Abstract
CD14-expressing Chinese hamster ovary (CD14-CHO) cells, established by transfection of human CD14 DNA, acquired high responsiveness to lipopolysaccharide (LPS) through membrane-bound CD14 expression. LPS induced DNA synthesis and activated a series of mitogen-activated protein (MAP) kinases, extracellular signal-regulated kinase 1/2 (Erk1/2), p38, and c-Jun N-terminal kinase/stress-activated protein kinase, in CD14-CHO cells but not in mock-transfected CHO cells. Anti-CD14 antibody completely abrogated both LPS-induced DNA synthesis and LPS-induced phosphorylation of those MAP kinases, suggesting a critical role of membrane-bound CD14 in LPS signaling. A p38 MAP kinase inhibitor, SB203580, markedly augmented LPS-induced DNA synthesis in CD14-CHO cells, whereas an Erk1/2 inhibitor, PD98059, had no affect. On the other hand, SB203580 exhibited no effect on epidermal growth factor-induced DNA synthesis in CD14-CHO cells, although PD98059 inhibited it significantly. The activation and inactivation of p38 MAP kinase with dominant negative and dominant positive mutants also suggested the participation of p38 MAP kinase in LPS-induced DNA synthesis. It was therefore suggested that the activation of p38 MAP kinase can negatively regulate LPS-induced cell proliferation in CD14-CHO cells.
Lipopolysaccharide (LPS) is present on the outer membranes of all gram-negative bacteria and known to cause the systemic inflammatory response syndrome septic shock and disseminated intravascular coagulation, leading finally to multiorgan failure (1, 21, 24, 33). The activation of monocytes/macrophages by LPS leads to inflammatory response via various cellular signaling events. LPS binds to monocytes/macrophages via membrane-bound CD14, which is the glycosylphosphatidylinositol-linked glycoprotein (37). The integration of membrane-bound CD14 renders various cell types highly sensitive to LPS. In fact, Chinese hamster ovary (CHO) cells which are transfected with the CD14 gene and express membrane-bound CD14 acquire the high responsiveness to LPS (7–9, 13–15, 18, 20, 22, 31, 38, 39). Membrane-bound CD14-expressing CHO (CD14-CHO) cells can respond to a low concentration of LPS and exhibit various responses, such as release of arachidonic acid metabolites (8), translocation of nuclear factor kappa B (NF-κB) (5, 9, 23) and production of interleukin 6 (10, 15), like LPS-responsive monocytes/macrophages. CD14-CHO cells may provide an experimental system useful for LPS signaling. LPS signaling is transduced by intracellular signal pathways using NF-κB and a series of mitogen-activated protein (MAP) kinases. In particular, the phosphorylation of three major MAP kinases i.e., extracellular signal-regulated kinase 1/2 (Erk1/2), p38, and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK), is rapidly induced by LPS in target cells such as macrophages (34, 35). There is no report on the activation of MAP kinases in LPS-stimulated CD14-CHO cells. In the present study, we examined whether MAP kinases were activated in LPS-stimulated CD14-CHO cells and what function the activation of MAP kinases had in those cells. Here we discuss the relationship between the activation of p38 MAP kinases and cell proliferation in LPS-stimulated CD14-CHO cells.
MATERIALS AND METHODS
Materials.
LPS from Escherichia coli O55:B5 and epidermal growth factor (EGF) were obtained from Sigma Chemical Co., St. Louis, Mo. SB203580, PD98059, and 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) were purchased from Calbiochem, San Diego, Calif. They were dissolved in dimethyl sulfoxide and diluted in the culture medium. Anti-human CD14 antibody CLB-MON/1 was purchased from Nichirei (Tokyo, Japan).
Establishment of CD14-CHO cells.
CHO-K1 fibroblasts, obtained from the American Type Culture Collection (Manassas, Va.), were maintained in Ham's F-12 (Sigma) containing 5% heat-inactivated fetal calf serum and antibiotics. The plasmid carrying human CD14 DNA was a kind gift from R. J. Ulevitch, The Scripps Research Institute, La Jolla, Calif. CHO cells were transfected with the CD14 plasmid by the lipofection method (8). CD14-CHO cells were selected positively by using anti-CD14 antibody-coated beads and further cultured with the addition of Geneticin (750 μg/ml). CD14-CHO cells were maintained in Ham's F-12 with 5% fetal calf serum. CHO cells transfected by the control vector plasmid served as mock-transfected control CHO cells.
Laser flow cytometric analysis of CD14 expression and LPS binding.
CD14-CHO cells were incubated with a 1:200 dilution of fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 monoclonal antibody (Coulter, Miami, Fla.) or 1 μg of FITC-conjugated LPS (Sigma) per ml at 4°C for 1 h. The cells were washed and suspended in phosphate-buffered saline. Fluorescence was analyzed by a laser flow cytometer (FACScaliber; Becton Dickinson, San Jose, Calif.).
DNA synthesis.
DNA synthesis in CD14-CHO cells was assayed by [3H]thymidine incorporation into the nucleus. Cells (3 × 104/100 μl) were plated in 96-well plates and incubated with LPS (1 μg/ml or various concentrations) in the presence or absence of anti-CD14 antibody (10 μg/ml) for 24 h. [3H]thymidine (1 μCi/well; Amersham, London, England) was added to the cultures. Eighteen hours later, the cells were harvested on glass fiber filters Radioactivity was determined as counts per minute in a Beckman β counter. In some experiments, CD14-CHO or mock-transfected control CHO cells were also pretreated with 20 μM SB203580 or PD98059, or various concentrations of SB203580, for 1 h before LPS treatment.
Immunoblotting.
CD14-CHO cells were seeded in 35-mm-diameter plastic dishes (4 × 105 cells/dish) and incubated with LPS (0.1 μg/ml) for 30 min. Cells were lysed in the lysis buffer and boiled for 5 min at 100°C as described previously (3). Aliquots (20 μg/lane) containing equal amounts of protein were electrophoresed under reducing conditions in a 4 to 20% gradient polyacrylamide gel and transferred to a polyvinylidene difluoride membrane filter. The membranes were treated with 5% bovine serum albumin for 1 h to block nonspecific binding, rinsed, and incubated with a panel of rabbit polyclonal antibodies against Erk1/2, phospho-Erk1/2, p38, phospho-p38, JNK/SAPK, and phospho-JNK/SAPK (New England Biolabs, Beverly, Mass.) for 1 h. The membranes were further treated with a 1:3,000 dilution of horseradish peroxidase-conjugated protein G for 1 h. Immune complexes were detected with an enhanced chemiluminescence substrate (New England Nuclear, Boston, Mass.) and exposed to Kodak XAR X-ray film. A prestained molecular weight standard kit (Nippon Bio-Rad, Tokyo, Japan) was used as a reference. In some experiments, CD14-CHO cells were pretreated with 20 μM SB203580 or PD98059 for 1 h before LPS treatment.
Transfection of dominant negative mutants of Erk1/2, p38, and JNK/SAPK and a dominant positive mutant of MKK6.
Dominant negative mutants of Erk1/2 (17), p38 (17), and JNK/SAPK (16) and a dominant positive mutant of MAP kinase kinase 6 (MKK6) (16) were kind gifts from J. D. Lee, The Scripps Research Institute. CD14-CHO cells were transfected with 5 μg of dominant negative mutants by the Lipofectamine (GIBCO-BRL, Gaithersburg, Md.) method (16, 17) for 8 h, and then the culture medium was replaced by Ham's F-12 containing 5% fetal calf serum. After 48 h of incubation, transfected cells were used for experiments. CD14-CHO cells were also transfected with a dominant positive mutant of MKK6 (5 μg/ml). To confirm overexpression of transfected MKK6, phospho-p38 was analyzed by immunoblotting using anti-phospho-p38 antibody. Anti-Flag (M2) antibody (Sigma) was also used to test transfection efficiency.
Statistical analysis.
Results are expressed as means ± standard deviations of triplicate determinations. Statistical significance was determined by Student's t test.
RESULTS
Establishment of CHO cells constitutively expressing membrane-bound CD14.
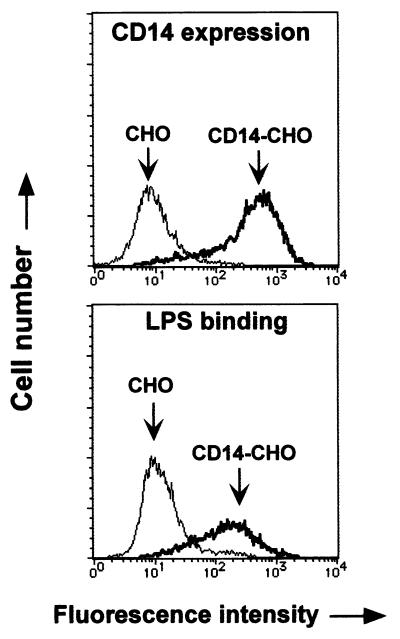
The expression of membrane-bound CD14 on CD14-CHO cells, which were transfected with human CD14 DNA, was analyzed by laser flow cytometry (Fig. 1). CD14-CHO cells expressed a high level of membrane-bound CD14. However, no significant CD14 expression was detected on mock-transfected control CHO cells. In an assay of the binding of FITC-conjugated LPS, the fluorescence intensity on CD14-CHO cells was approximately 10 times as high as that on mock-transfected CHO cells, suggesting enhanced binding of LPS to CD14-CHO cells.
FIG. 1.
Expression of membrane-bound CD14 and binding of LPS in CD14-CHO cells. CD14-CHO cells and mock-transfected control CHO cells were treated with FITC-conjugated anti-CD14 antibody (A) or LPS (B). Fluorescence intensity in CD14 expression and LPS binding was analyzed by laser flow cytometry.
Augmented DNA synthesis in LPS-stimulated CD14-CHO cells and its inhibition by anti-CD14 antibody.
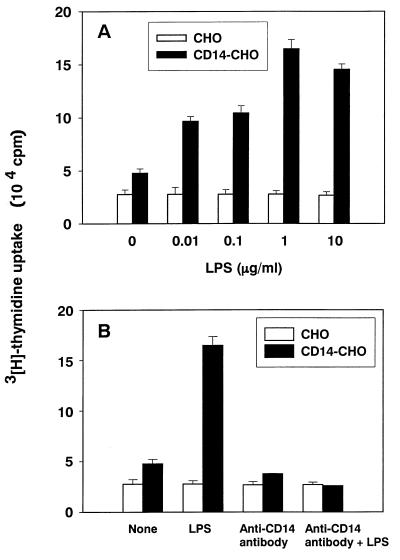
LPS at a concentration of 0.01 to 10 μg/ml augmented [3H]thymidine uptake in CD14-CHO cells; on the other hand, LPS at any concentration tested did not enhance DNA synthesis in mock-transfected control CHO cells (Fig. 2A). LPS was suggested to augment DNA synthesis via membrane-bound CD14 expression. To confirm the participation of membrane-bound CD14, the effect of anti-CD14 antibody on LPS-induced DNA synthesis in CD14-CHO cells was studied by [3H]thymidine incorporation (Fig. 2B). Anti-CD14 antibody abolished the augmentation of DNA synthesis in LPS-stimulated CD14-CHO cells. DNA synthesis in mock-transfected control CHO cells was not altered in the presence or absence of anti-CD14 antibody, thus confirming that the enhancement of DNA synthesis in CD14-CHO cells by LPS was mediated with membrane-bound CD14. Anti-CD14 antibody at a concentration of 5 μg/ml completely abrogated LPS-induced augmentation of DNA synthesis in CD14-CHO cells. The inhibition was roughly dependent on the concentration of anti-CD14 antibody added (data not shown).
FIG. 2.
Augmented DNA synthesis in LPS-stimulated CD14-CHO cells and its inhibition by anti-CD14 antibody. (A) Augmented DNA synthesis in LPS-stimulated CD14-CHO cells. [3H]thymidine incorporation was determined in CD14-CHO cells or mock-transfected control CHO cells cultured with various concentrations of LPS. (B) Inhibition of LPS-induced DNA synthesis by anti-CD14 antibody. [3H]thymidine incorporation was determined in CD14-CHO cells or mock-transfected control CHO cells cultured with LPS (1 μg/ml) in the presence of anti-CD14 antibody (10 μg/ml).
Activation of a series of MAP kinases in LPS-stimulated CD14-CHO cells and its inhibition by anti-CD14 antibody.
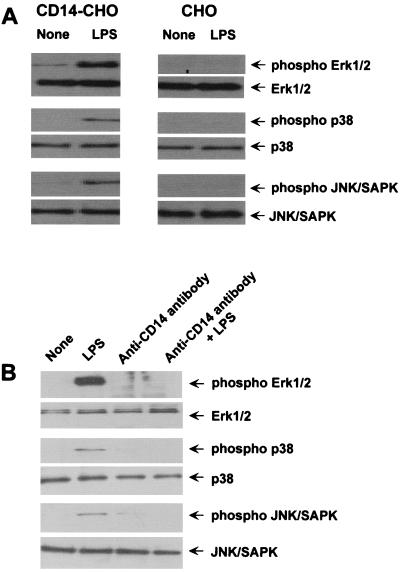
We have demonstrated that LPS augments DNA synthesis in CD14-CHO cells. Based on the fact that the MAP kinases Erk1/2, p38, and JNK/SAPK are reported to be involved in signal transduction of cell growth (6, 26–28, 40), it was of interest to determine the activation of MAP kinases in LPS-stimulated CD14-CHO cells. The phosphorylation of Erk1/2, p38, and JNK/SAPK in LPS-stimulated CD14-CHO cells was studied by immunoblotting using anti-phospho-MAP kinase antibodies (Fig. 3A). LPS induced the phosphorylation of Erk1/2, p38, and JNK/SAPK in CD14-CHO cells but not in mock-transfected control CHO cells. In addition, LPS did not affect constitutive levels of Erk1/2, p38, and JNK/SAPK expression. Next, we examined whether LPS-induced phosphorylation of MAP kinases in CD14-CHO cells was dependent on membrane-bound CD14 (Fig. 3B). The addition of anti-CD14 antibody (5 μg/ml) completely blocked the phosphorylation of all the three MAP kinases. Treatment with anti-CD14 antibody alone did not affect the phosphorylation of MAP kinases. This suggested that LPS activated Erk1/2, p38, and JNK/SAPK via membrane-bound CD14.
FIG. 3.
Phosphorylation of Erk1/2, p38, and JNK/SAPK MAP kinases in LPS-stimulated CD14-CHO cells and its inhibition by anti-CD14 antibody. (A) Phosphorylation of the MAP kinases in LPS-stimulated CD14-CHO cells. CD14-CHO cells and mock-transfected control CHO cells were cultured with LPS (0.1 μg/ml) for 30 min. (B) Inhibition LPS-induced phosphorylation of the MAP kinases by anti-CD14 antibody. CD14-CHO cells were pretreated with anti-CD14 antibody (5 μg/ml) for 4 h and then treated with LPS (0.1 μg/ml) for 30 min. The cells were lysed, and the lysates were analyzed by immunoblotting using an antibody to Erk1/2, phospho-Erk1/2, p38, phospho-p38, JNK/SAPK, or phospho -JNK/SAPK.
Enhancement of LPS-induced DNA synthesis in CD14-CHO cells by the p38 inhibitor SB203580.
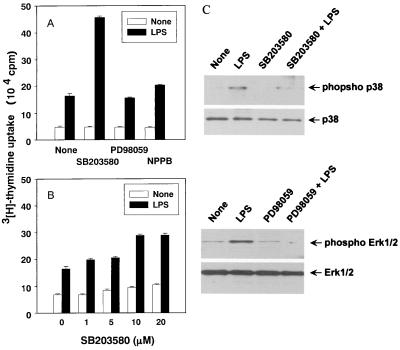
We tried to clarify the relationship between LPS-induced DNA synthesis and LPS-induced MAP kinase activation in CD14-CHO cells (Fig. 4). The effects of the p38 inhibitor SB203580, the Erk1/2 inhibitor PD98059, and the JNK/SAPK and Erk1/2 inhibitor (4) NPPB on DNA synthesis in LPS-stimulated CD14-CHO cells were assessed by [3H]thymidine incorporation. The addition of SB203580 resulted in striking enhancement of DNA synthesis in LPS-stimulated CD14-CHO cells, whereas neither PD98059 nor NPPB affected DNA synthesis (Fig. 4A). The inhibitor solvent, dimethyl sulfoxide, exhibited no effect on LPS-induced DNA synthesis. The concentration-dependent effect of SB203580 on DNA synthesis in LPS-stimulated CD14-CHO cells was studied (Fig. 4B). SB203580 at 10 μM exhibited a stronger enhancing effect on LPS-induced DNA synthesis than SB203580 at 1 μM did. The enhancing effect of SB203580 on DNA synthesis was also seen in the absence of LPS (>5 μM, P < 0.01). In addition, we confirmed that SB203580 and PD98059 completely abrogated the phosphorylation of p38 and Erk1/2, respectively (Fig. 4C). Results of the experiment using the p38 inhibitor SB203580 suggested that the inactivation of p38 MAP kinase might augment DNA synthesis in LPS-stimulated CD14-CHO cells.
FIG. 4.
Augmentation of LPS-induced DNA synthesis by a p38 inhibitor, SB203580. CD14-CHO cells or mock-transfected control CHO cells were pretreated with SB203580 (20 μM), PD98059 (20 μM), or NPPB (10 μM) (A) or with various concentrations of SB203580 (B) for 1 h and then treated with LPS (0.1 μg/ml). DNA synthesis was determined by [3H]thymidine incorporation. (C) Inhibitory action of SB203580 or PD98059 on the phosphorylation of p38 and Erk1/2 was confirmed by immunoblotting using an antibody to p38, phospho-p38, Erk1/2, or phospho-Erk1/2.
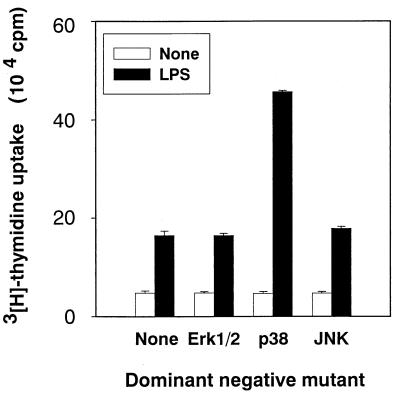
Enhancement of LPS-induced DNA synthesis by a dominant negative mutant of p38 MAP kinase.
Since the inhibition of p38 MAP kinase by SB203580 resulted in the enhancement of LPS-induced DNA synthesis, we examined the effect of a dominant negative mutant of p38 MAP kinase on LPS-induced DNA synthesis in CD14-CHO cells (Fig. 5). The dominant negative mutant of p38, but not that of Erk1/2 or JNK/SAPK, markedly enhanced LPS-induced DNA synthesis in CD14-CHO cells. No dominant negative mutants affected DNA synthesis in the absence of LPS.
FIG. 5.
Effect of the dominant negative mutant of Erk1/2, p38, or JNK/SAPK on DNA synthesis in LPS-stimulated CD14-CHO cells. CD14-CHO cells were transfected with a dominant negative mutant of Erk1/2, p38, or JNK/SAPK, and LPS-induced DNA synthesis was determined by [3H]thymidine incorporation.
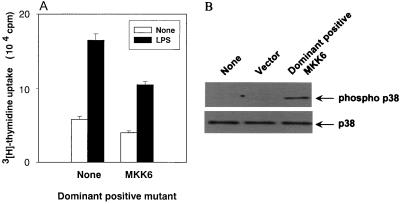
Inhibition of LPS-induced DNA synthesis by p38 activation via a dominant positive mutant of MKK6.
The effect of p38 activation on LPS-induced DNA synthesis in CD14-CHO cells was examined by the overexpression of MKK6, an upstream kinase of p38. The introduction of dominant positive mutants of MKK6 into CD14-CHO cells led to reduced DNA synthesis in LPS-stimulated CD14-CHO cells (Fig. 6A). The treatment also inhibited DNA synthesis in the absence of LPS (P < 0.01). The phosphorylation of p38 by transfection of a dominant positive mutant of MKK6 was confirmed by immunoblotting using anti-phospho-p38 antibody (Fig. 6B).
FIG. 6.
Effect of the dominant positive mutant of MKK6 on LPS-induced DNA synthesis in CD14-CHO cells. (A) CD14-CHO cells were transfected with a dominant positive mutant of MKK6, and DNA synthesis was determined in the presence or absence of LPS. (B) Phosphorylation of p38 MAP kinase in CD14-CHO cells transfected with a dominant positive mutant of MKK6 was confirmed by immunoblotting.
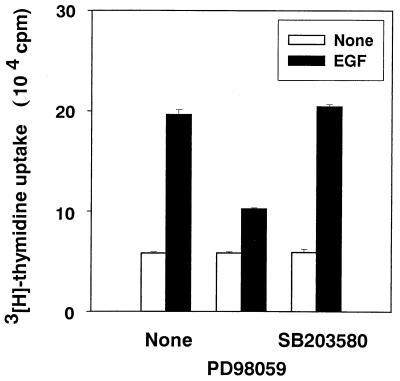
Effect of SB203580 on DNA synthesis in EGF-stimulated CD14-CHO cells.
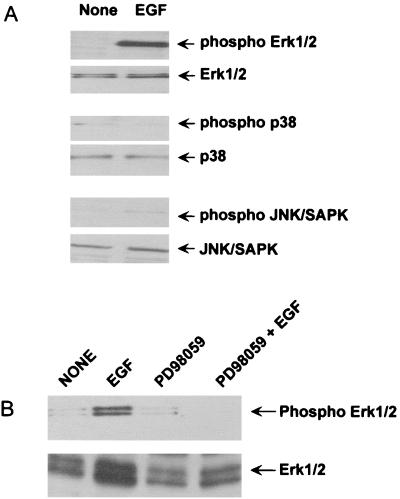
We determined whether p38 MAP kinase was involved in DNA synthesis in CD14-CHO cells stimulated with agents other than LPS. The effect of SB203580 on DNA synthesis in EGF-stimulated CD14-CHO cells was assessed by [3H]thymidine incorporation (Fig. 7). EGF-induced DNA synthesis in CD14-CHO cells was markedly inhibited by PD98059 but was not affected by SB203580. Next, the effect of EGF on phosphorylation of MAP kinases was studied (Fig. 8A). EGF induced the phosphorylation of Erk1/2 but not p38 and JNK/SAPK in CD14-CHO cells, and PD98059 completely inhibited EGF-induced Erk1/2 phosphorylation (Fig. 8B). In addition, there was no significant difference between CD14-CHO cells and mock-transfected control CHO cells in response to EGF (data not shown). This finding suggested that Erk1/2 and p38 MAP kinases might be involved in the signal transduction of EGF- and LPS-induced DNA synthesis in CD14-CHO cells, respectively.
FIG. 7.
Effect of SB203580 on DNA synthesis in EGF-stimulated CD14-CHO cells. CD14-CHO cells were pretreated with SB203580 (20 μM) or PD98059 (20 μM) for 1 h and then treated with EGF (50 ng/ml).
FIG. 8.
Effect of EGF on the phosphorylation of Erk1/2, p38, and JNK/SAPK MAP kinases in CD14-CHO cells and inhibition by PD98059. (A) Phosphorylation of Erk1/2 MAP kinase by EGF. CD14-CHO cells were cultured with EGF (50 ng/ml) for 30 min. The cells were lysed, and the lysates were analyzed by immunoblotting using an antibody to Erk1/2, phospho-Erk1/2, p38, phospho-p38, JNK/SAPK, or phospho-JNK/SAPK. (B) Inhibition of EGF-induced Erk1/2 phosphorylation by PD98059. CD14-CHO cells were pretreated with 20 μM PD98059 for 1 h and then treated with EGF for 30 min. The cells were lysed, and the lysates were analyzed by immunoblotting.
DISCUSSION
In this study we have demonstrated that LPS augments DNA synthesis and activates a series of MAP kinases, Erk1/2, p38, and JNK/SAPK, in CD14-CHO cells via membrane-bound CD14. Furthermore, a p38-specific inhibitor, SB203580, strikingly enhanced LPS-induced DNA synthesis in CD14-CHO cells. This suggests that the activation of p38 MAP kinase inhibits LPS-induced DNA synthesis in CD14-CHO cells and that the inhibition of p38 MAP kinase removes the inhibitory effect. This idea is also supported by the results of experiments with the dominant negative mutant of p38 and the dominant positive mutant of MKK6. Thus, LPS-activated p38 MAP kinase may negatively regulate the proliferative activity of CD14-CHO cells. This is to our knowledge the first report on the inhibitory action of p38 MAP kinase on LPS-induced cell proliferation. On the other hand, Erk1/2 did not seem to be involved in LPS-induced DNA synthesis of CD14-CHO cells, although Erk1/2 plays a pivotal role in EGF-induced DNA synthesis of CD14-CHO cells. Therefore, p38 MAP kinase is a key molecule in regulation of LPS-induced cell growth in CD14-CHO cells.
The p38 MAP kinase signal pathway plays an essential role in regulation of many cellular processes, including proliferation, differentiation, and cell death (6, 27–29, 40). Previously we reported that LPS reduced DNA synthesis in LPS-sensitive bovine aortic endothelial cells (3) and that SB203580 prevented LPS-induced reduction of DNA synthesis in those cells (3). The activation of p38 also regulates DNA synthesis negatively in bovine aortic endothelial cells. Although different cell types respond differentially to LPS in DNA synthesis, the inhibition of p38 MAP kinase essentially seems to have an enhancing effect on DNA synthesis. It has been reported that p38 MAP kinase provides a negative signal on cell proliferation of various cell types by various stimuli other than LPS (25–27, 30). On the other hand, the constitutive activation of p38 is reported to inhibit DNA synthesis in various cell types (6, 32). The exact role of p38 in cell proliferation requires further characterization.
Cell surface expression of CD14 provides the high responsiveness to LPS on CD14-CHO cells (7–9, 13–15, 18, 20, 22, 31, 38, 39). CD14-CHO fibroblasts become responsive to an extremely low concentration of LPS (8). Therefore, CD14-CHO cells have been studied as a model of LPS-responsive phagocytic and nonphagocytic cells since they appear to contain many elements responsible for LPS-induced signaling. In particular, CD14-CHO cells have been used for LPS signaling for NF-κB activation (5, 9), interleukin 6 production (10, 15), arachidonic acid release (8), and endotoxin tolerance (11). In the present study, we found that LPS induced the activation of a series of MAP kinases, Erk1/2, p38, and JNK/SAPK, in CD14-CHO cells. There is the first report on the activation of MAP kinases in LPS-stimulated CD14-CHO cells. Our experimental system using CD14-CHO cells might provide a new model for characterization of LPS-induced MAP kinase activation.
LPS modulates the growth of gingival fibroblasts in the presence of growth factors (12). LPS also acts as a mitogen in chicken embryo (36) and intestinal (2) fibroblasts. In CD14-CHO cells, LPS induces the up-regulation of an apparently extracellular protein, H411, which contains EGF-like repeats and promotes growth (10). Microinjection of mRNA of its highly homologous protein led to an autocrine/paracrine stimulation of DNA synthesis (19). LPS may induce DNA synthesis in CD14-CHO cells by up-regulation of H411 proteins. Although the biological significance of LPS-induced DNA synthesis in CD14-CHO cells is unknown, this system might be useful for characterization of the growth and proliferation of macrophages by stimulation of LPS.
ACKNOWLEDGMENTS
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and Daiko Foundation.
We thank M. Naruse for laser flow cytometric analysis.
REFERENCES
- 1.Bone R C. Gram-negative sepsis: a dilemma of modern medicine. Clin Microbiol Rev. 1993;6:57–68. doi: 10.1128/cmr.6.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakravortty D, Kumar K S. Induction of cell proliferation and collagen synthesis in human small intestinal lamina propria fibroblasts by lipopolysaccharide: possible involvement of nitric oxide. Biochem Biophys Res Commun. 1997;240:458–463. doi: 10.1006/bbrc.1997.7680. [DOI] [PubMed] [Google Scholar]
- 3.Chakravortty D, Kato Y, Koide N, Sugiyama T, Kawai M, Fukada M, Yoshida T, Yokochi T. Extracellular matrix components prevent lipopolysaccharide-induced bovine arterial endothelial cell injury by inhibiting p38 mitogen-activated protein kinase. Thromb Res. 2000;98:187–193. doi: 10.1016/s0049-3848(99)00226-1. [DOI] [PubMed] [Google Scholar]
- 4.Chan E D, Riches D W. Potential role of the JNK/SAPK signal transduction pathway in the induction of iNOS by TNF-alpha. Biochem Biophys Res Commun. 1998;253:790–796. doi: 10.1006/bbrc.1998.9857. [DOI] [PubMed] [Google Scholar]
- 5.Delude R L, Fenton M J, Savedra R, Jr, Perera P Y, Vogel S N, Thieringer R, Golenbock D T. CD14-mediated translocation of nuclear factor-kappa B induced by lipopolysaccharide does not require tyrosine kinase activity. J Biol Chem. 1994;269:22253–22260. [PubMed] [Google Scholar]
- 6.Diehl N L, Enslen H, Fortner K A, Merritt C, Stetson N, Charland C, Flavell R A, Davis R J, Rincon M. Activation of the p38 mitogen-activated protein kinase pathway arrests cell cycle progression and differentiation of immature thymocytes in vivo. J Exp Med. 2000;191:321–334. doi: 10.1084/jem.191.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durieux J J, Vita N, Popescu O, Guette F, Calzada-Wack J, Munker R, Schmidt R E, Lupker J, Ferrara P, Ziegler-Heitbrock H W. The two soluble forms of the lipopolysaccharide receptor, CD14: characterization and release by normal human monocytes. Eur J Immunol. 1994;24:2006–2012. doi: 10.1002/eji.1830240911. [DOI] [PubMed] [Google Scholar]
- 8.Golenbock D T, Liu Y, Millham F H, Freeman M W, Zoeller R A. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 9.Hamann L, Schumann R R, Flad H D, Brade L, Rietschel E T, Ulmer A J. Binding of lipopolysaccharide (LPS) to CHO cells does not correlate with LPS-induced NF-kappaB activation. Eur J Immunol. 2000;30:211–216. doi: 10.1002/1521-4141(200001)30:1<211::AID-IMMU211>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 10.Heine H, Delude R L, Monks B G, Espevik T, Golenbock D T. Bacterial lipopolysaccharide induces expression of the stress response genes hop and H411. J Biol Chem. 1999;274:21049–21055. doi: 10.1074/jbc.274.30.21049. [DOI] [PubMed] [Google Scholar]
- 11.Heine H, Kirschning C J, Lien E, Monks B G, Rothe M, Golenbock D T. Cells that carry A null allele for toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 12.Hill S J, Ebersole J L. The effect of lipopolysaccharide on growth factor-induced mitogenesis in human gingival fibroblasts. J Periodontol. 1996;67:1274–1280. doi: 10.1902/jop.1996.67.12.1274. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 14.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwagaki A, Porro M, Pollack M. Influence of synthetic antiendotoxin peptides on lipopolysaccharide (LPS) recognition and LPS-induced proinflammatory cytokine responses by cells expressing membrane-bound CD14. Infect Immun. 2000;68:1655–1663. doi: 10.1128/iai.68.3.1655-1663.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato Y, Kravchenko V V, Tapping R I, Han J, Ulevitch R J, Lee J-D. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J. 1997;16:7054–7066. doi: 10.1093/emboj/16.23.7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato Y, Zhao M, Morikawa A, Sugiyama T, Chakravortty D, Koide N, Yoshida T, Tapping R I, Young Y, Yokochi T, Lee J-D. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J Biol Chem. 2000;275:18534–18540. doi: 10.1074/jbc.M001573200. [DOI] [PubMed] [Google Scholar]
- 18.Kirschning C J, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091–2097. doi: 10.1084/jem.188.11.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecka-Czernik B, Lumpkin C K, Jr, Goldstein S. An overexpressed gene transcript in senescent and quiescent human fibroblasts encoding a novel protein in the epidermal growth factor-like repeat family stimulates DNA synthesis. Mol Cell Biol. 1995;15:120–128. doi: 10.1128/mcb.15.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lien E, Means T K, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton M J, Oikawa M, Qureshi N, Monks B, Finberg R W, Ingalls R R, Golenbock D T. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Investig. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin M A. Epidemiology and clinical impact of gram-negative sepsis. Infect Dis Clin North Am. 1991;5:739–752. [PubMed] [Google Scholar]
- 22.Means T K, Lien E, Yoshimura A, Wang S, Golenbock D T, Fenton M J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–6755. [PubMed] [Google Scholar]
- 23.Medvedev A E, Flo T, Ingalls R R, Golenbock D T, Teti G, Vogel S N, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-kappaB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;16:4535–4542. [PubMed] [Google Scholar]
- 24.Morrison D C, Ryan J L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 25.Murakami-Mori K, Mori S, Nakamura S. p38 MAP kinase is a negative regulator for ERK1/2-mediated growth of AIDS-associated Kaposi's sarcoma cells. Biochem Biophys Res Commun. 1999;264:676–682. doi: 10.1006/bbrc.1999.1574. [DOI] [PubMed] [Google Scholar]
- 26.Nagata Y, Tadokoro K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood. 1999;94:853–863. [PubMed] [Google Scholar]
- 27.Oh C D, Chang S H, Yoon Y M, Lee S J, Lee Y S, Kang S S, Chun J S. Opposing role of mitogen-activated protein kinase subtypes, erk-1/2 and p38, in the regulation of chondrogenesis of mesenchymes. J Biol Chem. 2000;275:5613–5619. doi: 10.1074/jbc.275.8.5613. [DOI] [PubMed] [Google Scholar]
- 28.Orsini M J, Krymskaya V P, Eszterhas A J, Benovic J L, Panettieri R A, Jr, Penn R B. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol. 1999;277:L479–L488. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]
- 29.Page K, Hershenson M B. Mitogen-activated signaling and cell cycle regulation in airway smooth muscle. Front Biosci. 2000;5:D258–D267. doi: 10.2741/page. [DOI] [PubMed] [Google Scholar]
- 30.Philips A, Roux P, Coulon V, Bellanger J M, Vie A, Vignais M L, Blanchard J M. Differential effect of Rac and Cdc42 on p38 kinase activity and cell cycle progression of nonadherent primary mouse fibroblasts. J Biol Chem. 2000;275:5911–5917. doi: 10.1074/jbc.275.8.5911. [DOI] [PubMed] [Google Scholar]
- 31.Pollack M, Ohl C A, Golenbock D T, Di Padova F, Wahl L M, Koles N L, Guelde G, Monks B G. Dual effects of LPS antibodies on cellular uptake of LPS and LPS-induced proinflammatory functions. J Immunol. 1997;159:3519–3530. [PubMed] [Google Scholar]
- 32.Puri P L, Wu Z, Zhang P, Wood L D, Bhakta K S, Han J, Feramisco J R, Karin M, Wang J Y. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 2000;14:574–584. [PMC free article] [PubMed] [Google Scholar]
- 33.Rietschel E T, Brade H. Bacterial endotoxins. Sci Am. 1992;67:54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- 34.Sanghera J S, Weinstein S L, Aluwalia M, Girn J, Pelech S L. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–4465. [PubMed] [Google Scholar]
- 35.Swantek J L, Cobb M H, Geppert T D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaheri A, Ruoslahti E, Sarvas M, Nurminen M. Mitogenic effect by lipopolysaccharide and pokeweed lectin on density-inhibited chick embryo fibroblasts. J Exp Med. 1973;138:1356–1364. doi: 10.1084/jem.138.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto H, Hanada K, Nishijima M. Involvement of diacylglycerol production in activation of nuclear factor kappaB by a CD14-mediated lipopolysaccharide stimulus. Biochem J. 1997;325:223–228. doi: 10.1042/bj3250223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang R B, Mark M R, Gray A, Huang A, Xie M H, Zhang M, Goddard A, Wood W I, Gurney A L, Godowski P J. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 1998;395:284–288. doi: 10.1038/26239. [DOI] [PubMed] [Google Scholar]
- 40.Yoon Y M, Oh C D, Kim D Y, Lee Y S, Park J W, Huh T L, Kang S S, Chun J S. Epidermal growth factor negatively regulates chondrogenesis of mesenchymal cells by modulating the protein kinase C-alpha, Erk-1, and p38 MAPK signaling pathways. J Biol Chem. 2000;275:12353–12359. doi: 10.1074/jbc.275.16.12353. [DOI] [PubMed] [Google Scholar]