induction.
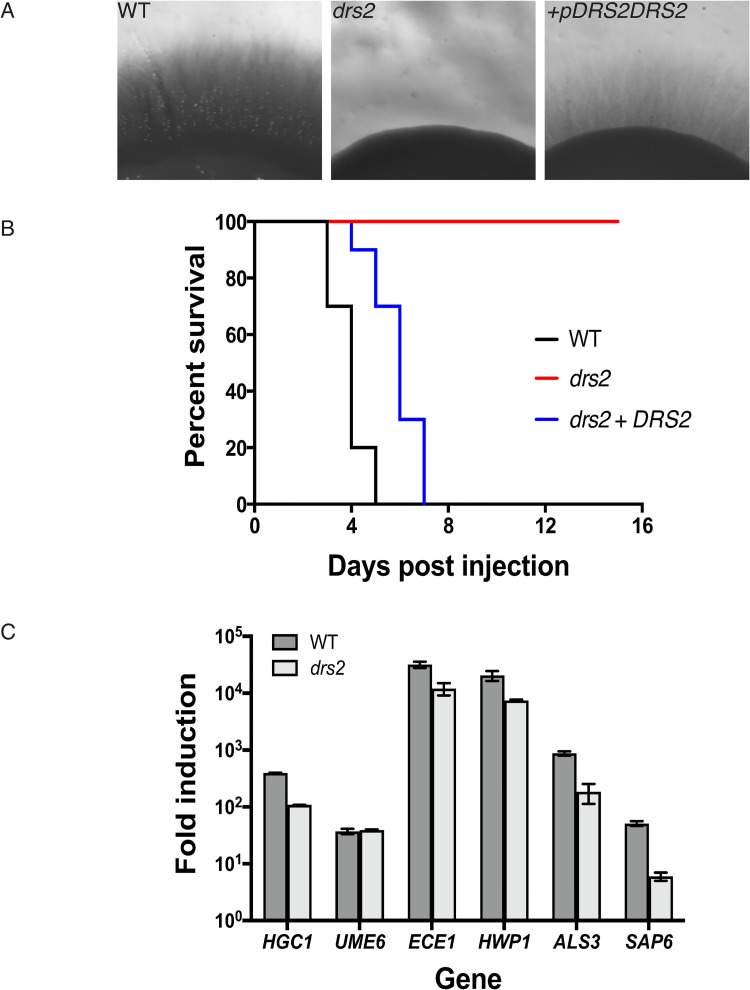
Fig 3. Drs2 is critical for virulence in a mouse model of systemic candidiasis.
A) Reintroduction of DRS2 complements the invasive growth defect of the drs2 mutant. The indicated strains WT (PY4861), drs2/drs2 (drs2, PY3375), and drs2/drs2 + pDRS2DRS2 (drs2 + DRS2, PY5042), were grown as in Fig 1A and 1B) The drs2 mutant has attenuated virulence in a mouse model of hematogenously disseminated candidiasis. Balb/C mice (n = 10) were injected with an inoculum (5 x 105 cells) of the indicated strains and the survival was assessed. C) Hyphal specific gene are induced in drs2 cells upon serum exposure. mRNA and cDNA were prepared from the indicated strains grown 120 min in the presence of serum. HGC1, ECE1, HWP1, ALS3 and SAP6 transcripts were determined by qRT-PCR using primer pairs described in [89], and UME6 transcripts were determined using UME6.pTm/UME6.mTm (89 bp) primer pair. Bars indicate the mean ± SD (n = 3 determinations). Similar results were observed in an additional biological replicate. NAD-linked Glyceraldehyde-3-phosphate dehydrogenase (TDH3) transcript levels were used for normalization.
To further characterize the drs2 mutant at the molecular level, we used time-lapse microscopy to follow cells expressing fluorescent reporters for different cellular compartments. Fig 4A illustrates representative time courses of hyphal growth both in WT and drs2 cells expressing a fluorescently tagged septin Cdc10 [47]. Measurements of the diameters of filament compartments, apical (pre-septum) and distal (post-septum) to the septin ring (Fig 4B), show that, while it remained constant in wild-type cells (1.8 ± 0.2 μm), the diameter increased in the apical compartments of drs2 cells by about 40% (to 2.5 ± 0.3 μm). This increase was associated with a reduced extension rate (on average 0.09 μm/min in drs2 cells, compared to 0.34 μm/min in WT cells; Fig 4C). These data are consistent with a defect in polarized growth in the drs2 mutant after the first septin ring forms. In agreement with this, we observed that, upon filament extension, active Cdc42, visualized with the CRIB (Cdc42 Rac1 Interactive Binding domain) reporter [48], became depolarized in drs2 cells and the SPK (visualized with the myosin light chain Mlc1, [49,50]), was not maintained at the filament tip following cell division, compared to the control cells (Fig 4D–4E). Furthermore, comparison of the wild-type and drs2 cells expressing both reporters for active Cdc42 and the Spitzenkörper, indicates that depolarization of Cdc42 occurred prior to the SPK delocalization from the filament tip (S1 and S2 Movies). Together, these data indicate that, subsequent to septin ring formation, the drs2 mutant is unable to redirect or reinitiate growth to the apex, which ultimately results in growth arrest and/or pseudohyphal growth.
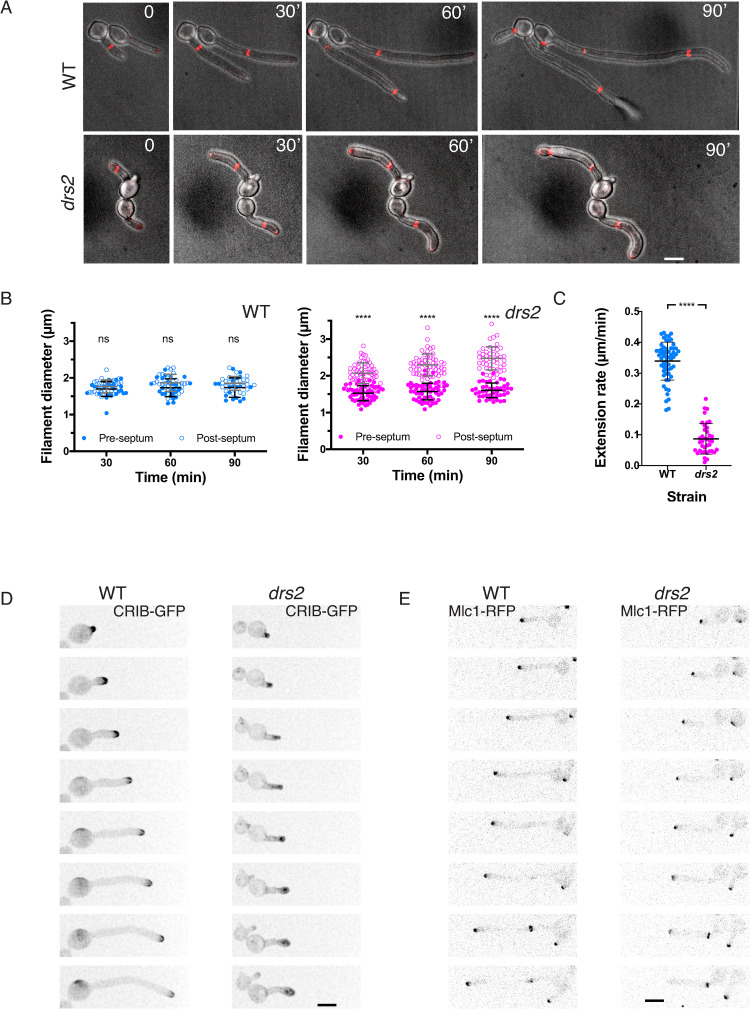
Fig 4. Drs2 is critical for maintaining polarized filament extension.
A) Both cell morphology and filament extension rate are altered in the drs2 mutant, after septin ring formation. Time lapse of wild-type (WT, PY5613) and drs2/drs2 (drs2, PY5615) cells expressing Cdc10-mScarlet, incubated in the presence of serum. Images were taken every 10 min and merges between DIC and sum projections of 21 z-sections are shown. Bars are 5 μm. B) Graphs represent the filament diameter before (open circles) and after (solid circles) the first septin ring, as a function of the times from the first image in which the septin ring is observed in wild-type (blue) and drs2 (magenta) cells. Means ± SD of 25–50 cells are shown. **** P < 0.0001; ns, not significant. C) The graph shows the filament extension rate in wild-type (blue) and drs2 (magenta) cells. Means ± SD of 30–60 cells are shown. D) and E) Polarized growth is altered in the drs2 mutant. D) Time lapse of wild-type (WT, PY2263) and drs2 cells (drs2, PY4972) expressing CRIB-GFP, incubated in the presence of serum. Images were taken every 10 min and maximum projections of 21 z-sections are shown. E) Time lapse of wild-type (WT, PY5349) and drs2/drs2 cells (drs2, PY5218) expressing Mlc1-mScarlet, incubated in the presence of serum. Images were taken every 5 min and sum projections of 23 z-sections are shown.
Drs2 localizes to the hyphal tip
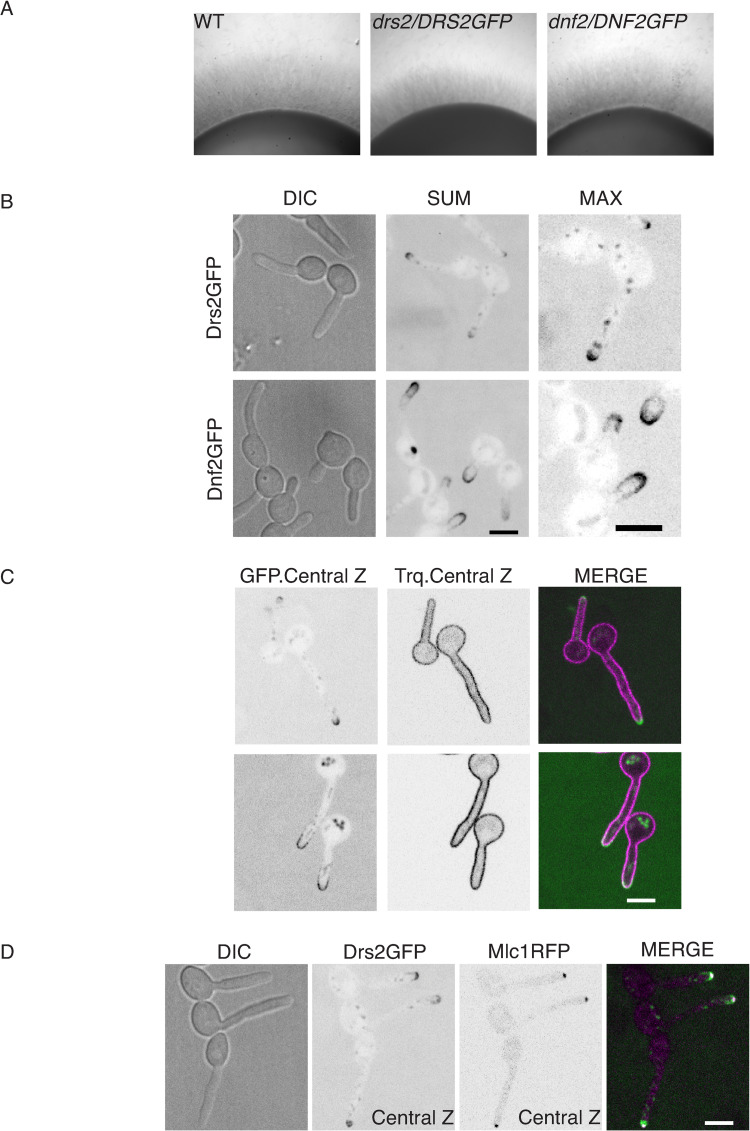
In S. cerevisiae, Drs2 localizes to the late Golgi during budding [20] and mating [24], while its homolog in A. nidulans, DnfB, localizes within the SPK core [25]. To examine the distribution of Drs2 in C. albicans, we generated a strain that expresses a functional DRS2-GFP fusion (Fig 5A). Given that the homolog of Dnf1-2 in A. nidulans, DnfA, also localizes to the Spitzenkörper, albeit to the outer layer macrovesicles compared to DnfB [25], we also generated a strain that expressed a functional DNF2-GFP fusion (Fig 5A). Fig 5B shows that Drs2 was restricted to the apical region of the hyphal tip, as well as in internal structures, likely Golgi cisternae by analogy with S. cerevisiae [20]. Dnf2 was essentially localized at the tip crescent, similar to its localization in S. cerevisiae at bud tips and mating projections [24]. Both Dnf2 and Drs2 co-localized with a PM marker (a prenylated RFP fusion, RFP-CtRac1, [51]), although Drs2 localized to a more restricted region of the hyphal tip than Dnf2 (Fig 5C). Drs2 also partially co-localized with the SPK marker, Mlc1 (Fig 5D), which could be due to the proximity (less than 100 nm) of this structure to the PM [52]. These data indicate that Drs2 and Dnf2 have distinct distributions at the filament apex.
Fig 5. Drs2 localizes preferentially to the filament apex.
A) The Drs2-GFP and Dnf2-GFP fusions are functional. The indicated strains, WT (PY4861), drs2/DRS2GFP (PY4665) and dnf2/DNF2GFP (PY5746) were grown as in Fig 1A and 1B) and C) Drs2 and Dnf2 localize differently to the filament apex. Sum projections and zoom images of Max projections of 16 z-sections of representative cells expressing Drs2-GFP and Dnf2-GFP after 90 min serum induction are shown (B). Central z-sections and merge of representative cells expressing mScarlet-CtRac1, together with either Drs2-GFP (PY6241) or Dnf2-GFP (PY6239), after 90 min serum induction are shown (C). D) Drs2 partially overlaps with Mlc1. Central z-section and merge of representative cells expressing Drs2-GFP together with Mlc1-mScarlet (PY4928), after 90 min serum induction are shown. Bars are 5 μm.
The drs2 mutant is altered for PI(4)P distribution
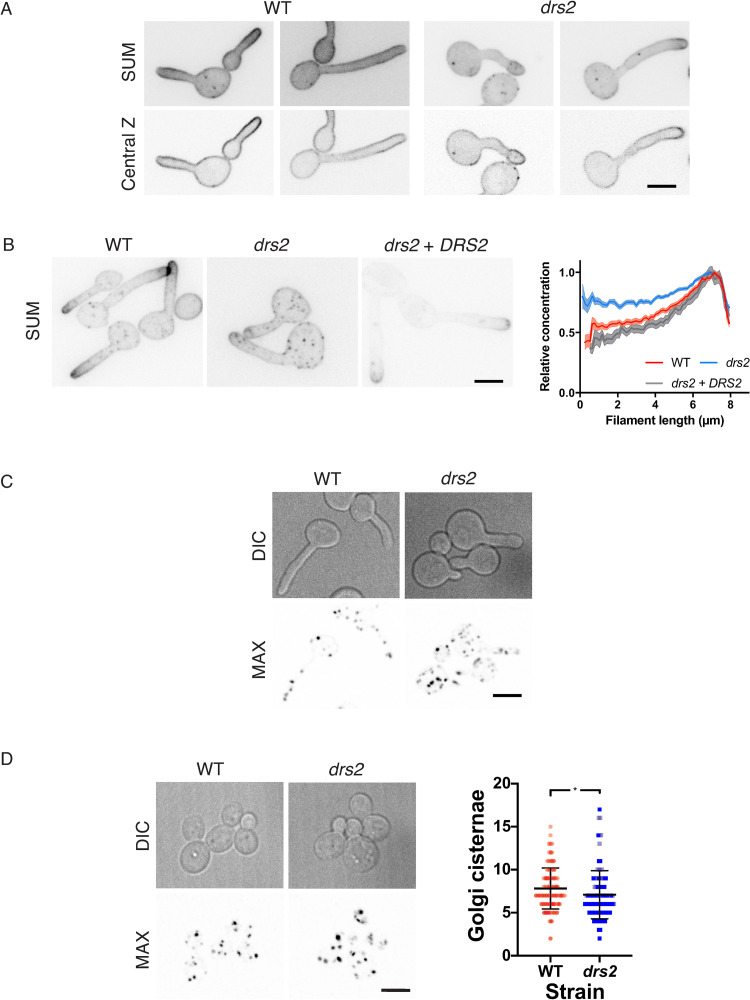
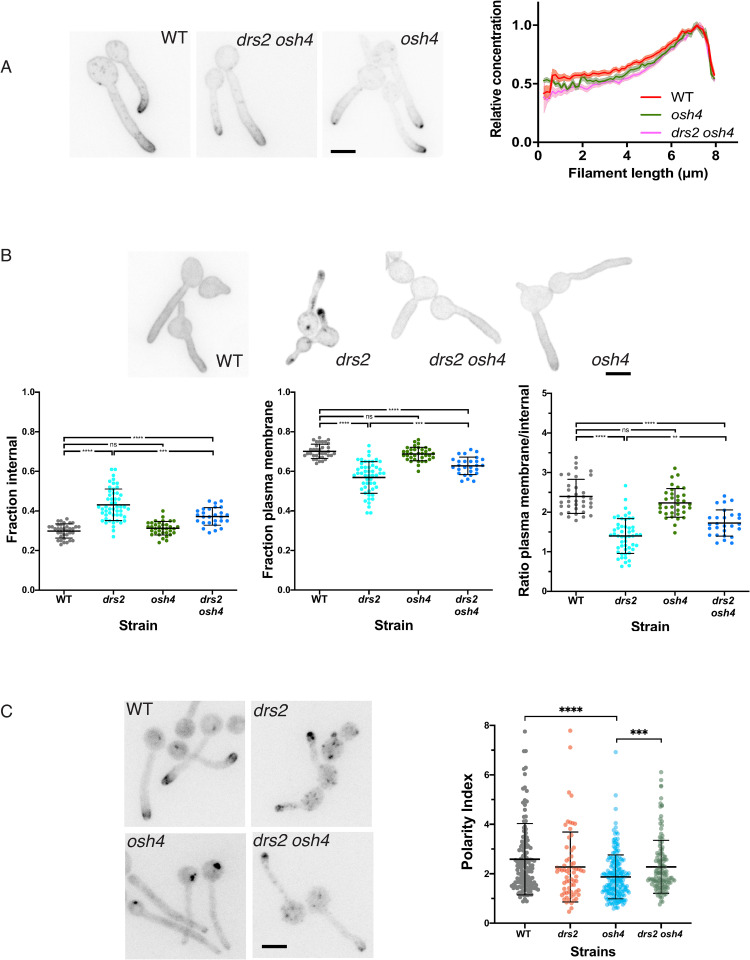
Drs2 is a P4-ATPase that flips PS selectively across the lipid bilayer in vitro and in vivo in S. cerevisiae [17,18]. In C. albicans, using the reporter Lactadherin C2 (LactC2) [53–55], we observed that the distribution of PS was altered during hyphal growth in the drs2 mutant, as the reporter was visible in an intracellular punctate pattern [36]. To determine the impact of the DRS2 deletion on the distribution of other lipids, shown to be critical for hyphal growth, such as the phosphatidylinositol phosphates PI(4)P and PI(4,5)P2 [56,57], as well as ergosterol [58], we used specific fluorescent reporters. The distribution of PI(4,5)P2 appears to be similar in wild-type cells and drs2 filamentous cells (Fig 6A), yet the distribution of PI(4)P was substantially less polarized in the mutant, compared to the wild-type or complemented strains (Fig 6B). This depolarization is further illustrated by the graph in Fig 6B, which shows the relative concentration of PI(4)P, as a function of filament length. In contrast, PI(4)P at the Golgi was largely unaffected in drs2, compared to WT cells, both during filamentous growth (Fig 6C), and budding growth (mean of 7.1 ± 2.8 Golgi cisternae per cell in drs2 cells compared to 7.8 ± 2.4 in the wild-type control, Fig 6D). These data indicate that deletion of DRS2 results in altered distribution of PM PI(4)P, but not PI(4,5)P2.
Fig 6. Plasma membrane PI(4)P distribution is altered in the drs2 mutant.
A) PM PI(4,5)P2 distribution is not altered in the drs2 mutant. Wild-type (PY1206) and drs2/drs2 cells (drs2, PY4050) expressing GFP-(PHPLCδ1)2-GFP were incubated for 60 or 90 min, respectively, in the presence of serum. Sum projections (21 z-sections) and central z-sections of representative cells are shown. B) PM PI(4)P distribution is altered in the drs2 mutant. The indicated cells, WT (PY5619), drs2/drs2 (drs2, PY5568) and drs2/drs2 + pDRS2DRS2 (drs2 + DRS2, PY6407) expressing GFP-(PHOSH2[H340R])2-GFP were incubated for 90 min in the presence of FCS. Sum projections of (21 z-sections) of representative cells are shown. The graph illustrates the means ± the SEM of the relative concentration of PM PI(4)P as a function of filament length, normalized to the maximal signal for each cell (n = 25–60 cells). C) and D) The number of Golgi cisternae is not substantially affected in the drs2 mutant. DIC and maximum projections (21 deconvolved z-sections) of representative WT (PY2578) and drs2/drs2 cells (drs2, PY3873) expressing FAPP1[E50A,H54A]-GFP incubated in the presence of serum are shown (C). The number of Golgi cisternae per cell was determined in budding cells of the indicated strains from maximum projections of deconvolved images (21 z-sections). (D). Bars indicate the mean ± the SD of 3 independent biological samples (n = 100 cells and ~700–800 cisternae for each strain). * P < 0.05.
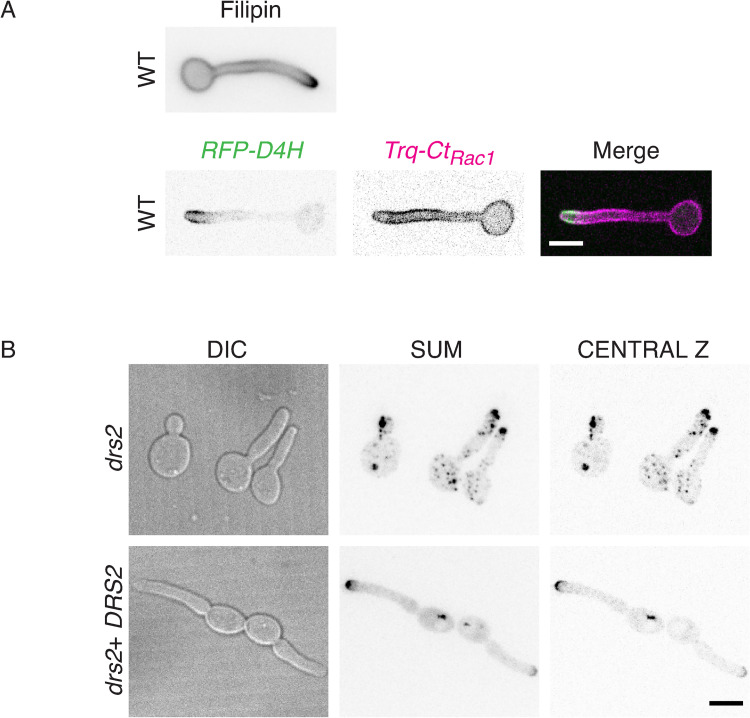
Using filipin staining, it was shown that membrane sterols are highly concentrated at the apex during C. albicans hyphal growth, with such a polarization not observed in budding and pseudohyphal cells [58]. We examined sterol distribution, using both filipin staining and the genetically encoded biosensor D4H. This biosensor consists of the Domain 4 of perfringolysin O, a toxin produced by Clostridium perfringens, used to monitor sterols in vivo [59–62]. Fig 7A shows that sterols are highly concentrated at the apex of WT hyphal cells, irrespective of the reporter used. In contrast, in the drs2 mutant, the D4H reporter preferentially labeled internal structures and reintroduction of a copy of DRS2 restored the ergosterol distribution to the PM (Fig 7B). Together, these data indicate that deletion of DRS2 results not only in altered distribution of PM PS, but also of PI(4)P and ergosterol.
Fig 7. Distribution of ergosterol is altered in the drs2 mutant.
A) The ergosterol reporters Filipin and D4H localize similarly at the apex of the filament in wild-type cells. Top panel: Wild-type cells (PY4861) were induced serum prior to staining with filipin, as described [58]. Images were taken with a wide-field fluorescence microscope. Bottom panel: Wild-type cells expressing mScarlet-D4H together with Trq-CtRac1 (PY6237) were induced with serum as in Fig 1B and images were taken with a spinning disk confocal microscope and central z-section, as well as merge images, are shown. B) Ergosterol distribution is altered in the drs2 mutant. Images of drs2/drs2 cells (drs2, PY6083) and drs2/drs2 + pDRS2DRS2 cells (drs2 + DRS2, PY6218) expressing mScarlet-D4H were taken as in Fig 6A bottom panel.
Deletion of OSH4 in the drs2 mutant restores invasive filamentous growth and plasma membrane PI(4)P distribution
LTPs can bind specific ligands such as PI(4)P, PS and sterol and, in S. cerevisiae, it was shown that Osh6/Osh7 transports PS in counter-exchange with PI(4)P [29], while Kes1 transports sterol in counter-exchange with PI(4)P [30]. C. albicans has 4 Osh proteins (Osh2-4 and Osh7), with Osh4 and Osh7 sharing ~ 60% identity with their S. cerevisiae counterparts, however their function is unknown in this organism. Note that the genes encoding OSH7 (called OBPA and OBPALPHA) are located at the mating-type-like locus (MTL), similar to those encoding the Golgi phosphatidylinositol kinase Pik1 (PIKA and PIKALPHA).
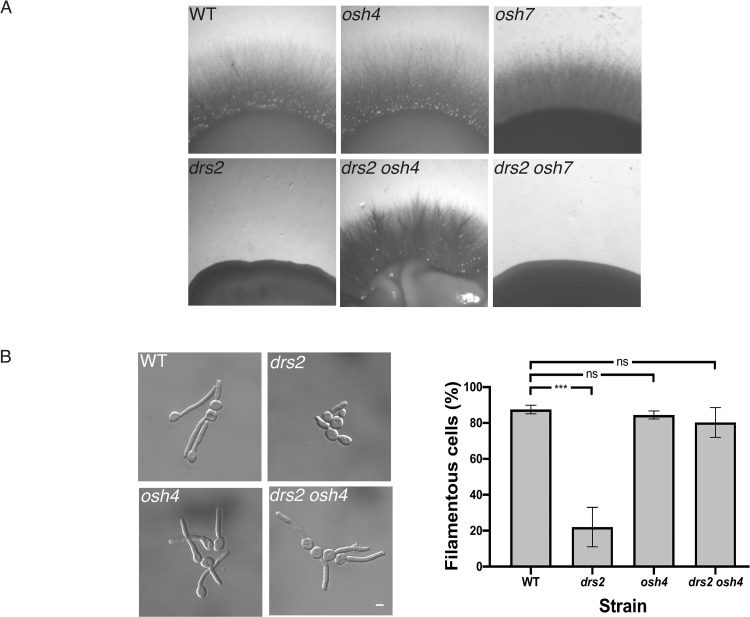
To investigate the importance of Osh proteins in C. albicans invasive hyphal growth, we first generated osh4 and osh7 deletion mutants, as well as drs2 osh4 and drs2 osh7 double deletion mutants (S1B Fig), and examined these mutants for invasive hyphal growth in response to serum. While deletion of OSH4 or OSH7 alone did not alter invasive growth, deletion of OSH4, but not OSH7, in the drs2 mutant restored invasive growth to a level similar to that of WT cells (Fig 8A). In serum-containing liquid media, we also observed a rescue of hyphal growth upon deletion of OSH4 in the drs2 mutant (Fig 8B). In this drs2 osh4 mutant, hyphal length was only slightly reduced compared to that of the wild-type and osh4 cells (17 ± 3 μm, compared to 19 ± 1 μm for WT and osh4 cells). To confirm the specificity of the OSH4 deletion in restoring invasive growth in the drs2 mutant, we also generated drs2 osh2 and drs2 osh3 double deletion mutants (S2A Fig). Candida albicans OSH3 has been reported to be important for invasive growth in nutrient poor media (Spider media), but not in the presence of serum [63]. Similarly, we observed that mutants deleted for either OSH2 or OSH3 alone exhibited invasive growth in the presence of serum (S2B Fig). In contrast to OSH4 deletion, absence of either OSH2 or OSH3 did not rescue the drs2 invasive growth defect, although colonies from the drs2 osh3 mutant were somewhat crenelated compared to the control strains (S2B Fig). Furthermore, while the osh2 and osh3 mutants formed hyphae similar to those of the wild-type (83 ± 5%, 73 ± 9% and 86 ± 6% for the WT, osh2 and osh3 strains, respectively), the percent of hyphae in the drs2 osh2 and drs2 osh3 mutants was similar to that of drs2 (8 ± 6% and 21 ± 7%, respectively, compared to 19 ± 8% in drs2) (S2C Fig). These data indicate that, while Osh proteins (Osh2-4 and Osh7) are not required per se for serum-induced invasive filamentous growth, deletion of OSH4 specifically bypasses the drs2 requirement in this process. Notably, OSH4 expression was not significantly altered in drs2 cells, compared to the WT cells (S1C Fig).
Fig 8. Deletion of OSH4 rescues hyphal invasive growth in the drs2 mutant.
A) Invasive filamentous growth is specifically restored in the drs2 mutant upon deletion of OSH4. The indicated strains, WT (PY4861), osh4/osh4 (osh4, PY3974), osh7/osh7 (osh7, PY5256), drs2/drs2 (drs2, PY3375), drs2/drs2 osh4/osh4 (drs2 osh4, PY5539) and drs2/drs2 osh7/osh7 (drs2 osh7, PY5297), were grown as in Fig 1A and images were taken after 6 days. Similar results were observed in 2 independent experiments. B) Hyphal growth is restored in the drs2 mutant upon deletion of OSH4. Cells from the indicated strains were incubated with serum as in Fig 1B. Bars are 5 μm. The graph represents the percentage of hyphae in the indicated strains, calculated as in Fig 1C. The percentage of hyphae was 82 ± 8%, 80 ± 6% and 76 ± 9% for the wild-type, osh4 and drs2 osh4 cells, respectively. ***, P < 0.0005; ns, not significant.
To better characterize the mechanisms underlying the rescue of hyphal growth in the drs2 osh4 mutant, we examined whether deletion of OSH4 also restored the lipid distribution in the drs2 mutant. Fig 9A shows that the distribution of PI(4)P was polarized in the drs2 osh4 double mutant, similar to that in wild-type and osh4 cells. Similarly, deletion of OSH4 in the drs2 mutant significantly restored PS distribution, as internal punctae were absent in drs2 osh4 cells, similar to the wild-type, complemented and osh4 strains (Fig 9B). Quantification of the LactC2 signal in the drs2 mutant shows that the fraction of PS was reduced at the PM and increased internally, compared to wild-type and osh4 cells, resulting in a decreased ratio of PM to intracellular signals (Fig 9B). Deletion of OSH4 in drs2 significantly increased this ratio, with an increase in PM signal, as well as a decrease in internal signal (Fig 9B). In contrast, the overall distribution of ergosterol appears similar in drs2 and drs2 osh4, with the presence of a number of internal punctae, compared to wild-type and osh4 cells (Fig 9C). The ergosterol tip polarization, determined as the apical versus sub-apical D4H signal, was also similar in these two mutants and not substantially different from that of wild-type cells. Notably, the ergosterol tip polarization was significantly reduced in the osh4 mutant alone, which exhibits hyphal growth similar to wild-type cells, suggesting that there is not a direct correlation between ergosterol tip polarization and hyphal growth. Together, these data indicate that Drs2 per se is not required for hyphal invasive growth, but rather that a balance in the activities of Drs2 and Osh4 regulate this process, likely via regulation of the PM PI(4)P gradient.
Fig 9. Deletion of OSH4 rescues PI(4)P distribution in the drs2 mutant.
A) PI(4)P distribution is restored in the drs2 mutant upon OSH4 deletion. Indicated cells expressing GFP-(PHOSH2[H340R])2-GFP, WT (PY5619), drs2/drs2 osh4/osh4 (drs2 osh4, PY5630) and osh4/osh4 (osh4, PY5626) were incubated as in Fig 6B, with sum projections of representative cells shown. The graph illustrates the means ± the SEM of the relative concentration of PM PI(4)P as a function of filament length, normalized to the maximal signal for each cell (n = 100 cells). B) PS distribution is partially restored in the drs2 mutant upon OSH4 deletion. Indicated cells expressing GFP-LactC2, WT (PY3239), drs2/drs2 (drs2, PY5134), drs2/drs2 osh4/osh4 (drs2 osh4, PY5364) and osh4/osh4 (osh4, PY5336) were incubated as in Fig 6B, with sum projections of representative cells shown. The graphs represent the relative PM and internal PS fractions, as well as the ratio of the signal at the PM over the internal signal for the indicated strains (n = 30–50 cells each), and bars mean ± the SD. ****, P < 0.0001; ***, P < 0.001; **, P < 0.01; ns, not significant. C) OSH4 deletion does not substantially restore ergosterol distribution in the drs2 mutant. Images of the indicated strains expressing mScarlet-D4H, WT (PY6037), drs2/drs2 osh4/osh4 (drs2 osh4, PY6286) and osh4/osh4 (osh4, PY6076) were taken as in Fig 7B. The graph represents the ergosterol tip polarization, i.e. the ratio of apical to sub-apical D4H signals, in the indicated strains.
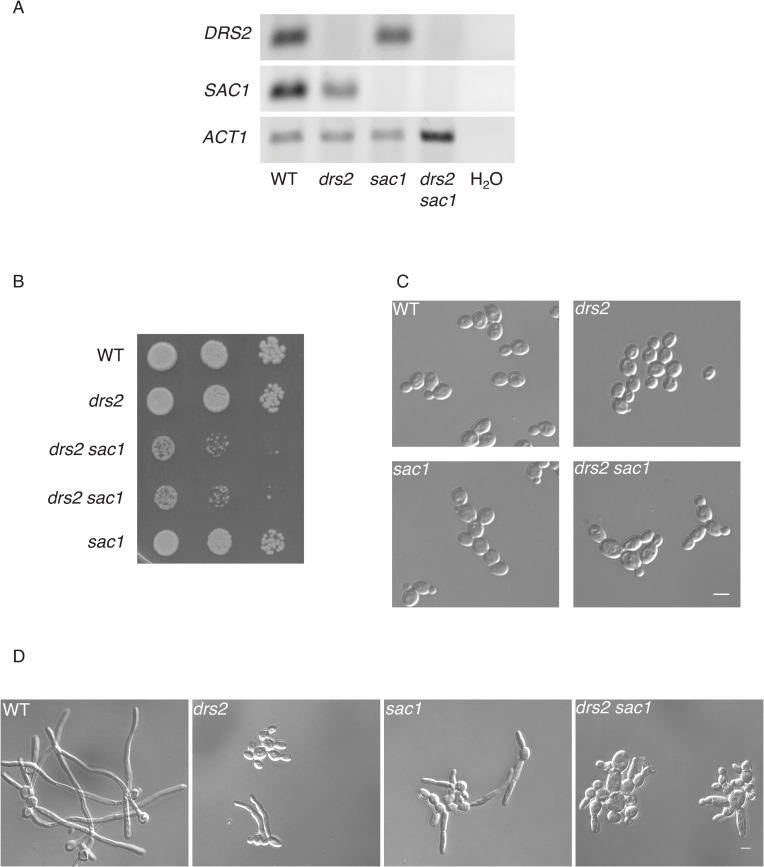
Deletion of both DRS2 and SAC1 results in a synthetic growth defect
To further examine the importance of the PM PI(4)P gradient, we generated mutants deleted for the lipid phosphatase Sac1, which dephosphorylates PI(4)P, and is responsible for regulating PM PI(4)P, both in yeast and mammalian cells [64,65]. In C. albicans, Sac1 is critical for the steep PM PI(4)P gradient [56] and hyphal growth maintenance [56,66]. In S. cerevisiae, deletion of either KES1 or SAC1 is synthetically lethal in cells largely devoid of ER-PM contact sites [67], and cold-sensitive growth of a drs2 deletion mutant is partially suppressed by deletion of either SAC1 or KES1 [31]. As deletion of OSH4 restored PI(4)P polarized distribution in drs2, as well as hyphal growth, we investigated the effect of SAC1 deletion in the drs2 mutant and RT-PCR confirmed the absence of DRS2 and/or SAC1 in the respective mutants (Fig 10A). Fig 10B and 10C show that budding growth was altered in such a drs2 sac1 mutant, with a doubling time of 270 min compared to 90 min for WT, drs2 and sac1 strains. Furthermore, in serum-containing liquid media, while the sac1 mutant formed filamentous cells, albeit shorter than the wild-type cells (Fig 10D; [56,66]), the drs2 sac1 mutant was unable to form filamentous cells, even after 270 min incubation. Together, these data show that DRS2 and SAC1 show a synthetic negative interaction in C. albicans, indicating that they operate in parallel pathways.
Fig 10. DRS2 and SAC1 interact during C. albicans development.
A) DRS2 and SAC1 transcript levels. Transcripts of the indicated strains, as in Fig 9B, were determined by RT-PCR, using SAC1.pTm/SAC1.mTm (90 bp) and DRS2.pTm/DRS2.mTm (62 bp) primer pairs. B) Deletion of both DRS2 and SAC1 results in a synthetic growth defect. Serial dilutions of indicated strains, WT (PY4861), drs2/drs2 (drs2, PY3375), drs2/drs2 sac1/sac1 (drs2 sac1, PY6431 & PY6432) and sac1/sac1 (sac1, PY6436), were spotted on YEPD and images were taken after 3 days. Similar results were observed in 2 independent experiments. C) Morphology of drs2 sac1 budding cells. Cells from the indicated strains WT (PY4861), drs2/drs2 (drs2, PY3375), drs2/drs2 sac1/sac1 (drs2 sac1, PY6432) and sac1/sac1 (sac1, PY6436), were grown to exponential phase in YEPD. D) The drs2 sac1 mutant does not generate hyphae in response to serum. Indicated strains, as in Fig 9B, were grown in the presence of serum for 180 min. At 90 min, the percent of filamentous cells was 83 ± 5%, 19 ± 8%, 38 ± 4% and 2 ± 1% for the WT, drs2, sac1 and drs2 sac1 strains, respectively; n ~ 100 cells. Bars are 5 μm.
Drs2 is important for plasma membrane organization
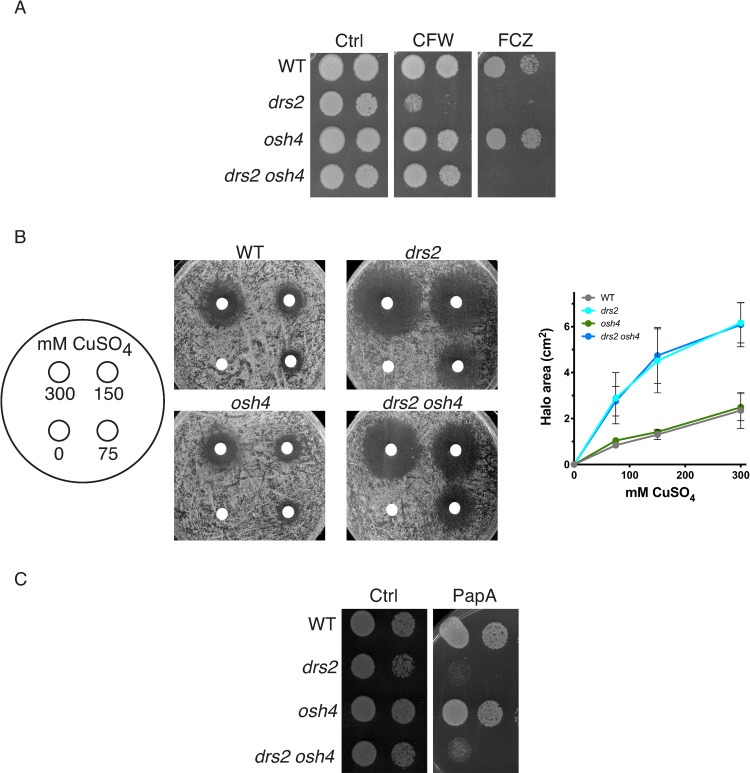
In addition to a defect in filamentous hyphal growth, the drs2 mutant also exhibits a hypersensitivity to CFW and FCZ, compared to wild-type cells (Fig 2A). We investigated whether a balance in the activities of Drs2 and Osh4 is also critical for growth on these compounds, and, while deletion of OSH4 in the drs2 mutant rescued the growth defect on CFW, it did not rescue that on FCZ (Fig 11A). Furthermore, as the drs2 mutant was reported to be hypersensitive to copper ions [35], we investigated whether deletion of OSH4 could restore growth in this condition. Fig 11B shows that both drs2 and drs2 osh4 mutants were similarly reduced for growth on CuSO4, compared to wild-type and osh4 cells, indicating that Drs2 has specific roles that are not linked to Osh4 activity.
Fig 11. Deletion of OSH4 does not rescue growth on fluconazole or copper in the drs2 mutant.
A) Deletion of OSH4 rescues growth on calcofluor white but not that on fluconazole in the drs2 mutant. Serial dilutions of indicated strains as in Fig 2B were spotted on YEPD media (Ctrl) containing 25 μg/ml calcofluor white (CFW) or 5 μg/ml fluconazole (FCZ). Images were taken after 2 days. Similar results were observed in 2 experiments. B) Deletion of OSH4 does not restore growth on copper in the drs2 mutant. The indicated strains, WT (PY4861), drs2/drs2 (drs2, PY3375), drs2/drs2 osh4/osh4 (drs2 osh4, PY5539) and osh4/osh4 (osh4, PY3974), were spread on YEPD and filter disks contained 10 μl of 0, 75, 150, or 300 mM CuSO4, were added as in [35]. The zone of growth inhibition surrounding the filter discs was recorded after 1 day at 30°C and graphs represent averages of 3 independent experiments, with standard deviations indicated. C) Deletion of OSH4 does not restore growth on papuamide A of the drs2 mutant. Serial dilutions of indicated strains, WT (PY4861), drs2/drs2 (drs2, PY3375), drs2/drs2 osh4/osh4 (drs2 osh4, PY5539) and osh4/osh4 (osh4, PY3974), were spotted on YEPD media (Ctrl) containing 1 μg/ml papuamide A (PapA) and images were taken after 2 days. Similar results were observed in 2 experiments.
As shown above, using the LactC2 reporter, we observed that the distribution of PS was altered during hyphal growth in the drs2 mutant [36]. Papuamide A (PapA) is a depsipeptide toxin that binds PS in the outer leaflet of the PM [68]. In C. albicans the cho1 deletion mutant, which lacks the PS synthase, is less sensitive to PapA than WT cells [69]. Fig 11C shows that the drs2 mutant was hypersensitive to PapA, compared to the wild-type strain, reflecting increased PS in the PM outer leaflet. Interestingly, the drs2 osh4 mutant did not grow on PapA, similar to drs2, while the osh4 mutant grew similar to the wild-type (Fig 11C). These results indicate that in this drs2 osh4 mutant, the PM PS bilayer asymmetry is not re-established.
The distinct roles of Drs2 can be associated with different localizations
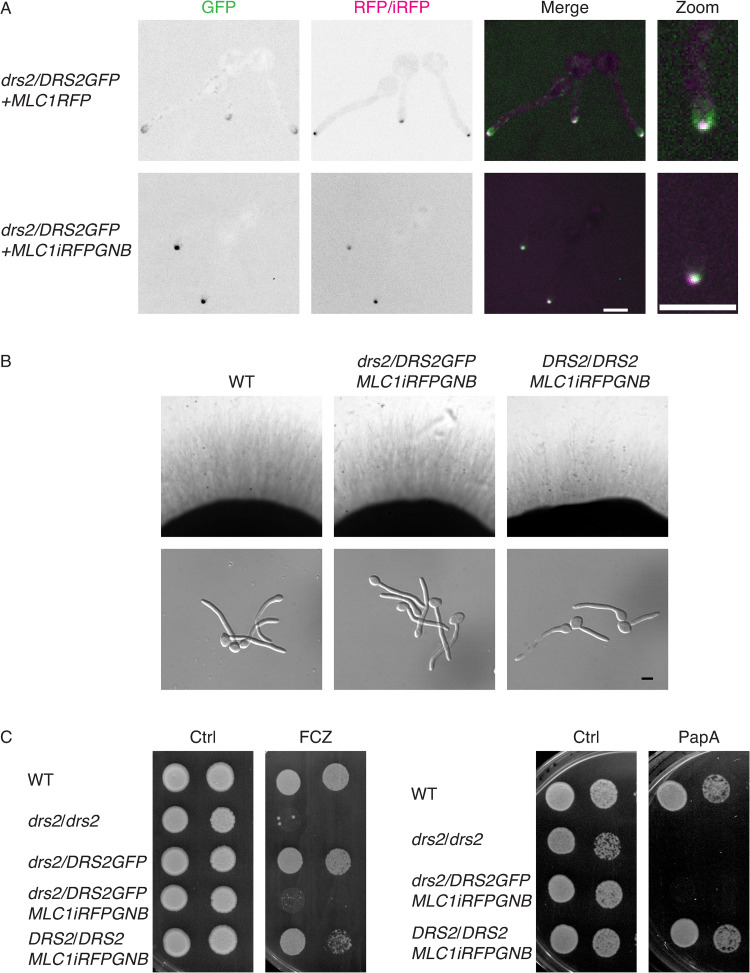
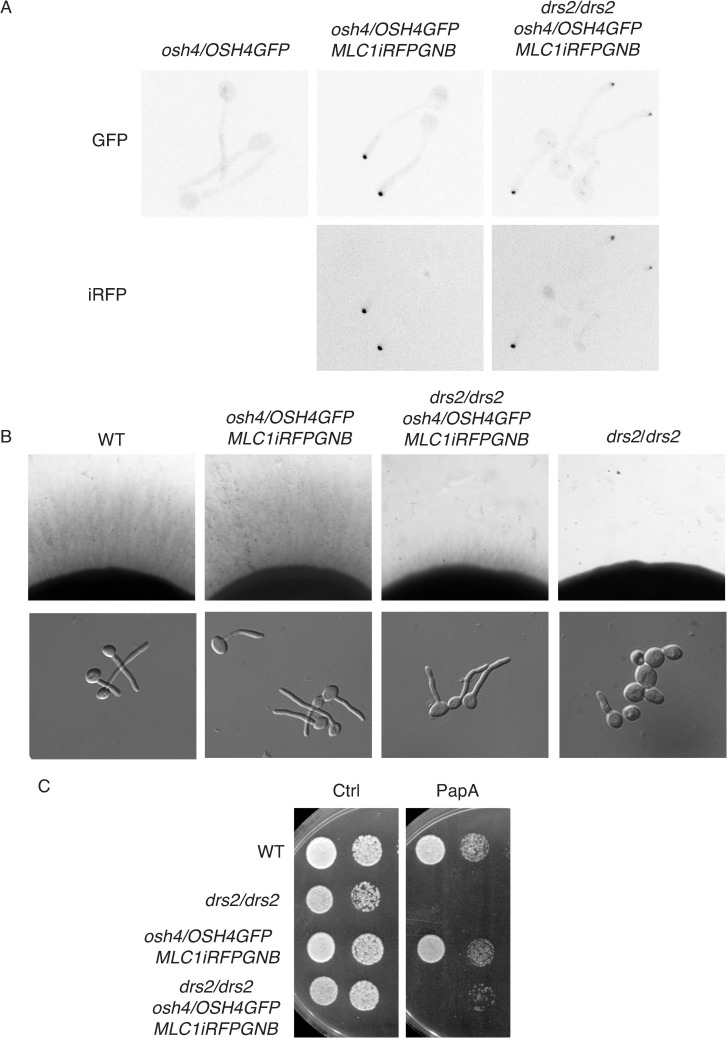
Our data indicate that while filamentous growth was restored in the drs2 mutant by OSH4 deletion, growth on fluconazole and papuamide A was not. On the other hand, Fig 5 shows that, in C. albicans, Drs2 localizes to different structures, including the SPK. Hence, we investigated whether a specific localization of Drs2 is critical for distinct functions, by using a synthetic physical interaction (SPI) approach to stabilize Drs2 at secretory vesicles, which accumulate at the SPK [50]. To generate a mutant with Drs2 restricted/stabilized at the SPK, we used a strain expressing a GFP nanobody (GNB) fused to one copy of Mlc1-iRFP, together with Drs2GFP as the sole expressed copy of DRS2. Fig 12A (bottom panel) shows that, in such a mutant, Drs2 and Mlc1 co-localized predominantly at the SPK, with the Drs2 signal strikingly increased in the strain expressing Mlc1-GNB, compared to a control strain (top panel), consistent with Drs2 stabilized at this structure. In this strain expressing Mlc1-GNB, Drs2 was not detected along the PM, compared to what we observed in the absence of the GNB construct (Figs 5 and 12A, top panel). In such a mutant expressing Mlc1-GNB, invasive filamentous growth was not altered (Fig 12B), with 85% of the cells forming hyphae in serum-containing liquid media after 90 min. In contrast, this mutant did not grow on fluconazole or papuamide A (Fig 12C). These data indicate that different roles of Drs2 are associated with its distinct cellular localizations, and suggest that C. albicans sensitivity to papuamide A is associated with Drs2 localization at the PM.
Fig 12. Stabilization of Drs2 at the Spitzenkörper alters growth on papuamide A and fluconazole.
A). Synthetic physical interaction (SPI) between Drs2 and Mlc1 stabilizes Drs2 at the Spitzenkörper. Representative images (central z-sections) of cells expressing Drs2-GFP, as a sole copy of Drs2, with either Mlc1RFP (PY4928) or Mlc1-iRFP-GNB (PY5885) are shown; iRFP stands for near-infrared fluorescent protein and GNB for GFP nanobody. Right panels are enlargement of merge images. B) Stabilization of Drs2 at the SPK does not alter invasive filamentous growth. The indicated strains, WT (PY4861), drs2/DRS2GFP MLC1-iRFP-GNB (PY5885) and DRS2/DRS2 MLC1-iRFP-GNB (PY5385) were grown on agar-containing serum media and images were taken after 6 days (top panels) or grown in the presence of serum for 90 min (bottom panels). The percent of filamentous cells was 85–86% for the wild-type, drs2/DRSGFP MLC1-iRFP-GNB and DRS2/DRS2 MLC1-iRFP-GNB strains; 2 experiments, n ~ 100 cells. C) Stabilization of Drs2 at the SPK alters growth on fluconazole and papuamide A. Serial dilutions of WT (PY4861), drs2/drs2 (PY3375), drs2/DRS2GFP (PY4665), drs2/DRS2GFP MLC1-iRFP-GNB (PY5885) and DRS2/DRS2 MLC1-iRFP-GNB (PY5385) strains spotted on YEPD media (Ctrl) containing either 2.5 μg/ml fluconazole (FCZ) or 2 μg/ml papuamide A (PapA) and images were taken after 2 days.
Given that deletion of OSH4 restored filamentous growth in a drs2 mutant, but not growth on fluconazole or papuamide A, we investigated the effect of recruiting Osh4 to the SPK in these growth conditions. In S. cerevisiae, it was shown that the majority of Osh4 was cytoplasmic, with some punctae concentrated in small buds and mother–daughter necks [70]. In C. albicans hyphal cells, we did not detect a distinct localization of Osh4 (Fig 13A, top left panel), similar to what was reported for the A. nidulans Osh4 homolog, OshC [71]. However, in cells expressing both Mlc1-GNB and Osh4-GFP, as the sole expressed copy of OSH4, Osh4 co-localized with Mlc1 at the SPK, indicating that Mlc1-GNB recruited this LTP (Fig 13A). Such a recruitment of Osh4 in a wild-type strain background did not alter filamentous growth, yet it restored filamentous growth in liquid media and partially restored that on solid media in a drs2 strain background (Fig 13B). In contrast, growth on papuamide A was not restored (Fig 13C). Together, these data indicate that recruitment of Osh4 to the SPK has a similar affect as deleting OSH4 in drs2 cells for filamentous growth.
Fig 13. The recruitment of Osh4 to the Spitzenkörper in drs2 phenocopies drs2 osh4.
A). Synthetic physical interaction between Osh4 and Mlc1 recruits Osh4 to the Spitzenkörper. Representative images (Max projections) of cells expressing Osh4-GFP, as a sole copy of Osh4, without (PY5875) or with Mlc1-iRFP-GNB, in the wild-type (PY5931) or the drs2 deletion (PY6447) strains are shown. B) Recruitment of Osh4 at the SPK restores invasive filamentous growth in a drs2 deletion strain. Indicated strains, wild-type (PY4861), drs2/drs2 (PY3375), drs2/drs2 osh4/OSH4GFP MLC1-iRFP-GNB (PY6447) and DRS2/DRS2 osh4/OSH4GFP MLC1-iRFP-GNB (PY5931) were grown on agar-containing YEPD with serum and images were taken after 6 days (top panels) or grown in the presence of serum for 90 min (bottom panels). At 90 min, the percent of filamentous cells was on average 85%, 78%, 78% and 22% for PY4861, PY5931, PY6447 and PY3375 strains, respectively; 2–3 independent experiments with n ~ 100 cells. C) Recruitment of Osh4 at the SPK does not restore growth on papuamide A in the drs2 mutant. Serial dilutions of indicated strains as in Fig 13B, were spotted on YEPD media (Ctrl) containing 2μg/ml papuamide A (PapA) and images were taken after 2 days.
Discussion
Our results show that Drs2 is critical for C. albicans invasive filamentous growth and that a drs2 deletion mutant exhibits increased sensitivity to calcofluor white and fluconazole, as well as papuamide A. The drs2 mutant also has an altered PS, PI(4)P and ergosterol distribution, but not that of PI(4,5)P2. The requirement for Drs2 in invasive filamentous growth, as well as growth in the presence of the chitin binding dye calcofluor white, can be specifically bypassed by the deletion of the lipid transfer protein Osh4, but not by that of other Osh proteins. Deletion of OSH4 in drs2 also restores the PI(4)P distribution and significantly the PS distribution, but not growth on papuamide A. Given that papuamide A binds PS in the outer leaflet of the PM, this indicates that the level of PS in the PM outer leaflet remains altered. In C. albicans, Drs2 localizes to different compartments, i.e. the Golgi, the Spitzenkörper and the PM. Such a localization of Drs2 is reminiscent of that of ChsB and SynA in A. nidulans [72], suggesting that this flippase also follows the endocytic recycling pathway. We propose that distinct roles of Drs2 are associated with different localizations.
Role of Drs2 in plasma membrane organization
In S. cerevisiae, it was shown that the DRS2/DNF1-3 genes have some redundancy during budding growth [20]. Similarly, in the filamentous fungus A. nidulans, a double deletion of the Dnf1 and Drs2 homologs (DnfA and DnfB, respectively) is lethal [25,73], indicating that these flippases are redundant, in particular for growth. Yet, they appear to also have unique functions and/or localizations in different fungi. For example, in contrast to the case in C. albicans, deletion of the Drs2 homolog DnfB alone does not drastically alter A. nidulans hyphal growth [25]. With respect to localization, Drs2 is associated to the Golgi apparatus in S. cerevisiae, even in shmoos [24], while its homolog in A. nidulans DnfB localizes to the SPK [25]. We show here that CaDrs2 localizes to the filament apex (SPK and PM), in addition to the Golgi apparatus, and our data indicate that these distinct localizations are associated with different Drs2 functions. Indeed, upon stabilization of Drs2 at secretory vesicles that accumulate at the SPK, in which this flippase is no longer detected at the PM, cells are similar to that of the wild-type for invasive filamentous growth and calcofluor white sensitivity, yet unable to grow on papuamide A. Furthermore, deletion of Osh4, which restores hyphal growth and calcofluor white sensitivity, does not restore drs2 growth on papuamide A, hence the level of PS in the PM external leaflet. Copper binds PS with high affinity and a drs2 mutant is hypersensitive to copper, linking PS and copper sensitivity [35]. Given that drs2 osh4 grows similar to drs2 on copper and papuamide A, we suggest that Drs2 is required for regulating PS asymmetry, via its ability to flip PS across the PM.
Role of lipids in invasive filamentous growth
A drs2 deletion mutant is defective in invasive filamentous growth in response to serum, and deletion of OSH4 can specifically overcome these defects. Upon serum exposure, the drs2 cells exhibit an alteration in the PM lipid distribution, i.e. PS, PI(4)P and ergosterol, which is at least partially compensated by OSH4 deletion, raising the question of the respective contribution of these lipids to hyphal growth.
Sterol-rich membrane domains, have been visualized, by using filipin, at the tips of mating projections in S. cerevisiae [74], at cell poles and division site in S. pombe [75], as well as at the hyphal tips of C. albicans [58] and A. nidulans [76], where they are thought to contribute to polarized growth. In S. cerevisiae drs2 cells, 20% of ergosterol appears to mislocalize to internal membranes, as assessed by filipin staining and DHE fluorescence, and deletion of KES1 suppresses the accumulation of intracellular ergosterol in this drs2 mutant [77]. Our data, using the D4H reporter for free ergosterol within the cell, indicates that 96 ± 7% and 78 ± 12% of cells had multiple internal punctae in the drs2 and drs2 osh4 mutants, respectively, compared to 1 ± 2% and 9 ± 6% in wild-type and osh4 cells, respectively, indicating that removal of Osh4 did not substantially suppress internal ergosterol accumulation in C. albicans drs2 cells. Polarization of ergosterol to the growth tip also appears similar in the drs2 and drs2 osh4 mutants, at a level intermediate between the wild-type and osh4 cells. Whether this polarized ergosterol signal is associated with the tip crescent and/or the SPK is unclear. In S. pombe, ergosterol associated D4H internal signals, which are enriched upon Arp2/3 inhibition, correspond to membrane-enclosed compartments, referred to as sterol-rich compartments [62]. Both this ergosterol movement to internal structures and the anterograde transport appear to be independent of vesicular trafficking [62]. Although the mechanism of sterol transfer is still to be determined, it is unlikely that the filamentous growth defect in drs2 directly results from the observed alteration of ergosterol distribution.
In C. albicans, the phosphatidylinositol 4-kinase stt4 deletion mutant, in which PM PI(4)P is no longer detectable, and the PS synthase cho1 mutant, which has little to no PS, exhibit defective filamentous growth and increased sensitivity to the antifungal drug caspofungin [9,78]. Both these mutants have increased exposure of cell wall β(1,3)-glucan and, given that 50% of PS is still detectable at the PM of stt4, we proposed that PM PI(4)P levels are associated with virulence by masking of cell wall β(1–3)-glucan [78]. In contrast to these mutants, the drs2 mutant grows similar to the wild-type cells on caspofungin and congo red, making it unlikely that the glucan synthase pathway is affected. As PM PS and PI(4)P levels are not drastically altered compared to the cho1 and stt4 deletion mutants, why the virulence is altered in drs2 is unclear. Similar to the drs2 mutant, the ratio of mean PM PS to internal PS decreases in a C. albicans stt4 mutant, with increased internal PS levels compared to wild-type cells [78], yet, as for stt4, our results are inconsistent with a correlation between PS distribution and filamentous growth defect in the drs2 mutant. Indeed, while invasive filamentous growth was restored upon deletion of Osh4, the PM to internal PS ratio was only partially restored, and deletion of Osh4 did not restore growth on papuamide A. Rather, PM PI(4)P appears to be critical. In S. cerevisiae, the cold-sensitive growth of a drs2 deletion mutant is partially suppressed by deletion of either the Osh4 homolog, Kes1, or the PI(4)P phosphatase Sac1 [31]. In C. albicans, deletion of Sac1 results in cells with altered PM PI(4)P gradient, as well as filamentous growth [56,66]. While the apical PI(4)P gradient as well as filamentous growth are restored upon deletion of Osh4 in drs2 cells, deletion of Sac1 substantially affects growth in drs2, presumably by increasing the level of PM PI(4)P. Together, these results strongly suggest that the PM PI(4)P gradient, rather than the level of PI(4)P, is critical for filamentous growth.
Role of Drs2 in cell wall integrity and fluconazole sensitivity
Lipid transport through vesicular and non-vesicular trafficking is well established [79] and PS flipping activity of Drs2 is critical for the vesicular transport of a number of PM proteins. For example, ScDrs2 is required at the Golgi for efficient segregation of cargo into exocytic vesicles, as the PM proteins Pma1 and Can1 are missorted to the vacuole in drs2 cells, and deletion of KES1 suppresses this defect [77]. Hence, it is likely that the hypersensitivity of the drs2 mutant both for calcofluor white and fluconazole results from an alteration of membrane traffic in specific pathways. Interestingly, the drs2 mutant exhibits increased sensitivity to calcofluor white but not to caspofungin, suggesting that the chitin synthase pathway is selectively altered. Consistently, it was proposed that PS translocation to cytosolic leaflet of the Golgi by a flippase is critical for the S. cerevisiae chitin synthase (Chs3) trafficking [80]. The growth defect of drs2 on fluconazole may result from a mislocalization of multi-drug transporters [39] and, consistent with such a scenario, we observe that ~ 80% of the drs2 cells had internal Cdr1 signal (Fig 2C). It would be interesting to investigate the importance of this flippase in pathogenic non albicans Candida species, known for their resistance to the antifungal drug, such as C. glabrata or C. auris [81].
Altogether our results are consistent with the notion that in C. albicans Drs2 down-regulates Osh4 activity, similar to S. cerevisiae [77], as wild-type filamentous growth and cell wall integrity were rescued in drs2, either by deleting or targeting Osh4 to the SPK. Furthermore, our data suggest that Osh4 does not function together with Drs2 at the SPK for filamentous growth, as targeting Osh4 to this organelle has the same effect as deleting Osh4. Strikingly, our data show that the genetic interaction between homologs of two different lipid transporters identified in S. cerevisiae budding growth [31], occurs during hyphal growth, a distinct growth mode in filamentous fungi. C. albicans diverged from S. cerevisiae ∼250 million years ago [82], hence an attractive possibility is that such an interaction is a conserved feature of the fungal kingdom, and filamentous fungi, with a large diversity in size and growth rate, represent useful models to understand how membrane expansion is regulated by lipid transport mechanisms.
Materials and methods
Ethics statement
Animal procedures were approved by the Bioethical Committee and Animal Welfare of the Instituto de Salud Carlos III (CBA2014_PA51) and of the Comunidad de Madrid (PROEX 330/14) and followed the current Spanish legislation (Real Decreto 53/2013) along with Directive 2010/63/EU.
Growth conditions
Yeast extract-peptone dextrose (YEPD) or synthetic complete (SC) medium was used and strains were grown at 30°C, unless indicated otherwise. Filamentous growth induction was carried out as described previously either with 50% serum in liquid media and agar plates, or 75% on agarose pads for microscopy [83]. For filipin staining experiments, 10% of serum was used. Growth on YEPD plates containing Congo red, calcofluor white, caspofungin or fluconazole was examined as described [84]. Copper sensitivity was investigated as described [30]. Congo red, calcofluor white, filipin, hygromycin and fluconazole were from Fluka, Sigma-Aldrich, Saint Quentin Fallavier, France. Papuamide A was from University British Columbia.
Strains and plasmids
Strains and oligonucleotides used are listed in Tables 1 and 2, respectively. All strains were derived from BWP17 [85]. The deletion mutants were generated by homologous recombination. Each copy was replaced by either HIS1, URA3, ARG4, SAT1 or HYGB, using knockout cassettes generated by amplification of pGem-HIS1, pGem-URA3, pGem-CdARG4, pFa-ARG4, pFa-SAT1 and pBH1S [85–88] with primer pairs DNF1.P1/DNF1.P2, DNF2.P1/DNF2.P2, DNF2.P3/DNF2.P4 DNF3.P1/DNF3.P2, OSH4.P1/OSH4.P2, OSH7Α.P1/OSH7Α.P2, OSH7α.P1/OSH7α.P2, OSH2.P1/OSH2.P2, OSH3.P1/OSH3.P2 and SAC1.P1/SAC1.P2. The drs2Δ/drs2Δ osh4Δ/osh4Δ, drs2Δ/drs2Δ osh2Δ/osh2Δ, drs2Δ/drs2Δ osh3Δ/osh3Δ, drs2Δ/drs2Δ osh7AΔ/osh7αΔ and drs2Δ/drs2Δ sac1Δ/sac1Δ strains were generated from the drs2Δ/drs2Δ strain (PY3310 [36]) and the dnf1Δ/dnf1Δ dnf2Δ/dnf2Δ strain from the dnf2Δ/dnf2Δ strain (PY5804).
Table 1. Strains used in the study.
| STRAIN | RELEVANT GENEOTYPE | REFERENCE |
|---|---|---|
| BWP17 | ura3Δ::λimm434/ura3Δ::λimm434 his1Δ::hisG/his1Δ::hisG arg4::hisG/arg4Δ::hisG | [85] |
| PY1206 | Same as BWP17 with RP10::ARG4-pADH1-GFP-PHPLCδ1-PHPLCδ1-GFP | [57] |
| PY2263 | Same as BWP17 with RP10::ARG4 pACT1-CRIBGFP | [48] |
| PY2578 | Same as BWP17 with RP10::pADH1-FAPP1[E50A,H54A]GFP | [56] |
| PY3239 | Same as BWP17 with RP10::ARG4- pACT1GFP-yeLactC2 | [36] |
| PY3310 | Same as BWP17 with drs2Δ::HIS1/ drs2Δ::URA3 | [36] |
| PY3375 | Same as PY3310 with RP10::ARG4 | [36] |
| PY3873 | Same as PY3310 with RP10::pADH1-FAPP1[E50A,H54A]GFP | [56] |
| PY3921 | Same as BWP17 with osh4Δ::URA3/ osh4Δ::HIS1 | This study |
| PY3945 | Same as BWP17 with osh3Δ::HIS1/ osh3Δ::URA3 | This study |
| PY3974 | Same as PY3921 with RP10::ARG4 | This study |
| PY3977 | Same as PY3945 with RP10::ARG4 | This study |
| PY3995 | Same as BWP17 with osh2Δ::HIS1/ osh2Δ::URA3 | This study |
| PY4002 | Same as PY3995 with RP10::ARG4 | This study |
| PY4050 | Same as PY3310 with RP10::ARG4-pADH1-GFP-PHPLCδ1-PHPLCδ1-GFP | This study |
| PY4665 | Same as PY3303 (URA3) with DRS2:: DRS2-GFPγ-HIS1 | This study |
| PY4671 | Same as PY3310 with drs2Δ::HIS1/ drs2Δ::SAT1 | This study |
| PY4861 | ura3Δ::λ imm434/ura3Δ::λ imm434 his1::hisG/HIS1::his1::hisG arg4::hisG/URA3::ARG4::arg4::hisG | [83] |
| PY4928 | Same as PY4665 with MLC1 /MLC1-mScarlet-ARG4 | This study |
| PY4946 | Same as BWP17 with dnf1Δ::SAT1/ dnf1Δ::HYGB | This study |
| PY4972 | Same as PY3310 with RP10:: ARG4 pACT1-CRIBGFP | This study |
| PY5003 | Same as PY3375 with NEUT5L::SAT1- pADHDNF2 | This study |
| PY5005 | Same as PY4861 with NEUT5L::SAT1- pADHDNF2 | This study |
| PY5042 | Same as PY3310 with RP10::ARG4-pDRS2DRS2 | [36] |
| PY5134 | Same as PY3310 with NEUT5L::SAT1-pACT1GFP-yeLactC2 | This study |
| PY5218 | Same as PY4676 with MLC1/MLC1-mScarlet-ARG4 | This study |
| PY5246 | Same as BWP17 with osh7aΔ::SAT1/ osh7αΔ::HIS1 | This study |
| PY5256 | Same as PY5246 with RP10::URA3-ARG4 | This study |
| PY5296 | Same as PY4671 with osh4Δ::URA3/ osh4Δ::HYGB | This study |
| PY5297 | Same as PY4671 with osh7αΔ::HYGB/osh7aΔ::URA3 | This study |
| PY5336 | Same as PY3921 with RP10::ARG4- pACT1GFP-yeLactC2 | This study |
| PY5349 | Same as PY4677 with MLC1/MLC1-mScarlet-ARG4 | This study |
| PY5364 | Same as PY5296 with RP10::ARG4- pACT1GFP-yeLactC2 | This study |
| PY5385 | Same as BWP17 with MLC1/MLC1-miRFP670-GNB-URA3 | [50] |
| PY5539 | Same as PY5296 with RP10::ARG4 | This study |
| PY5568 | Same as PY3310 with RP10::ARG4- pADH1- GFP-(PHOSH2[H340R])2-GFP | This study |
| PY5613 | Same as BWP17 with CDC10/CDC10::CDC10-mScarlet-ARG4 and NEUT5L::SAT1-pADH1miRFP670CtRac1 | This study |
| PY5615 | Same as PY3310 with CDC10/CDC10::CDC10-mScarlet-ARG4 and NEUT5L::SAT1-pADH1miRFP670CtRac1 | This study |
| PY5619 | Same as BWP17 with RP10::ARG4-pADH1- GFP-(PHOSH2[H340R])2-GFP | [56] |
| PY5626 | Same as PY3921 with RP10::ARG4- pADH1- GFP-(PHOSH2[H340R])2-GFP | This study |
| PY5630 | Same as PY5296 with RP10::ARG4- pADH1- GFP-(PHOSH2[H340R])2-GFP | This study |
| PY5677 | Same as BWP17 with DNF2Δ::HIS1/ DNF2 | This study |
| PY5741 | Same as BWP17 with dnf3Δ::URA3/ dnf3Δ::SAT1 | This study |
| PY5746 | Same as PY5677 with DNF2::DNF2-GFPγ-URA3 | This study |
| PY5801 | Same as PY5741 with RP10::ARG4-pExp and his1Δ::HIS1 | This study |
| PY5804 | Same as BWP17 with dnf2Δ::HIS1/ dnf2Δ::ARG4 | This study |
| PY5814 | Same as PY5804 with NEUT5L::URA3 | This study |
| PY5875 | Same as BWP17 with osh4Δ::URA3/ OSH4-GFPγ-ARG4 | This study |
| PY5885 | Same as PY5385 with drs2Δ::CdARG4/DRS2-GFPγ-HIS1 | This study |
| PY5919 | Same as PY5814 with NEUT5L::SAT1-pADHDNF2 | This study |
| PY5922 | Same as PY5871 with NEUT5L:: SAT1-pADHDNF2 | This study |
| PY5931 | Same as PY5385 with osh4Δ::HYGB/ OSH4-GFPγ-ARG4 | This study |
| PY6037 | Same as BWP17 with RP10::ARG4- pACT1-mScarlet-D4H | This study |
| PY6076 | Same as PY3921 with RP10::ARG4- pACT1-mScarlet-D4H | This study |
| PY6083 | Same as PY3310 with RP10::ARG4- pACT1-mScarlet-D4H | This study |
| PY6213 | Same as PY6083 with NEUT5L:: pACT1-Turquoise-CtRAC1-SAT1 | This study |
| PY6218 | Same as PY6083 with NEUT5L::SAT1-pDRS2DRS2 | This study |
| PY6235 | Same as PY4946 with RP10::ARG4 | This study |
| PY6237 | Same as PY6037 with NEUT5L::pACT1-Turquoise-CtRAC1-SAT1 | This study |
| PY6239 | Same as PY5746 with NEUT5L:: mScarlet-CtRAC1-SAT1 | This study |
| PY6241 | Same as PY4665 with NEUT5L:: mScarlet-CtRAC1-SAT1 | This study |
| PY6286 | Same as PY6083 with osh4Δ::HYGB/ osh4Δ::SAT1 | This study |
| PY6350 | Same as PY4972 with drs2Δ::HIS1/ drs2Δ::SAT1 | This study |
| PY6389 | Same as PY6350 with MLC1/MLC1-mScarlet-URA3 | This study |
| PY6393 | Same as PY4861 with CDR1/CDR1::CDR1-GFP-SAT1 | This study |
| PY6395 | Same as PY3375 with CDR1/CDR1::CDR1-GFP-SAT1 | This study |
| PY6400 | Same as PY5804 with dnf1Δ::URA3/ dnf1Δ::HYGB | This study |
| PY6407 | Same as PY5568 with NEUT5L::SAT1-pDRS2DRS2 | This study |
| PY6408 | Same as PY4671 with osh2Δ::URA3/ osh2Δ::HYGB | This study |
| PY6431 | Same as PY4671 with osh3Δ::URA3/ osh3Δ::ARG4 | This study |
| PY6432 | Same as PY4671 with sac1Δ::ARG4/ sac1Δ::HYGB | This study |
| PY6436 | Same as BWP17 with sac1Δ::ARG4/ sac1Δ::HYGB | This study |
| PY6447 | Same as PY4671 with osh4Δ::HYGB/ OSH4- GFPγ-ARG4 and MLC1/MLC1-miRFP670-GNB-URA3 | This study |
| PY6450 | Same as PY2263 with MLC1/MLC1-mScarlet-ARG4 | This study |
| PY6646 | Same as PY5042 with CDR1/CDR1::CDR1-GFP-SAT1 | This study |
Table 2. Primer sequences.
| PRIMER | SEQUENCE |
|---|---|
| DNF1.P1 | CAAGTGACAACTCTCCAGTTAGCTCCTATTATTTGCATCGACCAAATCTAGAAGATCTTACTAGAACATCGAAAGTTGGAGTGACCATTTGCTGAAGCTTCGTACGCTGCAGGTC |
| DNF1.P2 | CGAAAAATGTAAAACCGAAATCAAATGCAATGATACAATAAACTGAAACCCAATTACCACATAAAGTAGGTTAAGGATTCAAAGCTACACTATTCCTCTGATATCATCGATGAATTCGAG |
| DNF2.P1 | CACCTAATTCTACTTTATCCAATTTCGAATTGCTATCCACACTCTTATGACTAAAAATAAGCACAATTTCACGCGAAGCTTCGTACGCTGCAGGTC |
| DNF2.P2 | CTTGTATGTGTGTTTGTTGTCTATAATGTTTTCTTTTATGTTATGCAAATGAAAATTGCTGTTAATTCATTGAAGATACTCTCTGATATCATCGATGAATTCGAG |
| DNF2.P3 | CACCTAATTCTACTTTATCCAATTTCGAATTGCTATCCACACTCTTATGACTAAAAATAAGCACAATTTCACGCTAAATAATAGTAGTGATGGAGTGTGGAATTGTGAGCGGATA |
| DNF2.P4 | GTATGTGTGTTTGTTGTCTATAATGTTTTCTTTTATGTTATGCAAATGAAAATTGCTGTTAATTCATTGAAGATACTCTTTTTTGATTTTCGTAGCTTATTCTAGATGCTCTTTCCCAGTCACGACGTT |
| DNF3.P1 | CTACAGATTCAACTTTATAGAATCCTACATTGAGTCACCTGTCTCATCATTTGGTCATTAATTTACAACTACAGTGAATCATACAATTAGATTTGGAAGCTTCGTACGCTGCAGGTC |
| DNF3.P2 | GCTCGGGTGAATTGAGACAATTTTGATGTATCTTGTATATTTGTATAAATACTTAGATGTACATTTCTTTTGGTTTTGGAAACTCAATAGACACCTCTGATATCATCGATGAATTCGAG |
| OSH4.P1 | CTATATTTTAAAATAACACATCGTCTTCAAGCCAACAGTATATTTTTTTGTCATGTCAAACTCCAAATCCAGTTCTGAAGTGTGGAATTGTGAGCGGATA |
| OSH4.P2 | GATGTACTACTATTATAAACATTATTTTACGAAATTGAAAAAACCTACTCTAAATTCATTTAAAAGACTATTTCTTTCCCAGTCACGACGTT |
| OSH7A.P1 | CCCTTCCATTAAATCTCCCTGAGATTTGTTGTTTTTTATCTGTATTCAGTACGAAGTCAAGTTCACCATGGGTTTAACTGTGGAATTGTGAGCGGATA |
| OSH7A.P2 | CGATTTCTAATCTAAACTAAAGATAAATATATAAAGAAGTACATTAAATTGCAATATATCAAAAACGAATTAAGTTTCTTCTTTCCCAGTCACGACGTT |
| OSH7F061.P1 | CCAGCGAACATGCACTTTTCTTTCGTTGAAGGTTCAGGTAATCAGTATGGGATTAACAGCTAAATTAGATAAGTGTGGAATTGTGAGCGGATA |
| Osh7F061.p2 | TAAATTAGACAATACTACTTGATTCTAATTCAAAATCTATTAATATGACCGTATATTAAAAGTCCAACAATTAATTACTTTCCCAGTCACGACGTT |
| OSH2.P1 | CCATAATAAATTACTGATAAAACACTAACAACTTTAGAATAAACAGCTAAAATCATGAATGATTCACCCCAACTAAGTGCGAAGCTTCGTACGCTGCAGGTC |
| OSH2.P2 | CAATCAACAATTAAAACAGTCTAAAATATGTCTCCCGTGTTCGCCAAATCTTGTGCCTTTCTTCTAGGCCAATACTCTGATATCATCGATGAATTCGAG |
| OSH3.P1 | CCCACTCAATCCCATTATTTCTTTCCCTTGACAGTTGAACTTTCCCATACCAACTTGAAATATGGAAACTTTAGAAGGAAGCTTCGTACGCTGCAGGTC |
| OSH3.P2 | GGATTCTATGATCTAAATAATATAACGTCTAATAGATATGAAAAAGACAAAGAAATAGTAAAATTTACCACAATTTAATCTCTGATATCATCGATGAATTCGAG |
| SAC1.P1 | AATATAAACACTATTTTTTCCACTTTCTTTAATCTAACCTTATACGCAACTATACAATTATATAACAGAAAATGGTGTTAGAAGCTTCGTACGCTGCAGGTC |
| SAC1.P2 | ATCTATTATATAAAATAAGCAGCTACAACTAAAGACAACTTTCTAAAAAAAAAAACAACCCCCACAGCTAATCAATTCTTTCTGATATCATCGATGAATTCGAG |
| DNF2.P5 | GGCGCGCCATGACTAAAAATAAGCACAATTTCACGC |
| DNF2.P6 | ATACGCGTTTAATTCATTGAAGATACTCTTTTTTG |
| DRS2.P1 | TAACTAGTCTTTTAATATAGCCGAATTCCCCG |
| DRS2.P2 | TATAGCGGCCGCGAGTTGGTTAATAACAAAATATCAG |
| OSH4.P3 | GGAACTTTGAAAGACTCAAAGTATGACCATGGTGAAGCCAAGCATTGGAGAGTTAACTTGAAAAAGTTGGAAGATGAAAAAGAAATAGTCTTTGGTGCTGGCGCAGGTGCTTC |
| OSH4.P4 | GTAATCCTTCTATAGTAAACTGTTTATATTTAAATGATGTACTACTATTATAAACATTATTTTACGAAATTGAAAAAACCTACTCTAAATTCATTTATCTGATATCATCGATGAATTCGAG |
| DRS2.P3 | CAAGTTGAAGGTCAAGATCAAGATAAAATTGTTAGATTATATGATACTACTAAAAAAAGAGGAGTTTTTGGGGAATTATCAGAAAGTAAGGGTGCTGGCGCAGGTGCTTC |
| DRS2.P4 | GGGAGTGAGATAGTTGTATATCAAACAGTTCTATAGCTTTAAATAAATAAAAAAAAACATTCATTGATTGCATTAATATACTACTCATACTCTATTTATCTGATATCATCGATGAATTCGAG |
| DNF2.P7 | GGTAGCTACAGAAGAAATTCCATTAGAAGATTTTGATGATGAACAAAAAAGAGCATCTAGAATAAGCTACGAAAATCAAAAAAGAGTATCTTCAATGAATGGTGCTGGCGCAGGTGCTTC |
| DNF2.P8 | CCTCTCATTCTTTATATAAATATGCTTGAAATTCTTGTATGTGTGTTTGTTGTCTATAATGTTTTCTTTTATGTTATGCAAATGAAAATTGCTGTTATCTGATATCATCGATGAATTCGAG |
| MLC1.P1 | GATGAGTTATTAAAAGGGGTCAATGTAACTTCTGATGGAAATGTGGATTATGTTGAATTTGTCAAATCAATTTTAGACCAAGGTGCTGGCGCAGGTGCTTC |
| MLC1.P2 | CGAACAAGACTATACAATAACTATAATTTGTAAAACTTGTAGTATATATATTTCAATGGTTAATTGTAAATTTTCTTTTATTCTGATATCATCGATGAATTCGAG |
| CDC10.P1 | CAATCAAACCAAGATTTGAAGAACACCTCTGGTGTGCCAAATGCTCCTATGTTCCAATCAACTACAGGTACTGCTGCTGCTAGAGGTGCTGGCGCAGGTGCTTC |
| CDC10.P2 | CGCGTTTTGCTTTTCAACAAACACACAAAAGAAGAGGAATACAAAAAAGTAAAATCACATTATATCAATAACAAACCTGATATCATCGATGAATTCGAG |
| D4H.P1 | ACGGCGCGCCGACTAGTGGATCCAAGGGAAAAATAAACTTAGATCATAGTGGAGCC |
| D4H.P2 | GATACGCGTTAATTGTAAGTAATACTAGATCCAGGG |
| RT.PCR | |
| DRS2.pTm | CGGCATTAGTGGTTACCATGT |
| DRS2.mTm | GCAGGATACCAACCTAACCATAA |
| DNF1.pTm | GCACAATCAACGGGAAGTCATAC |
| DNF1.mTm | AATGCCTGCACGTTTATCCAA |
| DNF2.pTm | TGACAAGACCGGGACATTA |
| DNF2.mTm | TCCGTAACCCAGCCAAT |
| DNF3.pTm | AAGCATCAATGGCAGGTCATC |
| DNF3.mTm | GCTGCCGTGCTTGTTCTTG |
| OSH4.pTm | TTTGATTACTGCTGCTCCTT |
| OSH4.mTm | AATGTGACCACTGGATGCTT |
| OSH7a.pTm | TGAAGCAGCTGTCAAATCTAATGTG |
| OSH7a.mTm | CTGTCCCTCGTTATCCATTTCATC |
| OSH7α.pTm | GTAACTCGTCTGCAGCAATCATG |
| OSH7 α.mTm | CCTCGTTGTCCCATTTACCAA |
| OSH2.pTm | AAGCCAGAAAACAGAGGGAAGAT |
| OSH2.mTm | TCACTGGATGCTTTCTCTTAACAAA |
| OSH3.pTm | GGGTAAACTTGCACCTACGGATT |
| OSH3.mTm | GCAGTGTCTGTATCTCCCTTTTCA |
| UME6.pTm | TGGCTCCACTTACAAATCATAGTGA |
| UME6.mTm | GCTTTACCAATCCTAGTCCCAACTC |
| SAC1.pTm | CCCATTCCACAGCAACAGATG |
| SAC1.mTm | TTCAACACCACCTCCCAGTGT |
| ACT1.pTm | ATGTTCCCAGGTATTGCTGA |
| ACT1.mTm | ACATTTGTGGTGAACAATGG |
| TDH3.pTm | ATCCCACAAGGACTGGAGA |
| TDH3.mTm | GCAGAAGCTTTAGCAACGTG |
Plasmid pDUP3-pADH1DNF2 was constructed by amplification from gDNA of the DNF2 ORF, using primers with a unique AscI site at the 5’ end (DNF2.P5) and a unique MluI site at the 3’ end (DNF2.P6) and pDUP3-pDRS2DRS2 by amplification from pExp-pDRS2DRS2 [36] of the DRS2 ORF together with 1 kb upstream and downstream, using primers with a unique SpeI site at the 5’ end (DRS2.P1) and a unique NotI site at the 3’ end (DRS2.P2). The fragments were subsequently cloned into pDUP3-SAT1 [89], yielding to pDUP3-pADH1DNF2 and pDUP3-pDRS2DRS2, respectively. To visualize Drs2, Dnf2 and Osh4, GFPγ was inserted by homologous recombination at the 3’ end of DRS2, DNF2 or OSH4 ORF in strains heterozygous for these genes, after amplification of GFPγ from the plasmid pFA-GFPγ-HIS1 [90], using the primers DRS2.P3/DRS2.P4, DNF2.P7/DNF2.P8 or OSH4.P3/OSH4.P4. pExp-pACT1CRIBGFP [48] was used to transform the WT (BWP17) and drs2/drs2 (PY3310) strains. Cdr1-GFP, PH-FAPP1[E50A,H54A]-GFP, GFP-(PHOSH2[H340R])2-GFP and GFP-PHPLCδ1-PHPLCδ1-GFP expressing strains were generated as described [56,57]. pDUP5-mScarlet-CtRac1 [91] was used to transform the drs2/DRS2GFP (PY4665) and dnf2/DNF2GFP (PY5746) strains. Mlc1-mScarlet and Cdc10-mScarlet were generated by amplification of mScarlet-ARG4 from pFA-mScarlet-ARG4 [92], using the primer pairs MLC1.P1/MLC1.P2 and CDC10.P1/CDC10.P2, respectively. mTurquoise-CtRac1 (mTrq-CtRac1) was constructed by replacing mScarlet in pDUP5-pADH1-mScarlet-CtRac1 [91] with mTrq, and subsequently cloning mTrq-CtRac1 into pDUP3. To visualize the distribution of phosphatidylserine, a fusion of GFP with the discoidin-like C2 domain of lactadherin (GFP-LactC2) was used, as previously described [36]. Strains expressing Mlc1-iRFP-GNB were generated by homologous recombination, using pFA-iRFP670-GNB-URA3, as in [50]. To visualize the distribution of ergosterol, we used filipin staining, as described [58], as well as the genetically encoded biosensor D4H [62]. D4H was amplified from plasmid pSM2244 [62], using primer pair D4H.P1 and D4H.P2 and cloned into plasmid pExp-pACT1-mScarlet-CtRac1 [92], using AscI and MluI unique restriction sites, yielding pExp-pACT1-mScarlet-D4H. This plasmid was linearized with NcoI and integrated into the RP10 locus. All other pExp plasmids were linearized with StuI and integrated into the RP10 locus. pDUP3 and pDUP5 plasmids were digested with NgoMIV to release the cassette to be integrated into the NEUTL5 locus.
Two independent clones of each strain were generated and confirmed by PCR. RT-PCR was also performed, where relevant, using the primers (GENE.pTm and GENE.mTm) listed in Table 2 or previously described [93] and RNA extraction was carried out using Master Pure yeast RNA extraction purification kit (Epicentre). All PCR amplified products were confirmed by sequencing (Eurofins MWG Operon, Ebersberg, Germany).
Microscopy analyses
Colony and cell morphology imaging were performed as described [83]. Briefly, plates were incubated for 3–6 days prior to imaging with a Leica MZ6 binocular (x20) and cells (budding or serum-induced) were imaged by differential interference contrast with a microscope Leica DMR, using an ImagingSource DMK 23UX174 sCMOS camera.
Time lapses and fluorescent images were obtained using a spinning disk confocal microscope (inverted IX81 Olympus microscope with a 100X objective and a numerical aperture 1.45) and an EMCCD camera (Andor technology, UK). Z-stacks (images of 0.4 μm sections) were acquired every 5 min, as described [56]. Maximum or sum intensity projections were generated from 21 z-sections with ImageJ software. Laser illuminations of 445 nm (Turquoise), 488 nm (GFP), 561 nm (mScarlet) and 640 nm (iRFP) were used. CRIBGFP distribution experiments were carried out as described [48]. For filipin analyses, widefield images were acquired on an inverted ZEISS Axio Observer Z1 microscope with a 100X (1.3 NA) objective. Huygens professional software version 18.04 (Scientific-Volume Imaging) was used to deconvolve z-stack images of cells expressing PH-FAPP1[E50A,H54A]-GFP. Bars are 5 μm.
Filament lengths and diameters were measured from DIC images, using ImageJ. Golgi cisternae were quantitated from deconvolved MAX projection images. Quantitation of PI(4)P distribution was performed on SUM projections, using the Matlab program HyphalPolarity [56]. Quantitation of PS PM and internal mean signals was performed on central z-sections, also using the Matlab program HyphalPolarity. Central Z sections are middle planes of the cells. Unless indicated otherwise, error bars represent the standard deviations. Statistical significance was determined with Student’s unpaired two-tailed t test.
Virulence assays
HDC was induced in 10 Balb/C mice (Charles Rivers, Italy) per group by injecting the lateral tail vein with an inoculum of 5 x 105 cells [94]. Animal body weight was monitored daily and animals were sacrificed by cervical dislocation when they had lost more than 20% of their weight.
Supporting information
A) DRS2 and DNF1-3 transcript levels. mRNA and cDNA were prepared from the indicated strains, as in Fig 1A. DRS2 and DNF1-3 transcripts were determined by RT-PCR, using DRS2.pTm/DRS2.mTm (62 bp), DNF1.pTm/DNF1.mTm (73 bp), DNF2.pTm/DNF2.mTm (106 bp) and DNF3.pTm/DNF3.mTm (104 bp) primer pairs, respectively. Actin (ACT1) transcript levels (ACT1.pTm/ACT1.mTm primer pair) were used for normalization. B) OSH4 and OSH7 transcript levels. mRNA and cDNA were prepared from the indicated strains, as in Fig 8A. Transcripts were determined as in S1A Fig, using OSH4.pTm/OSH4.mTm (69 bp), OSH7A.pTm/OSH7A.mTm (84 bp), OSH7α.pTm/ OSH7α.mTm (65 bp) primer pairs, respectively. C) Deletion of DRS2 does not result in a significant alteration of OSH4 expression. mRNA and cDNA were prepared from the indicated strains grown without (0) or with serum for 120 min (120). OSH4 and DRS2 transcripts were determined by qRT-PCR using primer pairs OSH4.pTm/OSH4.mTm and DRS2.pTm/DRS2.mTm as above. Bars indicate the mean ± SD of 2 biological samples (n = 3 determinations each). ACT1 transcript levels were used for normalization.
(TIF)
A) DRS2 and OSH2-3 transcript levels. mRNA and cDNA were prepared from the indicated strains, and transcripts were determined as in S1A Fig, using OSH2.pTm/OSH2.mTm (74 bp), OSH3.pTm/OSH3.mTm (74 bp) primer pairs, respectively. B) Invasive growth is not restored in the drs2 mutant upon deletion of OSH2 or OSH3. The indicated strains, WT (PY4861), osh2/osh2 (osh2, PY3977), osh3/osh3 (osh3, PY4002), drs2/drs2 (drs2, PY3375), drs2/drs2 osh2/osh2 (drs2 osh2, PY6408) and drs2/drs2 osh3/osh3 (drs2 osh3, PY6431), were grown on agar-containing serum media and images were taken after 6 days. Similar results were observed in 2 independent experiments. C) Hyphal growth is not restored in the drs2 mutant upon deletion of OSH2 or OSH3. At 90 min, the percent of filamentous cells, was determined from 3 independent biological samples for the WT, drs2, osh2, osh3, drs2 osh2 and drs2 osh3 strains; n ~ 100 cells.
(TIF)
Time lapse of wild-type (PY6450) cells expressing Mlc1-mScarlet, together with CRIB-GFP incubated in the presence of serum. Images were taken every 10 min and maximum projections of 21 z-sections are shown.
(AVI)
Time lapse of drs2/drs2 cells (PY6389) expressing Mlc1-mScarlet, together with CRIB-GFP incubated in the presence of serum. Images were taken every 10 min and maximum projections of 21 z-sections are shown.
(AVI)
Acknowledgments
We thank J. Berman, S. Martin, A. Mitchell, J.C. Shieh and J. Wendland, for plasmids and strains. We thank the Platform Resources in Imaging and Scientific Microscopy facility (PRISM; M. Mondin, S. Lachambre, and B. Monterroso), and Microscopy Imaging Côte d’Azur (MICA) for microscopy support.
Data Availability
All relevant data are within the manuscript and its supporting information files.
Funding Statement
This work was supported by the CNRS, INSERM, Université Côte d’Azur, and ANR (ANR-11-LABX-0028-01, ANR-16-CE13-0010-01 and ANR-19-CE13-0004-01) grants to RAA, MB, and grant SAF2017-86192 from the Spanish Ministry for Science and Innovation to RG, OZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Das A, Slaughter BD, Unruh JR, Bradford WD, Alexander R, Rubinstein B, et al. Flippase-mediated phospholipid asymmetry promotes fast Cdc42 recycling in dynamic maintenance of cell polarity. Nat Cell Biol. 2012;14(3):304–10. Epub 2012/02/22. doi: 10.1038/ncb2444 ; PubMed Central PMCID: PMC3534761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fairn GD, Hermansson M, Somerharju P, Grinstein S. Phosphatidylserine is polarized and required for proper Cdc42 localization and for development of cell polarity. Nat Cell Biol. 2011;13(12):1424–30. Epub 2011/10/04. doi: 10.1038/ncb2351 . [DOI] [PubMed] [Google Scholar]
- 3.Meca J, Massoni-Laporte A, Martinez D, Sartorel E, Loquet A, Habenstein B, et al. Avidity-driven polarity establishment via multivalent lipid-GTPase module interactions. EMBO J. 2019;38(3). Epub 2018/12/19. doi: 10.15252/embj.201899652 ; PubMed Central PMCID: PMC6356062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haupt A, Minc N. Gradients of phosphatidylserine contribute to plasma membrane charge localization and cell polarity in fission yeast. Mol Biol Cell. 2017;28(1):210–20. Epub 2016/11/18. doi: 10.1091/mbc.E16-06-0353 ; PubMed Central PMCID: PMC5221626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kay JG, Fairn GD. Distribution, dynamics and functional roles of phosphatidylserine within the cell. Cell Commun Signal. 2019;17(1):126. Epub 2019/10/17. doi: 10.1186/s12964-019-0438-z ; PubMed Central PMCID: PMC6792266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noack LC, Jaillais Y. Functions of Anionic Lipids in Plants. Annu Rev Plant Biol. 2020;71:71–102. Epub 2020/05/23. doi: 10.1146/annurev-arplant-081519-035910 . [DOI] [PubMed] [Google Scholar]
- 7.Cassilly CD, Reynolds TB. PS, It’s Complicated: The Roles of Phosphatidylserine and Phosphatidylethanolamine in the Pathogenesis of Candida albicans and Other Microbial Pathogens. J Fungi (Basel). 2018;4(1). Epub 2018/02/21. doi: 10.3390/jof4010028 ; PubMed Central PMCID: PMC5872331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo J, Stanchev LD, da Silva VKA, Nimrichter L, Pomorski TG, Rodrigues ML. Role of lipid transporters in fungal physiology and pathogenicity. Comput Struct Biotechnol J. 2019;17:1278–89. Epub 2020/01/11. doi: 10.1016/j.csbj.2019.09.001 ; PubMed Central PMCID: PMC6944739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YL, Montedonico AE, Kauffman S, Dunlap JR, Menn FM, Reynolds TB. Phosphatidylserine synthase and phosphatidylserine decarboxylase are essential for cell wall integrity and virulence in Candida albicans. Mol Microbiol. 2010;75(5):1112–32. Epub 2010/02/06. doi: 10.1111/j.1365-2958.2009.07018.x . [DOI] [PubMed] [Google Scholar]
- 10.Chen T, Jackson JW, Tams RN, Davis SE, Sparer TE, Reynolds TB. Exposure of Candida albicans beta (1,3)-glucan is promoted by activation of the Cek1 pathway. PLoS Genet. 2019;15(1):e1007892. Epub 2019/02/01. doi: 10.1371/journal.pgen.1007892 ; PubMed Central PMCID: PMC6372213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenoir G, D’Ambrosio JM, Dieudonne T, Copic A. Transport Pathways That Contribute to the Cellular Distribution of Phosphatidylserine. Front Cell Dev Biol. 2021;9:737907. Epub 2021/09/21. doi: 10.3389/fcell.2021.737907 ; PubMed Central PMCID: PMC8440936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyons JA, Timcenko M, Dieudonne T, Lenoir G, Nissen P. P4-ATPases: how an old dog learnt new tricks—structure and mechanism of lipid flippases. Curr Opin Struct Biol. 2020;63:65–73. Epub 2020/06/04. doi: 10.1016/j.sbi.2020.04.001 . [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Marques RL. Lipid flippases in polarized growth. Curr Genet. 2021;67(2):255–62. Epub 2021/01/04. doi: 10.1007/s00294-020-01145-0 . [DOI] [PubMed] [Google Scholar]
- 14.Lenoir G, Williamson P, Puts CF, Holthuis JC. Cdc50p plays a vital role in the ATPase reaction cycle of the putative aminophospholipid transporter Drs2p. J Biol Chem. 2009;284(27):17956–67. Epub 2009/05/05. doi: 10.1074/jbc.M109.013722 ; PubMed Central PMCID: PMC2709398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saito K, Fujimura-Kamada K, Furuta N, Kato U, Umeda M, Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15(7):3418–32. Epub 2004/04/20. doi: 10.1091/mbc.e03-11-0829 ; PubMed Central PMCID: PMC452594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timcenko M, Lyons JA, Januliene D, Ulstrup JJ, Dieudonne T, Montigny C, et al. Structure and autoregulation of a P4-ATPase lipid flippase. Nature. 2019;571(7765):366–70. Epub 2019/06/28. doi: 10.1038/s41586-019-1344-7 . [DOI] [PubMed] [Google Scholar]
- 17.Natarajan P, Wang J, Hua Z, Graham TR. Drs2p-coupled aminophospholipid translocase activity in yeast Golgi membranes and relationship to in vivo function. Proc Natl Acad Sci U S A. 2004;101(29):10614–9. Epub 2004/07/14. doi: 10.1073/pnas.0404146101 ; PubMed Central PMCID: PMC489982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, Graham TR. Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc Natl Acad Sci U S A. 2009;106(39):16586–91. Epub 2009/10/07. doi: 10.1073/pnas.0904293106 ; PubMed Central PMCID: PMC2757829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Best JT, Xu P, Graham TR. Phospholipid flippases in membrane remodeling and transport carrier biogenesis. Curr Opin Cell Biol. 2019;59:8–15. Epub 2019/03/22. doi: 10.1016/j.ceb.2019.02.004 ; PubMed Central PMCID: PMC6726550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua Z, Fatheddin P, Graham TR. An essential subfamily of Drs2p-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol Biol Cell. 2002;13(9):3162–77. Epub 2002/09/11. doi: 10.1091/mbc.e02-03-0172 ; PubMed Central PMCID: PMC124150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen CY, Ingram MF, Rosal PH, Graham TR. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function. J Cell Biol. 1999;147(6):1223–36. Epub 1999/12/22. doi: 10.1083/jcb.147.6.1223 ; PubMed Central PMCID: PMC2168089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pomorski T, Lombardi R, Riezman H, Devaux PF, van Meer G, Holthuis JC. Drs2p-related P-type ATPases Dnf1p and Dnf2p are required for phospholipid translocation across the yeast plasma membrane and serve a role in endocytosis. Mol Biol Cell. 2003;14(3):1240–54. Epub 2003/03/13. doi: 10.1091/mbc.e02-08-0501 ; PubMed Central PMCID: PMC151593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frosig MM, Costa SR, Liesche J, Osterberg JT, Hanisch S, Nintemann S, et al. Pseudohyphal growth in Saccharomyces cerevisiae involves protein kinase-regulated lipid flippases. J Cell Sci. 2020;133(15). Epub 2020/07/15. doi: 10.1242/jcs.235994 . [DOI] [PubMed] [Google Scholar]
- 24.Sartorel E, Barrey E, Lau RK, Thorner J. Plasma membrane aminoglycerolipid flippase function is required for signaling competence in the yeast mating pheromone response pathway. Mol Biol Cell. 2015;26(1):134–50. Epub 2014/11/08. doi: 10.1091/mbc.E14-07-1193 ; PubMed Central PMCID: PMC4279224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultzhaus Z, Yan H, Shaw BD. Aspergillus nidulans flippase DnfA is cargo of the endocytic collar and plays complementary roles in growth and phosphatidylserine asymmetry with another flippase, DnfB. Mol Microbiol. 2015;97(1):18–32. Epub 2015/04/08. doi: 10.1111/mmi.13019 . [DOI] [PubMed] [Google Scholar]
- 26.Yun Y, Guo P, Zhang J, You H, Guo P, Deng H, et al. Flippases play specific but distinct roles in the development, pathogenicity, and secondary metabolism of Fusarium graminearum. Mol Plant Pathol. 2020;21(10):1307–21. Epub 2020/09/04. doi: 10.1111/mpp.12985 ; PubMed Central PMCID: PMC7488471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong LH, Gatta AT, Levine TP. Lipid transfer proteins: the lipid commute via shuttles, bridges and tubes. Nat Rev Mol Cell Biol. 2019;20(2):85–101. Epub 2018/10/20. doi: 10.1038/s41580-018-0071-5 . [DOI] [PubMed] [Google Scholar]
- 28.Maeda K, Anand K, Chiapparino A, Kumar A, Poletto M, Kaksonen M, et al. Interactome map uncovers phosphatidylserine transport by oxysterol-binding proteins. Nature. 2013;501(7466):257–61. Epub 2013/08/13. doi: 10.1038/nature12430 . [DOI] [PubMed] [Google Scholar]
- 29.Moser von Filseck J, Vanni S, Mesmin B, Antonny B, Drin G. A phosphatidylinositol-4-phosphate powered exchange mechanism to create a lipid gradient between membranes. Nat Commun. 2015;6:6671. Epub 2015/04/08. doi: 10.1038/ncomms7671 . [DOI] [PubMed] [Google Scholar]
- 30.de Saint-Jean M, Delfosse V, Douguet D, Chicanne G, Payrastre B, Bourguet W, et al. Osh4p exchanges sterols for phosphatidylinositol 4-phosphate between lipid bilayers. J Cell Biol. 2011;195(6):965–78. Epub 2011/12/14. doi: 10.1083/jcb.201104062 ; PubMed Central PMCID: PMC3241724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthusamy BP, Raychaudhuri S, Natarajan P, Abe F, Liu K, Prinz WA, et al. Control of protein and sterol trafficking by antagonistic activities of a type IV P-type ATPase and oxysterol binding protein homologue. Mol Biol Cell. 2009;20(12):2920–31. Epub 2009/05/01. doi: 10.1091/mbc.e08-10-1036 ; PubMed Central PMCID: PMC2695799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilbert MJ, Thornton CR, Wakley GE, Talbot NJ. A P-type ATPase required for rice blast disease and induction of host resistance. Nature. 2006;440(7083):535–9. Epub 2006/03/24. doi: 10.1038/nature04567 . [DOI] [PubMed] [Google Scholar]
- 33.Li B, Dong X, Zhao R, Kou R, Zheng X, Zhang H. The t-SNARE protein FgPep12, associated with FgVam7, is essential for ascospore discharge and plant infection by trafficking Ca2+ ATPase FgNeo1 between Golgi and endosome/vacuole in Fusarium graminearum. PLoS Pathog. 2019;15(5):e1007754. Epub 2019/05/09. doi: 10.1371/journal.ppat.1007754 ; PubMed Central PMCID: PMC6527245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Yun Y, Lou Y, Abubakar YS, Guo P, Wang S, et al. FgAP-2 complex is essential for pathogenicity and polarised growth and regulates the apical localisation of membrane lipid flippases in Fusarium graminearum. Cell Microbiol. 2019;21(8):e13041. Epub 2019/05/16. doi: 10.1111/cmi.13041 . [DOI] [PubMed] [Google Scholar]
- 35.Douglas LM, Konopka JB. Plasma membrane architecture protects Candida albicans from killing by copper. PLoS Genet. 2019;15(1):e1007911. Epub 2019/01/12. doi: 10.1371/journal.pgen.1007911 ; PubMed Central PMCID: PMC6345494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Labbaoui H, Bogliolo S, Ghugtyal V, Solis NV, Filler SG, Arkowitz RA, et al. Role of Arf GTPases in fungal morphogenesis and virulence. PLoS Pathog. 2017;13(2):e1006205. Epub 2017/02/14. doi: 10.1371/journal.ppat.1006205 ; PubMed Central PMCID: PMC5325608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu D, Zhang X, Zhang B, Zeng X, Mao H, Xu H, et al. The lipid flippase subunit Cdc50 is required for antifungal drug resistance, endocytosis, hyphal development and virulence in Candida albicans. FEMS Yeast Res. 2019;19(3). Epub 2019/04/21. doi: 10.1093/femsyr/foz033 . [DOI] [PubMed] [Google Scholar]
- 38.Jain BKW A. S.; Reynolds T. B.; Graham T. R. Lipid transport by Candida albicans Dnf2 is required for hyphal growth and virulence. bioRxiv. 2022: doi: 10.1101/2022.06.10.495726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prasad R, De Wergifosse P, Goffeau A, Balzi E. Molecular cloning and characterization of a novel gene of Candida albicans, CDR1, conferring multiple resistance to drugs and antifungals. Curr Genet. 1995;27(4):320–9. Epub 1995/03/01. doi: 10.1007/BF00352101 . [DOI] [PubMed] [Google Scholar]
- 40.Carlisle PL, Kadosh D. Candida albicans Ume6, a filament-specific transcriptional regulator, directs hyphal growth via a pathway involving Hgc1 cyclin-related protein. Eukaryot Cell. 2010;9(9):1320–8. Epub 2010/07/27. doi: 10.1128/EC.00046-10 ; PubMed Central PMCID: PMC2937344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Banerjee M, Thompson DS, Lazzell A, Carlisle PL, Pierce C, Monteagudo C, et al. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell. 2008;19(4):1354–65. Epub 2008/01/25. doi: 10.1091/mbc.e07-11-1110 ; PubMed Central PMCID: PMC2291399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng X, Wang Y, Wang Y. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J. 2004;23(8):1845–56. Epub 2004/04/09. doi: 10.1038/sj.emboj.7600195 ; PubMed Central PMCID: PMC394249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naglik JR, Gaffen SL, Hube B. Candidalysin: discovery and function in Candida albicans infections. Curr Opin Microbiol. 2019;52:100–9. Epub 2019/07/10. doi: 10.1016/j.mib.2019.06.002 ; PubMed Central PMCID: PMC6687503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuchimori N, Sharkey LL, Fonzi WA, French SW, Edwards JE Jr., Filler SG. Reduced virulence of HWP1-deficient mutants of Candida albicans and their interactions with host cells. Infect Immun. 2000;68(4):1997–2002. Epub 2000/03/18. doi: 10.1128/IAI.68.4.1997-2002.2000 ; PubMed Central PMCID: PMC97378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cleary IA, Reinhard SM, Miller CL, Murdoch C, Thornhill MH, Lazzell AL, et al. Candida albicans adhesin Als3p is dispensable for virulence in the mouse model of disseminated candidiasis. Microbiology (Reading). 2011;157(Pt 6):1806–15. Epub 2011/03/26. doi: 10.1099/mic.0.046326-0 ; PubMed Central PMCID: PMC3167918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hube B, Monod M, Schofield DA, Brown AJ, Gow NA. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14(1):87–99. Epub 1994/10/01. doi: 10.1111/j.1365-2958.1994.tb01269.x . [DOI] [PubMed] [Google Scholar]
- 47.Gonzalez-Novo A, Jimenez J, Garcia MJ, Rios-Serrano I, Pla J, Jimenez A, et al. Dynamics of CaCdc10, a septin of Candida albicans, in living cells and during infection. Int Microbiol. 2004;7(2):105–12. Epub 2004/07/13. . [PubMed] [Google Scholar]
- 48.Corvest V, Bogliolo S, Follette P, Arkowitz RA, Bassilana M. Spatiotemporal regulation of Rho1 and Cdc42 activity during Candida albicans filamentous growth. Mol Microbiol. 2013;89(4):626–48. Epub 2013/06/26. doi: 10.1111/mmi.12302 . [DOI] [PubMed] [Google Scholar]
- 49.Crampin H, Finley K, Gerami-Nejad M, Court H, Gale C, Berman J, et al. Candida albicans hyphae have a Spitzenkorper that is distinct from the polarisome found in yeast and pseudohyphae. J Cell Sci. 2005;118(Pt 13):2935–47. Epub 2005/06/25. doi: 10.1242/jcs.02414 . [DOI] [PubMed] [Google Scholar]
- 50.Puerner C, Serrano A, Wakade RS, Bassilana M, Arkowitz RA. A Myosin Light Chain Is Critical for Fungal Growth Robustness in Candida albicans. mBio. 2021;12(5):e0252821. Epub 2021/10/06. doi: 10.1128/mBio.02528-21 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vauchelles R, Stalder D, Botton T, Arkowitz RA, Bassilana M. Rac1 dynamics in the human opportunistic fungal pathogen Candida albicans. PLoS One. 2010;5(10):e15400. Epub 2010/11/10. doi: 10.1371/journal.pone.0015400 ; PubMed Central PMCID: PMC2965673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weiner A, Orange F, Lacas-Gervais S, Rechav K, Ghugtyal V, Bassilana M, et al. On-site secretory vesicle delivery drives filamentous growth in the fungal pathogen Candida albicans. Cell Microbiol. 2019;21(1):e12963. Epub 2018/10/16. doi: 10.1111/cmi.12963 . [DOI] [PubMed] [Google Scholar]
- 53.Del Vecchio K, Stahelin RV. Investigation of the phosphatidylserine binding properties of the lipid biosensor, Lactadherin C2 (LactC2), in different membrane environments. J Bioenerg Biomembr. 2018;50(1):1–10. Epub 2018/02/11. doi: 10.1007/s10863-018-9745-0 ; PubMed Central PMCID: PMC5820145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–27. Epub 2010/03/03. doi: 10.1146/annurev.biophys.093008.131234 . [DOI] [PubMed] [Google Scholar]
- 55.Yeung T, Gilbert GE, Shi J, Silvius J, Kapus A, Grinstein S. Membrane phosphatidylserine regulates surface charge and protein localization. Science. 2008;319(5860):210–3. Epub 2008/01/12. doi: 10.1126/science.1152066 . [DOI] [PubMed] [Google Scholar]
- 56.Ghugtyal V, Garcia-Rodas R, Seminara A, Schaub S, Bassilana M, Arkowitz RA. Phosphatidylinositol-4-phosphate-dependent membrane traffic is critical for fungal filamentous growth. Proc Natl Acad Sci U S A. 2015;112(28):8644–9. Epub 2015/07/01. doi: 10.1073/pnas.1504259112 ; PubMed Central PMCID: PMC4507248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vernay A, Schaub S, Guillas I, Bassilana M, Arkowitz RA. A steep phosphoinositide bis-phosphate gradient forms during fungal filamentous growth. J Cell Biol. 2012;198(4):711–30. Epub 2012/08/15. doi: 10.1083/jcb.201203099 ; PubMed Central PMCID: PMC3514036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin SW, Konopka JB. Lipid raft polarization contributes to hyphal growth in Candida albicans. Eukaryot Cell. 2004;3(3):675–84. Epub 2004/06/11. doi: 10.1128/EC.3.3.675-684.2004 ; PubMed Central PMCID: PMC420133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kishimoto T, Mioka T, Itoh E, Williams DE, Andersen RJ, Tanaka K. Phospholipid flippases and Sfk1 are essential for the retention of ergosterol in the plasma membrane. Mol Biol Cell. 2021;32(15):1374–92. Epub 2021/05/27. doi: 10.1091/mbc.E20-11-0699 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koselny K, Mutlu N, Minard AY, Kumar A, Krysan DJ, Wellington M. A Genome-Wide Screen of Deletion Mutants in the Filamentous Saccharomyces cerevisiae Background Identifies Ergosterol as a Direct Trigger of Macrophage Pyroptosis. mBio. 2018;9(4). Epub 2018/08/02. doi: 10.1128/mBio.01204-18 ; PubMed Central PMCID: PMC6069111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maekawa M, Yang Y, Fairn GD. Perfringolysin O Theta Toxin as a Tool to Monitor the Distribution and Inhomogeneity of Cholesterol in Cellular Membranes. Toxins (Basel). 2016;8(3). Epub 2016/03/24. doi: 10.3390/toxins8030067 ; PubMed Central PMCID: PMC4810212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marek M, Vincenzetti V, Martin SG. Sterol biosensor reveals LAM-family Ltc1-dependent sterol flow to endosomes upon Arp2/3 inhibition. J Cell Biol. 2020;219(6). Epub 2020/04/23. doi: 10.1083/jcb.202001147 ; PubMed Central PMCID: PMC7265315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hur HS, Ryu JH, Kim KH, Kim J. Characterization of Osh3, an oxysterol-binding protein, in filamentous growth of Saccharomyces cerevisiae and Candida albicans. J Microbiol. 2006;44(5):523–9. Epub 2006/11/04. . [PubMed] [Google Scholar]
- 64.Stefan CJ, Manford AG, Baird D, Yamada-Hanff J, Mao Y, Emr SD. Osh proteins regulate phosphoinositide metabolism at ER-plasma membrane contact sites. Cell. 2011;144(3):389–401. Epub 2011/02/08. doi: 10.1016/j.cell.2010.12.034 . [DOI] [PubMed] [Google Scholar]
- 65.Zewe JP, Wills RC, Sangappa S, Goulden BD, Hammond GR. SAC1 degrades its lipid substrate PtdIns4P in the endoplasmic reticulum to maintain a steep chemical gradient with donor membranes. Elife. 2018;7. Epub 2018/02/21. doi: 10.7554/eLife.35588 ; PubMed Central PMCID: PMC5829913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang B, Yu Q, Jia C, Wang Y, Xiao C, Dong Y, et al. The actin-related protein Sac1 is required for morphogenesis and cell wall integrity in Candida albicans. Fungal Genet Biol. 2015;81:261–70. Epub 2015/01/13. doi: 10.1016/j.fgb.2014.12.007 . [DOI] [PubMed] [Google Scholar]
- 67.Quon E, Sere YY, Chauhan N, Johansen J, Sullivan DP, Dittman JS, et al. Endoplasmic reticulum-plasma membrane contact sites integrate sterol and phospholipid regulation. PLoS Biol. 2018;16(5):e2003864. Epub 2018/05/22. doi: 10.1371/journal.pbio.2003864 ; PubMed Central PMCID: PMC5983861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parsons AB, Lopez A, Givoni IE, Williams DE, Gray CA, Porter J, et al. Exploring the mode-of-action of bioactive compounds by chemical-genetic profiling in yeast. Cell. 2006;126(3):611–25. Epub 2006/08/12. doi: 10.1016/j.cell.2006.06.040 . [DOI] [PubMed] [Google Scholar]
- 69.Cassilly CD, Maddox MM, Cherian PT, Bowling JJ, Hamann MT, Lee RE, et al. SB-224289 Antagonizes the Antifungal Mechanism of the Marine Depsipeptide Papuamide A. PLoS One. 2016;11(5):e0154932. Epub 2016/05/18. doi: 10.1371/journal.pone.0154932 ; PubMed Central PMCID: PMC4868317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ling Y, Hayano S, Novick P. Osh4p is needed to reduce the level of phosphatidylinositol-4-phosphate on secretory vesicles as they mature. Mol Biol Cell. 2014;25(21):3389–400. Epub 2014/08/29. doi: 10.1091/mbc.E14-06-1087 ; PubMed Central PMCID: PMC4214785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buhler N, Hagiwara D, Takeshita N. Functional Analysis of Sterol Transporter Orthologues in the Filamentous Fungus Aspergillus nidulans. Eukaryot Cell. 2015;14(9):908–21. Epub 2015/06/28. doi: 10.1128/EC.00027-15 ; PubMed Central PMCID: PMC4551590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hernandez-Gonzalez M, Bravo-Plaza I, Pinar M, de Los Rios V, Arst HN Jr., Penalva MA. Endocytic recycling via the TGN underlies the polarized hyphal mode of life. PLoS Genet. 2018;14(4):e1007291. Epub 2018/04/03. doi: 10.1371/journal.pgen.1007291 ; PubMed Central PMCID: PMC5880334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schultzhaus Z, Cunningham GA, Mourino-Perez RR, Shaw BD. The phospholipid flippase DnfD localizes to late Golgi and is involved in asexual differentiation in Aspergillus nidulans. Mycologia. 2019;111(1):13–25. Epub 2019/01/31. doi: 10.1080/00275514.2018.1543927 . [DOI] [PubMed] [Google Scholar]
- 74.Bagnat M, Simons K. Cell surface polarization during yeast mating. Proc Natl Acad Sci U S A. 2002;99(22):14183–8. Epub 2002/10/11. doi: 10.1073/pnas.172517799 ; PubMed Central PMCID: PMC137858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wachtler V, Rajagopalan S, Balasubramanian MK. Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 2003;116(Pt 5):867–74. Epub 2003/02/07. doi: 10.1242/jcs.00299 . [DOI] [PubMed] [Google Scholar]
- 76.Takeshita N, Higashitsuji Y, Konzack S, Fischer R. Apical sterol-rich membranes are essential for localizing cell end markers that determine growth directionality in the filamentous fungus Aspergillus nidulans. Mol Biol Cell. 2008;19(1):339–51. Epub 2007/11/16. doi: 10.1091/mbc.e07-06-0523 ; PubMed Central PMCID: PMC2174190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hankins HM, Sere YY, Diab NS, Menon AK, Graham TR. Phosphatidylserine translocation at the yeast trans-Golgi network regulates protein sorting into exocytic vesicles. Mol Biol Cell. 2015;26(25):4674–85. Epub 2015/10/16. doi: 10.1091/mbc.E15-07-0487 ; PubMed Central PMCID: PMC4678023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Garcia-Rodas R, Labbaoui H, Orange F, Solis N, Zaragoza O, Filler SG, et al. Plasma Membrane Phosphatidylinositol-4-Phosphate Is Not Necessary for Candida albicans Viability yet Is Key for Cell Wall Integrity and Systemic Infection. mBio. 2022:e0387321. Epub 2022/02/16. doi: 10.1128/mbio.03873-21 ; PubMed Central PMCID: PMC8942462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stefan CJ, Trimble WS, Grinstein S, Drin G, Reinisch K, De Camilli P, et al. Membrane dynamics and organelle biogenesis-lipid pipelines and vesicular carriers. BMC Biol. 2017;15(1):102. Epub 2017/11/02. doi: 10.1186/s12915-017-0432-0 ; PubMed Central PMCID: PMC5663033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu P, Baldridge RD, Chi RJ, Burd CG, Graham TR. Phosphatidylserine flipping enhances membrane curvature and negative charge required for vesicular transport. J Cell Biol. 2013;202(6):875–86. Epub 2013/09/11. doi: 10.1083/jcb.201305094 ; PubMed Central PMCID: PMC3776346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berkow EL, Lockhart SR. Fluconazole resistance in Candida species: a current perspective. Infect Drug Resist. 2017;10:237–45. Epub 2017/08/18. doi: 10.2147/IDR.S118892 ; PubMed Central PMCID: PMC5546770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen XX, Opulente DA, Kominek J, Zhou X, Steenwyk JL, Buh KV, et al. Tempo and Mode of Genome Evolution in the Budding Yeast Subphylum. Cell. 2018;175(6):1533–45 e20. Epub 2018/11/13. doi: 10.1016/j.cell.2018.10.023 ; PubMed Central PMCID: PMC6291210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bassilana M, Blyth J, Arkowitz RA. Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans. Eukaryot Cell. 2003;2(1):9–18. Epub 2003/02/13. doi: 10.1128/EC.2.1.9-18.2003 ; PubMed Central PMCID: PMC141177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ram AF, Klis FM. Identification of fungal cell wall mutants using susceptibility assays based on Calcofluor white and Congo red. Nat Protoc. 2006;1(5):2253–6. Epub 2007/04/05. doi: 10.1038/nprot.2006.397 . [DOI] [PubMed] [Google Scholar]
- 85.Wilson RB, Davis D, Mitchell AP. Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol. 1999;181(6):1868–74. Epub 1999/03/12. doi: 10.1128/JB.181.6.1868-1874.1999 ; PubMed Central PMCID: PMC93587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bijlani S, Thevandavakkam MA, Tsai HJ, Berman J. Autonomously Replicating Linear Plasmids That Facilitate the Analysis of Replication Origin Function in Candida albicans. mSphere. 2019;4(2). Epub 2019/03/08. doi: 10.1128/mSphere.00103-19 ; PubMed Central PMCID: PMC6403455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai WC, Sun HF, Lin PH, Ho Lin HL, Shieh JC. A new rapid and efficient system with dominant selection developed to inactivate and conditionally express genes in Candida albicans. Curr Genet. 2016;62(1):213–35. Epub 2015/10/27. doi: 10.1007/s00294-015-0526-6 . [DOI] [PubMed] [Google Scholar]
- 88.Schaub Y, Dunkler A, Walther A, Wendland J. New pFA-cassettes for PCR-based gene manipulation in Candida albicans. J Basic Microbiol. 2006;46(5):416–29. Epub 2006/09/30. doi: 10.1002/jobm.200510133 . [DOI] [PubMed] [Google Scholar]
- 89.Gerami-Nejad M, Zacchi LF, McClellan M, Matter K, Berman J. Shuttle vectors for facile gap repair cloning and integration into a neutral locus in Candida albicans. Microbiology (Reading). 2013;159(Pt 3):565–79. Epub 2013/01/12. doi: 10.1099/mic.0.064097-0 ; PubMed Central PMCID: PMC3709822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li L, Zhang C, Konopka JB. A Candida albicans temperature-sensitive cdc12-6 mutant identifies roles for septins in selection of sites of germ tube formation and hyphal morphogenesis. Eukaryot Cell. 2012;11(10):1210–8. Epub 2012/08/14. doi: 10.1128/EC.00216-12 ; PubMed Central PMCID: PMC3485918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Puerner C, Kukhaleishvili N, Thomson D, Schaub S, Noblin X, Seminara A, et al. Mechanical force-induced morphology changes in a human fungal pathogen. BMC Biol. 2020;18(1):122. Epub 2020/09/12. doi: 10.1186/s12915-020-00833-0 ; PubMed Central PMCID: PMC7488538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silva PM, Puerner C, Seminara A, Bassilana M, Arkowitz RA. Secretory Vesicle Clustering in Fungal Filamentous Cells Does Not Require Directional Growth. Cell Rep. 2019;28(8):2231–45 e5. Epub 2019/08/23. doi: 10.1016/j.celrep.2019.07.062 . [DOI] [PubMed] [Google Scholar]
- 93.Bassilana M, Hopkins J, Arkowitz RA. Regulation of the Cdc42/Cdc24 GTPase module during Candida albicans hyphal growth. Eukaryot Cell. 2005;4(3):588–603. Epub 2005/03/10. doi: 10.1128/EC.4.3.588-603.2005 ; PubMed Central PMCID: PMC1087799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sanchez AA, Johnston DA, Myers C, Edwards JE Jr., Mitchell AP, Filler SG. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect Immun. 2004;72(1):598–601. Epub 2003/12/23. doi: 10.1128/IAI.72.1.598-601.2004 ; PubMed Central PMCID: PMC344013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A) DRS2 and DNF1-3 transcript levels. mRNA and cDNA were prepared from the indicated strains, as in Fig 1A. DRS2 and DNF1-3 transcripts were determined by RT-PCR, using DRS2.pTm/DRS2.mTm (62 bp), DNF1.pTm/DNF1.mTm (73 bp), DNF2.pTm/DNF2.mTm (106 bp) and DNF3.pTm/DNF3.mTm (104 bp) primer pairs, respectively. Actin (ACT1) transcript levels (ACT1.pTm/ACT1.mTm primer pair) were used for normalization. B) OSH4 and OSH7 transcript levels. mRNA and cDNA were prepared from the indicated strains, as in Fig 8A. Transcripts were determined as in S1A Fig, using OSH4.pTm/OSH4.mTm (69 bp), OSH7A.pTm/OSH7A.mTm (84 bp), OSH7α.pTm/ OSH7α.mTm (65 bp) primer pairs, respectively. C) Deletion of DRS2 does not result in a significant alteration of OSH4 expression. mRNA and cDNA were prepared from the indicated strains grown without (0) or with serum for 120 min (120). OSH4 and DRS2 transcripts were determined by qRT-PCR using primer pairs OSH4.pTm/OSH4.mTm and DRS2.pTm/DRS2.mTm as above. Bars indicate the mean ± SD of 2 biological samples (n = 3 determinations each). ACT1 transcript levels were used for normalization.
(TIF)
A) DRS2 and OSH2-3 transcript levels. mRNA and cDNA were prepared from the indicated strains, and transcripts were determined as in S1A Fig, using OSH2.pTm/OSH2.mTm (74 bp), OSH3.pTm/OSH3.mTm (74 bp) primer pairs, respectively. B) Invasive growth is not restored in the drs2 mutant upon deletion of OSH2 or OSH3. The indicated strains, WT (PY4861), osh2/osh2 (osh2, PY3977), osh3/osh3 (osh3, PY4002), drs2/drs2 (drs2, PY3375), drs2/drs2 osh2/osh2 (drs2 osh2, PY6408) and drs2/drs2 osh3/osh3 (drs2 osh3, PY6431), were grown on agar-containing serum media and images were taken after 6 days. Similar results were observed in 2 independent experiments. C) Hyphal growth is not restored in the drs2 mutant upon deletion of OSH2 or OSH3. At 90 min, the percent of filamentous cells, was determined from 3 independent biological samples for the WT, drs2, osh2, osh3, drs2 osh2 and drs2 osh3 strains; n ~ 100 cells.
(TIF)
Time lapse of wild-type (PY6450) cells expressing Mlc1-mScarlet, together with CRIB-GFP incubated in the presence of serum. Images were taken every 10 min and maximum projections of 21 z-sections are shown.
(AVI)
Time lapse of drs2/drs2 cells (PY6389) expressing Mlc1-mScarlet, together with CRIB-GFP incubated in the presence of serum. Images were taken every 10 min and maximum projections of 21 z-sections are shown.
(AVI)
Data Availability Statement
All relevant data are within the manuscript and its supporting information files.