Abstract
The purpose of this study was to examine the suitability of fibronectin-binding protein FBP54 as a putative vaccine for Streptococcus pyogenes infections. When the distribution of the fbp54 gene among the clinical isolates representing various M serotypes was tested by PCR and Southern blot assays, it was found that all of the strains possess this gene. Furthermore, a significant increase in immunoglobulin G (IgG) antibody titers against FBP54 was observed in sera from patients with S. pyogenes infections compared with those from healthy volunteers (P < 0.005). Mice were immunized with FBP54 subcutaneously, orally, or nasally. An enzyme-linked immunosorbent assay revealed that antigen-specific IgG antibodies were induced in the sera of immunized mice, while high salivary levels of IgA antibodies were detected after oral and nasal immunizations. Mice subcutaneously or orally immunized with FBP54 survived significantly longer following the challenge with S. pyogenes than did nonimmunized mice (P < 0.001). These results indicate that FBP54 is a promising vaccine for the prevention of S. pyogenes infections.
Streptococcus pyogenes causes a number of diseases, such as uncomplicated pharyngitis, impetigo, acute rheumatic fever, and poststreptococcal glomerulonephritis. Acute rheumatic fever has decreased markedly in developed countries; however, it is still a leading cause of heart diseases among children in developing countries (3, 40). An acute type of severe S. pyogenes infection called streptococcal toxic shock-like syndrome (TSLS) has been reported in the United States, Europe, and Japan since the mid-1980s (9, 16, 21, 28). TSLS is usually associated with severe infections like necrotizing fasciitis and septicemia. Thus, a safe and effective vaccine against S. pyogenes would be highly desirable worldwide, and in this context, streptococcal surface proteins which are involved in the adhesion and invasion of host cells may be reasonable targets in the development of a new vaccine for protection against S. pyogenes infection.
A major virulence factor located on the streptococcal cell surface may be the M protein that confers resistance to phagocytosis and acts as an adhesin (11, 15). Although many studies have revealed that M protein elicits protective immune responses against the infecting serotypes (6, 26, 27), there are two major problems in the development of this vaccine. One is that more than 100 different M serotypes have been identified and a number of clinical isolates have been M nontypeable (19, 35). The other is that some M proteins share structural homology with cardiac proteins such as myosin and tropomyosin (10, 17). The M19 protein possesses both a protective epitope and an autoimmune epitope in the amino terminus (2).
Immunological aspects of M proteins have been extensively studied; however, the other streptococcal protein antigens have not been well assessed as vaccine candidates. In this regard, there are studies showing that immunization with proteases such as C5a peptidase and streptococcal cysteine protease elicit a non-type-specific immunity irrespective of the M serotype (20, 22). More recently, several cell surface proteins have been reported to induce protective immune responses in mice (8, 13, 39). However, it is still unclear whether these proteins induce protective immune responses to different M serotypes.
Many studies have revealed that the fibronectin-binding proteins of S. pyogenes, SfbI (identical to protein F1) and FBP54, function as epithelial cell adhesins and/or invasins (4, 14, 18, 31). We demonstrate here that all of the clinical isolates of S. pyogenes possess the gene fbp54, which encodes fibronectin-binding protein FBP54 (5), and that the deduced amino acid sequences of this protein are highly (>98%) conserved among different M serotypes. Thus, the ultimate goal of this study was to determine whether the FBP54 protein is a promising candidate for use as a vaccine against S. pyogenes infection.
MATERIALS AND METHODS
Animals.
Female BALB/c and CD1 mice were obtained from Charles River Inc. (Yokohama, Japan) and were provided sterile food and water ad libitum. All of the mice used in this study were 6 weeks old.
Bacterial strains and growth conditions.
S. pyogenes strains isolated from Japanese patients with TSLS and with uncomplicated pharyngitis were obtained from T. Murai (Toho University, Tokyo, Japan) and Y. Shimizu (Asahi Central Hospital, Chiba, Japan), and the other clinical isolates were identified in our laboratory. Streptococcus mutans MT8148 and Streptococcus gordonii ATCC 10558 were employed as negative controls to assess the production of FBP54. Streptococcal strains were grown in Todd-Hewitt broth (Becton Dickinson, Cockeysville, Md.) supplemented with 0.2% yeast extract (THY) or on THY agar plates. emm genotyping was performed as described previously (1). Escherichia coli XL1-Blue (Stratagene, La Jolla, Calif.) served as the host for DNA manipulation experiments and was cultured in Luria-Bertani (LB) medium or on LB agar supplemented with ampicillin (100 μg/ml).
DNA manipulations.
Isolation of chromosomal DNA and plasmid DNA, transformation, Southern blotting, and PCR were performed as described previously (38). Nucleotide sequences of target genes were determined by a DNA sequencer (model 310; PE Applied Biosystems, Foster City, Calif.) and analyzed with the GeneWorks program (IntelliGenetics, Campbell, Calif.). Other DNA manipulations were carried out in accordance with the manufacturers' instructions.
Preparation of FBP54 and anti-FBP54 specific antibody.
The fbp54 gene was amplified by PCR from strain SSI-1 genomic DNA as a template with primers containing EcoRI and XbaI sites. The PCR product was ligated with the pTrc99A (Pharmacia) expression vector, and the resultant plasmid was transformed into E. coli XL-1 Blue. The organisms were grown in LB broth to mid-log phase and incubated for another 2 h following the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 0.3 mM. Recombinant FBP54 (rFBP54) protein was purified from this cell lysate. FBP54-specific antiserum was obtained from rabbits immunized subcutaneously (s.c.) four times with rFBP54 protein.
Measurement of FBP54 expression on streptococcal strains by enzyme-linked immunosorbent assay (ELISA).
Streptococcal strains were grown overnight in THY broth with or without bovine testis hyaluronidase (final concentration; 100 μg/ml; Wako Pure Chemical, Osaka, Japan). The cells were harvested by centrifugation and suspended in 0.1 M carbonate buffer (pH 9.6). The samples (100 μl) were placed into 96-well ELISA plates (Sumitomo Bakelite, Tokyo, Japan) and incubated for 2 h at 37°C. The wells were blocked with phosphate-buffered saline (PBS) containing skim milk for 2 h at 37°C. The wells were washed three times with PBS and incubated with rabbit anti-FBP54 serum for 2 h at 37°C. The plates were washed and incubated with horseradish peroxidase (HRPO)-conjugated goat anti-rabbit immunoglobulin G (IgG) antibody (Southern Biotechnology Associates, Birmingham, Ala.) for 2 h at 37°C. The wells were then washed and developed with TMB (3, 3′, 5, 5′-tetramethylbenzidine; Moss Inc., Pasadena, Md.) solution. After a 15-min incubation, the enzyme reaction was stopped by adding 0.5 N HCl and the A450 of the plates was read using a microplate reader (Titertek MK11; Flow Laboratories, McLean, Va.).
Immunization of mice.
Female 6-week-old BALB/c mice were separated into five groups for immunization with rFBP54 as shown in Table 1. We performed three separate experiments. Two groups were orally immunized with cholera toxin (CT; 10 μg)-FBP54 (10 μg) or CT (10 μg)-PBS by using an intubation needle. The third group was nasally immunized with CT (10 μg)-FBP54 (10 μg). The fourth group was s.c. immunized with FBP54 (10 μg) in Freund's complete adjuvant (FCA; Difco Laboratories, Detroit, Mich.). The fifth group was s.c. injected with PBS. Immunization was performed four times, on days 0, 14, 28, and 42.
TABLE 1.
Experimental protocol for immunization with FBP54
| Groupa | Vaccine/adjuvant used | Procedureb on postimmunization day:
|
||||
|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 42 | 51 | ||
| I | FBP54/FCA | s.c. | s.c. | s.c. | s.c. | Challenge |
| II | FBP54/CT | i.n. | i.n. | i.n. | i.n. | Challenge |
| III | FBP54/CT | p.o. | p.o. | p.o. | p.o. | Challenge |
| IV | CT | p.o. | p.o. | p.o. | p.o. | Challenge |
| V | None | Challenge | ||||
Each group consisted of five female six-week-old BALB/c mice.
Each vaccine was injected biweekly for a total of four inoculations. Blood and saliva samples were collected on days 0, 14, 28, and 49. Mice were challenged with S. pyogenes on day 51. s.c., s.c. injection with an emulsion of FBP54 and FCA; i.n., intranasal immunization with 10 μg of FBP54 and 10 μg of CT; p.o., peroral inoculation with 20 μg of FBP54 and 10 μg of CT or with 10 μg of CT alone.
Sample collection.
Human sera were obtained from 10 healthy volunteers (age, 26.4 ± 2.8 years) and from 12 patients (age, 33.2 ± 18.2 years) with S. pyogenes infection with informed consent. Blood and saliva samples of mice were obtained on days 0, 14, 28, and 49. Blood samples were collected from the inferior ophthalmic vein by using a capillary glass tube. Saliva samples were collected by treatment with pilocarpine hydrochloride (Wako Pure Chemical). These samples were divided into aliquots and stored at −20°C until used.
Antigen-specific ELISA.
Serum samples were analyzed for FBP54-specific IgA and IgG antibodies by ELISA using a modified version of the procedures described previously (23, 24). Briefly, 96-well ELISA plates (Sumitomo Bakelite) were coated with FBP54 (5 μg/ml) in 0.1 M carbonate buffer (pH 9.6) and incubated at 4°C. The wells were blocked with PBS containing skim milk overnight at 4°C. Duplicate twofold serial dilutions of samples in an appropriate range for the particular analysis were incubated in the wells overnight at 4°C. The wells were washed and incubated overnight at 4°C with HRPO-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch Labs, West Grove, Pa.) or HRPO-conjugated goat anti-mouse IgA or IgG antibody (Southern Biotechnology Associates). The wells were then washed and developed with TMB solution. After 15 min of incubation, the enzyme reaction was stopped by adding 0.5 N HCl and the A450 of the plates was read using a microplate reader (Titertek MK11; Flow Laboratories). The results were presented as the difference between the absorbances of the pre- and postimmunization samples. The reciprocal endpoint titers of antigen-specific IgA and IgG antibodies were defined as the highest dilutions giving an A450 of 0.1.
Opsonophagocytosis assays.
The opsonization assay was done, with minor modifications, as described previously (8). Briefly, 80 μl of heparinized whole blood was mixed with 10 μl of bacteria (∼5 × 106 CFU) and 20 μl of rabbit anti-FBP54 serum or nonimmunized serum for 5 to 10 min at 37°C. The sample fixed on a glass slide was stained with Giemsa stain, and the percentage of bacterium-associated leukocytes was determined by counting 100 cells per slide under a microscope.
The bactericidal assay was performed, with minor modifications, as described previously (41). Briefly, 80 μl of heparinized whole blood was mixed with 10 μl of bacteria (∼30 CFU) and 20 μl of rabbit anti-FBP54 serum or nonimmunized serum and gently shaked for 3 h at 37°C. The mixture was serially diluted and plated on THY agar. Following incubation, the number of CFU was determined.
Mouse protection assays.
Actively immunized mice were intraperitoneally challenged with an appropriate number of CFU of S. pyogenes SSI-1 (M3), SSI-9 (M1), or S42 (M12) on day 51 following immunization. On the other hand, CD1 mice were passively immunized with 100 μl of rabbit anti-FBP54 serum intraperitoneally 4 h before the challenge with S. pyogenes. Mortality was recorded every 24 h.
Statistical evaluations.
Data are presented as the mean ± the standard error of the mean (SEM). Statistical analyses were determined by χ2 analysis for the survival studies and by a nonparametric Mann-Whitney U test for all other analyses using Statview for MacIntosh. All conclusions were based on a significance level of P < 0.05.
RESULTS
Expression of the fbp54 gene and FBP54 protein among S. pyogenes isolates.
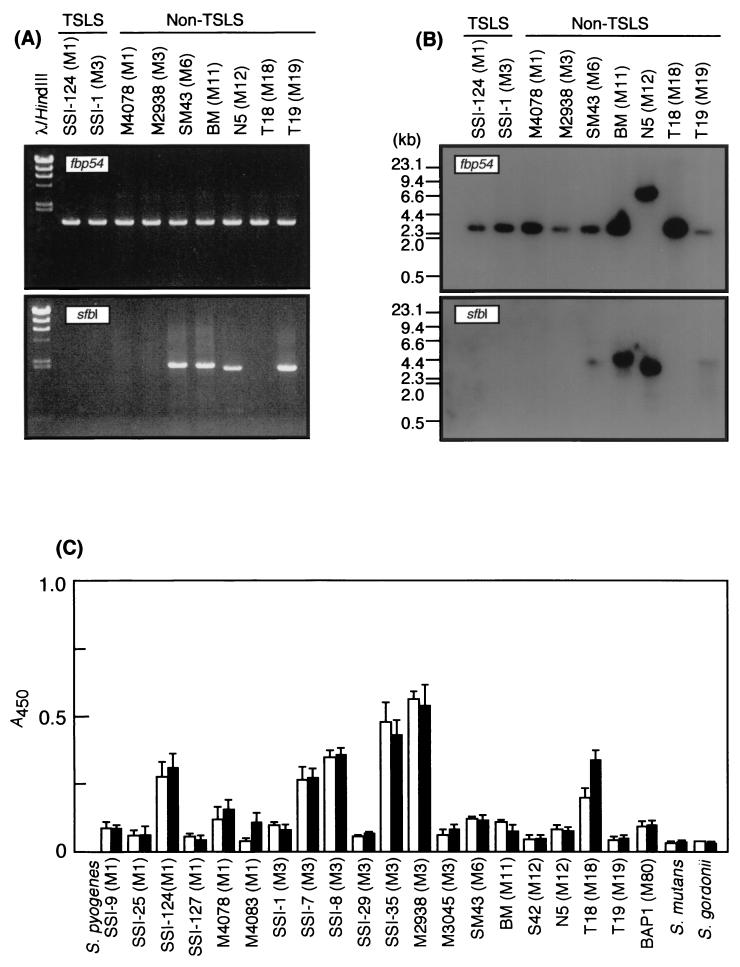
To determine the distribution of fibronectin-binding protein-genes encoding sfbI and fbp54, PCR and Southern hybridization assays were done. All of the test strains of S. pyogenes were found to possess the fbp54 gene but not the sfbI gene by PCR analysis (Fig. 1A). Southern blotting showed the same observation (Fig. 1B). The deduced amino acid sequences of the fbp54 genes revealed that they are highly conserved (>98%) among the test strains, and the FBP54 protein was found to consist of 474 amino acids. Differences in the expression of this protein among heterologous S. pyogenes isolates were recognized by ELISA using rabbit anti-FBP54 serum (Fig. 1C).
FIG. 1.
Distribution of the fbp54 and sfbI genes among and expression of the FBP54 protein by S. pyogenes strains. Nine isolates from patients with TSLS and from non-TSLS patients were employed for PCR (A) and Southern blot (B) analyses. (C) Measurement of FBP54 protein from S. pyogenes by ELISA after growth with (■) or without (□) hyaluronidase. One hundred microliters of bacteria (A550 = 1.0) was added to each well of a 96-well microtiter plate and incubated for 1 h at 37°C. Following the addition of rabbit anti-FBP54 serum, the wells were incubated with HRPO-conjugated goat anti-rabbit IgG antibody. The enzyme reaction was developed with TMB solution and stopped by adding 0.5 N HCl, and the A450 of the plates was read. Values represent the means ± the SEM.
Immunogenicity of FBP54 and opsonic activity of anti-FBP54 serum.
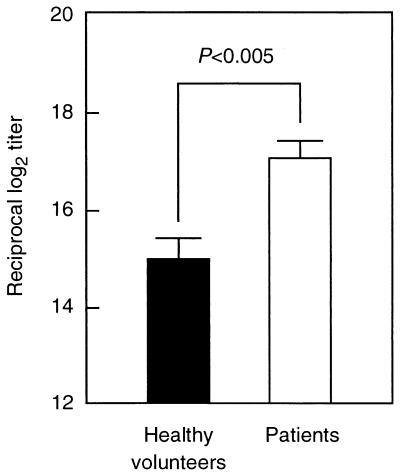
To evaluate the possibility of using FBP54 as an immunogen, levels of FBP54-specific IgG antibodies in sera from healthy volunteers without any symptoms of S. pyogenes infection and from patients with streptococcal pharyngitis and impetigo were measured (Fig. 2). Higher levels of FBP54-specific IgG antibodies were observed in sera from patients with pharyngitis than in those from the healthy control group (P < 0.005).
FIG. 2.
FBP54-specific antibodies in sera from healthy volunteers and patients with S. pyogenes infection. Antigen-specific antibody titers in sera from the patients (n = 12) were significantly higher than those in sera from the healthy control group (n = 10) (P < 0.005). Values represent the means ± the SEM.
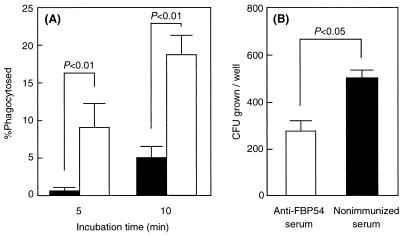
To examine the opsonic activity of anti-FBP54 serum, strain SSI-1 was employed in the in vitro opsonization and bactericidal assays. Association of strain SSI-1 with polymorphonuclear leukocytes was accelerated in human whole blood and rabbit FBP54-specific antiserum (P < 0.01; Fig. 3A), and bacterial growth in whole blood containing the antiserum was significantly reduced (P < 0.05; Fig. 3B) compared with that of the strain incubated with nonimmunized rabbit serum.
FIG. 3.
Effect of rabbit anti-FBP54 serum on opsonization and killing of S. pyogenes. (A) Heparinized whole blood (80 μl) was mixed with bacteria (10 μl, ∼5 × 106 CFU) and with rabbit anti-FBP54 serum (20 μl) or nonimmunized serum and incubated for 5 to 10 min at 37°C. The sample fixed on a glass slide was stained with Giemsa stain, and the percentage of phagocytosed leukocytes was determined by counting 100 cells per slide under a microscope. (B) Heparinized whole blood (80 μl) was mixed with bacteria (10 μl, ∼30 CFU) and with rabbit anti-FBP54 serum (20 μl) or nonimmunized serum and then gently shaken for 3 h at 37°C. The mixture was serially diluted and plated on THY agar. Following incubation, the number of CFU was determined. Data are expressed as the mean ± the SEM of four independent determinations with a total of three wells.
Humoral immune response to FBP54 in mice.
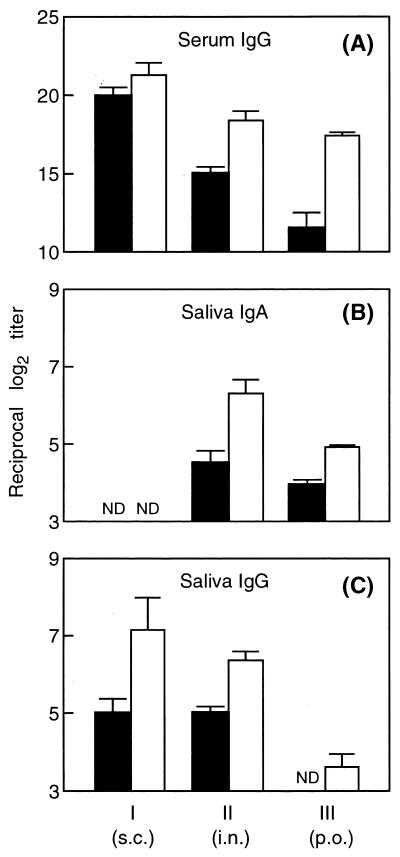
Following the four immunizations with rFBP54, anti-FBP54 IgG antibodies were markedly induced in the sera of mice immunized s.c., nasally, or orally (Fig. 4A). CT-immunized and nonimmunized control groups did not show any detectable humoral immune responses to FBP54 in sera (data not shown). Nasal and oral immunizations induced antigen-specific serum IgG and salivary IgA antibodies, whereas s.c. immunization did not elicit IgA production in saliva (Fig. 4A and B). However, s.c. immunization strongly induced FBP54-specific IgG antibody in serum and in saliva in comparison with the nasal and oral administrations (Fig. 4A and C).
FIG. 4.
Production of anti-FBP54 antibody by BALB/c mice immunized with FBP54 via the s.c., intranasal (i.n.), and peroral (p.o.) routes. Groups I, II, and III correspond to those in Table 1. Serum and saliva samples were obtained on days 28 (■) and 49 (□) following the primary immunization. Values represent the mean ± the SEM of four or five mice. ND, not detected at an eightfold dilution of saliva.
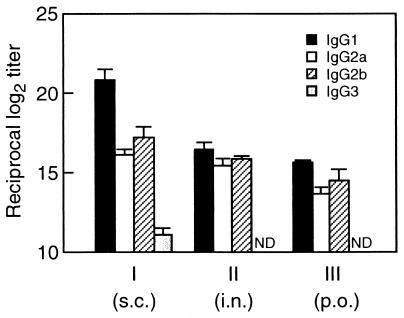
We next examined the profile of FBP54-specific IgG subclasses induced by these three kinds of immunizations (Fig. 5). Immunization via the s.c. route showed an IgG1-dominant profile (IgG1 > IgG2b ≥ IgG2a > IgG3), suggesting that systemic administration of FBP54 and FCA preferentially induced a Th2-type response. In contrast, nasal and oral immunizations with FBP54 and CT produced similar patterns of IgG subclasses, with similar titers of IgG1, IgG2a, and IgG2b antibodies, indicating that the mucosal administrations lead to neither a typical Th1 nor a Th2 immune response.
FIG. 5.
FBP54-specific IgG subclass profiles in sera (day 49) of mice immunized with FBP54. Groups I, II, and III correspond to those in Table 1. Values represent the mean ± the SEM of log2 ELISA titers of four or five mice. ND, not detected at an ∼1,000-fold dilution of serum. i.n., intranasal; p.o., peroral.
Protection of mice immunized with FBP54 against intraperitoneal challenge with S. pyogenes isolates.
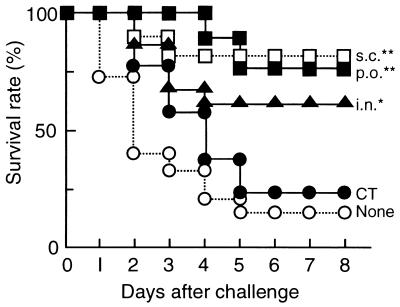
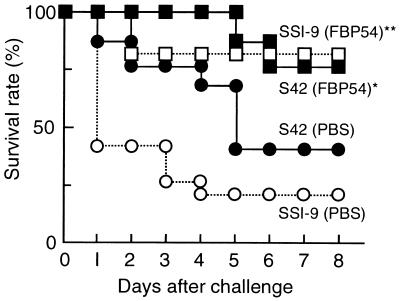
On day 51 after the first immunization, all of the mice were challenged intraperitoneally with 106 CFU of S. pyogenes SSI-1 obtained from a patient with TSLS (Fig. 6). Eighty percent of the nonimmunized mice were killed within 5 days. In contrast, most of the mice immunized either orally or s.c. survived. To examine the protection of FBP54-immunized mice from lethal infection with heterologous isolates obtained from TSLS patients, we chose the s.c. route for immunization. Twenty percent and 40% of the nonimmunized mice escaped death after i.p. infection with SSI-9 (M1) and S42 (M12), respectively, while 80% of the FBP54-immunized mice survived (Fig. 7). These results suggest that FBP54 is an effective vaccine against S. pyogenes infection.
FIG. 6.
Lethal challenge of FBP54-immunized mice with S. pyogenes strain SSI-1. Mice (n = 15) were injected intraperitoneally with 106 CFU of strain SSI-1 (M3) on day 51 after the first immunization. Mortality was monitored every day. χ2 analysis was employed to analyze the significance of differences between the means of FBP54-immunized and nonimmunized mice on day 8. ∗, P < 0.05; ∗∗, P < 0.001. p.o., peroral; i.n., intranasal.
FIG. 7.
Protection of mice immunized s.c. with FBP54 against lethal infection with heterologous S. pyogenes strains. Groups of mice (n = 15) were injected intraperitoneally with strain SSI-9 (M1; 107 CFU) or strain S42 (M12; 3 × 107 CFU) on day 51 after the first immunization. Mortality was monitored every day. χ2 analysis was employed to analyze the significance of differences between the means of FBP54-immunized and nonimmunized mice on day 8. ∗, P < 0.05; ∗∗, P < 0.001.
We then examined the effect of passive immunization with anti-FBP54 serum on mouse lethality. A significant reduction in lethality was observed in 15 mice immunized passively with the antigen in comparison with 15 nonimmunized mice when all of the mice were infected with strains SSI-9 and S42 (P < 0.05 and P < 0.01, respectively). In contrast, no significant difference in mortality was observed between immunized and nonimmunized mice following the challenge with strain SSI-1 whereas the immunized mice survived longer than the nonimmunized ones.
DISCUSSION
It has been previously reported that the distribution of various fibronectin-binding proteins (e.g., SfbI [protein F1], SfbII [SOF], protein F2, and PFBP) correlates with M serotypes of S. pyogenes (25, 33, 34, 37). The most common serotypes of strains isolated worldwide from pharyngitis and TSLS patients are M1 and M1/M3, respectively. However, these strains seemed not to possess fibronectin-binding proteins and/or their genes (12, 30, 32). In this study, we showed that all of the test strains from TSLS and non-TSLS patients possess the fbp54 gene with different serotypes of M protein (Fig. 1A and B). It was also found that FBP54 is expressed in different quantities on the cell surface of S. pyogenes, as revealed by ELISA using rabbit anti-FBP54 antibody (Fig. 1C). Since common epitopes or proteins are better targets for the production of a universal vaccine, the FBP54 protein could be a better candidate than the M protein. Therefore, we chose to evaluate the efficacy of FBP54 as a vaccine in this study.
Sera from human volunteers were found to contain high levels of antibodies specific for FBP54 and low levels of SPE-B-specific antibodies. Regarding this finding, two hypotheses could be proposed. One is that FBP54 is a better immunogen than SPE-B in human beings, and the other is that some microorganisms induce antibodies cross-reactive to FBP54, as they seem to possess an epitope similar to the FBP54 protein. A homology search of the GenBank database revealed that S. pneumoniae PavA and S. gordonii FlpA possess amino acid sequence similarities of 62 and 63%, respectively.
Nasal, s.c., and oral immunizations with FBP54 resulted in induction of FBP54-specific IgG antibodies in sera (Fig. 4). The immune responses elicited by s.c. immunization with FBP54 clearly protect the mice against intraperitoneal challenges with a lethal dose of S. pyogenes (P < 0.001; Fig. 7). These results indicate that serum IgG antibody is the most important for protection against S. pyogenes infection. Induction of salivary IgA following nasal and oral immunizations may also be beneficial in protection against S. pyogenes infection, in addition to the serum IgG-dominant systemic immunity, since S. pyogenes initially adheres to and subsequently propagates on the mucosal surface of the oropharynx.
Nasal and oral immunizations with FBP54 alone resulted in an induction of FBP54-specific IgG and IgA antibodies in saliva on day 49 following the first immunization (results not shown). This phenomenon suggests that FBP54 has mucosal adjuvanticity. Medina et al. (29) have reported that nasal immunization of mice with SfbI (fibronectin-binding protein)-ovalbumin conjugant elicited ovalbumin-specific IgA antibody in lung wash. Bacterial fibronectin-binding proteins may exhibit mucosal adjuvant activity, and fibronectin-binding domains may be responsible for this particular activity. Investigations are ongoing in our laboratory to determine the functional domain.
Although rabbit anti-M protein serum promoted almost complete killing of S. pyogenes during the 3-h incubation with whole blood (2), significant but not complete inhibition of bacterial growth was seen with rabbit anti-FBP54 serum (Fig. 3). An explanation for this could be that, of the M3-type strains used in this study, strains SSI-35, M2938, SSI-7, and SSI-8 possess a high level of cell-associated protein which reacted with anti-FBP54 serum, compared with strain SSI-1, used in Fig. 3 (Fig. 1). If a high titer of anti-FBP54 serum or strains with high FBP54 expression had been employed for this bactericidal assay, S. pyogenes growth might have been inhibited more strongly than in our assay or even completely.
M protein-specific antibodies have been reported to be involved in serotype-specific opsonization and to protect against group A streptococcal infection; however, these antibodies cross-react with some host tissue components, such as myosin, tropomyocin, and vimentin (36). Dale et al. (7) constructed a recombinant multivalent M protein vaccine containing amino-terminal subunits with protective epitopes alone, but it was found to be tissue cross-reactive. Isolates from patients with TSLS mainly show the M1 and M3 types worldwide. Furthermore, passive immunization with antibodies to FBP54 results in rescue of mice infected with a lethal dose of S. pyogenes. Therefore, from a therapeutical point of view, a vaccine that contains M1- and M3-specific and FBP54-specific antibodies, along with the administration of antibiotics may rescue TSLS patients from death.
The fibronectin-binding domain of FBP54 has been suggested to be the N-terminal 1 to 89 residues of the molecule (5). Furthermore, the antibody to the N-terminal residue 1 to 89 peptide segment of FBP54 binds to S. pyogenes (14), suggesting that this repeat region is expressed on the surface of the cell. Thus, this functional domain within the N-terminal region may be a useful, short segment for a peptide vaccine against S. pyogenes in the future. In conclusion, FBP54 possesses considerable antigenicity in humans and immunization of mice with this protein induces protective immune responses irrespective of different M types.
ACKNOWLEDGMENT
This research was supported by grants from the Japan Society for the Promotion of Science and the Ministry of Health and Welfare.
REFERENCES
- 1.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronze M S, Beachey E H, Dale J B. Protective and heart-crossreactive epitopes located within the NH2 terminus of type 19 streptococcal M protein. J Exp Med. 1988;167:1849–1859. doi: 10.1084/jem.167.6.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carapetis J R, Currie B J, Kaplan E L. Epidemiology and prevention of group A streptococcal infections: acute respiratory tract infections, skin infections, and their sequelae at the close of the twentieth century. Clin Infect Dis. 1999;28:205–210. doi: 10.1086/515114. [DOI] [PubMed] [Google Scholar]
- 4.Courtney H S, Dale J B, Hasty D L. Differential effects of the streptococcal fibronectin-binding protein, FBP54, on adhesion of group A streptococci to human buccal cells and HEp-2 tissue culture cells. Infect Immun. 1996;64:2415–2419. doi: 10.1128/iai.64.7.2415-2419.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale J B, Beachey E H. Localization of protective epitopes of the amino terminus of type 5 streptococcal M protein. J Exp Med. 1986;163:1191–1202. doi: 10.1084/jem.163.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dale J B, Chiang E Y, Lederer J W. Recombinant tetravalent group A streptococcal M protein vaccine. J Immunol. 1993;151:2188–2194. [PubMed] [Google Scholar]
- 8.Dale J B, Chiang E Y, Liu S, Courtney H S, Hasty D L. New protective antigen of group A streptococci. J Clin Investig. 1999;103:1261–1268. doi: 10.1172/JCI5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demers B, Simor A E, Vellend H, Schlievert P M, Byrne S, Jamieson F, Walmsley S, Low D E. Severe invasive group A streptococcal infections in Ontario, Canada: 1987–1991. Clin Infect Dis. 1993;16:792–800. doi: 10.1093/clind/16.6.792. [DOI] [PubMed] [Google Scholar]
- 10.Fenderson P G, Fischetti V A, Cunningham M W. Tropomyosin shares immunologic epitopes with group A streptococcal M proteins. J Immunol. 1989;142:2475–2481. [PubMed] [Google Scholar]
- 11.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaworzewska E, Colman G. Changes in the pattern of infection caused by Streptococcus pyogenes. Epidemiol Infect. 1988;100:257–269. doi: 10.1017/s095026880006739x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzmán C A, Talay S R, Molinari G, Medina E, Chhatwal G S. Protective immune response against Streptococcus pyogenes in mice after intranasal vaccination with the fibronectin-binding protein SfbI. J Infect Dis. 1999;179:901–906. doi: 10.1086/314655. [DOI] [PubMed] [Google Scholar]
- 14.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasty D L, Ofek I, Courtney H S, Doyle R J. Multiple adhesins of streptococci. Infect Immun. 1992;60:2147–2152. doi: 10.1128/iai.60.6.2147-2152.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoge C W, Schwartz B, Talkington D F, Breiman R F, MacNeill E M, Englender S J. The changing epidemiology of invasive group A streptococcal infections and the emergence of streptococcal toxic shock-like syndrome. JAMA. 1993;269:384–389. [PubMed] [Google Scholar]
- 17.Hosein B, McCarty M, Fischetti V A. Amino acid sequence and physicochemical similarities between streptococcal M protein and mammalian tropomyosin. Proc Natl Acad Sci USA. 1979;76:3765–3768. doi: 10.1073/pnas.76.8.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis. 1998;178:147–158. doi: 10.1086/515589. [DOI] [PubMed] [Google Scholar]
- 19.Jamal F, Pit S, Johnson D R, Kaplan E L. Characterization of group A streptococci isolated in Kuala Lumpur, Malaysia. J Trop Med Hyg. 1995;98:343–346. [PubMed] [Google Scholar]
- 20.Ji Y, Carlson B, Kondagunta A, Cleary P P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect Immun. 1997;65:2080–2087. doi: 10.1128/iai.65.6.2080-2087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson D R, Stevens D L, Kaplan E L. Epidemiologic analysis of group A streptococcal serotypes associated with severe systemic infections, rheumatic fever, or uncomplicated pharyngitis. J Infect Dis. 1992;166:374–382. doi: 10.1093/infdis/166.2.374. [DOI] [PubMed] [Google Scholar]
- 22.Kapur V, Maffei J T, Greer R S, Li L L, Adams G J, Musser J M. Vaccination with streptococcal extracellular cysteine protease (interleukin-1β convertase) protects mice against challenge with heterologous group A streptococci. Microb Pathog. 1994;16:443–450. doi: 10.1006/mpat.1994.1044. [DOI] [PubMed] [Google Scholar]
- 23.Kawabata S, Miller C J, Lehner T, Fujihashi K, Kubota M, McGhee J R, Imaoka K, Hiroi T, Kiyono H. Induction of Th2 cytokine expression for p27-specific IgA B cell responses after targeted lymph node immunization with simian immunodeficiency virus antigens in rhesus macaques. J Infect Dis. 1998;177:26–33. doi: 10.1086/513811. [DOI] [PubMed] [Google Scholar]
- 24.Kawabata S, Terao Y, Fujiwara T, Nakagawa I, Hamada S. Targeted salivary gland immunization with plasmid DNA elicits specific salivary immunoglobulin A and G antibodies and serum immunoglobulin G antibodies in mice. Infect Immun. 1999;67:5863–5868. doi: 10.1128/iai.67.11.5863-5868.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreikemeyer B, Talay S R, Chhatwal G S. Characterization of a novel fibronectin-binding surface protein in group A streptococci. Mol Microbiol. 1995;17:137–145. doi: 10.1111/j.1365-2958.1995.mmi_17010137.x. [DOI] [PubMed] [Google Scholar]
- 26.Lancefield R C. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959;110:271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lancefield R C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962;89:307–313. [PubMed] [Google Scholar]
- 28.Martin P R, Hoiby E A. Streptococcal serogroup A epidemic in Norway 1987–1988. Scand J Infect Dis. 1990;22:421–429. doi: 10.3109/00365549009027073. [DOI] [PubMed] [Google Scholar]
- 29.Medina E, Talay S R, Chhatwal G S, Guzmán C A. Fibronectin-binding protein I of Streptococcus pyogenes is a promising adjuvant for antigens delivered by mucosal route. Eur J Immunol. 1998;28:1069–1077. doi: 10.1002/(SICI)1521-4141(199803)28:03<1069::AID-IMMU1069>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 30.Mencarelli M, Corbisiero R, Marzocchi B, Gistri A, Signori R, Rossolini A, Cellesi C. M genotyping and DNA fingerprinting of Streptococcus pyogenes isolated from an area of central Italy. Epidemiol Infect. 1998;121:77–84. doi: 10.1017/s0950268898008802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molinari G, Talay S R, Valentin-Weigand P, Rohde M, Chhatwal G S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musser J M, Hauser A R, Kim M H, Schlievert P M, Nelson K, Selander R K. Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc Natl Acad Sci USA. 1991;88:2668–2672. doi: 10.1073/pnas.88.7.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natanson S, Sela S, Moses A E, Musser J M, Caparon M G, Hanski E. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis. 1995;171:871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 34.Rakonjac J V, Robbins J C, Fischetti V A. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding repeat domain. Infect Immun. 1995;63:622–631. doi: 10.1128/iai.63.2.622-631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Relf W A, Martin D R, Sriprakash K S. Identification of sequence types among the M-nontypeable group A streptococci. J Clin Microbiol. 1992;30:3190–3194. doi: 10.1128/jcm.30.12.3190-3194.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson J H, Kehoe M A. Group A streptococcal M proteins: virulence factors and protective antigens. Immunol Today. 1997;13:362–367. doi: 10.1016/0167-5699(92)90173-5. [DOI] [PubMed] [Google Scholar]
- 37.Rocha C L, Fischetti V A. Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect Immun. 1999;67:2720–2728. doi: 10.1128/iai.67.6.2720-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Stålhammar-Carlemalm M, Areschoug T, Larsson C, Lindahl G. The R28 protein of Streptococcus pyogenes is related to several group B streptococcal surface proteins, confers protective immunity and promotes binding to human epithelial cells. Mol Microbiol. 1999;33:208–219. doi: 10.1046/j.1365-2958.1999.01470.x. [DOI] [PubMed] [Google Scholar]
- 40.Stollerman G H. Rheumatic fever. Lancet. 1997;349:935–942. doi: 10.1016/S0140-6736(96)06364-7. [DOI] [PubMed] [Google Scholar]
- 41.Wessels M R, Goldberg J B, Moses A E, DiCesare T S. Effects on virulence of mutations in a locus essential for hyaluronic acid capsule expression in group A streptococci. Infect Immun. 1994;62:433–441. doi: 10.1128/iai.62.2.433-441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]