Abstract
Mitochondrial dysfunction has been implicated as a key player in the pathogenesis of Parkinson’s disease (PD). The MitoPark mouse, a transgenic mitochondrial impairment model developed by specific inactivation of TFAM in dopaminergic neurons, spontaneously exhibits progressive motor deficits and neurodegeneration, recapitulating several features of PD. Since nonmotor symptoms are now recognized as important features of the prodromal stage of PD, we comprehensively assessed the clinically relevant motor and nonmotor deficiencies from ages 8–24 wk in both male and female MitoPark mice and their littermate controls. As expected, motor deficits in MitoPark mice began around 12–14 wk and became severe by 16–24 wk. Interestingly, MitoPark mice exhibited olfactory deficits in the novel and social scent tests as early as 10–12 wk as compared to age-matched littermate controls. Additionally, male MitoPark mice showed spatial memory deficits before female mice, beginning at 8 wk and becoming most severe at 16 wk, as determined by the Morris water maze. MitoPark mice between 16–24 wk spent more time immobile in forced swim and tail suspension tests, and made fewer entries into open arms of the elevated plus maze, indicating a depressive and anxiety-like phenotype, respectively. Importantly, depressive behavior as determined by immobility in forced swim test was reversible by antidepressant treatment with desipramine. Neurochemical and mechanistic studies revealed significant changes in CREB phosphorylation, BDNF, and catecholamine levels as well as neurogenesis in key brain regions. Collectively, our results indicate that MitoPark mice progressively exhibit deficits in olfactory discrimination, cognitive learning and memory, and anxiety- and depression-like behaviors as well as key neurochemical signaling associated with nonmotor deficits in PD. Thus, MitoPark mice can serve as an invaluable model for studying motor and nonmotor deficits in addition to studying the motor deficits related to pathology in PD.
Keywords: Parkinson’s disease, behavior, nonmotor, MitoPark
Introduction
Parkinson’s disease (PD) is a chronic, progressive neurodegenerative disorder affecting about 5 million people worldwide. The neuropathology of this disease is characterized by a loss of dopaminergic neurons in the substantia nigra (SN) of the brain, leading to a functional loss of dopamine in the striatum and severe motor deficits. Additionally, accumulations of abnormal alpha-synuclein (αSyn) proteins form Lewy bodies and Lewy neurites, both pathologic hallmarks of PD. Several genes have been linked with PD including PINK1, Parkin, DJ-1, and LRRK2; however, the vast majority of PD cases are considered idiopathic, implicating an etiologic role of environmental factors such as metals, pesticides, and other toxins in the development of the disease. Cardinal motor symptoms such as bradykinesia, tremor, rigidity, and postural instability are still classically used for the clinical diagnosis of PD. Neuroinflammation, oxidative stress and mitochondrial dysfunction are thought to contribute to the neurodegenerative processes of this disease (Kanthasamy et al., 2010; Subramaniam and Chesselet, 2013; Varcin et al., 2012). Current therapies, including levodopa (L-DOPA), monoamine oxidase inhibitors, and dopamine agonists, treat the symptoms yet ultimately cannot interrupt or slow down the neurodegenerative process. Furthermore, most commonly prescribed treatments do not address the full scope of symptomology in PD patients and may even pose an elevated risk of developing nonmotor symptoms (Marinus et al., 2018).
In addition to the characteristic motor symptoms, nonmotor symptoms such as hyposmia, sleep disturbances, gastrointestinal (GI) dysfunction, autonomic and cognitive deficits negatively affect the quality of life and cost of living for PD patients (Schapira et al., 2017). Although often overlooked, nonmotor symptoms are a frequent cause of hospitalization and diminished quality of life for PD patients (Chaudhuri and Schapira, 2009). More than 70% of PD patients present nonmotor symptoms, according to a recent cross-sectional observational study (Zhang et al., 2016a). Disease onset in areas of the brain outside of the substantia nigra (SN) or even peripheral onset of PD is supported by the idea that PD patients experience a variety of nonmotor symptoms before classical motor signs are observed (Mahlknecht et al., 2015). Certain symptoms are now considered to be early warning signs of PD, including hyposmia, constipation, rapid eye movement behavior disorder, and depression (Abbott et al., 2005; Ishihara and Brayne, 2006; Kang et al., 2016; Postuma and Berg, 2016). Neuropsychiatric symptoms in PD include depression, dementia, anxiety, apathy, and cognitive dysfunction. Olfactory deficits are observed in more than 95% of those affected by PD, while depression is estimated to affect more than one-third of PD patients (Chaudhuri and Schapira, 2009; Haehner et al., 2011; Meyer et al., 2014).
Although behavioral tests are available to study various nonmotor phenotypes in rodent species, large data gaps still remain in understanding the nonmotor phenotype of many toxin-based and genetic models of PD (Taylor et al., 2010). Significant overlap and co-morbidity exist between nonmotor symptoms (Postuma and Berg, 2016), yet combined effects or interdependency of behavioral phenotypes have not yet been addressed.
In many toxin-based models of PD, rodent species either do not suffer from PD-related nonmotor symptomology or their complete behavioral phenotyping has not yet been performed. For example, animals receiving intraperitoneal injections of MPTP display inconsistent olfactory impairment across studies (Doty et al., 1992; Kurtenbach et al., 2013; Schintu et al., 2009), although intranasal MPTP adminitration can functionally damage the olfactory epithelium (Kurtenbach et al., 2013). Although gastric emptying and small intestine transit are unaffected by MPTP, the toxin-induced loss of enteric dopaminergic neurons increases colon motility (Anderson et al., 2007). Several studies using rodent models reported that exposure to the neurotoxic pesticide paraquat or paraquat/maneb co-administration only induced anxiety- and depression-like behaviors (Campos et al., 2013; Litteljohn et al., 2009; Tinakoua et al., 2015). Interestingly, a recent study revealed that rotenone-treated zebrafish display motor, olfactory, and neuropsychiatric changes (Wang et al., 2017). In the 6-OHDA lesion model, olfactory discrimination, neuropsychiatric effects, memory impairment, and gut microbiota changes have been observed (Bonito-Oliva et al., 2014a; Bonito-Oliva et al., 2014b; Faivre et al., 2019; Koutzoumis et al., 2020).
In transgenic mouse models, only a few αSyn mutant animal models reportedly show olfactory and GI functional changes (Dawson et al., 2010; Fleming et al., 2008; Wang et al., 2008). A bacterial artificial chromosome (BAC) synuclein transgenic rat model with human SNCA displayed progressive motor impairments and alterations in olfaction by 3 months of age along with an increase in new olfactory bulb neurons (Nuber et al., 2013). Parkin knockout mice have spatial memory impairments but do not show evidence of olfactory dysfunction, anxiety, depression, or motor deficits (Rial et al., 2014). Dranka et. al. identified olfactory dysfunction in the LRRK2R1441G mouse model (Dranka et al., 2014). The presence of nonmotor behavioral impairments in PD models would be particularly useful if they can be characterized as early-onset and progressive similar to clinical PD. It is imperative that we develop and characterize models that recapitulate a broad range of nonmotor abnormalities and their associated neurochemical mechanisms. These models could then be utilized in the development of therapies to treat nonmotor symptoms and also to screen for adverse effects resulting from dopaminergic therapies.
The MitoPark mouse model, which recapitulates many of the hallmark features of PD, was created by selectively inactivating the mitochondrial transcription factor A (TFAM) in the nigrostriatal pathway, creating a conditional knockout driven by the dopamine transporter (DAT) promoter. MitoPark mice exhibit adult-onset progressive dopaminergic neurodegeneration, protein aggregation in nigral tissues, and L-dopa-responsive motor deficits (Ekstrand and Galter, 2009; Ekstrand et al., 2007). More recently, MitoPark mice were discovered to display certain nonmotor deficits such as all-light- or all-dark-induced circadian rhythm dysfunction and early cognitive deficits (Fifel and Cooper, 2014; Li et al., 2013). The overarching hypothesis of this present study is that MitoPark mice display nonmotor deficits characteristic of PD. Thus, we chronologically characterized the nonmotor behavioral phenotype of the MitoPark mouse model of PD in both sexes throughout the disease progression.
Materials and Methods
Chemicals
Dopamine hydrochloride, 3–4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were all purchased from Sigma (St Louis, MO). Halt protease and phosphatase inhibitor cocktail was obtained from Thermo Fisher (Waltham, MA). Bradford assay reagent and Western blotting buffers were purchased from Bio-Rad (Hercules, CA). Anti-4-hydroxynonenal antibody was purchased from R&D Systems (MAB3249, Minneapolis, MN (Ghosh et al., 2016)), while anti-BDNF was purchased from Santa Cruz Biotechnology (sc-546, Dallas, TX). CREB and p-CREB (Ser133) antibodies were obtained from Cell signaling (9104, 87G3, Boston, MA (Jin et al., 2011)). The anti-mouse and anti-rabbit secondary antibodies (Alexa Fluor 680 conjugated anti-mouse IgG and IRdye 800 conjugated anti-rabbit IgG) were purchased from Invitrogen and Rockland Inc., respectively.
Experimental design
The experimenter was blinded to mouse genotype for each behavioral test by flipping cage cards and randomizing mouse order prior to beginning the experiment. A third party was used to conceal treatment and vehicle solution identity prior to drug administration. Genotype and administration blinding was decoded after data acquisition was complete. Power analysis using the “fpower” function in SAS was used to determine the following animal requirements based on 80% power, alpha=0.05, and four groups. Clinically significant differences and standard deviations used to determine delta were taken from ANOVA analyses of previous studies in our lab. Based on preliminary forced swim test data, a minimum of 11 animals per group were used for behavioral animal studies to detect an effect size of 1.5. Male and female mice were combined, for a total of 19 MitoParks and 25 Littermate control mice used for all behavioral experiments. Mice unable to swim to a visible platform were to be excluded from Morris water maze (MWM) on day one, however, no such mice were observed in our study.
Animal treatment
MitoPark mice were originally kindly provided and generated by Dr. Nils-Goran Larson at the Karolinska Institute in Stockholm (Ekstrand et al., 2007). All mice for this study were bred, maintained, genotyped, and further characterized at ISU. MitoPark mice (DAT +/Cre, Tfam LoxP/LoxP) and their littermate controls (DAT +/+, Tfam +/LoxP) were fed ad libitum (Teklad S-2335 #7004 from Envigo) and housed in standard conditions (constant temperature (22 ± 1°C), humidity (relative, 30%), and a 12-h light/dark cycle) approved and supervised by the Institutional Animal Care and Use Committee (IACUC) at ISU. Mice were weighed and subjected to behavioral tests every two weeks to minimize effects on other behavioral experiments (See Figure 1A). Neurochemical, biochemical, and histological studies were performed after sacrificing mice at age 24 wk.
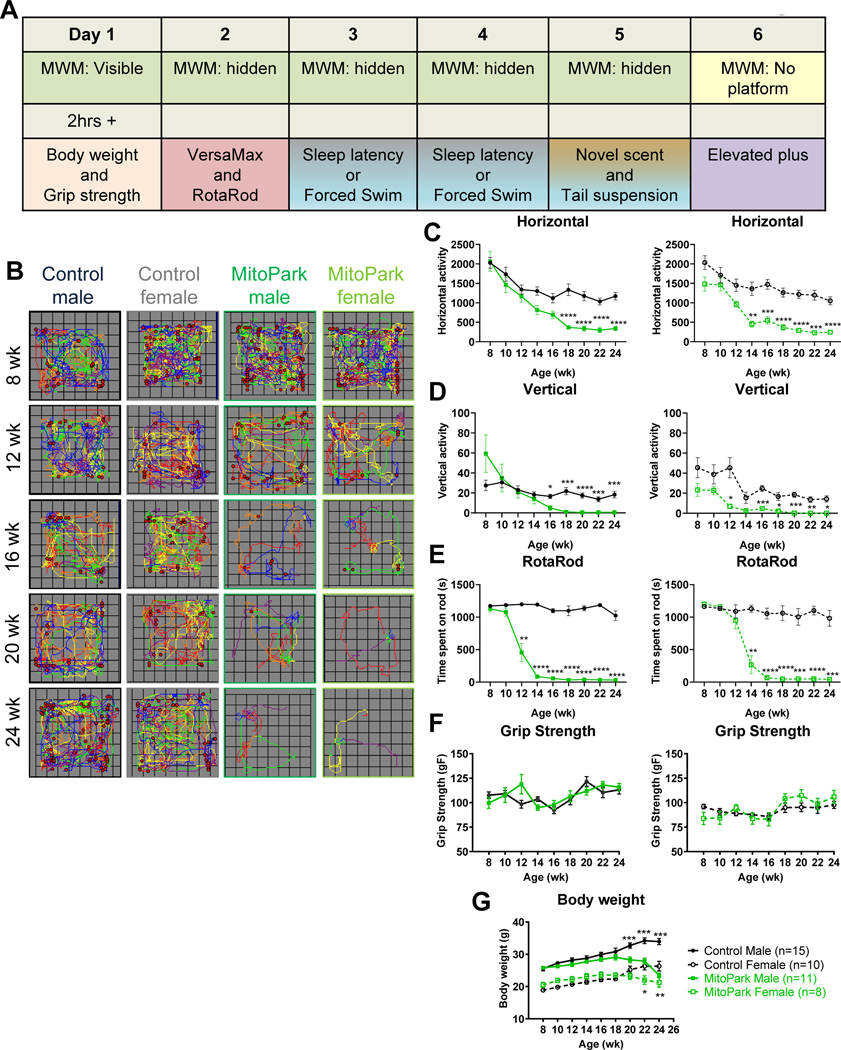
Figure 1. Progressive Motor Deficits in MitoPark Mice.
A, Schematic of the behavioral test battery and the order in which tests were performed. B, Representative VersaPlots of individual mice subjected to the VersaMax open-field test showing horizontal (lines) and vertical (red dots) locomotion during a 10-min testing interval. Quantification of (C) horizontal and (D) vertical activities as determined by VersaMax analyzer during 10-min open-field test. E, Average time (in seconds) spent on RotaRod at 20 rpm during 5 trials. F, Grip strength and (G) body weights from 8- to 24-wk-old littermate control and MitoPark mice. *, p≤0.05; ***, p<0.001. n=15 LC males, n=10 LC females, n=11 MP males, and n=8 MP females.
Motor function test
For the open-field test, a VersaMax system (VersaMax monitor, model RXYZCM-16, and analyzer, model VMAUSB, AccuScan, Columbus, OH) was used for monitoring locomotor activity. For horizontal and vertical activity and corresponding plots, mice were acclimated for 2 min prior to recording for 10 min using the VersaMax system. RotaRod equipment (AccuScan) was used to test movement coordination as previously described (Ghosh et al., 2012). Briefly, time spent on rod rotating at 20 rpm was measured for a maximum of either 20 min or five trials, each of which ended with a mouse falling from the rod.
Neuromuscular function and muscular strength
Each mouse was lifted over the grip strength meter (GSM)’s baseplate by the tail so that its forepaws could grasp onto the steel grip. Each mouse was then gently pulled backward by the tail until its grip released. The GSM measures the maximal force before the mouse releases the bar (Danilov and Steward, 2015). Three trials were performed for each mouse with a 1-minute resting period between trials. Latency to release (sec) and gram-force (gF) were recorded.
Social discrimination and novel scent tests
To determine the olfactory function of control and MitoPark mice, we used a social discrimination test as previously described (Ngwa et al., 2014). However, this procedure was adapted to use ANY-maze tracking software (AMS, Stoelting Co., Wood Dale, IL ) to determine time spent sniffing based on the animal’s head being within a defined zone (1-cm perimeter around dish) surrounding the bedding. Total time spent sniffing the opposite sex’s bedding (from a group-housed cage) was recorded during a 3-min trial. Similarly, AMS was used to determine time sniffing a novel scent as described by Taylor et al. (Taylor et al., 2009). During a 3-min trial, time spent sniffing scented and non-scented zones was recorded using AMS. Scents used were lemon, peppermint and vanilla, whereas water served as the non-scent.
Cognitive testing
A six-day MWM protocol was used as described previously in an Alzheimer’s disease mouse model (Bromley-Brits et al., 2011). Briefly, each mouse gets five 1-min trials per day. On the first day, the platform is visible and its position changes between trials to show that the ability to see and swim to the platform is not impaired by visual or motor deficits. On days 2–5, mice are placed into the MWM tank filled with white (Tempera paint), opaque water to learn to find a hidden platform whose position does not change between trials. The time required for a mouse to find and mount the platform is reported here as escape latency. Finally, the platform is removed on the sixth day when mice performed a single 1-min probe trial to show memory retention of the previously located platform, reported here as time spent searching in the quadrant that contained the platform during Days 2–5. Each trial was monitored using AMS. Each inter-trial interval was a minimum of 20 min, during which time mice were warmed and dried on heating pads placed under cages. Water temperature in the MWM tank was maintained at 23±1°C.
Forced swim and tail suspension tests
For depressive-like phenotyping, tail suspension and forced swim tests were used to measure behavioral despair or stress-coping response during inescapable tasks (Commons et al., 2017). During tail suspension trials, mice were individually suspended at a height of 30 cm by attaching the tail to a horizontal ring stand bar using adhesive tape. Each 6-min test session was videotaped and scored using AMS for escape-oriented behavior/mobility and bouts of immobility. The time spent immobile was recorded for each mouse as a correlate of depression-like behavior (Can et al., 2012b; Taylor et al., 2010).
For the Porsolt “forced swim” test, mice were placed individually in a glass cylinder (24 × 16 cm) with 15 cm of water maintained at 25°C as previously described (Can et al., 2012a; Porsolt et al., 1979). Mice were left in the cylinder and their behavior was videotaped from the side of the cylinder for 6 min. After the first 2 min, the total duration of time spent immobile was recorded during a 4-min test. A mouse was deemed immobile when it was floating 65% passively for at least 2.5 sec according to AMS. A separate cohort of mice underwent the forced swim test with intraperitoneal administration of the antidepressant desipramine or saline 30 min prior to testing (n=7). These mice were immediately sacrificed for dissection and Western blotting, so were not subjected to any additional behavioral testing.
Elevated plus maze
Time spent in the open arms is inversely correlated with an anxious phenotype (Lister, 1987), which in rodents emerges as the trade-off between risk avoidance and spontaneous exploration of novel environments (Crawley, 2008). Mice were placed into the center of the elevated plus maze (Stoelting) and video-recorded for 10 min as previously described (Komada et al., 2008). Time spent in open arms was determined by AMS.
Sleep latency test
Animals were allowed to acclimate 4 h in the VersaMax monitor, and were then video-recorded from above after being awakened by gentle handling. Latency to sleep was determined by observer video-monitoring behavioral signs of sleep. Sleep was defined as 2 min of uninterrupted sleep behavior and 75% of the next ten minutes spent in sleep behavior as previously described (Taylor et al., 2009).
High-performance liquid chromatography (HPLC)
Striatum, hippocampus, and olfactory bulb samples were prepared and processed for HPLC as described previously (Gordon et al., 2016). Briefly, dissected brain regions were placed in a buffer comprising 0.2 M perchloric acid, 0.05% Na2EDTA, 0.1% Na2S2O5 and isoproterenol (internal standard) to extract monoamine neurotransmitters. Monoamine lysates were placed in a refrigerated automatic sampler (model WPS-3000TSL) until being separated isocratically by a reversed-phase C18 column with a flow rate of 0.6 mL/ min using a Dionex Ultimate 3000 HPLC system (pump ISO-3100SD, Thermo Scientific, Bannockburn, IL). Electrochemical detection was achieved using a CoulArray model 5600A coupled with a guard cell (model 5020) and an analytical cell (microdialysis cell 5014B) with cell potentials set at −350, 0, 150, and 220 mV. Data acquisition and analysis were performed using Chromeleon 7 and ESA CoulArray 3.10 HPLC Software and quantified data were normalized to wet tissue weight.
BrdU treatment paradigm
Littermate control and MitoPark mice were injected intraperitoneally with 100 mg/kg BrdU daily for 3 days and sacrificed 12 h past the last injection as previously described (Fu et al., 2016). For each age, 6–8 mice were used per genotype. Mouse brains were perfused with PFA, and then cryoprotected in sucrose before being cryo-embedded in OCT and cryosectioned at 30 μm. Sections were treated with HCl to denature DNA prior to IHC to allow binding of BrdU antibody. DAB immunostaining was performed for BrdU and hematoxylin was used to counterstain the nuclei. Prior to antigen retrieval in citrate buffer, DNA was denatured by keeping sections in 1N of ice-cold HCl for 10 min, 2N HCl at 37°C, followed by 2 washes (15 min) in borate buffer at pH 8.5. Color deconvolution and cell counting were performed in Image J Software.
Western blot
Protein lysates from the striatum and SN were prepared in RIPA buffer with protease and phosphatase inhibitors and ran on a 12–15% SDS-PAGE as previously described (Jin et al., 2014) before being transferred to a nitrocellulose membrane. After blocking for 1 h, membranes were incubated with primary antibodies at 4ºC overnight. Next, membranes were incubated with secondary antibodies (Alexa Fluor 680 and Rockland IR800) at RT for 1 h, and images were captured via a LI-COR Odyssey imager. Densitometric analysis was done using ImageJ software.
Statistical analysis
Behavioral tests were analyzed by 3-way ANOVA to determine any significant effect of sex or an interaction between sex and genotype. For behavioral tests where sex was not a significant source of variation, data were combined and analyzed by two-way ANOVA with Bonferroni post-tests in GraphPad Prism software. Data in which sex and/or the interaction between genotype and sex was a significant source of variation were separated by sex, graphed, and analyzed by two-way ANOVA with Bonferroni post-tests. Similarly, biochemical and neurochemical analyses were performed by 2-way ANOVA to check for the effect of sex before combining and analyzing by two-tailed Student’s t-test. No data sets analyzed for this study violated the normality assumption, so nonparametric tests were not used. For ANOVA and t-tests, p≤0.05 was used to determine significance. Plotted data represent mean ± SEM.
Data Availability
Raw data supporting the results reported in this article are in the figure source data files available upon request.
Results
Progressive Motor Deficits in MitoPark Mice
Previous studies have characterized motor deficits in MitoPark mice (Ekstrand et al., 2007). In our laboratory, some female mice exhibit poor condition after age 24 wk. Therefore, we decided to sacrifice at 24 wk instead of the previously reported 40 wk (Ekstrand and Galter, 2009; Ekstrand et al., 2007). A comprehensive behavioral battery of motor and nonmotor tests was performed on the same mice in a strategic order with breaks in between activities to avoid confounding effects of other tests as much as possible without using individual cohorts of mice for each test (Fig. 1A). Briefly, MWM trials were performed first and mice were acclimated for a minimum of 2 h prior to a second behavioral testing period, which varied by day in the sequence as depicted in Fig. 1A. Female MitoPark mice revealed decreased horizontal activity at 14 wk and vertical activity as early as 12 wk (Fig. 1B–D, right panels), which progressively worsened over time. Male mice first showed deficits by 16–18 wk in the open-field test (Fig. 1B–D, right panels), yet male MitoPark mice spent significantly less time on the RotaRod from 12 wk onward (Fig. 1E, right panel). Female MitoPark mice began spending less time on the RotaRod at 14 wk (Fig. 1E, right panel). No significant changes were observed for grip strength at any age between MitoPark mice and littermate controls (Fig. 1F), indicating forelimb neuromuscular function remained intact. Similar to previous reports, MitoPark mice showed a significant reduction in body weight at 20 wk for males and at 22 wk for females (Fig. 1G). Because males and females differed significantly overall for certain behavioral parameters, including body weight and the learning portion of MWM, they were graphed and statistically analyzed separately instead of combined. The overall statistical analysis and additional graphs of combined or separated data are also available in Supplementary Figures 1–3 and Supplementary Table 1.
Cognitive dysfunction in MitoPark Mice
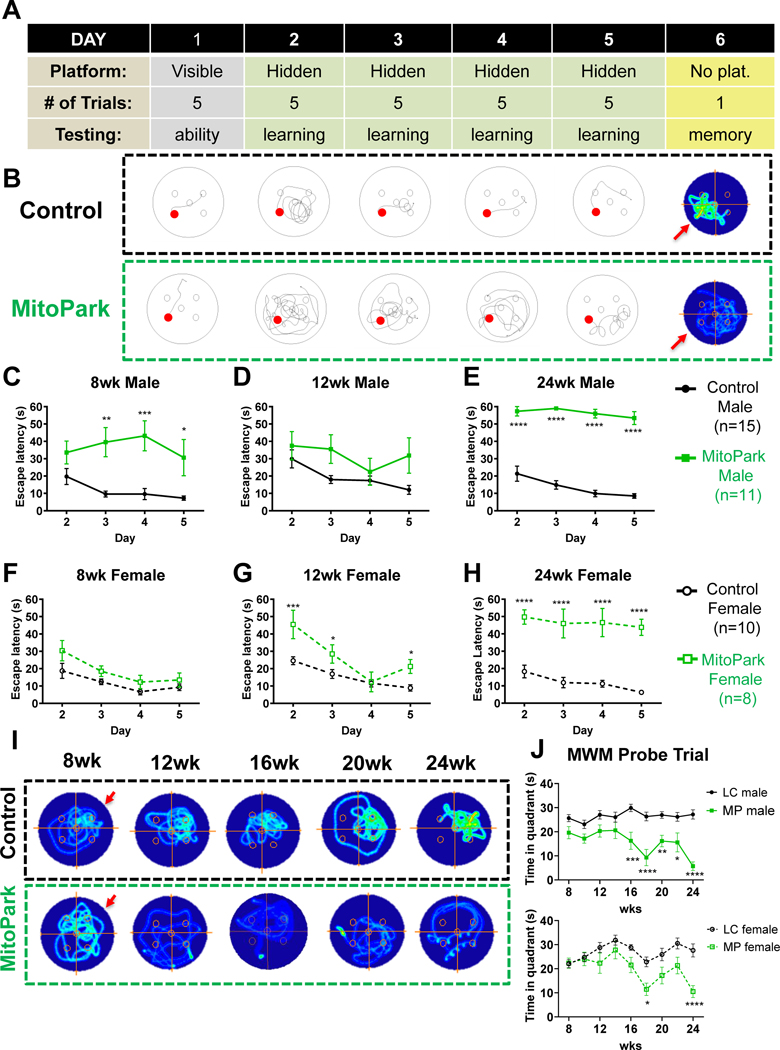
To screen for cognitive deficits associated with spatial learning and memory, we next applied a six-day MWM protocol as described in Fig. 2A and depicted at 24 wk in Fig. 2B. Days 1–5 are track plots of an animal’s path to the visible (day1) or hidden (days 2–5) platform. Day 6 occupancy plots reveal time spent in each location during a one-minute retention trial with the platform removed. Importantly, average speed (m/s) was determined to not significantly differ between genotypes, and all animals were able to find the visible platform (day 1) at each age assessed (Supplemental Fig. 2). Recently, Li et al. (Li et al., 2013) showed that cognitive dysfunction precedes motor deficits in MitoPark mice using the Barnes Maze. Similarly, we report that 8-wk MitoPark males exhibited impairments in the learning phase of the MWM (Fig. 2C). To our surprise, female MitoPark mice did not show an increase in escape latency until 12 wk of age (Fig. 2F–G). At 24 wk, both male and female MitoPark mice were unable to find the platform during the MWM learning phase (Fig. 2E, H). Deficits in the memory retention testing phase of MWM were apparent by 16 wk of age in males and 18 wks of age in females as depicted and quantified in Fig. 2I and 2J, respectively. Overall, these results not only confirm previous findings that learning deficits precede motor dysfunction in the MitoPark mouse model, but also further describe advanced spatial memory problems after 16 wk of age and reveal sex differences in learning the MWM platform location.
Figure 2. Cognitive dysfunction in MitoPark Mice.
A, Schematic describing 6-day Morris water maze (MWM) protocol. B, Representative track plots from learning and occupancy plots from retention trials of MWM for one littermate control (n=15 males; n=10 females) and one MitoPark (n=11 males; n=8 females) mouse. C, 8-, (D) 12-, and (E) 24-wk MWM learning period for male mice. F, 8-, (G) 12-, and (H) 24-wk MWM learning period for female mice. I, Representative occupancy plots of individual mice during retention trial with arrow indicating the previous platform location. J, Duration of retention trial spent in the quadrant where the platform had been located during the learning phase. *, p≤0.05; **, p<0.01; ***, p<0.001.
Behavioral despair and anxiety-like behavior in MitoPark mice
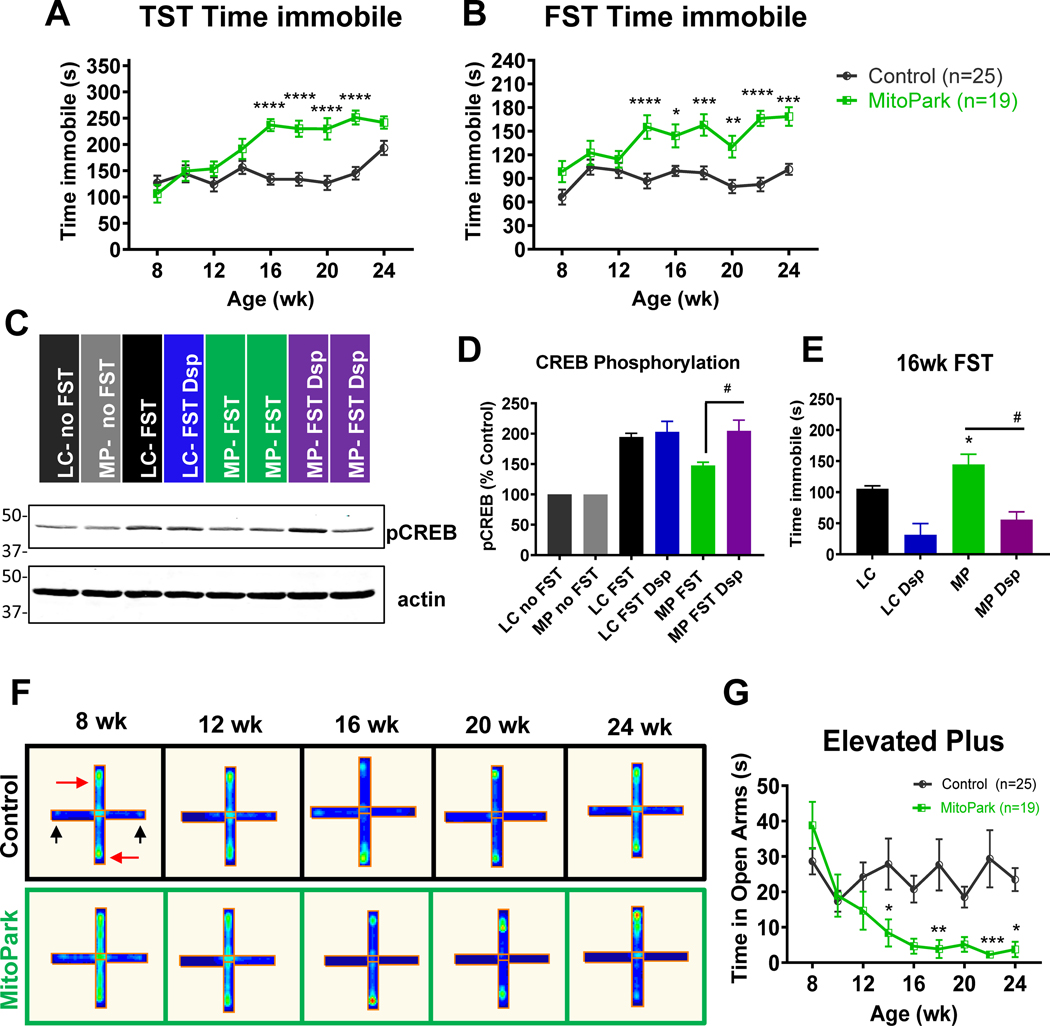
Depression is estimated to affect more than half of Parkinson’s patients and largely impacts patients’ quality of life (Balestrino and Martinez-Martin, 2017). MitoPark mice were monitored every two weeks for depressive and anxiety-like phenotypes from 8–24 wk of age. The tail suspension test (TST) revealed depressive-like behavior as indicated by increased immobility time in MitoPark mice at 16 weeks when compared to age-matched littermate control mice (Fig 3A). Control mice also showed increased immobility during TST at 24 wk. During the Porsolt forced swim test (FST), a significant increase in immobility occurred from 14 wk onward in MitoPark mice, while immobility in control mice remained relatively constant over time (Fig 3B).
Figure 3. Behavioral despair and anxiety-like behavior in MitoPark mice.
MitoPark mice were monitored at 2-wk intervals for neuropsychiatric deficits from 8–24 weeks of age. A, Tail suspension test (TST) and (B) forced swim test (FST) reveal depressive behavior in MitoPark mice at 16 and 14 weeks, respectively, when compared to age-matched control mice. n=25 control and n=19 MitoPark mice. C-D, Western blotting reveals increased CREB phosphorylation in FST mice versus untested controls and increased CREB phosphorylation in MitoPark mice administered desipramine. E, Desipramine treatment (Dsp, 5 mg/kg, i.p., 30 min prior to FST, n=7 per group) significantly reduced immobility time during the FST in MitoPark mice (#, p <0.01 versus saline-injected MitoPark). F, Representative occupancy plots of closed (red arrow) and open (black arrow) arms of elevated plus maze and (G) corresponding time in open arms. *, p≤0.05; **, p<0.01; ***, p<0.001.
To further support that this finding was due to behavioral despair and not motor dysfunction, we treated a subset of 16- and 24-wk mice with desipramine (5 mg/kg, i.p.), an antidepressant that increases neurogenesis, and performed the FST 30 min post-treatment. In accordance with other studies showing antidepressant efficacy through neurogenesis, our Western blotting revealed increased CREB phosphorylation in the hippocampus of FST-tested mice versus untested controls (Fig. 3C–D and Supplemental Fig. 4). However, MitoPark mice did not show significant induction of pCREB unless treated with desipramine. Importantly, antidepressant treatment restored CREB phosphorylation to the levels in littermate control mice (Fig. 3C–D) and reduced immobility during the FST at 16 and 24 wk of age (Fig. 3E and Supplemental Fig. 5A). Neurochemical data showing significant increases in norepinephrine and serotonin in desipramine-treated MitoPark mice are available in Supplemental Figs. 5B and 6. Our data show that neurochemical restoration and increasing pCREB in the hippocampus in desipramine-treated MitoPark mice attenuated behavioral despair. Furthermore, the fact that immobility was reduced by antidepressant treatment suggests that motor dysfunction in the MitoPark model is not the cause of immobility observed during the FST.
We also performed a 10-min elevated plus maze trial to test for anxiety-like behavior in MitoPark mice. The open arms are indicated by black arrows and closed arms by red arrows in Fig. 3F. Due to their natural preference for darker, enclosed spaces, mice in both groups spent less than 5–10% of their time exploring open arms. Despite the overwhelming preference for closed arms, clear behavioral differences emerged between groups. The time spent in the open arms decreased significantly in MitoPark mice beginning at 14 wk of age (Fig. 3G), suggesting a progressive increase in anxious behavior. Taken together, we have identified anxiety-like and depression-like changes present starting from 14 wk in MitoPark mice, concurrent with the onset of motor deficits in this model. The neuropsychiatric effects observed seem to not be mediated by sleep deprivation, since both sleep latency (Supplemental Fig. 7A) and total time sleeping (Supplemental Fig. 7B) during the sleep latency test remain unchanged between control and MitoPark mice.
Olfactory dysfunction in MitoPark mice
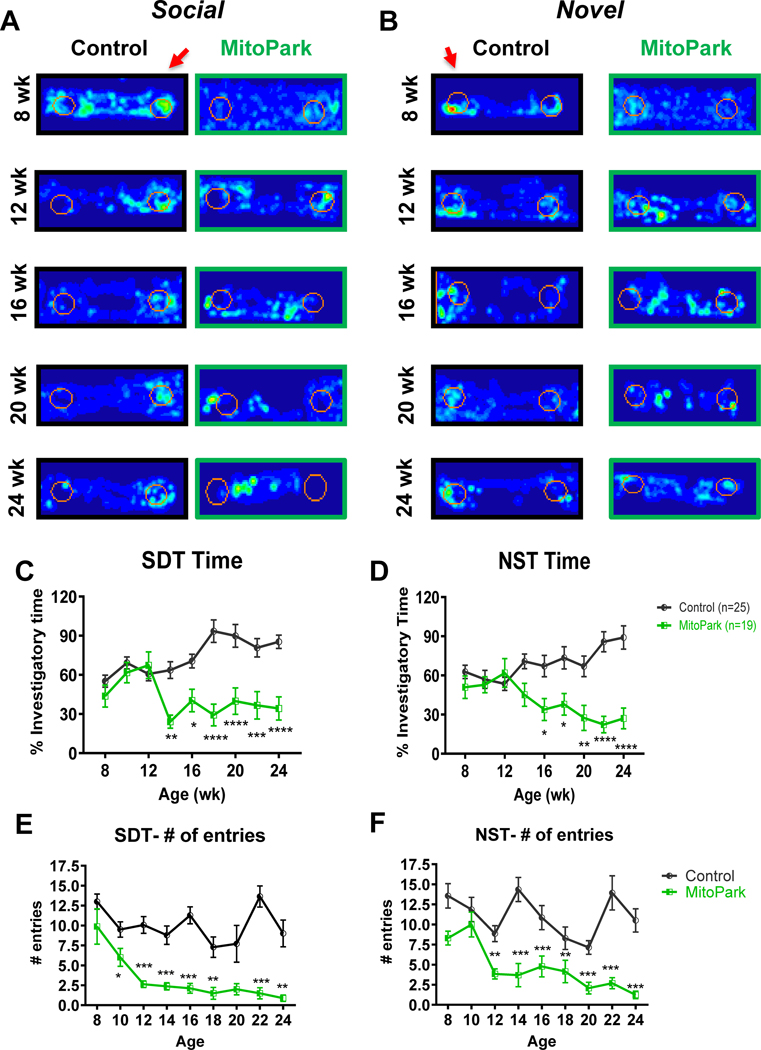
Some degree of hyposmia is highly prevalent in PD patients and may occur decades prior to the onset of motor dysfunction, making screening of olfactory deficits a potential prognostic tool in early PD (Ottaviano et al., 2016; Visanji and Marras, 2015). Representative occupancy plots from a 3-min trial of the social discrimination test (Fig. 4A) and novel scent test (Fig. 4B) at ages 8–24 wk reveal a reduced preference for the scented region (arrow) over time in MitoPark mice but not age-matched controls. Olfactory deficits, as indicated by significant differences in percent investigatory time during the social discrimination and novel scent tests, emerged at 14 and 16 wk, respectively (Fig. 4C–D). Also, fewer entries into the scented region during the social discrimination test occurred as early as 10 wk (Fig. 4E), while during the novel scent test, a significant reduction in entries began at 12 wk of age (Fig. 4F). Our results indicate that olfactory deficits begin prior to the onset of motor dysfunction in MitoPark mice.
Figure 4. Olfactory dysfunction in MitoPark mice.
MitoPark mice were monitored at 2-wk intervals for olfactory deficits from 8–24 weeks of age. n=25 control and n=19 MitoPark mice. A-B, Occupancy plots and quantification of (C-F) time in scented zones are shown. Red arrows indicate scented zones. Olfactory deficits as determined by (A, C, E) social discrimination test (SDT) and (B, D, F) novel scent test (NST) were present as soon as 14 weeks of age in MitoPark mice versus controls. *, p≤0.05; **, p<0.01; ***, p<0.001.
Biochemical changes parallel observed behavioral impairments in MitoPark mice
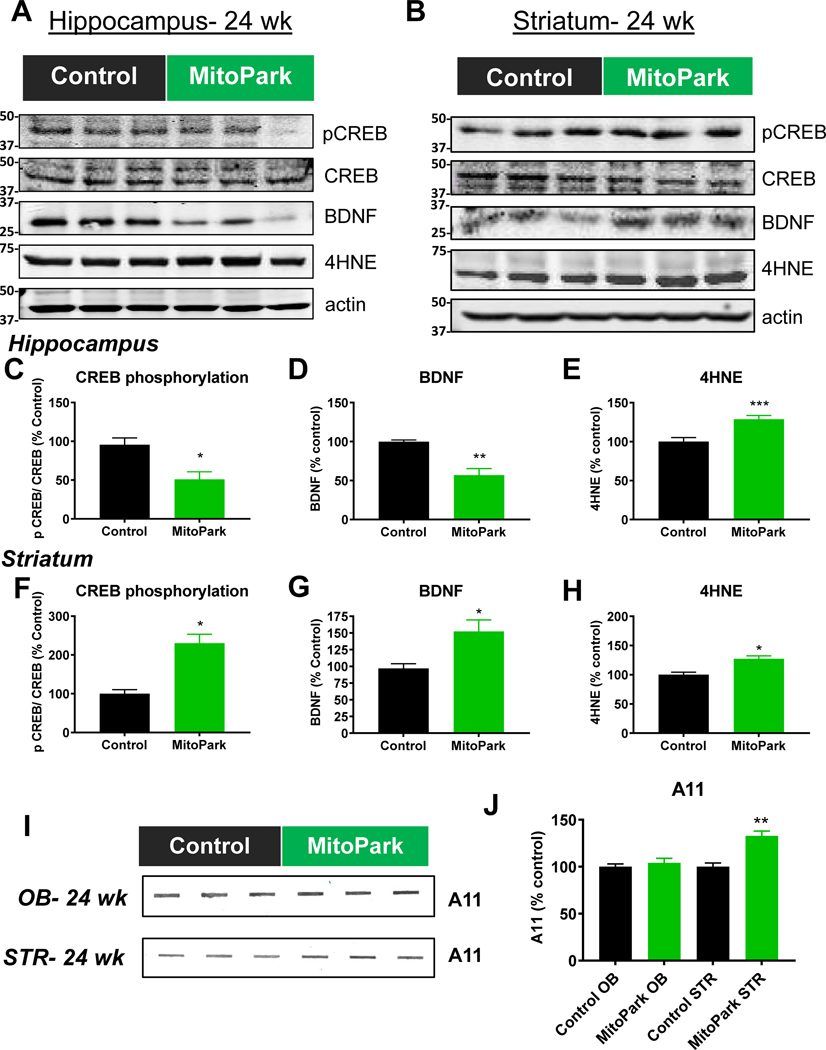
Next, we tested for specific biochemical changes potentially corresponding to the occurrence of key nonmotor behavioral impairments. Since CREB phosphorylation and BDNF levels increase in mice post-MWM in a time-dependent manner (Cho et al., 2013; Lee et al., 2015; Min et al., 2015), we sacrificed mice 10–30 min after the last MWM retention trial and performed Western blotting for CREB, phospho-CREB, and BDNF protein levels. The significant reductions in CREB phosphorylation (Fig. 5A, C; all full western blot images available in Supplemental Fig. 8) and BDNF (Fig. 5A, D) protein levels in the hippocampus may be associated with the observed cognitive deficits.
Figure 5. Biochemical changes parallel the observed behavioral impairments in MitoPark mice.
A-B, Western blots and (C-H) densitometric analysis of proteins related to neuropsychiatric and cognitive changes. n=6 control and n=7 MitoPark mice. I, A11 slot blot and (J) densitometric analysis from striatum and olfactory bulb tissues from littermate control (n=3) and MitoPark mice (n=3). *, p≤0.05; **, p<0.01; ***, p<0.001.
Conversely, the increases in CREB phosphorylation and BDNF in the striatum may be related to the depressive phenotype observed (Fig. 5B, F, and G). In both striatal and hippocampal tissues, we observed a significant increase in 4HNE, a lipid peroxidation product that results from oxidative damage (Fig. 5A, B, E, and H).
Researchers have attempted to link olfactory dysfunction to αSyn deposition since both occur early in PD pathogenesis (Reichmann et al., 2016). However, we did not see an increase in oligomeric protein in the olfactory bulb of MitoPark mice as determined by slot blot for A11 anti-oligomeric protein antibody (Fig. 5I–J). Similar to what Ekstrand et al.(Ekstrand et al., 2007) reported in the substantia nigra of MitoPark mice, we did see significantly increased protein aggregation in striatal tissues.
Altered neurogenesis in MitoPark mice
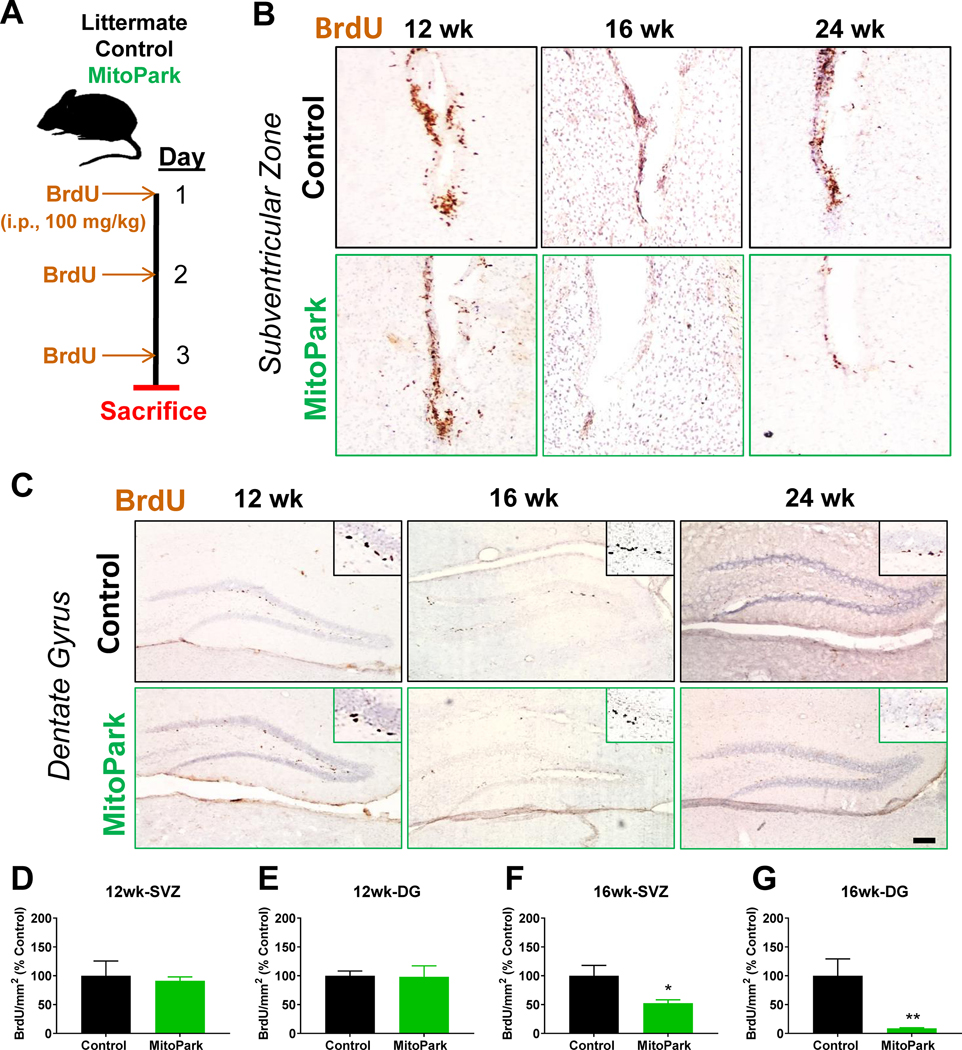
The subventricular zone (SVZ) to rostral migratory stream to olfactory bulb pathway is responsible for olfactory function and maintenance, while alterations in hippocampal neurogenesis in the subgranular zone (SGZ) has been linked to both depression-like behavior and cognition. Therefore, we injected MitoPark and control mice with BrdU to see if neurogenesis was affected in this model of chronic, progressive dopaminergic neurodegeneration. Littermate control and MitoPark mice were injected i.p. with 100 mg/kg BrdU daily for 3 days and sacrificed 12 h past the last injection as shown in the treatment paradigm (Fig. 6A). DAB immunohistochemistry for anti-BrdU was performed on sections containing the SVZ (Fig. 6B) and SGZ (Fig. 6C) to label proliferative cells counterstained with hematoxylin. Although no significant changes in BrdU+ cells were observed at 12 wk of age (Fig. 6D and E), MitoPark mice had fewer BrdU+ cells in the SVZ in contrast to littermate control mice at ages 16 and 24 wk (Fig. 6F). Similarly, a significant decrease in BrdU+ cells is observed in the SGZ in hippocampal sections from 16-wk-old MitoPark mice (Fig. 6G). Overall, BrdU staining revealed fewer proliferating cells in both the SVZ and SGZ of aged MitoPark mice by 16 wk of age, when multiple nonmotor deficits are observed in this model.
Figure 6. Altered neurogenesis in MitoPark mice.
(A) Schematic of BrdU treatment paradigm for assessment of neurogenesis in MitoPark (MP) and littermate control (LC) mice (n=6 LC and n=6 MP mice at 12 wk; n=8 LC and n=8 MP mice at 16 wk). BrdU immunostaining of subventricular zone (SVZ, B) and subgranular zone (SGZ, C) reveals no differences at 12 wk of age (D, E). By 16 wk, the number of BrdU+ cells is significantly reduced in both the SVZ (F) and SGZ (G). *, p≤0.05; **, p<0.01.
Neurochemical changes in MitoPark mice
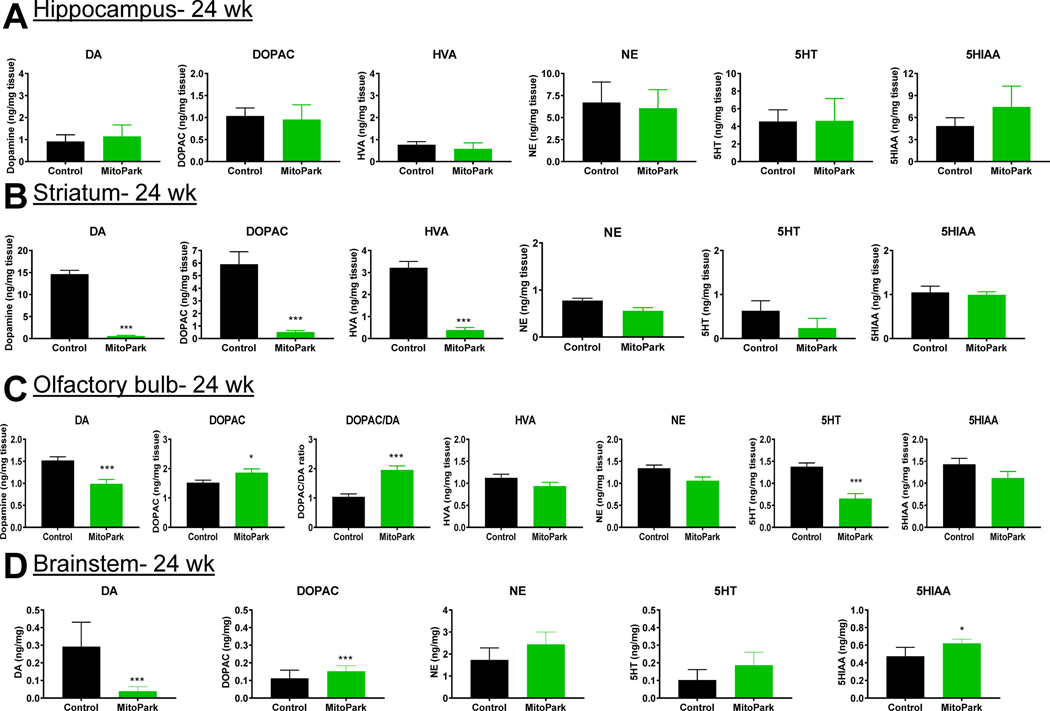
Depression-like behaviors in toxin-based models of PD have been predominantly associated with reductions in hippocampal serotonin and striatal dopamine (Santiago et al., 2010). However, no significant changes were observed in hippocampal neurotransmitters (Fig. 7A), indicating that observed changes in depressive behavior are instead possibly due to neurogenesis or plasticity modifications. As anticipated, strong reductions in dopamine and its metabolites were observed in the striatum of MitoPark mice, corresponding to the motor phenotype of the model (Fig. 7B). In our study, levels of dopamine and serotonin were reduced in the olfactory bulbs of 24-wk MitoPark mice (Fig. 7C), which may help explain the hyposmia in this model. However, Branch et. al. reported a dopamine reduction in the olfactory bulb that did not occur until a later age (Branch et al., 2016). Because DOPAC also increased (Fig. 7C), we examined whether enhanced dopamine turnover occurred in the olfactory bulbs of MitoPark mice. Indeed, a similar decrease in dopamine occurred despite an increase in DOPAC in 24-wk MitoPark brainstem samples (Figure 7D). Since oligomeric protein did not significantly increase in the olfactory bulb (Fig. 5I–J), the hyposmia most likely resulted from neurochemical changes rather than protein aggregation. Future studies should explore the role of specific olfactory receptors in this model at various stages to better elucidate the mechanism of hyposmia in MitoPark mice.
Figure 7. Neurochemical changes in MitoPark mice.
Analysis of monoamine neurotransmitters from the hippocampus (A, n=7 LC and n=7 MP mice), striatum (B, n=7 LC and n=7 MP mice), olfactory bulb (C, n=9 LC and n=9 MP mice), and brainstem (D, n=4 LC and n=4 MP mice) tissues from MitoPark mice and their littermate controls at 24 wk of age. *, p≤0.05; ***, p<0.001.
Discussion
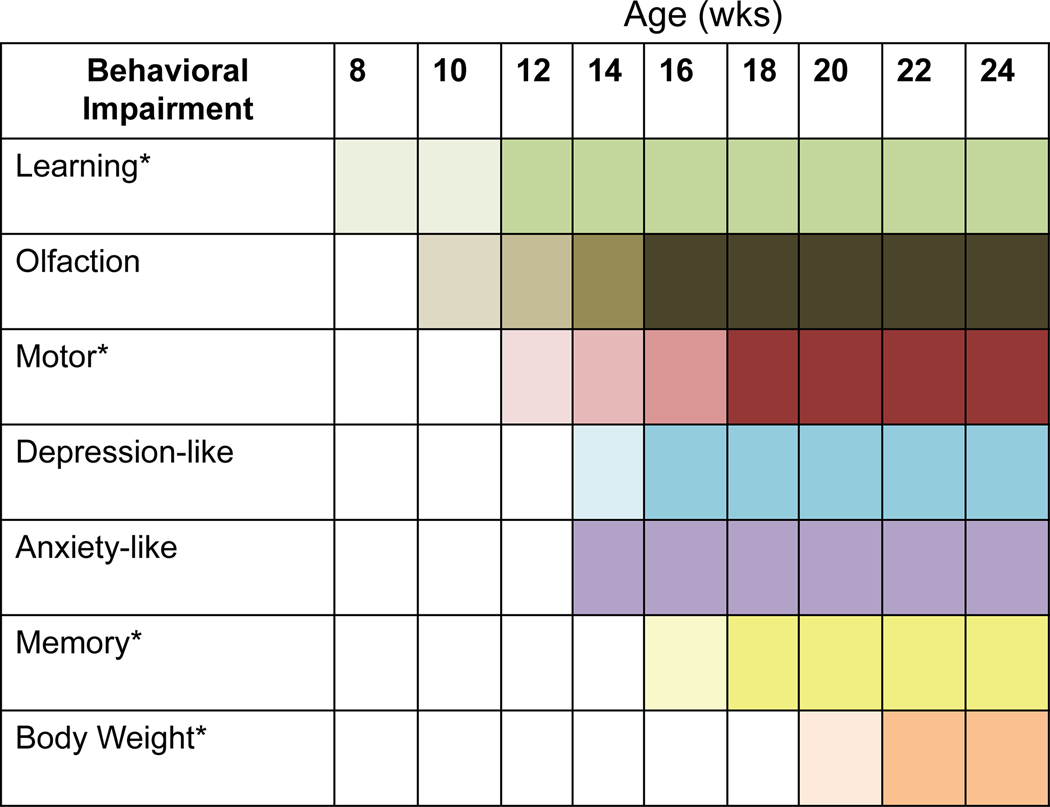
MitoPark mice recapitulate several features of PD in humans, including a progressive course of the phenotypic manifestations and neurodegeneration, protein inclusions in nigral tissues, motor deficits that are ameliorated by L-DOPA administration, an altered response to L-DOPA treatment, and adult-onset of disease. However, the full range of nonmotor deficits in the MitoPark model has not been characterized. In this study, we demonstrate that similar to human PD, many nonmotor behavioral impairments including olfactory dysfunction, learning and memory deficits, and anxiety- and depressive-like problems are also evident in MitoPark mice prior to or concurrent with the occurrence of motor deficits (Summary in Figure 8).
Figure 8. Summary of nonmotor behavioral impairments in MitoPark mice.
Asterisks (*) indicate sex-specific differences in a behavioral task. Changes in color shading represent additional parameters that were significant in that behavioral task.
We found that while some behavioral impairments in MitoPark mice occur before the onset of motor dysfunction (i.e., learning and olfactory deficits), many did not appear until after. The temporal overlap between certain nonmotor deficits and motor dysfunction is likely due to the fast onset of the motor phenotype once the MitoPark mice reach 12 wk of age. For this reason, it is particularly important to consider parallel motor task parameters when interpreting the neurobehavioral results. For example, tasks which require horizontal movements (i.e. elevated plus, novel scent test) should be observed alongside open field test data which suggest motor deficits at 14 wk of age; whereas motor ability assessed during MWM trials as average swimming speed (m/s) suggested no significant impairment in swimming. Interestingly, dual task performance has been shown in certain situations to improve performance for PD patients, thought to be due to increased production of catecholamines and arousal of additional brain regions (Altmann et al., 2015; Hazamy et al., 2017). Similarly, paradoxical kinesia can enhance motor performance during a perceived stressful event, such as an earthquake, and has also been observed to occur in PD (Bonanni et al., 2010). These phenomena could potentially explain the enhanced ability of MitoPark mice to swim during MWM during times when their locomotor activity is diminished in other types of motor tasks.
Accordingly, we chose the MWM for spatial learning and memory assessment over Barnes maze since the average swim speed of mice (Supplementary Fig. 2) remained unchanged while horizontal activity in the open-field test was progressively affected from an early age. However, this has its own set of considerations including an increased potential for stress response during both the MWM and subsequent tests performed (Harrison et al., 2009) as well as task repetition potentially influencing performance in later testing periods. To minimize any effects of stress, parameters such as water temperature, lighting, and the duration of task acclimation prior to testing were held consistent between genotypes and time points. Moreover, when compared to other published studies, our data were in line with values reported in naive mice (Can et al., 2012a; Komada et al., 2008; Taylor et al., 2010) or have even been previously identified in MitoPark mice and confirmed independently by another research group (Cong et al., 2016; Langley et al., 2018; Li et al., 2013; Paß et al., 2020). Therefore, we do not believe this to have significantly affected our interpretation. Ideally, completely naive cohorts of MitoPark mice should be subjected to each behavioral test and at each time point. However, this requires a large number of the transgenic mouse model.
For example, our cognitive function data are also supported by a recent study showing spatial learning and memory deficits in the Barnes maze and object recognition deficits that preceded the appearance of motor deficits in these animals (Fifel and Cooper, 2014; Li et al., 2013). However, our study expanded their findings by showing various ages of mice, sex differences, and progressive worsening. An important caveat of our model assessment is that it was not designed to discriminate temporal differences between male and female mice regarding the onset and incidence of nonmotor symptoms. Future time-course experiments could help to better associate the initial pathological findings with the onset of behavioral deficits to add to our nonmotor study in which both males and females were already symptomatic prior to their initial behavioral assessments at 8 wk. In terms of sex differences, role environmental and occupational exposures such as metal and pesticide exposures should be considered since the chemical exposure is a major risk factor for PD pathogenesis (Erikson and Aschner, 2019; Harischandra et al., 2019; Horning et al., 2015). It is noteworthy to mention that chemical industrial occupation is a male dominated field, suggesting that this may play role in higher incidence of PD in males than females.
The olfactory system provides sensory information from our environment that can be key to determining palatability of food and identifying the presence of dangerous fumes and other indicators of toxins (Doty, 2012). The prevalence of hyposmia is about 90% in sporadic PD cases, and 68% of patients surveyed described alterations in their quality of life due to impaired olfactory function (Doty, 2012). Olfactory disturbances do not occur in all neurodegenerative diseases, making this nonmotor symptom particularly valuable in differential diagnosis (Doty, 2009). Dopaminergic cells can be found in the periglomerular layer of the olfactory bulb, but do no degenerate in PD (Doty, 2012; Huisman et al., 2008; Tong et al., 2000). Unlike the SN and VTA, tyrosine hydroxylase (TH) actually increases in the olfactory bulb (OB) of patients with PD and in experimental PD animal models (Belzunegui et al., 2007; Doty, 2012; Huisman et al., 2008; Lelan et al., 2011; Yamada et al., 2004). In an intranasal MPTP model, dopaminergic and noradrenergic deficits were seen in the brain, but dopamine therapy does not help the olfactory deficits in PD patients (Prediger et al., 2010). Interestingly, although dopaminergic therapy does not improve olfaction in PD patients, we observe olfactory deficits in this model of mitochondrial dysfunction in dopaminergic cells, implicating the complex role dopamine has in the olfactory system prior to the onset of motor impairment. Non-dopaminergic neurotransmitter systems are thought to contribute to or cause olfactory loss in PD (Doty, 2012). Thy1-aSyn mice were shown to have olfactory deficits following social recognition deficits, indicating the deficits are not exclusively due to olfaction impairment in this model (Magen et al., 2015).
Although the social discrimination scent test revealed changes slightly before the novel scent test in MitoPark mice, simple scent discrimination versus any social recognition deficits cannot be deciphered from our data. Changes in response to social stimuli in young adult MitoPark mice should be explored in future studies. Corroborating our findings in the novel and social discrimination tests, Pass et al. (2020) recently identified differences in the novel scent test but not the buried food detection test. Interestingly, different scents were used in our study versus their study, so the lack of detecting buried food could show decreased interest in eating (Ghaisas et al., 2019) or lack of motivation to dig to retrieve the food rather than the ability to discriminate between odors. Since no changes in neurogenesis or olfactory bulb neurotransmitter or protein aggregation levels were observed prior to dopamine loss in this model, changes in other components involved in olfactory discrimination should also be explored including odor receptors in the nasal epithelium, individual glomerular and mitral layers of the olfactory bulb, piriform cortex, hippocampus, and thalamus (Chen et al., 2016; Griffanti et al., 2016; Ravi et al., 2017).
Animal models and clinical PD studies have shown that reduced dopamine levels correlate with the reduced proliferation of cells in neurogenic regions of the brain, which is thought to contribute to the nonmotor symptoms observed in PD (Chiu et al., 2015; Kuipers et al., 2014; Regensburger et al., 2014). However, for most mouse models of PD, either nonmotor behavioral impairments do not emerge or else nonmotor performance was never characterized, thereby limiting our understanding of the therapeutic potential that can be gleaned from current genetic and toxin-based models (Taylor et al., 2010). Interestingly, mice deficient in monoamine storage capacity display a progressive loss of dopaminergic cells in the SN, loss of striatal dopamine, motor deficits, αSyn accumulation and nonmotor deficits (Taylor et al., 2009). In the MitoPark model, just the dopaminergic cells are targeted rather than other neuronal systems, yet we see a very similar set of nonmotor behavioral deficits. This particularly highlights the importance of mitochondrial dysfunction in dopaminergic cells in the development of these nonmotor behavioral impairments in MitoPark mice as well as nonmotor aspects of PD.
Since the MitoPark mouse model was developed, more detailed pathological features have been identified by various groups, some of which may help explain mechanisms underlying the nonmotor deficits observed. At 6–10 wk, MitoPark mice exhibit impaired electrophysiological parameters in dopaminergic neurons and increased expression of L-type calcium channel mRNA and PK2 protein when compared to their littermate controls (Branch et al., 2016; Good et al., 2011; Gordon et al., 2016). At 28–30 wk, increased astrocyte marker GFAP, greater striatal glutamate release, and white matter MRI changes were identified (Farrand et al., 2016). Also at 30 wk, MRI changes indicative of iron accumulation were found in the SN (Farrand et al., 2016). Mitochondrial dysfunction is expected to lead to oxidative damage in neurodegenerative disorders (Islam, 2017), potentially representing the mechanism underlying the occurrence of nonmotor behavioral impairments in the MitoPark model. In our previous publications, we have observed a significant loss of mitochondrial protein expression by 12 wk in the MitoPark striatum and SN, but no significant changes in the oxidative stress marker 4HNE until 24 wk (Langley et al., 2017; Langley et al., 2018). Neuroinflammation is another mechanism implicated in the pathophysiology of PD and nonmotor symptoms, given that inflammatory cytokine levels have been positively correlated with nonmotor deficits (Lindqvist et al., 2012; Menza et al., 2010). We recently identified morphological changes indicative of microglia activation and increased IBA1+ microglia in the SN of 24-wk MitoPark mice, however, this was not yet observed in 12-wk MitoPark mice (Langley et al., 2017; Langley et al., 2018).
We also observed strong neurochemical and biochemical changes that corresponded well with key nonmotor behavioral impairments in the present study. For example, reduced levels of dopamine and serotonin in the olfactory bulb of 24-wk MitoPark mice may help explain the hyposmia at later ages in this model (Ferrer et al., 2012; Taylor et al., 2009). Delayed dopamine dysfunction was recently observed in female MitoPark mice in another study (Chen et al., 2019); however, we did not test neurotransmitter levels in our study prior to age 24 wk, when both males and females exhibit severe deficits in both motor and cognitive-behavioral tasks. The female mice in our experiments demonstrated impairment in sensorimotor coordination on the RotaRod and learning in the MWM later than male mice, so delayed dopamine dysfunction could certainly contribute to these behavioral findings.
Many nonmotor deficits closely correlate with Lewy body deposition and begin in the prodromal stage of PD, prior to the classical motor deficits used for clinical diagnosis (Pellicano et al., 2007; Schneider and Obeso, 2015; Shulman et al., 2001). First, GI and olfactory disturbances are observed while αSyn pathology is evident in the olfactory bulb and the motor nucleus of the vagus nerve, which provides parasympathetic innervation to the GI tract (Burke et al., 2008). Next, synucleopathy in the hypothalamus, locus coeruleus, and raphe nucleus correlates with sleep disorders and neuropsychiatric symptoms such as depression and anxiety. Motor symptoms and clinical diagnosis then begin during Braak stages 3–4, when the midbrain becomes involved. Finally, cognitive decline and dementia are found in association with cortical deposits of αSyn, similar to what is seen in dementia with Lewy bodies (Jellinger, 2009).
In addition to protein aggregate deposition, alterations in adult neurogenesis and neurochemical changes are implicated in the development of nonmotor symptoms in PD (Lamm et al., 2014; Schlachetzki et al., 2016). Recent studies suggest that dopamine depletion and changes in αSyn may even synergistically contribute to the altered neurogenesis associated with nonmotor deficits (Schlachetzki et al., 2016). Recently, when exogenous mutant αSyn was overexpressed in the olfactory bulb of rats, dopamine depletion and olfactory deficits were observed followed by spreading to other brain regions and subsequent motor coordination deficits, suggesting an interesting prodromal model of PD (Niu et al., 2018). In our studies, we did observe increased striatal protein aggregation by anti-oligomeric (A11) slot blot analysis but not in the olfactory bulb. Despite immunoreactivity to an anti-αSyn antibody, protein inclusions found in dopaminergic neurons of MitoPark mice were convincingly determined not to contain αSyn by crossing with knockout mice (Ekstrand et al., 2007). Since electrophysiological parameters are altered very early in MitoPark mice, we believe that more advanced electrophysiological or subcellular fraction studies in additional brain regions may provide a better understanding of the more subtle deficits preceding the nigrostriatal dopamine depletion, but were outside the scope of this study.
It is estimated that up to 50% of PD patients are affected by mild cognitive impairment, while longitudinal studies reveal that dementia will eventually affect 80% (Goldman et al., 2018). Among other genetic and environmental risk factors, polymorphisms in BDNF and COMT genes have been implicated in PD cognitive impairments (Gao et al., 2010; Goldman et al., 2018). Within the mesolimbic dopamine circuit, increased BDNF through CREB activation mediates susceptibility to stress (Krishnan and Nestler, 2008; Vaidya and Duman, 2001). In contrast, stress decreases hippocampal levels of BDNF and neurogenesis through CREB activity and cortisol concentrations (Krishnan and Nestler, 2008). Oxidative stress has also been implicated in a variety of neuropsychiatric disorders, with particularly strong evidence in depression and anxiety studies (Balmus et al., 2016; Ng et al., 2008). Also, reductions in CREB phosphorylation and BDNF in the hippocampus may be associated with the observed cognitive deficits and decreased neurogenesis. Importantly, increased BDNF and pCREB resulting from noradrenergic or serotonergic antidepressants can promote neurogenesis and improve cognition (Sugimoto et al., 2008; Zhang et al., 2016b). Conversely, increases in CREB phosphorylation, BDNF, and oxidative damage marker 4-HNE in the striatum may be related to the depression-like behavior observed. Although animal models and post-mortem studies do indicate adult neurogenesis is affected in PD, the exact mechanism of the changes and the correlation with nonmotor deficiencies have not been directly explored. Importantly, we have shown that following dopamine depletion in MitoPark mice, proliferating cells are significantly reduced in both neurogenic niches of the brain, namely the subventricular and subgranular zones. The possibility that the migration or differentiation of the neural stem and progenitor cells is impaired at earlier ages in the olfactory bulb should be examined in future studies.
Interestingly, no significant changes in sleep latency were observed (Fig. S2); although, a recent paper found that MitoPark mice display circadian rhythm dysfunction following an all-light or all-dark cycle (Fifel and Cooper, 2014). A recent systematic review of sleep and wakefulness disorders suggests that PD medications can have positive or negative effects on sleep symptoms, indicating a particularly complex role of dopaminergic therapies in the sleep-related disorders associated with PD (Chahine et al., 2017). Because constipation and other GI problems are associated with early stages of PD, further studies should be done to comprehensively characterize GI changes in MitoPark mice. GI dysfunction can even affect motor symptoms by impairing drug bioavailability and, when left untreated, autonomic changes can contribute to falls common in PD patients (Palma and Kaufmann, 2018). Based on MPTP studies resulting in loss of dopaminergic neurons in the enteric nervous system, changes in colon motility should specifically be explored (Anderson et al., 2007). A recent study in our laboratory has demonstrated that MitoPark mice recapitulate the chronology and development of GI dysfunction when compared to their littermate controls (Ghaisas et al., 2019). We also showed that oral administration of quercetin, one of the major flavonoids in plants, effectively reversed behavioral deficits, striatal dopamine depletion, and TH neuronal cell loss in MitoPark mice (Ay et al., 2017), suggesting this model as an attractive translational animal model for pre-clinical assessment of the efficacy of new anti-Parkinsonian drugs.
Collectively, our study demonstrates that in addition to progressive motor deficits, MitoPark mice also exhibit nonmotor deficiencies including olfactory dysfunction, learning and memory deficits, and neuropsychological problems. The presence of these nonmotor deficits in combination with the progressive motor dysfunction makes the MitoPark mouse model of PD particularly valuable for mechanistic and drug discovery studies.
Supplementary Material
Highlights.
MitoPark mice display clinically-relevant nonmotor behavioral impairments.
Cognitive and olfactory dysfunction precedes dopaminergic depletion in MitoPark mice.
Behavioral despair and anxiety-like behavior are present in MitoPark mice.
Altered neurogenesis in MitoPark mice is observed from 16 wk of age.
Neurochemical and biochemical changes parallel observed behavioral deficits in MitoPark mice.
Acknowledgments
This study was supported by National Institute of Health grants ES026892, ES027245, NS100090, and NS088206. The W. Eugene and Linda Lloyd Endowed Chair and Armbrust endowment to A.G.K. and the Salisbury Endowed Professorship to A.K. are also acknowledged. We would like to thank Gary Zenitsky for his help in preparing the manuscript.
Abbreviations
- αSyn
Alpha-synuclein
- AMS
ANY-maze software
- ANOVA
Analysis of variance
- CREB
cAMP response element-binding protein
- DG
dentate gyrus
- DAT
dopamine transporter
- DOPAC
3–4-dihydroxyphenylacetic acid
- FST
forced swim test
- GI
gastrointestinal
- GSM
grip strength meter
- HPLC
high-performance liquid chromatography
- HVA
homovanillic acid
- IHC
immunohistochemistry
- ISU
Iowa State University
- LRRK2
leucine-rich repeat kinase 2
- L-DOPA1
levodopa
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MWM
Morris water maze
- OB
olfactory bulb
- 6-OHDA
6-hydroxydopamine
- PD
Parkinson’s disease
- PINK1
PTEN-induced putative kinase 1
- qPCR
quantitative polymerase chain reaction
- RMS
rostral migratory stream
- SN
substantia nigra
- SVZ
subventricular zone
- SGZ
subgranular zone
- STR
striatum
- TST
tail suspension test
- TFAM
mitochondrial transcription factor A
Footnotes
Competing Interests
A.G.K. and V.A. are shareholders of PK Biosciences Corporation (Ames, IA) and Probiome Therapeutics, which are interested in identifying novel biomarkers and potential therapeutic targets for PD. A.G.K. and V.A. do not have any commercial interests in the present work. All other authors declare no potential conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott RD, Ross GW, White LR, Tanner CM, Masaki KH, Nelson JS, Curb JD, Petrovitch H, 2005. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology 65, 1442–1446. [DOI] [PubMed] [Google Scholar]
- Altmann LJ, Stegemoller E, Hazamy AA, Wilson JP, Okun MS, McFarland NR, Wagle Shukla A, Hass CJ, 2015. Unexpected dual task benefits on cycling in Parkinson disease and healthy adults: a neuro-behavioral model. PloS one 10, e0125470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Noorian AR, Taylor G, Anitha M, Bernhard D, Srinivasan S, Greene JG, 2007. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Experimental neurology 207, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ay M, Luo J, Langley M, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG, 2017. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J Neurochem 141, 766–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrino R, Martinez-Martin P, 2017. Neuropsychiatric symptoms, behavioural disorders, and quality of life in Parkinson’s disease. J Neurol Sci 373, 173–178. [DOI] [PubMed] [Google Scholar]
- Balmus IM, Ciobica A, Antioch I, Dobrin R, Timofte D, 2016. Oxidative Stress Implications in the Affective Disorders: Main Biomarkers, Animal Models Relevance, Genetic Perspectives, and Antioxidant Approaches. Oxidative medicine and cellular longevity 2016, 3975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzunegui S, San Sebastian W, Garrido-Gil P, Izal-Azcarate A, Vazquez-Claverie M, Lopez B, Marcilla I, Lanciego JL, Luquin MR, 2007. The number of dopaminergic cells is increased in the olfactory bulb of monkeys chronically exposed to MPTP. Synapse 61, 1006–1012. [DOI] [PubMed] [Google Scholar]
- Bonanni L, Thomas A, Anzellotti F, Monaco D, Ciccocioppo F, Varanese S, Bifolchetti S, D’Amico MC, Di Iorio A, Onofrj M, 2010. Protracted benefit from paradoxical kinesia in typical and atypical parkinsonisms. Neurol Sci 31, 751–756. [DOI] [PubMed] [Google Scholar]
- Bonito-Oliva A, Masini D, Fisone G, 2014a. A mouse model of non-motor symptoms in Parkinson’s disease: focus on pharmacological interventions targeting affective dysfunctions. Frontiers in behavioral neuroscience 8, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonito-Oliva A, Pignatelli M, Spigolon G, Yoshitake T, Seiler S, Longo F, Piccinin S, Kehr J, Mercuri NB, Nistico R, Fisone G, 2014b. Cognitive impairment and dentate gyrus synaptic dysfunction in experimental parkinsonism. Biol Psychiatry 75, 701–710. [DOI] [PubMed] [Google Scholar]
- Branch SY, Chen C, Sharma R, Lechleiter JD, Li S, Beckstead MJ, 2016. Dopaminergic Neurons Exhibit an Age-Dependent Decline in Electrophysiological Parameters in the MitoPark Mouse Model of Parkinson’s Disease. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 4026–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley-Brits K, Deng Y, Song W, 2011. Morris water maze test for learning and memory deficits in Alzheimer’s disease model mice. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke RE, Dauer WT, Vonsattel JP, 2008. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Annals of neurology 64, 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FL, Carvalho MM, Cristovao AC, Je G, Baltazar G, Salgado AJ, Kim YS, Sousa N, 2013. Rodent models of Parkinson’s disease: beyond the motor symptomatology. Frontiers in behavioral neuroscience 7, 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD, 2012a. The mouse forced swim test. J Vis Exp, e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD, 2012b. The tail suspension test. Journal of visualized experiments : JoVE, e3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahine LM, Amara AW, Videnovic A, 2017. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev 35, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH, 2009. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. The Lancet. Neurology 8, 464–474. [DOI] [PubMed] [Google Scholar]
- Chen J, Ho SL, Lee TM, Chang RS, Pang SY, Li L, 2016. Visuomotor control in patients with Parkinson’s disease. Neuropsychologia 80, 102–114. [DOI] [PubMed] [Google Scholar]
- Chen YH, Wang V, Huang EY, Chou YC, Kuo TT, Olson L, Hoffer BJ, 2019. Delayed Dopamine Dysfunction and Motor Deficits in Female Parkinson Model Mice. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu WH, Depboylu C, Hermanns G, Maurer L, Windolph A, Oertel WH, Ries V, Hoglinger GU, 2015. Long-term treatment with L-DOPA or pramipexole affects adult neurogenesis and corresponding non-motor behavior in a mouse model of Parkinson’s disease. Neuropharmacology 95, 367–376. [DOI] [PubMed] [Google Scholar]
- Cho N, Lee KY, Huh J, Choi JH, Yang H, Jeong EJ, Kim HP, Sung SH, 2013. Cognitive-enhancing effects of Rhus verniciflua bark extract and its active flavonoids with neuroprotective and anti-inflammatory activities. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 58, 355–361. [DOI] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG, 2017. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci 8, 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Muir ER, Chen C, Qian Y, Liu J, Biju KC, Clark RA, Li S, Duong TQ, 2016. Multimodal MRI Evaluation of the MitoPark Mouse Model of Parkinson’s Disease. PloS one 11, e0151884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, 2008. Behavioral phenotyping strategies for mutant mice. Neuron 57, 809–818. [DOI] [PubMed] [Google Scholar]
- Danilov CA, Steward O, 2015. Conditional genetic deletion of PTEN after a spinal cord injury enhances regenerative growth of CST axons and motor function recovery in mice. Experimental neurology 266, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL, 2010. Genetic animal models of Parkinson’s disease. Neuron 66, 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, 2009. Symposium overview: Do environmental agents enter the brain via the olfactory mucosa to induce neurodegenerative diseases? Ann N Y Acad Sci 1170, 610–614. [DOI] [PubMed] [Google Scholar]
- Doty RL, 2012. Olfactory dysfunction in Parkinson disease. Nat Rev Neurol 8, 329–339. [DOI] [PubMed] [Google Scholar]
- Doty RL, Singh A, Tetrud J, Langston JW, 1992. Lack of major olfactory dysfunction in MPTP-induced parkinsonism. Annals of neurology 32, 97–100. [DOI] [PubMed] [Google Scholar]
- Dranka BP, Gifford A, McAllister D, Zielonka J, Joseph J, O’Hara CL, Stucky CL, Kanthasamy AG, Kalyanaraman B, 2014. A novel mitochondrially-targeted apocynin derivative prevents hyposmia and loss of motor function in the leucine-rich repeat kinase 2 (LRRK2(R1441G)) transgenic mouse model of Parkinson’s disease. Neuroscience letters 583, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand MI, Galter D, 2009. The MitoPark Mouse - an animal model of Parkinson’s disease with impaired respiratory chain function in dopamine neurons. Parkinsonism Relat Disord 15 Suppl 3, S185–188. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG, 2007. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A 104, 1325–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson KM, Aschner M, 2019. Manganese: Its Role in Disease and Health. Metal ions in life sciences 19. [DOI] [PubMed] [Google Scholar]
- Faivre F, Joshi A, Bezard E, Barrot M, 2019. The hidden side of Parkinson’s disease: Studying pain, anxiety and depression in animal models. Neurosci Biobehav Rev 96, 335–352. [DOI] [PubMed] [Google Scholar]
- Farrand AQ, Gregory RA, Backman CM, Helke KL, Boger HA, 2016. Altered glutamate release in the dorsal striatum of the MitoPark mouse model of Parkinson’s disease. Brain Res 1651, 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Lopez-Gonzalez I, Carmona M, Dalfo E, Pujol A, Martinez A, 2012. Neurochemistry and the non-motor aspects of PD. Neurobiology of disease 46, 508–526. [DOI] [PubMed] [Google Scholar]
- Fifel K, Cooper HM, 2014. Loss of dopamine disrupts circadian rhythms in a mouse model of Parkinson’s disease. Neurobiology of disease 71, 359–369. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF, 2008. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. The European journal of neuroscience 28, 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Jiang W, Gao X, Zeng A, Cholger D, Cannon J, Chen J, Zheng W, 2016. Aberrant Adult Neurogenesis in the Subventricular Zone-Rostral Migratory Stream-Olfactory Bulb System Following Subchronic Manganese Exposure. Toxicol Sci 150, 347–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L, Diaz-Corrales FJ, Carrillo F, Diaz-Martin J, Caceres-Redondo MT, Carballo M, Palomino A, Lopez-Barneo J, Mir P, 2010. Brain-derived neurotrophic factor G196A polymorphism and clinical features in Parkinson’s disease. Acta Neurol Scand 122, 41–45. [DOI] [PubMed] [Google Scholar]
- Ghaisas S, Langley MR, Palanisamy BN, Dutta S, Narayanaswamy K, Plummer PJ, Sarkar S, Ay M, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG, 2019. MitoPark transgenic mouse model recapitulates the gastrointestinal dysfunction and gut-microbiome changes of Parkinson’s disease. Neurotoxicology 75, 186–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Kanthasamy A, Joseph J, Anantharam V, Srivastava P, Dranka BP, Kalyanaraman B, Kanthasamy AG, 2012. Anti-inflammatory and neuroprotective effects of an orally active apocynin derivative in pre-clinical models of Parkinson’s disease. J Neuroinflammation 9, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Langley MR, Harischandra DS, Neal ML, Jin H, Anantharam V, Joseph J, Brenza T, Narasimhan B, Kanthasamy A, Kalyanaraman B, Kanthasamy AG, 2016. Mitoapocynin Treatment Protects Against Neuroinflammation and Dopaminergic Neurodegeneration in a Preclinical Animal Model of Parkinson’s Disease. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology 11, 259–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Vernaleo BA, Camicioli R, Dahodwala N, Dobkin RD, Ellis T, Galvin JE, Marras C, Edwards J, Fields J, Golden R, Karlawish J, Levin B, Shulman L, Smith G, Tangney C, Thomas CA, Troster AI, Uc EY, Coyan N, Ellman C, Ellman M, Hoffman C, Hoffman S, Simmonds D, 2018. Cognitive impairment in Parkinson’s disease: a report from a multidisciplinary symposium on unmet needs and future directions to maintain cognitive health. NPJ Parkinsons Dis 4, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CH, Hoffman AF, Hoffer BJ, Chefer VI, Shippenberg TS, Backman CM, Larsson NG, Olson L, Gellhaar S, Galter D, Lupica CR, 2011. Impaired nigrostriatal function precedes behavioral deficits in a genetic mitochondrial model of Parkinson’s disease. FASEB J 25, 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R, Neal ML, Luo J, Langley MR, Harischandra DS, Panicker N, Charli A, Jin H, Anantharam V, Woodruff TM, Zhou QY, Kanthasamy AG, Kanthasamy A, 2016. Prokineticin-2 upregulation during neuronal injury mediates a compensatory protective response against dopaminergic neuronal degeneration. Nature communications 7, 12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffanti L, Rolinski M, Szewczyk-Krolikowski K, Menke RA, Filippini N, Zamboni G, Jenkinson M, Hu MTM, Mackay CE, 2016. Challenges in the reproducibility of clinical studies with resting state fMRI: An example in early Parkinson’s disease. Neuroimage 124, 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haehner A, Hummel T, Reichmann H, 2011. Olfactory loss in Parkinson’s disease. Parkinson’s disease 2011, 450939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harischandra DS, Ghaisas S, Zenitsky G, Jin H, Kanthasamy A, Anantharam V, Kanthasamy AG, 2019. Manganese-Induced Neurotoxicity: New Insights Into the Triad of Protein Misfolding, Mitochondrial Impairment, and Neuroinflammation. Frontiers in neuroscience 13, 654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP, 2009. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behavioural brain research 198, 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazamy AA, Altmann LJP, Stegemoller E, Bowers D, Lee HK, Wilson J, Okun MS, Hass CJ, 2017. Improved cognition while cycling in Parkinson’s disease patients and healthy adults. Brain Cogn 113, 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horning KJ, Caito SW, Tipps KG, Bowman AB, Aschner M, 2015. Manganese Is Essential for Neuronal Health. Annu Rev Nutr 35, 71–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman E, Uylings HB, Hoogland PV, 2008. Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson’s disease patients. Mov Disord 23, 1407–1413. [DOI] [PubMed] [Google Scholar]
- Ishihara L, Brayne C, 2006. What is the evidence for a premorbid parkinsonian personality: a systematic review. Mov Disord 21, 1066–1072. [DOI] [PubMed] [Google Scholar]
- Islam MT, 2017. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurological research 39, 73–82. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, 2009. Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Frontiers of neurology and neuroscience 24, 114–125. [DOI] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Anantharam V, Rana A, Kanthasamy AG, 2011. Transcriptional regulation of pro-apoptotic protein kinase Cdelta: implications for oxidative stress-induced neuronal cell death. The Journal of biological chemistry 286, 19840–19859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Harischandra DS, Kondru N, Ghosh A, Panicker N, Anantharam V, Rana A, Kanthasamy AG, 2014. Histone hyperacetylation up-regulates protein kinase Cdelta in dopaminergic neurons to induce cell death: relevance to epigenetic mechanisms of neurodegeneration in Parkinson disease. The Journal of biological chemistry 289, 34743–34767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SH, Lee HM, Seo WK, Kim JH, Koh SB, 2016. The combined effect of REM sleep behavior disorder and hyposmia on cognition and motor phenotype in Parkinson’s disease. J Neurol Sci 368, 374–378. [DOI] [PubMed] [Google Scholar]
- Kanthasamy A, Jin H, Mehrotra S, Mishra R, Kanthasamy A, Rana A, 2010. Novel cell death signaling pathways in neurotoxicity models of dopaminergic degeneration: relevance to oxidative stress and neuroinflammation in Parkinson’s disease. Neurotoxicology 31, 555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komada M, Takao K, Miyakawa T, 2008. Elevated plus maze for mice. Journal of visualized experiments : JoVE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutzoumis DN, Vergara M, Pino J, Buddendorff J, Khoshbouei H, Mandel RJ, Torres GE, 2020. Alterations of the gut microbiota with antibiotics protects dopamine neuron loss and improve motor deficits in a pharmacological rodent model of Parkinson’s disease. Experimental neurology 325, 113159. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ, 2008. The molecular neurobiology of depression. Nature 455, 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers SD, Bramham CR, Cameron HA, Fitzsimons CP, Korosi A, Lucassen PJ, 2014. Environmental control of adult neurogenesis: from hippocampal homeostasis to behavior and disease. Neural plasticity 2014, 808643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach S, Wewering S, Hatt H, Neuhaus EM, Lubbert H, 2013. Olfaction in three genetic and two MPTP-induced Parkinson’s disease mouse models. PloS one 8, e77509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm O, Ganz J, Melamed E, Offen D, 2014. Harnessing neurogenesis for the possible treatment of Parkinson’s disease. The Journal of comparative neurology 522, 2817–2830. [DOI] [PubMed] [Google Scholar]
- Langley M, Ghosh A, Charli A, Sarkar S, Ay M, Luo J, Zielonka J, Brenza T, Bennett B, Jin H, Ghaisas S, Schlichtmann B, Kim D, Anantharam V, Kanthasamy A, Narasimhan B, Kalyanaraman B, Kanthasamy AG, 2017. Mito-Apocynin Prevents Mitochondrial Dysfunction, Microglial Activation, Oxidative Damage, and Progressive Neurodegeneration in MitoPark Transgenic Mice. Antioxid Redox Signal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley MR, Ghaisas S, Ay M, Luo J, Palanisamy BN, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG, 2018. Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson’s disease: Relevance to gene and environment interactions in metal neurotoxicity. Neurotoxicology 64, 240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kim HG, Lee HW, Han JM, Lee SK, Kim DW, Saravanakumar A, Son CG, 2015. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Scientific reports 5, 9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelan F, Boyer C, Thinard R, Remy S, Usal C, Tesson L, Anegon I, Neveu I, Damier P, Naveilhan P, Lescaudron L, 2011. Effects of Human Alpha-Synuclein A53T-A30P Mutations on SVZ and Local Olfactory Bulb Cell Proliferation in a Transgenic Rat Model of Parkinson Disease. Parkinson’s disease 2011, 987084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Redus L, Chen C, Martinez PA, Strong R, Li S, O’Connor JC, 2013. Cognitive dysfunction precedes the onset of motor symptoms in the MitoPark mouse model of Parkinson’s disease. PloS one 8, e71341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O, 2012. Non-motor symptoms in patients with Parkinson’s disease - correlations with inflammatory cytokines in serum. PloS one 7, e47387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG, 1987. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92, 180–185. [DOI] [PubMed] [Google Scholar]
- Litteljohn D, Mangano E, Shukla N, Hayley S, 2009. Interferon-gamma deficiency modifies the motor and co-morbid behavioral pathology and neurochemical changes provoked by the pesticide paraquat. Neuroscience 164, 1894–1906. [DOI] [PubMed] [Google Scholar]
- Magen I, Torres ER, Dinh D, Chung A, Masliah E, Chesselet MF, 2015. Social Cognition Impairments in Mice Overexpressing Alpha-Synuclein Under the Thy1 Promoter, a Model of Pre-manifest Parkinson’s Disease. J Parkinsons Dis 5, 669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht P, Seppi K, Poewe W, 2015. The Concept of Prodromal Parkinson’s Disease. J Parkinsons Dis 5, 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ, 2018. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol 17, 559–568. [DOI] [PubMed] [Google Scholar]
- Menza M, Dobkin RD, Marin H, Mark MH, Gara M, Bienfait K, Dicke A, Kusnekov A, 2010. The role of inflammatory cytokines in cognition and other non-motor symptoms of Parkinson’s disease. Psychosomatics 51, 474–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Zimmermann R, Gschwandtner U, Hatz F, Bousleiman H, Schwarz N, Fuhr P, 2014. Apathy in Parkinson’s disease is related to executive function, gender and age but not to depression. Frontiers in aging neuroscience 6, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min AY, Doo CN, Son EJ, Sung NY, Lee KJ, Sok DE, Kim MR, 2015. N-palmitoyl serotonin alleviates scopolamine-induced memory impairment via regulation of cholinergic and antioxidant systems, and expression of BDNF and p-CREB in mice. Chemico-biological interactions 242, 153–162. [DOI] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI, 2008. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. The international journal of neuropsychopharmacology 11, 851–876. [DOI] [PubMed] [Google Scholar]
- Ngwa HA, Kanthasamy A, Jin H, Anantharam V, Kanthasamy AG, 2014. Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. Neurotoxicology 43, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Shen L, Li T, Ren C, Ding S, Wang L, Zhang Z, Liu X, Zhang Q, Geng D, Wu X, Li H, 2018. Alpha-synuclein overexpression in the olfactory bulb initiates prodromal symptoms and pathology of Parkinson’s disease. Transl Neurodegener 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nuber S, Harmuth F, Kohl Z, Adame A, Trejo M, Schonig K, Zimmermann F, Bauer C, Casadei N, Giel C, Calaminus C, Pichler BJ, Jensen PH, Muller CP, Amato D, Kornhuber J, Teismann P Yamakado H Takahashi R, Winkler J, Masliah E, Riess O 2013. A progressive dopaminergic phenotype associated with neurotoxic conversion of alpha-synuclein in BAC-transgenic rats. Brain 136, 412–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviano G, Frasson G, Nardello E, Martini A, 2016. Olfaction deterioration in cognitive disorders in the elderly. Aging clinical and experimental research 28, 37–45. [DOI] [PubMed] [Google Scholar]
- Palma JA, Kaufmann H, 2018. Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord 33, 372–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paß T, Aßfalg M, Tolve M, Blaess S, Rothermel M, Wiesner RJ, Ricke KM, 2020. The Impact of Mitochondrial Dysfunction on Dopaminergic Neurons in the Olfactory Bulb and Odor Detection. Molecular neurobiology 57, 3646–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pass T, Assfalg M, Tolve M, Blaess S, Rothermel M, Wiesner RJ, Ricke KM, 2020. The Impact of Mitochondrial Dysfunction on Dopaminergic Neurons in the Olfactory Bulb and Odor Detection. Mol Neurobiol 57, 3646–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano C, Benincasa D, Pisani V, Buttarelli FR, Giovannelli M, Pontieri FE, 2007. Prodromal non-motor symptoms of Parkinson’s disease. Neuropsychiatric disease and treatment 3, 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Blavet N, Deniel M, Jalfre M, 1979. Immobility induced by forced swimming in rats: effects of agents which modify central catecholamine and serotonin activity. European journal of pharmacology 57, 201–210. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, 2016. Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12, 622–634. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Aguiar AS Jr., Rojas-Mayorquin AE, Figueiredo CP, Matheus FC, Ginestet L, Chevarin C, Bel ED, Mongeau R, Hamon M, Lanfumey L, Raisman-Vozari R, 2010. Single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotox Res 17, 114–129. [DOI] [PubMed] [Google Scholar]
- Ravi N, Sanchez-Guardado L, Lois C, Kelsch W, 2017. Determination of the connectivity of newborn neurons in mammalian olfactory circuits. Cell Mol Life Sci 74, 849–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regensburger M, Prots I, Winner B, 2014. Adult hippocampal neurogenesis in Parkinson’s disease: impact on neuronal survival and plasticity. Neural plasticity 2014, 454696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann H, Brandt MD, Klingelhoefer L, 2016. The nonmotor features of Parkinson’s disease: pathophysiology and management advances. Current opinion in neurology 29, 467–473. [DOI] [PubMed] [Google Scholar]
- Rial D, Castro AA, Machado N, Garcao P, Goncalves FQ, Silva HB, Tome AR, Kofalvi A, Corti O, Raisman-Vozari R, Cunha RA, Prediger RD, 2014. Behavioral phenotyping of Parkin-deficient mice: looking for early preclinical features of Parkinson’s disease. PloS one 9, e114216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago RM, Barbieiro J, Lima MM, Dombrowski PA, Andreatini R, Vital MA, 2010. Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Progress in neuropsychopharmacology & biological psychiatry 34, 1104–1114. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, Chaudhuri KR, Jenner P, 2017. Non-motor features of Parkinson disease. Nat Rev Neurosci 18, 435–450. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR, 2009. Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson’s disease. Neurotox Res 16, 127–139. [DOI] [PubMed] [Google Scholar]
- Schlachetzki JC, Grimm T, Schlachetzki Z, Ben Abdallah NM, Ettle B, Vohringer P, Ferger B, Winner B, Nuber S, Winkler J, 2016. Dopaminergic lesioning impairs adult hippocampal neurogenesis by distinct modification of alpha-synuclein. Journal of neuroscience research 94, 62–73. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Obeso JA, 2015. Clinical and pathological features of Parkinson’s disease. Current topics in behavioral neurosciences 22, 205–220. [DOI] [PubMed] [Google Scholar]
- Shulman LM, Taback RL, Bean J, Weiner WJ, 2001. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord 16, 507–510. [DOI] [PubMed] [Google Scholar]
- Subramaniam SR, Chesselet MF, 2013. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Progress in neurobiology 106-107, 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto Y, Kajiwara Y, Hirano K, Yamada S, Tagawa N, Kobayashi Y, Hotta Y, Yamada J, 2008. Mouse strain differences in immobility and sensitivity to fluvoxamine and desipramine in the forced swimming test: analysis of serotonin and noradrenaline transporter binding. European journal of pharmacology 592, 116–122. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW, 2009. Nonmotor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. The Journal of neuroscience : the official journal of the Society for Neuroscience 29, 8103–8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Greene JG, Miller GW, 2010. Behavioral phenotyping of mouse models of Parkinson’s disease. Behavioural brain research 211, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinakoua A, Bouabid S, Faggiani E, De Deurwaerdere P, Lakhdar-Ghazal N, Benazzouz A, 2015. The impact of combined administration of paraquat and maneb on motor and non-motor functions in the rat. Neuroscience 311, 118–129. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Kingsbury AE, Foster OJ, 2000. Up-regulation of tyrosine hydroxylase mRNA in a sub-population of A10 dopamine neurons in Parkinson’s disease. Brain Res Mol Brain Res 79, 45–54. [DOI] [PubMed] [Google Scholar]
- Vaidya VA, Duman RS, 2001. Depresssion--emerging insights from neurobiology. British medical bulletin 57, 61–79. [DOI] [PubMed] [Google Scholar]
- Varcin M, Bentea E, Michotte Y, Sarre S, 2012. Oxidative stress in genetic mouse models of Parkinson’s disease. Oxidative medicine and cellular longevity 2012, 624925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visanji N, Marras C, 2015. The relevance of pre-motor symptoms in Parkinson’s disease. Expert review of neurotherapeutics 15, 1205–1217. [DOI] [PubMed] [Google Scholar]
- Wang L, Fleming SM, Chesselet MF, Tache Y, 2008. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport 19, 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu W, Yang J, Wang F, Sima Y, Zhong ZM, Wang H, Hu LF, Liu CF, 2017. Parkinson’s disease-like motor and non-motor symptoms in rotenone-treated zebrafish. Neurotoxicology 58, 103–109. [DOI] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H, 2004. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson’s disease. J Neurochem 91, 451–461. [DOI] [PubMed] [Google Scholar]
- Zhang TM, Yu SY, Guo P, Du Y, Hu Y, Piao YS, Zuo LJ, Lian TH, Wang RD, Yu QJ, Jin Z, Zhang W, 2016a. Nonmotor symptoms in patients with Parkinson disease: A cross-sectional observational study. Medicine 95, e5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Mu JW, Wang HB, Jolkkonen J, Liu TT, Xiao T, Zhao M, Zhang CD, Zhao CS, 2016b. Increased protein expression levels of pCREB, BDNF and SDF-1/CXCR4 in the hippocampus may be associated with enhanced neurogenesis induced by environmental enrichment. Mol Med Rep 14, 2231–2237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data supporting the results reported in this article are in the figure source data files available upon request.