Abstract
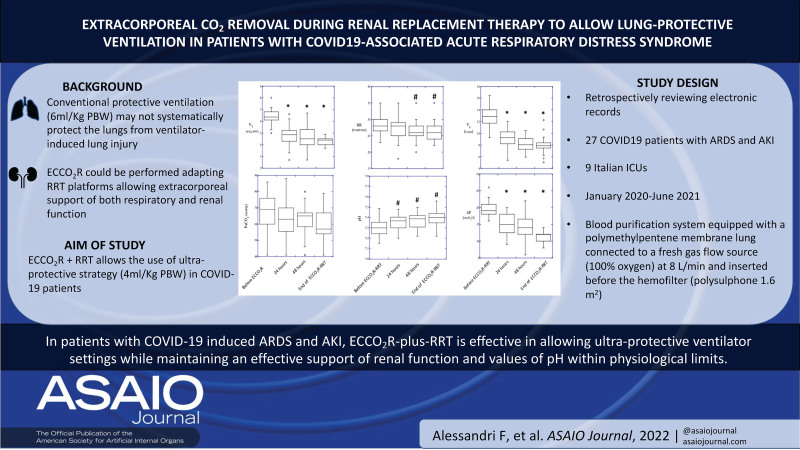
The aim of this retrospective multicenter observational study is to test the feasibility and safety of a combined extracorporeal CO2 removal (ECCO2R) plus renal replacement therapy (RRT) system to use an ultraprotective ventilator setting while maintaining (1) an effective support of renal function and (2) values of pH within the physiologic limits in a cohort of coronavirus infectious disease 2019 (COVID-19) patients. Among COVID-19 patients admitted to the intensive care unit of 9 participating hospitals, 27 patients with acute respiratory distress syndrome (ARDS) and acute kidney injury (AKI) requiring invasive mechanical ventilation undergoing ECCO2R-plus-RRT treatment were included in the analysis. The treatment allowed to reduce VT from 6.0 ± 0.6 mL/kg at baseline to 4.8 ± 0.8, 4.6 ± 1.0, and 4.3 ± 0.3 mL/kg, driving pressure (ΔP) from 19.8 ± 2.5 cm H2O to 14.8 ± 3.6, 14.38 ± 4.1 and 10.2 ± 1.6 cm H2O after 24 hours, 48 hours, and at discontinuation of ECCO2R-plus-RRT (T3), respectively (p < 0.001). PaCO2 and pH remained stable. Plasma creatinine decreased over the study period from 3.30 ± 1.27 to 1.90 ± 1.30 and 1.27 ± 0.90 mg/dL after 24 and 48 hours of treatment, respectively (p < 0.01). No patient-related events associated with the extracorporeal system were reported. These data show that in patients with COVID-19–induced ARDS and AKI, ECCO2R-plus-RRT is effective in allowing ultraprotective ventilator settings while maintaining an effective support of renal function and values of pH within physiologic limits.
Keywords: ECCO2R, RRT, COVID-19, ultraprotective ventilation
Although most of the patients affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have a favorable outcome, pneumonia and severe hypoxemia can lead to acute respiratory distress syndrome (ARDS), which is associated with a high mortality rate.1
Lung-protective strategies are the mainstay of mechanical ventilation in patients with ARDS, as the use of a tidal volume (VT) of 6 mL/kg predicted body weight (PBW) and end-inspiratory plateau pressures (PPLAT) <30 cm H2O improves survival.2 However, several studies showed that conventional protective ventilatory settings may not systematically protect the lungs from ventilator-induced lung injury (VILI).3–5 Ultraprotective strategies (i.e., VT as low as 4 mL/kg and PPLAT ≤25 cm H2O), integrated by extracorporeal CO2 removal (ECCO2R) to minimize the risk of severe respiratory acidosis caused by the reduction in minute ventilation, have therefore been proposed to further minimize the risk of VILI.6 It has been recently proposed that ECCO2R could be performed adapting conventional renal replacement platforms to incorporate a membrane lung to allow CO2 elimination allowing extracorporeal support of both respiratory and renal function.7,8 This may be of interest for patients with COVID-19 ARDS because (1) acute kidney injury (AKI) is common among critically ill COVID-19 patients, with ~20% of the patients requiring renal replacement therapy (RRT)9; (2) mechanical ventilation is an independent risk factor for mortality in patients with AKI10,11; (3) high dead space and low compliance of the respiratory system often occurring in patients with COVID-19–associated ARDS may limit the efficacy of conventional protective ventilatory settings.1,12,13
The current study set out to examine whether in patients with COVID-19–induced ARDS and AKI, the use of ECCO2R during RRT allows the use of ultraprotective ventilator settings while maintaining (1) an effective support of renal function and (2) values of pH within the physiologic limits.
Methods
The study was conducted retrospectively reviewing electronic records of patients enrolled in clinical database collected in the period January 2020–June 2021. Institutional Review Boards of nine Italian hospitals (Policlinico Umberto I [Sapienza Università di Roma]; Azienda Ospedaliera Universitaria [Università di Sassari]; Ospedale Papa Giovanni XXIII Bergamo; Ospedale Sant’Eugenio Roma; IRCCS Policlinico di Sant’Orsola [Alma Mater Studiorum, Università di Bologna], Policlinico di Modena [Università di Modena e Reggio Emilia]; AOU San Giovanni di Dio e Ruggi D’Aragona, [University of Salerno]; Ospedali Riuniti Marche Nord Pesaro; Spedali Civili Brescia) approved the study protocol. Consent was obtained according to institutional indications.
Among patients admitted to the intensive care unit (ICU) of participating hospitals, patients were enrolled if they met the following inclusion criteria: worsening respiratory symptoms caused by COVID-19; mild or moderate ARDS14 requiring invasive mechanical ventilation; ∆P (i.e. the difference between end-inspiratory plateau pressure [PPLAT] minus positive end-expiratory pressure [PEEP]) ≥15 cm H2O15 despite the use of conventional protective ventilatory settings2; AKI requiring continuous venovenous hemodiafiltration. Exclusion criteria were duration of mechanical ventilation <48 hours, patients eligible for extracorporeal membrane oxygenation (ECMO) following Extracorporeal Life Support Organization (ELSO) criteria,16 end-stage renal disease requiring dialysis, decompensated heart failure or acute coronary syndrome; severe chronic obstructive pulmonary disease; acute brain injury; and severe liver insufficiency (Child–Pugh scores >7) or fulminant hepatic failure, heparin-induced thrombocytopenia, contraindication for systemic anticoagulation, platelet <50 g/L, catheter access to femoral vein or jugular vein impossible, pneumothorax, incomplete records for the variables of interest, “do not intubate/do not resuscitate” order.
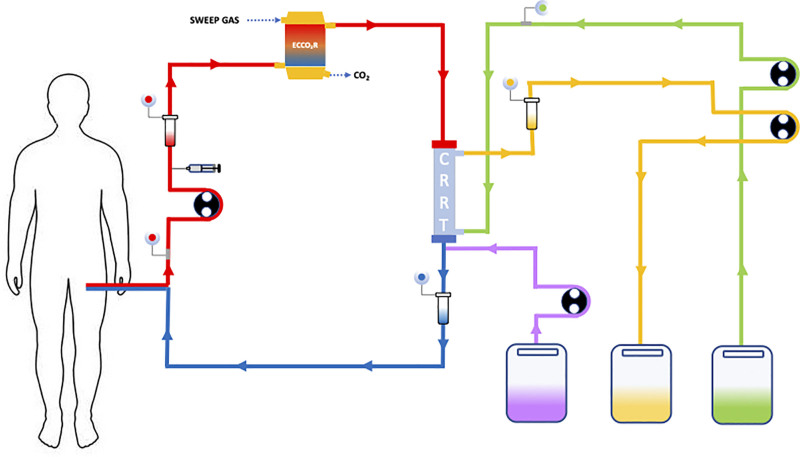
All patients matching inclusion and exclusion criteria received ECCO2R during RRT with the OMNI blood purification system (B.Braun Avitum AG, Melsungen, Germany) available for clinical use in all sites. This blood purification system is equipped with a polymethylpentene membrane lung (1.81 m2; Eurosets, Medolla, Italy) connected to a fresh gas flow source (100% oxygen) at a suggested rate of 8 L/min and inserted before the hemofilter (polysulphone 1.6 m2) (Figure 1). Vascular access (internal jugular vein or femoral vein or subclavian veins) was performed using a 14 French double lumen catheter (OMNIcath; B.Braun Avitum AG) inserted with the Seldinger technique. Anticoagulation was ensured by continuous infusion of heparin to maintain values of activated partial thromboplastin time (aPTT) ratio at 1.5–2.0 of baseline.
Figure 1.
Venovenous extracorporeal removal of carbon dioxide (ECCO2R) associated with a continuous renal replacement therapy (CRRT) circuit. The blood flows from the venous vascular access through the ECCO2R filter where the sweep gas flow removes the CO2 and immediately thereafter through the CRRT filter. In the continuous hemodialysis mode a counter-current dialysate flow (first narrow from the right) favors the diffusion of small molecules The continuous hemofiltration mode is based on convection and removes median molecules: the substitution flow (second narrow from the right) replaces the convective flow in postdilution. Continuous hemodiafiltration combines these two CRRT modes. The effluent flows away from the loop (second narrow from the right). The syringe infuses heparin into the circuit. Manholes and pressure valves regulate and control the flow. Roller pumps generate the flow.
Patients were treated with an ultraprotective ventilatory strategy simultaneously with continuous venovenous hemodiafiltration.7,17 This strategy was applied with the following protocol: RRT was commenced at a blood flow of 300 mL/min and sweep gas was set at 0 L/min. VT was reduced to 4 mL/kg PBW in three steps (from 6.0 to 5.0, from 5.0 to 4.5, and from 4.5 to 4 mL/kg PBW). Once the lowest value of VT was reached, sweep gas was switched on (10 L/min) to obtain PaCO2 values similar to baseline (±20%). To optimize oxygenation PEEP and FiO2 were titrated to maintain SpO2 ≥92%. Patients received neuromuscular blocking agents when it was difficult to provide adaptation to controlled ventilation with deep sedation as directed by the attending physician.
Data were analyzed for feasibility (achieve and maintain a VT of 4 mL/kg ideal body weight [IBW] and PPLAT ≤25 cm H2O with a PaCO2 not increasing more than 20% from baseline and a value of arterial pH >7.30 while providing effective RRT) and safety (occurrence of severe adverse events and of mechanical/clinical ECCO2R-related adverse events [ECCO2R-AE]) as previously described.6
Static compliance of the respiratory system (CRS) was calculated as tidal volume/(PPLAT – total PEEP).18 Driving pressure (ΔP) was calculated as VT/CRS.15 Oxygenation was quantified as the ratio of partial pressure of arterial oxygen to fractional concentration of oxygen in inspired air (PaO2/FiO2). Ventilatory ratio (VR) was calculated as minute ventilation × PaCO2/(predicted minute ventilation × predicted PaCO2) and used as a surrogate of dead space.19
Clinical variables were collected before the start of ECCO2R-plus-RRT (T0), after 24 (T1), and 48 (T2) hours and at discontinuation of ECCO2R-plus-RRT (T3). Creatinine concentrations at ICU admission, T0, T1, T3, length of hospital stay, and mortality at 28 days were recorded.
Data, unless otherwise stated, are presented as mean with standard deviation (±SD) for continuous variables and as frequencies, proportions, and percentages for categorical variables. Repeated measures for continuous variables were compared with Kruskal–Wallis test (each row represents matched observations); categorical variables were compared by Fisher’s exact tests or chi-square test. Longitudinal data were analyzed by jointly considering all four follow-up measurements (i.e., baseline, 24 hours, 48 hours, and at the end of treatment). Multiple imputation was used to account for missing values, using chained equations that fill in missing values in multiple variables iteratively. Data were analyzed using the SPSS statistical software packages (SPSS Statistics for Mac, 22.0; IBM Corp., Armonk, NY). Methods of unsupervised clustering, statistical tests, and regression analyses were implemented utilizing R statistics software.
Results
Twenty-seven patients were treated with the ECCO2R-plus-RRT. It was initiated 11 ± 9 days after ICU admission and discontinued after 4.3 ± 2.2 days for death in 14 patients and for normalization of renal function in the remaining 13 patients.
Clinical variables before initiating the ECCO2R-plus-RRT treatment are reported in Table 1. All patients were ventilated in volume-controlled mode and received a propofol and opiate-based analog-sedation regime. Creatine amounted to 3.30 ± 1.27 mg/dL and all patients had a KDIGO (Kidney Disease Improving Global Outcome) class of 3. Modes of RRT implemented were continuous venovenous hemodiafiltration (15 patients), continuous venovenous hemodialysis (6 patients), and continuous venovenous hemofiltration (6 patients). PaO2/FiO2, CRS, ΔP, and VR amounted to 108 ± 29, 23.2 ± 2.7 mL/cm H2O, 19.8 ± 2.5 cm H2O, and 2.9 ± 1.1, respectively.
Table 1.
Baseline Clinical, Renal, and Respiratory Variables Before Initiating ECCO2R plus RRT
| Age (years) | 64 (±11) |
| Gender, male/female | 24/3 |
| SAPS II | 34 (±14) |
| SOFA at ICU admission | 6 (±3) |
| SOFA at T0 | 7 (±2) |
| ICU length of stay (days) | 28 (±14) |
| Mortality at 28 days (%) | 63% (17/27) |
| Hypertension, n (%) | 13 (50%) |
| COPD, n (%) | 6 (23%) |
| Diabetes, n (%) | 6 (23%) |
| Coronary artery disease, n (%) | 5 (19%) |
| Peripheral vascular disease, n (%) | 4 (15%) |
| Pulmonary embolism, n (%) | 2 (7.7%) |
| Atrial fibrillation, n (%) | 1 (3.9%) |
| Plasma creatinine (mg/dL) | 3.30 ± 1.27 |
| VT (mL) | 454 ± 59 |
| VT/PBW (mL/kg) | 6 ± 0.6 |
| RR (breath/min) | 28.6 ± 3.2 |
| Minute ventilation (L/min) | 13.0 ± 1.7 |
| PEEP (cm H2O) | 9.3 ± 2.6 |
| PPLAT (cm H2O) | 28.9 ± 2.8 |
| pH | 7.30 ± 0.08 |
| PaCO2 (mmHg) | 68.1 ± 11.2 |
| HCO3– (mmol/L) | 30.5 ± 9.11 |
| BE | 5.7 ± 8 |
| PaO2/FiO2 | 108 ± 29 |
| CRS (mL/cm H2O) | 23.2 ± 2.7 |
| ∆P (cm H2O) | 19.8 ± 2.5 |
| Ventilatory ratio | 2.9 ± 1.1 |
Data are mean ± standard deviation.
∆P, driving pressure; BE, base excess; COPD, chronic obstructive pulmonary disease; CRS, static compliance of the respiratory system; ECCO2R, extracorporeal CO2 removal; HCO3–, bicarbonate; ICU, intensive care unit; PaCO2, arterial partial pressure of carbon dioxide; PaO2/FiO2, ratio between arterial partial pressure of oxygen (PaO2) and fraction of inspired oxygen (FiO2); PBW, predictive body weight; PEEP, positive end-expiratory positive pressure; PPLAT, plateau pressure; RR, respiratory rate; RRT, renal replacement therapy; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; VT, tidal volume.
Blood flow and sweep gas set on the platform ranged between 186 and 393 mL/min and 9–11 L/min, respectively. Infusion of heparin maintained aPTT ratio at 1.12 ± 0.3, 1.64 ± 0.9, 2.02 ± 1.2, and 2.08 ± 0.5 at T0, T1, T2, and T3, respectively. Renal dose amounted to 32 ± 3.3, 32 ± 4.4, 31 ± 4.3, and 31 ± 4.6 mL/kg/h at T0, T1, T2, and T3, respectively.
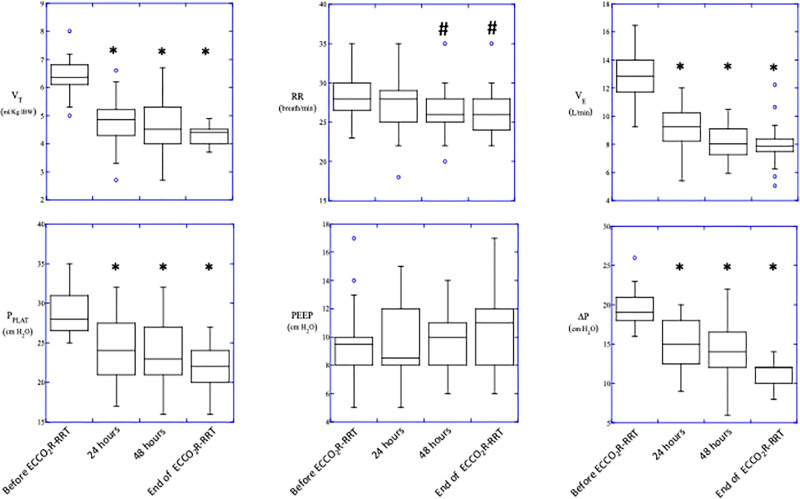
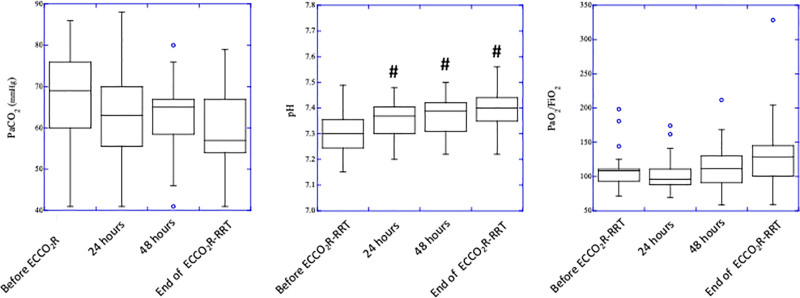
Time course of ventilatory variables is reported in Figure 2. Initiation of treatment allowed to reduce VT from 6.0 ± 0.6 mL/kg to 4.8 ± 0.8, 4.6 ± 1.0, and 4.3 ± 0.3 mL/kg; PPLAT from 28.9 ± 2.7 cm H2O to 24.4 ± 3.9, 23.9 ± 3.9, and 21.6 ± 2.8 cm H2O; and ΔP from 19.8 ± 2.5 cm H2O to 14.8 ± 3.6, 14.38 ± 4.1, and 10.2 ± 1.6 cm H2O at baseline, T1, T2, and T3, respectively (p < 0.001). Despite a ~30% reduction in minute ventilation and ~10% reduction in respiratory rate (p < 0.01), PaCO2 remained stable whereas pH slightly but significantly (p < 0.01) increased (7.30 ± 0.08 at baseline, 7.35 ± 0.07 at T1, 7.37 ± 0.07 at T2, and 7.39 ± 0.08 at T3, p < 0.05). No change in systemic oxygenation was observed (Figure 3).
Figure 2.
Time course of ventilatory variables. VT, tidal volume; RR, respiratory rate; VE, minute ventilation; PPLAT, end-inspiratory plateau pressure; PEEP, positive end-expiratory pressure; ∆P, driving pressure (ΔP = PPLAT minus PEEP). *p < 0.001 vs. baseline, #p < 0.05 vs. baseline.
Figure 3.
Time course of ventilatory variables. PaCO2, partial pressure of arterial CO2; PaO2/FiO2, ratio of arterial-to-inspiratory oxygen fraction. *P < 0.001 vs. baseline, #P < 0.05 vs. baseline.
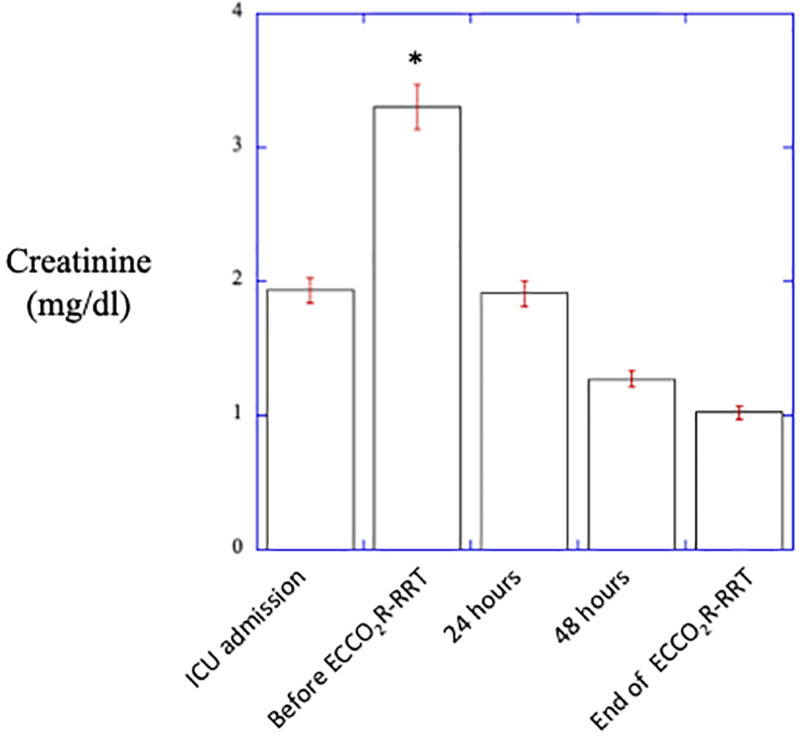
Creatinine at ICU admission amounted to 1.93 ± 0.47 mg/dL and significantly increased to 3.30 ± 1.27 leading to initiation of RRT that resulted in a significant decrease in plasma creatinine over the study period to 1.90 ± 1.30 and 1.27 ± 0.90 mg/dL after 24 and 48 hours of treatment, respectively (p <0.01). At the end of study treatment, plasma creatinine amounted to 1.02 ± 0.70 mg/dL (Figure 4).
Figure 4.
Changes in creatinine on ICU admission and during the study period. ICU, intensive care unit. *P < 0.001 vs. baseline, #P < 0.05 vs. baseline.
No patient-related events directly attributable to the combined extracorporeal circuit and no other adverse events associated with the extracorporeal system were reported, except for four episodes of premature circuit clotting which required circuit replacement. The small cohort of patients undergoing ECCO2R-plus-RRT did not report any major bleeding episode, hemolysis, or infections related to the system.
Discussion
Results of this retrospective observational multicenter study show that in patients with COVID-19–induced ARDS and AKI, ECCO2R-plus-RRT is effective in allowing ultraprotective ventilator settings while maintaining (1) an effective support of renal function and (2) values of pH within the physiologic limits.
Mechanical ventilation may cause a form of injury that is clinically and morphologically indistinguishable from ARDS.20 A seminal randomized clinical trial demonstrated that limiting VT to 6 mL/kg and PPLAT to ≤30 cm H2O improves survival.2 Amato and coworkers showed that these protective ventilatory settings were effective if associated with a decrease in driving pressure (PPLAT – PEEP) and that protective ventilator settings are associated to a lower risk of death only for values of ΔP <15 cm H2O.15 Several studies demonstrated that conventional protective ventilatory settings may not be always protective because some patients may still present morphological or physiologic evidence of VILI with VT of 6 mL/kg PBW and PPLAT lower than 30 cm H2O.5,21,22 Reduction of VT to 3–4 mL/kg and PPLAT ≤25 cm H2O has been proposed to further minimize the risk of VILI, but this entails a significant risk of severe respiratory acidosis.23 ECCO2R can minimize this risk by clearing CO2 enabling strategies that are more protective and might improve outcomes by (1) using VT as low as 3–4 mL/kg and further decreasing PPLAT below 30 cm H2O (often termed ultraprotective5,24), (2) decreasing respiratory rates, (3) minimizing driving pressures and mechanical power.25–27 A recent multicenter, randomized clinical trial conducted by McNamee and coworkers was stopped early for futility because tidal volume reduction facilitated by ECCO2R did not reduce mortality.28 It should be noted that conventional protective ventilator settings failed to obtain values of ∆P <15 cm H2O in ~ 50% of patients included in McNamee, whereas all patients included in the current study had values of ∆P >15 cm H2O (19.8 ± 2.5 cm H2O).
Several single center studies reported the possible allocation of a membrane lung within a conventional RRT circuit to allow simultaneous removal of fluids and metabolites (with the hemofilter) and CO2 (with the membrane lung).7,29–31 Forster and coworkers modified a commercially available RRT device (bm11/14; Edwards-Lifescience, Irvine, CA) with a standard setup and adjustment for continuous venovenous hemodialysis adding downstream to the high-flux polysulfone capillary hemofilter (Polyflux 140H; Gambro, Hechingen, Germany; membrane surface area of 1.4 m2), a small standard hollow-fiber gas exchanger (D902 Liliput 2 ECMO; Sorin Group Milan, Milan, Italy, surface area of 0.67 m2).29 They found that in 10 ventilated critically ill patients with ARDS and AKI undergoing RRT and respiratory replacement therapy, this simple device was feasible and safe and led to a significant CO2 removal and rapid correction of arterial pH with a positive impact on hemodynamic stability. Concomitant RRT was in no way compromised, and alarm functions of the RRT system ensured safety control for the gas-exchange device.29 Similarly, Allardet-Servent and coworkers modified a commercially available RRT device (PrismaFlex v6.0 monitor, Gambro, Lund, Sweden) set in continuous venovenous hemofiltration mode adding a polymethylpentene heparin-coated hollow fiber membrane oxygenator (MEDOS HILITE 2400 LT; MEDOS Medizintechnik AG, Stolberg, Germany; 0.65 m2) either upstream and downstream of the hemofilter (AN69 membrane, M150; Hospal, Meyzieu, France; 1.5 m2).30 The study enrolled 11 patients and confirmed that combined RRT with ECCO2R was safe and allowed sustained blood purification together with enhanced lung-protective ventilation during the early phase of ARDS and AKI.30 Blood flow through the membrane lung (p < 0.001) and CO2 removal rate were significantly higher when the membrane oxygenator was placed upstream than when the membrane oxygenator was placed downstream of the hemofilter.30 Both studies did not report adverse events.30,31 Consistently with these data, Fanelli and coworkers using a propensity score analysis compared patients with ARDS and AKI treated with conventional protective ventilation and RRT with patients treated with ECCO2R-plus-RRT (VT of 7.04 ± 0.5 mL/kg PBW and ∆P of 19.2 ± 2.2 cm H2O vs. VT of 4.84 ± 0.4 mL/kg PBW and ∆P of 14.1 ± 2.1 cm H2O, respectively).7 Recovery of renal function was more pronounced, and concentrations of inflammatory and proapoptotic mediators were lower when ultraprotective ventilation was allowed by ECCO2R-plus-RRT. Moreover, a multicenter pilot study from Schmidt and coworkers reported the use conventional RRT circuit equipped with a membrane lung to allow CO2 removal without providing RRT.32 A polymethylpentene, hollow-fiber gas-exchanger membrane (surface area 0.32 m2) was added to a conventional RRT platform (Prismaflex [Gambro-Baxter]) to allow low-flow (421 ± 40 mL/min; sweep gas 10 ± 0.3 L/min), stand-alone CO2-removal treatments. In 20 patients with mild or moderate ARDS, the study showed how this approach allowed to safely reach low VT (from 6.10 ± 0.30 to 3.98 ± 0.18 mL/kg PBW), PPLAT (from 26.3 ± 3.5 to 22.8 ± 2.6 cm H2O), and ∆P (from 13.0 ± 4.8 to 7.9 ± 3.2 cm H2O). However, this occurred with a ~20% increase in PaCO2 (from 43 ± 8 to 53 ± 9 mmHg).32
The relatively large number of patients included in the study (N = 27) and the multicenter design (nine centers) represent the major strength of this study. Our data confirm that the combined use of ECCO2R and RRT allows to safely (only four episodes of premature circuit clotting) reach and maintain (for 4.3 ± 2.2 days) a low VT (from ~6 to ~4 mL/kg PBW), a low PPLAT (from ~30 to ~20 cm H2O), and reduce ∆P (from ~20 to ~10 cm H2O) while maintaining constant values of PaCO2 (from ~70 to ~60 mmHg) and pH (from ~7.30 to ~7.40) at T0 and T3, respectively, and normalizing creatinine values (from ~3.0 mg/dL at the onset of RRT to ~2.0 mg/dL at the end of RRT, respectively). However, there are several important limitations that should be taken into account in interpreting our results. First, we included only patients with COVID-19 ARDS in whom ECCO2R-plus-RRT was started 11 ± 9 days after ICU admission; this could represent a problem in generalizing to ARDS from other causes and earlier admission. Second, these patients represent the most severe patients since CRS, ΔP, and VR amounted to 23.2 ± 2.7 mL/cm H2O, 19.8 ± 2.5 cm H2O, and 2.9 ± 1.1. This profound alteration of the respiratory function may explain the value of PaCO2 observed at study inclusion (~70 mmHg) that is higher than the PaCO2 values observed in some of the previous studies performed (~50 mmHg).6,7,17,28,30,32 Third, our sample may have intrinsic heterogeneity since it is a retrospective analysis. Fourth, mortality in these patients was quite high; however, this cohort of critically ill COVID-19 patients was burdened with a particularly poor prognosis and met the inclusion criteria in a late phase of the disease. Also still not proven, ECCO2R could be beneficial to reduce VILI in the early phase of ARDS3 and has been suggested as a tool to maintain a protective level of mechanical ventilation.33 A combined technique should be indicated in case of severe acute renal failure (KDIGO3); therefore, the right timing of a combined treatment should be clarified, taking into account the possible role on metabolic and fluid balance in promoting a faster compensation of acidosis. Further studies are needed to compare ECCO2R-plus-RRT vs. ECCO2R stand alone.
In conclusion, our study shows that combination of ECCO2R and RRT in patients mechanically ventilated for COVID-19–induced ARDS with AKI and in whom conventional ventilator settings failed to obtain effective protection from VILI (∆P >15 cm H2O) was able to ensure ultraprotective ventilatory setting (VT of ~4 mL/kg PBW; PPLAT of ~20 cm H2O; ∆P of ~10 cm H2O) while maintaining constant values of PaCO2 and pH and providing effective RRT. Further studies are needed to assess overall benefits of this approach.
∥∥RETROSPECTIVE STUDY ON COVID-19 PATIENTS UNDERGOING CO2 REMOVAL AND DIALYSIS (RECORD STUDY): Giorgio Aldegheri, Francesco Alessandri , Claus Bernd, Cristian Borrazzo, Matteo Brivio, Stefano Busani, Nuno Catorze, Sergio Cattaneo, Antonio Corcione, Lorenzo Dall’Ara, Mario Dauri, Marina Di Luca, Vito Fanelli, Roberto Fumagalli, Giulia Gianni, Giovanni Giordano, Massimo Girardis, Salvatore Grasso, Lorenzo Grazioli, Martina Novelli, Tomás Lamas, Luca Lorini, Alessio Margola, Roberto Palumbo, Ornella Piazza, Mario Piazzolla, Laura Pistidda, Valentina Pistolesi, Mauro Polzoni, Valeria Possick, Francesco Pugliese, Inês Ribeiro, Davide Ricci, V. Marco Ranieri, Filipa Oliveira Enrico Storti, Davide Salaris, Pierpaolo Terragni, Tommaso Tonetti, Robert Ravholt Winding, Michele Tempesta, Rosario Urbino, Andrea Zanoni.
Footnotes
Disclosure: The authors have no conflicts of interest to declare.
Contributor Information
Collaborators: Giorgio Aldegheri, Francesco Alessandri, Claus Bernd, Cristian Borrazzo, Matteo Brivio, Stefano Busani, Nuno Catorze, Sergio Cattaneo, Antonio Corcione, Lorenzo Dall’Ara, Mario Dauri, Marina Di Luca, Vito Fanelli, Roberto Fumagalli, Giulia Gianni, Giovanni Giordano, Massimo Girardis, Salvatore Grasso, Lorenzo Grazioli, Martina Novelli, Tomás Lamas, Luca Lorini, Alessio Margola, Roberto Palumbo, Ornella Piazza, Mario Piazzolla, Laura Pistidda, Valentina Pistolesi, Mauro Polzoni, Valeria Possick, Francesco Pugliese, Inês Ribeiro, Davide Ricci, V. Marco Ranieri, Filipa Oliveira, Enrico Storti, Davide Salaris, Pierpaolo Terragni, Tommaso Tonetti, Robert Ravholt Winding, Michele Tempesta, Rosario Urbino, and Andrea Zanoni
References
- 1.Grasselli G, Tonetti T, Protti A, et al. ; collaborators: Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. 8: 1201–1208, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Terragni PP, Del Sorbo L, Mascia L, et al. : Tidal volume lower than 6 ml/kg enhances lung protection: Role of extracorporeal carbon dioxide removal. Anesthesiology. 111: 826–835, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Fanelli V, Ranieri MV, Mancebo J, et al. : Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care. 20: 36, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terragni PP, Rosboch G, Tealdi A, et al. : Tidal hyperinflation during low tidal volume ventilation in acute respiratory distress syndrome. Am J Respir Crit Care Med. 175: 160–166, 2017. [DOI] [PubMed] [Google Scholar]
- 6.Combes A, Fanelli V, Pham T, Ranieri VM; European Society of Intensive Care Medicine Trials Group and the “Strategy of Ultra-Protective lung ventilation with Extracorporeal CO2 Removal for New-Onset moderate to severe ARDS” (SUPERNOVA) investigators: Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: The SUPERNOVA study. Intensive Care Med. 45: 592–600, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Fanelli V, Cantaluppi V, Alessandri F, et al. : Extracorporeal CO2 removal may improve renal function of patients with acute respiratory distress syndrome and acute kidney injury: an open-label, interventional clinical trial. Am J Respir Crit Care Med. 198: 687–690, 2018. [DOI] [PubMed] [Google Scholar]
- 8.Ronco C, Bagshaw SM, Bellomo R, et al. : Extracorporeal blood purification and organ support in the critically ill patient during COVID-19 pandemic: Expert review and recommendation. Blood Purif. 50: 17–27, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ronco C, Reis T, Husain-Syed F: Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 8: 738–742, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchino S, Kellum JA, Bellomo R, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 294: 813–818, 2005. [DOI] [PubMed] [Google Scholar]
- 11.McNicholas BA, Rezoagli E, Pham T, et al. ; ESICM Trials Group and the Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) Investigators: Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: A secondary analysis of a multicenter observational study. Crit Care Med. 47: 1216–1225, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Tonetti T, Grasselli G, Rucci P, et al. : Synergistic effect of static compliance and d-dimers to predict outcome of patients with COVID-19-ARDS: A prospective multicenter study. Biomedicines. 9: 1228, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuckton TJ, Alonso JA, Kallet RH, et al. : Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med. 346: 1281–1286, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ; ARDS Definition Task Force: Acute respiratory distress syndrome: the Berlin definition. JAMA. 307: 2526–2533, 2012. [DOI] [PubMed] [Google Scholar]
- 15.Amato MB, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 372: 747–755, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Badulak J, Antonini MV, Stead CM, et al. ; ELSO COVID-19 Working Group Members: Extracorporeal membrane oxygenation for COVID-19: Updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. 67: 485–495, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain-Syed F, Birk HW, Wilhelm J, et al. : Extracorporeal carbon dioxide removal using a renal replacement therapy platform to enhance lung-protective ventilation in hypercapnic patients with coronavirus disease 2019-associated acute respiratory distress syndrome. Front Med (Lausanne). 7: 598379, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranieri VM, Eissa NT, Corbeil C, et al. : Effects of positive end-expiratory pressure on alveolar recruitment and gas exchange in patients with the adult respiratory distress syndrome. Am Rev Respir Dis. 144(3 pt 1): 544–551, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Sinha P, Calfee CS, Beitler JR, et al. : Physiologic analysis and clinical performance of the ventilatory ratio in acute respiratory distress syndrome. Am J Respir Crit Care Med. 199: 333–341, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slutsky AS, Ranieri VM: Ventilator-induced lung injury. N Engl J Med. 369: 2126–2136, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Bellani G, Guerra L, Musch G, et al. : Lung regional metabolic activity and gas volume changes induced by tidal ventilation in patients with acute lung injury. Am J Respir Crit Care Med. 183: 1193–1199, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasso S, Stripoli T, De Michele M, et al. : ARDSnet ventilatory protocol and alveolar hyperinflation: Role of positive end-expiratory pressure. Am J Respir Crit Care Med. 176: 761–767, 2007. [DOI] [PubMed] [Google Scholar]
- 23.Fanelli V, Costamagna A, Ranieri VM: Extracorporeal support for severe acute respiratory failure. Semin Respir Crit Care Med. 35: 519–527, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Bein T, Weber-Carstens S, Goldmann A, et al. : Lower tidal volume strategy (≈3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: The prospective randomized Xtravent-study. Intensive Care Med. 39: 847–856, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grasso S, Stripoli T, Mazzone P, et al. : Low respiratory rate plus minimally invasive extracorporeal CO2 removal decreases systemic and pulmonary inflammatory mediators in experimental acute respiratory distress syndrome. Crit Care Med. 42: e451–460, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Goligher EC, Combes A, Brodie D, et al. ; SUPERNOVA investigators (European Society of Intensive Care Medicine trials group) and for the International ECMO Network (ECMONet): Determinants of the effect of extracorporeal carbon dioxide removal in the SUPERNOVA trial: Implications for trial design. Intensive Care Med. 45: 1219–1230, 2019. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L, Tonetti T, Cressoni M, et al. : Ventilator-related causes of lung injury: The mechanical power. Intensive Care Med. 42: 1567–1575, 2016. [DOI] [PubMed] [Google Scholar]
- 28.McNamee JJ, Gillies MA, Barrett NA, et al. ; REST Investigators: Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: The REST randomized clinical trial. JAMA. 326: 1013–1023, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forster C, Schriewer J, John S, Eckardt KU, Willam C: Low-flow CO₂ removal integrated into a renal-replacement circuit can reduce acidosis and decrease vasopressor requirements. Crit Care. 17: R154, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allardet-Servent J, Castanier M, Signouret T, Soundaravelou R, Lepidi A, Seghboyan JM: Safety and efficacy of combined extracorporeal CO2 removal and renal replacement therapy in patients with acute respiratory distress syndrome and acute kidney injury: The pulmonary and renal support in acute respiratory distress syndrome study. Crit Care Med. 43: 2570–2581, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nentwich J, Wichmann D, Kluge S, Lindau S, Mutlak H, John S: Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: A pilot study. Ann Intensive Care. 9: 3, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A: Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care. 22: 122, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Combes A, Auzinger G, Capellier G, et al. : ECCO2R therapy in the ICU: Consensus of a European round table meeting. Crit Care. 24: 490, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]