Abstract
Background:
Cancer patients who exhibit cachexia lose weight and have low treatment tolerance and poor outcomes compared to cancer patients without weight loss. Despite the clear increased risk for patients, diagnosing cachexia still often relies on self-reported weight loss. A reliable biomarker to identify patients with cancer cachexia would be a valuable tool to improve clinical decision making and identification of patients at risk of adverse outcomes.
Methods:
Targeted metabolomics, that included panels of amino acids, tricarboxylic acids, fatty acids, acylcarnitines, and sphingolipids, were conducted on plasma samples from patients with confirmed pancreatic ductal adenocarcinoma (PDAC) with and without cachexia and control patients without cancer (n=10/group, equally divided by sex). Additional patient samples were analyzed (total n=95) and Receiver Operating Characteristic (ROC) analyses were performed to establish if any metabolite could effectively serve as a biomarker of cachexia.
Results:
Targeted profiling revealed that cachectic patients had decreased circulating levels of three sphingolipids compared to either non-cachectic PDAC patients or patients without cancer. The ratio of C18-ceramide to C24-ceramide (C18:C24) outperformed a number of other previously proposed biomarkers of cachexia (area under ROC = 0.810). It was notable that some biomarkers, including C18:C24, were only altered in cachectic males.
Conclusions:
Our findings identify C18:C24 as a potentially new biomarker of PDAC-induced cachexia that also highlight a previously unappreciated sexual dimorphism in cancer cachexia.
Introduction
Cachexia is a common clinical syndrome of cancer patients and is characterized by involuntary weight loss due to depletion of skeletal muscle and adipose tissue [1]. Although anorexia is a component of the cachexia syndrome, supplemental nutrition is generally ineffective at preventing or reversing weight loss [2, 3]. Patients with cachexia have higher morbidity and mortality, due in part to decreased tolerance of chemo- and radiotherapy and worse surgical outcomes [4–8]. To date, there remains no approved treatment for cancer cachexia in most parts of the world.
Pancreatic ductal adenocarcinoma (PDAC) patients have amongst the highest incidence of cachexia, with estimates as high as 70% of patients affected [8–10]. Cachexia also tends to be severe in PDAC patients, with body weight losses averaging ~14% of pre-illness weight [10]. Furthermore, while an increased incidence of cachexia is associated with advanced disease, a significant proportion of PDAC patients already meet cachexia criteria at the time of cancer diagnosis [8, 11].
Although significant advances have been made in identifying some potential underlying mechanisms leading to muscle wasting and weight loss, cachexia remains surprising challenging to diagnose. Especially at the time of diagnosis, providers rely on often unreliable self-reported weight loss, likely leading to an underappreciation of the incidence of cachexia [12–14]. Moreover, although an international consensus defines cachexia as a 5% loss of pre-illness body weight, recent studies in PDAC patients indicate that only greater losses are associated with poor outcomes [8, 15]. Therefore, there remains an urgent need for a biomarker of cancer cachexia to more accurately identify patients at greater risk of morbidity and mortality.
Recently, we performed a multiplex analysis on a targeted panel of circulating cytokines, chemokines, and growth factors in early-stage PDAC patients. We were surprised to find in our study that a number of classical inflammatory cytokines that historically have been considered as biomarkers of cancer cachexia, including tumor necrosis factor (TNF), interleukin 1β (IL-1β), interleukin-6 (IL-6), and interferon-γ (IFN-γ) were not associated with weight loss in these patients [16]. Given that cachexia may be ultimately a metabolic syndrome, we took a different approach to search for a cachexia biomarker using metabolomic profiling, which has been similarly examined by other investigators [17–22]. However, unlike these previous findings, we identified that the ratio of C18-ceramide to C24-ceramide (C18:C24) showed a strong association with cachexia. In head-to-head comparisons, C18:C24 outperformed a number of circulating factors previously proposed as biomarkers of cancer cachexia, and unexpectedly was associated with cachexia only in male PDAC patients.
Results
Patients undergoing an abdominal operation for suspected or confirmed PDAC or other benign conditions were eligible for enrollment into our Cancer Cachexia Tissue Registry [15, 16, 23, 24]. Patients electing to participate were asked about their history of weight loss at time of their pre-operative clinic visit. When possible, weight loss data were confirmed by existing medical records.
Plasma samples from three groups of patients (n=10/group; 5 males and 5 females) were chosen for analysis from our Pancreatic Cancer Cachexia tissue registry biobank. These groups included: 1) Control patients without active cancer undergoing abdominal operations for a variety of diagnoses with no recent history of weight loss; 2) Weight-stable (non-cachectic) patients with histologically confirmed PDAC; and 3) PDAC patients with cachexia, defined as more than 5% weight loss over the previous 6-month period [1]. Notably, at time of sample collection, PDAC cohorts were treatment-naïve. Clinical data from each group appear in Supplemental Table 1. To the best of our ability, patients were matched based on age and body mass index. PDAC patients with cachexia exhibited a mean weight loss of 11.2%. Plasma from these cohorts were screened by targeted metabolomic profiling for pre-established panels of amino compounds, tricarboxylic acids, fatty acids, acylcarnitines, and sphingolipids.
Cachectic PDAC patients have altered plasma sphingolipid content
Following data normalization and correction for multiple hypothesis testing (false discovery rate <0.05), no statistically significant differences were identified in the panels of amino compounds, fatty acids, or acylcarnitines between PDAC cachectic, non-cachectic, or control patients. In targeted profiling of tricarboxylic acid cycle metabolites, only fumarate was significantly decreased in non-cachectic PDAC patients compared to cachectic PDAC and control patients (data not shown).
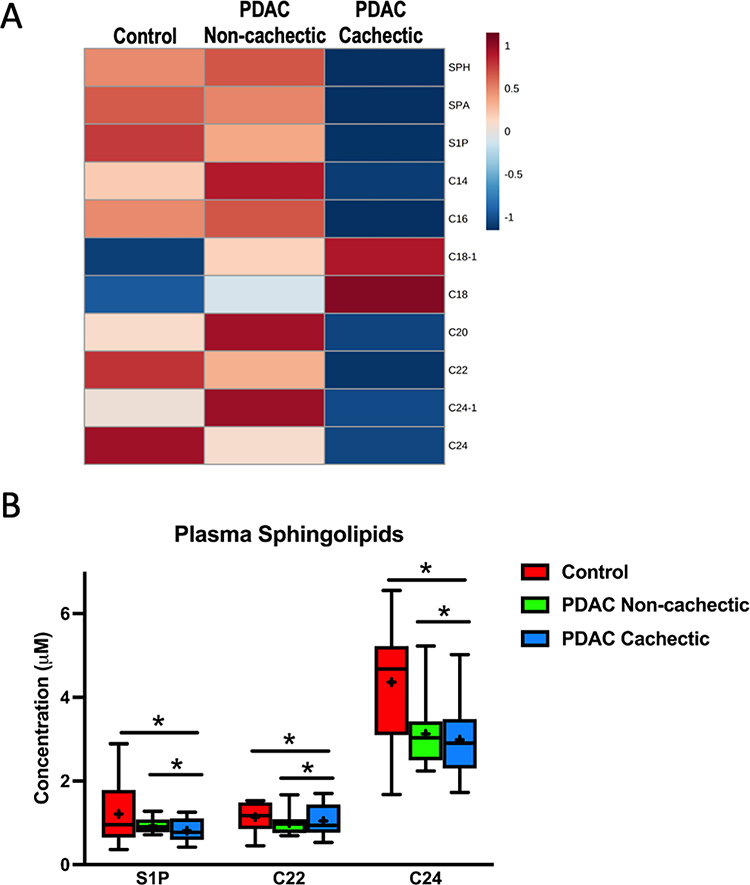
With regards to targeted profiling of plasma sphingolipids, Sphingosine (SPH), Sphinganine (SPA), Sphingosine-1-phosphate (S1P), C14-Ceramide (C14), C16-Ceramide (C16), C18:1-Ceramide (C18–1), C18-Ceramide (C18, C20-Ceramide (C20), C22-Ceramide (C22), C24:1-Ceramide (C24–1), and C24-Ceramide (C24) were all detected in our plasma samples. C8-Ceramide was not detected in any samples and thus excluded from further analysis. Plasma from cachectic patients had a distinct sphingolipid signature compared to plasma from control and non-cachectic PDAC patients (Fig. 1A). In particular, the concentrations of S1P, C22, and C24 were decreased in cachectic patients compared to both other groups (Fig. 1B, Supplemental Fig. 1). Given these trends, we sought to confirm our findings by repeating our analysis in additional patient samples (n=37, clinical data for selected patients appears in Supplemental Table 2). Importantly, a similar pattern of plasma ceramides was observed in cachectic patients (Supplemental Fig. 2A).
Figure 1. Plasma sphingolipid content is altered in cachectic PDAC patients.
(A) Heatmap of plasma sphingolipids in control patients without cancer (n=10), non-cachectic PDAC patients (n=10), and cachectic PDAC patients (n=10). The heatmap was generated in MetaboAnalyst using Euclidean distant measurements, auto-scaled by samples, and colored according to the normalized values. (B) Plasma levels of S1P, C22, and C24 are significantly decreased in cachectic PDAC patients compared to both control patients and non-cachectic PDAC patients. In the box-and-whiskers plot, the box is the 25th/75th percentile with minimum-to-maximum whiskers. The line represents the median with the + representing the mean. * represents p < 0.05 by one-way ANOVA with Fisher’s LSD post-hoc using a false discovery rate of 0.05.
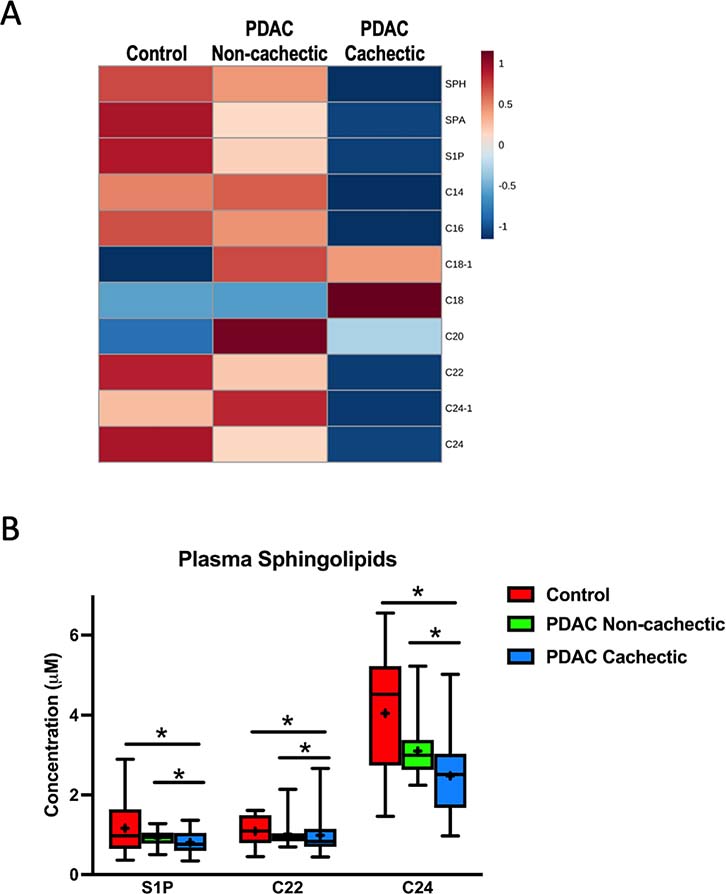
Because ceramide values are measured in comparison to known references and thus are quantitative, data from the first and second cohorts were collapsed into a single analysis (clinical data for the combined cohort appears in Table 1). Similar to our initial analysis, S1P, C22, and C24 remained significantly decreased in cachectic PDAC patients compared to non-cachectic PDAC patients and non-cancer controls (Fig. 2 and Supplemental Fig. 2B). Of note, alterations in ceramides have been previously associated with lymph node-positive PDAC [25]. To ensure that our observed alterations in sphingolipids in cachectic patients were not simply a reflection of gross metastasis, we repeated our analysis excluding the six patients in the cachectic group with metastatic (M1) disease. All three previously identified differences in plasma ceramide content remained (Supplemental Fig. 3A and 3B), suggesting that the decreases in blood sphingolipid content of cachectic PDAC patients are not simply a consequence of metastatic disease.
Table 1.
Clinical Characteristics of the combined cohort.
| Pre-operative Variables | No Cancer Surgical Control (n=14) |
PDAC Non-Cachectic (n=18) |
PDAC Cachectic (n=35) |
p-value | |
|---|---|---|---|---|---|
|
| |||||
| Age | 60.7 (52.7–68.7) | 64.4 (59.0–69.8) | 66.8 (63.2–70.3) | 0.235 | |
| Sex Males:Females | 6:8 | 9:9 | 18:17 | 0.861 | |
| Ethnicity, % White | 86% | 94% | 97% | 0.311 | |
| Pre-illness BMI | 27.5 (25.0–30.0) | 29.6 (26.7–32.5) | 30.9 (28.4–33.3) | 0.243 | |
| BMI | 27.5 (25.0–30.0) | 29.4 (26.6–32.1) | 28.0 (25.7–30.3) | 0.651 | |
| % weight loss | 0 | 0.8 (0.0–1.6) | 9.3 (8.4–10.2) | <0.001 | |
| Hypertension | 43% | 61% | 71% | 0.172 | |
| Diabetes | 14% | 17% | 34% | 0.213 | |
| Current Smoker | 7% | 22% | 20% | 0.488 | |
| PDAC Stage | 1 | - | 17% | 6% | 0.155 |
| 2 | - | 83% | 74% | ||
| 3 | - | 0% | 3% | ||
| 4 | - | 0% | 17% | ||
| M Stage | 0 | - | 100% | 83% | 0.062 |
| 1 | - | 0% | 17% | ||
Values are presented as mean (95% CI of the mean)
Figure 2. Plasma sphingolipid content is altered in an expanded cohort of cachectic PDAC patients.
(A) Heatmap of plasma sphingolipids in control patients without cancer (n=14), non-cachectic PDAC patients (n=18), and cachectic PDAC patients (n=35). The heatmap was generated in MetaboAnalyst using Euclidean distant measurements, auto-scaled by samples, and colored according to the normalized values. (B) Plasma levels of S1P, C22, and C24 are significantly decreased in cachectic PDAC patients compared to both control patients and non-cachectic PDAC patients. In the box-and-whiskers plot, the box is the 25th/75th percentile with minimum-to-maximum whiskers. The line represents the median with the + representing the mean. * represents p < 0.05 by one-way ANOVA with Fisher’s LSD post-hoc using a false discovery rate of 0.05.
Plasma ceramides as biomarkers for cancer cachexia
To determine whether alterations in circulating sphingolipid content could serve as a biomarker of PDAC-induced cachexia, the Biomarker Analysis feature of MetaboAnalyst was used to perform Receiver Operating Characteristic (ROC) analysis. This analysis tested the ability of a given sphingolipid to distinguish cachectic from non-cachectic cancer patients, thus excluding non-cancer controls. Of our targeted metabolites, only C24 ceramide was able to significantly separate cachectic patients, with an area under the ROC curve (AUROC) of 0.730 (95% CI 0.598–0.858, p= 0.007, Fig. 3A).
Figure 3. C24, ceramide ratios, and classical circulating factors as biomarkers of cancer cachexia.
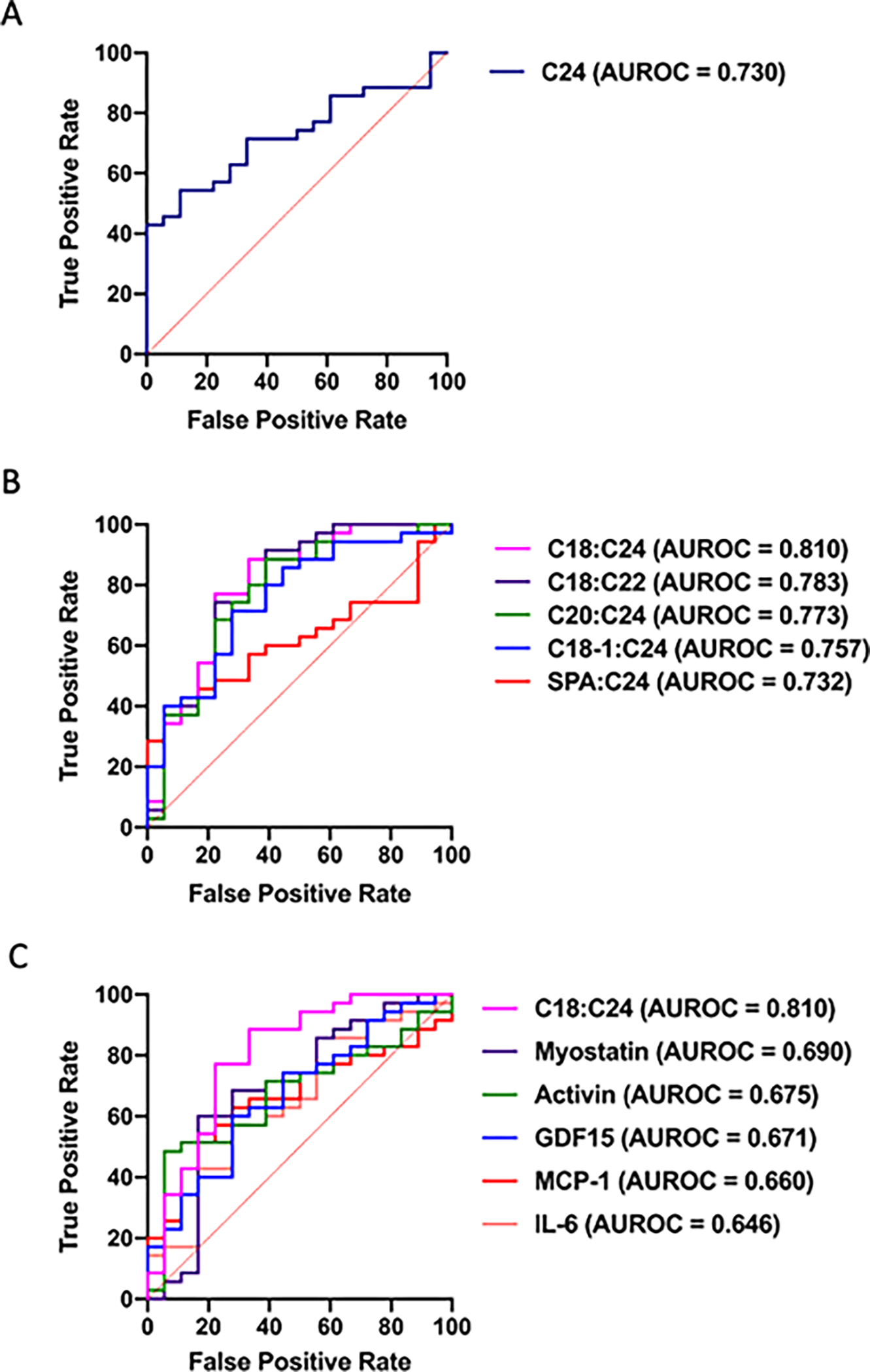
(A) ROC curve for distinguishing non-cachectic from cachectic PDAC patients by C24. (B) ROC curves for distinguishing non-cachectic from cachectic PDAC patients by ratios of sphingolipids (C) ROC curve for C18:C24 distinguishing non-cachectic from cachectic PDAC patients compared to ROC curves for other previously proposed biomarkers of cancer cachexia. ROC curves are displayed as sensitivity vs. (1-specificity) value. n=18 treatment-naïve, non-cachectic PDAC patients, n=35 treatment naïve, cachectic PDAC patients.
Because a ratio of plasma ceramides has been previously established as an indicator of mortality risk marker in individuals with cardiovascular disease [26], we posited that a ratio of plasma ceramides might serve as a more effective marker of cancer cachexia than a single ceramide. Indeed, 18 ratios of plasma sphingolipids were able to effectively identify cachectic PDAC patients. Five of these ratios had AUROCs greater than that of C24 alone, with C18:C24 exhibiting the highest AUROC of 0.810, p= 0.0087 (Table 2, Fig. 3B).
Table 2.
Plasma ceramide ratios are able to distinguish cachectic from non-cachectic PDAC patients.
| Non-Cachectic (n=18) |
Cachectic (n=35) |
AUROC (95% CI) | Optimal Cutoff | p value | |
|---|---|---|---|---|---|
| C18:C24 | 0.0933 ± 0.0387 | 0.2009 ± 0.0467 | 0.810 (0.679–0.921) | >0.055 | 0.0087 |
| C18:C22 | 0.2236 ± 0.0495 | 0.3757 ± 0.0546 | 0.783 (0.633–0.910) | >0.203 | 0.0075 |
| C20:C24 | 0.1042 ± 0.0404 | 0.1923 ± 0.0478 | 0.773 (0.630–0.903) | >0.062 | 0.0196 |
| C18–1:C24 | 0.0021 ± 0.0005 | 0.0064 ± 0.0015 | 0.757 (0.615–0.8) | >0.0015 | 0.0048 |
| SPA:C24 | 0.0028 ± 0.0003 | 0.0042 ± 0.0005 | 0.732 (0.593–0.863) | >0.0043 | 0.0098 |
| C24 alone | 3.097 ± 0.1577 | 2.474 ± 0.1627 | 0.730 (0.598–0.858) | <2.55 | 0.0074 |
Mean ± SEM, AUROC (95% CI of the mean), p values are from ROC analysis.
C18:C24 outperforms previously proposed biomarkers of cancer cachexia
To test the robustness of C18:C24 as a cachexia biomarker, we performed a head-to-head comparison with a number of other circulating factors that have been proposed as biomarkers of cancer cachexia [16, 27–29]. High-sensitivity ELISAs for IL-6, activin, Growth Differentiation Factor 15 (GDF15), myostatin, and monocyte chemoattractant protein-1 (MCP-1) were performed from the same plasma samples used in our metabolomic analysis. Similar to previous findings [15, 16], IL-6 only tended to distinguish non-cachectic and cachectic PDAC patients (p=0.12, Table 3). Also consistent with previous findings [28], trends for decreased myostatin and increased activin levels existed between cachectic and non-cachectic patients, but did not reach statistical significance (p=0.12 and p=0.08, respectively, Table 3). Both GDF15 and MCP-1 were able to effectively distinguish cachectic PDAC patients from non-cachectic PDAC patients (Table 3 and Fig. 3C). Significantly, C18:C24 exhibited a greater AUROC compared to each of the other proposed biomarkers that we tested (Table 3 and Fig. 3C).
Table 3.
ROC Curve Analysis of Proposed Cachexia Biomarkers
| Non-Cachectic (n=18) |
Cachectic (n=35) |
AUROC (95% CI) | Optimal Cutoff | p value | |
|---|---|---|---|---|---|
| C18:C24 | 0.0933 ± 0.0387 | 0.201 ± 0.0467 | 0.810 (0.679–0.921) | >0.055 | 0.0087 |
| MCP-1 (pg/mL) | 290.4 ± 38.9 | 482.5 ± 57.9 | 0.660 (0.513–0.797) | >311 | 0.0410 |
| GDF15 (pg/mL) | 1233 ± 235 | 2097 ± 309 | 0.671 (0.489–0.800) | >1280 | 0.0431 |
| Activin (pg/mL) | 314.7 ± 48.5 | 456.7 ± 77.2 | 0.675 (0.521–0.832) | >386 | 0.0868 |
| IL-6 (pg/mL) | 6.36 ± 2.44 | 26.47 ± 10.76 | 0.646 (0.470–0.818) | >1.6 | 0.1259 |
| Myostatin (pg/mL) | 1647 ± 162 | 1213 ± 77 | 0.690 (0.505–0.856) | <1210 | 0.1293 |
Mean ± SEM, AUROC (95% CI of the mean), p values are from ROC analysis.
In addition to the proposed biomarkers listed in Table 3, neutrophil-to-leukocyte ratio (NLR) has also been suggested to identify weight changes in cancer patients [30, 31]. For our combined treatment naïve population, 49 of the 53 patients had an available NLR in their medical record. In a ROC analysis similar to those presented in Figure 3 and Table 3, the AUROC for NLR was 0.624 (CI: 0.437–0.791) and did not reach statistical significance (p=0.10). Additionally, an absolute ratio of 3.5 has been proposed as a cut-off value for high NLR [30, 32]. Using this cut point, we found that 20/33 (61%) were cachectic, while 11/16 (69%) of patients with an NLR above 3.5 had lost more than 5% of their pre-illness body weight.
Furthermore, all 53 treatment naïve patients had laboratory values in their medical record for the pancreatic cancer associated prognostic marker CA19–9. ROC analysis found that CA19–9 was unable to distinguish cachectic from non-cachectic patients in this population, with an AUROC of 0.503 (CI: 0.342–0.677, p=0.89).
C18:C24 ratio is affected by neo-adjuvant treatment and does not predict survival
In a previous study, we showed that the association between MCP-1 and cancer cachexia was lost in patients who had received neoadjuvant treatment. To determine if the C18:C24 ratio was similarly affected, we selected a cohort of cachectic and non-cachectic PDAC patients who had received chemotherapy with or without radiotherapy prior to their attempted tumor resection and plasma collection (n=28, clinical data appear in Supplemental Table 3). Similar to our findings with MCP-1, C18:C24 ratio was similar to control patients in both non-cachectic and cachectic PDAC patients having undergone treatment (Supplemental Fig. 4A). Consistent with this finding, C18:C24 was unable to distinguish treated cachectic patients from non-cachectic patients (AUROC = 0.579, p = 0.949, Supplemental Fig. 4B).
To test if elevated C18:C24 was associated with decreased survival of PDAC patients, we performed a survival analysis on all PDAC patients in which C18:C24 was assessed (n=81). Above-median C18:C24 was not associated with decreased survival, although a distinct trend existed (p= 0.109, Supplemental Fig. 5A). Similar results were obtained in treatment-naïve PDAC patients (n=53, p=0.14, Supplemental Fig. 5B). A Cox survival model was fit for all PDAC patients with C18:C24 and relevant clinical variables and confirmed that only clinical stage associated with decreased survival (Supplemental Table 4), which was in keeping with our previous findings [15].
In an attempt to associate an increased C18:C24 ratio to other clinical variables, we fit models to data from all PDAC patients (n=81). We first attempted to construct a linear regression model for C18:C24 ratio and were unsuccessful. We were instead successful in constructing a binary logistic model for above-median C18:C24. Consistent with our findings, above-median C18:C24 was associated with cachexia and treatment naivety, but not other clinical variables of interest such as age, sex, BMI, cancer stage, or diabetes (Supplemental Table 5).
C18:C24 and other proposed biomarkers are sexually dimorphic
Finally, since recent studies have pointed to potential sex differences in cancer cachexia [33], we sought to determine if a sexual dimorphism existed with our newly identified C18:C24 biomarker. Interestingly, results showed that plasma levels of C18:C24 associated with PDAC-induced cachexia only in male patients (Fig. 4A). By ROC analysis, C18:C24 was able to separate male cachectic PDAC patients from non-cachectic patients, but not female patients (Table 4). We observed that a similar, albeit more modest, dimorphism occurred with MCP-1 and GDF15 (Fig. 4B, 4C, and Table 4). For comparison purposes, ROC curves for both males and females are overlaid for C18:C24 (Fig. 4D), and MCP-1 (Fig. 4E), and GDF15 (Fig. 4F). In contrast, there was no apparent sexual dimorphism in plasma levels of activin (Fig. 4G), IL-6 (Fig. 4H), and myostatin (Fig. 4I).
Figure 4. C18:C24 and other biomarkers of cancer cachexia are sexually dimorphic.
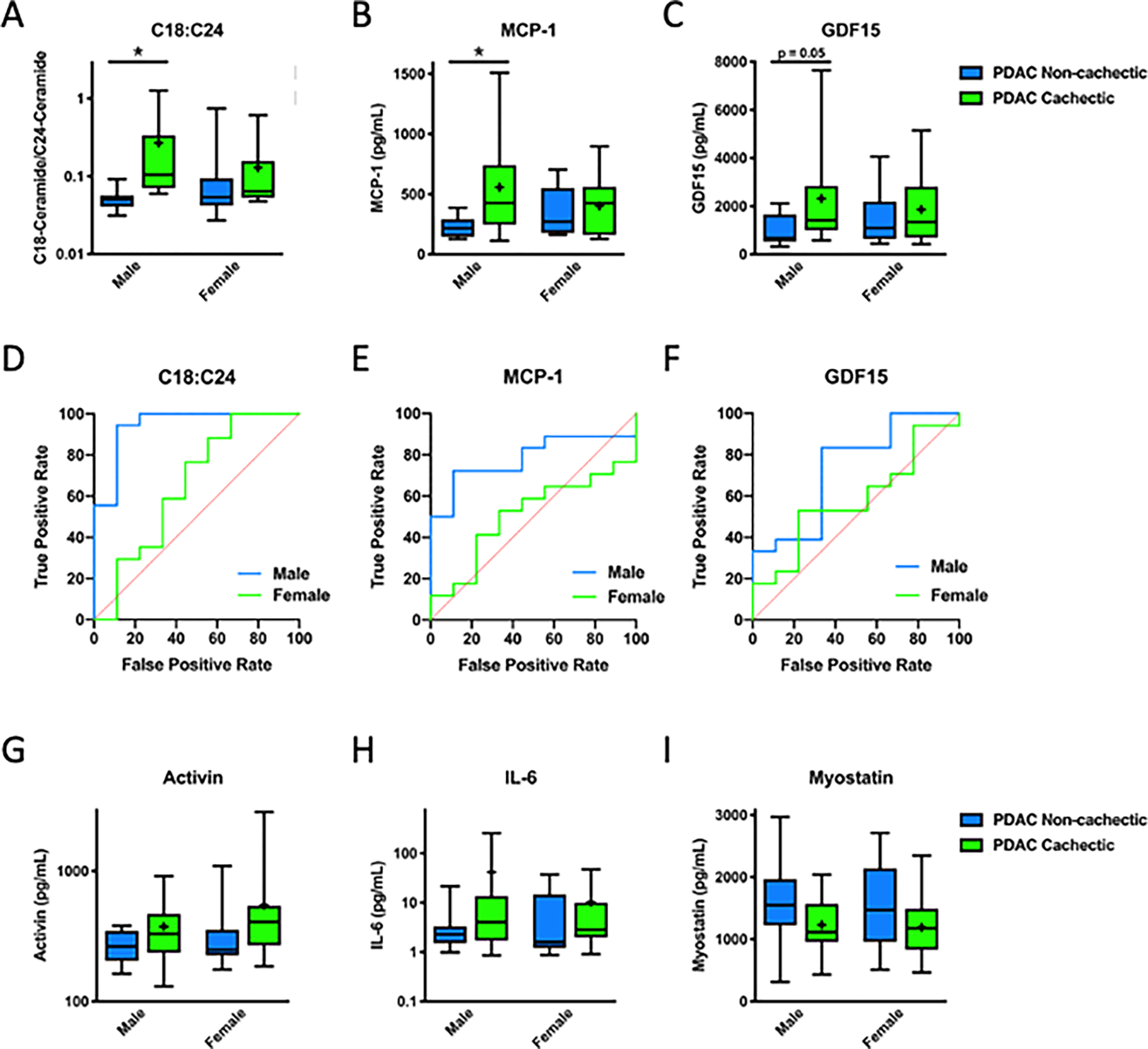
(A) C18:C24 and (B) MCP-1 are increased in cachectic male PDAC patients, but not female patients, and (C) GDF15 tends to be increased in cachectic males but not females. Clear differences exist between ROC curves for males and females for (D) C18:C24, (E) MCP-1, and (F) GDF15. Circulating levels of (G) activin, (H) IL-6, or (I) myostatin were not increased in cachectic male or female patients. (G-I) In box-and-whiskers plots, the box is the 25th/75th percentile with minimum-to-maximum whiskers. The line represents the median with the + representing the mean. ROC curves are displayed as sensitivity vs. (1-specificity) value. n=18 treatment-naïve, non-cachectic PDAC patients, n=35 treatment naïve, cachectic PDAC patients * represents significantly different from control of the same sex, p < 0.05 by two-way ANOVA with sex and cachexia as variables.
Table 4.
Proposed biomarkers can distinguish male but not female cachectic PDAC patients.
| Male n = 9 Non-cachectic n = 18 Cachectic |
Female n = 9 Non-cachectic n = 17 Cachectic |
|||
|---|---|---|---|---|
|
| ||||
| AUROC (95% CI) | p value | AUROC (95% CI) | p value | |
| C18:C24 | 0.951 (0.828–1.000) | 0.0031 | 0.694 (0.425–0.907) | 0.4722 |
| MCP-1 (pg/mL) | 0.796 (0.602–0.948) | 0.0131 | 0.510 (0.278–0.719) | 0.7754 |
| GDF15 (pg/mL) | 0.744 (0.506–0.935) | 0.0250 | 0.588 (0.350–0.791) | 0.5612 |
AUROC (95% CI of the mean), p values are from ROC analysis.
We were curious if this potential sexual dimorphism extended to survival. We assessed the ability of above-median C18:C24 to predict survival separately in treatment-naïve male (Supplemental Figure 5C) and female (Supplemental Figure 5D) PDAC patients. Although insignificant, likely due to small sample size, our results are consistent with our hypothesis of a sexual dimorphism, with clear separation apparent in males, but not females.
Discussion
Our results reported here identify plasma ceramide ratios, and particularly C18:C24, as a potential biomarker for PDAC-induced cachexia. Further, our results indicate that patient characteristics such as sex should be considered when new biomarkers are assessed.
Our data are consistent with previous work in which decreases in C24-ceramide and S1P were found to be part of a metabolic signature capable of distinguishing patients with PDAC from patients with other GI disorders [34]. While this study did not consider weight loss, it is tempting to speculate that the high incidence of weight loss in PDAC patients may have contributed to this finding. Alterations in plasma ceramide levels have also been reported with obesity and type 2 diabetes [35]. Although we did not find diabetes or BMI to be predictive of above-median levels of C18:C24 (Supplemental Table 5), future biomarker studies should more carefully consider such factors.
Several caveats should be noted in our study. First, while we used the international consensus definition for cancer cachexia to stratify our patients [1], recent work suggests that weight loss of 5% may not be associated with decreased survival in PDAC patients [8, 15]. Furthermore, because our study was performed at a single institution, our results reflect the patient demographics of that site, which were disproportionally non-Hispanic White individuals of European descent. Thus, our data are not reflective of the more diverse population that develops PDAC-induced cachexia. Moreover, our dataset is limited to PDAC patients undergoing a surgical procedure and thus not representative of the majority of patients diagnosed with advanced PDAC. Thus, we do not know if the C18:C24 ratio identified in cachectic patients with early stage PDAC would be generalizable to patients with more advance disease or cachexia induced by other cancers, although the recent work by Morigny et al. suggests that alterations in circulating ceramides are common in cachectic patients [36]. Such factors need to be addressed in larger subsequent studies. Finally, in our study, we used the current International Consensus definition for cancer cachexia of greater than 5% weight loss over the preceding six months [1]. We did not associate our findings to body composition, as a substantial portion of our population of patients did not have pre-surgical CT scan available for body composition analysis. Reasons for unavailable CT scans included patients instead having undergone an MRI for surgical planning and scans taken outside of the institution that were not archived for long-term storage.
Our data uncovered an interesting finding related to differences in circulating C18:C24 between cachectic and non-cachectic PDAC patients that appear to be primarily driven by differences in male patients. It is increasingly recognized that in addition to varying by age, metabolite profiles also differ between sexes [37]. However, we note that this sexual dimorphism is not specific to ceramides, as we measured similar, albeit more modest, differences in MCP-1 and GDF15. A similar sexual dimorphism in the relationship of circulating levels of GDF15 to muscle mass has been previously reported [38]. While it is certainly possible that this apparent sex difference in cachexia-associated biomarkers is tied to basal differences in sphingolipid levels or the accuracy of self-reported weight loss between males and females [39–41], our data make a compelling argument for the necessity of considering sex as a biological variable in future efforts to identify a biomarker of cancer cachexia. This is particularly important as evidence emerges for potential differential responses to anti-cachexia therapy between sexes and should be considered in future studies.
Methods
Biobank
Patients 18 years of age and older undergoing an abdominal operation for pancreatic cancer or other benign conditions were eligible for enrollment into the Ohio State Pancreatic Cancer Cachexia tissue registry [15, 16, 23, 24]. A detailed patient history as well as height and weight measurements were taken at the pre-operative surgical clinic visit to determine the history of weight loss. When possible, weight loss data were confirmed by existing medical records. All other variables were abstracted from the pre-operative history and physical, as well as the electronic medical record. Consistent with the international definition, cachexia was defined as more than 5% loss of body weight over the previous 6 months [1]. Patients with >2% but <5% loss of pre-illness body weight and were confirmed to have a body mass index (BMI) of less than 20 kg/m2 were considered cachectic. Because not every enrolled patient had a CT scan available for analysis, patients were not assessed for cachexia based upon muscle volume. Cancers were staged based on the 7th edition AJCC Cancer Staging Manual for pancreatic cancer [42]. Patients were enrolled from November 2013 to June 2017. Survival cutoff date was November 30, 2019.
Preparation of plasma
For patients who elected to contribute to the biobank, approximately 30 cc peripheral blood was collected intraoperatively following induction of anesthesia in heparinized tubes. Following centrifugation at 500 g for 10 minutes, plasma was aliquoted and stored at −80 °C until use.
Study design
Our initial cross-sectional study design involved choosing three patient cohorts for analysis from our institutional tissue bank: 1) control patients without active cancer or inflammatory conditions undergoing elective abdominal operations for a variety of diagnoses and with no recent history of weight loss; 2) weight-stable (non-cachectic) patients with pathology-confirmed pancreatic adenocarcinoma; and 3) cachectic patients with pathology-confirmed pancreatic adenocarcinoma. Groups consisted of five males and five females. To the best of our ability, patients were matched based on age and body mass index. We elected to limit our cachectic patient cohort to patients with weight loss between 5 and 15% weight loss to avoid including patients with refractory cachexia [1]. Following our initial results, additional samples were analyzed to confirm our initial findings. In our second cohort of treatment-naïve patients, we allowed patients with weight loss of less than 5% to be included in our study. Our cohort of treated patients received standard of care chemotherapy – generally FOLFIRINOX or gemcitabine/nab-paclitaxel with or without chemoradiation. No patient received immune therapy. All together, our analysis included 14 control patients without cancer, 18 treatment-naïve non-cachectic PDAC patients, 35 treatment-naïve cachectic PDAC patients, 15 treated non-cachectic PDAC patients, and 13 treated cachectic PDAC patients, for a total n of 95. We did not pre-register our experimental plan.
Metabolomic sample processing and analysis
Frozen plasma samples were shipped to the Mayo Clinic for analysis by ultra performance liquid chromatography followed by mass spectrometry (UPLC-MS) to determine absolute metabolite concentrations were based on reference standards, with the exception of tricarboxylic acid cycle intermediates, which were analyzed by gas chromatograph mass spectrometry (GC/MS), by methods that have been described previously [43–48]. Samples were run in random order to minimize drift, and technical staff were blinded to the sample groups.
Enzyme-linked immunosorbent assay (ELISA)
Plasma levels of IL-6, activin, GDF15, myostatin, and MCP-1 were determined by ELISA. Kits for myostatin, GDF15, and activin were purchased from R&D Systems, while high sensitivity IL-6 and MCP-1 kits were purchased from eBioscience. Samples were analyzed in duplicate and results averaged.
Statistical analysis
Statistical analyses were conducted and graphs constructed with GraphPad Prism 8.3, with the exception of statistical models, which were fit with SPSS 27. For comparisons of clinical data with three groups, One-way Analysis of Variance (ANOVA) was used for continuous variables, and Chi-square or Fisher’s exact test was used for binary or nominal variables, as appropriate. Comparisons of clinical data with two groups were performed using Student’s t-tests for continuous variables Chi-square or Fisher’s exact test was used for binary or nominal variables, as appropriate.
Metabolite analysis was performed using MetaboAnalyst [49]. Samples were normalized to the median of each metabolite analyzed, and then data were transformed by cube-root transformation due to the substantial number of near-zero concentrations of ceramides [50]. Pareto scaling was then performed. Differences between groups were then assessed using one-way ANOVA with Fisher’s LSD post-hoc using a false discovery rate of 0.05. Heatmaps were generated in MetaboAnalyst using Euclidean distant measurements, auto-scaled by features, and colored according to the normalized values.
Ability to discriminate between pancreatic cancer patients with and without cachexia was determined by Receiver Operating Characteristic (ROC) analysis using the Biomarker Analysis feature of MetaboAnalyst 4.0 [49]. Area Under the Receiver Operating Characteristic Curve (AUROC) is reported as a measure of a biomarker’s potential as a circulating marker of pancreatic cancer-induced cachexia. MetaboAnalyst 4.0 was utilized for this analysis, with data log transformed and auto-scaled as suggested by Chong et al. [49]. However, similar results were obtained without transformation and scaling. Graphical depictions of ROC curves were generated using GraphPad Prism and may vary slightly from those generated by MetaboAnalyst 4.0, as they lack normalization. However, AUROC and p values were similar by both methods. The ROC curves are displayed as sensitivity vs. (1-specificity) value. Optimal cutoff value was determined as the point farthest from the line of equivalency.
Construction of a multivariate linear model was attempted both by hand and with the automatic linear modeling function of SPSS. Binary logistic modeling for C18:C24 ratio was conducted with pre-determined clinical variables of interest - age, BMI, sex, cancer stage (early/late), cachexia, neoadjuvant treatment, and diabetes.
Univariate survival was assessed with log-rank statistics. A Cox survival model was fit with age (above/below median), sex, cachexia, BMI (above/below median), cancer stage (early/ late), neoadjuvant treatment, and C18:C24 ratio (above/below median). The assumption of proportionality was assessed by adding time-dependent interaction variables to the model and comparing this model to our initial model by a Chi-square test, which was not significant (p=0.882), indicating that none of our covariates are time-dependent.
Differences in levels of circulating factors between sexes were assessed by two-way ANOVAs with sex and cachexia as factors on log-transformed data, followed by Sidak’s multiple comparisons test.
Study approval
All aspects of the study were approved by The Ohio State University Institutional Review Board and conducted in accordance with both the approved study and all relevant guidelines and regulations. Written informed consent was obtained from each patient prior to enrollment in the tissue bank.
Supplementary Material
Acknowledgements
This work was supported by a pilot grant from the Mayo Clinic Metabolomics Resource Core through grant number U24DK100469 from the National Institute of Diabetes and Digestive and Kidney Diseases, which originates from the National Institutes of Health Director’s Common Fund. The authors would like to thank the Mayo Clinic Metabolomics Core, and particularly Xuan-Mai T. Petterson, for sample processing and coordination. Additional support was provided by the National Cancer Institute P30 CA016058 (Ohio State University) and R01CA180057 (DCG), a Weiss Postdoctoral Fellowship (EET), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases R00AR071508 (EET) and R0AR072714 (DCG).
Footnotes
Competing Interests
JMC, MED, CRS, PVR, TMW, and EET declare that they have no conflicts of interest. DCE declares that he has received research support and has a speaking and consulting agreement with Abbott Laboratories. DCG declares that he receives research support from Pfizer and consults for Immuneering and Catabasis.
Data availability
Informed consent was obtained for the publication of data in aggregate, but not as individual and therefore potentially identifiable datapoints. Therefore, the datasets have not been publicly deposited; however, the datasets can be made available from DCG through a non-human subjects research request.
References
- 1.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12(5):489–95. [DOI] [PubMed] [Google Scholar]
- 2.Evans WK, Makuch R, Clamon GH, et al. Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res 1985;45(7):3347–53. [PubMed] [Google Scholar]
- 3.Bozzetti F Effects of artificial nutrition on the nutritional status of cancer patients. JPEN J Parenter Enteral Nutr 1989;13(4):406–20. [DOI] [PubMed] [Google Scholar]
- 4.Andreyev HJ, Norman AR, Oates J, et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer 1998;34(4):503–9. [DOI] [PubMed] [Google Scholar]
- 5.Moningi S, Walker AJ, Hsu CC, et al. Correlation of clinical stage and performance status with quality of life in patients seen in a pancreas multidisciplinary clinic. J Oncol Pract 2015;11(2):e216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pausch T, Hartwig W, Hinz U, et al. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012;152(3 Suppl 1):S81–8. [DOI] [PubMed] [Google Scholar]
- 7.Bachmann J, Heiligensetzer M, Krakowski-Roosen H, et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12(7):1193–201. [DOI] [PubMed] [Google Scholar]
- 8.Nemer L, Krishna SG, Shah ZK, et al. Predictors of Pancreatic Cancer-Associated Weight Loss and Nutritional Interventions. Pancreas 2017;46(9):1152–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozola Zalite I, Zykus R, Francisco Gonzalez M, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology 2015;15(1):19–24. [DOI] [PubMed] [Google Scholar]
- 10.Baracos VE, Martin L, Korc M, et al. Cancer-associated cachexia. Nat Rev Dis Primers 2018;4:17105. [DOI] [PubMed] [Google Scholar]
- 11.Hendifar AE, Chang JI, Huang BZ, et al. Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J Gastrointest Oncol 2018;9(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niedhammer I, Bugel I, Bonenfant S, et al. Validity of self-reported weight and height in the French GAZEL cohort. Int J Obes Relat Metab Disord 2000;24(9):1111–8. [DOI] [PubMed] [Google Scholar]
- 13.Lin CJ, DeRoo LA, Jacobs SR, et al. Accuracy and reliability of self-reported weight and height in the Sister Study. Public Health Nutr 2012;15(6):989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villanueva EV. The validity of self-reported weight in US adults: a population based cross-sectional study. BMC Public Health 2001;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsey ML, Talbert E, Ahn D, et al. Circulating interleukin-6 is associated with disease progression, but not cachexia in pancreatic cancer. Pancreatology 2019;19(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talbert EE, Lewis HL, Farren MR, et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naive pancreatic cancer patients. J Cachexia Sarcopenia Muscle 2018; 10.1002/jcsm.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara Y, Kobayashi T, Chayahara N, et al. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS One 2014;9(11):e113259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller J, Alshehri A, Ramage MI, et al. Plasma Metabolomics Identifies Lipid and Amino Acid Markers of Weight Loss in Patients with Upper Gastrointestinal Cancer. Cancers (Basel) 2019;11(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang QJ, Zhao JR, Hao J, et al. Serum and urine metabolomics study reveals a distinct diagnostic model for cancer cachexia. J Cachexia Sarcopenia Muscle 2018;9(1):71–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisner R, Stretch C, Eastman T, et al. Learning to predict cancer-associated skeletal muscle wasting from 1H-NMR profiles of urinary metabolites. Metabolomics 2011;7(1):25–34. [Google Scholar]
- 21.Stretch C, Eastman T, Mandal R, et al. Prediction of skeletal muscle and fat mass in patients with advanced cancer using a metabolomic approach. J Nutr 2012;142(1):14–21. [DOI] [PubMed] [Google Scholar]
- 22.Cala MP, Agullo-Ortuno MT, Prieto-Garcia E, et al. Multiplatform plasma fingerprinting in cancer cachexia: a pilot observational and translational study. J Cachexia Sarcopenia Muscle 2018;9(2):348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis HL, Chakedis JM, Talbert E, et al. Perioperative cytokine levels portend early death after pancreatectomy for ductal adenocarcinoma. J Surg Oncol 2017; 10.1002/jso.24940. [DOI] [PubMed] [Google Scholar]
- 24.Talbert EE, Cuitino MC, Ladner KJ, et al. Modeling Human Cancer-induced Cachexia. Cell Rep 2019;28(6):1612–1622 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, DiVittore NA, Young MM, et al. Altered sphingolipid metabolism in patients with metastatic pancreatic cancer. Biomolecules 2013;3(3):435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laaksonen R, Ekroos K, Sysi-Aho M, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 2016;37(25):1967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lerner L, Hayes TG, Tao N, et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J Cachexia Sarcopenia Muscle 2015;6(4):317–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loumaye A, de Barsy M, Nachit M, et al. Role of Activin A and myostatin in human cancer cachexia. J Clin Endocrinol Metab 2015;100(5):2030–8. [DOI] [PubMed] [Google Scholar]
- 29.Okada S, Okusaka T, Ishii H, et al. Elevated serum interleukin-6 levels in patients with pancreatic cancer. Jpn J Clin Oncol 1998;28(1):12–5. [DOI] [PubMed] [Google Scholar]
- 30.Derman BA, Macklis JN, Azeem MS, et al. Relationships between longitudinal neutrophil to lymphocyte ratios, body weight changes, and overall survival in patients with non-small cell lung cancer. BMC Cancer 2017;17(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Song MM, Zhang X, et al. Association of systemic inflammation with survival in patients with cancer cachexia: results from a multicentre cohort study. J Cachexia Sarcopenia Muscle 2021; 10.1002/jcsm.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin GN, Peng JW, Liu PP, et al. Elevated neutrophil-to-lymphocyte ratio predicts poor outcome in patients with advanced non-small-cell lung cancer receiving first-line gefitinib or erlotinib treatment. Asia Pac J Clin Oncol 2017;13(5):e189–e194. [DOI] [PubMed] [Google Scholar]
- 33.Zhong X, Zimmers TA. Sex Differences in Cancer Cachexia. Curr Osteoporos Rep 2020;18(6):646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayerle J, Kalthoff H, Reszka R, et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut 2017; 10.1136/gutjnl-2016-312432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boon J, Hoy AJ, Stark R, et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 2013;62(2):401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morigny P, Zuber J, Haid M, et al. High levels of modified ceramides are a defining feature of murine and human cancer cachexia. J Cachexia Sarcopenia Muscle 2020;11(6):1459–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Audano M, Maldini M, De Fabiani E, et al. Gender-related metabolomics and lipidomics: From experimental animal models to clinical evidence. J Proteomics 2018;178:82–91. [DOI] [PubMed] [Google Scholar]
- 38.Herpich C, Franz K, Ost M, et al. Associations between serum GDF15 concentrations, muscle mass and strength show sex-specific differences in older hospital patients. Rejuvenation Res 2020; 10.1089/rej.2020.2308. [DOI] [PubMed] [Google Scholar]
- 39.Lemon SC, Rosal MC, Zapka J, et al. Contributions of weight perceptions to weight loss attempts: differences by body mass index and gender. Body Image 2009;6(2):90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc 2001;101(1):28–34; quiz 35–6. [DOI] [PubMed] [Google Scholar]
- 41.Weir JM, Wong G, Barlow CK, et al. Plasma lipid profiling in a large population-based cohort. J Lipid Res 2013;54(10):2898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. In. 7th ed. New York, NY.: Springer; 2010. [Google Scholar]
- 43.Dutta T, Kudva YC, Persson XM, et al. Impact of Long-Term Poor and Good Glycemic Control on Metabolomics Alterations in Type 1 Diabetic People. J Clin Endocrinol Metab 2016;101(3):1023–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkins J, Sakrikar D, Petterson XM, et al. A comprehensive protocol for multiplatform metabolomics analysis in patient-derived skin fibroblasts. Metabolomics 2019;15(6):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanza IR, Zhang S, Ward LE, et al. Quantitative metabolomics by H-NMR and LC-MS/MS confirms altered metabolic pathways in diabetes. PLoS One 2010;5(5):e10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blachnio-Zabielska AU, Persson XM, Koutsari C, et al. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun Mass Spectrom 2012;26(9):1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savica R, Murray ME, Persson XM, et al. Plasma sphingolipid changes with autopsy-confirmed Lewy Body or Alzheimer’s pathology. Alzheimers Dement (Amst) 2016;3:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Persson XM, Blachnio-Zabielska AU, Jensen MD. Rapid measurement of plasma free fatty acid concentration and isotopic enrichment using LC/MS. J Lipid Res 2010;51(9):2761–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr Protoc Bioinformatics 2019;68(1):e86. [DOI] [PubMed] [Google Scholar]
- 50.van den Berg RA, Hoefsloot HC, Westerhuis JA, et al. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 2006;7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Informed consent was obtained for the publication of data in aggregate, but not as individual and therefore potentially identifiable datapoints. Therefore, the datasets have not been publicly deposited; however, the datasets can be made available from DCG through a non-human subjects research request.