PURPOSE
Neoadjuvant gemcitabine and cisplatin (GC) followed by radical cystectomy (RC) is standard for patients with muscle-invasive bladder cancer (MIBC). On the basis of the activity of atezolizumab (A) in metastatic BC, we tested neoadjuvant GC plus A for MIBC.
METHODS
Eligible patients with MIBC (cT2-T4aN0M0) received a dose of A, followed 2 weeks later by GC plus A every 21 days for four cycles followed 3 weeks later by a dose of A before RC. The primary end point was non–muscle-invasive downstaging to < pT2N0.
RESULTS
Of 44 enrolled patients, 39 were evaluable. The primary end point was met, with 27 of 39 patients (69%) < pT2N0, including 16 (41%) pT0N0. No patient with < pT2N0 relapsed and four (11%) with ≥ pT2N0 relapsed with a median follow-up of 16.5 months (range: 7.0-33.7 months). One patient refused RC and two developed metastatic disease before RC; all were considered nonresponders. The most common grade 3-4 adverse event (AE) was neutropenia (n = 16; 36%). Grade 3 immune-related AEs occurred in five (11%) patients with two (5%) requiring systemic steroids. The median time from last dose of chemotherapy to surgery was 7.8 weeks (range: 5.1-17 weeks), and no patient failed to undergo RC because of AEs. Four of 39 (10%) patients had programmed death-ligand 1 (PD-L1)–positive tumors and were all < pT2N0. Of the patients with PD-L1 low or negative tumors, 23 of 34 (68%) achieved < pT2N0 and 11 of 34 (32%) were ≥ pT2N0 (P = .3 for association between PD-L1 and < pT2N0).
CONCLUSION
Neoadjuvant GC plus A is a promising regimen for MIBC and warrants further study. Patients with < pT2N0 experienced improved relapse-free survival. The PD-L1 positivity rate was low compared with published data, which limits conclusions regarding PD-L1 as a predictive biomarker.
INTRODUCTION
The majority of patients with muscle-invasive bladder cancer (MIBC) will develop metastases after radical cystectomy and pelvic lymph node dissection (RC-PLND).1,2 Neoadjuvant cisplatin-based chemotherapy before RC-PLND improves long-term survival compared with RC-PLND alone.3-5 Although the pivotal phase III neoadjuvant trial used methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC),4 subsequent efforts include regimens with improved toxicity, such as gemcitabine and cisplatin (GC),6,7 and dose-dense (dd) administration, such as ddMVAC and ddGC.8-10 A consistent finding across studies is that non–muscle-invasive downstaging (< pT2) and negative lymph nodes (N0) at the time of RC-PLND correlate with improved survival.4,7-14 This correlation between pathologic response and clinical benefit from neoadjuvant chemotherapy is similar to observations in other malignancies such as breast cancer and non–small-cell lung cancer.15-19 However, unlike patients with localized breast cancer in whom pathologic complete response (pCR) after neoadjuvant therapy is the most robust end point,15-17 pCR and < pT2N0 appear to similarly correlate with improved survival in MIBC.13,20,21 Unfortunately, only 36%-49% of patients with MIBC will achieve non–muscle-invasive downstaging with GC,7,12,13,22 which is a widely used neoadjuvant regimen.12,23
CONTEXT
Key Objective
In patients with muscle-invasive bladder cancer, neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy is a standard of care. The objective of this phase II trial was to evaluate the efficacy and safety of adding atezolizumab to gemcitabine and cisplatin (GC).
Knowledge Generated
Compared with the historical control of neoadjuvant chemotherapy alone and despite low programmed death-ligand 1 positivity (10%) in the cohort, the addition of atezolizumab to GC resulted in a high rate of non–muscle-invasive downstaging (69%), which correlated with improved relapse-free survival and overall survival. No patients failed to undergo timely radical cystectomy because of toxicity, and no unexpected safety signals were observed during treatment or postoperatively.
Relevance
This trial demonstrated that the addition of atezolizumab to neoadjuvant GC is a promising approach in muscle-invasive bladder cancer. The need for improved biomarkers beyond programmed death-ligand 1 and the association of non–muscle-invasive downstaging and improved outcomes have implications for future trial design.
To improve outcomes in MIBC, a combinatorial approach with GC and immune checkpoint blockade (ICB) of the programmed death 1 (PD-1)/programmed death-ligand 1 (PD-L1) axis is attractive for several reasons. The absence of clinical cross-resistance is demonstrated by remarkably durable responses in some patients with platinum-resistant metastatic bladder cancer treated with ICB.24-28 There are also potential immunomodulatory effects of GC that may enhance antitumor immunity.29-32 Finally, ICB without chemotherapy can result in non–muscle-invasive downstaging at the time of surgery in cisplatin-ineligible patients.33,34 However, the glucocorticoids given to attenuate chemotherapy-associated nausea and the nontargeted genotoxic effects of GC may suppress an antitumor immune response.30,35 Therefore, we assessed the efficacy and safety of an atezolizumab lead-in dose followed by four cycles of GC with atezolizumab and one additional dose of atezolizumab after chemotherapy completion and before RC-PLND.
METHODS
Patients
Eligible patients were candidates for RC-PNLD and had cT2N0M0-cT4aN0M0 disease as determined by cystoscopy and transurethral resection of bladder tumor (TURBT) within 60 days of treatment initiation as well as cross-sectional imaging (computed tomography of the chest, abdomen, and pelvis with intravenous [IV] contrast) within 30 days of treatment initiation. TURBT at enrolling sites was not mandated, but pathologic confirmation of MIBC was required. Cisplatin eligibility was defined as an estimated glomerular filtration rate ≥ 50 mL/min per 1.73 m2 (Chronic Kidney Disease Epidemiology Collaboration formula); Eastern Cooperative Oncology Group performance status of 0 or 1; no pre-existing grade ≥ 2 peripheral neuropathy or hearing impairment; and no New York Heart Association class III or IV heart failure, or recent cardiovascular event. Major exclusion criteria included active infection, prior use of ICB, and autoimmune disease.
Trial Oversight
This study was approved by the institutional review boards of participating sites and performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients provided written informed consent before study entry.
Study Procedures
This was a nonrandomized, multi-institutional, open-label phase II study (ClinicalTrials.gov identifier: NCT02989584). Patients were treated with an atezolizumab lead-in dose followed 2 weeks later by four 21-day cycles of GC with atezolizumab on day 8 and one additional dose of atezolizumab 3 weeks after chemotherapy completion. Atezolizumab was administered as a flat dose of 1,200 mg IV once every 3 weeks, gemcitabine was administered at a dose of 1,000 mg/m2 IV once on days 1 and 8, and cisplatin was administered as either 70 mg/m2 IV once on day 1 or as split-dose at 35 mg/m2 IV once on days 1 and 8. Split-dose cisplatin was recommended if the estimated glomerular filtration rate was 50 to < 60 or by treating physician's discretion. Hydration, mannitol, and antiemetics were administered in accordance with institutional protocols with the exception of dexamethasone, which was given as either 12 mg IV on day 1 and 4 mg orally on day 2 with cisplatin 70 mg/m2 dosing or 12 mg IV on days 1 and 8 with split-dose cisplatin. Reductions in dexamethasone dose were allowed at the treating physician's discretion.
Patients underwent a history, physical examination, and toxicity assessment using National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 on all treatment days and on a post-treatment follow-up visit before RC-PLND. Imaging was performed at the completion of protocol therapy and RC-PLND recommended within approximately 4-8 weeks from the last dose of chemotherapy. Postoperative complications up to 90 days after surgery were retrospectively recorded.
PD-L1 immunohistochemical staining on pretreatment TURBT specimens was prospectively assessed centrally (Targos, Kassel, Germany) with the SP142 assay (Ventana, AZ). Staining was performed within 60 days of slides being cut and was defined as ≥ 5% of tumor-infiltrating immune cells staining positive (IC2/3).34,36
Statistical Considerations
The primary end point was non–muscle-invasive downstaging, defined as the absence of muscle-invasive disease (< pT2) and lymph node metastases (N0) within the RC-PLND specimen as assessed by institutional pathologists using the American Joint Committee on Cancer 7th edition criteria.37 We used a Simon's two-stage minimax design with the probabilities of a type I error and type II error set at .05 and .2, respectively. A total of 39 patients were required to detect an improvement in the non–muscle-invasive downstaging rate to 55% from a historical rate of 35% with conventional-dose, cisplatin-based chemotherapy alone.3,4,7 Twenty-one patients were planned to be accrued in the first stage. If ≥ 9 were < pT2N0, then an additional 18 would be accrued. The combination would be considered promising if at least 19 of 39 patients achieved non–muscle-invasive downstaging. Patients who developed progressive and/or metastatic disease while on neoadjuvant therapy, those who were unable or unwilling to undergo RC-PLND, and those who discontinued protocol therapy because of treatment-related delays and/or treatment-related toxicity were considered nonresponders. Patients who received < 2 cycles of protocol therapy because of withdrawal of consent or unrelated adverse events (AEs) were evaluable for toxicity assessment but were not evaluable for the primary end point and replaced.
Secondary end points included the proportion of patients with a complete pathologic response (pT0N0), safety (Common Terminology Criteria for Adverse Events v4.0), time to cystectomy, overall survival (OS), and relapse-free survival (RFS). RFS was evaluated using the Kaplan-Meier method and measured from treatment initiation until disease recurrence, which was defined as investigator-determined clinical or radiographic progression. Patients without documented recurrence were censored at the last follow-up for the purpose of OS and at the time of last cross-sectional imaging for the purpose of RFS. Association between non–muscle-invasive downstaging and PD-L1 status was analyzed using the Fisher's exact test.
RESULTS
Study Patients
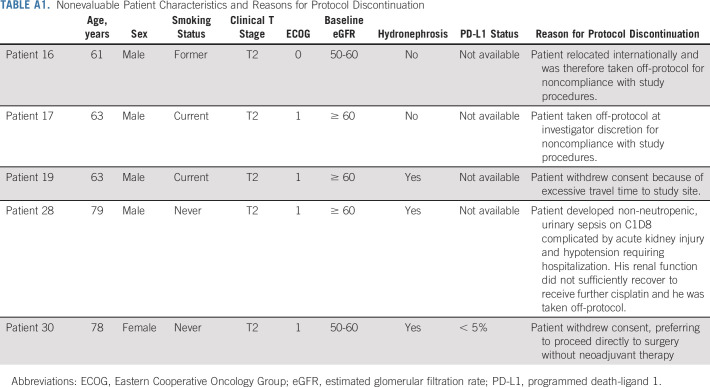
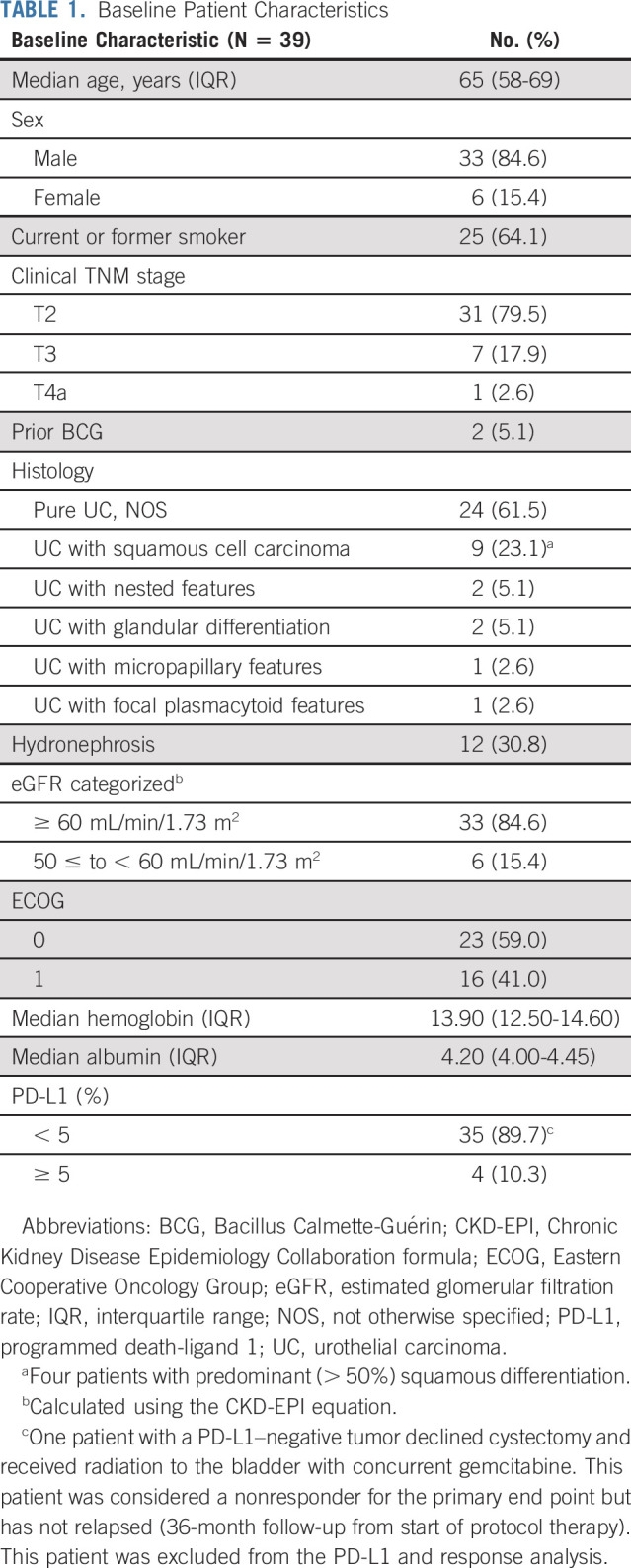
From February 2018 to May 2020, a total of 44 patients were enrolled across five institutions. Five patients came off protocol before completing two cycles of therapy and were evaluable for safety but not for efficacy (Appendix Table A1, online only). Table 1 summarizes the baseline characteristics of the 39 response-evaluable patients. The median age was 65 years and most patients were male (85%), current or former smokers (64%), Eastern Cooperative Oncology Group 0 (59%), and clinical T2N0M0 (80%). Six patients (15%) had an estimated pretreatment glomerular filtration rate < 60 mL/min. Hydronephrosis was present in 12 (31%) patients. Tumors were predominantly histologically pure urothelial carcinoma, not otherwise specified (n = 24, 62%). Only four (10%) pretreatment tumors were PD-L1–positive.
TABLE 1.
Baseline Patient Characteristics
Efficacy
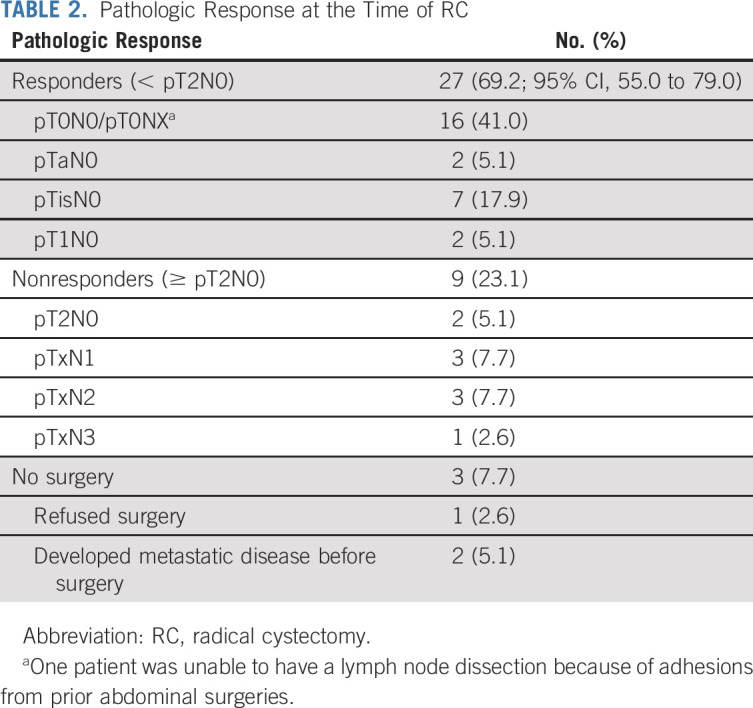
The study met its primary end point with 27 of 39 response-evaluable patients achieving < pT2N0 disease for an overall non–muscle-invasive downstaging rate of 69% (95% CI, 55 to 79), including 16 (41%) patients with a pCR (Table 2). Two patients (5%) exhibited pT2N0 disease at RC-PLND, and seven (18%) nonresponders had node-positive disease at RC-PLND. Two patients (5%) developed metastatic disease prior to RC-PLND and were considered nonresponders. A single patient refused RC-PLND and instead received radiotherapy to the bladder with concurrent gemcitabine. This patient was considered a nonresponder but has not relapsed (36-month follow-up from the start of protocol therapy).
TABLE 2.
Pathologic Response at the Time of RC
Among the 39 response-evaluable patients, four have died from disease and six have experienced disease progression (including the two who progressed before RC-PLND). Median follow-up from time of treatment start was 23.6 months (range: 12.0-38.2 months). Median RFS and OS were not reached (Fig 1; data cutoff May 1, 2021). Thirty-six patients underwent RC-PLND with a median follow-up of 16.5 months (range: 7.0-33.7 months) from the time of RC-PLND to last follow-up among those alive at the end of study. Among these 36 patients, no responding patient (< pT2N0) has relapsed, and four nonresponding patients (≥ pT2N0) subsequently relapsed. Responders had significantly better RFS compared with nonresponders (log-rank P < .001; Fig 2).
FIG 1.
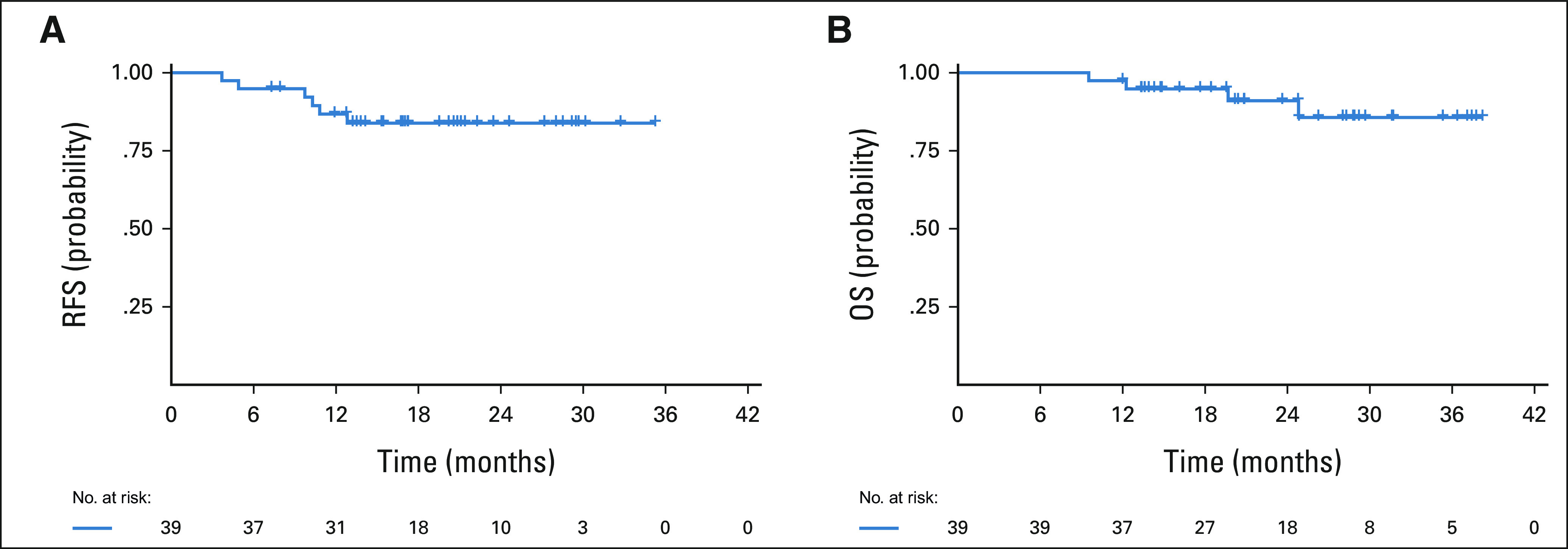
(A) RFS and (B) OS in 39 response-evaluable patients who were treated with neoadjuvant GC with atezolizumab. GC, gemcitabine and cisplatin; OS, overall survival; RFS, relapse-free survival.
FIG 2.
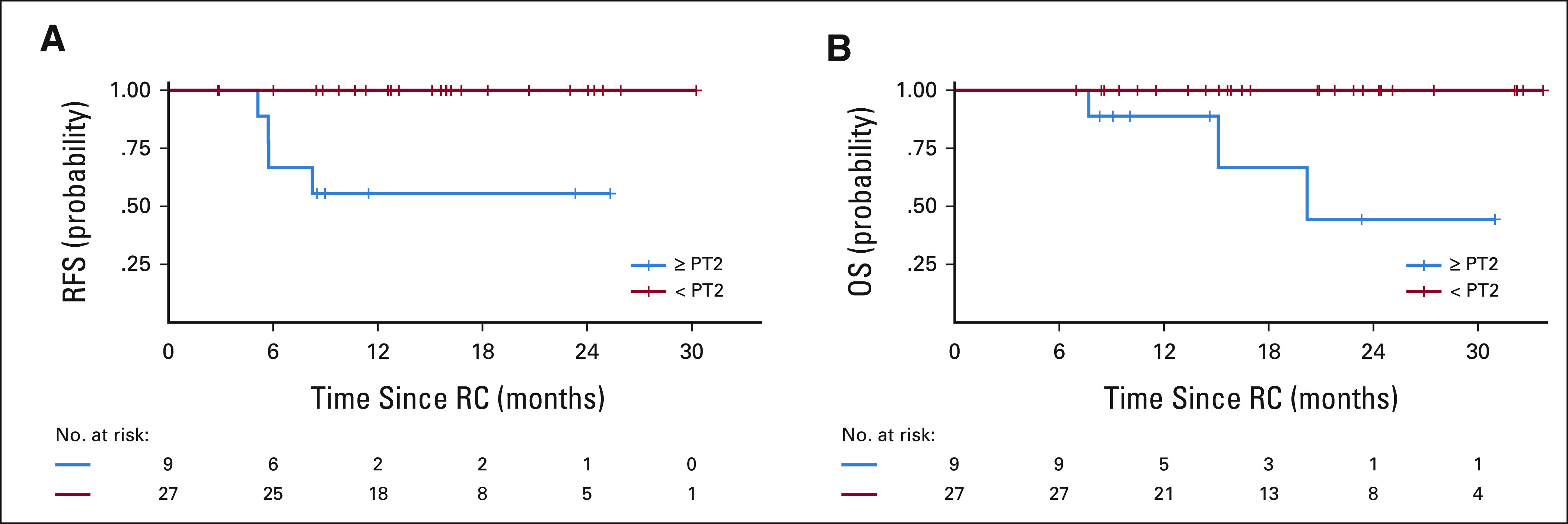
(A) RFS and (B) OS stratified by pathologic response (< pT2 v ≥ pT2N0) for 36 patients who underwent RC. OS, overall survival; RC, radical cystectomy; RFS, relapse-free survival.
All patients with PD-L1–positive tumors achieved < pT2N0 (4 of 4; 100%). Of the patients with PD-L1 low and negative tumors (excluding the one patient who refused surgery), 23 of 34 (68%) achieved < pT2N0 and 11 of 34 (32%) were ≥ pT2N0 (P = .3 for association between PD-L1 and < pT2N0).
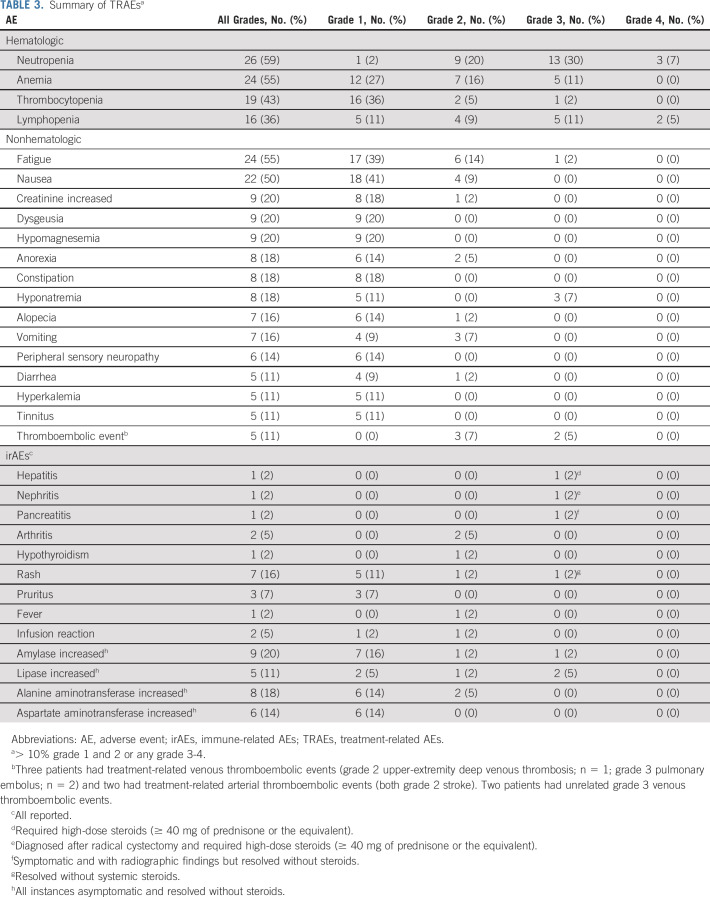
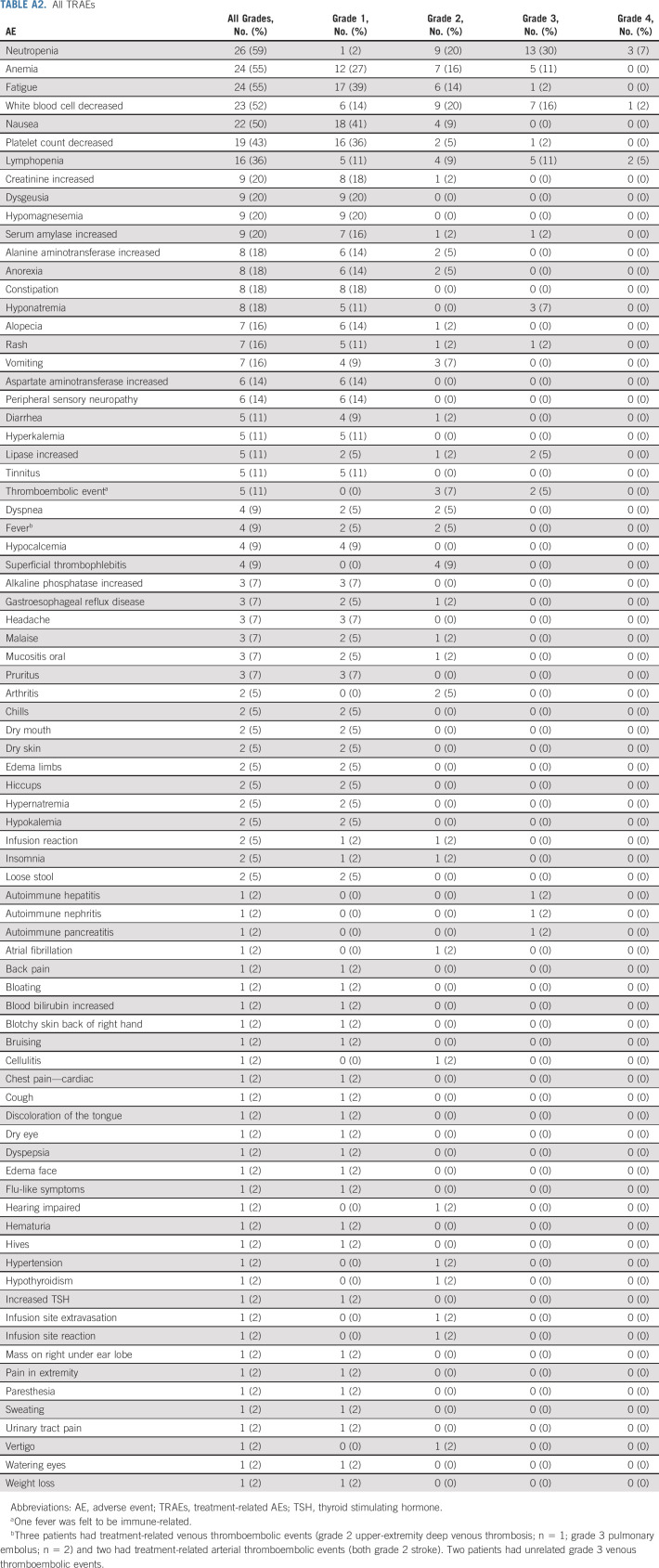
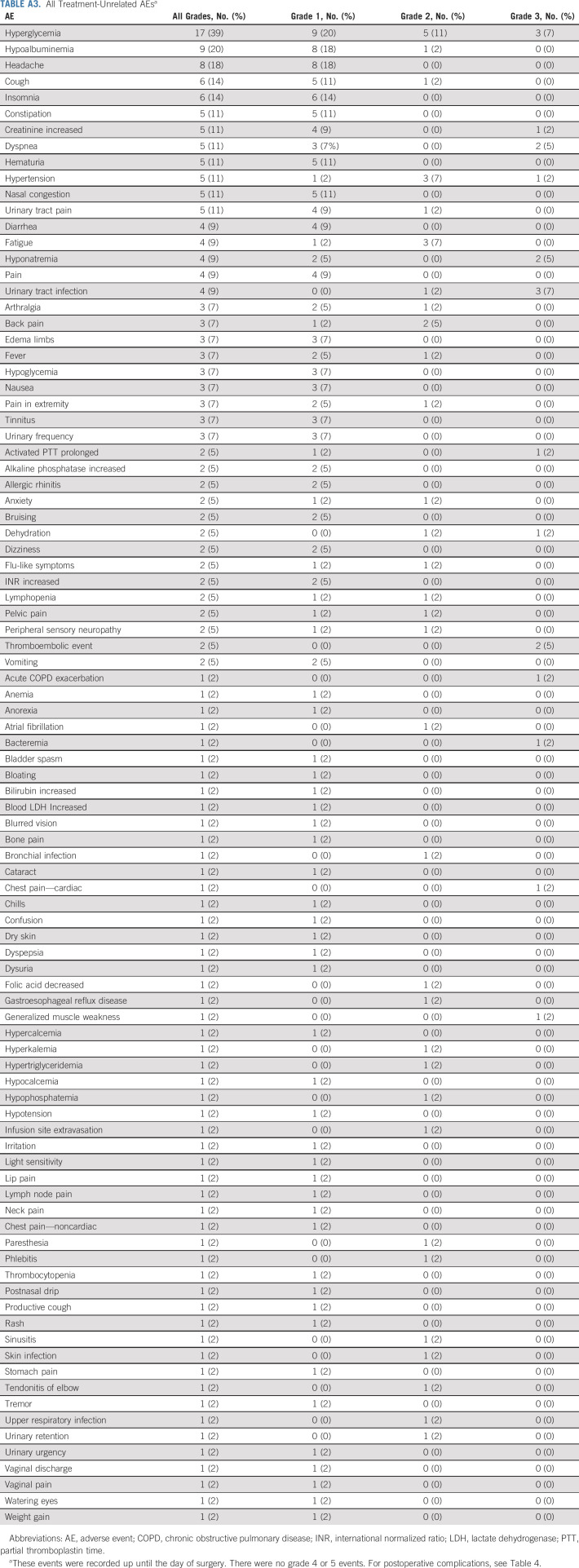
Safety
Among the 44 patients evaluable for safety, 98% experienced treatment-related AEs (TRAEs; Table 3; Appendix Tables A2 and A3, online only). The most common TRAEs of any grade were neutropenia (n = 26; 59%), fatigue (n = 24; 55%), anemia (n = 24; 55%), and nausea (n = 22; 50%). A total of 26 patients (59%) experienced a grade ≥ 3 TRAE. The most common grade 3-4 TRAE was neutropenia (n = 16, 36%), although no patients developed neutropenic fever. Three patients experienced treatment-related venous thromboembolic events (grade 2 upper-extremity deep venous thrombosis; n = 1; grade 3 pulmonary embolus; n = 2) and two experienced a treatment-related arterial thromboembolic event (grade 2 stroke; n = 2). Grade 3 immune-related AEs (irAEs) occurred in five (11%) patients including hepatitis (n = 1), asymptomatic elevation in lipase during treatment and nephritis after RC-PLND (n = 1), pancreatitis (n = 1), rash (n = 1), and asymptomatic elevations in amylase/lipase (n = 1). The patient with hepatitis and the patient with nephritis required high-dose systemic steroids (≥ 40 mg of prednisone or the equivalent).
TABLE 3.
Summary of TRAEsa
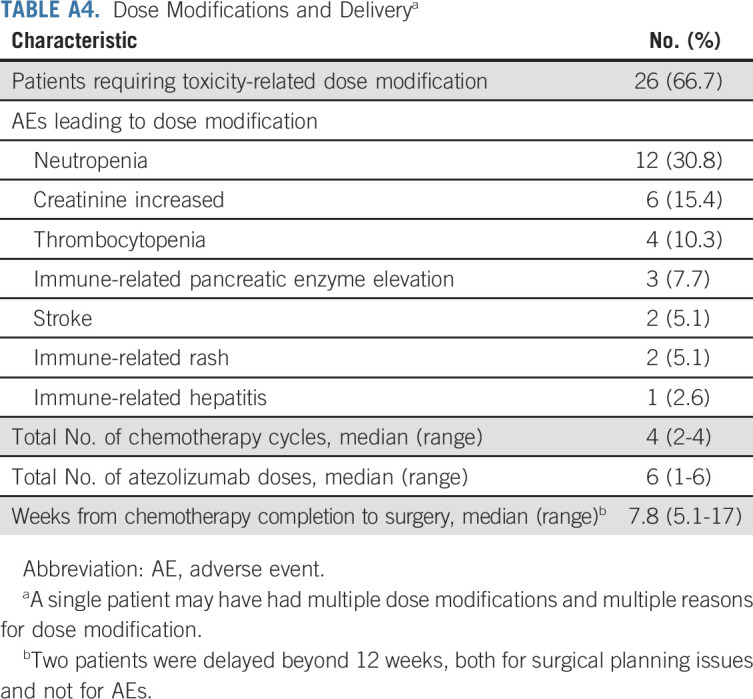
Among the 39 response-evaluable patients, 26 (67%) required dose modifications (Appendix Table A4, online only). The most common reasons for dose modifications were chemotherapy-related and included neutropenia (n = 12), creatinine increase (n = 6), and thrombocytopenia (n = 4). Thirty-six (92%) patients received four cycles of cisplatin-based chemotherapy and 29 (74%) received all six planned doses of atezolizumab. Two patients discontinued chemotherapy after experiencing grade 2 stroke and one patient discontinued chemotherapy after experiencing a grade 2 creatinine increase; all patients underwent RC-PLND.
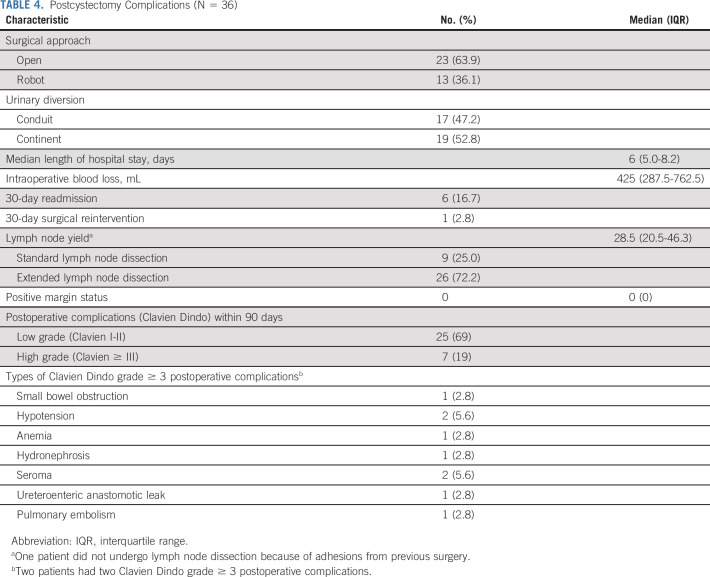
None of the 39 response-evaluable patients failed to undergo surgery owing to AEs and the median number of weeks from last chemotherapy to RC was 7.8 (range, 5.1-17.0). Two patients had delays in surgery beyond 12 weeks from chemotherapy, and neither was because of AEs. The quality of surgery as well as postoperative complications are listed in Table 4.
TABLE 4.
Postcystectomy Complications (N = 36)
DISCUSSION
In this single-arm multicenter phase II study, neoadjuvant GC with atezolizumab demonstrated promising activity in MIBC, with 27 patients (69%) found to be < pT2N0 and 16 (41%) found to be pT0N0 at the time of RC-PLND. Based upon these results, the study met its primary end point of at least 19 of 39 patients achieving < pT2N0 to consider GC with atezolizumab worthy of further investigation. Grade 3-4 TRAEs were observed in 59% of patients and were primarily because of chemotherapy. No patient experienced AE-related delays to RC-PLND.
The 69% < pT2N0 and 41% pT0N0 rates observed with GC plus atezolizumab compare favorably to those reported for GC and dd-MVAC, which are the neoadjuvant regimens preferred by the National Comprehensive Cancer Network.38 Several large retrospective analyses of neoadjuvant GC have reported < pT2N0 rates of 36%-46% and pT0N0 rates of 21%-31%.7,12,13,39 Two prospective single-arm trials of ddMVAC found < pT2N0 rates of 49%-53%, including a pT0N0 rate of 26%-38%.8,9 A recent randomized phase III study (VESPER) prospectively compared neoadjuvant ddMVAC with GC.22 This study reported < pT2N0 rates among patients treated with ddMVAC and GC of 63% and 49% (P = .007), respectively, as well as pCR rates of 42% and 36% (P = .2), respectively. Patients who underwent RC-PLND and achieved < pT2N0 after GC with atezolizumab experienced excellent RFS and OS, which is consistent with the neoadjuvant cisplatin-based chemotherapy experience in patients with MIBC.4,7-14
The future of combination anti–PD-1/L1 immunotherapy with GC in patients with metastatic bladder cancer remains uncertain. Two randomized phase III trials were conducted to test the combination of pembrolizumab (KEYNOTE-361) or atezolizumab (IMvigor130) with platinum doublet chemotherapy in the frontline setting.40,41 In KEYNOTE-361, the progression-free survival and OS benefits of chemotherapy with pembrolizumab versus chemotherapy alone did not reach statistical significance.40 In IMvigor130, chemotherapy with atezolizumab demonstrated a statistically significant improvement in progression-free survival compared with chemotherapy alone (8.2 months v 6.3 months, HR 0.82, P = .007), although OS data are not yet mature.41 Importantly, these trials included both cisplatin-ineligible and cisplatin-eligible patients, and the majority in both studies were treated with carboplatin-based therapy. Thus, these results cannot necessarily be extrapolated to the treatment of cisplatin-eligible patients with MIBC. Indeed, a subgroup analysis of IMvigor130 revealed a larger effect size with the addition of atezolizumab in the subset of patients who received cisplatin (hazard ratio for OS, 0.73; 95% CI, 0.55 to 0.97).41 Furthermore, in addition to our study of GC with atezolizumab, other trials of neoadjuvant GC with anti–PD-1/L1 ICB for patients with MIBC have reported < pT2N0 rates of 56%-66% and pT0N0 rates of 34%-44%, which also compare favorably to historical data with GC alone.42-45 Finally, the administration of atezolizumab before and after GC is unique to this study and may have facilitated enhanced antitumor immunity.
The toxicity of GC with atezolizumab observed in our study is consistent with prior chemotherapy with ICB studies in that no new safety signals emerged and the combination AE profile was consistent with those of the individual therapeutic modalities.41,42,46-49 Grade 3 treatment-related venous thromboembolic events occurred in three (7%) patients and stroke occurred in two (5%) patients, which are consistent with rates reported with cisplatin-based chemotherapy across multiple malignancies.50 Two patients (5%) required high-dose systemic steroids (≥ 40 mg of prednisone or the equivalent) for irAEs, one with hepatitis and the other with nephritis. The nephritis manifested after RC-PLND, underscoring the need for continued vigilance for irAEs in the postoperative period. We found GC with atezolizumab to be associated with moderate rates of grade ≥ 3 cytopenias (36% neutropenia, 11% anemia, and 2% thrombocytopenia), no neutropenic fever, only 2% grade 3 fatigue, and 0% grade ≥ 3 nausea. The VESPER trial demonstrated ddMVAC is associated with more grade ≥ 3 anemia (22% v 8%), nausea (10% v 3%), and fatigue (14% v 4%) than GC.22 Both ddMVAC and GC were associated with moderate rates of grade ≥ 3 neutropenia (39% and 46%) and thrombocytopenia (20% and 17%) with neutropenic fever occurring in 7% and 2% of patients receiving ddMVAC and GC, respectively.22 Although larger comparative trials are needed, the < pT2N0 rate of 69% observed in this study with GC with atezolizumab mirrors the higher < pT2N0 rate of ddMVAC versus GC (63% and 49%; P = .007) in the VESPER trial but with a toxicity profile more similar to GC, with the exception of uncommon irAEs.
There are limitations to this study. The median follow-up may be insufficient to capture all recurrences. In particular, the correlation between pathologic response and long-term OS needs further study in the context of neoadjuvant ICB for MIBC, although initial data from single-arm, phase II trials of neoadjuvant chemotherapy plus PD-1/L1 blockade in non–small-cell lung cancer as well as pembrolizumab in MIBC suggest that the correlation is maintained.42,46,51-53 Furthermore, the 15% rate of pCR with TURBT alone presents challenges to the interpretation of response rates in all single-arm MIBC trials.4 In addition, the higher proportion of cT2 cases and variant histology as well as the low rate of PD-L1 positivity may limit generalizability. However, the rate of cT2N0 (80%) is similar to the 65% and 72% rates reported in the neoadjuvant ddGC and GC with pembrolizumab studies, respectively, and less than the > 90% rate reported in VESPER.13,22,42 Among the eight patients who were ≥ cT3 in our study, five achieved non–muscle-invasive downstaging (pTisN0, n = 3; pT0N0, n = 2), which is not markedly different from the overall cohort. Finally, in single-arm phase II trials reported to date of neoadjuvant GC with anti–PD-1/L1 ICB for patients with MIBC,42-45 PD-L1 positivity was not predictive of non–muscle-invasive downstaging. Thus, additional interrogation of the genomic and host immune factors mediating response and resistance to GC with atezolizumab is ongoing.
In summary, the addition of atezolizumab to neoadjuvant GC was safe and associated with a 69% non–muscle-invasive downstaging rate and a 41% complete response rate in patients with MIBC. The combination of GC and ICB, therefore, compares favorably with historical data and warrants additional investigation. Indeed, there are ongoing phase III trials evaluating GC versus GC combined with pembrolizumab (anti–PD-1),54 nivolumab (anti–PD-1) with or without the indoleamine 2,3-dioxygenase inhibitor linrodostat,55 and durvalumab (anti–PD-L1).56 In addition, enfortumab vedotin, an antibody-drug conjugate targeting nectin-4 that is approved for patients with metastatic bladder cancer who previously received platinum-based treatment and a PD-1 or PD-L1 inhibitor,57,58 is being investigated in phase III trials as neoadjuvant therapy for patients with MIBC.59,60 Of note, these phase III trials include adjuvant therapy irrespective of the pathologic response at the time of RC-PLND, which data from our study and from studies of neoadjuvant cisplatin-based chemotherapy suggest may be overtreatment for those patients with non–muscle-invasive downstaging. Furthermore, as novel therapies become incorporated into the perioperative treatment armamentarium for patients with MIBC, a prospective biomarker-based approach to identify those patients most likely to benefit from these therapies is crucial. For example, the RETAIN (NCT02710734) and HCRN GU16-257 (NCT03558087) trials61,62 and the ongoing A031701 (NCT03609216) trial incorporate assessment for tumor DNA damage repair mutations as part of the qualifying criteria for pursing active surveillance instead of RC-PLND following neoadjuvant therapy. Additional coordinated efforts will be needed apply both immune-profiling and genomic-profiling technologies to personalize therapeutic combinations while preserving efficacy and minimizing toxicity.
ACKNOWLEDGMENT
The clinical trial protocol was designed, in part, at the 2015 ASCO/AACR Methods in Clinical Cancer Research Workshop supported by the National Cancer Institute of the National Institutes of Health under Award Number R25CA068647.
APPENDIX
TABLE A1.
Nonevaluable Patient Characteristics and Reasons for Protocol Discontinuation
TABLE A2.
All TRAEs
TABLE A3.
All Treatment-Unrelated AEsa
TABLE A4.
Dose Modifications and Deliverya
Samuel A. Funt
Employment: ByHeart (I)
Stock and Other Ownership Interests: Kite, a Gilead company, Urogen pharma, Allogene Therapeutics, Neogene Therapeutics, Kronos Bio, Vida Ventures, IconOVir Bio
Consulting or Advisory Role: Merck, Immunai
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Decibel Therapeutics (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca/MedImmune
Hikmat Al-Ahmadie
Consulting or Advisory Role: Bristol Myers Squibb, EMD Serono, AstraZeneca/MedImmune, Janssen Biotech, PAIGE.AI
Min Yuen Teo
Consulting or Advisory Role: Janssen Oncology
Research Funding: Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Pharmacyclics (Inst)
Chung-Han Lee
Honoraria: Intellisphere, Research to Practice, American Institute of Continuing Medical Education
Consulting or Advisory Role: Eisai, Bristol Myers Squibb, Merck, Pfizer/EMD Serono, Exelixis
Research Funding: Eisai (Inst), Bristol Myers Squibb (Inst), Calithera Biosciences (Inst), Exelixis (Inst), Merck (Inst)
David Aggen
Consulting or Advisory Role: Boehringer Ingelheim, Seattle Genetics, Astellas Pharma
Patents, Royalties, Other Intellectual Property: University of Illinois—Urbana Champaign
(OPTIONAL) Open Payments Link: https://openpaymentsdata.cms.gov/physician/4226107
Deaglan McHugh
Consulting or Advisory Role: Progenics
Arlyn Apollo
Employment: Covera Health (I)
Leadership: Covera Health (I)
Stock and Other Ownership Interests: Covera Health (I)
Consulting or Advisory Role: Covera Health (I)
Travel, Accommodations, Expenses: Covera Health (I)
Kaitlyn Francese
Consulting or Advisory Role: Seattle Genetics, Astellas Pharma
Yuanquan Yang
Stock and Other Ownership Interests: Bristol Myers Squibb, Pfizer, AstraZeneca
Consulting or Advisory Role: The Whiteoak Group
Eugene Pietzak
Honoraria: UpToDate
Consulting or Advisory Role: Merck, Chugai Pharma, QED Therapeutics, Janssen
Alvin C. Goh
Consulting or Advisory Role: Medtronic
Travel, Accommodations, Expenses: Medtronic
William C. Huang
Honoraria: Urogen pharma
Consulting or Advisory Role: Intuitive Surgical
Research Funding: SonaCare Medical, photocure, Storz, Storz, Merck (Inst), Intuitive Surgical (Inst)
Travel, Accommodations, Expenses: Photocure
Dean F. Bajorin
Consulting or Advisory Role: Merck, Dragonfly Therapeutics, Fidia Farmaceutici S. p. A, Bristol Myers Squibb Foundation
Research Funding: Novartis (Inst), Merck (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst)
Travel, Accommodations, Expenses: Merck
Gopa Iyer
Consulting or Advisory Role: Bayer, Janssen, Mirati Therapeutics, Basilea, Flare Therapeutics, Loxo/Lilly
Speakers' Bureau: Gilead Sciences, The Lynx Group
Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Debiopharm Group (Inst), Bayer (Inst), Janssen (Inst), Seattle Genetics (Inst)
Bernard H. Bochner
Consulting or Advisory Role: Olympus
Arjun V. Balar
Leadership: GT Biopharma
Stock and Other Ownership Interests: GT Biopharma
Honoraria: Merck, Genentech/Roche, AstraZeneca/MedImmune
Consulting or Advisory Role: Genentech/Roche, Merck, Cerulean Pharma, AstraZeneca/MedImmune, Pfizer/EMD Serono, Incyte, Seattle Genetics/Astellas, Nektar, Dragonfly Therapeutics, GlaxoSmithKline, Bristol Myers Squibb/Celgene
Research Funding: Merck (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Seattle Genetics, Gilead Sciences (Inst)
Amir Mortazavi
Honoraria: Motive Medical Intelligence
Consulting or Advisory Role: Seattle Genetics, Debiopharm Group, Pfizer
Research Funding: Acerta Pharma (Inst), Genentech/Roche (Inst), Merck (Inst), Novartis (Inst), Seattle Genetics (Inst), Mirati Therapeutics (Inst), Bristol Myers Squibb (Inst), Roche (Inst), Astellas Pharma (Inst), Debiopharm Group (Inst), Debiopharm Group (Inst)
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Intellisphere, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seattle Genetics, Bayer, BioClin Therapeutics, QED Therapeutics, Adicet Bio, Pharmacyclics, western oncolytics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
No other potential conflicts of interest were reported.
See accompanying Oncology Grand Rounds on page 1275
PRIOR PRESENTATION
Presented at the Annual ASCO Meeting, June 4-8, 2021, virtual.
SUPPORT
Supported by Genentech/Roche, the NIH/NCI Cancer Center Support Grant P30 CA008748, and the NIH/NCI P50 CA221745 SPORE in Bladder Cancer.
CLINICAL TRIAL INFORMATION
S.A.F. and M.L. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Samuel A. Funt, Irina Ostrovnaya, Timothy F. Donahue, Dean F. Bajorin, Gopa Iyer, Jonathan E. Rosenberg
Financial support: Jonathan E. Rosenberg
Administrative support: Samuel A. Funt, Colleen Quinlan, Ashley Regazzi, Marwah Jihad, Abigail Boswell, Timothy F. Donahue, Gopa Iyer, Jonathan E. Rosenberg
Provision of study materials or patients: Samuel A. Funt, Min Yuen Teo, Chung-Han Lee, Danielle Zimmerman, Deaglan McHugh, Arlyn Apollo, Jeffrey Kamradt, Maged Khalil, Bradley Lash, Kaitlyn Francese, Harry W. Herr, S. Machele Donat, Timothy F. Donahue, Alvin C. Goh, William C. Huang, Gopa Iyer, Bernard H. Bochner, Arjun V. Balar, Amir Mortazavi, Jonathan E. Rosenberg
Collection and assembly of data: Samuel A. Funt, Michael Lattanzi, Karissa Whiting, Hikmat Al-Ahmadie, Colleen Quinlan, Min Yuen Teo, Chung-Han Lee, Danielle Zimmerman, Arlyn Apollo, Trey D. Durdin, Hong Truong, Jeffrey Kamradt, Asia S. McCoy, Grace Hettich, Ashley Regazzi, Marwah Jihad, Neha Ratna, Abigail Boswell, Kaitlyn Francese, Yuanquan Yang, Edmund Folefac, Eugene Pietzak, Eugene K. Cha, Alvin C. Goh, William C. Huang, Gopa Iyer, Bernard H. Bochner, Amir Mortazavi, Jonathan E. Rosenberg
Data analysis and interpretation: Samuel A. Funt, Michael Lattanzi, Karissa Whiting, Hikmat Al-Ahmadie, Min Yuen Teo, Chung-Han Lee, David Aggen, Deaglan McHugh, Trey D. Durdin, Hong Truong, Maged Khalil, Bradley Lash, Irina Ostrovnaya, Harry W. Herr, S. Machele Donat, Timothy F. Donahue, Alvin C. Goh, William C. Huang, Dean F. Bajorin, Gopa Iyer, Arjun V. Balar, Jonathan E. Rosenberg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Neoadjuvant Atezolizumab With Gemcitabine and Cisplatin in Patients With Muscle-Invasive Bladder Cancer: A Multicenter, Single-Arm, Phase II Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Samuel A. Funt
Employment: ByHeart (I)
Stock and Other Ownership Interests: Kite, a Gilead company, Urogen pharma, Allogene Therapeutics, Neogene Therapeutics, Kronos Bio, Vida Ventures, IconOVir Bio
Consulting or Advisory Role: Merck, Immunai
Research Funding: Genentech/Roche (Inst), AstraZeneca (Inst), Decibel Therapeutics (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca/MedImmune
Hikmat Al-Ahmadie
Consulting or Advisory Role: Bristol Myers Squibb, EMD Serono, AstraZeneca/MedImmune, Janssen Biotech, PAIGE.AI
Min Yuen Teo
Consulting or Advisory Role: Janssen Oncology
Research Funding: Bristol Myers Squibb (Inst), Clovis Oncology (Inst), Pharmacyclics (Inst)
Chung-Han Lee
Honoraria: Intellisphere, Research to Practice, American Institute of Continuing Medical Education
Consulting or Advisory Role: Eisai, Bristol Myers Squibb, Merck, Pfizer/EMD Serono, Exelixis
Research Funding: Eisai (Inst), Bristol Myers Squibb (Inst), Calithera Biosciences (Inst), Exelixis (Inst), Merck (Inst)
David Aggen
Consulting or Advisory Role: Boehringer Ingelheim, Seattle Genetics, Astellas Pharma
Patents, Royalties, Other Intellectual Property: University of Illinois—Urbana Champaign
(OPTIONAL) Open Payments Link: https://openpaymentsdata.cms.gov/physician/4226107
Deaglan McHugh
Consulting or Advisory Role: Progenics
Arlyn Apollo
Employment: Covera Health (I)
Leadership: Covera Health (I)
Stock and Other Ownership Interests: Covera Health (I)
Consulting or Advisory Role: Covera Health (I)
Travel, Accommodations, Expenses: Covera Health (I)
Kaitlyn Francese
Consulting or Advisory Role: Seattle Genetics, Astellas Pharma
Yuanquan Yang
Stock and Other Ownership Interests: Bristol Myers Squibb, Pfizer, AstraZeneca
Consulting or Advisory Role: The Whiteoak Group
Eugene Pietzak
Honoraria: UpToDate
Consulting or Advisory Role: Merck, Chugai Pharma, QED Therapeutics, Janssen
Alvin C. Goh
Consulting or Advisory Role: Medtronic
Travel, Accommodations, Expenses: Medtronic
William C. Huang
Honoraria: Urogen pharma
Consulting or Advisory Role: Intuitive Surgical
Research Funding: SonaCare Medical, photocure, Storz, Storz, Merck (Inst), Intuitive Surgical (Inst)
Travel, Accommodations, Expenses: Photocure
Dean F. Bajorin
Consulting or Advisory Role: Merck, Dragonfly Therapeutics, Fidia Farmaceutici S. p. A, Bristol Myers Squibb Foundation
Research Funding: Novartis (Inst), Merck (Inst), Bristol Myers Squibb (Inst), AstraZeneca (Inst), Astellas Pharma (Inst), Seattle Genetics/Astellas (Inst)
Travel, Accommodations, Expenses: Merck
Gopa Iyer
Consulting or Advisory Role: Bayer, Janssen, Mirati Therapeutics, Basilea, Flare Therapeutics, Loxo/Lilly
Speakers' Bureau: Gilead Sciences, The Lynx Group
Research Funding: Mirati Therapeutics (Inst), Novartis (Inst), Debiopharm Group (Inst), Bayer (Inst), Janssen (Inst), Seattle Genetics (Inst)
Bernard H. Bochner
Consulting or Advisory Role: Olympus
Arjun V. Balar
Leadership: GT Biopharma
Stock and Other Ownership Interests: GT Biopharma
Honoraria: Merck, Genentech/Roche, AstraZeneca/MedImmune
Consulting or Advisory Role: Genentech/Roche, Merck, Cerulean Pharma, AstraZeneca/MedImmune, Pfizer/EMD Serono, Incyte, Seattle Genetics/Astellas, Nektar, Dragonfly Therapeutics, GlaxoSmithKline, Bristol Myers Squibb/Celgene
Research Funding: Merck (Inst), Genentech/Roche (Inst), AstraZeneca/MedImmune (Inst), Seattle Genetics, Gilead Sciences (Inst)
Amir Mortazavi
Honoraria: Motive Medical Intelligence
Consulting or Advisory Role: Seattle Genetics, Debiopharm Group, Pfizer
Research Funding: Acerta Pharma (Inst), Genentech/Roche (Inst), Merck (Inst), Novartis (Inst), Seattle Genetics (Inst), Mirati Therapeutics (Inst), Bristol Myers Squibb (Inst), Roche (Inst), Astellas Pharma (Inst), Debiopharm Group (Inst), Debiopharm Group (Inst)
Jonathan E. Rosenberg
Honoraria: UpToDate, Medscape, Peerview, Research To Practice, Intellisphere, Clinical Care Options, Physicans' Education Resource, MJH Life Sciences, EMD Serono
Consulting or Advisory Role: Lilly, Merck, Roche/Genentech, AstraZeneca/MedImmune, Bristol Myers Squibb, Seattle Genetics, Bayer, BioClin Therapeutics, QED Therapeutics, Adicet Bio, Pharmacyclics, western oncolytics, GlaxoSmithKline, Janssen Oncology, Astellas Pharma, Boehringer Ingelheim, Pfizer/EMD Serono, Mirati Therapeutics, Immunomedics, Tyra Biosciences, Infinity Pharmaceuticals
Research Funding: Genentech/Roche (Inst), Seattle Genetics (Inst), Bayer (Inst), AstraZeneca (Inst), QED Therapeutics (Inst), Astellas Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Predictor of platinum sensitivity (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Stein JP, Lieskovsky G, Cote R, et al. : Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol 19:666-675, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Yafi FA, Aprikian AG, Chin JL, et al. : Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: A Canadian multicentre experience. BJU Int 108:539-545, 2011 [DOI] [PubMed] [Google Scholar]
- 3.International Collaboration of Trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group) , European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group, Australian Bladder Cancer Study Group, et al. : International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: Long-term results of the BA06 30894 trial. J Clin Oncol 29:2171-2177, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman HB, Natale RB, Tangen CM, et al. : Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349:859-866, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Vale CL: Neoadjuvant chemotherapy in invasive bladder cancer: Update of a systematic review and meta-analysis of individual patient data: Advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 48:202-206, 2005 [DOI] [PubMed] [Google Scholar]
- 6.von der Maase H, Hansen SW, Roberts JT, et al. : Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18:3068-3077, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Dash A, Pettus JA, Herr HW, et al. : A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder. Cancer 113:2471-2477, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. : Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: Results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 32:1895-1901, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choueiri TK, Jacobus S, Bellmunt J, et al. : Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: Pathologic, radiologic, and biomarker correlates. J Clin Oncol 32:1889-1894, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyer G, Balar AV, Milowsky MI, et al. : Multicenter prospective phase II trial of neoadjuvant dose-dense gemcitabine plus cisplatin in patients with muscle-invasive bladder cancer. J Clin Oncol 36:1949-1956, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Splinter TA, Scher HI, Denis L, et al. : The prognostic value of the pathological response to combination chemotherapy before cystectomy in patients with invasive bladder cancer. European Organization for Research on Treatment of Cancer—Genitourinary Group. J Urol 147:606-608, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Zargar H, Espiritu PN, Fairey AS, et al. : Multicenter assessment of neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol 67:241-249, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer G, Tully CM, Zabor EC, et al. : Neoadjuvant gemcitabine-cisplatin plus radical cystectomy-pelvic lymph node dissection for muscle-invasive bladder cancer: A 12-year experience. Clin Genitourin Cancer 18:387–394, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhindi B, Frank I, Mason RJ, et al. : Oncologic outcomes for patients with residual cancer at cystectomy following neoadjuvant chemotherapy: A pathologic stage-matched analysis. Eur Urol 72:660-664, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Rastogi P, Anderson SJ, Bear HD, et al. : Preoperative chemotherapy: Updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol 26:778-785, 2008 [DOI] [PubMed] [Google Scholar]
- 16.von Minckwitz G, Untch M, Blohmer J-U, et al. : Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol 30:1796-1804, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Pataer A, Kalhor N, Correa AM, et al. : Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 7:825-832, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pataer A, Weissferdt A, Vaporciyan AA, et al. : Evaluation of pathologic response in lymph nodes of patients with lung cancer receiving neoadjuvant chemotherapy. J Thorac Oncol 16:1289–1297, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Schultz PK, Herr HW, Zhang Z-F, et al. : Neoadjuvant chemotherapy for invasive bladder cancer: Prognostic factors for survival of patients treated with M-VAC with 5-year follow-up. J Clin Oncol 12:1394-1401, 1994 [DOI] [PubMed] [Google Scholar]
- 21.Sonpavde G, Goldman BH, Speights VO, et al. : Quality of pathologic response and surgery correlate with survival for patients with completely resected bladder cancer after neoadjuvant chemotherapy. Cancer 115:4104-4109, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfister C, Gravis G, Fléchon A, et al. : Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin, or gemcitabine and cisplatin as perioperative chemotherapy for patients with muscle-invasive bladder cancer. Analysis of the GETUG/AFU V05 VESPER trial secondary endpoints: Chemotherapy toxicity and pathological responses. Eur Urol 79:214-221, 2021 [DOI] [PubMed] [Google Scholar]
- 23.Porter MP, Kerrigan MC, Donato BMK, et al. : Patterns of use of systemic chemotherapy for Medicare beneficiaries with urothelial bladder cancer. Urol Oncol 29:252-258, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg JE, Hoffman-Censits J, Powles T, et al. : Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 387:1909-1920, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharma P, Retz M, Siefker-Radtke A, et al. : Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol 18:312-322, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Bellmunt J, de Wit R, Vaughn DJ, et al. : Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 376:1015-1026, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel MR, Ellerton J, Infante JR, et al. : Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN solid tumor): Pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 19:51-64, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massard C, Gordon MS, Sharma S, et al. : Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 34:3119-3125, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hato SV, Khong A, de Vries IJM, et al. : Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res 20:2831-2837, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Zitvogel L, Apetoh L, Ghiringhelli F, et al. : Immunological aspects of cancer chemotherapy. Nat Rev Immunol 8:59-73, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Suzuki E, Kapoor V, Jassar AS, et al. : Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11:6713-6721, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ramakrishnan R, Assudani D, Nagaraj S, et al. : Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest 120:1111-1124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Necchi A, Anichini A, Raggi D, et al. : Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): An open-label, single-arm, phase II study. J Clin Oncol 36:3353–3360, 2018 [DOI] [PubMed] [Google Scholar]
- 34.Powles T, Kockx M, Rodriguez-Vida A, et al. : Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med 25:1706-1714, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Rozkova D, Horvath R, Bartunkova J, et al. : Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of Toll-like receptors. Clin Immunol 120:260-271, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Powles T, Durán I, van der Heijden MS, et al. : Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 391:748-757, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Edge SB, Compton CC: The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 17:1471-1474, 2010 [DOI] [PubMed] [Google Scholar]
- 38.National Comprehensive Cancer Network . Bladder Cancer (Version 3.2021). https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf [Google Scholar]
- 39.Galsky MD, Pal SK, Chowdhury S, et al. : Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer 121:2586-2593, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Alva A, Csőszi T, Ozguroglu M, et al. : LBA23 pembrolizumab (P) combined with chemotherapy (C) vs C alone as first-line (1L) therapy for advanced urothelial carcinoma (UC): KEYNOTE-361. Ann Oncol 31:S1155, 2020 [Google Scholar]
- 41.Galsky MD, Arija JÁA, Bamias A, et al. : Atezolizumab with or without chemotherapy in metastatic urothelial cancer (IMvigor130): A multicentre, randomised, placebo-controlled phase 3 trial. Lancet 395:1547-1557, 2020 [DOI] [PubMed] [Google Scholar]
- 42.Rose TL, Harrison MR, Deal AM, et al. : Phase II study of gemcitabine and split-dose cisplatin plus pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive bladder cancer. J Clin Oncol 39:3140-3148, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta S, Sonpavde GP, Weight C, et al. : Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. J Clin Oncol 38, 2020. (suppl 6; abstr 439) [Google Scholar]
- 44.Cathomas R, Rothschild SI, Hayoz S, et al. : Safety and efficacy of perioperative cisplatin/gemcitabine (cis/gem) and durvalumab (durva) for operable muscle-invasive urothelial carcinoma (MIUC): Sakk 06/17. J Clin Oncol 39, 2021. (suppl 6; abstr 430) [Google Scholar]
- 45.Hoimes CJ, Adra N, Fleming MT, et al. : Phase Ib/II neoadjuvant (N-) pembrolizumab (P) and chemotherapy for locally advanced urothelial cancer (laUC): Final results from the cisplatin (C)- eligible cohort of HCRN GU14-188. J Clin Oncol 38, 2020. (suppl 15; abstr 5047) [Google Scholar]
- 46.Shu CA, Gainor JF, Awad MM, et al. : Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:786-795, 2020 [DOI] [PubMed] [Google Scholar]
- 47.Socinski MA, Jotte RM, Cappuzzo F, et al. : Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378:2288-2301, 2018 [DOI] [PubMed] [Google Scholar]
- 48.Schmid P, Adams S, Rugo HS, et al. : Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108-2121, 2018 [DOI] [PubMed] [Google Scholar]
- 49.Horn L, Mansfield AS, Szczęsna A, et al. : First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379:2220-2229, 2018 [DOI] [PubMed] [Google Scholar]
- 50.Moore RA, Adel N, Riedel E, et al. : High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J Clin Oncol 29:3466-3473, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Provencio M, Nadal E, Insa A, et al. : Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol 21:1413-1422, 2020 [DOI] [PubMed] [Google Scholar]
- 52.Rothschild SI, Zippelius A, Eboulet EI, et al. : Sakk 16/14: Durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer—A multicenter single-arm phase II trial. J Clin Oncol 39:2872-2880, 2021 [DOI] [PubMed] [Google Scholar]
- 53.Bandini M, Gibb EA, Gallina A, et al. : Does the administration of preoperative pembrolizumab lead to sustained remission post-cystectomy? First survival outcomes from the PURE-01 study☆. Ann Oncol 31:1755-1763, 2020 [DOI] [PubMed] [Google Scholar]
- 54.Siefker-Radtke AO, Steinberg GD, Bedke J, et al. : Phase III study of perioperative pembrolizumab (pembro) plus neoadjuvant chemotherapy (chemo) versus placebo plus neoadjuvant chemo in cisplatin-eligible patients (pts) with muscle-invasive bladder cancer (MIBC): KEYNOTE-866. J Clin Oncol 38, 2020. (suppl 6; abstr TPS599) [Google Scholar]
- 55.Sonpavde G, Necchi A, Gupta S, et al. : ENERGIZE: A phase III study of neoadjuvant chemotherapy alone or with nivolumab with/without linrodostat mesylate for muscle-invasive bladder cancer. Future Oncol 16:4359-4368, 2019 [DOI] [PubMed] [Google Scholar]
- 56.Powles T, Meeks JJ, Galsky MD, et al. : A phase III, randomized, open-label, multicenter, global study of efficacy and safety of durvalumab in combination with gemcitabine plus cisplatin for neoadjuvant treatment followed by durvalumab alone for adjuvant treatment in muscle-invasive bladder cancer (NIAGARA). J Clin Oncol 39, 2021. (suppl 6; abstr TPS505) [Google Scholar]
- 57.Rosenberg JE, O’Donnell PH, Balar AV, et al. : Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 37:2592-2600, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powles T, Rosenberg JE, Sonpavde GP, et al. : Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 384:1125-1135, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galsky MD, Necchi A, Shore ND, et al. : KEYNOTE-905/EV-303: Perioperative pembrolizumab or pembrolizumab plus enfortumab vedotin (EV) and cystectomy compared to cystectomy alone in cisplatin-ineligible patients with muscle-invasive bladder cancer (MIBC). J Clin Oncol 39, 2021. (suppl 6; abstr TPS507) [Google Scholar]
- 60.A Phase 3, Randomized, Open-Label Study to Evaluate Perioperative Enfortumab Vedotin Plus Pembrolizumab (MK-3475) Versus Neoadjuvant Gemcitabine and Cisplatin in Cisplatin-Eligible Participants With Muscle-Invasive Bladder Cancer (KEYNOTE-B15/EV-304). clinicaltrials.gov, 2021 [Google Scholar]
- 61.Geynisman DM, Abbosh P, Ross EA, et al. : A phase II trial of risk enabled therapy after initiating neoadjuvant chemotherapy for bladder cancer (RETAIN BLADDER): Interim analysis. J Clin Oncol 39, 2021. (suppl 6; abstr 397) [Google Scholar]
- 62.Galsky MD, Daneshmand S, Chan KG, et al. : Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle- invasive bladder cancer (MIBC): HCRN GU 16-257. J Clin Oncol 39, 2021. (suppl 15; abstr 4503) [Google Scholar]